Abstarct
Because of its involvement in a wide variety of cardiovascular, metabolic and behavioural functions, the hypothalamus constitutes a potential target for neuromodulation in a number of treatment-refractory conditions. The precise neural substrates and circuitry subserving these responses, however, are poorly characterized to date. We sought to retrospectively explore the acute sequelae of hypothalamic region deep brain stimulation and characterize their neuroanatomical correlates. To this end we studied—at multiple international centres—58 patients (mean age: 68.5 ± 7.9 years, 26 females) suffering from mild Alzheimer’s disease who underwent stimulation of the fornix region between 2007 and 2019. We catalogued the diverse spectrum of acutely induced clinical responses during electrical stimulation and interrogated their neural substrates using volume of tissue activated modelling, voxel-wise mapping, and supervised machine learning techniques. In total 627 acute clinical responses to stimulation—including tachycardia, hypertension, flushing, sweating, warmth, coldness, nausea, phosphenes, and fear—were recorded and catalogued across patients using standard descriptive methods. The most common manifestations during hypothalamic region stimulation were tachycardia (30.9%) and warmth (24.6%) followed by flushing (9.1%) and hypertension (6.9%). Voxel-wise mapping identified distinct, locally separable clusters for all sequelae that could be mapped to specific hypothalamic and extrahypothalamic grey and white matter structures. K-nearest neighbour classification further validated the clinico-anatomical correlates emphasizing the functional importance of identified neural substrates with area under the receiving operating characteristic curves between 0.67 and 0.91. Overall, we were able to localize acute effects of hypothalamic region stimulation to distinct tracts and nuclei within the hypothalamus and the wider diencephalon providing clinico-anatomical insights that may help to guide future neuromodulation work.
Keywords: hypothalamus, deep brain stimulation, autonomic nervous system, Alzheimer’s disease, metabolic diseases
Neudorfer et al. investigate the acute effects of hypothalamic deep brain stimulation. Based on an analysis of 627 recorded autonomic and behavioural responses, they identify symptom-specific, spatially separable clusters providing voxel-wise insights into the clinico-anatomical relationships within this complex region.
Introduction
Autonomic nervous system dysfunction is a common neurological manifestation that may occur in a wide range of cardiovascular, metabolic and behavioural disorders and is characterized by impaired autonomic control and loss of homeostatic variability. Increased sympathetic nerve activity in particular has been associated with increased morbidity and mortality in the developed world.1 For example, it is estimated that neurogenic hypertension may account for up to 40–65% of patients diagnosed with high blood pressure,2 while hypothalamic-pituitary-adrenal (HPA) axis dysfunction—manifesting as elevated plasma cortisol levels—is thought to affect ∼50% of patients with major depression.3
Located at the interface of autonomic, endocrine, and behavioural function, the hypothalamus is a key area involved in the maintenance of homeostasis.4 Through highly interconnected networks, hypothalamic nuclei integrate internal and environmental stressors and broadcast them across organ systems to coordinate appropriate physiological responses. Interference with these networks by means of lesioning or electrical stimulation consequently has the potential to drastically alter homeostatic states. Indeed, hypothalamic neuromodulation has been previously reported to induce acute changes in cardiovascular performance, thermoregulation, mood, and behaviour.5,6 Furthermore, putative effects of stimulation on the HPA axis have been proposed.7 Along with an increased recognition of autonomic nervous system dysfunction in disease, harnessing the therapeutic potential of the hypothalamus has thus become a growing focus in functional neurosurgery and related disciplines.8-11 However, despite extensive study in animals and clinical characterization of stimulation-induced responses, the neuroanatomical substrates governing these effects at the hypothalamic level have been poorly characterized to date.
Growing interest in the application of forniceal deep brain stimulation (fx-DBS) for treatment of Alzheimer’s disease has led to a sizable cohort of patients undergoing electrode implantation in the hypothalamic region, offering a unique opportunity to explore its functional neuroanatomy.12–15 We took advantage of this unique large patient cohort (n = 58) to map the incidental autonomic and mood-related effects of electrical stimulation at specific hypothalamic locations along the length of the implanted DBS leads. To do so, we performed volume of tissue activated (VTA) modelling and voxel-wise mapping in order to localize specific diencephalic ‘hotspots’ potentially associated with higher incidence or probability of certain stimulation-induced effects. In addition, we applied supervised machine learning to further tease apart which structures may be important in driving prominent stimulation-induced responses. Certain aspects of these patients, including their clinical course with respect to Alzheimer’s disease13,14 and the experiential phenomena they experienced with stimulation16 have been reported elsewhere and were therefore not addressed.
Materials and methods
Patient population
This retrospective study analysed a total of 58 patients (mean age: 68.5 ± 7.9 years, 26 females) with mild Alzheimer’s disease who underwent fx-DBS between March 2007 and January 2019 at seven international centres (Fig. 1A). All patients had been enrolled in prior prospective clinical trials (NCT00658125, NCT01608061, ‘clinical and imaging biomarkers’ trial) (Supplementary Fig. 1) and were diagnosed based on standardized criteria and expert examination. Specifically, patients were offered DBS if they achieved a score of 0.5 or 1 on the Clinical Dementia Ratings scale and scores of 12–24 on the Alzheimer’s Disease Assessment Scale Cognitive subscale 11 (ADAS-Cog 11).17 A detailed description of inclusion and exclusion criteria is outlined in Supplementary Tables 1–3. For analysis, only complete datasets featuring (i) pre- and postoperative MRI scans with a slice thickness of 1 mm or less; (ii) intra- and postoperative test stimulation observations; and (iii) the associated stimulation parameters including electrode contact, stimulation voltage, frequency, and pulse width were included. The retrospective evaluation of the data was approved by the institutional research ethics board (IRB #15–9777 University Health Network).
Figure 1.
Summary of stimulation-induced clinical responses and anatomical distribution of implanted electrodes. (A) A total of 58 patients suffering from mild Alzheimer’s disease were included for analysis. Intra- and postoperative stimulation testing yielded clinical manifestations in all patients; of the 422 investigated stimulation settings 382 evoked at least one side effect. Electrical stimulation was most frequently associated with autonomic nervous system disruption, namely cardiovascular function. (B) Bar graph featuring the number of stimulations at each contact (‘0’: most ventral, ‘3’: most dorsal) that did (blue) or did not (orange) yield a clinical response. (C) Box plot displaying the voltage thresholds required to elicit clinical responses (blue) and maximal voltages that did not yield side effects (orange). (D) Reconstructions of all investigated leads (n = 116) featured in sagittal (left), coronal (middle), and axial (right) plane. The atlas of the human hypothalamus39 is superimposed on slices of a 100-μm, 7 T brain scan in MNI 152 space93 and depicts the neural substrates that each respective contact impinged on following surgical implantation. AN = arcuate nucleus; LH = lateral hypothalamus; Pa = paraventricular nucleus; Pe = periventricular nucleus; PH = posterior hypothalamus; SCh = suprachiasmatic nucleus; SO = supraoptic nucleus; TM = tuberomammillary nucleus.
Surgical procedure and clinical assessment
The surgical techniques associated with fx-DBS have been described in detail elsewhere.18 In brief, direct targeting methods were used to identify the descending columns of the fornix on preoperative T1- and T2-weighted MRI scans. The preliminary target was located within the hypothalamus 2 mm anterior to the postcommissural fornix, ∼5 mm from the midline and above the dorsal surface of the optic tract. The trajectory was then adjusted in a manner to maximize electrical stimulation of the target structure, placing the proximal lead contacts along the descending columns of the fornix. Intraoperatively, quadripolar electrodes (model 3387, Medtronic) featuring circular (i.e. non-directional) contacts were advanced bilaterally into the predetermined target under fluoroscopic guidance and awake condition. To evaluate stimulation-induced effects, intraoperative test stimulation was performed in all patients. Specifically, we sought to induce acute recollections of memory16,19 and survey adverse side effects associated with hypothalamic stimulation to verify accurate lead placement. The frequency of observed clinical responses at each contact served as a surrogate for implantation depth. Electrodes were then internalized and connected to an internal pulse generator (IPG) under general anaesthesia. Postoperative MRI scans were obtained on the following day to verify accurate electrode placement.
Postoperative test stimulation
Monopolar review test stimulation was carried out 2 weeks after lead implantation during the initial programming visit. Contacts were surveyed in a standardized manner (distal to proximal), activating leads unilaterally (one side at a time) fixing the stimulation parameters at 90 µs and 130 Hz, and gradually increasing voltages in increments of 1.0 V. During stimulation testing, objective numerical changes in vital signs (heart rate, blood pressure), objective changes in physical appearance (flushing, sweating) or behaviour (fear), and subjective symptoms (warmth, coldness, pleasantness, nausea, phosphenes, dizziness, pressure, memory recollections) reported spontaneously by the patient were recorded. If stimulation reached a maximum of 10.0 V or patients reported intolerable side effects, stimulation was discontinued and patients were given a recovery period before testing was resumed at the next contact. In some cases, certain contacts were not stimulated on account of patient discomfort or unwillingness to continue testing. For data analysis, observed responses were dichotomized into two categories: ‘side-effect inducing’ and ‘not side-effect inducing’. To this end, continuous variables such as heart rate and blood pressure were only deemed ‘side-effect inducing’ in cases where a 30% change from baseline was observed that reverted back to baseline upon cessation of stimulation. This threshold was chosen a priori as it accounted for heart rate and blood pressure variability, which is typically in the order of 10–15% in the investigated age group.20
Imaging acquisition
The sequences and protocols used for the acquisition of structural MRI scans were adopted from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocols for multi-scanner acquisition.21,22 The imaging core at Johns Hopkins University School of Medicine was responsible for the establishment of protocols, data organization, and quality control of the MRI data.14 For analysis, T1- and T2-weighted volumetric pre- and postoperative scans obtained at 1.5 T across seven sites were used.
DBS lead localization and volume of tissue activated modelling
Lead localization and VTA modelling of active electrode contacts were performed using Lead-DBS v2 (https://www.lead-dbs.org/).23 First, each patient’s post- and preoperative MRI scans were rigidly co-registered using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12). The postoperative scans were then non-linearly normalized to MNI152 (ICBM 2009 b NLIN asymmetric) space using ‘low variance’ ANTS (http://stnava.github.io/ANTs/). Each normalization was validated visually and, if necessary, refined using an additional subcortical transformation step.24 This normalization pipeline was chosen to ensure the highest possible registration accuracy at the hypothalamic level.25
Localization of DBS electrodes was performed manually on postoperative MRI scans by an experienced user (A.H.) and confirmed by a second (G.J.B.E.). The electrode models were then warped to MNI space using the transforms described above. The VTAs associated with stimulation settings at each active contact as employed during intra- and postoperative stimulation testing were then modelled. Specifically, a volume conductor model was first generated to segregate the peri-electrode space by tissue type. The potential distribution arising from electrical stimulation was then computed using the FieldTrip-SimBio pipeline (https://www.mrt.uni-jena.de/simbio/index.php/; http://fieldtriptoolbox.org).26 This modelling approach was adapted from work that identified computationally simplified estimates of gold standard axon cable models used to study DBS activation volumes—the same work that underpins the FDA-approved SureTune 3 software.27,28 For analysis, only the lowest voltage that was associated with an acute symptomatic response was selected, along with the voltage setting(s) below, that did not induce any objective or subjective changes. For contacts that did not yield any clinically observable responses, the largest tested voltage setting was utilized. This conservative selection approach was chosen to avoid false-positive results. In keeping with previous publications,29–31 all VTAs were pooled to the right hemisphere by flipping left-sided VTAs (n = 203) along the mid-sagittal plane.23 This was done under the assumption of functional non-dissimilarity across hemispheres to facilitate group-level analysis and increase statistical power.
Statistical analysis
The VTAs sampled for analysis were initially stratified by lead contact, total number of observed clinical effects at each contact, and stimulation voltage to investigate potential effects of these variables independent of VTA location. Simple linear models were fitted to relate the changes between contact position and voltage, and voltage and clinical responses. Multivariate regression analysis was performed to analyse the effect of demographic (age at surgery, disease duration) and clinical quantitative variables (dementia severity) on reported clinical observations.
Probabilistic frequency mapping
Supplementary Fig. 2 provides an overview of the statistical methods used in the generation and masking of maps. To investigate the spatial distribution of stimulation-induced side effects, frequency maps reflecting the number of clinical responses at each voxel were generated (in-house MATLAB script, The MathWorks, Inc., Version R2017b. Natick, MA, USA).32,33 First, patient-specific VTAs were clinically weighted by the number of clinical responses observed at each respective VTA. Clinically weighted VTAs were then overlapped, and a mean score was calculated by averaging the sum of clinical responses at each voxel—this process yielded a raw average frequency map. To control for outlier voxels that were only encompassed by a minority of VTAs, an unweighted n-map was computed. Each voxel value within the n-map reflected the number of VTAs overlapping the respective voxel. The n-map was conservatively thresholded at 5% and then used to mask the raw average map. This process removed all voxels that were touched by <21 of the 422 VTAs. Finally, given that the dataset did not follow a normal distribution (P < 0.001), Wilcoxon signed-rank tests were performed to compute a P-map indicating the degree of confidence that DBS was associated with clinical change at each voxel. The null-hypothesis that stimulation was not associated with a change in clinical outcome was rejected if P < 0.05. To reflect this, the final P-map was thresholded at P < 0.05 and used to mask the n-masked average map, causing any voxels not significantly associated with clinical change to be discarded.
Investigation of neural substrates underlying stimulation-induced responses
To explore the topography of individual stimulation-induced responses, composite maps were generated using voxel-wise odds ratio (VOR) mapping and whole-brain logistic regression (for a detailed description of the workflow, see Supplementary Fig. 3). These maps were intended to provide voxel-wise insight into how likely a given brain area was to yield a given response when stimulated. VOR maps were obtained by separately summing ‘side-effect inducing’ and ‘not side-effect inducing’ VTAs at each voxel.34,35 The total number of overlapping VTAs was reflected in the voxel value. For instance, if 10 side-effect inducing and five not side-effect inducing VTAs overlapped at a specific voxel, the corresponding voxel value in the sum maps would be 10 and 5, respectively (Supplementary Fig. 3). VOR maps were then generated by comparing the summed values of each (‘side-effect inducing’ and ‘not side-effect inducing’) map using the following formula:
| (1) |
where VP = number of side-effect inducing VTAs overlapping a specific voxel; VC = number of control VTAs without side effect overlapping a specific voxel; NP = overall number of side-effect inducing VTAs; NC = overall number of control VTAs without side effect. The ensuing VOR maps were thresholded at a lower boundary of 2.0 to confine identified voxels to areas of relevance.
The same dataset was then used to perform whole-brain voxel-wise logistic regression (https://github.com/Mouse-Imaging-Centre/RMINC) (Supplementary Fig. 3). This approach compared VTAs associated with a particular clinical response to VTAs unrelated to that same response at each voxel while controlling for voltage and repeated stimulation within each patient. Resulting t-values were corrected for multiple comparison using a false discovery rate (FDR) of PFDR < 0.05 to confine brain regions to areas of significance. This approach was chosen as it has been shown to produce reliable results for both voxel and cluster-wise inference.36–38
P FDR < 0.05 correctly rejects the null hypothesis at the defined value of 95%, while controlling the rate of potentially false positive voxels at 5%. Thus, for every cluster of 20 voxels, 19 are certain to be true positives. To account for potential false positives and confirm the identified voxels, the final FDR-corrected t-maps were overlaid and masked by their corresponding thresholded VOR maps (Supplementary Fig. 3). This yielded the final composite map only containing voxels that were shared across both maps. Finally, to study the neuroanatomical relationships between composite maps and diencephalic structures of interest, each map was overlaid onto digital atlases including hypothalamic and surrounding extrahypothalamic structures,39 nucleus accumbens,40 caudate nucleus,41 superolateral branch of the medial forebrain bundle (slMFB),42 anterior limb of the internal capsule (ALIC),43 and optic tract.44
K-nearest neighbours classification
Voxel-wise mapping identified distinct substrates associated with each clinical manifestation, but did not provide insights into the extent of their contribution to the evoked response. Thus, to validate the diencephalic structures driving stimulation-induced clinical responses and to determine the degree to which their involvement could predict each side effect, a k-nearest neighbours (k-NN) classifier was implemented in a final analysis.45 K-NN is a supervised, instance-based machine learning classifier that is able to assign unclassified data-points to a specific label (e.g. ‘side-effect inducing’ or ‘not side-effect inducing’). The data structure underlying classification is ‘learned’ on a training dataset during the training period; during testing, k-NN then finds the optimal solution of parameters to classify a set of previously ‘unseen’ data-points based on the information learned during the training period. Specifically, k-NN assigns an ‘unseen’ data-point to a specific label based on a majority vote of its (learned) neighbours, whereby k determines the number of neighbours to be considered for the vote. In doing so k-NN is able to draw non-parametric decision boundaries in feature space.
In the present study, the absolute volume overlaps between each individual VTA and diencephalic structures of interest were used to classify VTAs into ‘side-effect inducing’ and ‘not side-effect inducing’. Structures of interest were determined based on the previously generated composite maps i.e. only structures identified by a specific VOR/t-map were considered for modelling this symptom. Following feature selection, the performance of the k-NN classifier was evaluated using 10-fold cross-validation. To this end the dataset was randomly split into 10-folds. The k-NN was then trained on 9/10-folds and validated on the remaining fold, repeating the process for each of the 10 datasets. As model performance is highly dependent on the predominant k closest samples in feature space, hyperparameter tuning of k was performed using grid search (min = 1; max = 50). This approach optimized the classifier by independently determining the number of neighbouring data-points to be considered for classifying unlabelled VTAs and ultimately drawing the decision boundary in feature space. The most parsimonious model that best classified clinical responses (‘best model’) was then chosen based on the model’s accuracy, precision, and recall.
To validate the performance of the computed model, an alternative k-NN (‘alternative model’) was deployed that performed classification of VTAs based on contact position and voltage only. These features were chosen to determine how the model would perform based on stimulation location and intensity, two purely topological descriptors, as compared to neuroanatomical substrates for clinical responses.
Data availability
The final thresholded composite maps associated with each clinical observation have been uploaded to ZENODO and are publicly available for download (https://doi.org/10.5281/zenodo.4643689). The data that support the findings of this study are available from the corresponding author upon request.
Results
Clinical characteristics
A total of 58 Alzheimer’s disease patients who underwent fx-DBS were enrolled (Fig. 1). Patient demographics and baseline characteristics are featured in Table 1. At the time of surgery, patients had a mean age of 68.5 ± 7.9 years and were recently diagnosed with mild Alzheimer’s disease (mean ADAS-Cog 11 score: 19.5 ± 5.6; range: 10 – 35), with a mean disease duration of 2.2 years. There was no significant correlation between the sum of reported clinical observations and age at surgery (R = −0.019, P = 0.921), disease duration (R = −0.015, P = 0.540), or preoperative baseline dementia severity (R = 0.097, P = 0.301).
Table 1.
Patient demographics and baseline characteristics of the Alzheimer’s disease cohort undergoing bilateral forniceal DBS
| Characteristic | Total |
|---|---|
| Number of patients | 58 |
| Female:male ratio, % | 45:55 |
| Age at disease onset, years, mean ± SD | 65.8 ± 7.9 |
| Disease duration prior to surgery, years, mean ± SD | 2.2 ± 1.7 |
| Age at surgery, years, mean ± SD | 68.5 ± 7.9 |
| ADAS-Cog 11 at surgery, mean ± SD | 19.5 ± 5.6 |
| Number of electrodes investigated | 116 |
| Number of VTAs yielding clinical responses | 422 |
| Number of observed clinical responses to stimulation | 624 |
| Number of observed clinical responses per contact, mean ± SD | 1.4 ± 0.9 |
| Stimulation parameters | |
| Amplitude,V, mean ± SD | 5.2 ± 1.6 |
| Pulse width, µs | 90 |
| Frequency, Hz | 130 |
ADAS-Cog 11 = Alzheimer’s Disease Assessment Scale Cognitive subscale 11; SD = standard deviation.
Aggregated analysis of electrode locations and volumes of tissue activated
Following normalization of individual patient scans to MNI space, broadly dispersed lead distributions were observed across the ventral diencephalon (Fig. 1). In the axial plane, contacts primarily clustered around fornix (22.7%) and bed nucleus of the stria terminalis (BNST) (20.2%). The most frequently overlapping hypothalamic structure was the lateral hypothalamus (14.1%). Intra- and postoperative test stimulation of 116 leads (314 contacts) yielded clinical manifestations in all patients. In total, 422 VTAs—each reflecting a different stimulation setting—were sampled, of which 382 (90.5%) evoked at least one side effect (Fig. 1). Given that individual VTAs induced up to four different clinical observations, the total number of recorded responses (n = 627) exceeded the number of symptom-inducing VTAs.
Stratification of VTAs by contact, number of side effects per VTA, and stimulation voltage revealed a ventrodorsal gradient of stimulation-induced effects along electrode trajectories (R = 0.18, P = 3.55 × 10−18) (Fig. 1B and C). The majority of clinical observations occurred at the most distal contacts [contact ‘0’: n = 250 side effects (37.3%); contact ‘1’: n = 212 side effects (31.6%)], while stimulation of proximal sites was associated with significantly fewer side effects [contact ‘2’: n = 152 side effects (22.7%); contact ‘3’: n = 56 side effects (8.4%)] (P = 1.6 × 10−3). Finally, significant differences were observed between contact location and voltage (P = 3.2 × 10−17), with mean voltages incrementally increasing at more dorsal contacts (Fig. 1C). This indicates that stimulation was less tolerable at distal contacts, where autonomic responses were evoked more frequently and at lower voltage thresholds.
The most common acute responses during hypothalamic region stimulation were autonomic responses; specifically, tachycardia (30.9%) and warmth (24.6%) followed by flushing (9.1%) and hypertension (6.9%) (Fig. 1A). Mood-related responses, such as fear and pleasantness were only observed in a minority (4.7%) of stimulations. After controlling for contact location, we identified a significant effect of voltage thresholds on the type of clinical response (R = 0.245, P = 4.3 × 10−33), with the evocation of nausea (P = 8.7 × 10−3), sweating (P = 3.8 × 10−3), and acute memory recollections (P = 4.6 × 10−6) requiring significantly larger amplitudes. For a detailed description of the findings associated with memory flashbacks, see Deeb et al.16 and Germann et al.19
The frequency of clinical responses is spatially dependent
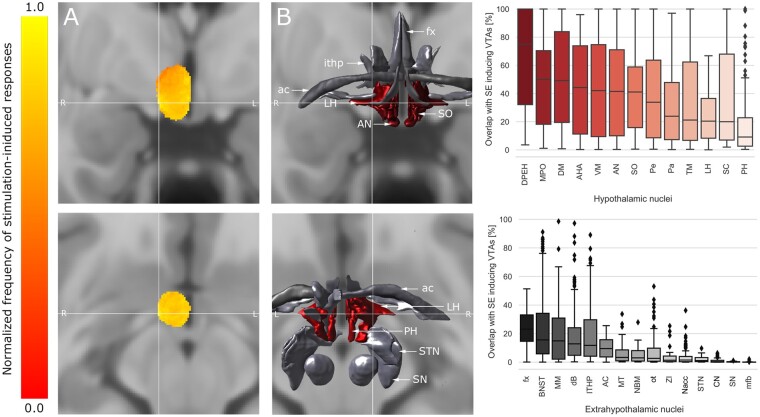
We identified hypothalamic areas associated with increased prevalence of clinical responses using probabilistic frequency mapping (Fig. 2). The aggregated volume encompassed by all sampled VTAs amounted to 7.1 cm3 and occupied a considerable portion of the diencephalon as well as the anterior aspect of the mesencephalon. In accordance with the results of the contact site analysis, the largest number of autonomic responses was observed at the most ventral aspect of the hypothalamus. The peak intensity (i.e. the area associated with the most clinical responses) was identified at the following MNI coordinates: x = 8.94, y = −3.08, z = −12.01, encompassing the ventroposterolateral aspect of lateral hypothalamus (Fig. 2). Stratification of response-inducing VTAs by hypothalamic overlap corroborated these findings, revealing an overlap with lateral hypothalamus in 93.1% of stimulations. Interestingly, stimulation of a fraction of the lateral hypothalamus volume (mean: 22.1 ± 16.2%) was sufficient to induce clinically observable side-effects (mean volume overlap with other hypothalamic nuclei: 35.6 ± 33.6%) (Fig. 2B), indicating the overall eloquence of this hypothalamic structure. Supplementary Fig. 4 features an overview of the anatomic relationships between hypothalamic and extrahypothalamic nuclei.
Figure 2.
Frequency of stimulation-induced clinical responses across the hypothalamic region. (A) Probabilistic frequency map superimposed on a T1-weighted template (MNI 152 NLIN 2009 b) featured in coronal (top) and axial (bottom) sections. All voxel values are normalized to 1 with respect to the highest value voxels (representing the largest number of stimulation-induced manifestations) for visualization purposes. The map is centred on the peak intensity (cross hair) at the following MNI coordinates: x = 9.0, y = −4.7, z = −12.5. (B) The neural substrate underlying the peak intensity was located within the hypothalamus (red), namely the lateral hypothalamic area (LH).39 Box plots feature the extent of overlap between patient-specific, side-effect inducing VTAs and their underlying hypothalamic (top, red) and extrahypothalamic (bottom, grey) substrates. AC = anterior commissure; AHA = anterior hypothalamic area; AN = arcuate nucleus; BNST = bed nucleus of the stria terminalis; CN = caudate nucleus; dB = diagonal band of Broca; DPEH = dorsal periventricular nucleus; DM = dorsomedial hypothalamic nucleus; fx = fornix; ithp = inferior thalamic peduncle; MM = mammillary bodies; sl-mfb = superolateral branch of medial forebrain bundle; MPO = medial preoptic nucleus; MT = mammillothalamic tract; Nacc = nucleus accumbens; NBM = nucleus basalis of Meynert; ot = optic tract; Pa = paraventricular nucleus; Pe = periventricular nucleus; PH = posterior hypothalamus; RN = red nucleus; SC = suprachiasmatic nucleus; SN = substantia nigra; SO = supraoptic nucleus; STN = subthalamic nucleus; TM = tuberomammillary nucleus; VM = ventromedial nucleus; ZI = zona incerta.
Individual hypothalamic effects are related to stimulation location and size
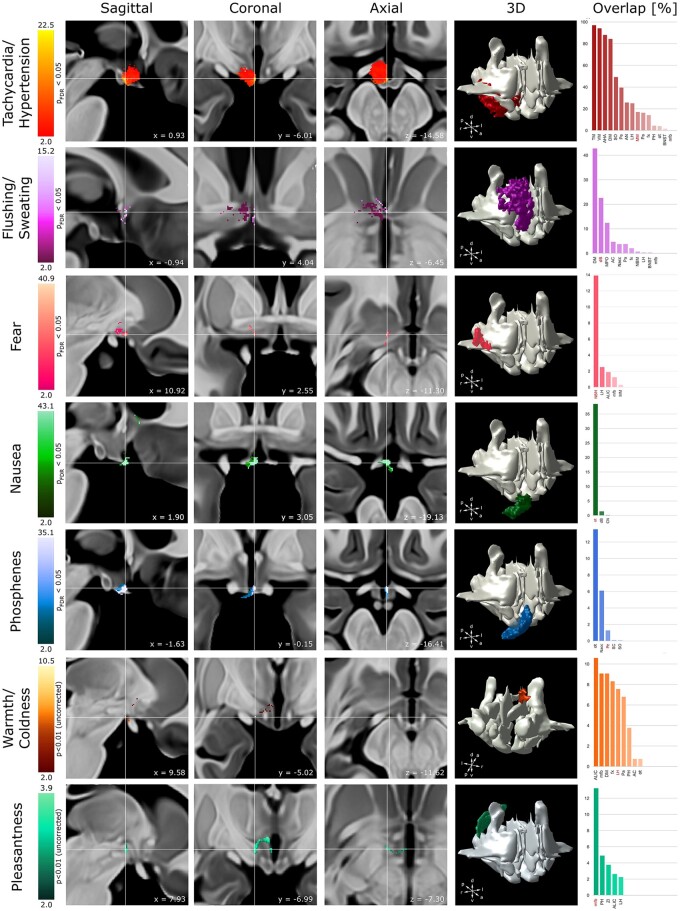
Clinical effects associated with electrical stimulation of hypothalamic and surrounding structures were mapped using VOR and logistic regression (Fig. 3). For analysis, changes in heart rate and blood pressure were analysed jointly as both symptoms frequently cooccurred. The same approach was taken for subjective (warmth, coldness) and objective (flushing, sweating) changes in thermoregulation, respectively. FDR correction at PFDR<0.05 yielded clusters associated with tachycardia/hypertension, flushing/sweating, fear, nausea, and phosphenes (Fig. 3). Pleasantness and warmth/coldness did not pass FDR correction and are reported uncorrected with t-values thresholded at T > 2.6 (equivalent to P < 0.01). Dizziness and pressure are commonly reported sequelae during DBS and are considered non-localizing adverse effects.46 Hence, these symptoms were not subjected to voxel-wise mapping. To confirm that these results were not driven by patient-specific or hemispheric differences, linear mixed-effect models were generated controlling for non-independence of stimulation effects within individual leads, patients and hemispheres, respectively. These supplementary analyses reaffirmed our previous findings identifying nearly identical clusters of voxels associated with the respective symptoms (Supplementary Figs 5 and 6).
Figure 3.
Hypothalamic and extrahypothalamic substrates of acute clinical responses evoked during acute hypothalamic stimulation. Composite VOR/t-maps (see ‘Materials and methods’ section for details) obtained from 58 patients are projected on sagittal (left), coronal (middle left), and axial (middle) T1-weighted MRI sections. Each section is centred on the peak intensity (cross hair) of the composite map associated with the respective clinical observation. Each map features the voxels that are significantly associated with the respective symptom (t-maps: PFDR < 0.05) and was thresholded at a lower boundary of 2.0 (VOR maps) to confine identified voxels to areas of relevance; brighter colours indicate a higher degree of confidence that the underlying voxel associated with a clinical response. Note the spatially distinct clusters associated with each clinical response. To identify the neural substrates subserving each symptom composite maps were superimposed on digital atlases (middle right). Bar plots (right) feature the overlap of each map with the underlying diencephalic structures. The structure encompassing the peak intensity is highlighted in red.
Inspection of the composite maps revealed the spatial distribution of each clinical response and its relationship to neuroanatomical substrates across the hypothalamic region. Importantly, symptoms formed spatially separable clusters (one-way ANOVA; P = 2.2 × 10−16, n = 7 symptom clusters) that occupied distinct hypothalamic and extrahypothalamic grey and white matter loci. Post hoc analysis revealed statistically significant differences in location between each symptom cluster pair (PBonferroni ≤ 8 × 10−11) (Supplementary Fig. 7). Peak intensities and coordinates, as well as the size of each identified cluster can be obtained from Fig. 3 and Supplementary Table 4. Tachycardia/hypertension overlapped with the ventral aspect of the diencephalon and encompassed a multitude of hypothalamic nuclei. The peak intensity was located at the anterior border of the mammillary bodies in close proximity to lateral hypothalamus. Flushing/sweating formed a distinct cluster within the preoptic area of the hypothalamus occupying the space between anterior commissure (AC) and optic chiasm. Flushing/sweating shared voxels with warmth/coldness further emphasizing the role of the preoptic area in thermoregulation. However, while the former only occupied the rostral aspect of the hypothalamus, warmth/coldness extended further caudal, forming an additional cluster at the level of the postcommissural fornix that encapsulated lateral hypothalamus, posterior hypothalamus, and slMFB.
Neural substrates implicated in fear were distributed across the ventral striatum, encompassing the nucleus basalis of Meynert (NBM) as well as the ventrolateral aspect of lateral hypothalamus. A minority of voxels additionally touched ALIC and slMFB. In contrast, pleasantness formed a distinct cluster in the dorsocaudal aspect of the hypothalamus, encompassing slMFB, posterior hypothalamus, and to a lesser extent ALIC and lateral hypothalamus. Nausea and phosphene associated composite maps were both confined to the most anteroventral aspect of the hypothalamus, primarily impinging on the optic chiasm and its related fibres.
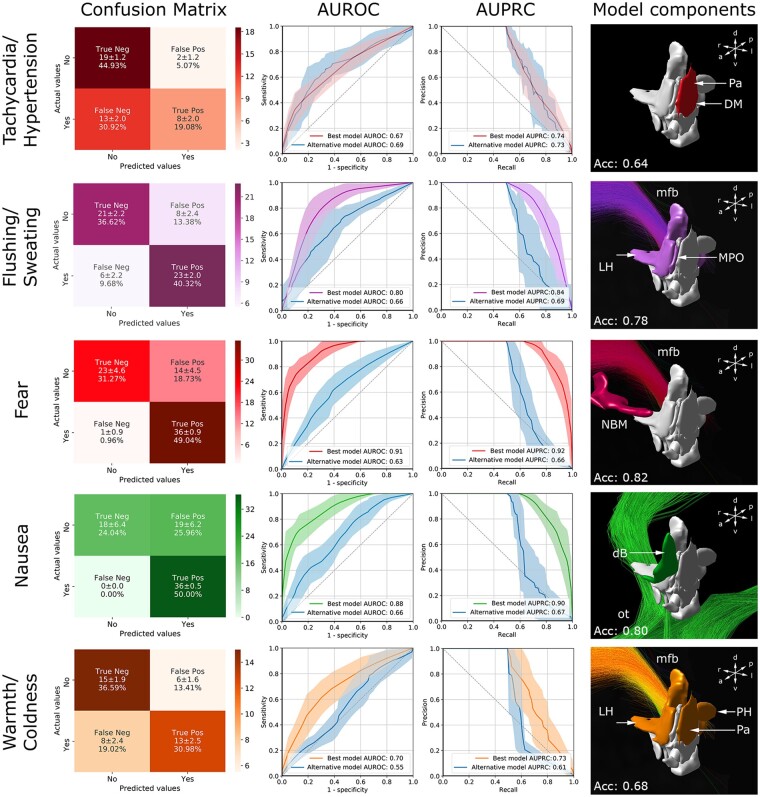
K-nearest neighbours classification validates diencephalic ‘hotspots’
K-NN classification was performed on two models: the ‘best model’ incorporated the absolute volume-overlaps between VTAs and diencephalic structures as identified by the composite maps, while the ‘alternative model’ comprised the two topological descriptors, contact position and voltage (Fig. 4 and Supplementary Fig. 8). With the exception of tachycardia/hypertension, the occurrence of clinical responses could not be fully explained by the ‘alternative model’; instead, the ‘best model’ yielded more accurate predictions emphasizing the role of distinct neural substrates in the generation of clinical responses. The addition of control variables (patient, electrode, and hemisphere) into the model build did not alter model performance (Supplementary Table 5).
Figure 4.
K-NN classification. Confusion matrices (left), AUROC curves (middle left), and AUPRC curves (middle right) feature the performance of the k-NN in the binary (symptom inducing versus not symptom inducing) classification of clinical responses. The diencephalic nuclei and white matter bundles used for the generation of the ‘best model’ are featured on the right. For the ‘alternative model’ (blue) the purely topological descriptors ‘contact position’ and ‘voltage’ were selected. The graphs feature the mean (± standard deviation) of each k-NN following 10-fold cross-validation. With the exception of tachycardia/hypertension, the ‘best model’ yielded an overall better performance. The confusion matrices associated with the ‘alternative model’ can be obtained from Supplementary Fig. 8. dB = diagonal band of Broca; DM = dorsomedial nucleus; LH = lateral hypothalamus; sl-mfb = superolateral branch of medial forebrain bundle; MPO = medial preoptic nucleus; NBM = nucleus basalis of Meynert; ot = optic tract; Pa = paraventricular nucleus; PH = posterior hypothalamus.
Ten-fold cross-validation yielded the strongest predictive accuracy for fear and nausea (Fig. 4). For fear, a model using VTA-overlap with NBM and slMFB was found to be most successful at classification, yielding an accuracy of 0.82 and an area under the receiver operating curve (AUROC) of 0.91, respectively. While the t-map associated with nausea primarily impinged on the optic tract, a model featuring both the optic tract and the diagonal band of Broca performed better (accuracy: 0.80, AUROC: 0.88, AUPRC: 0.90) than one utilizing the optic tract alone [accuracy: 0.65, AUROC: 0.70, area under the precision recall curve (AUPRC): 0.79]. Using volume overlap with slMFB, lateral hypothalamus, and medial preoptic nucleus, k-NN was able to predict flushing/sweating with an accuracy of 0.78 (AUROC: 0.80, AUPRC: 0.84). Using the same neural substrates, classification of warmth/coldness yielded poorer predictions (accuracy: 0.60, AUROC: 0.66, AUPRC: 0.70); the most parsimonious model for the symptom was achieved using slMFB, lateral hypothalamus, paraventricular nucleus, and posterior hypothalamus (accuracy: 0.68, AUROC: 0.70, AUPRC: 0.73). The reduced predictive accuracy associated with warmth/coldness may reflect poor associations with the neuroanatomical substrates identified by t-maps that did not pass FDR correction. Tachycardia/hypertension yielded the overall lowest predictions across the observed symptom spectrum. Both the alternative model (accuracy: 0.68, AUROC: 0.69, AUPRC: 0.73) and a k-NN comprising paraventricular nucleus and dorsomedial hypothalamic nucleus (accuracy: 0.64, AUROC: 0.67, AUPRC: 0.74) performed classification with similar accuracy; both models were more prone to false negatives (Fig. 4 and Supplementary Fig. 8). The lack of observations associated with phosphenes and pleasantness, which were only observed in a minority of stimulations during monopolar review testing (phosphenes: n = 6; pleasantness: n = 3), precluded the employment of a classifier for these symptoms. The associated maps were thus excluded from analysis.
Overall, k-NN classification allowed the association of stimulus-response phenomena with distinct hypothalamic nuclei. This data-driven approach was validated in two additional analyses. First, the substrates associated with the most parsimonious models were permuted across symptoms to compare model performance (Supplementary Fig. 9). With one exception the particular combination of nuclei that best classified each symptom in the data-driven analysis yielded poorer performances when applied to other symptoms. Second, classification accuracy of our data-driven approach was compared to hypothesis-driven models that classified symptoms based on diencephalic structures identified by the literature (Supplementary Table 6). In all but one case, the derived combination of nuclei was very similar to the data-driven models and yielded comparable performances. Both of these investigations reinforce our initial data-driven results and further corroborate that the nuclei implicated in these side effects by our analyses are legitimately involved. Conversely, these findings also suggest that a comprehensive association of symptoms to functionally relevant structures may not be captured by our model. This holds especially true for ‘fear’, which was the exception in both analyses and could be predicted equally well by the nuclei derived from our data-driven approach, different literature-based nuclei, and nuclei that were found to optimally predict other symptoms.
Discussion
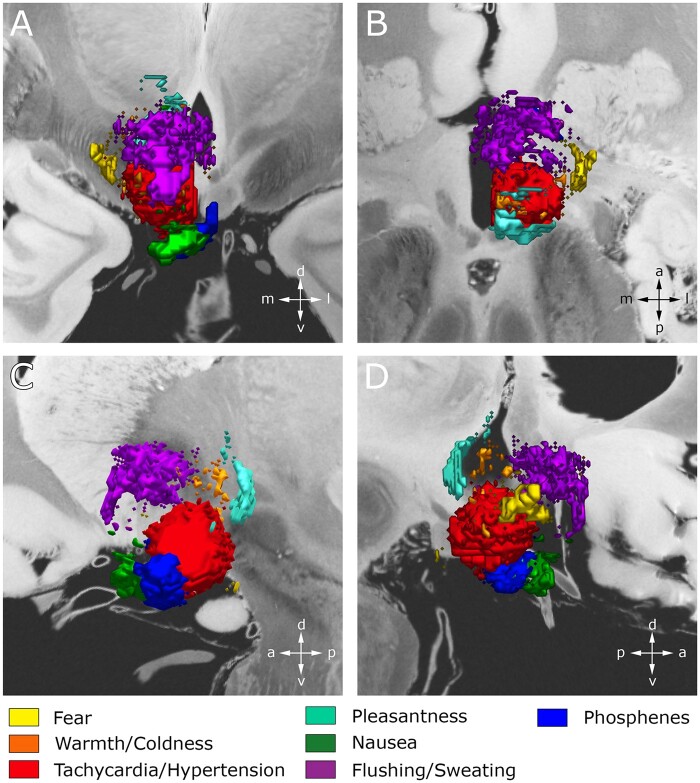
This study used VTA modelling, voxel-wise mapping techniques, and machine learning to systematically interrogate the neuroanatomical correlates of the acute responses arising from hypothalamic region stimulation in the context of fx-DBS for Alzheimer’s disease. Most autonomic and behavioural sequelae identified here have previously been reported in the literature, albeit only in the context of small case series and case reports that did not include detailed clinico-anatomical correlations.47–51 In contrast, the present study was able to draw data from a multicentre cohort of 58 fx-DBS patients. This enabled a comprehensive, data-driven exploration of clinical responses and, more importantly, facilitated the voxel-wise mapping of symptoms across the hypothalamic region (Fig. 3). Statistical analysis revealed symptom-specific clusters that were spatially separable and overlapped with distinct hypothalamic and extrahypothalamic grey and white matter structures. In particular, reference to a novel high resolution, MRI-based hypothalamic atlas39 helped to connect these symptom clusters to known tracts and nuclei, allowing meaningful links to be drawn to the pre-existing literature (Fig. 5). Finally, classification of VTAs into ‘symptom-inducing’ and ‘not symptom-inducing’ regions by means of a k-NN classifier allowed us to validate the identified clinico-anatomical correlates and emphasized the functional importance of certain neural substrates over others with respect to specific stimulation-induced responses. Importantly, the present manuscript marks a step beyond causal evidence underlying hypothalamic function in humans, which—by necessity—has primarily been derived from animal work.
Figure 5.
3D representation featuring the spatial relationship between individual composite maps at the hypothalamic level. Composite VOR/t-maps, each representing a distinct clinical response, are projected on coronal (A), axial (B), and sagittal (C and D) slices of a 100-μm resolution, 7 T brain scan in MNI 152 space.93 Clinical responses formed distinct clusters that were spatially separable, encapsulating both hypothalamus and surrounding grey and white matter structures.
Tachycardia and hypertension were among the most frequently observed responses to hypothalamic stimulation and localized to the largest spatial cluster, reflecting the extensive intrahypothalamic network implicated in cardiovascular function.52 Indeed, the hypothalamus is known to alter autonomic reflex responses from peripheral vasculature, directly monitor humoral factors in the extracellular fluid environment, and integrate complex behavioural states to induce appropriate cardiovascular responses. These functions are processed within dedicated hypothalamic nuclei, which were identified here: voxel-wise mapping implicated the paraventricular and supraoptic nucleus, both of which exert top-down regulatory control over sympathetic and parasympathetic reflex responses (Supplementary Fig. 4). Along with autoregulatory fibres from periventricular and arcuate nucleus, these nuclei have efferents that converge in the caudal aspect of the hypothalamus to form a distinct fibre bundle, the dorsal longitudinal fasciculus (DLF). The DLF descends to the nucleus of the solitary tract (NTS) and related autonomic centres in the medulla where hypothalamic input is believed to reset baro- and chemo-receptor reflexes, such that sympathetic outflow is regulated at higher operating ranges.53,54 The crucial role of this hypothalamic outflow tract in the regulation of cardiovascular function is evidenced by DBS-induced tachycardia and hypertension observed in the vicinity of the DLF. Indeed, electrical stimulation of the posterior hypothalamus,55 the more caudally located ‘ergotropic triangle’,56 as well as anteromedial subthalamic nucleus (STN)-DBS51,57 have been associated with cardiovascular changes. While sympathetic manifestations as a result of direct nuclear stimulation cannot be excluded,58 the similar symptom characteristics suggest at least partial DLF involvement in the overall cardiovascular response observed in this study.57,59,60
Machine learning emphasized the role of paraventricular nucleus in cardiovascular control, which is exerted not only through direct axonal projections to the brainstem as described above, but also via the neuroendocrine release of hormones. Indeed, preliminary evidence of incidental hypothalamic stimulation implicated the release of thyrotropin-releasing hormone (TRH) from paraventricular nucleus and subsequent activation of the HPA axis as a potential mechanism underlying acute mood elevations during OCD-DBS.7 Similarly, reductions in plasma cortisol levels that corresponded with symptom improvement have been noted.61 While an effect of paraventricular nucleus hormones on cardiovascular function is well established and could perhaps explain the rapid sympathetic alterations, the extent to which electrical stimulation alters sympathetic activity by neuroendocrine release is unclear. A body of literature measuring hormone levels during electrical stimulation suggests a minor contribution, as changes from baseline were not significant.50,62,63
The ability to anticipate and rapidly respond to external environmental threats is critical for survival. It is consequently not surprising that highly complex brain systems have evolved to effectively mediate defensive responses and alter mood states accordingly. One hub for emotional processing is the amygdala, which integrates conditioned and unconditioned cues from the environment and promotes emotional salience in response.64 When exposed to an immediate threat, the amygdala promotes immediate fear responses via subcortical pathways that elicit neuroendocrine responses through the paraventricular nucleus, induce startle responses via midbrain pathways, and alter autonomic processing through lateral hypothalamus and dorsomedial nuclei (Fig. 4).65 Direct engagement of subcortical elements by means of electrical stimulation consequently has the potential to mimic these neurobiological processes. Indeed, DBS studies in humans have demonstrated the evocation of fear, panic, and associated autonomic responses by electrically stimulating the amygdala,66 hypothalamus,67,68 nucleus accumbens,47 and inferior thalamic peduncle.69 Importantly, these structures are located along the ventral amygdalofugal pathway, a major amygdala outflow tract that traverses the ventral striatum along the NBM, and sends projections to brainstem, hypothalamus, and nucleus accumbens prior to ascending to the thalamus.70–72 While our data-driven approach emphasized the key role of ventral amygdalofugal pathway in stimulation-induced fear responses (using NBM as surrogate) (Fig. 4), supplementary analyses suggested a greater involvement of hypothalamic nuclei (Supplementary Fig. 9 and Supplementary Table 6) that was not captured by our data-driven model.
While unconditioned stimuli are typically processed via direct pathways that readily induce autonomic and behavioural responses, conditioned stimuli undergo additional processing in distributed cortical areas.73 Based on contextual information, they exert top-down regulatory control over diencephalic and brainstem structures, thus modulating mood and reward responses.74 As an integral part of the mesolimbic pathway, the medial forebrain bundle (MFB) links orbital and prefrontal cortex to downstream dopaminergic centres such as ventral tegmental area and nucleus accumbens.75 Specifically, the slMFB has been implicated in the neural encoding of mood-related behaviours, promoting anti-anhedonic and motivational effects by controlling the release of dopamine from its effector nuclei.76 Electrical stimulation of the fibre bundle has been associated with immediate antidepressant effects including relaxation, increased motivation, and positive changes in mood.77,78 Consistent with this notion, our own results suggest that slMFB stimulation likely contributed to the—albeit rare—observations of patient-reported pleasantness observed during hypothalamic DBS.
Temperature-related symptoms including, warmth, coldness, flushing and sweating are autonomic effectors that are critically involved in the maintenance of homeothermy, the preservation of a constant body core temperature.79 Voxel-wise mapping identified distinct neuroanatomical substrates associated with thermoregulation in the rostral and caudal aspects of the hypothalamus (Fig. 5). However, while subjective sensations of warmth and coldness localized to both the rostral and caudal hypothalamus, the objectively observed signs of flushing and sweating exclusively occupied the rostral hypothalamus. This finding corroborates clinical and physiological evidence suggesting the presence of rostral and caudal heat control centers.80
The rostral heat control centre is located in the preoptic area, which encompasses the periventricular and medial preoptic (MPO) nuclei, the latter of which was identified by voxel-wise mapping (Fig. 3) and emphasized during k-NN classification. As the primary integrative site for thermoregulation, preoptic area receives sensory neural input from the periphery, but also features warm-sensitive neurons that directly respond to increases in body core temperature, as evidenced by direct thermal stimulation.81,82 Through hypothalamic relay stations, the autonomic response is then generated and orchestrated across organ systems. First, the preoptic area exerts direct inhibitory control over the dorsomedial nucleus, which promotes cutaneous vasoconstriction and shivering via the medullary rostral raphe pallidus nucleus (rRPA).83 This effect is antagonized by the paraventricular nucleus and lateral hypothalamus, which reach rRPA via the MFB. Insights from machine learning emphasized the contribution of these structures to heat loss and sweating via the sympathetic cholinergic system that promotes cutaneous vasodilation and sudomotor activity.84,85 Transections of paraventricular nucleus and lateral hypothalamus consequently suppresses heat loss, yielding rapid increases in non-shivering thermogenesis and rectal temperature.86 Finally, paraventricular nucleus-induced flushing and sweating via the release of TRH and increased energy expenditure through the hypothalamic-pituitary-thyroid axis have been proposed.87
Apart from clusters in the preoptic area, voxel-wise mapping identified distinct voxels in the caudal heat control centre. This area encompasses the posterior hypothalamus and is temperature-blind i.e. their neurons do not respond to direct thermal stimulation. The posterior hypothalamus has been implicated in cold responses mediating shivering, vasoconstriction, and increased metabolic heat production.85 This is corroborated by lesional studies demonstrating impaired heat production following posterior hypothalamus transection.88 K-NN classification emphasized the role of the posterior hypothalamus in subjective sensations of warmth/coldness, but not flushing/sweating.
Voxel-wise mapping results indicated that both nausea and phosphenes could be localized to the anteroventral hypothalamic area and in particular were elicited by stimulation that impinged on the optic chiasm. This pattern of response is well described in the context of internal globus pallidus (GPi) DBS; current spread ventral to the GPi target may encroach on the optic tract provoking characteristic phosphenes and nausea.89 While nausea and phosphenes did not necessarily co-occur here, this overlapping localization might suggest that the nausea observed here was in part triggered by stimulation-induced visual percepts. However, stimulation may also have provoked nausea directly. The neurocircuitry underpinning nausea and emesis is complex and known to involve both the brainstem—particularly the area postrema and NTS—and hypothalamus.90 Our finding that nausea was particularly located in the anterior aspect of the hypothalamus is supported by earlier work, such as a PET study that found severe nausea associated with increased regional cerebral blood flow to the anterior hypothalamus.91 Machine learning not only reinforced the relevance of the optic tract to nausea but further hinted at a role for the diagonal band of Broca in subserving this response. This is consistent with recent work that has implicated certain populations of basal forebrain and specifically diagonal band of Broca neurons in the integration of environmental sensory cues and—through projections to lateral hypothalamus—the promotion of food avoidance and hypophagia.92
This study does have limitations underlying the exploration of neural substrates during hypothalamic region DBS. First, it is important to note that the present study was conducted in a convenience sample of Alzheimer’s disease patients and an effect of the disease on the stimulation-induced responses observed here cannot be excluded. Hence, further research is necessary to determine whether our findings in this Alzheimer’s disease cohort are generalizable and transferable to other patient populations. Second, the data associated with acute stimulation-induced responses were gathered retrospectively during unblinded stimulation testing, meaning both patient/clinician expectations (as well as variation in patient verbalization of symptoms) and inter-study recording differences may have exerted an effect. Third, while the methods used in the present study have been published before, it is important to acknowledge that each imaging analysis step, from electrode localization to normalization and electric field modelling is associated with distinct geometrical errors that may have been exacerbated by the cerebral atrophic changes commonly observed in this patient cohort. Furthermore, variability in lead localization may have arisen from factors related to the sampling of data from multicentre trials, the relative novelty of the surgical target, disease-related factors such as brain atrophy that complicated trajectory planning and electrode implantation, as well as the need to penetrate the lateral ventricles during surgery. Fourth, because VTAs were pooled to a single side to increase statistical power, we forfeited our ability to possibly find asymmetric results. Finally, the employed significance threshold of PFDR < 0.05 may have yielded false-positive voxels during logistic regression. This could have led to the incorrect identification of the neural substrates of various responses, especially given the small size of the hypothalamus, its nuclei, and surrounding structures. However, the use of a second statistical method (VOR mapping), the fact that symptoms with well established correlates (e.g. phosphenes) localized to expected areas, and the general corroboration of identified substrates by the prior literature all reinforce our findings and validate our approach. A comprehensive discussion of the limitations is featured in the Supplementary material.
Conclusion
In this analysis of a large, multicentre patient cohort, we identified the neuroanatomical underpinnings of the acute sequelae of hypothalamic region DBS. We identified distinct, spatially separable clusters associated with each symptom, providing clinico-anatomical insights that may help guide future neuromodulation work targeting this intricate brain region. In particular, utilizing a previously described high-resolution atlas in conjunction with the response-specific voxel clusters identified here may provide immediate guidance for surgical targeting refinement, helping future studies avoid unwanted side-effects or attain preferred behavioural or autonomic outcomes. This holds true for novel stimulation paradigms such as directional DBS, which may allow more sophisticated shaping of the electric field towards the desired clinical response. Overall, these results strengthen the case that direct stimulation of the hypothalamus and other regions can reproducibly and predictably yield specific autonomic and behavioural responses—responses that may potentially harbour therapeutic value in autonomic nervous system dysfunction.
Funding
This work was supported by the RR Tasker Chair in Functional Neurosurgery at University Health Network (A.M.L.), the Canadian Institutes of Health Research (reference # 164235: G.J.B.E.) and the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG NE 2276/1–1: CN; DFG grants 410169619 and 424778381: AH).
Competing interests
A.M.L. is scientific director of Functional Neuromodulation and reports consultant fees from Medtronic, Abbott, and Boston Scientific. C.G.L. reports consultant fees from Avanir, Eli Lilly, and the NFL benefits office. Z.M. reports consultant fees from Biogen, AbbVie, Global Kinetics Corporation, nQ Medical, NeuroReserve, and Sensory Cloud. M.S.O. serves as a consultant for the Parkinson’s Foundation, and has received research grants from NIH, Parkinson’s Foundation, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. His DBS research is supported by: NIH R01 NR014852 and R01NS096008. M.S.O. is PI of the NIH R25NS108939 Training Grant and has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, Perseus, Robert Rose, Oxford and Cambridge (movement disorders books). M.S.O. is an associate editor for New England Journal of Medicine Journal Watch Neurology. He has participated in CME and educational activities on movement disorders sponsored by the Academy for Healthcare Learning, PeerView, Prime, QuantiaMD, WebMD/Medscape, Medicus, MedNet, Einstein, MedNet, Henry Stewart, American Academy of Neurology, Movement Disorders Society and by Vanderbilt University. The institution and not M.S.O. receive grants from Medtronic, Abbvie, Boston Scientific, Abbott and Allergan and the PI has no financial interest in these grants. M.S.O. has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. Research projects at the University of Florida receive device and drug donations. P.B.R. reports consultant fees from GLG, Leerink, Otsuka, Avanir, Bionomics, ITI, IQVIA, and the Food and Drug Administration. S.S. reports research support and consultant fees from Biogen, Lilly, Eisai, Genentech, Roche, Novartis, and Avid. D.A.W. reports consultant fees from Lilly, Merck, Jannsen, GE Healthcare, and Neuronix. M.N.S. reports consultant fees from Allergan, Biogen, Roche, Genentech, Cortexyme, Bracket, and Sanofi. All other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
- AUPRC
area under the precision recall curve
- AUROC
area under the receiver operating curve
- DBS
deep brain stimulation
- k-NN
k-nearest neighbour
- slMFB
superolateral branch of the medial forebrain bundle
- VOR
voxel-wise odds ratio
- VTA
volume of tissue activated
References
- 1. Fisher JP, Young CN, Fadel PJ.. Central sympathetic overactivity: Maladies and mechanisms. Auton Neurosci Basic Clin. 2009;148(1–2):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Esler M. The 2009 Carl Ludwig Lecture: Pathophysiology of the human sympathetic nervous system in cardiovascular diseases: The transition from mechanisms to medical management. J Appl Physiol. 2010;108(2):227–237. [DOI] [PubMed] [Google Scholar]
- 3. Musselman DL, Evans DL, Nemeroff CB.. The relationship of depression to cardiovascular disease: Epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55(7):580–592. [DOI] [PubMed] [Google Scholar]
- 4. Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9(3):399–431. [Google Scholar]
- 5. Halpern CH, Wolf JA, Bale TL, et al. Deep brain stimulation in the treatment of obesity: A review. J Neurosurg. 2008;109(4):625–634. [DOI] [PubMed] [Google Scholar]
- 6. Franzini A, Messina G, Cordella R, Marras C, Broggi G.. Deep brain stimulation of the posteromedial hypothalamus: Indications, long-term results, and neurophysiological considerations. Neurosurg Focus. 2010;29(2):E13. [DOI] [PubMed] [Google Scholar]
- 7. de Koning PP, Figee M, Endert E, et al. Rapid effects of deep brain stimulation reactivation on symptoms and neuroendocrine parameters in obsessive-compulsive disorder. Transl Psychiatry. 2016;6: E722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hyam JA, Kringelbach ML, Silburn PA, Aziz TZ, Green AL.. The autonomic effects of deep brain stimulation-a therapeutic opportunity. Nat Rev Neurol. 2012;8(7):391–400. [DOI] [PubMed] [Google Scholar]
- 9. Lozano AM, Lipsman N, Bergman H, et al. Deep brain stimulation: Current challenges and future directions. Nat Rev Neurol. 2019;15(3):148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navaneethan SD, Lohmeier TE, Bisognano JD.. Baroreflex stimulation: A novel treatment option for resistant hypertension. J Am Soc Hypertens. 2009;3(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esler M. Harnessing the autonomic nervous system for therapeutic intervention. In: Robertson D, Biaggioni I, Low P, Paton J, eds. Primer on the autonomic nervous system. 3rd ed. Elsevier Academic Press; 2012: 649–652. [Google Scholar]
- 12. Hamani C, McAndrews MP, Cohn M, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63(1):119–123. [DOI] [PubMed] [Google Scholar]
- 13. Laxton AW, Tang-Wai DF, McAndrews MP, et al. A Phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68(4):521–534. [DOI] [PubMed] [Google Scholar]
- 14. Lozano AM, Fosdick L, Chakravarty MM, et al. A phase II study of fornix deep brain stimulation in mild Alzheimer’s disease. J Alzheimer’s Dis. 2016;54(2):777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leoutsakos J, Yan H, Anderson W, et al. Deep brain stimulation targeting the fornix for mild Alzheimer Dementia (The ADvance Trial): A two year follow-up including results of delayed activation. J Alzheimers Dis. 2018;64(2):597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deeb W, Salvato B, Almeida L, et al. Fornix-region deep brain stimulation–induced memory flashbacks in Alzheimer’s disease. N Engl J Med. 2019;381(8):783–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jack CR, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ponce FA, Asaad WF, Foote KD, et al. ; ADvance Research Group. Bilateral deep brain stimulation of the fornix for Alzheimer’s disease: Surgical safety in the ADvance trial. J Neurosurg. 2016;125(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Germann J, Elias GJB, Boutet A, et al. Brain structures and networks responsible for stimulation-induced memory flashbacks during forniceal deep brain stimulation for Alzheimer’s Disease. Alzheimer’s Dement. Published online 21 January 2021. doi:10.1002/alz.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aune D, Sen A, ó'Hartaigh B, et al. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality – A systematic review and dose–response meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2017;27(6):504–517. [DOI] [PubMed] [Google Scholar]
- 21. Jack CR, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jack CR, Bernstein MA, Borowski BJ, et al. ; Alzheimer's Disease Neuroimaging Initiative. Update on the magnetic resonance imaging core of the Alzheimer’s disease neuroimaging initiative. Alzheimer’s Dement. 2010;6(3):212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horn A, Li n, Dembek TA, et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage. 2019;184:293–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC.. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ewert S, Horn A, Finkel F, Li N, Kühn AA, Herrington TM.. Optimization and comparative evaluation of nonlinear deformation algorithms for atlas-based segmentation of DBS target nuclei. Neuroimage. 2019;184:586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vorwerk J, Oostenveld R, Piastra MC, Magyari L, Wolters CH.. The FieldTrip-SimBio pipeline for EEG forward solutions. Biomed Eng Online. 2018;17(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Åström M, Diczfalusy E, Martens H, Wårdell K.. Relationship between neural activation and electric field distribution during deep brain stimulation. IEEE Trans Biomed Eng. 2015;62(2):664–672. [DOI] [PubMed] [Google Scholar]
- 28. Duffley G, Anderson DN, Vorwerk J, Dorval AD, Butson CR.. Evaluation of methodologies for computing the deep brain stimulation volume of tissue activated. J Neural Eng. 2019;16(6):066024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elias GJB, Boutet A, Joel SE, et al. Probabilistic mapping of deep brain stimulation: Insights from 15 years of therapy. Ann Neurol. 2021;89(3):426–443. [DOI] [PubMed] [Google Scholar]
- 30. Al-Fatly B, Ewert S, Kübler D, Kroneberg D, Horn A, Kühn AA.. Connectivity profile of thalamic deep brain stimulation to effectively treat essential tremor. Brain. 2019;142(10):3086–3098. [DOI] [PubMed] [Google Scholar]
- 31. Petry-Schmelzer JN, Krause M, Dembek TA, et al. EUROPAR and the IPMDS Non-Motor PD Study Group. Non-motor outcomes depend on location of neurostimulation in Parkinson’s disease. Brain. 2019;142(11):3592–3604. [DOI] [PubMed] [Google Scholar]
- 32. Eisenstein SA, Koller JM, Black KD, et al. Functional anatomy of subthalamic nucleus stimulation in Parkinson disease. Ann Neurol. 2014;76(2):279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dembek TA, Barbe MT, Åström M, et al. Probabilistic mapping of deep brain stimulation effects in essential tremor. NeuroImage Clin. 2017;13:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sprenger T, Seifert CL, Valet M, et al. Assessing the risk of central post-stroke pain of thalamic origin by lesion mapping. Brain. 2012;135(Pt 8):2536–2545. [DOI] [PubMed] [Google Scholar]
- 35. Boutet A, Ranjan M, Zhong J, et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain. 2018;141(12):3405–3414. [DOI] [PubMed] [Google Scholar]
- 36. Genovese CR, Lazar NA, Nichols T.. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. [DOI] [PubMed] [Google Scholar]
- 37. Eklund A, Nichols TE, Knutsson H.. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113(28):7900–E7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kessler D, Angstadt M, Sripada CS.. Reevaluating "cluster failure" in fMRI using nonparametric control of the false discovery rate. Proc Natl Acad Sci U S A. 2017;114(17):E3372–E3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neudorfer C, Germann J, Elias G, Gramer R, Boutet A, Lozano A.. A high-resolution in-vivo MRI atlas of the human hypothalamus. Sci Data. 2020;7(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pauli WM, Nili AN, Tyszka JM.. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci Data. 2018;5:180063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prodoehl J, Yu H, Little DM, Abraham I, Vaillancourt DE.. Region of interest template for the human basal ganglia: Comparing EPI and standardized space approaches. Neuroimage. 2008;39(3):956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anthofer JM, Steib K, Fellner C, Lange M, Brawanski A, Schlaier J.. DTI-based deterministic fibre tracking of the medial forebrain bundle. Acta Neurochir (Wien). 2015;157(3):469–477. [DOI] [PubMed] [Google Scholar]
- 43. Ilinsky I, Horn A, Paul-Gilloteaux P, Gressens P, Verney C, Kultas-Ilinsky K.. Human motor thalamus reconstructed in 3D from continuous sagittal sections with identified subcortical afferent territories. eNeuro. 2018;5(3):ENEURO.0060-18.2018-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yeh FC, Panesar S, Fernandes D, et al. Population-averaged atlas of the macroscale human structural connectome and its network topology. Neuroimage. 2018;178:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ashby F. 568-Decoding via multivoxel pattern analysis. In: Ashby F, ed. Statistical Analysis of fMRI Data. 2nd ed. MIT Press; 2019. [Google Scholar]
- 46. Marks W, ed. Deep Brain Stimulation Management. 1st ed.Cambridge University Press; 2010. [Google Scholar]
- 47. Okun MS, Mann G, Foote KD, et al. Deep brain stimulation in the internal capsule and nucleus accumbens region: Responses observed during active and sham programming. J Neurol Neurosurg Psychiatry. 2007;78(3):310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leone M, Franzini A, Broggi G, Bussone G.. Hypothalamic stimulation for intractable cluster headache: Long-term experience. Neurology. 2006;67(1):150–152. [DOI] [PubMed] [Google Scholar]
- 49. Kuhn J, Lenartz D, Mai JK, Huff W, Klosterkoetter J, Sturm V.. Disappearance of self-aggressive behaviour in a brain-injured patient after deep brain stimulation of the hypothalamus: Technical case report. Neurosurgery. 2008;62(5):E1182; discussion E1182. [DOI] [PubMed] [Google Scholar]
- 50. Franco RR, Fonoff ET, Alvarenga PG, et al. Assessment of safety and outcome of lateral hypothalamic deep brain stimulation for obesity in a small series of patients with Prader-Willi syndrome. JAMA Netw Open. 2018;1(7):E185275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ludwig J, Remien P, Guballa C, et al. Effects of subthalamic nucleus stimulation and levodopa on the autonomic nervous system in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(7):742–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dampney RAL. Central neural control of the cardiovascular system: Current perspectives. Adv Physiol Educ. 2016;40(3):283–296. [DOI] [PubMed] [Google Scholar]
- 53. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–346. [DOI] [PubMed] [Google Scholar]
- 54. Rahmouni K. Cardiovascular regulation by the arcuate nucleus of the hypothalamus: neurocircuitry and signaling systems. Hypertension. 2016;67(6):1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cortelli P, Guaraldi P, Leone M, et al. Effect of deep brain stimulation of the posterior hypothalamic area on the cardiovascular system in chronic cluster headache patients. Eur J Neurol. 2007;14(9):1008–1015. [DOI] [PubMed] [Google Scholar]
- 56. Sano K, Mayanagi Y, Sekino H, Ogashiwa M, Ishijima B.. Results of stimulation and destruction of the posterior hypothalamus in man. J Neurosurg. 1970;33(6):689–707. [DOI] [PubMed] [Google Scholar]
- 57. Lipp A, Tank J, Trottenberg T, Kupsch A, Arnold G, Jordan J.. Sympathetic activation due to deep brain stimulation in the region of the STN. Neurology. 2005;65(5):774–775. [DOI] [PubMed] [Google Scholar]
- 58. Sauleau P, Raoul S, Lallement F, et al. Motor and non motor effects during intraoperative subthalamic stimulation for Parkinson’s disease. J Neurol. 2005;252(4):457–464. [DOI] [PubMed] [Google Scholar]
- 59. Torres C, Blasco G, Navas GM, et al. Deep brain stimulation for aggressiveness: Long-term follow-up and tractography study of the stimulated brain areas. J Neurosurg. Published online 7 February 2020. doi:10.3171/2019.11.JNS192608 [DOI] [PubMed] [Google Scholar]
- 60. Dantas SAF, Alho EJL, da Silva JJ, Mendes NNN, Fonoff ET., Hamani C.. Deep brain stimulation modulates hypothalamic-brainstem fibers in cluster headache: Case report. J Neurosurg. 2019;132(3):717–720. [DOI] [PubMed] [Google Scholar]
- 61. de Koning PP, Figee M, Endert E, Storosum JG, Fliers E, Denys D.. Deep brain stimulation for obsessive-compulsive disorder is associated with cortisol changes. Psychoneuroendocrinology. 2013;38(8):1455–1459. [DOI] [PubMed] [Google Scholar]
- 62. Whiting DM, Tomycz ND, Bailes J, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: A pilot study with preliminary data on safety, body weight, and energy metabolism: Clinical article. J Neurosurg. 2013;119(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Leone M, Proietti Cecchini A, Franzini A, Broggi G, Bussone G.. Hypothalamic deep brain stimulation in the treatment of chronic cluster headache. Ther Adv Neurol Disord. 2010;3(3):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci. 1993;16(8):328–333. [DOI] [PubMed] [Google Scholar]
- 65. Davis M. The role of the Amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. [DOI] [PubMed] [Google Scholar]
- 66. Chapman WP, Schroeder HR, Geyer G, et al. Physiological evidence concerning importance of the amygdaloid nuclear region in the integration of circulatory function and emotion in man. Science. 1954;120(3127):949–950. [DOI] [PubMed] [Google Scholar]
- 67. Wilent WB, Oh MY, Buetefisch CM, et al. Induction of panic attack by stimulation of the ventromedial hypothalamus: Case report. J Neurosurg. 2010;112(6):1295–1298. [DOI] [PubMed] [Google Scholar]
- 68. Elias GJB, Giacobbe P, Boutet A, et al. Probing the circuitry of panic with deep brain stimulation: Connectomic analysis and review of the literature. Brain Stimul. 2020;13(1):10–14. [DOI] [PubMed] [Google Scholar]
- 69. Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585–592. [DOI] [PubMed] [Google Scholar]
- 70. Peuskens D, Van Loon J, Van Calenbergh F, Van Den Berg R, Goffin J, Plets C.. Anatomy of the anterior temporal lobe and the frontotemporal region demonstrated by fiber dissection. Neurosurgery. 2004;55(5):1174–1184. [DOI] [PubMed] [Google Scholar]
- 71. Neudorfer C, Maarouf M.. Neuroanatomical background and functional considerations for stereotactic interventions in the H fields of Forel. Brain Struct Funct. 2018;223(1):17–30. [DOI] [PubMed] [Google Scholar]
- 72. Kamali A, Sair HI, Blitz AM, et al. Revealing the ventral amygdalofugal pathway of the human limbic system using high spatial resolution diffusion tensor tractography. Brain Struct Funct. 2016;221(7):3561–3569. [DOI] [PubMed] [Google Scholar]
- 73. Phelps EA, LeDoux JE.. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48(2):175–187. [DOI] [PubMed] [Google Scholar]
- 74. Miller EK, Cohen JD.. An Integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 75. Haber SN, Yendiki A, Jbabdi S.. Four deep brain stimulation targets for obsessive-compulsive disorder: Are they different? Biol Psychiatry. Published online 25 July 2020. doi:10.1016/j.biopsych.2020.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schlaepfer TE, Bewernick BH, Kayser S, Hurlemann R, Coenen VA.. Deep brain stimulation of the human reward system for major depression - Rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39(6):1303–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fenoy AJ, Schulz P, Selvaraj S, et al. Deep brain stimulation of the medial forebrain bundle: Distinctive responses in resistant depression. J Affect Disord. 2016;203:143–151. [DOI] [PubMed] [Google Scholar]
- 78. Schlaepfer TE, Bewernick BH, Kayser S, Mädler B, Coenen VA.. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204–1212. [DOI] [PubMed] [Google Scholar]
- 79. Morrison SF, Nakamura K.. Central mechanisms for thermoregulation. Annu Rev Physiol. 2019;81:285–308. [DOI] [PubMed] [Google Scholar]
- 80. Nieuwenhuys R, Voogd J, van Huijzen C. Diencephalon: Hypothalamus H. In: The human central nervous system. A synopsis and atlas. 4th ed. Springer; 2008:289–336. [Google Scholar]
- 81. Nakamura K, Morrison SF.. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11(1):62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tan CL, Cooke EK, Leib DE, et al. Warm-sensitive neurons that control body temperature. Cell. 2016;167(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. DiMicco JA, Zaretsky DV.. The dorsomedial hypothalamus: A new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R47–R63. [DOI] [PubMed] [Google Scholar]
- 84. Madden CJ, Morrison SF.. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R831–R843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nagashima K, Nakai S, Tanaka M, Kanosue K.. Neuronal circuitries involved in thermoregulation. Auton Neurosci Basic Clin. 2000;85(1–3):18–25. [DOI] [PubMed] [Google Scholar]
- 86. Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K.. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol. 1998;512(3):883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Miao Y, Wu W, Dai Y, et al. Liver X receptor β controls thyroid hormone feedback in the brain and regulates browning of subcutaneous white adipose tissue. Proc Natl Acad Sci U S A. 2015;112(45):14006–14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Swaab DF. The human hypothalamus: Basic and clinical aspects. Part II, neuropathology of the human hypothalamus and adjacent brain structures. In: Aminoff MJ, Boller F, Swaab DF, eds. Handbook of Clinical Neurology. Elsevier; 2004. [Google Scholar]
- 89. Isaias I, Tagliati M.. Managing dystonia patients treated with DBS. In: Marks W, ed. Deep brain stimulation management. Cambridge University Press; 2010:83–90. [Google Scholar]
- 90. Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001;111(8):106–112. [DOI] [PubMed] [Google Scholar]
- 91. Fredrikson M, Hursti TJ, Wik G.. Neural networks in chemotherapy-induced delayed nausea: a pilot study using positron emission tomography. Oncol Rep. 1995;2(6):1001–1003. [DOI] [PubMed] [Google Scholar]
- 92. Patel JM, Swanson J, Ung K, et al. Sensory perception drives food avoidance through excitatory basal forebrain circuits. Elife. 2019;8:e44548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Edlow B, Mareyam A, Horn A, et al. 7 Tesla MRI of the ex vivo human brain at 100 micron resolution. Sci Data. 2019;6(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The final thresholded composite maps associated with each clinical observation have been uploaded to ZENODO and are publicly available for download (https://doi.org/10.5281/zenodo.4643689). The data that support the findings of this study are available from the corresponding author upon request.