Abstract
Although CSF analysis routinely enables the diagnosis of neurological diseases, it is mainly used for the gross distinction between infectious, autoimmune inflammatory, and degenerative disorders of the CNS. To investigate, whether a multi-dimensional cellular blood and CSF characterization can support the diagnosis of clinically similar neurological diseases, we analysed 546 patients with autoimmune neuroinflammatory, degenerative, or vascular conditions in a cross-sectional retrospective study. By combining feature selection with dimensionality reduction and machine learning approaches we identified pan-disease parameters that were altered across all autoimmune neuroinflammatory CNS diseases and differentiated them from other neurological conditions and inter-autoimmunity classifiers that subdifferentiate variants of CNS-directed autoimmunity. Pan-disease as well as diseases-specific changes formed a continuum, reflecting clinical disease evolution. A validation cohort of 231 independent patients confirmed that combining multiple parameters into composite scores can assist the classification of neurological patients. Overall, we showed that the integrated analysis of blood and CSF parameters improves the differential diagnosis of neurological diseases, thereby facilitating early treatment decisions.
Keywords: CNS autoimmunity, multiple sclerosis, differential diagnosis, CSF, immune profile
By investigating patients with neuroinflammatory, -degenerative, and -vascular disorders, Gross et al. identify cellular parameters in blood and CSF that can improve the differential diagnosis of neurological diseases, and that provide insights into shared and distinct pathophysiological processes.
Introduction
Inflammatory, degenerative, and vascular disorders of the CNS affect millions of people worldwide. Autoimmune neuroinflammatory CNS diseases comprise a large and heterogeneous spectrum of disorders; some diseases such as neuromyelitis optica spectrum disorders (NMOSD)1-3 and autoimmune encephalitis (AIE)4,5 are characterized by disease-specific (auto)antibodies, while others such as Susac syndrome6 feature alterations in the T cell compartment or alterations in both the adaptive and innate immune compartment, as in multiple sclerosis.7–32 Multiple sclerosis is the most prevalent autoimmune disease of the CNS. With 2.3 million people affected worldwide, multiple sclerosis is a paradigmatic example of a chronic neuroinflammatory disease, comprising typical features of CNS autoimmunity, including (i) a clinically heterogeneous disease course; (ii) continuous disease evolution over time; and (iii) putative pathophysiological heterogeneity within one disease (i.e. inflammatory versus neurodegenerative components).
CSF is an ultrafiltrate of the serum surrounding and protecting the CNS parenchyma.33 Since pathophysiological changes in the CNS are reflected in the CSF,34 its analysis, concomitant with radiological, (neuro-)physiological and neuropsychological examinations, facilitates the diagnosis of neurological diseases. However, CSF parameters such as total cell counts, lactate, glucose quotient, integrity of the blood–CSF barrier, total protein and intrathecal immunoglobulin (Ig) synthesis enable only a gross differentiation between infectious, autoimmune and degenerative CNS disorders.35 Whether multi-dimensional cellular peripheral blood and CSF characterization by flow cytometry can support a more fine-grained distinction between clinically similar disease entities remains unknown.36,37 By investigating patients with neuroinflammatory, degenerative, and vascular disorders (Fig. 1A), we identified distinct cellular parameters in the peripheral blood and CSF that can improve the classification of neurological diseases and provide insights into shared and distinct pathophysiological processes (Fig. 1B).
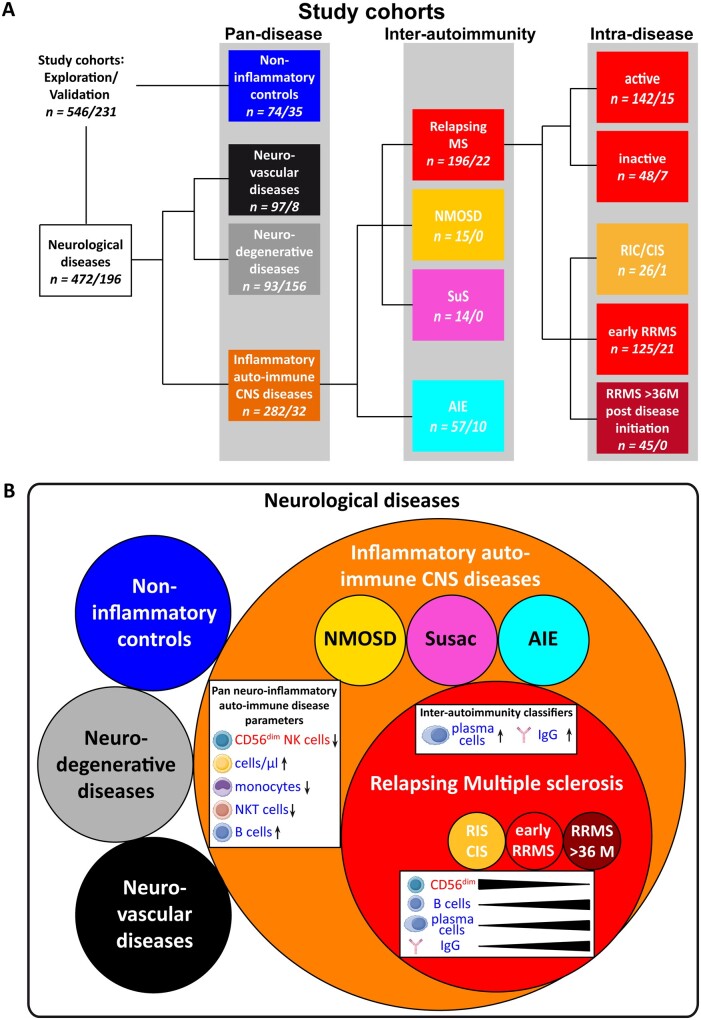
Figure 1.
Study cohorts and conclusion. (A) Data derived from patients undergoing routine as well as flow cytometric CSF analysis were investigated to identify factors assisting the classification of neurological diseases. The results from this discovery cohort (first n numbers) were validated in a comparable validation cohort (second n numbers). From a total of 546/231 patients undergoing routine as well as flow cytometric CSF analysis, a group of 74/35 individuals was identified as non-inflammatory controls (blue box). Furthermore, 97/8 patients with neuro-vascular (ischaemic stroke, black boxes) and 93/156 patients with neurodegenerative diseases (grey boxes, amyotrophic lateral sclerosis, n = 52/0; mild Alzheimer’s disease, n = 41/156) were included in the study. The orange box details 282/32 patients who suffered from inflammatory (auto-)immune CNS diseases, which could be further differentiated into patients with multiple sclerosis (MS; n = 196/22, red box), NMOSD (n = 15/0, yellow box), Susac syndrome (SuS; n = 14/0, magenta box), or AIE (n = 57/10, cyan box). Patients with RRMS could be further divided into patients with radiologically or clinically isolated symptoms (RIC/CIS; n = 26/1), early RRMS (≤36 months since onset, n = 125/21) and those in the later stages >36 months since disease onset (n = 45/0) or into patients with active (n = 142/15) or inactive (n = 48/7) disease at the time of lumbar puncture. (B) Visualization of multi-level discrimination of neurological diseases by flow cytometric immune profiling between (i) inflammatory autoimmune CNS diseases and other neurological diseases; (ii) RRMS and other inflammatory autoimmune CNS diseases; and (iii) alteration of involved parameters during the disease course.RRMS)
Materials and methods
Ethics statement
The study was conducted according to the Declaration of Helsinki and approved by the ethics committee of the University of Münster (registration nos. 2010-262-f-S, 2011-665-f-S, 2013-350-f-S, 2014-068-f-S and 2016-053-f-S). All patients provided written informed consent.
Patient characteristics
In total, 3424 files of patients who received routine CSF diagnostics and multi-parameter flow cytometry following our standard operating procedures between 2011 and 2019 were retrospectively screened for the discovery cohort. From these, we selected 546 manually curated patients for the discovery cohort including: (i) 74 non-inflammatory control subjects; (ii) 282 patients with neuroinflammatory diseases, including 196 patients with relapsing multiple sclerosis, 15 with NMOSD, 14 with Susac syndrome and 57 with AIE; (iii) 93 patients with neurodegenerative diseases, including 52 with amyotrophic lateral sclerosis and 41 with mild Alzheimer’s disease; and (iv) 97 patients with ischaemic stroke, exemplary of neurovascular disease (Fig. 1A and Supplementary Table 1). In addition, we screened an additional 1448 files of patients and identified an independent validation cohort of 231 patients [(i) 35; (ii) 32; (iii) 156; and (iv) eight] to verify the results generated based on data from the discovery cohort (Fig. 1A and Supplementary Table 1). All patients were treatment-naïve for immune-modulating drugs at the time of sampling. Patients were diagnosed according to current guidelines37–42 by experienced neurologists. Non-inflammatory controls included patients with somatoform disorders or patients who donated CSF during the course of spinal anaesthesia. Non-inflammatory control subjects presented with intrathecal leucocyte counts <5 cells/µl, intrathecal lactate <2 mmol/l, an intact blood–CSF barrier as identified by the age-adjusted albumin quotient, no intrathecal Ig-synthesis according to Reiber criteria and oligoclonal band pattern type 1.43 Multiple sclerosis disease activity at the time of lumbar puncture was defined as a clinical relapse within the preceding 4 weeks and/or new T2 or gadolinium-enhanced lesions on brain MRI within the preceding 12 weeks. MRI scans were performed with a field strength of 1.5 or 3.0 T. In addition to 777 untreated patients, 105 patients with relapsing-remitting multiple sclerosis (RRMS) under disease-modifying therapy (interferon-β n = 9, glatiramer acetate n = 5, dimethyl fumarate n = 5, fingolimod n = 15, natalizumab n = 41 or alemtuzumab 10–14 months post-last injection n = 30) were included solely for Fig. 2D.
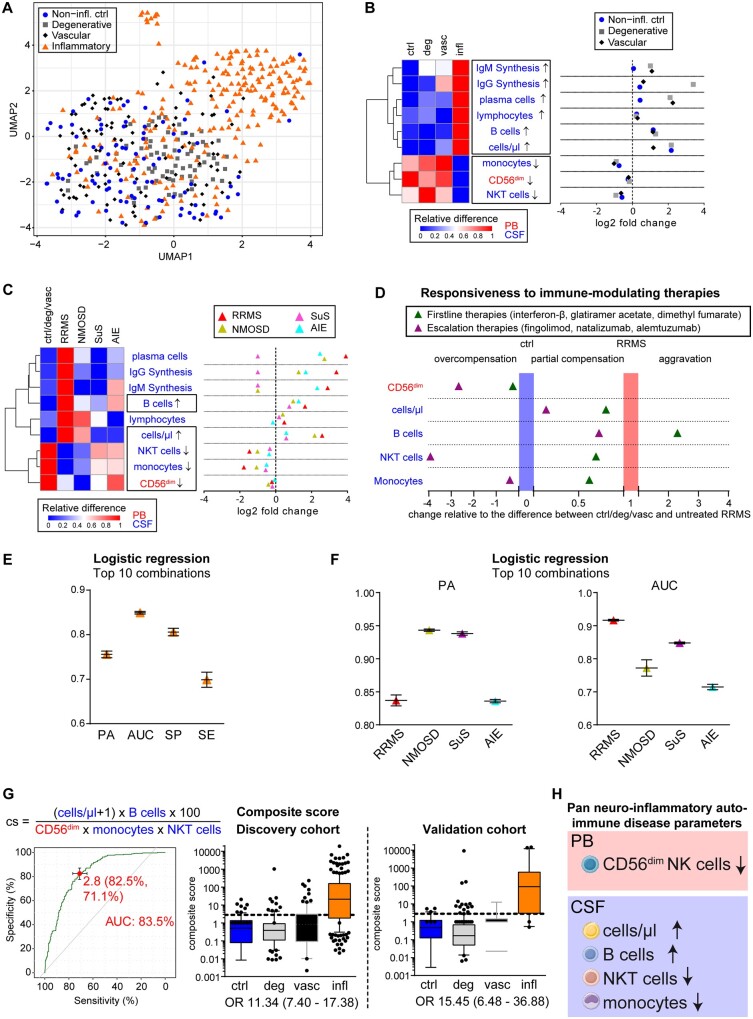
Figure 2.
Identification of pan-disease parameters characterizing CNS neuroinflammation. (A) Patients from the discovery cohort under homeostatic conditions (non-inflammatory controls, blue circle, n = 74), neurodegenerative [grey square, n = 93: amyotrophic lateral sclerosis (ALS), mild Alzheimer’s disease (mAD)], neurovascular (red diamond, n = 97) or inflammatory autoimmune CNS disorders [inflammatory, orange triangle, n = 229: Early RRMS including radiologically or clinically isolated syndrome (RIS/CIS), NMOSD, Susac syndrome (SuS) and AIE] were mapped based on immunological data from the peripheral blood and CSF following dimensionality reduction with uniform manifold approximation and prediction (UMAP). (B and C) Heat map (left) illustrating the relative changes and dot plot (right) displaying the fold changes of the nine parameters differentiating CNS neuroinflammation (infl) from non-inflammatory controls (ctrl), neurodegenerative (deg) and neurovascular (vasc) conditions (B) as well as in distinct inflammatory autoimmune diseases of the CNS (early RRMS including RIS/CIS, red triangle, n = 143; NMOSD, yellow triangle, n = 15; Susac syndrome, pink triangle, n = 14; AIE, blue triangle, n = 57) in comparison to control cohorts (C). In the heat maps, red indicates the highest expression, whereas blue indicates the lowest expression. Parameter labelling provides information on the respective compartment (peripheral blood; CSF). The black boxes highlight the commonly altered parameters. (D) Realignment plot illustrating the change in pan neuroinflammatory parameters as a consequence of first line and escalation immune therapies. The median height of each parameter was summed up and averaged as described for volcano plots by division of the result from the subtraction of the control (non-inflammatory control, degenerative, and vascular cohort) median from the respective median by the result of the subtraction of the control median from the median of treatment-naïve patients with RRMS. For CD56dim NK cells for example, controls were at 12.22%, naïve RRMS at 10.55% and escalation therapies at 17.62%, resulting in (17.62%–12.22%)/(10.55%–12.22%) = −3.23, indicating an overcompensation (<0) beyond control levels. Thus, parameters overcompensated by immunotherapy [baseline therapies (green triangle): interferon-β n = 9, glatiramer acetate n = 5 and dimethyl fumarate n = 5; escalation therapies (purple triangle): fingolimod n = 15, natalizumab n = 41 and alemtuzumab (10–14 months post last injection) n = 30 are in the left part of the graph, whereas parameters with partial compensation for neuroinflammatory alterations are in the middle. Parameters exhibiting aggravated levels more different from control cohorts than untreated RRMS patients are represented in the right of the graph. (E) Mean and standard deviation of prediction accuracy (PA), area under the curve (AUC), specificity (SP) and sensitivity (SE) from the top 10 combinations of up to five pan-disease parameters as calculated by logistic regression to differentiate all neuroinflammatory diseases from non-inflammatory controls, neurodegenerative, and neurovascular diseases. (F) Mean and standard deviation of prediction accuracy and AUC for differentiating distinct neuroinflammatory CNS diseases (RRMS, NMOSD, Susac syndrome and AIE) from control cohorts. (G) Predictive score composed by division of parameters consistently increased by parameters decreased in neuroinflammation (top). Receiver operating characteristic (ROC) analysis (left) identified a cut-off of 2.8 distinguishing (right) patients with neuroinflammatory diseases (infl) from non-inflammatory controls (ctrl), neurodegenerative (deg) and neurovascular (vasc) patients with an AUC of 83.5 and odds ratio (OR) of 11.34. This composite score was verified in the validation cohort, resulting in the identification of neuroinflammatory patients with an OR of 15.45. (H) Pan neuroinflammatory auto-immune disease parameters commonly altered in all neuroinflammatory autoimmune diseases of the CNS in comparison with other neurological diseases as well homeostatic conditions.
Biomaterials
CSF was collected by lumbar puncture and processed within 1 h, simultaneously to serum collection in serum-gel tubes (Sarstedt) and peripheral blood collection in EDTA tubes (Sarstedt). Further details on the generation of conventional CSF data and multi-parametric flow cytometric data are provided in the Supplementary material.
Data processing
By combining conventional CSF analysis with multi-parameter flow cytometry, we obtained a total of 113 cellular and molecular features in the peripheral blood and CSF per patient (Supplementary Fig. 1A). To eliminate colinear features while retaining as much information as possible, we computed pairwise correlations using complete observations and calculated their statistical significance using the corr.test function from the psych package in R3.5.2 via RStudio 1.1.442, including adjustment for multiple comparisons using the ‘Holm’ approach (Supplementary material). Variables showing a high absolute correlation (>0.9) with statistically significant P-values (<0.05) were considered to be correlated (Supplementary Fig. 2). From the correlated variables, only one was included in further analyses (Supplementary Table 2). Furthermore, of the closely related biological features (e.g. CD4+ T cells/ml and CD4+ T cells as percentage of lymphocytes), only one was retained. Features excluded in one compartment were also excluded in the other respective compartment (e.g. replacement of CD4+ T cells/ml in peripheral blood and CSF). Using this approach, correlated parameters (r2 > 90%, P-value < 0.05) were condensed into a single feature for further analysis. Thereby, the number of features was reduced to 34 (Supplementary Fig. 1A and Supplementary Table 2). The missingness in the remaining dataset (Supplementary Fig. 1A) was <1%. With the non-colinear variables, missing data were imputed groupwise, because observations from different groups could differ widely across variables using the median imputation method (Supplementary material). Therefore, the median of each variable at each group was calculated and the missing values for that variable and group were assigned their median value adding a tiny jitter to make these imputed values different from each other. For categorical parameters, imputed values were rounded to the next categorical value. Intrathecal plasma cells were categorized as plasma cell positive if there was at least one plasma cell per millilitre of CSF, since the detection of intrathecal plasma cells per se indicates pathophysiological processes and plasma cells are absent in non-pathological controls. For machine learning approaches as well as uniform manifold approximation and prediction (UMAP) dimensionality reduction, the resulting data were additionally centred and scaled along the variables (Supplementary material) to bring all the variables to a similar scale and set the data to a normal distribution.
Composite scores
In general, composite scores assisting the differentiation of distinct pathologies on a pan-disease or inter-disease level were created by dividing the parameters identified to be consistently increased by parameters exhibiting reduced frequencies. Parameters frequently taking a value of zero—e.g. categorical parameters—were added one by one to avoid errors while preserving the information content.
Statistical analysis
Statistical analyses were performed using R3.5.2 via RStudio 1.1.442, GraphPad Prism V6.01, and Excel 2016. The tests used are indicated in the respective figure legend. Further details on the statistical methods associated with machine learning, UMAP, volcano plots, heat maps, realignment plots, receiver operating characteristic and correlation analyses are provided in the Supplementary material.
Data availability
Data are available from the corresponding author upon reasonable request. Information on the R codes used is available from https://doi.org/10.17605/OSF.IO/EQRF5.
Results
To identify the soluble and cellular parameters in peripheral blood and CSF that can improve the diagnosis of neurological diseases, we performed a comprehensive retrospective study in a discovery cohort of 546 patients with autoimmune neuroinflammatory, neurodegenerative, neurovascular and non-inflammatory conditions (Fig. 1A and Supplementary Table 1). Patients received no immune-modulating drugs at the time of sampling. Feature selection followed by dimensionality reduction with unsupervised cluster analysis using UMAP44 revealed that patients with autoimmune neuroinflammatory diseases cluster together (Fig. 2A), suggesting that their profiles generally differ from patients with neurodegenerative, neurovascular and non-inflammatory conditions (Supplementary Fig. 3). Machine learning and exploratory data analysis identified parameters that differentiate autoimmune neuroinflammatory from other conditions (Supplementary Fig. 4A and B and Supplementary Table 3). Nine of the identified parameters were consistently altered compared with the three control cohorts (Fig. 2B and Supplementary Table 4). These nine parameters were further investigated to identify pan-disease parameters commonly altered across all studied neuro-inflammatory CNS diseases. Using this strategy, we identified cells/µl, monocytes, natural killer (NK)T cells and B cells as pan-disease parameters in the CSF and, more strikingly, CD56dim NK cells in the peripheral blood (Fig. 2C). Interestingly, these pan-disease parameters were susceptible to immune-modulating therapies (Fig. 2D). The five identified pan-disease parameters discriminated neuro-inflammatory CNS diseases from non-pathological controls and patients with neurodegenerative and neurovascular conditions with a high prediction accuracy of 76%, i.e. correctly assigning 76% of the patients without any further diagnostic information, an area under the curve (AUC) of 85%, a specificity of 81% and a sensitivity of 70% (Fig. 2E and Supplementary Table 3). Importantly, our strategy for the identification of pan-inflammatory markers ensured that the values for prediction accuracy and AUC were between 84%–94% and 71%–92%, respectively, for all investigated neuro-inflammatory diseases (Fig. 2F). Alteration of the pan-disease blood parameter CD56dim NK cells was confirmed in an independent validation cohort of 115 patients with RRMS, exemplary of neuroinflammatory autoimmune diseases, in comparison with 71 healthy individuals (Supplementary Fig. 4C). While some of these changes have been described in multiple sclerosis,11–13,30,45,46 the relevance of those pan-disease parameters as a general feature of neuroinflammation is novel and hints at their pathophysiological role in CNS autoimmunity. Although B cells and NKT cells in the CSF were affected by age under non-inflammatory conditions (Supplementary Fig. 5A) and patients with neurodegenerative and neurovascular diseases were on average older than those with neuroinflammatory conditions (Supplementary Table 1), differences in these parameters still persisted in a smaller age-matched subcohort (Supplementary Fig. 5B). To facilitate differential diagnosis, we summarized the identified pan-disease parameters into a composite score; thus, translating our results into clinical practice (Fig. 2G). Receiver operating characteristic (ROC) analysis provided a cut-off of 2.8 differentiating autoimmune neuroinflammatory from other conditions with an odds ratio (OR) of 11.34. An independent validation cohort of 231 patients (Fig. 1A and Supplementary Table 1) confirmed this result with an OR of 15.45 (Fig. 2G). In summary, we have identified distinct peripheral and intrathecal cellular pan-disease parameters commonly altered across all neuro-inflammatory diseases (Fig. 2H).
Differential diagnosis between distinct CNS autoimmune diseases can be difficult and often lacks specific biomarkers. Therefore, we sought to identify inter-autoimmunity parameters to improve the differentiation of RRMS as the prototype of an autoimmune disease of the CNS from its differential diagnoses NMOSD, Susac syndrome and AIE (Fig. 1A and Supplementary Table 1). In addition to established parameters, including the occurrence of intrathecal plasma cells concomitant with IgG synthesis, logistic regression identified intrathecal IgA and IgM synthesis as additional inter-autoimmunity classifiers (Supplementary Fig. 6A). Furthermore, disease-specific parameters such as alterations in (i) circulating peripheral blood CD56bright NK cells and intrathecal lactate concentrations in NMOSD; (ii) circulating CD4+ and CD8+ T cells and activation of circulating and intrathecal activated HLA-DR+ CD8+ T cells in Susac syndrome; and (iii) circulating and intrathecal lymphocytes, intrathecal NKT cells, monocytes and CD14+CD16+ monocytes in AIE were identified. These parameters differentiate RRMS from other neuroinflammatory diseases with high prediction accuracy (NMOSD: 90.1, Susac syndrome: 98.1, AIE: 90.9) and AUCs (NMOSD: 92.4, Susac syndrome: 94.9, AIE: 82.6) (Supplementary Fig. 6A and Supplementary Table 3). Further exploratory analysis validated this result by revealing that, in accordance with the literature,13,22,23,26–28 the occurrence of plasma cells in the CSF together with intrathecal IgG synthesis is an immune-pathogenic trait characteristic for RRMS (Supplementary Fig. 6B, red labels). Strikingly, those two inter-autoimmunity classifiers were sufficient to distinguish RRMS from the other neuroinflammatory diseases with a comparably high prediction accuracy (NMOSD: 87.3, Susac syndrome: 95.3, AIE: 89.4) and AUC (NMOSD: 91.5, Susac syndrome: 90.7, AIE: 82.7) (Fig. 3A and Supplementary Table 3), compared with the additional parameters. ROC analysis of a composite score derived by the combination of intrathecal plasma cells and IgG synthesis revealed a cut-off of 1.5 differentiating RRMS from NMOSD, Susac syndrome and AIE with ORs of 18.9 and 35.8 in the discovery and validation cohort, respectively (Fig. 3B). Taken together, we identified the occurrence of intrathecal plasma cells concomitant with IgG synthesis as sufficient inter-autoimmunity parameters defining RRMS-specific immune-pathogenic traits (Fig. 3C).
Figure 3.
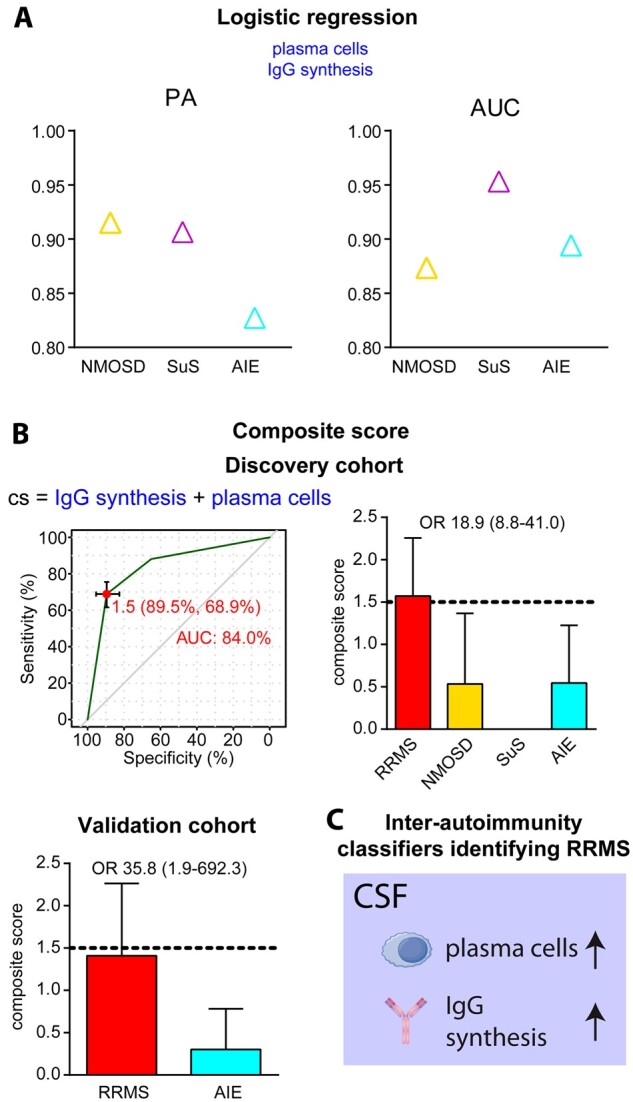
Inter-autoimmunity classifiers characterizing distinct neuro-inflammatory diseases. (A) Prediction accuracy and AUC of plasma cell occurrence and intrathecal IgG synthesis as determined by logistic regression for differentiating early RRMS, including radiologically or clinically isolated syndrome (RIS/CIS) from NMOSD (yellow triangle), Susac syndrome (SuS, pink triangle) and AIE (blue triangle). (B) Composite score (top left) derived by addition of plasma cell positivity (=1) and intrathecal IgG synthesis (=1) allowing differentiation (top right) of patients with RRMS in the discovery cohort from patients with NMOSD, Susac syndrome and AIE by a cut-off of 1.5 as determined by ROC analysis (top left) with an AUC of 84.0 and an OR of 18.9. Inter-autoimmunity classification was verified in the validation cohort, showing classification with an odds ratio of 35.8 (bottom left). (C) Inter-autoimmunity classifiers distinguishing RRMS from other neuroinflammatory autoimmune diseases.
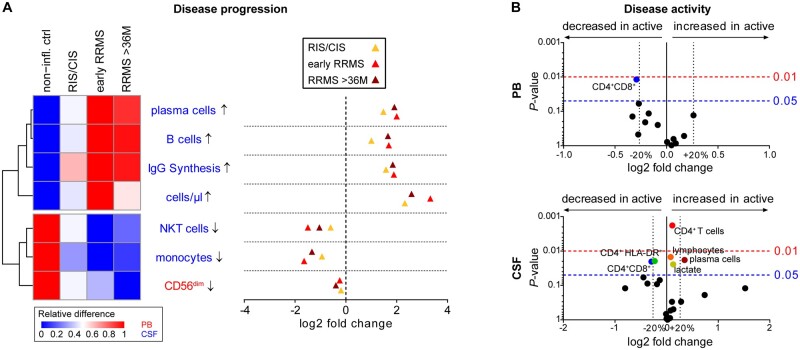
Multiple sclerosis is characterized by the dissemination of inflammatory demyelinating lesions in space and time. Thus, we reasoned that this may be a paradigmatic example that can be used to study how immune profiles develop during disease evolution as a trajectory of time since disease manifestation. We analysed the alterations in cellular parameters involved in differentiating inflammatory autoimmune CNS diseases in therapy-naïve patients with radiologically or clinically isolated syndrome, early RRMS (disease onset ≤ 36 months) and RRMS at later stages (disease onset > 36 months) (Fig. 4A). Strikingly, while pan-disease and inter-autoimmunity parameters were present at all stages of disease progression, thereby supporting their diagnostic value, disease-related alterations in the circulating proportions of CD56dim NK cells as well as intrathecal B cells, plasma cells, and IgG synthesis gradually increased during disease progression. This indicated that both pan-disease parameters and RRMS-specific alterations progress with disease evolution. Dichotomization of patients with RRMS based on disease activity revealed minor effects on intrathecal proportions of lymphocytes, CD4+ T cells, CD4+ HLA-DR+ T cells and lactate levels (Fig. 4B). In contrast, changes in peripheral and intrathecal CD4+CD8+ T cells as well as intrathecal plasma cells were more pronounced (Fig. 4B).
Figure 4.
Factors describing RRMS disease evolution and activity. (A) Heat map (left) illustrating the relative changes and dot plot (right) displaying the fold changes in pan- and inter-autoimmunity classifiers between patients with non-inflammatory diseases as well as patients from the discovery cohort with radiologically or clinically isolated syndrome (RIS/CIS, yellow triangles, n = 26) or RRMS within (early, light red triangles, n = 125) and after 36 months (>36 M, dark red triangles, n = 45) from disease manifestation. On the heat map, red indicates the highest expression, whereas blue indicates the lowest expression. Parameter labelling provides information on the respective compartment (peripheral blood or CSF). (B) Volcano plots representing the median fold change in parameters between patients with RRMS without (inactive, n = 48) and with (active, n = 142) clinical and/or radiological disease activity within 4 weeks before lumbar puncture. P-values were calculated by Mann-Whitney test. Only significantly (P < 0.05) altered parameters are labelled.
Overall, our study revealed that cellular parameters in the peripheral blood and CSF can be employed to improve differential diagnosis between neurological diseases and within CNS autoimmune conditions with a high prediction accuracy while providing novel information about pathophysiological processes.
Discussion
Our study revealed that distinct cellular parameters in the peripheral blood and CSF improve differential diagnosis with high prediction accuracy and provided insights into shared and distinct pathophysiological processes allowing the classification of neurological diseases.
By combining feature selection with dimensionality reduction and machine learning, we identified pan-disease parameters in the CSF (cells/µl, monocytes, NKT cells and B cells) and more strikingly in the peripheral blood (CD56dim NK cells) that were altered across all of the investigated inflammatory autoimmune diseases of the CNS and susceptible to immune-modulating therapies, while discriminating from neurodegenerative and neurovascular conditions. While these changes were already expected for multiple sclerosis,11–13,30,45,46 the relevance of those pan-disease parameters as common immune-pathogenic traits of neuroinflammation is novel as is the relevance of intrathecal NKT cells in AIE and a potential role of CD56dim NK cells in NMOSD. This suggests a pathophysiological role for these cell subsets in CNS autoimmunity. CD56dim NK cells have been shown to play a crucial role in controlling the activation of antigen-specific T cells in multiple sclerosis,30 and it is therefore tempting to speculate that impaired immune regulation by NK cells may be a general feature driving neuroinflammation. Likewise, our study did not only validate several reports demonstrating disease-specific changes in the intrathecal B-cell compartment of patients with multiple sclerosis,13,22,23,26–28 but also highlighted enhanced frequencies of intrathecal B cells as a common immune-pathogenic trait driving neuroinflammation. To facilitate the differential diagnosis of patients with CNS-autoimmune disease from other neurological disorders, especially in cases where MRI and neurophysiological parameters are non-pathological, we constructed a composite score of the pan-disease parameters. This may be relevant to the differentiation of either patients with demyelinating white matter MRI lesions of unclear inflammatory origin (e.g. multiple sclerosis, NMOSD or Susac syndrome) from those with an ischaemic origin (i.e. stroke) or patients with non-demyelinating grey matter lesions of inflammatory origin (i.e. AIE) from those with a degenerative origin (e.g. mild Alzheimer’s disease). The identified pan-disease parameters discriminate neuroinflammatory CNS diseases from other CNS conditions with a prediction accuracy of 76%, i.e. from 1000 patients with suspected inflammatory CNS disease, 760 would be assigned correctly without any further diagnostic information. Of note, our strategy ensures that this is true for each of the investigated neuroinflammatory CNS diseases.
Differential diagnosis between distinct CNS autoimmune diseases can be hampered by specific clinical and/or MRI features and the lack of specific biomarkers. A notable example is antibody-seronegative NMOSD, which can be indistinguishable from multiple sclerosis in its clinical presentation, despite distinct disease mechanisms.47 Another example is Susac syndrome, in which the classical clinical triad of encephalopathy, branch-retinal-artery-occlusions and sensory-neural hearing loss is only seen at disease onset in 13% of cases.48 Misdiagnosis as multiple sclerosis can be detrimental, since some therapeutics such as interferon-β, dimethyl fumarate, fingolimod, natalizumab and alemtuzumab in NMOSD (reviewed in Jarius et al.49) and interferon-β in Susac syndrome50 have adverse effects. We were able to demonstrate that established parameters including the occurrence of intrathecal plasma cells and IgG synthesis are sufficient to differentiate patients with multiple sclerosis from those suffering from NMOSD, Susac syndrome and AIE with prediction accuracy values of of 92%, 91% and 83%, respectively. In addition, we identified distinct cellular parameters that improve differential diagnosis with implications for personalized therapeutic decisions. For instance, differential diagnosis between Susac syndrome and multiple sclerosis can be improved by including HLA-DR expression on CD8 T cells in the peripheral blood and CSF as additional parameters. To further strengthen the differential diagnosis of neurological CNS diseases, several studies investigated the power of various soluble parameters in serum and CSF.51,52 A combination of cellular and soluble parameters should further improve the predictive power.
Taken together, our study demonstrates the power of multi-dimensional CSF analysis in the classification of neurological diseases and endophenotypes within a disease spectrum (e.g. multiple sclerosis) with implications for personalized medicine. Future studies including both, more detailed cellular (B/T-cell subsets) and additional soluble parameters such as interferon-γ, tumour necrosis factor-α, amyloid-β, neurofilament light chain, CXCL13 and a proliferation-inducing ligand,51,53–55 analysed by state-of-the-art deep learning techniques may further improve differential diagnosis. Identification of other discriminating parameters with a potentially lower effect size requires larger cohorts, especially of patients with orphan diseases such as NMOSD and Susac syndrome. Moreover, the inclusion of additional neurological diseases may reveal further parameters assisting differential diagnosis. Additional data from longitudinal studies over 10–20 years may identify parameters involved in the progression from relapsing-remitting to secondary progressive multiple sclerosis or within other neuroinflammatory CNS diseases. Individual patterns and dominance types of immune signatures not only reveal novel insights in the compartment-specific changes associated with different diseases, but also regarding their underlying pathogenesis. Thus, individual immune signatures may be used to select therapeutic options best suited to normalize immune profiles, thereby potentially reducing adverse events and increasing treatment efficacy.
Supplementary Material
Acknowledgements
We are especially grateful to all patients participating in this study. Furthermore, the authors would like to thank Kirsten Weiss, Gabriele Berens, Arne Seeger, Tobias Schilling, Tanja Wandelt, Christiane Schulze-Weppel, and Lena Schünemann for providing expert services in clinical laboratory diagnostics and the handling of patient material.
Funding
This study was supported by the German Research Council (DFG) through SFB-CRC TR128 ‘Initiating/Effector versus Regulatory Mechanisms in Multiple Sclerosis—Progress towards Tackling the Disease’, projects A09 (to C.C.G. and H.W.) and Z02 (to H.W. and L.K.), GR3946_3/1 ‘Susac syndrome (SuS) as a paradigm of a CD8+ T-cell mediated endotheliopathy’ (to C.C.G.), the Interdisciplinary Center for Clinical Studies (IZKF) grant Kl3/010/19 ‘Antigen-specific CD8 T-cell responses in Neuromyelitis Optica and Susac syndrome’ (to C.C.G. and L.K.), and the Federal Ministry of Education and Research funded Disease Related Competence Network for Multiple Sclerosis (KKNMS, project FKZ01GI1603A to H.W., L.K. and C.C.G.).
Competing interests
C.C.G. received speaker honoraria from Mylan, Bayer Healthcare, and Sanofi-Genzyme and travel/accommodation/meeting expenses from Bayer Healthcare, Biogen, EUROIMMUN, Novartis, and Sanofi-Genzyme. She also received research support from Biogen, Roche, and Novartis. A.S.-M. received travel expenses and research support from Novartis. M.P. received travel/accommodation/meeting expenses from Novartis. J.K. received honoraria for lecturing from Biogen, Novartis, Sanofi Genzyme, Merck, Mylan, and Teva and financial research support from Sanofi Genzyme and Novartis. L.R. received travel reimbursements from Novartis and Sanofi Genzyme. T.R. reports grants from German Ministry of Education, Science, Research and Technology, grants and personal fees from Sanofi-Genzyme and Alexion; personal fees from Biogen, Roche and Teva; personal fees and nonfinancial support from Merck Serono, outside the submitted work. T.S.-H. received research and travel support from Biogen and Novartis Pharma. N.S. received travel support from Biogen and Novartis, as well as research support from Biogen outside the submitted work. J.M. receives grants from EVER Pharma Jena GmbH, and Ferrer International, travel grants from Boehringer Ingelheim and speaking fees from Bayer Vital and Chugai Pharma. N.M. has received honoraria for lecturing and travel expenses for attending meetings from Biogen Idec, GlaxoSmith Kline, Teva, Novartis Pharma, Bayer Healthcare, Genzyme, Alexion Pharamceuticals, Fresenius Medical Care, Diamed and BIAL, and has received financial research support from EUROIMMUN, Fresenius Medical Care, Diamed, Alexion Pharamceuticals and Novartis Pharma. L.K. received honoraria for lecturing, and travel expenses for attending meetings from Alexion, Bayer Health Care, Biogen, Genzyme, Grifols, Janssen Cilag Pharma, Merck Serono, Novartis, Roche, Sanofi-Aventis, Santhera and Teva. S.G.M. received honoraria for lecturing, and travel expenses for attending meetings, and research support from Alexion, Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, HERZ Burgdorf, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, Quintiles IMS and Teva. G.M. has received speaker honoraria from Alexion and PFB pharma. S.E.B. received honoraria for acting as a member of Scientific Advisory Boards of Biogen, Genzyme, EMD Serono, Novartis, Genentech and Sanofi-Aventis. H.W. received honoraria for acting as a member of Scientific Advisory Boards of Biogen, Evgen, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG and Sanofi-Aventis as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd, Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Genzyme, TEVA and WebMD Global. He is acting as a paid consultant for Abbvie, Actelion, Biogen, IGES, Johnson & Johnson, Novartis, Roche, Sanofi-Aventis and the Swiss Multiple Sclerosis Society. L.M., C.S., C.B., A.S.-P., L.L. and S.R. report no competing interests. Information presented in this manuscript is part of a patent application in preparation.
Supplementary material
Supplementary material is available at Brain online.
Glossary
- AIE
autoimmune encephalitis
- NMOSD
neuromyelitis optica spectrum disorder
- RRMS
relapsing-remitting multiple sclerosis
References
- 1. Jarius S, Wildemann B, Paul F.. Neuromyelitis optica: Clinical features, immunopathogenesis and treatment. Clin Exp Immunol. 2014;176(2):149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gruter T, Ott A, Meyer W, et al. Effects of IVIG treatment on autoantibody testing in neurological patients: Marked reduction in sensitivity but reliable specificity. J Neurol. 2020;267(3):715–720. [DOI] [PubMed] [Google Scholar]
- 3. Ringelstein M, Harmel J, Zimmermann H, et al. ; Neuromyelitis Optica Study Group (NEMOS). Longitudinal optic neuritis-unrelated visual evoked potential changes in NMO spectrum disorders. Neurology. 2020;94(4):e407–e418. [DOI] [PubMed] [Google Scholar]
- 4. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dalmau J, Graus F.. Antibody-mediated encephalitis. N Engl J Med. 2018;378(9):840–851. [DOI] [PubMed] [Google Scholar]
- 6. Gross CC, Meyer C, Bhatia U, et al. CD8(+) T cell-mediated endotheliopathy is a targetable mechanism of neuro-inflammation in Susac syndrome. Nat Commun. 2019;10(1):5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiendl H. Neuroinflammation: The world is not enough. Curr Opin Neurol. 2012;25(3):302–305. [DOI] [PubMed] [Google Scholar]
- 8. Lassmann H. The changing concepts in the neuropathology of acquired demyelinating central nervous system disorders. Curr Opin Neurol. 2019;32(3):313–319. [DOI] [PubMed] [Google Scholar]
- 9. Scolozzi R, Boccafogli A, Tola MR, et al. T-cell phenotypic profiles in the cerebrospinal fluid and peripheral blood of multiple sclerosis patients. J Neurol Sci. 1992;108(1):93–98. [DOI] [PubMed] [Google Scholar]
- 10. Stinissen P, Vandevyver C, Medaer R, et al. Increased frequency of gamma delta T cells in cerebrospinal fluid and peripheral blood of patients with multiple sclerosis. Reactivity, cytotoxicity, and T cell receptor V gene rearrangements. J Immunol. 1995;154:4883–4894. [PubMed] [Google Scholar]
- 11. Kraus J, Oschmann P, Engelhardt B, et al. Soluble and cell surface ICAM-3 in blood and cerebrospinal fluid of patients with multiple sclerosis: Influence of methylprednisolone treatment and relevance as markers for disease activity. Acta Neurol Scand. 2000;101(2):135–139. [DOI] [PubMed] [Google Scholar]
- 12. Kraus J, Oschmann P, Engelhardt B, et al. CD45RA+ ICAM-3+ lymphocytes in cerebrospinal fluid and blood as markers of disease activity in patients with multiple sclerosis. Acta Neurol Scand. 2000;102(5):326–332. [DOI] [PubMed] [Google Scholar]
- 13. Cepok S, Jacobsen M, Schock S, et al. Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain. 2001;124(Pt 11):2169–2176. [DOI] [PubMed] [Google Scholar]
- 14. Misu T, Onodera H, Fujihara K, et al. Chemokine receptor expression on T cells in blood and cerebrospinal fluid at relapse and remission of multiple sclerosis: Imbalance of TH1/TH2-associated chemokine signaling. J Neuroimmunol. 2001;114(1-2):207–212. [DOI] [PubMed] [Google Scholar]
- 15. Kivisakk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: Evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100(14):8389–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okuda Y, Okuda M, Apatoff BR, Posnett DN.. The activation of memory CD4(+) T cells and CD8(+) T cells in patients with multiple sclerosis. J Neurol Sci. 2005;235(1-2):11–17. [DOI] [PubMed] [Google Scholar]
- 17. Heinrich A, Ahrens N, Schmidt S, Khaw AV.. Immunophenotypic patterns of T-cell activation in neuroinflammatory diseases. Acta Neurol Scand. 2006;113(4):248–255. [DOI] [PubMed] [Google Scholar]
- 18. Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H.. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol. 2007;147(3):412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E.. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132(Pt 12):3329–3341. [DOI] [PubMed] [Google Scholar]
- 20. Mullen KM, Gocke AR, Allie R, et al. Expression of CCR7 and CD45RA in CD4+ and CD8+ subsets in cerebrospinal fluid of 134 patients with inflammatory and non-inflammatory neurological diseases. J Neuroimmunol. 2012;249(1-2):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beltran E, Gerdes LA, Hansen J, et al. Early adaptive immune activation detected in monozygotic twins with prodromal multiple sclerosis. J Clin Invest. 2019;129(11):4758–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han S, Lin YC, Wu T, et al. Comprehensive immunophenotyping of cerebrospinal fluid cells in patients with neuroimmunological diseases. J Immunol. 2014;192(6):2551–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schafflick D, Xu CA, Hartlehnert M, et al. Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nat Commun. 2020;11(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oksaranta O, Tarvonen S, Ilonen J, et al. Influx of nonactivated t lymphocytes into the cerebrospinal fluid during relapse of multiple sclerosis. Ann Neurol. 1995;38(3):465–468. [DOI] [PubMed] [Google Scholar]
- 25. Schirmer L, Rothhammer V, Hemmer B, Korn T.. Enriched CD161high CCR6+ γδ T cells in the cerebrospinal fluid of patients with multiple sclerosis. JAMA Neurol. 2013;70(3):345–351. [DOI] [PubMed] [Google Scholar]
- 26. Cepok S, von Geldern G, Grummel V, et al. Accumulation of class switched IGD-IGM-memory B cells in the cerebrospinal fluid during neuroinflammation. J Neuroimmunol. 2006;180(1-2):33–39. [DOI] [PubMed] [Google Scholar]
- 27. Sellebjerg F, Bornsen L, Khademi M, et al. Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology. 2009;73(23):2003–2010. [DOI] [PubMed] [Google Scholar]
- 28. Kowarik MC, Cepok S, Sellner J, et al. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflamm. 2012;9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haman KH, Norton TM, Ronconi RA, et al. Great shearwater (Puffinus gravis) mortality events along the eastern coast of the United States. J Wildl Dis. 2013;49(2):235–245. [DOI] [PubMed] [Google Scholar]
- 30. Gross CC, Schulte-Mecklenbeck A, Runzi A, et al. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc Natl Acad Sci U S A. 2016;113(21):E2973–E2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pashenkov M, Huang YM, Kostulas V, Haglund M, Söderström M, Link H.. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124(Pt 3):480–492. [DOI] [PubMed] [Google Scholar]
- 32. Longhini A, von Glehn F, Brandão C, et al. Plasmacytoid dendritic cells are increased in cerebrospinal fluid of untreated patients during multiple sclerosis relapse. J Neuroinflamm. 2011;8(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lleo A, Cavedo E, Parnetti L, et al. Cerebrospinal fluid biomarkers in trials for Alzheimer and Parkinson diseases. Nat Rev Neurol. 2015;11(1):41–55. [DOI] [PubMed] [Google Scholar]
- 35. Tumani H, Petereit HF, Gerritzen A, et al. S1-leitlinie: Lumbalpunktion und liquordiagnostik. DGNeurol. 2019;2(6):456–480. [Google Scholar]
- 36. Alvermann S, Hennig C, Stuve O, Wiendl H, Stangel M.. Immunophenotyping of cerebrospinal fluid cells in multiple sclerosis: In search of biomarkers. JAMA Neurol. 2014;71(7):905–912. [DOI] [PubMed] [Google Scholar]
- 37. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. [DOI] [PubMed] [Google Scholar]
- 38. Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: Revising the Nincds-Adrda criteria. Lancet Neurol. 2007;6(8):734–746. [DOI] [PubMed] [Google Scholar]
- 39. Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299. [DOI] [PubMed] [Google Scholar]
- 40. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kleffner I, Dorr J, Ringelstein M, et al. ; European Susac Consortium (EuSaC). Diagnostic criteria for Susac syndrome. J Neurol Neurosurg Psychiatry. 2016;87(12):1287–1295. [DOI] [PubMed] [Google Scholar]
- 42. Wingerchuk DM, Banwell B, Bennett JL, et al. ; International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lueg G, Gross CC, Lohmann H, et al. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer's disease. Neurobiol Aging. 2015;36(1):81–89. [DOI] [PubMed] [Google Scholar]
- 44. Becht E, McInnes L, Healy J, et al. Dimensionality reduction for visualizing single-cell data using Umap. Nat Biotechnol. Published online 3 December 2018. doi:10.1038/nbt.4314 [DOI] [PubMed] [Google Scholar]
- 45. Munschauer FE, Hartrich LA, Stewart CC, Jacobs L.. Circulating natural killer cells but not cytotoxic t lymphocytes are reduced in patients with active relapsing multiple sclerosis and little clinical disability as compared to controls. J Neuroimmunol. 1995;62(2):177–181. [DOI] [PubMed] [Google Scholar]
- 46. Vranes Z, Poljakovic Z, Marusic M.. Natural killer cell number and activity in multiple sclerosis. J Neurol Sci. 1989;94(1-3):115–123. [DOI] [PubMed] [Google Scholar]
- 47. Toledano M, Weinshenker BG, Solomon AJ.. A clinical approach to the differential diagnosis of multiple sclerosis. Curr Neurol Neurosci Rep. 2015;15(8):57. [DOI] [PubMed] [Google Scholar]
- 48. Dorr J, Krautwald S, Wildemann B, et al. Characteristics of Susac syndrome: A review of all reported cases. Nat Rev Neurol. 2013;9(6):307–316. [DOI] [PubMed] [Google Scholar]
- 49. Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B.. Neuromyelitis optica. Nat Rev Dis Primers. 2020;6(1):85. [DOI] [PubMed] [Google Scholar]
- 50. Algahtani H, Shirah B, Amin M, Altarazi E, Almarzouki H.. Susac syndrome misdiagnosed as multiple sclerosis with exacerbation by interferon beta therapy. Neuroradiol J. 2018;31(2):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Magliozzi R, Scalfari A, Pisani AI, et al. The CSF profile linked to cortical damage predicts multiple sclerosis activity. Ann Neurol. 2020;88(3):562–573. [DOI] [PubMed] [Google Scholar]
- 52. Lepennetier G, Hracsko Z, Unger M, et al. Cytokine and immune cell profiling in the cerebrospinal fluid of patients with neuro-inflammatory diseases. J Neuroinflamm. 2019;16(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lycke JN, Karlsson J-E, Andersen O, Rosengren LE.. Neurofilament protein in cerebrospinal fluid: A potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64(3):402–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hesse C, Rosengren L, Vanmechelen E, et al. Cerebrospinal fluid markers for Alzheimer's disease evaluated after acute ischemic stroke. J Alzheimers Dis. 2000;2(3-4):199–206. [DOI] [PubMed] [Google Scholar]
- 55. Santos AN, Ewers M, Minthon L, et al. Amyloid-β oligomers in cerebrospinal fluid are associated with cognitive decline in patients with Alzheimer's disease. J Alzheimers Dis. 2012;29(1):171–176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request. Information on the R codes used is available from https://doi.org/10.17605/OSF.IO/EQRF5.