Abstract
Aims
Despite well-known gender differences in heart failure, it is unknown if clinical markers and symptoms differ between women and men after left ventricular assist device (LVAD) implantation. Our aim was to examine gender differences in trajectories of clinical markers (echocardiographic markers and plasma biomarkers) and symptoms from pre- to post-LVAD implantation.
Methods and results
This was a secondary analysis of data collected from a study of patients from pre- to 1, 3, and 6 months post-LVAD implantation. Data were collected on left ventricular internal end-diastolic diameter (LVIDd) and ejection fraction (LVEF), plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP), and soluble suppressor of tumorigenicity (sST2). Physical and depressive symptoms were measured using the Heart Failure Somatic Perception Scale and Patient Health Questionnaire-9, respectively. Latent growth curve modelling was used to compare trajectories between women and men. The average age of the sample (n = 98) was 53.3 ± 13.8 years, and most were male (80.6%) and had non-ischaemic aetiology (65.3%). Pre-implantation, women had significantly narrower LVIDd (P < 0.001) and worse physical symptoms (P = 0.041) compared with men. Between pre- and 6 months post-implantation, women had an increase in plasma sST2 followed by a decrease, whereas men had an overall decrease (slope: P = 0.014; quadratic: P = 0.011). Between 1 and 6 months post-implantation, women had a significantly greater increase in LVEF (P = 0.045) but lesser decline in plasmoa NT-proBNP compared with men (P = 0.025).
Conclusion
Trajectories of clinical markers differed somewhat between women and men, but trajectories of symptoms were similar, indicating some physiologic but not symptomatic gender differences in response to LVAD.
Keywords: Gender, Biomarker, Symptoms, Ventricular assist device
Introduction
Left ventricular assist devices (LVADs) are a common treatment for women and men with advanced heart failure (HF).1 The development of smaller, more durable continuous flow LVADs2 has increased eligibility for implantation among smaller patients, many of whom are women. However, the ratio of implants remains notably disproportionate (only ∼20% of LVAD implants are women),3 and there is still on-going debate about where in the referral process the scales are tipped to create this pre-implantation gender imbalance.4 Moreover, post-implantation, some studies have noted gender differences in clinical events and transplant rates. For example, some studies have shown that women have higher rates of neurological adverse events, are more likely to be readmitted, and are less likely to receive a heart transplant after implantation compared with men.5–7 A recent study, however, showed that there was no significant gender difference in the combined outcome of death, durable mechanical circulatory support, or heart transplantation among an advanced HF cohort.8 Given that women are implanted less frequently and may possibly experience worse clinical events post-implantation, it would be helpful to understand how women and men respond clinically and symptomatically to LVAD therapy, providing anticipatory guidance from pre-implant through post-implant stages.
Gender differences in the pathogenesis and response to treatment have been explored in HF, but we have a limited understanding of these differences with LVAD support. Women have smaller ventricular dimensions and reduced chronotropic and contractile reserves compared with men, often translating into different phenotypic HF profiles.9 Among LVAD patients, ejection fraction10 increases overall, and it was recently shown that women had a greater reduction in left ventricular dimensions and greater increase in ejection fraction compared with men.11 It has been noted that women have higher levels of natriuretic peptides12 and lower inflammatory and remodelling biomarkers in HF.13 Biomarkers of myocardial stress, fibrosis, and inflammation decrease overall following LVAD implantation,10,14,15 but it is unknown if gender differences in biomarkers in HF translate to differences in response to LVAD therapy. Moreover, women often have worse HF symptoms, including fatigue, dyspnoea, and lower extremity oedema,16,17 and there are gender differences in the descriptors of dyspnoea.18 Prior to and after LVAD implantation, women have more depression and anxiety compared with men;8,19,20 however, no studies have examined gender differences in both physical HF symptoms and depressive symptoms post-implantation. Thus, we chose common echocardiographic markers, biomarkers, and symptoms to comprehensively capture the mechanical, biochemical, and symptomatic responses to LVAD implantation. Accordingly, the purpose of this study is to examine gender differences in trajectories of clinical markers (echocardiographic markers and plasma biomarkers) and symptoms from pre- to 6 months post-LVAD implantation to shed light on how women and men respond to LVAD therapy.
Methods
Study design and sample
This was a secondary analysis of data collected as part of a U.S. National Institutes of Health-funded cohort study examining biobehavioural responses after continuous-flow LVAD implantation.21 Clinical and symptom data and plasma samples were collected pre-LVAD implantation (median of 4 days pre-implant) and at 1, 3, and 6 months post-LVAD implantation. Inclusion criteria for the study were age 21 years or older, ability to read and comprehend 5th grade English or Spanish, and eligibility for implantation of a continuous-flow LVAD. Exclusion criteria were documented major cognitive impairment (e.g. Alzheimer’s disease), major psychiatric illness (i.e. psychosis), prior heart transplantation or durable mechanical circulatory support, or a concomitant terminal illness. Participants were recruited through a single centre between April 2012 and May 2016. Our Institutional Review Board approved the study (#7907 and #16952). Written informed consent was obtained from all participants; 98 participants consented to having their data and samples stored in a biorepository for future research. Only one person (male) declined participation in the parent study. The investigation conforms with the principles outlined in the Declaration of Helsinki.22
Measurement
Sociodemographic and clinical data
Participants completed a sociodemographic questionnaire at baseline to collect data on age, gender, marital status, race/ethnicity, and education. Baseline history, aetiology, treatment of HF, New York Heart Association functional class, and clinical and laboratory data were collected from medical records. The Charlson Comorbidity Index23 was used to summarize comorbid conditions. At 1, 3, and 6 months post-implantation, treatment characteristics (i.e. medications) were collected.
Clinical markers
Data were derived from standard-of-care echocardiograms and plasma biomarkers tested specifically for this study.21 Echocardiographic data [i.e. left ventricular internal end-diastolic diameter (LVIDd) and left ventricular ejection fraction (LVEF)] were extracted from the electronic medical record at baseline and 1, 3, and 6 months post-implantation. We selected these echocardiographic metrics given their importance in HF prognostication.24,25
At pre- and 1, 3, and 6 months post-implantation, whole blood was collected from participants, transported directly to the research core laboratory per study procedures, and centrifuged for 15 min at 1000 × g to separate plasma, which was aliquoted and stored at −80°C. Samples were thawed once for assay. We measured plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) as a marker of myocardial stress using an enzyme-linked immunosorbent assay (Cusabio Technology, Houston, TX, USA) with a sensitivity of 11.8 pg/mL, detection limit of 47 pg/mL, and intra- and inter-assay coefficients of variation of 8% and 9%, respectively. We measured plasma soluble suppressor of tumorigenicity 2 (sST2) as a marker of hypervolumetric stress and fibrosis using an enzyme-linked immunosorbent assay (Critical Diagnostics, San Diego, CA, USA) with a sensitivity of 2.35 ng/mL, detection limit of 1.31 ng/mL, and intra- and inter-assay coefficients of variation of 6% and 9%, respectively. We chose these two biomarkers as they reflect common, but distinct, pathophysiological processes,26 and when used together, have better prognostication than either alone.27
Symptoms
Participants completed symptom questionnaires at baseline and at 1, 3, and 6 months post-implantation, either in hospital or at home. Physical symptoms were assessed with the 18-item HF Somatic Perception Scale (HFSPS), a valid and reliable measure of perceived severity of both non-specific (e.g. fatigue and weight gain) and acute HF (e.g. orthopnoea and dyspnoea) symptoms.28 The HFSPS has 6 response options ranging from 0 (not at all) to 5 (extremely bothersome). Scores on the HFSPS range from 0 to 90, with higher scores indicating worse perceived symptom severity. The Cronbach’s alpha of the HFSPS in this sample was 0.87.
Depressive symptoms were assessed with the 9-Item Patient Health Questionnaire (PHQ9).29 The PHQ9 scores each of the 9 related DSM-IV criteria for depression with 4 response options ranging from 0 (not at all) to 3 (nearly every day). Scores on the PHQ9 range from 0 to 27 with higher scores indicating worse depressive symptoms; a score of ≥10 is indicative of the need for mental health assessment.29 The PHQ9 is a valid and reliable screen of depressive symptoms in HF.30 The Cronbach’s alpha of the PHQ9 in this sample was 0.81.
Statistical analysis
Descriptive statistics of frequency, central tendency, and dispersion [standard deviation or interquartile range (IQR)] were used to describe the sample. Raw values of plasma NT-proBNP and sST2 were natural log-transformed to approximate normality for analyses. Comparative statistics (i.e. Student’s t, Mann–Whitney U, Fisher’s exact, or Pearson’s chi-square tests) were used to compare pre-implantation sociodemographic and clinical characteristics between women and men.
Latent growth curve modelling was used to estimate change in clinical markers and symptoms from pre- to 1, 3, and 6 months post-implantation. Latent growth curve modelling allows for the estimation of inter-individual variability in intra-individual patterns of change using latent intercepts (i.e. pre-implantation values) and latent slopes (i.e. rate of change from pre- to post-implantation).31 It is particularly robust in situations with missing data, unequally spaced time points, non-linear trajectories, and non-normally distributed repeated measures; it can also accommodate small sample sizes of <100.32 Non-linear latent growth models (i.e. intercept, linear change, and quadratic change) and multiphase latent growth models (i.e. intercept and two major linear phases of change) were generated to identify the model that optimally represented the shape of the observed change.31 Similar to our previous work,33 the two phases of change were: (i) between pre- and 1-month post-implantation and (ii) between 1 and 6 months post-implantation. Based on the best fit between observed data and growth models, multiphase latent growth models were selected for change in LVIDd, LVEF, plasma lnNT-proBNP, HFSPS scores, and PHQ9 scores. A quadratic latent growth model was selected for change in plasma lnsST2. To test for differences in trajectories between women and men, we quantified the effect of gender on intercepts and each shape or phase of change. Additionally, we also examined the within-gender trajectory of change using the known class approach in mixture modelling, similar to our previous work.33
In each of the models, the missing data were as follows: LVIDd (2%), LVEF (1%), lnNT-proBNP (3%), lnsST2 (2%), HFSPS scores (3%), and PHQ9 scores (3%). All 19 women were included in all of the trajectory models except the PHQ9 model in which data were missing for one woman. Post-implantation, 92% of participants (95% of women) had at least 1 LVIDd measurement, and 89% (89% of women) had at least 1 LVEF measurement. Because echocardiograms were only performed if clinically indicated, there were more missing data for LVEF and LVIDd. Most participants had measurements of plasma NT-proBNP (80% overall; 79% of women), plasma sST2 (78% overall; 79% of women), and HFSPS (70% overall; 84% of women) and PHQ9 scores (70% overall; 79% of women) across all four time points. Full information maximum likelihood estimation was used to handle missing data post-implantation, as this method produces less biased parameter estimates than other methods of handling missing data.34 Significance was set at α < 0.05. Analyses were performed using Stata/MP v.15 (College Station, TX, USA) and MPlus v.8 (Los Angeles, CA, USA). GraphPad Prism 8.2 (San Diego, CA, USA) was used to prepare the figures. The corresponding author will make the data and methods used in the analysis available to any qualified researcher upon reasonable request for purposes of reproducing the results.
Results
There were a few statistically significant differences in baseline clinical characteristics between women (n = 19) and men (n = 79) in this predominantly middle-aged and white cohort (Table 1). Namely, fewer women had an intra-aortic balloon pump prior to implant, and women had lower serum haemoglobin compared with men. Also, while not statistically significant, women had more non-ischaemic HF aetiologies and a shorter duration of time living with HF. There were no significant differences in comorbidities or other clinical markers. By 6 months post-implantation, there were no significant differences between women and men in treatment with a diuretic (P = 0.354), a beta-blocker (P = 0.051), or an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (P = 0.64). No participants were lost to follow-up.
Table 1.
Characteristics of the sample at baseline (pre-implantation) ( n = 98)
| Mean±SD, n (%), or median [IQR] |
|||
|---|---|---|---|
| Women (n = 19) | Men (n = 79) | P-value | |
| Patient characteristics | |||
| Age (years) | 54.3 ± 14.2 | 53.1 ± 13.8 | 0.740 |
| Non-Hispanic Caucasian | 16 (84.2) | 63 (82.9) | 1.00 |
| Married/living with partner | 14 (73.7) | 46 (60.5) | 0.288 |
| Education level | 0.443 | ||
| Less than high school | 3 (15.8) | 7 (9.2) | |
| >High school but < college | 9 (47.4) | 46 (60.5) | |
| College degree | 7 (36.8) | 23 (30.3) | |
| Body mass index (kg/m2) | 27.5 ± 5.8 | 29.4 ± 5.3 | 0.215 |
| Charlson Comorbidity Index (weighted) | 2.9 ± 2.1 | 2.5 ± 1.5 | 0.448 |
| Atrial fibrillation | 9 (47.4) | 38 (48.1) | 0.954 |
| Stage 3 chronic kidney disease | 7 (36.8) | 29 (36.7) | 0.991 |
| Type II diabetes mellitus | 7 (36.8) | 34 (43.0) | 0.623 |
| General heart failure characteristics | |||
| Time with heart failure in years: median [IQR] | 4.0 [1.8–10.0] | 7.0 [1.2–14.3] | 0.596 |
| NYHA functional class | 0.832 | ||
| Class II | 0 (0) | 4 (5.1) | |
| Class III | 9 (47.4) | 38 (48.7) | |
| Class IV | 10 (52.6) | 36 (46.2) | |
| Non-ischaemic etiology | 16 (84.2) | 48 (60.8) | 0.054 |
| Prescribed a β-blocker | 8 (42.1) | 37 (46.8) | 0.710 |
| Prescribed an ACE-I or ARB | 17 (89.5) | 55 (69.6) | 0.090 |
| Prescribed an aldosterone antagonist | 14 (73.7) | 55 (69.6) | 0.728 |
| Prescribed an inotrope | 15 (79.0) | 55 (69.6) | 0.419 |
| ICD or biventricular ICD | 17 (89.5) | 65 (82.3) | 0.728 |
| Intra-aortic balloon pump | 4 (21.1) | 39 (49.4) | 0.026 |
| Serum sodium (mEq/L) | 135.1 ± 3.5 | 134.1 ± 4.2 | 0.288 |
| Serum haemoglobin (g/dL) | 11.1 ± 1.2 | 12.4 ± 2.0 | <0.001 |
| Serum BUN:creatinine ratio | 25.5 ± 8.9 | 22.0 ± 8.0 | 0.127 |
| Heart rate | 94.7 ± 16.1 | 92.6 ± 17.4 | 0.618 |
| Systolic blood pressure | 102.7 ± 16.6 | 102.1 ± 12.9 | 0.882 |
| Diastolic blood pressure | 63.6 ± 7.7 | 64.7 ± 8.4 | 0.567 |
| Pulmonary capillary wedge pressure (mmHg) | 21.9 ± 7.7 | 24.0 ± 8.6 | 0.317 |
| Right atrial pressure (mmHg) | 8.6 ± 4.3 | 9.6 ± 4.9 | 0.359 |
| Cardiac index (L/min/m2 by Fick equation) | 1.8 ± 0.6 | 1.9 ± 0.5 | 0.416 |
| LVAD characteristics | |||
| INTERMACS categorya | 0.891 | ||
| 1 | 1 (5.9) | 4 (5.3) | |
| 2 | 12 (70.6) | 57 (75.0) | |
| 3 | 4 (23.5) | 15 (19.7) | |
| Implant strategy | 0.276 | ||
| Bridge to transplantation | 12 (63.2) | 53 (67.1) | |
| Bridge to decision | 4 (21.1) | 22 (27.9) | |
| Destination therapy | 3 (15.8) | 4 (5.1) | |
| Device | 0.679 | ||
| Heartmate II | 13 (68.4) | 57 (72.2) | |
| Heartmate III | 1 (5.3) | 2 (2.5) | |
| Heartware | 5 (26.3) | 20 (25.3) | |
INTERMACS category not available for all participants.
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; ICD, implantable cardioverter-defibrillator; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; IQR, interquartile range; LVAD, left ventricular assist device; NYHA, New York Heart Association; SD, standard deviation.
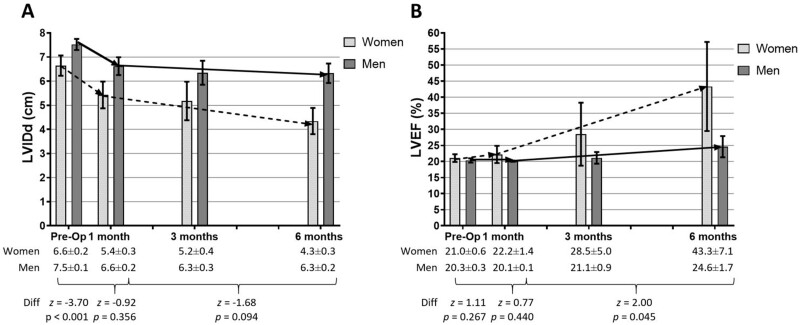
Baseline LVIDd was significantly narrower for women compared with men (Figure 1A). Women had a significant reduction in LVIDd between 1 and 6 months post-implantation; however, there was no significant gender difference in the rate of change. Both women and men had significant improvements in LVEF between 1 and 6 months post-implantation, and notably, LVEF increased significantly more in women than men (Figure 1B).
Figure 1.
Trajectories of echocardiographic parameters in women and men pre- to post- left ventricular assist device implantation. Left ventricular internal end-diastolic diameter. (A) and left ventricular ejection fraction (B) (shown as mean and 95% confidence interval on the graph and mean and standard error of the mean in the table) were measured in women (dashed line) and men (solid line) from pre- to 6 months post-implantation. In the first month post-implantation, both women (n = 19) and men (n = 77) had a significant decrease in left ventricular internal end-diastolic diameter (women: z = −5.21, P < 0.001; men: z = −7.45, P < 0.001); between 1 and 6 months post-implantation, only women had a significant decrease (women: z = −2.88, P = 0.004; men: z = −0.97, P = 0.333). Comparing trajectories, pre-implantation left ventricular internal end-diastolic diameter was significantly different between women and men, but the changes thereafter were not significantly different. In the first month post-implantation, neither women (n = 19) nor men (n = 78) had a significant change in left ventricular ejection fraction (women: z = 0.63, P = 0.528; men: z = −0.62, P = 0.537); between 1 and 6 months post-implantation, both women and men had a significant increase in left ventricular ejection fraction (women: z = 2.44, P = 0.015; men: z = 2.55, P = 0.011). Comparing trajectories, neither pre-implantation left ventricular ejection fraction nor changes in the first month were significantly different between women and men; however, women had a significantly greater increase in left ventricular ejection fraction between 1 and 6 months post-implantation compared with men. LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal end-diastolic diameter.
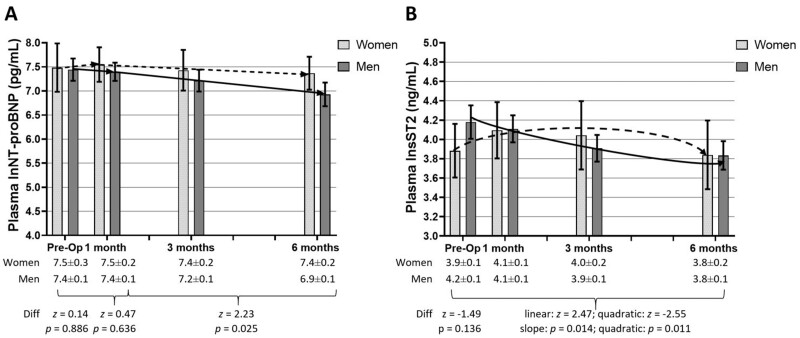
Between 1 and 6 months post-implantation, men had a significant reduction in plasma lnNT-proBNP, and there was a significant gender difference in the rate of change (Figure 2A). There were significant differences in both the linear and quadratic aspects of change in plasma lnsST2 comparing women and men (Figure 2B). Women had an increase in plasma lnsST2 through 1 month followed by an eventual decrease through 6 months post-implantation; in contrast, men had an initial and overall decrease in plasma lnsST2 post-implantation. Trajectories of change in raw values of biomarkers are displayed in Supplementary material online, Figure.
Figure 2.
Trajectories of plasma biomarkers in women and men pre- to post-left ventricular assist device implantation. Plasma natural log of N-terminal pro-B-type natriuretic peptide (A) and natural log of soluble supressor of tumorigenicity levels (B) (shown as mean and 95% confidence interval on the graph and mean and standard error of the mean in the table) were measured in women (dashed line) and men (solid line) from pre- to 6 months post-implantation. In the first month post-implantation, neither women (n = 19) nor men (n = 76) had significant changes in plasma natural log of N-terminal pro-B-type natriuretic peptide (women: z = 0.29, P = 0.772; men: z = −0.60, P = 0.550); between 1 and 6 months post-implantation, only men had a significant decrease (women: z = −0.96, P = 0.339; men: z = −6.03, P < 0.001). Comparing trajectories, neither pre-implantation plasma natural log of N-terminal pro-B-type natriuretic peptide nor changes in the first month were significantly different between women and men; between 1 and 6 months post-implantation, however, men had a greater decline in plasma natural log of N-terminal pro-B-type natriuretic peptide compared with women. Between pre- and 6-months post-implantation, women (n = 19) did not have significant changes in plasma natural log of soluble suppressor of tumorigenicity (linear: z = 1.14, P = 0.253; quadratic: z = −1.71, P = 0.088) but men (n = 77) did (linear: z = −3.80, P < 0.001; quadratic: z = 2.69, P = 0.007). Comparing trajectories, pre-implantation plasma natural log of soluble suppressor of tumorigenicity was not significantly different between women and men, but both the linear and quadratic changes were significantly different. lnNT-pro-BNP, natural log of N-terminal pro-B-type natriuretic peptide; lnsST2, natural log of soluble suppressor of tumorigenicity.
Baseline physical symptoms were significantly worse in women compared with men (Figure 3A). Physical symptoms improved significantly for women and men; however, there were no significant gender differences in the rate of change. Both women and men had significant improvements in depressive symptoms in the first month post-implantation with no significant gender difference (Figure 3B). Men continued to have significant improvements; however, there was no significant gender difference, and average scores for women and men were well below 10. Overall, depressive symptoms decreased by about half for both women and men between baseline and 6 months post-implantation.
Figure 3.
Trajectories of symptoms in women and men pre- to post-left ventricular assist device implantation. Physical symptoms (A) and depressive symptoms (B) (shown as mean and 95% confidence interval on the graph and mean and standard error of the mean in the table) were measured in women (dashed line) and men (solid line) from pre- to 6 months post- implantation. In the first month post-implantation, both women (n = 19) and men (n = 76) had significant decreases in physical symptoms (women: z = −4.32, P < 0.001; men: z = −7.16, P < 0.001); between 1 and 6 months post-implantation, women and men continued to have significant decreases (women: z = −2.46, P = 0.014; men: z = −2.79, P = 0.005). Comparing trajectories, pre-implantation physical symptoms were significantly different between women and men; however, changes in the first month and between 1 and 6 months post-implantation were not significantly different. In the first month post-implantation, both women (n = 19) and men (n = 76) had significant decreases in depressive symptoms (women: z = −2.78, P = 0.005; men: z = −6.28, P < 0.001); between 1 and 6 months post-implantation, only men continued to have significant decreases (women: z = −0.27, P = 0.788; men: z = −2.44, P = 0.015). Comparing trajectories, there was no significant difference in pre-implantation depressive symptoms nor in changes thereafter between women and men.
Discussion
In our study of 98 patients who received an LVAD, we found significant gender differences in trajectories of change in clinical markers from pre- to 6 months post-implantation but fairly similar trajectories in symptoms between women and men. While women had narrower left ventricular diameters and similar ejection fraction compared with men pre-implantation, ejection fraction improved more dramatically among women than men post-implantation. Moreover, while baseline values of plasma NT-proBNP and sST2 were similar, women had lesser improvement in plasma NT-proBNP and a significantly different trajectory of change in plasma sST2 compared with men post-implantation. Lastly, while women had significantly worse physical symptoms compared with men pre-implantation, there were no significant gender differences in trajectories of physical and depressive symptoms post-implantation, indicating that women and men respond similarly in terms of symptoms. Taken together, while women have slightly worse biomarker trajectories, they have better echocardiographic trajectories and similar symptoms compared with men post-implantation. Our study builds on previous research by reinforcing the gender disparity in LVAD implantation rates and shows that women respond favourably to LVAD therapy.
The differences in echocardiographic parameters between women and men were of interest. In both health and disease, women have slightly different cardiac structure and function, including smaller left ventricles and higher LVEF but greater concentric hypertrophy and left ventricular stiffness compared with men.9 Several studies have shown that gender influences myocardial performance using multiple imaging modalities.35,36 Our findings provide evidence that although women started out with smaller diameters, reduction of LVIDd following LVAD implantation decreases similarly among women and men with advanced HF. Kenigsberg et al. demonstrated that women had a greater reduction in LVIDd, independent of body size, compared with men in a larger sample size. Although a similar trend was observed in our study, it failed to reach statistical significance; the small sample of women in our study may have contributed to this difference in our findings. Adding to previous research showing that LVEF significantly improves after implantation10 and similar to findings by Kenigsberg et al.,11 we found that women had a greater increase in LVEF between 1 and 6 months post-implantation compared with men. In sum, mechanical unloading with an LVAD may reveal some inherent sex differences in cardiovascular pathophysiological mechanisms.9,35
Gender differences in trajectories of plasma biomarkers were notable. Men demonstrated a significant decrease in plasma NT-proBNP post-implantation but women did not despite having better improvement in mean LVEF and a similar reduction in LVIDd. There is evidence from previous research that plasma NT-proBNP improves among both women and men following LVAD implantation,10 so it is unclear why our findings differed. It has been noted that women have higher plasma NT-proBNP across the HF ejection fraction spectrum,12 which may explain our findings. Moreover, women had a different, albeit non-significant, trajectory of change (i.e. n-shaped) in plasma sST2 compared with men (i.e. a significant overall decline). Women had higher rates of infection at 3 months post-implant, which could partly explain the differences in trajectories of plasma sST2 given that sST2 has been linked with inflammatory processes and higher C-reactive protein levels.15 Taken together, known gender differences in biomarkers may persist after LVAD implantation, reflecting different aetiologies, progression, and responses to mechanical offloading, as well as implications for prognostication based on the two biomarkers.
We showed that symptoms generally improved for both women and men after implantation, extending our previous findings on changes in symptoms from pre- to post-LVAD implantation.37 Although women had greater physical symptom burden pre-implantation compared with men, both women and men experienced improvements in physical symptoms post-implantation. We also showed that both women and men reported similar significant improvements in depressive symptoms post-implantation. Previous research showed that women are more likely to have combined depression and anxiety pre-implantation compared with men19 and that there may be gender differences post-implantation in the depression and anxiety dimension of health-related quality of life.20 Findings from our study did not support these differences, however. We used the PHQ9 as a measure of depressive symptoms and did not connect anxiety with depression, which may explain the differences in findings. Despite these encouraging findings, it will be important to continue to screen for depression given the high prevalence of depression in HF,38 coupled with the added burden of LVAD maintenance.
Taken together, these findings may provide clinical guidance for women and men scheduled for LVAD implantation. Importantly, findings from our study provide preliminary evidence that women respond favourably to LVAD therapy with similar overall improvement in physical and depressive symptoms compared with men. Furthermore, findings from our study indicate that women experience substantial improvements in left ventricular structure and function following LVAD implantation. Our sample of women was small; however, so the associations will have to be re-evaluated in larger studies to determine the clinical utility of the findings. Understanding typical clinical and symptom trajectories for women and men may provide context for shared decision-making, especially given the gender imbalance in implantation rates.3 Similar to our previous research,33 understanding differences and similarities in symptom trajectories and cardiac function between subgroups of LVAD patients will inform decisions to implant an LVAD and post-implantation expectations.
Our study has limitations. First, while our sample was relatively large compared with other prospective LVAD studies, we had substantially more men than women, which unfortunately reflects the gender imbalance that pervades advances therapies.3 Thus, the small sample size of women may have hindered the identification of some differences at baseline and in trajectories of change. Second, this racially homogenous sample was drawn from one medical centre in the Pacific Northwest in the US and, as such, may not be generalizable to the advanced HF patient population at large. Third, while this study focused on responses to mechanical unloading using continuous flow devices, this sample was primarily comprised of Heartmate II implants, which should be taken into consideration when applying these data to future iterations of LVADs. Finally, given the complexity of maintaining and managing an LVAD, there may have been other contextual characteristics (e.g. caregiver support) not captured in these data that could explain our findings.
Future research should focus on a few key areas. First, future research will be needed to test these relationships in larger clinical samples, especially in studies with more equal gender representation. Second, there is a need to examine gender differences in physiological changes that occur post-implantation that may explain observed differences in LVEF (e.g. possible left ventricular recovery39) and NT-proBNP (e.g. possible right ventricular failure40). Third, while we observed similar improvements in physical and depressive symptoms in both women and men post-implantation, these findings should be explored further to identify factors that help or hinder symptom burden in women and men, particularly in the context of noted gender differences in symptom biology.41 Additionally, while the HFSPS captures multiple physical HF symptoms (e.g. oedema, dyspnoea) together, future research should explore gender differences in trajectories of specific symptoms plus others (e.g. pain) post-implantation. Finally, the reason for substantial gender differences in LVAD use remains unknown. There are known gender differences in aetiology, comorbidities, and age in HF;3,9 however, these differences alone do not fully explain why only about one out of every five LVAD recipients are women. Differences in referral patterns and/or refusal of this advanced surgical treatment option may be factors.
Implications for practice
Women respond well to left ventricular assist device therapy in terms of echocardiographic markers but had lesser improvements in biomarkers compared with men post-implantation.
Women and men had similar improvements in physical and depressive symptoms post-implantation.
However, more men (∼80%) than women (∼20%) are implanted with left ventricular assist devices, which limits our ability to fully understand response among women.
Conclusions
In our sample of LVAD patients prospectively studied over 4 time points, we found significant gender differences in trajectories of clinical markers, but fairly similar trajectories in symptoms, from pre- to 6 months post-implantation. These findings signal potential differences and similarities in parameters of physiologic, pathophysiologic, and responses to intervention that should be taken into consideration in the clinical management of this growing patient population. The sample of women in our study is small, however, yet it is reflective of the known gender imbalance in LVAD implantation rates. Given the positive response in symptoms and cardiac function among women post-implantation, it is unclear why the gender imbalance in LVAD implantation rates exists. Future research should seek to understand the gender imbalance that pervades advanced therapies and inform clinical decision-making to ensure equitable allocation of advanced therapies.
Supplementary material
Supplementary material is available at European Journal of Cardiovascular Nursing.
Funding
The Office of Research on Women’s Health and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (K12HD043488 to Q.E.D); the National Institutes of Health/National Institute of Nursing Research (1R01NR013492 to C.S.L.); and an Innovations Grant through Oregon Health & Science University School of Nursing (to C.S.L.). Additional support was received by the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000128); the Achievement Rewards for College Scientists Scholar Award and the Oregon Health & Science University School of Nursing Dean’s Stipend to M.R.D. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest: C.V.C. is a consultant for Abbott and on the Boston Scientific Steering Committee.
Supplementary Material
References
- 1. Waqas M, Cowger JA.. Role of durable mechanical circulatory support for the management of advanced heart failure. Heart Fail Clin 2016;12:399–409. [DOI] [PubMed] [Google Scholar]
- 2. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH.. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241–2251. [DOI] [PubMed] [Google Scholar]
- 3. Hsich EM. Sex differences in advanced heart failure therapies. Circulation 2019;139:1080–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khazanie P. REVIVAL of the sex disparities debate: are women denied, never referred, or ineligible for heart replacement therapies? JACC Heart Fail 2019;7:612–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeFilippis EM, Truby LK, Garan AR, Givens RC, Takeda K, Takayama H, Naka Y, Haythe JH, Farr MA, Topkara VK.. Sex-related differences in use and outcomes of left ventricular assist devices as bridge to transplantation. JACC: Heart Fail 2019;7:250–257. [DOI] [PubMed] [Google Scholar]
- 6. Vidula H, Kutyifa V, Johnson BA, Strawderman RL, Harrington D, Polonsky B, Papernov A, Alexis JD.. Readmission patterns during long-term follow-up after left ventricular assist device implantation. Am J Cardiol 2018;122:1021–1027. [DOI] [PubMed] [Google Scholar]
- 7. Morris AA, Pekarek A, Wittersheim K, Cole RT, Gupta D, Nguyen D, Laskar SR, Butler J, Smith A, Vega JD.. Gender differences in the risk of stroke during support with continuous-flow left ventricular assist device. J Heart Lung Transplant 2015;34:1570–1577. [DOI] [PubMed] [Google Scholar]
- 8. Stewart GC, Cascino T, Richards B, Khalatbari S, Mann DL, Taddei-Peters WC, Baldwin JT, Jeffries NO, Spino C, Stevenson LW, Aaronson KD; REVIVAL Investigators. Ambulatory advanced heart failure in women: a report from the REVIVAL registry. JACC Heart Fail 2019;7:602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM.. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation 2018;138:198–205. [DOI] [PubMed] [Google Scholar]
- 10. Muthiah K, Humphreys DT, Robson D, Dhital K, Spratt P, Jansz P, Macdonald PS, Hayward CS.. Longitudinal structural, functional, and cellular myocardial alterations with chronic centrifugal continuous-flow left ventricular assist device support. J Heart Lung Transplant 2017;36:722–731. [DOI] [PubMed] [Google Scholar]
- 11. Kenigsberg BB, Majure DT, Sheikh FH, Afari-Armah N, Rodrigo M, Hofmeyer M, Molina EJ, Wang Z, Boyce S, Najjar SS, Mohammed SF.. Sex-associated differences in cardiac reverse remodeling in patients supported by contemporary left ventricular assist devices. J Card Fail 2020;26:494–504. [DOI] [PubMed] [Google Scholar]
- 12. Faxén UL, Lund LH, Orsini N, Strömberg A, Andersson DC, Linde C, Dahlström U, Savarese G.. N-terminal pro-B-type natriuretic peptide in chronic heart failure: the impact of sex across the ejection fraction spectrum. Int J Cardiol 2019;287:66–72. [DOI] [PubMed] [Google Scholar]
- 13. Meyer S, van der Meer P, van Deursen VM, Jaarsma T, van Veldhuisen DJ, van der Wal MHL, Hillege HL, Voors AA.. Neurohormonal and clinical sex differences in heart failure. Eur Heart J 2013;34:2538–2547. [DOI] [PubMed] [Google Scholar]
- 14. Ahmad T, Wang T, O'Brien EC, Samsky MD, Pura JA, Lokhnygina Y, Rogers JG, Hernandez AF, Craig D, Bowles DE, Milano CA, Shah SH, Januzzi JL, Felker GM, Patel CB.. Effects of left ventricular assist device support on biomarkers of cardiovascular stress, fibrosis, fluid homeostasis, inflammation, and renal injury. JACC: Heart Fail 2015;3:30–39. [DOI] [PubMed] [Google Scholar]
- 15. Tseng CCS, Huibers MMH, Gaykema LH, Siera-de Koning E, Ramjankhan FZ, Maisel AS, de Jonge N.. Soluble ST2 in end-stage heart failure, before and after support with a left ventricular assist device. Eur J Clin Invest 2018;48:e12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dewan P, Rørth R, Jhund PS, Shen L, Raparelli V, Petrie MC, Abraham WT, Desai AS, Dickstein K, Køber L, Mogensen UM, Packer M, Rouleau JL, Solomon SD, Swedberg K, Zile MR, McMurray JJV.. Differential impact of heart failure with reduced ejection fraction on men and women. J Am Coll Cardiol 2019;73:29–40. [DOI] [PubMed] [Google Scholar]
- 17. Heo S, Shin M-S, Hwang SY, An M, Park J-K, Kim SHwa, Shim JL, Kim J.. Sex differences in heart failure symptoms and factors associated with heart failure symptoms. J Cardiovasc Nurs 2019;34:306–312. [DOI] [PubMed] [Google Scholar]
- 18. Ekman I, Boman K, Olofsson M, Aires N, Swedberg K.. Gender makes a difference in the description of dyspnoea in patients with chronic heart failure. Eur J Cardiovasc Nurs 2005;4:117–121. [DOI] [PubMed] [Google Scholar]
- 19. Lundgren S, Poon CYM, Selim A, Lowes BD, Zolty R, Burdorf A, Potashnik-Peled Y, Moulton MJ, Um JY, Raichlin E.. Depression and anxiety in patients undergoing left ventricular assist device implantation. Int J Artificial Organs 2018;41:76–83. [DOI] [PubMed] [Google Scholar]
- 20. Grady KL, Sherri W, Naftel DC, et al. Age and gender differences and factors related to change in health-related quality of life from before to 6 months after left ventricular assist device implantation: findings from Interagency Registry for Mechanically Assisted Circulatory Support. J Heart Lung Transplant 2016;35:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee CS, Mudd JO, Gelow JM, Nguyen T, Hiatt SO, Green JK, Denfeld QE, Bidwell JT, Grady KL.. Background and design of the profiling biobehavioral responses to mechanical support in advanced heart failure study. J Cardiovasc Nurs 2014;29:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rickham PP. Human experimentation. Code of ethics of the world medical association. Declaration of Helsinki. Br Med J 1964;2:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. 1987/01/01. [DOI] [PubMed] [Google Scholar]
- 24. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M.. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006;113:1424–1433. [DOI] [PubMed] [Google Scholar]
- 25. Quiñones MA, Greenberg BH, Kopelen HA, Koilpillai C, Limacher MC, Shindler DM, Shelton BJ, Weiner DH.. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol 2000;35:1237–1244. [DOI] [PubMed] [Google Scholar]
- 26. Lee CS, Denfeld QE, Aouizerat BE, Jurgens CY, Chien CV, Aarons E, Gelow JM, Hiatt SO, Mudd JO.. Comparative symptom biochemistry between moderate and advanced heart failure. Heart Lung 2018;47:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bayes-Genis A, de Antonio M, Galán A, Sanz H, Urrutia A, Cabanes R, Cano L, González B, Díez C, Pascual T, Elosúa R, Lupón J.. Combined use of high-sensitivity ST2 and NTproBNP to improve the prediction of death in heart failure. Eur J Heart Fail 2012;14:32–38. [DOI] [PubMed] [Google Scholar]
- 28. Jurgens CY, Lee CS, Riegel B.. Psychometric analysis of the Heart Failure Somatic Perception Scale as a measure of patient symptom perception. J Cardiovasc Nurs 2017;32:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kroenke K, Spitzer RL, Williams JB.. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hammash MH, Hall LA, Lennie TA, Heo S, Chung ML, Lee KS, Moser DK.. Psychometrics of the PHQ-9 as a measure of depressive symptoms in patients with heart failure. Eur J Cardiovasc Nurs 2013;12:446–453. [DOI] [PubMed] [Google Scholar]
- 31. Ram N, Grimm K.. Using simple and complex growth models to articulate developmental change: matching theory to method. Int J Behav Dev 2007;31:303–316. [Google Scholar]
- 32. Curran PJ, Obeidat K, Losardo D.. Twelve frequently asked questions about growth curve modeling. J Cogn Dev 2010;11:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee CS, Gelow JM, Chien CV, Hiatt SO, Bidwell JT, Denfeld QE, Grady KL, Mudd JO.. Implant strategy-specific changes in symptoms in response to left ventricular assist devices. J Cardiovasc Nurs 2018;33:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Enders CK, Bandalos DL.. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Model 2001;8:430–457. [Google Scholar]
- 35. FöLl D, Jung B, Schilli E, Staehle F, Geibel A, Hennig JRGEN, Bode C, Markl M.. Magnetic resonance tissue phase mapping of myocardial motion new insight in age and gender. Circ Cardiovasc Imaging 2010;3:54–64. [DOI] [PubMed] [Google Scholar]
- 36. Andre F, Steen H, Matheis P, Westkott M, Breuninger K, Sander Y, Kammerer R, Galuschky C, Giannitsis E, Korosoglou G, Katus HA, Buss SJ.. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 2015;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee CS, Mudd JO, Lyons KS, Denfeld QE, Jurgens CY, Aouizerat BE, Gelow JM, Chien CV, Aarons E, Grady KL.. Heart failure symptom biology in response to ventricular assist device implantation. J Cardiovasc Nurs 2019;34:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Silver MA. Depression and heart failure: an overview of what we know and don't know. Cleveland Clin J Med 2010;77:S7–S11. [DOI] [PubMed] [Google Scholar]
- 39. Wever-Pinzon O, Drakos SG, McKellar SH, Horne BD, Caine WT, Kfoury AG, Li DY, Fang JC, Stehlik J, Selzman CH.. Cardiac recovery during long-term left ventricular assist device support. J Am Coll Cardiol 2016;68:1540–1553. [DOI] [PubMed] [Google Scholar]
- 40. Cogswell R, John R, Shaffer A.. Right ventricular failure after left ventricular assist device. Cardiol Clin 2020;38:219–225. [DOI] [PubMed] [Google Scholar]
- 41. Lee CS, Hiatt SO, Denfeld QE, Chien CV, Mudd JO, Gelow JM.. Gender-specific physical symptom biology in heart failure. J Cardiovasc Nurs 2015;30:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.