Abstract
Nucleoside analogue (NA) therapy for chronic hepatitis B (CHB) is associated with improved clinical outcomes, but usually requires long‐term use. Whether treatment can be safely withdrawn and the factors associated with post‐withdrawal outcome are not well defined. To assess long‐term outcomes after stopping antiviral therapy, patients with hepatitis B e antigen (HBeAg)–negative CHB who had received antiviral therapy for 4 or more years with hepatitis B virus (HBV) DNA (≤100 IU/mL) were prospectively withdrawn from antiviral therapy and monitored monthly for the initial 6 months and every 3 months thereafter. Those with clinical relapse were retreated according to severity of relapse. Fifteen patients were withdrawn from lamivudine (4), adefovir (5), or a combination of the two (6) after a mean treatment duration of 8.4 years. The mean age was 45 years, 13 were male, and 8 were initially HBeAg‐positive before treatment. After a mean follow‐up of 6.6 years, outcomes differed by pretreatment HBeAg status. All patients who were HBeAg+ before treatment experienced virological relapse (8 of 8); 6 of 8 experienced clinical relapse; 4 of 8 had ALT flares; 5 of 8 required re‐initiation of treatment, one of whom cleared hepatitis B surface antigen (HBsAg); and 3 of 8 remained off treatment, one of whom cleared HBsAg. In contrast, 4 of 7 patients who were HBeAg‐negative before treatment experienced virological relapse, 3 of 7 experienced clinical relapse, and 1 of 7 had an alanine aminotransferase (ALT) flare. None restarted treatment, and 4 of 7 cleared HBsAg. Low pre‐withdrawal HBsAg level was predictive of HBsAg loss. Conclusion: NA therapy can be safely withdrawn with long‐term remission and high rates of HBsAg loss in most HBeAg‐negative patients without cirrhosis. Patients who were initially HBeAg+ should not be withdrawn from treatment, because clinical relapse was frequent and often severe.
Abbreviations
- ALT
alanine aminotransferase
- anti‐HBe
antibody to HBeAg
- anti‐HBs
antibody to HBsAg
- AST
aspartate aminotransferase
- CHB
chronic hepatitis B
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- NA
nucleos(t)ide analgue
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NIH
National Institutes of Health
- NK
natural killer
- qHBsAg
quantitative HBsAg
- sTRAIL
soluble TRAIL
- ULN
upper limit of normal
- VCTE
vibration‐controlled transient elastography
The development of nucleos(t)ide analogues (NAs) has revolutionized treatment of chronic hepatitis B (CHB). These agents are safe, well‐tolerated, potent inhibitors of viral replication. Prolonged viral suppression results in a reduced incidence of cirrhosis, liver decompensation, hepatocellular carcinoma, and mortality.( 1 , 2 , 3 ) However, NAs are not curative, as they cannot eradicate covalently closed circular DNA, the viral template for transcription. Thus, virological relapse is observed in most patients if NAs are stopped.( 4 , 5 ) Consequently, treatment is administered long‐term, if not indefinitely. Long‐term use is associated with lower adherence rates, safety concerns, particularly renal and bone toxicity, and cost.
Current treatment guidelines suggest that NAs may be stopped among hepatitis B e antigen (HBeAg)–positive patients after HBeAg seroconversion, provided that patients receive a minimum of 1 year of consolidation therapy. Asian and European guidelines suggest that treatment may be stopped among HBeAg‐negative patients after a minimum of 2 or 3 years of virological suppression, respectively, but these recommendations are based on limited data. Hepatitis B surface antigen (HBsAg) loss is a more robust treatment endpoint but is infrequently achieved with NA therapy.( 6 ) Interest in stopping antiviral therapy among HBeAg‐negative patients was rekindled after a high rate of HBsAg loss, and sustained virological suppression was observed after stopping adefovir therapy.( 7 )
We conducted a prospective study of treatment withdrawal with predefined rules for re‐initiation of treatment with the primary goal of assessing rates of long‐term virological remission and clinical relapse after stopping oral antiviral therapy in patients with CHB.
Patients and Methods
Patients
This was a single‐arm, pilot study conducted at the Clinical Center of the National Institutes of Health (Bethesda, MD). Consecutive adult patients with CHB receiving antiviral therapy for 4 years were screened for enrollment into the study. All patients had to be on lamivudine, adefovir, or the combination, and be HBeAg‐negative at the time of treatment withdrawal. Patients who were HBeAg‐positive before treatment and lost HBeAg had to have received a minimum of 1 year of consolidation therapy. Patients were required to have either alanine aminotransferase (ALT) or aspartate aminotransferase (AST) within the upper limit of normal (ULN), HBV DNA ≤ 100 IU/mL for at least 4 years before study enrollment, be willing to reinitiate therapy if necessary, and provide informed consent. Patients were excluded if they had biopsy‐proven cirrhosis or, in the absence of histology, any three of the following variables: platelet count ≤100,000/mm3, reversal of ALT/AST ratio, total bilirubin >2.0 mg/dL, splenomegaly on ultrasound, and evidence of portal hypertension on endoscopy. Additional exclusion criteria were decompensated liver disease, co‐infection with hepatitis C virus (HCV), hepatitis D virus (HDV) and human immunodeficiency virus (HIV), renal insufficiency defined as a serum creatinine >1.5 mg/dL or an estimated glomerular filtration rate ≤50 mls/minute using the Cockroft and Gault formula, and prior or current therapy with entecavir, tenofovir, or tenofovir‐emtricitabine.
Study Design
Upon enrollment, each patient underwent a complete history and physical examination. Laboratory testing included hepatic panel, HBsAg, HBeAg, antibody to HBeAg (anti‐HBe), HBV DNA, antibody to HCV, antibody to HDV, and antibody to HIV. An abdominal ultrasound was obtained if not done in the 12 months preceding enrollment. Each patient underwent vibration‐controlled transient elastography (VCTE) at 6‐month intervals (more frequently in case of a flare). Patients were followed for a minimum of 4 years. The monitoring schedule was every 4 weeks for the first 24 weeks and then every 12 weeks thereafter. Patients were seen more frequently in the event of an ALT flare. After 4 patients experienced severe ALT flares (defined as an ALT > 20 ULN), all of whom were HBeAg‐negative (anti‐HBe‐negative), the protocol was amended to exclude such patients from withdrawal of antiviral therapy. The study was approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and registered under clinicaltrials.gov as NCT01581554. All subjects provided written, informed consent.
Criteria for Restarting Antiviral Therapy
Antiviral therapy was restarted in patients with a virological relapse at defined time‐points according to the following algorithm:
Immediately after virological relapse in patients with serum ALT above 20 ULN (>800 U/L) confirmed a minimum of 1 week apart, or total bilirubin ≥2.0 mg/dL, or any clinical decompensation (encephalopathy, ascites, variceal bleed).
Within 3 months after virological relapse in patients with HBV DNA persistently ≥1,000 IU/mL and serum ALT persistently above 5 ULN (>200 U/L). Patients were followed at 2‐week intervals until resolution of flare.
Within 6 months after virological relapse in patients with HBV DNA ≥ 1,000 IU/mL confirmed on three occasions a minimum of 1 month apart and serum ALT above 1.5 ULN (>60 U/L) confirmed on three occasions a minimum of 1 month apart.
Patients meeting restart rules were offered enrollment into a randomized clinical trial at the Clinical Center to receive therapy with either tenofovir versus tenofovir‐emtricitabine (Clinical trials.gov NCT00524173).
Primary Endpoint
The primary endpoint of the study was sustained suppression of HBV DNA ≤ 1,000 IU/mL and serum ALT < 1.5X ULN 1 year after stopping therapy.
Study Definitions
HBeAg reversion: reappearance of HBeAg in the serum of a HBeAg‐negative subject, on two occasions a minimum of 1 month apart.
HBsAg loss: undetectable HBsAg in the serum on two occasions a minimum of 1 month apart.
Virological relapse: HBV DNA level > 1,000 IU/mL confirmed on repeat testing a minimum of 1 month apart.
Clinical relapse: Virological relapse and ALT level > 1.5 ULN confirmed on repeat testing a minimum of 1 month apart.
Biochemical flare: ALT level > 10 ULN (400 U/L) at any time‐point after withdrawal of antiviral therapy.
Assays
HBV DNA was tested by COBAS TaqMan HBV real‐time polymerase chain reaction (Roche Molecular Systems, Inc., Branchburg, NJ), lower limit of quantification = 20 IU/mL. HBV serology was tested using the VITROS ECi/ECiQ Immunodiagnostic System (Ortho Clinical Diagnostics, Raritan, NJ). Quantitative HBsAg (qHBsAg) levels were tested using the Abbott Architect HBsAg QT assay (Abbott Diagnostics, Abbott Park, IL), and HBV‐RNA levels were tested using the HBV pgRNA Research Assay (Abbott Diagnostics). Soluble TRAIL (sTRAIL) and soluble CD14 (sCD14) were measured in serum using the human TRAIL/TNSF10 and human CD14 Quantikine ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The intra‐assay and interassay percent coefficient of variability were 1.3 and 3.9 for sTRAIL and 2.3 and 2.5 for sCD14.
Statistical Analysis
Data were summarized using means with ranges for continuous variables and proportions for categorical variables. Wilcoxon rank sum test was used to compare two groups of interest for continuous outcomes, and either chi‐squared or exact test was used for two‐group comparison for categorical variables. Kaplan‐Meier life table analysis was used to report rates of long‐term remission. Differences between those with a maintained response and failure after withdrawal was assessed by log rank test. All analyses were two‐tailed tests based on a significance level of 0.05 and conducted using SAS Version 9.4 (SAS Institute, Cary, NC). Correlations between sTRAIL and sCD14 levels with ALT and HBsAg levels were performed using R package “rmcorr.” ( 8 ) Student t test was used to compare means.
Results
Baseline Characteristics
Seventy‐three patients with CHB on antiviral therapy for 4 or more years were prescreened for the study. Fifty‐four were ineligible, 4 declined, and 15 agreed to enroll (Supporting Fig. S1). Baseline demographics are given in Table 1. Before treatment, 8 patients were HBeAg‐positive and 7 were HBeAg‐negative. Before withdrawal, there were no significant differences in baseline demographic and clinical features between the two groups. The mean duration of follow‐up for the cohort was 6.6 years. No patient had cirrhosis at time‐of‐treatment withdrawal.
TABLE 1.
Demographics and Clinical Characteristics at Time‐of‐Treatment Withdrawal
| Demographics Means, (%) | HBeAg‐Positive, n = 8 | HBeAg‐Negative, n = 7 |
|---|---|---|
| Age (range, years) | 41 (29‐62) | 49 (31‐64) |
| Male gender | 7 (88) | 6 (86) |
| Race | ||
| Caucasian | 3 (38) | 0 (0) |
| Asian | 5 (62) | 6 (86) |
| African American | 0 (0) | 1 (14) |
| Body mass index (kg/m2) | 24.4 (17‐31) | 28 (22‐45) |
| Clinical characteristics (%) | ||
| Duration of Rx (years) | 8.2 (6.1‐10.0) | 8.7 (4.8‐12.8) |
| Rx* | ||
| Lamivudine | 0 (0) | 4 (57) |
| Adefovir | 3 (38) | 1 (14) |
| Lamivudine and adefovir | 5 (62) | 2 (29) |
| Alkaline phosphatase (range, U/L) | 62 (39‐87) | 68 (51‐102) |
| ALT (range, U/L) | 45 (19‐74) | 41 (24‐74) |
| AST (range, U/L) | 23 (13‐33) | 24 (8‐52) |
| Total bilirubin (range, mg/dL) | 0.6 (0.2‐1) | 0.6 (0.4‐0.8) |
| Albumin (range, g/dL) | 4.4 (4‐5) | 4.1 (3.4‐4.6) |
| International normalized ratio | 1.0 (0.9‐1.1) | 1.1 (1‐1.2) |
| Platelets (range, K/uL) | 213 (161‐311) | 200 (161‐247) |
| HBV DNA undetectable | 8 (100) | 6 (86) |
| qHBsAg (range, IU/mL)* | 785 (0.3‐2,882) | 1924 (10‐10,251) |
| HBV RNA (range, log U/mL)* | 0.8 (0‐2.6) | 0.5 (0‐2) |
| Histology activity index score † | 2 (1‐5) | 2.8 (1‐5) |
| Ishak fibrosis score † | 0.7 (0‐2) | 1.3 (0‐3) |
Week 4 pre‐withdrawal serum was used for testing qHBsAg and HBV RNA in 1 patient.
n = 13. Two patients did not undergo baseline biopsy.
Primary Endpoint
One year off therapy, 6 of 15 (40%) patients met the primary endpoint of suppression of HBV DNA ≤ 1,000 IU/mL and serum ALT < 1.5 ULN, 5 of 15 (33%) patients met protocol criteria to restart therapy, 4 (80%) of whom experienced a clinically significant hepatitis flare, 2 of 15 (13%) had an elevated HBV DNA > 1,000 IU/mL (1,260 and 3,220 IU/mL) but normal ALT levels, and 1 of 15 (7%) had an elevated serum ALT level (115 U/L) but HBV DNA = 221 IU/mL, and 1 of 15 (7%) withdrew consent to participate and restarted treatment at week 45 off treatment, at which time HBV DNA was 29 IU/mL and serum ALT was 38 U/L (Fig. 1). In follow‐up, 9 of 14 (64%) met the primary endpoint.
FIG. 1.
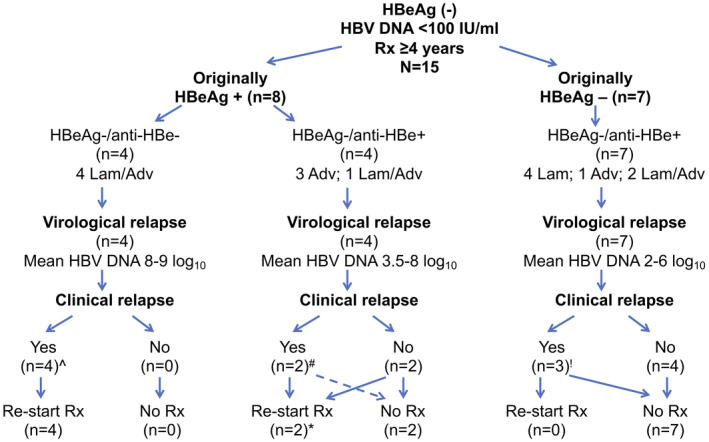
Outcome after stopping treatment among HBeAg‐positive and HBeAg‐negative patients. *One patient withdrew consent to participate at post‐withdrawal week 45 and restarted antiviral therapy. ^Three patients met the definition for an ALT flare. #One patient met the definition for an ALT flare. !One patient met the definition for an ALT flare.
Clinical Course After Stopping Antiviral Therapy
Patients Who Were HBeAg‐positive Before Treatment
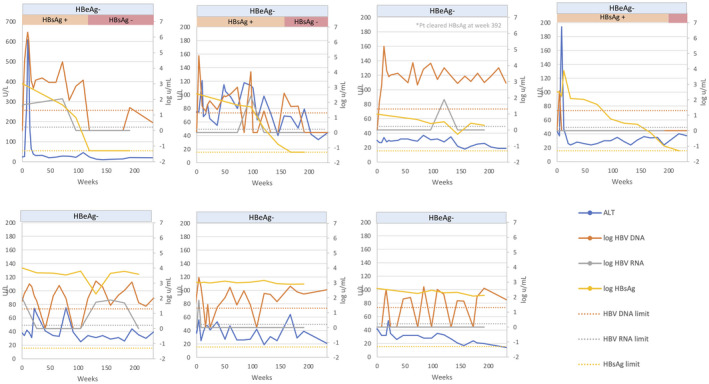
The 8 patients who were HBeAg‐positive before treatment received therapy for a mean of 8.2 (6‐10) years. Consolidation therapy was administered for a mean of 5.4 (2.8‐8.2) years after HBeAg loss. Four patients were anti‐HBe‐positive and 4 were anti‐HBe‐negative at the time of withdrawal of therapy. After stopping therapy, virological relapse (HBV DNA > 103 IU/mL) was observed in all patients, and HBV DNA exceeded a level > 105 IU/mL in 5 of 8 (63%) and >107 IU/mL in 4 of 8 (50%). Similarly, ALT levels became elevated in 8 of 8 (100%), were >2 ULN (80 U/L) in 6 of 8 (75%), were >5 ULN (200 U/L) in 6 of 8 (75%), and ALT flares (>10 ULN [400 U/L]) were observed in 4 of 8 (50%) within the first year of follow‐up. The course of patients who were HBeAg‐negative/anti‐HBe‐negative at time of withdrawal differed compared to those who were HBeAg‐negative/anti‐HBe‐positive. All HBeAg‐negative/anti‐HBe‐negative patients experienced HBeAg reversion within 2 months of withdrawal, with marked elevations in serum HBV DNA (mean peak HBV DNA = 8.7 [7.8‐9.2] log10 IU/mL) and ALT (mean peak ALT = 1,771 [366‐3,376] U/L) levels (Figs. 1 and 2), and a transient rise in hepatic stiffness = 14 (5.6‐31) kPa by VCTE. These 4 patients had lost HBeAg a mean of 7.4 (6‐8) years before withdrawal and received consolidation treatment for a mean of 5.1 (4.3‐5.9) years. One patient became jaundiced with peak bilirubin of 8.7 mg/dL; none experienced hepatic decompensation. Two were randomized to start tenofovir, and 2 to tenofovir‐emtricitabine. Two patients delayed restarting treatment. Nonetheless, upon restarting therapy, all responded with loss of HBeAg (mean = 7 months, range = 2‐12 months), suppression of HBV DNA to undetectable, and normalization of ALT level. VCTE scores returned to baseline following resolution of the hepatitis flare. HBsAg loss with development of anti‐HBs was observed in 1 patient 9 months after restarting treatment.
FIG. 2.
Changes in serum ALT, HBV DNA, HBV RNA, and qHBsAg levels after stopping treatment among patients who were HBeAg‐positive before treatment. Top row: Patients who were HBeAg‐negative/anti‐HBe‐negative at time of withdrawal. Bottom row: Patients who were HBeAg‐negative/anti‐HBe‐positive at time of withdrawal.
In contrast, among the 4 HBeAg‐negative/anti‐HBe‐positive patients, the mean peak HBV‐DNA level was 4.9 (3.5‐6.8) log10 IU/mL, and the mean peak ALT was 370 (70‐1,029) U/L after stopping treatment (Figs. 1 and 2). A single patient experienced HBeAg reversion accompanied by a withdrawal flare with a peak ALT of 1,029 U/L and peak HBV DNA of 6.8 log10 IU/mL 4 months after stopping treatment. This patient was restarted on tenofovir‐emtricitabine and responded with normalization of serum ALT and undetectable HBV DNA followed by loss of HBeAg 22 months after starting treatment. The other 3 patients experienced mild elevations in serum ALT and HBV‐DNA levels during follow‐up, but none met the protocol criteria for re‐initiation of treatment. One patient experienced HBsAg loss without development of anti‐HBs 5.9 years after stopping treatment. One patient remains in remission 7.4 years off therapy, with the last ALT being 14 U/L and last available HBV DNA being 319 IU/mL. The other patient wished to restart treatment despite not meeting protocol criteria (HBV DNA was 29 IU/mL and serum ALT was 38 U/L) at week 45 off treatment and withdrew consent to participate. This patient was excluded from subsequent analyses.
Patients Who Were HBeAg‐negative Before Treatment
The 7 HBeAg‐negative patients were on therapy for 8.7 (4.8‐12.8) years before withdrawal of treatment. Upon stopping therapy, HBV‐DNA levels rose transiently in all patients with a mean peak HBV DNA of 4 (2.5‐6.3) log10 IU/mL; virological relapse (HBV DNA > 103 IU/mL) was noted in 4 of 7 (57%), HBV DNA exceeded >105 IU/mL in 3 of 7 (43%) and >107 IU/mL in 0 of 7 (0%). Similarly, ALT levels became elevated in most patients: 6 of 7 (86%) with a mean peak ALT of 163 (34‐609) U/L. Serum ALT was >2 ULN (80 U/L) in 3 of 7 (43%), >5 ULN (200 U/L) in 1 of 7 (14%) and an ALT flare (>10 ULN [400 U/L]) was observed in 1 of 7 (14%) within the first year of follow‐up. This latter patient had a peak ALT of 609 U/L and HBV DNA of 6.3 log10 IU/mL. The flare was transient and resolved rapidly within 1.5 months. The 7 patients were followed for a mean of 6.3 (4.5‐7.9) years. None experienced HBeAg reversion. Intermittent, transient elevations in ALT and HBV‐DNA levels were noted (Figs. 1 and 3), but none met the protocol criteria for restarting antiviral therapy. Four of 7 (57%) cleared HBsAg at 2.3, 3.2, 4.2, and 7.5 years after treatment withdrawal. One patient died of non‐HBV‐related causes (complications of diabetes and renal failure).
FIG. 3.
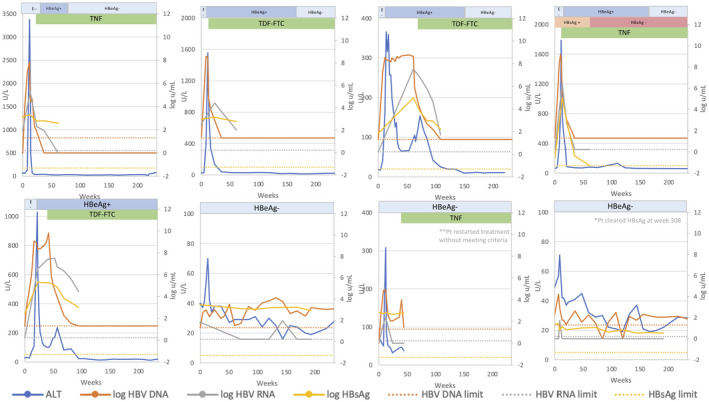
Changes in serum ALT, HBV DNA, HBV RNA, and qHBsAg levels after stopping treatment among patients who were HBeAg‐negative before treatment.
Predictors of HBsAg Loss
The qHBsAg levels were significantly higher at baseline among patients who were initially HBeAg‐negative before treatment (1,924 [9.6‐10,251] IU/mL compared with those who were initially HBeAg‐positive [785 (0.3‐2,882) IU/mL]). The annual decline in qHBsAg levels among patients not requiring retreatment was similar between patients initially HBeAg‐positive (n = 2) and those initially HBeAg‐negative (n = 7): 0.2 versus 0.3 log10 IU/mL/per year, respectively. Time to HBsAg loss is shown in the Kaplan‐Meier plot (Fig. 4) and kinetics of HBsAg (Fig. 5A). HBsAg loss was confirmed in all patients over a mean follow‐up of 3 (0.4‐6.5) years; anti‐HBs developed in 4 of 6 (67%) of patients.
FIG. 4.
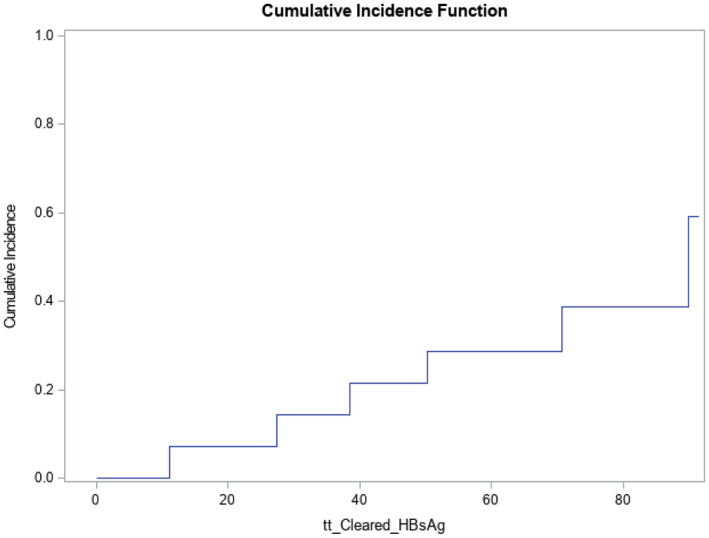
Kaplan‐Meier graph of time to HBsAg loss.
FIG. 5.
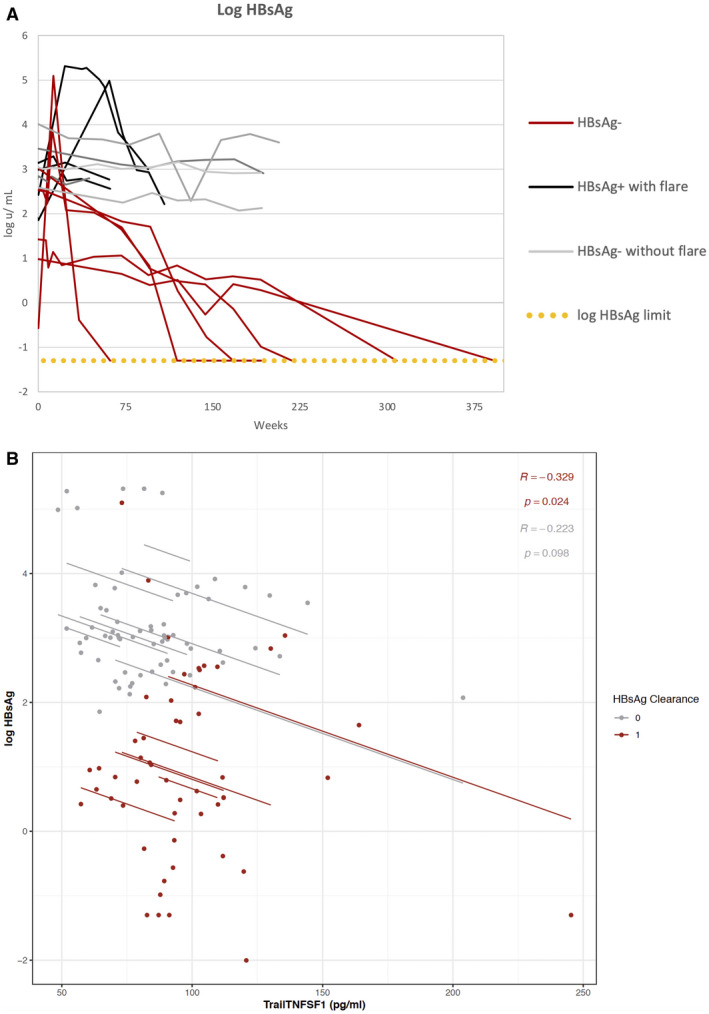
(A) Kinetics of qHBsAg levels after stopping treatment among patients with HBsAg loss with (red line) and without ALT flares (blue line) and without HBsAg loss with (black line) and without ALT flares (gray line). (B) Log10 HBsAg IU/mL levels by sTRAIL levels (pg/mL) among patients with (red line) and without HBsAg loss (gray line).
A low qHBsAg at treatment withdrawal was the only predictor of HBsAg loss. Mean qHBsAg at the time of treatment withdrawal among patients who lost HBsAg was 297 IU/mL compared with 1,991 IU/mL among those who did not lose HBsAg (P = 0.04; Table 2). Neither mean HBV‐RNA level nor the proportion with unquantifiable HBV RNA were significantly different in those with and without HBsAg loss (Fig. 6).
TABLE 2.
Demographic and Clinical Characteristics at Time‐of‐Treatment Withdrawal Between Patients With and Without HBsAg Loss
| Demographics | HBsAg‐Negative, n = 6 | HBsAg‐Positive, n = 9 | P Value |
|---|---|---|---|
| Age (years) | 45 (38‐59) | 45 (29‐64) | 0.72 |
| Male gender (%) | 6 (100) | 7 (78) | 0.49 |
| Race (%) | 1.00 | ||
| Caucasian | 1 (17) | 2 (22) | |
| Asian | 5 (83) | 6 (67) | |
| African American | 0 (0) | 1 (11) | |
| GT | 0.54 | ||
| A | 2 (33) | 2 (22) | |
| B | 1 (17) | 0 (0) | |
| C | 1 (17) | 5 (56) | |
| D | 1 (17) | 1 (11) | |
| Missing | 1 (17) | 1 (11) | |
| Mean body mass index (kg/m2) | 25.4 (22‐28.3) | 25.8 (23.3‐25.9) | 0.72 |
| Clinical characteristics | |||
| Duration of Rx (years) | 8.13 (4.8‐10.8) | 8.6 (6.1‐12.78) | 0.91 |
| Rx (%) | 0.46 | ||
| Lamivudine | 3 (50) | 1 (11) | |
| Adefovir | 1 (17) | 3 (33) | |
| Lamivudine and adefovir | 2 (33) | 5 (56) | |
| Alkaline phosphatase (U/L) | 66.7 (51‐102) | 63 (39‐87) | 0.95 |
| ALT (U/L) | 42 (30‐65) | 38 (28‐42) | 0.56 |
| AST U/L) | 23 (8‐33) | 24 (13‐52) | |
| 0.68 | |||
| Total bilirubin (mg/dL) | 0.63 (0.4‐0.8) | 0.58 (0.2‐1) | 0.59 |
| Albumin (g/dL) | 4.3 (4‐4.6) | 4.2 (3.4‐5) | 1.00 |
| International normalized ratio | 1.0 (0.95‐1.2) | 1.0 (0.91‐1.2) | 0.59 |
| Platelets (K/uL) | 202 (116‐228) | 210 (161‐311) | 0.91 |
| HBeAg‐positive at Rx initiation (%) | 2 (33) | 6 (67) | 0.31 |
| Anti‐HBe‐positive (%) | 5 (83) | 6 (67) | 0.6 |
| HBV DNA undetectable | 6 (100) | 8 (89) | |
| qHBsAg (IU/mL) | 296.47 (0.3‐1,036.1) | 1990.7 (71.5‐10,250.9) | 0.04 |
| HBV RNA (log U/mL) | 0.3 (0‐1.7) | 0.9 (0‐2.6) | 0.18 |
| Histology activity index score | 2.2 (1‐5)* | 2.5 (1‐5) † | 0.64 |
| Ishak fibrosis score | 1.0 (0‐2)* | 1.0 (0‐3) † | 0.81 |
One patient did not undergo liver biopsy.
One patient did not undergo liver biopsy.
Abbreviation: GT, genotype.
FIG. 6.
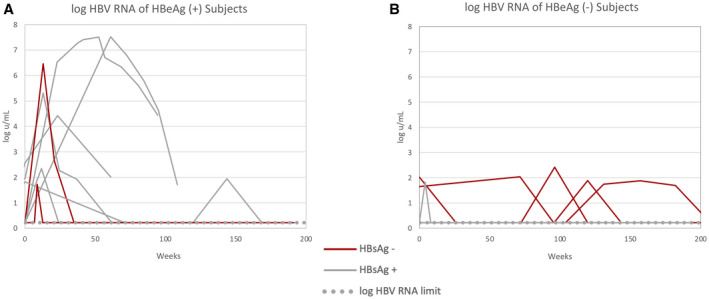
(A) Kinetics of HBV‐RNA levels after stopping treatment among patients who were HBeAg‐positive before treatment. (B) Kinetics of HBV‐RNA levels after stopping treatment among patients who were HBeAg‐negative before treatment.
Two cytokines representative of natural killer (NK)–cell activation (sTRAIL and macrophages/monocytes activation, sCD14) were assayed to assess the role of host immune response with HBsAg loss. sTRAIL and sCD14 were measured before withdrawal and at 24‐48‐week intervals thereafter for the duration of patient follow‐up depending on sample availability. For patients who restarted treatment, sTRAIL and sCD14 levels were measured until 24 weeks after re‐initiation of therapy. sTRAIL and sCD14 were assayed in a mean of 10 samples per patient (range 6‐19). Among all patients, there was a weak, negative correlation between sTRAIL with HBsAg levels (R = 0.27; P = 0.006). The association was significant among patients who cleared HBsAg (R = 0.33; P = 0.024) but not among those without HBsAg loss (R = 0.22; P = 0.1) (Fig. 5B). In addition, the association was significant among patients initially HBeAg‐positive before treatment (R = 0.45; P = 0.003) but not among those who were HBeAg‐negative (R = 0.2; P = 0.12). sTRAIL levels before stopping treatment were not different between those with and without HBsAg loss: 91 versus 84 pg/mL; P = 0.48. There was no correlation between sCD14 with HBsAg levels (R = 0.05; P = 0.65).
Predictors of ALT Flares
Five of 15 (33%) patients experienced a protocol defined ALT flare, (ALT ≥ 10 ULN). ALT flares were accompanied by a transient increase in VCTE from a pre‐withdrawal mean of 4.7 KPa to a mean peak of 13 KPa during the height of the ALT flare, which then declined to 6.7 KPa following resolution of the flare. The mean VCTE scores obtained on the day of withdrawal among those with a protocol defined flare was 4.7 KPa compared with 5.3 KPa (P = not significant), among those without a flare. ALT flares were more common among Caucasians and those with HBV genotype A. No other demographic or clinical factor was associated with ALT flares (Supporting Table S1). Neither mean HBV‐RNA level nor the proportion with unquantifiable HBV RNA were significantly different in those with and without an ALT flare.
The association between sTRAIL and sCD14 levels with ALT flares was examined. There was no correlation between sTRAIL (R = 0.12; P = 0.17) and sCD14 (R = 0.03; P = 0.7) with serum ALT levels. Moreover, sTRAIL and sCD14 levels were not significantly different before withdrawal of antiviral therapy among patients with and without an ALT flare (sTRAIL, 100.7 vs. 85.6 pg/mL, P = 0.3; and sCD14, 2.8 vs. 2.6 µg/mL, P = 0.6; respectively).
Discussion
Although a number of studies have investigated stopping NAs, only a few have been prospective but with limited follow‐up. A systematic review of 22 studies reported that at 1 year off therapy, virological relapse (HBV DNA > 2,000 IU/mL) occurred in about 70% of patients, clinical relapse (HBV DNA > 2,000 IU/mL and ALT elevation) occurred in about 50% of patients, and about 40% of patients required retreatment.( 9 ) Our findings yielded similar results to other prospective studies, which have reported that 55%‐74% of selected patients can remain off treatment but the frequency of HBsAg loss in our study was substantially higher (40%).( 7 , 10 , 11 , 12 , 13 , 14 ) Taken together, these data suggest that a sizable proportion of patients can remain off treatment, but whether clinical outcomes will differ between those who remain on treatment remains unknown.
In this study, pretreatment HBeAg status was associated with markedly different outcomes after withdrawal of treatment. Patients originally HBeAg‐negative had a lower incidence of ALT flares, higher rates of HBsAg loss, and none required retreatment compared with those originally HBeAg‐positive, despite similar treatment durations. This observation suggests that NA‐related HBeAg loss is not a durable endpoint. A potentially interesting finding was that patients who were anti‐HBe‐negative at treatment withdrawal experienced shorter time to HBeAg reversion and more severe ALT flares, suggesting a role for anti‐HBe in immune control. However, prior studies demonstrated no role for anti‐HBe in virus neutralization.( 15 ) It is possible that HBeAg‐negative/anti‐HBe‐negative patients may still have low‐level HBeAg that cannot be detected by current assays, and that dampens the host immune response.( 16 ) Regardless of the explanation, our data suggest that patients who do not develop anti‐HBe after HBeAg loss should not be withdrawn from therapy.
One of the primary reasons for the renewed interest in stopping antiviral therapy is the potential for HBsAg loss. Whether the rate of HBsAg loss after stopping therapy is higher compared with that of uninterrupted therapy is controversial. A randomized study comparing stopping to continuing therapy reported a significantly higher rate of HBsAg loss in patients who were withdrawn compared with those who continued therapy (19% vs. 0%) after a mean follow‐up of 3 years, whereas another reported no difference in the rate of HBsAg loss of about 1% in those who stopped or continued therapy, but follow‐up was shorter (1.5 years).( 10 , 14 ) The length of follow‐up is the likely explanation for the disparate results. In our study, HBsAg loss was not seen until 2 years off therapy.
Why HBsAg loss may occur at a higher rate after stopping compared with continuing treatment is unclear. It is possible that prolonged viral suppression may reset the immune response, leading to a more robust immune response when HBV replication returns, resulting in HBsAg loss. In support of this, a recent study reported a significant increase in multifunctional HBV core‐specific T‐cell responses after stopping therapy, especially for CD4+ T cells, but the magnitude of induced HBV‐specific T‐cell responses did not correlate with the subsequent HBsAg decline.( 17 ) However, patients who achieved HBsAg loss expressed lower levels of the exhaustion markers killer cell lectin like receptor G1 (KLRG1) and programmed cell death protein 1 (PD‐1) and displayed an increased frequency of Ki‐67+CD38+T cells after stopping NA treatment, suggesting that perhaps a less exhausted phenotype before treatment withdrawal may be associated with HBsAg loss. NK cells may also have a role, as shown in another analysis of the same cohort, reporting that cell cytotoxicity was increased after discontinuation of therapy and correlated with ALT levels, especially in patients with subsequent HBsAg loss.( 18 ) The observation that HBsAg loss is preceded by ALT flares in some but not all patients suggests that the host immune response at the time of withdrawal of treatment may be the critical factor in outcome after withdrawal of treatment. Patients with a high frequency of HBV‐specific T cells at the time of stopping treatment may rapidly control viremia, prevent ALT flares, and already be clearing HBsAg through noncytolytic mechanisms, resulting in a longer time to HBsAg clearance after stopping treatment.( 19 ) In contrast, patients with a more exhausted T‐cell response may have unchecked viral replication after stopping treatment, resulting in interferon/cytokine production and an augmented cytototoxic NK cell response, which leads to an ALT flare and resultant faster HBsAg loss.( 18 ) These are theoretical inferences, and the issue of ALT flares and timing of HBsAg loss after stopping treatment requires further investigation. In the current study, the observation that higher sTRAIL levels were associated with lower HBsAg levels, particularly among those with HBsAg loss, and might suggest that activated NK cells may also play a role in HBsAg clearance through apoptotic or noncytolytic mechanisms. However, HBsAg loss was not preceded by or temporally related to ALT flares. The only predictor of HBsAg loss was the HBsAg level at the time of withdrawal, which has been reported in other studies.( 20 , 21 )
The opportunity for HBsAg loss after treatment withdrawal must be balanced against the risk of ALT flares. In this study, the incidence of protocol‐defined flares (ALT > 10 ULN) was 5 of 15 (33%), a rate similar to that reported in the literature.( 7 , 10 , 13 , 14 ) ALT flares occurred within 2 to 5 months of stopping therapy and were more common among formerly HBeAg‐positive patients who lacked anti‐HBe. Importantly, most patients with elevated ALT levels < 10 ULN generally resolved the hepatitis without the need for antiviral therapy. Having a biomarker to identify patients at risk for ALT flares after stopping NA treatment would be of clinical utility. In this study, HBV‐RNA levels before withdrawal could not distinguish between patients with and without an ALT flare. Similarly, neither sTRAIL nor sCD14 were associated with ALT flares. Moreover, baseline levels were not different in those with and without flares. Another study reported that the frequency of HBV core and polymerase‐specific T cells were important for control viremia after treatment withdrawal, with a higher frequency being associated with a lower occurrence of flares.( 19 ) However, assessment of these immunological markers requires specialized methodology that is not widely available. Interestingly, a panel of five soluble immune markers (SIMs) that included sTRAIL, identified by machine learning, was able to detect early virological relapse (within the initial 24 weeks of stopping therapy).( 22 ) However, the SIM panel was not assessed for its role in predicting clinical relapse. At present, given the absence of markers to identify patients at risk for a withdrawal flare, close observation will be required. We recommend a tiered monitoring approach such as used in this study.
Our study had several limitations. This was an uncontrolled trial and we do not know whether the rate of HBsAg loss would have differed, had patients remained on treatment. The sample size was limited and did not include patients on first‐line agents entecavir and tenofovir because these agents were used as rescue therapy.
In conclusion, this study suggests that patients with HBeAg‐negative CHB who have received antiviral therapy for a minimum of 4 years and who do not have cirrhosis may be safely withdrawn from antiviral therapy and experience high rates of sustained virological suppression and HBsAg loss. Although severe flares occurred, this was more common among patients who were HBeAg‐positive before treatment and anti‐HBe‐negative at the time of withdrawal. Such patients should not discontinue treatment. Future studies are needed to elucidate the mechanism of HBsAg loss after treatment withdrawal.
Supporting information
Fig S1
Table S1
Acknowledgment
The authors thank the patients for their participation in this study. They also thank the nursing staff of the National Institutes of Health Clinical Center Outpatient Clinic‐9 for their efforts in conducting the study.
Supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Potential conflict of interest: Dr. Cloherty is employed by Abbott. Dr. Gara advises Gilead. He is on the speakers’ bureau for Abbvie.
References
- 1. Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5‐year open‐label follow‐up study. Lancet 2013;381:468‐475. [DOI] [PubMed] [Google Scholar]
- 2. Wong GL, Chan HL, Mak CW, Lee SK‐Y, Ip ZM‐Y, Lam AT‐H, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 2013;58:1537‐1547. [DOI] [PubMed] [Google Scholar]
- 3. Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, van Boemmel F, et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology 2017;66:1444‐1453. [DOI] [PubMed] [Google Scholar]
- 4. Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa‐2a, lamivudine, and the combination for HBeAg‐positive chronic hepatitis B. N Engl J Med 2005;352:2682‐2695. [DOI] [PubMed] [Google Scholar]
- 5. Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, et al. Peginterferon alfa‐2a alone, lamivudine alone, and the two in combination in patients with HBeAg‐negative chronic hepatitis B. N Engl J Med 2004;351:1206‐1217. [DOI] [PubMed] [Google Scholar]
- 6. Alawad AS, Auh S, Suarez D, Ghany MG. Durability of spontaneous and treatment‐related loss of hepatitis B s antigen. Clin Gastroenterol Hepatol 2019;18:700‐709.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg‐negative patients with chronic hepatitis B who stop long‐term treatment with adefovir. Gastroenterology 2012;143:629‐636.e1. [DOI] [PubMed] [Google Scholar]
- 8. Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol 2017;8:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang ML, Liaw YF, Hadziyannis SJ. Systematic review: cessation of long‐term nucleos(t)ide analogue therapy in patients with hepatitis B e antigen‐negative chronic hepatitis B. Aliment Pharmacol Ther 2015;42:243‐257. [DOI] [PubMed] [Google Scholar]
- 10. Berg T, Simon KG, Mauss S, Schott E, Heyne R, Klass DM, et al. Long‐term response after stopping tenofovir disoproxil fumarate in non‐cirrhotic HBeAg‐negative patients—FINITE study. J Hepatol 2017;67:918‐924. [DOI] [PubMed] [Google Scholar]
- 11. Cao J, Chi H, Yu T, Li Z, Hansen BE, Zhang X, et al. Off‐treatment hepatitis B virus (HBV) DNA levels and the prediction of relapse after discontinuation of nucleos(t)ide analogue therapy in patients with chronic hepatitis B: a prospective stop study. J Infect Dis 2017;215:581‐589. [DOI] [PubMed] [Google Scholar]
- 12. Su TH, Yang HC, Tseng TC, Liou J‐M, Liu C‐H, Chen C‐L, et al. Distinct relapse rates and risk predictors after discontinuing tenofovir and entecavir therapy. J Infect Dis 2018;217:1193‐1201. [DOI] [PubMed] [Google Scholar]
- 13. Papatheodoridis GV, Rigopoulou EI, Papatheodoridi M, Zachou K, Xourafas V, Gatselis N, et al. DARING‐B: discontinuation of effective entecavir or tenofovir disoproxil fumarate long‐term therapy before HBsAg loss in non‐cirrhotic HBeAg‐negative chronic hepatitis B. Antivir Ther 2018;23:677‐685. [DOI] [PubMed] [Google Scholar]
- 14. Liem KS, Fung S, Wong DK, Yim C, Noureldin S, Chen J, et al. Limited sustained response after stopping nucleos(t)ide analogues in patients with chronic hepatitis B: results from a randomised controlled trial (Toronto STOP study). Gut 2019;68:2206‐2213. [DOI] [PubMed] [Google Scholar]
- 15. Gerin JL, Wai‐Kuo Shih J, McAuliffe VJ, Purcell RH. Antigens of hepatitis B virus: failure to detect HBeAg on the surfaces of HBsAg forms. J Gen Virol 1978;38:561‐566. [DOI] [PubMed] [Google Scholar]
- 16. Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A 1990;87:6599‐6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rinker F, Zimmer CL, Höner zu Siederdissen C, Manns MP, Kraft ARM, et al. Hepatitis B virus‐specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg‐negative chronic hepatitis B. J Hepatol 2018;69:584‐593. [DOI] [PubMed] [Google Scholar]
- 18. Zimmer CL, Rinker F, Höner zu Siederdissen C, Manns MP, Wedemeyer H, Cornberg M, et al. Increased NK cell function after cessation of long‐term nucleos(t)ide analogue treatment in chronic hepatitis B is associated with liver damage and HBsAg loss. J Infect Dis 2018;217:1656‐1666. [DOI] [PubMed] [Google Scholar]
- 19. Rivino L, Le Bert N, Gill US, Kunasegaran K, Cheng Y, Tan DZM, et al. Hepatitis B virus‐specific T cells associate with viral control upon nucleos(t)ide‐analogue therapy discontinuation. J Clin Invest 2018;128:668‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeng WJ, Chen YC, Chien RN, Sheen IS, Liaw YF. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen‐negative chronic hepatitis B. Hepatology 2018;68:425‐434. [DOI] [PubMed] [Google Scholar]
- 21. Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, Lee CM. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol 2014;61:515‐522. [DOI] [PubMed] [Google Scholar]
- 22. Wubbolding M, Lopez Alfonso JC, Lin CY, Binder S, Falk C, Debarry J, et al. Pilot study using machine learning to identify immune profiles for the prediction of early virological relapse after stopping nucleos(t)ide analogues in HBeAg‐negative CHB. Hepatol Commun 2021;5:97‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1


