Dear Editor,
Recently, we published a preliminary report about the potential benefit of adjuvant use of local and systemic ozone therapy (O3T) in the management of refractory pelvic pain secondary to cancer treatment, at 3 months after ozone administration [1]. After that, several colleagues contacted us to ask for more details about the overall O3T schedule and an update of the results at longer follow-up times. The aim of this letter is to try to answer those questions.
Management of local pain after pelvic tumor treatment requires excluding the possibility of tumor relapse and, at the same time, starting analgesic and opioid treatment as needed. If recurrent tumor is not found, persistent pelvic pain can be secondary to cancer treatments. In those patients with potentially prolonged life expectancy, high doses of opioids are controversial because their long-term safety and effectiveness have not been well established [2]. This management can be even more difficult if neuropathic pain is present, which may happen in 34% to 40% of these patients [3]. We have previously described the potential clinical benefit of O3T, with a prolonged effect, in the management of several side effects of cancer treatments, the most relevant at the pelvic level [4].
Recently [1], we reported encouraging preliminary results during the first 3 months of O3T in a group of six cancer patients without evidence of tumor relapse but with refractory chronic pelvic pain secondary to cancer treatment (radiotherapy, chemotherapy, surgery, or a combination of them). All patients had previously received unsuccessful pain management with anti-inflammatory, co-adjuvants or opioid drugs, and half of them had also received treatment with interventional techniques. Moreover, two patients with pelvic pain secondary to cystitis (patients 2 and 4) requested cystectomy and had seriously contemplated suicide because of the pain. Table 1 summarizes patient details. Further clinical characteristics before O3T were previously described [1]. Patients were evaluated at our multidisciplinary Chronic Pain Unit for compassionate O3T. Ozone treatment was further approved by the Health Care Ethics Committee and by our Research Ethics Committee (BCV-OZO-2019-01 study). Informed written consent was obtained for all patients. Pain before, during, and after O3T was assessed on a visual analog scale (VAS) ranging from 0 (no pain) to 10 (worst imaginable pain).
Table 1.
Relevant clinical characteristics and patient-reported changes after ozone therapy in continuous and acute pelvic pain
| Patient Number | Age, years | Sex | Diagnosis | Months with Pain Before O3T | Weeks of treatment | Number of Sessions | Continuous Pelvic Pain Before/After O3T† | Acute Pelvic Pain Before/After O3T† |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | F |
Uterine cervix carcinoma. Vaginal pain, after RT+CT. Associated with vaginal dryness and ulceration. Tx: Antibiotics. |
4 | 11 | 12 | 7.5/0 | 7.5/0 |
| 2* | 54 | M |
Prostate carcinoma. Bladder pain, after RT (prostatic surgery 3 years earlier). Associated with hematuria. Tx: Opioids, interventional techniques. |
15 | 15 | 17 | 10/10 | 10/10 |
| 3 | 61 | M |
Prostate carcinoma. Rectal pain, after endoscopic treatment. Associated with tenesmus. Tx: Opioids. |
13 | 58 | 77 | 4.5/1 | 9/2 |
| 4* | 64 | M |
Follicular lymphoma Stage IV-A. Bladder pain, after CT. Associated with interstitial cystitis. Tx: Opioids, interventional techniques. |
30 | 46 | 64 | 10/0 | 10/0 |
| 5 | 60 | F |
Uterine cervix carcinoma. Vaginal and pelvic pain, after RT+CT. Associated with vaginal necrosis and recto-vaginal fistula. Tx: Opioids, antibiotics, hyperbaric oxygen. |
15 | 22 | 37 | 7/0 | 7/0 |
| 6 | 71 | F |
Rectal adenocarcinoma. Rectal pain, after S+RT+CT. Associated with tenesmus. Tx: Loperamide. Opioids were rejected. |
9 | 33 | 60 | 8/0 | 8/0 |
| Mean ± SD or median (range) | 14 (4–30) | 28 (11–58) | 49 (12–77) | 7.8 + 2.1/1.8 + 4 | 8.6 + 1.3/2 + 4.0 | |||
| P | 0.005‡ | |||||||
Patients 2 and 4 had previously requested cystectomy and had seriously contemplated suicide because of the pelvic pain. Patient 2: After further unsuccessful treatments, a cystectomy was performed 9 months after O3T, although cystectomy did not completely relieve pelvic pain. Patient 4: Intradetrusor infiltrations with botulinum toxin-A and low-pressure bladder hydrodistension were maintained every 6 months as treatment for its interstitial cystitis.
Pelvic pain according to the VAS before and at the end of ozone therapy.
Paired Student t test.
F = female; M = male; S = surgery; RT = radiotherapy; CT = chemotherapy; Tx = previous treatments other than anti-inflammatory and non-opioid drugs; SD = standard deviation.
On an outpatient basis, O3T was administered by rectal, intravesical, and/or intravaginal approaches. Systemic O3T by rectal insufflation was applied in five patients (patients 2, 3, 4, 5, and 6). Gas volume started at 180 mL/session and was slowly increased (depending on patient tolerance of bowel bloating) up to a maximum volume of 300 mL/session if tolerated. Initial O3/O2 concentration was 10 µg/mL (µg of O3 per mL of O2), which was increased by 5 µg/mL every two sessions until a final concentration of 30 µg/mL was reached. Intravesical O3T (by insufflation of O3/O2 or instillations of ozonated water) was applied in two patients (patients 2 and 4). Vaginal O3T (by O3/O2 insufflations or vaginal washing with ozonated water) was applied in two patients (patients 1 and 5). Further details were previously described [1]. When possible, the treatment schedule initially consisted of three sessions per week, and sessions were slowly reduced according to clinical improvement, from two sessions per week to one session per month at the very end of the treatment course. The length and final number of O3T sessions depended on clinical evolution. The median duration of O3T was 28 weeks (range: 11–58), and the median number of O3T sessions was 49 weeks (range: 12–77).
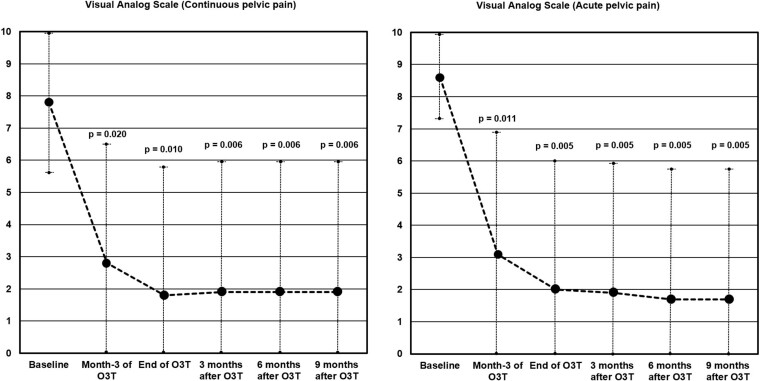
After several months of unsuccessful treatments before O3T, continuous pelvic pain was severe (VAS 7.8 ± 2.1). Pain significantly decreased after 3 months of O3T applications (VAS 2.8 ± 3.8, P = 0.020) and at the end of O3T (VAS 1.8 ± 4, P = 0.010). The benefit was maintained at 3 (VAS 1.9 ± 4, P = 0.006), 6 (VAS 1.9 ± 4, P = 0.006), and 9 (VAS 1.9 ± 4, P = 0.006) months’ follow-up after the end of O3T. All but one patient (patient 2) were able to decrease or even discontinue analgesic intake requirements. Patient 2 was treated once per week, which is not an appropriate frequency for the first weeks of O3T. Except in patient 2, episodes of acute pelvic pain (initial VAS 8.6 ± 1.3), spontaneous or during deposition or urination, also disappeared or decreased in frequency and intensity after 3 months of O3T applications (VAS 3.1 ± 3.8, P = 0.011) and at the end of O3T (VAS 2 ± 4, P = 0.005). The benefit was maintained at 3 (VAS 1.9 ± 4, P = 0.005), 6 (VAS 1.7 ± 4.1, P = 0.005), and 9 (VAS 1.7 ± 4.1, P = 0.005) months’ follow-up after the end of O3T. The improvement in associated symptoms (vaginal dryness, hematuria, rectal or vaginal wounds, tenesmus, and the number of bowel movements per day) described in the initial report [1] was also maintained and/or improved during O3T and after the end of O3T. Figure 1 shows the evolution of continuous and acute pelvic pain.
Figure 1.
Clinical evolution of continuous and acute pelvic pain during and after ozone therapy. Pain levels according to the VAS. All but one of the patients showed clinically relevant improvement in pain levels after O3T. (A) Continuous pelvic pain. Initial continuous pelvic pain (7.8 ± 2.1) was significantly decreased after 3 months of O3T (P = 0.020) and at the end of O3T (P = 0.010). The benefit was maintained at 3 (P = 0.006), 6 (P = 0.006), and 9 (P = 0.006) months’ follow-up after the end of O3T. (B) Acute pelvic pain. Initial acute pelvic pain (8.6 ± 1.3) was significantly decreased in frequency at intensity after 3 months of O3T (P = 0.011) and at the end of O3T (P = 0.005). The benefit was maintained at 3 (P = 0.005), 6 (P = 0.005), and 9 (P = 0.005) months’ follow-up after the end of O3T.
In this group of patients, we report for the first time the potential usefulness of ozone as adjuvant therapy in the management of chronic pelvic pain secondary to cancer treatment. Our data show that initial pain decreases during the first 3 months of O3T [1] and further improves during several months after the end of therapy. As previously reported, the main side effects during O3T were slight intestinal bloating for some hours after each session and occasional and moderate discomfort secondary to rectal tube placement [1, 4].
Oxidative stress, inflammation, and ischemia/hypoxia are the principal mechanisms involved in toxicity secondary to cancer treatments. Ozone therapy can modulate all those factors, inducing a controlled oxidative stress sufficient to induce an adaptive antioxidant and anti-inflammatory response of tissues at the local and/or systemic level, as we have previously described in detail [5].
To conclude, in our small cohort of patients, ozone therapy provided a clinically relevant improvement in patients with chronic pelvic pain secondary to cancer treatment. The potential addition of ozone therapy as adjuvant in the management of this clinical condition merits further research. A related randomized clinical trial is in progress (EudraCT: 2019-000821-37; ClinicalTrials.gov: NCT04299893).
Funding sources: The publication of this paper and the subsequent randomized clinical trial (EudraCT number: 2019-000821-37, ClinicalTrials.gov: NCT04299893) is supported by a grant (PI 19/00458) from the Instituto de Salud Carlos III (Spanish Ministry of Science and Innovation, Madrid, Spain); a grant (016/2019) from the Fundación DISA (Las Palmas, Spain); and a grant (BF1-19-13), from the Fundación Española del Dolor (Spanish Pain Foundation, Madrid, Spain).
Conflicts of interest: The ozone therapy device Ozonosan Alpha-plus® was provided by Dr. Renate Viebahn (Dr. Hänsler GmbH, Iffezheim, Germany). The authors have no conflicts of interest to disclose.
Trial registration: EudraCT (European Union Drug Regulating Authorities Clinical Trials Database) 2019-000821-37; ClinicalTrials.gov NCT04299893.
Prior presentation: Preliminary data were 1) presented as oral communication at the Second International Traditional and Complementary Medicine Congress (Istanbul, Turkey, April 2019), organized with technical sponsorship of the World Health Organization, and 2) published as a Brief Report in the Journal of Palliative Medicine: Clavo B, Navarro M, Federico M, et al. Ozone therapy in refractory pelvic pain syndromes secondary to cancer treatment: A new approach warranting exploration. J Palliat Med 2020;24(1):97–102.
References
- 1. Clavo B, Navarro M, Federico M, et al. Ozone therapy in refractory pelvic pain syndromes secondary to cancer treatment: A new approach warranting exploration. J Palliat Med 2021;24(1):97–102. [DOI] [PubMed] [Google Scholar]
- 2. Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2016;34(27):3325–45. [DOI] [PubMed] [Google Scholar]
- 3. Bennett MI, Rayment C, Hjermstad M, et al. Prevalence and aetiology of neuropathic pain in cancer patients: A systematic review. Pain 2012;153(2):359–65. [DOI] [PubMed] [Google Scholar]
- 4. Clavo B, Ceballos D, Gutierrez D, et al. Long-term control of refractory hemorrhagic radiation proctitis with ozone therapy. J Pain Symptom Manage 2013;46(1):106–12. [DOI] [PubMed] [Google Scholar]
- 5. Clavo B, Rodriguez-Esparragon F, Rodriguez-Abreu D, et al. Modulation of oxidative stress by ozone therapy in the prevention and treatment of chemotherapy-induced toxicity: Review and prospects. Antioxidants (Basel) 2019;8(12):588. [DOI] [PMC free article] [PubMed] [Google Scholar]