Abstract
Background and Aims
Many recent studies emphasize that mixed species is a promising silvicultural option for sustainable ecosystem management under uncertain and risky future environmental conditions. However, compared with monocultures, knowledge of mixed stands is still rather fragmentary. This comprehensive study analysed the most common Central European tree species combinations to determine the extent to which mono-layered species mixing (1) can increase stand productivity and stem diameter growth, (2) increase stand density or growth efficiency, and (3) reduce competition and attenuate the relationship between stand density and stem diameter growth compared with mono-specific stands.
Methods
The study was based on 63 long-term experimental plots in Germany with repeated spatially explicit stand inventories. They covered mono-specific and mixed species stands of Norway spruce (Picea abies), silver fir (Abies alba), Scots pine (Pinus sylvestris), European beech (Fagus sylvatica), sessile oak (Quercus petraea), European ash (Fraxinus excelsior) and sycamore maple (Acer pseudoplatanus). Based on spatially explicit measurement, we quantified for each tree the intra- or inter-specific neighbourhood, local stand density and growth. We applied mixed models to analyse how inter-specific neighbourhoods modify stand productivity, stand density, growth efficiency, individual tree growth and the trade-off between individual tree growth and stand productivity.
Key Results
We found stand productivity gains of 7–53 % of mixed versus mono-specific stands continuing over the entire rotation. All mixtures achieved a 3–36 % higher leaf area index until advanced stand age. Stem diameter growth increased by up to 31 % in mixed stands. The growth efficiency of the leaf area was up to 31 % higher, except in mixtures of sessile oak and European beech. The trade-off between stem diameter growth and stand productivity was attenuated by the mixture.
Conclusions
The increased productivity was mainly based on a density increase in the case of Norway spruce/silver fir/European beech and sessile oak/European beech and it was based on a more efficient resource use given the same stand density in the case of Scots pine/European beech and European ash/sycamore maple. In the other species assemblages the increased productivity was based on a combination of density and efficiency increase. We hypothesize that the density effect may be site-invariant and mainly depends on the structural species complementarity. The efficiency increase of growth may depend on the growth-limiting factor that is remedied by mixture and thus be co-determined by the site conditions. For forest management, the results indicate increased stand and tree size growth by species mixing. For the common mixtures examined in this study the results show that thinning for the acceleration of stem growth requires less density reduction and causes less stand growth losses than in monocultures. We discuss the consequences of our findings for silvicultural prescriptions for mixed-species stands.
Keywords: Tree species mixing, stand productivity, stand density, growth efficiency, competition reduction, facilitation, overyielding, overdensity, trade-off between stand productivity and tree growth
INTRODUCTION
Many recent studies emphasize that mixed species is a promising silvicultural option for sustainable ecosystem management under uncertain and risky future environmental conditions.
Recent studies suggest that mixed-species stands can overyield mono-specific stands (Mason and Connolly, 2014; Liang et al., 2016; Jactel et al., 2018) because they can be denser (Pretzsch, 2014; Jucker et al., 2015; Steckel et al., 2019) and more efficient in resource use (Khanna, 1997; Forrester, 2014). The superiority of mixed-species stands can even increase under drought (Lebourgeois et al., 2013; Pretzsch et al., 2013; Neuner et al., 2015; Dănescu et al., 2018), insect attacks (Jactel and Brockerhoff, 2007; Jactel et al., 2021) and other types of abiotic and biotic disturbances (Knoke et al., 2008; Griess et al., 2012). For successful management of mixed-species stands (Pretzsch and del Río, 2020), especially for species selection and stand density regulation, it is important to understand the overyielding of different species assemblages, i.e. whether overyielding is mainly an effect of increased density or higher efficiency of resource use and how any mixing effects change with progressing stand development. Better insight into the structure and functioning of mixed-species stands is crucial for the exploitation of overyielding to improve wood production, carbon uptake, and storage. However, compared with monocultures, the knowledge about mixed stands summarized in this introduction is still rather fragmentary.
Several recent studies have found overyielding in middle-aged stands. Jactel et al. (2018), for instance, reported overyielding of 15 % in a meta-analysis. The overyielding reported by Pretzsch et al. (2010, 2015, 2020), Thurm and Pretzsch (2016), Steckel et al. (2019) and Ruiz-Peinado et al. (2021) ranged between 2 and 59 % in terms of stand volume or mass growth. The course of individual tree growth has been well analysed for mono-specific stands (Kozlowski, 1962; Zeide, 1993) and also for rich structured selection forests where trees can remain for a long period in the understorey (Magin, 1959; Mitscherlich, 1970; Hilmers et al., 2019). Recent studies showed that tree mixing of species often (Brooks et al., 2002; Pretzsch et al., 2013) but not always (Grossiord, 2020; Gillerot et al., 2020) facilitates tree growth in drought years, and that the mixing effects depend on the species identity and combination (Pardos et al., 2021). However, how mixing modifies tree growth in the long term has been hardly addressed yet (Pretzsch and Schütze, 2009; Thurm et al., 2017; Pretzsch et al., 2021); this is probably due to the rarity of long-term observations.
So far, mixing effects on productivity have mainly been analysed in young or middle-aged stands. Young stands are often not yet closed, are far from maximum stand density, and have only a short time to acclimate to the intra- or inter-specific conditions (Bauhus et al., 2000; Amoroso and Turnblom, 2006; Forrester et al., 2006; Vanclay et al., 2013). With progressing stand development the acclimation of trees to their neighbourhood may proceed differently in mixed compared with mono-specific stands. Therefore, comparisons between young mixed stands and respective monocultures (commonly used as reference) can hardly be transferred to older or mature forest stands (Nichols et al., 2006). Most of the studies addressed in the previous paragraph and meta-analyses (Piotto, 2008; Pretzsch et al., 2017; Jactel et al., 2018) were based on medium-aged forest stands, which are often fully stocked, and represent the most productive phase of rotation. As long-term mixing experiments are rare, little is known about the mixing effects until advanced stand ages. Dieler et al. (2017) and Zeller and Pretzsch (2019) showed that in the advanced development stage, structural diversity may increase productivity (older stands may tend to open canopy space, caused by human and natural disturbances) and that when stands are mixed and structured, productivity losses may be better buffered and compensated than in mono-layered monocultures.
Several studies provide evidence that tree species mixing can increase stand density in terms of stand density index (Pretzsch and Biber, 2016; Williams et al., 2017), crown coverage (Pretzsch, 2014) and leaf area index (LAI) (Peng et al., 2017). Such an increase in packing density may result from the complementary use of space and resources above or below the ground (Forrester, 2014). Supposing there is mainly a density effect (competition reduction) of mixing on growth, the benefit of mixture could be exploited by keeping stands at a higher stand density level, whereas more widely planted or strongly thinned mixed stands would not generate more yield.
An increase in growth efficiency (growth per leaf area or per crown projection area), in contrast, means that at lower densities the inter-specific neighbourhood may increase the efficiency of the crown, similar to a fertilization effect (Khanna, 1997). In this case, benefits may emerge independently of stand density, but also under wide spacing and strong thinning. Thinning may cancel the density effect, but not the efficiency increase (Forrester et al., 2013; Brunner and Forrester, 2020). Forrester et al. (2013) showed that the efficiency effect may be amplified by density, i.e. complementarity effects may become stronger under high density, or complementarity enables higher densities, so that both are positive and can reinforce each other.
For mono-specific stands, it is well known that stand density lowering reduces stand growth and increases individual tree diameter growth (del Río et al., 2017), resulting in a well-known trade-off between stand productivity and tree size growth (Zeide, 2001; Mäkinen and Isomäki, 2004a, b; Pretzsch, 2020). In mixed stands, stem diameter growth and stand growth may be increased by competition reduction and facilitation (Kelty, 1992; Forrester, 2014). In mixed stands, larger stem diameters may be achieved even under higher stand densities than in mono-specific stands. Thus, a given target stem size may be achieved with higher density and less loss of stand productivity due to density reduction. The dilemma of the forest manager between maintaining stand density and growth at a maximum and providing tall high-quality stems by stand density reduction may be attenuated by tree species mixture.
The objective of this study was to comprehensively quantify common tree species combinations in Central Europe in terms of their tree and stand growth compared with mono-specific stands. Our study covered mono-specific and mixed species stands of Norway spruce (Picea abies), silver fir (Abies alba), Scots pine (Pinus sylvestris), European beech (Fagus sylvatica), sessile oak (Quercus petraea), European ash (Fraxinus excelsior), and sycamore maple (Acer pseudoplatanus).
We analysed 63 plots covering the species combinations of (1) Norway spruce/European beech, (2) Norway spruce/silver fir/European beech, (3) Norway spruce/Scots pine, (4) Scots pine/European beech, (5) sessile oak/European beech and (6) European ash/sycamore maple. The plots provided new insights as they represented, for each species combination, age series from young to mature stands at the same sites. All plots were fully stocked with the species growing in both inter- and intra-specific neighbourhoods. The plots represented medium- and high-quality site conditions; the range of site conditions was not wide enough for thoroughly exploring the dependency of the mixing effects on site quality. The measurements included tree coordinates, crown width and length, and up to five repeated surveys of stem diameter, and height of all trees. Tree heights and height to the crown base were measured at each survey but only of sample trees; crown sizes were measured only at one or two surveys but of all trees. The plots were used for analysis at the stand and tree levels and for testing the following hypotheses about mixed stands compared with mono-specific stands:
H I: Tree species mixing can increase stand productivity and stem diameter growth throughout the entire rotation.
H II: The mixing effects are based on both the increased stand density and growth efficiency.
H III: Tree species mixing can reduce competition and attenuate the relationship between stand density and stem diameter growth compared with mono-specific stands.
MATERIALS AND METHODS
Study plots
The study was based on 11 age series (see example in Fig. 1) with 63 long-term plots in Germany with repeated spatially explicit stand inventories. They were established in 18- to 238-year-old stands and covered the main tree species in Central Europe in intra- and inter-specific neighbourhoods throughout the whole rotation. The plots represented the most common medium- and high-quality site conditions (Table 1), were fully stocked, and the mixing patterns ranged from individual trees to cluster mixtures. Most of the stands were planted and even-aged; occasionally natural regeneration may have complemented them. On some of the plots moderate thinning from above was applied in the second half of the 20- to 30-year survey period.
Fig. 1.
Age series SON 814 with eight plots near Schongau/Bavaria as an example of the setup of the 11 age series included in this study. (A–H) Plots 7, 4, 9, 8, 5, 1, 2 and 3 ranked by age, increasing from 62 to 127 years in the case of Norway spruce and 92 to 142 years in the case of European beech (state of the last survey in autumn 2011). Crown sizes of Norway spruce and European beech are plotted in red and blue, respectively. The sizes of plots 7, 4, 9, 8, 5, 1, 2 and 3 were 0.20, 0.41, 0.39, 0.39, 0.56, 0.26, 0.63 and 1.00 ha, respectively.
Table 1.
Location, climate characteristics and site conditions of the eight long-term experiments included in this study
| Age series | Name | Species combination | Longitude | Latitude | Elevation a.s.l. | Annual precipitation | Mean temperature (°C) |
Soil type | Substrate | Geology | Ecoregion (see footnote) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | ° | ° | m | mm year−1 | °C | ||||||
| FRE 813 | Freising | N. sp., E. be. | 11·66 | 48·42 | 515 | 814 | 7·7 | Parabrown soil | Loam | Tertiary sand | 12.8 |
| SON 814 | Schongau | N. sp., E. be. | 10·77 | 47·87 | 790 | 1114 | 6·8 | Brown soil | Loam | Günz-Mindel lower moraine | 14.4.1 |
| NOR 811 | Nordhalben | N. sp., E. be. | 11·59 | 50·31 | 590 | 850 | 5·5 | Brown soil | Stony loam | Clay shale | 8.1 |
| KEH 804 | Kelheim | S. oak, E. be. | 11·76 | 48·93 | 455 | 721 | 7·5 | Brown soil | Silt loam | Tertiary sediments | 6.2 |
| ROT 801 | Rothenbuch | S. oak, E. be. | 9·44 | 49·95 | 375 | 960 | 7·0 | Brown soil | Silt loam | Lower sandstone | 2.2.1 |
| SWE 803 | Schweinfurt | S. oak, E. be. | 10·30 | 50·13 | 340 | 660 | 8·0 | Brown soil | Silt loam | Lower Trias | 4.1 |
| KRE 824 | Kreuth | N. sp., s. fir, E. be. | 11·69 | 47·63 | 1200 | 1800 | 4·5 | Brown soil | Loam | Main dolomite | 15.5 |
| GEI 832 | Geisenfeld | S. pi., E. be. | 11·22 | 48·57 | 430 | 725 | 7·6 | Brown soil | loamy sand | tertiary sand | 12.8 |
| AMB 833 | Amberg | S. pi., E. be. | 11·83 | 49·35 | 480 | 650 | 7·5 | Brown soil | Sandy loam | Chalkstone | 6.5 |
| NEU 841 | Neuburg | S. pi., N. sp. | 11·22 | 48·56 | 430 | 725 | 7·6 | Brown soil | Loamy sand | Tertiary sand | 12.8 |
| ARN 851 | Arnstein | E. ash, syc. map. | 9·94 | 49·99 | 260 | 670 | 8·0 | Parabrown soil | Silt loam | Shell limestone | 4.2 |
The ecoregion numbers indicate the following units (translation to English in brackets): 12.8 Oberbayerisches Tertiärhügelland (Upper Bavarian tertiary hills), 14.4.1 Westliche kalkalpine Jungmoräne (Western limestone young moraine region), 8.1 Frankenwald (Franconian Forest), 6.2 Südlicher Oberpfälzer Jura (Southern Upper Palatinate Jurassic region), 2.2.1 Hochspessart (Upper Spessart region), 4.1 Nördliche Fränkische Platte (Northern Franconian plateau region), 15.5 Mittlere Bayerische Kalkalpen (Middle Bavarian limestone Alps), 6.5 Oberpfälzer Jurarand (Upper Palatinate jurassic borderline region), 4.2 Südliche Fränkische Platte (Southern Franconian plateau region) (according to Arbeitskreis Standortskartierung (1985) Forstliche Wuchsgebiete und Wuchsbezirke in der Bundesrepublik Deutschland).
N. sp., Norway spruce; E. be. European beech; s. oak, sessile oak; s. fir, silver fir; S. pi., Scots pine; E. ash, European ash; syc. map., sycamore maple.
The age series were established in the 1990s for ad hoc data acquisition for parameterization of an individual tree simulator for mono-specific and mixed species stands in South Germany (Pretzsch et al., 2002). The plots within each age series were established on similar sites and close to each other. Since their establishment and first survey, chronosequences were remeasured up to five times, so that the original chronosequences became real-time series of long-term surveys. For example, the survey of a 20-year-old stand (first survey carried out 25 years ago) was repeated four times so that the original 20-year-old stand resulted in a 45-year-old one and overlapped with the survey data of the original 40-year-old stand. In this way, we covered, for all considered mixtures, an age span of a whole rotation or more.
The pseudo 3-D visualization of the age series SON 814 in Fig. 1 was based on the first inventory and tree coordinate measurements in 1991; the repeated measurements (survey 2011) of the stem diameters, tree heights and crown sizes are explained in detail in the next section. Supplementary Data Fig. S1 shows as an example the crown maps of SON 814/1–8, where the plots of the age series cover individual trees and group-mixture Norway spruce and European beech as well as mono-specific parts. For the sake of simplicity, we visualized the crown size as concentric circles calculated as the quadratic mean of the eight crown radius measurements recorded during the course of the repeated surveys.
The plot size increased from the young to the old stands (see 10-m scale at the bottom of each of the crown maps) in order to cover representative sections of the representative stand development phases. The range of plot sizes varied according to stand ages between 0.2 and 1.0 ha and was similar between age series. The plots within the age series covered stand ages from 17 to 238 years. The experiment KRE824 represents a large plot that comprises several age phases in close vicinity to each other.
Dendrometric measurements
Table 2 summarizes the abbreviations and explanations of the main measurement variables and metrics and the objective variables used in this study. From each tree that was higher than 1·30 m, we recorded the species identity, measured the x- and y-coordinates of the tree positions at the first survey, and all stem diameters at breast height in each of the up to five surveys (Fig. 2A, Table 3). Tree height (h) and height to crown base (hcb) of a subset of trees were measured in each survey. For this purpose, we sampled ~30 trees per species on each plot on each age series; at the plot level the sample trees were selected uniformly over the whole diameter range. In the course of the successive surveys we preferably used the same sample trees for the measurement of height and height to crown base. However, we replaced them by neighbours of similar stem diameter if they had been removed. Crown radii in the eight cardinal directions were measured only in one or two surveys, but for all trees. All these introduced tree characteristics were also measured for the ingrowth since the first survey. In addition, we inventoried the natural regeneration since the first survey; as the latter information was not used for this study we refrain from reporting details about the natural regeneration.
Table 2.
Overview of main measurements variables and metrics used in this study
| Variable and metric names | Abbreviation | Explanation and indication |
|---|---|---|
| (1) Tree-level variables | ||
| Stem diameter | d | Indication of tree present size |
| Tree height | h | Determination of radius for competition analysis |
| Height to crown base, to lowest branch | hcb | Indication of bole length, used for visualization |
| Crown radius | cr | for visualization |
| Crown length | cl | used for visualization |
| Tree leaf area | la | Estimated depending on stem diameter |
| Above-ground tree mass | ma | Estimated depending on stem diameter |
| Search radius for neighbourhood analysis | sr | for analysis |
| Annual stem diameter increment | id | Periodical diameter increment/period length |
| Local competition index | SDIci | Local SDI in circle calculated without centre tree |
| Local mixing proportion | mixport | Calculated using equivalence factors |
| (2) Variables related to sample circle | ||
| Stand density index on circle | SDIc | SDI standardized by equivalence coefficients |
| Mass growth on circle | IMc | Scaled up by stem diameter, tree mass >7 cm |
| Quadratic mean tree diameter on circle | dqc | Species overarching dq |
| Leaf area index at circle level | LAIc | LAI standardized by equivalence coefficients |
| Categorical variable indicating mono-specific versus mixed on circle | m |
m = 0, i.e. mixing proportion <10 % m = 1, i.e. mixing proportion ≥10 % |
| (3) Stand-level variables | ||
| Quadratic mean stem diameter | dq | Calculated species-overarching |
| Standing stem volume | V | Merchantable volume 7 cm at the smaller end |
| Leaf area index | LAI | Upscaled depending on d by allometric functions |
| Carbon stock at the stand level | C | Above-ground biomass × 0·5 |
| Stand stem volume growth | IV | Periodical mean annual stem volume growth |
Fig. 2.
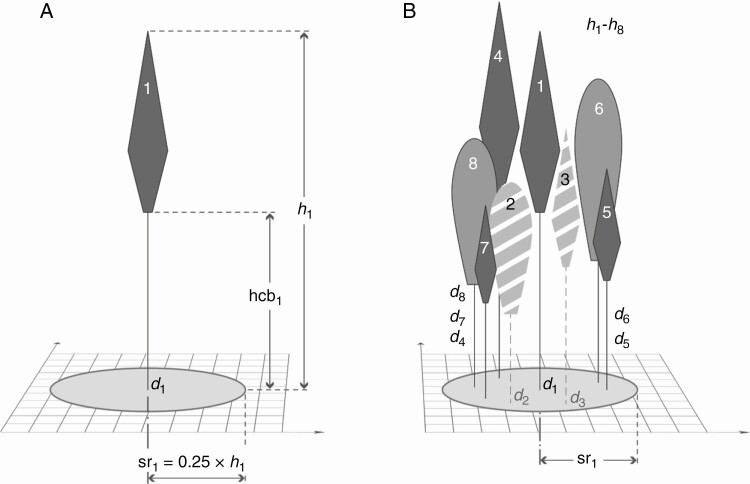
(A) Measurements at tree level. (B) Setup for the evaluation of the local neighbourhood of each tree. For variable explanation see footnote Table 2. The central tree in the circle has number 1 and the breast height diameter and tree height d1 and h1. The search radius around the central tree has the radius sr1. In this example there are the neighbouring trees numbered 2–8, with diameters d2–d8, and heights h1–h8 within the search radius. Trees 2 and 3 (hatched) represent removal trees.
Table 3.
Overview of the age 11 series with 63 plots included in this study. The number of measurements refers to the number of measured tree attributes, such as stem diameters, coordinates crown and characteristics at the first survey; remeasurements are only counted once. For explanation of species names see footnote of Table 1
| Age series | Name | Species combination | Site indexa ho age 100 | Age from tob | Number of plots | Total plot area | First survey | Last survey | Number of surveys | Number of trees measured for d including repeated | Number of trees measured for h including repeated | Number of trees measured, x, y coordinates | Number of trees measured, crowns including repeated |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | m | years | ha | m | Number | Number | Number | Number | |||||
| FRE 813 | Freising | N. sp., E. be. | 35·1 | 37–168 | 6 | 2·87 | 1994 | 2012 | 4 | 7939 | 2498 | 2725 | 2020 |
| SON 814 | Schongau | N. sp., E. be. | 37·4 | 50–142 | 8 | 3·87 | 1991 | 2011 | 5 | 14106 | 4204 | 3619 | 3538 |
| NOR 811 | Nordhalben | N. sp., E. be. | 33·3 | 36–126 | 5 | 2·19 | 1997 | 2008 | 2 | 3474 | 1494 | 1736 | 999 |
| KEH 804 | Kelheim | S. oak, E. be. | 32·7 | 17–165 | 7 | 3·18 | 1996 | 2015 | 4 | 14587 | 2743 | 4129 | 1426 |
| ROT 801 | Rothenbuch | S. oak, E. be. | 25·6 | 32–238 | 6 | 3·35 | 1994 | 2009 | 3 | 11282 | 1270 | 3911 | 1389 |
| SWE 803 | Schweinfurt | S. oak, E. be. | 27·3 | 21–186 | 6 | 2·78 | 1995 | 2005 | 2 | 6874 | 1813 | 4000 | 1339 |
| KRE 824 | Kreuth | N. sp., s. fir, E. be. | 20·5 | 50–218 | 1 | 0·87 | 1994 | 2012 | 4 | 2503 | 526 | 724 | 759 |
| GEI 832 | Geisenfeld | S. pi., E. be. | 32·0 | 18–214 | 6 | 2·07 | 1996 | 2010 | 3 | 7823 | 1837 | 2775 | 1029 |
| AMB 833 | Amberg | S. pi., E. be. | 31·5 | 26–136 | 5 | 0·69 | 1991 | 2016 | 5 | 5142 | 1942 | 1869 | 979 |
| NEU 841 | Neuburg | S. pi., N. sp. | 32·8 | 22–118 | 6 | 2·46 | 1997 | 2014 | 3 | 13433 | 4002 | 5135 | 1406 |
| ARN 851 | Arnstein | syc. map., E. ash | 35·0 | 20–94 | 7 | 1·93 | 1998 | 2014 | 3 | 5557 | 2864 | 5135 | 1241 |
aho is related to the leading tree species mentioned in the first place in column 3.
bRelates to the first survey of the youngest plot and the last survey of the oldest plot.
The stand age was read off from the historical documentation of stand establishment; if this was not available we derived the tree age by tree-ring counting on increment cores sampled at the foot of the trunks. Stand ages were assumed to be identical with mean tree age in cases of natural regenerated stands. In planted stands, stand age was assumed to be mean tree age minus 3 years to take into account the usual age of plants coming from the nursery.
Rationale of the descriptive evaluation
Testing the hypotheses H I to H III required dendrometric characteristics at the tree level, information about the neighbourhood of the trees, and growth and yield characteristics of the stands (see variables in Table 2). For completion of the tree-level data we used auxiliary relationships, e.g. for deriving the tree height, stem mass and leaf area of every tree. For analysing the competition and neighbourhood we constructed a sample circle around each tree and analysed, among others, the local stand density and the species composition within this circle. Stand-level growth and yield characteristics were derived on both the sample circle level and the whole plot level. In the following sections we describe the auxiliary relationships and methods which we applied for the derivation of the various characteristics.
Auxiliary relationships
By modelling the relationship between height, stem diameter and age (see section Results. Overview of tree and stand characteristics), we calculated the height of each tree. To estimate the individual tree height (h) depending on the stem diameter (d) and tree age (age) we parameterized the model below for each species in each of the 11 age series:
| (1) |
All regression coefficients were significant, at least at the level of P < 0.05; the R2 values ranged between 0.85 and 0.98. For the model parameters, see Supplementary Data Table S1.
To estimate the above-ground stem biomass (ma) and leaf area (la) we used the species-specific functions of Forrester et al. (2017):
| (2) |
| (3) |
Equations (2) and (3) result in the de-logarithmic models and . Factors CF were applied in order to correct for the bias that results from back-transforming ln-transformed predictions of the biomass (ma) and leaf area (la) (Snowdon, 1991). For the model parameters, see Supplementary Data Tables S2 and S3. For species with minor portions for which species-specific functions were not available, we used the generalized functions for conifer and broad-leaved species, respectively (see also Forrester et al., 2017). Above-ground tree carbon mass resulted from multiplication of above-ground tree dry biomass by 0·50 (Knigge and Schulz, 1966; Körner, 2002; Lamlom and Savidge, 2003).
Analysing tree competition and neighbourhood
For analysing the effects of intra- and inter-specific neighbourhoods on stem diameter growth of each tree we constructed a circle with radius sr1 = 0·25 × h1 around its stand point, where is the height of the central tree (Fig. 2B). Within the constructed circles, there were, on average, eight or nine trees and at least five or six neighbours with strong impact on the growth of the central tree (Prodan, 1968a, b). We fixed the search radius to a quarter of the height of the respective central tree as other search radii resulted in lower correlations between growth of the central tree and the respective competition index. By choosing the search radius depending on tree height we took into consideration that in even-aged stands the influence zone around a tree increases with progressing size development (Pretzsch, 2009, pp. 295–296). All trees within the constructed circle were used to quantify local competition, mixing status, local density, leaf area and productivity.
To quantify the competitive status of each individual tree we calculated the local stand density index (SDIci). This calculation was based on all trees in the search radius sr, except the central tree; SDIci served as a proxy for local density and competition. We used the concept of the stand density index (SDI) (Reineke, 1933). The SDI is a measure of relative density. It provides the stand density in terms of trees per hectare for a stand with an index mean tree diameter of 25 cm. All trees within the circle were used to calculate the local density n on circle area a. N = 10·000/a × n was the respective tree number upscaled to 1 ha. For the n trees, we calculated the quadratic mean stem diameter, dq; based on N and dq we then calculated the local density SDI = N × (25/dq) around each individual tree. The local SDI was calculated using species-specific allometric exponents derived by Pretzsch and Biber (2005). Note that the exponents α were derived on unthinned and A-grade plots of long-term experiments in South Germany that are located in the same area as the age series of this study. On A-grade plots the treatment is restricted to the measurement and removal of dead or dying trees (VDFV, 1902); they serve as reference for the thinned plots. The derived exponents α deviated from the species-overarching exponent of −1·605, as proposed by Reineke (1933), are species-specific and representative of South Germany. The resulting competition index SDIci is distant-dependent and easy to interpret. SDIci values were calculated with and without the removed trees for all circles and all surveys; the relationships reported in the Results section were based on the SDI values of the remaining stand at the end of each survey period.
The sampled trees were also used to calculate local mixing proportions. The mixing proportions should reflect the area proportions of two or more species in the observed mixed stands (Dirnberger et al., 2017; Pretzsch and del Río, 2020). Tree number, basal area or volume proportions are only appropriate for this purpose if the mixed species have similar growing area requirements (Pretzsch et al., 2017).
To standardize the density and to calculate the unbiased area-related mixing proportions and leaf area indices, we applied the equivalence factors of Pretzsch and Biber (2016). They take into consideration that the analysed species vary per se in the growing area requirement and maximum stand density in fully stocked stands. For example, a European beech with a stem diameter of 25 cm may require approximately double the growing space of a Norway spruce of the same diameter; i.e. the density in terms of trees per hectare is only half of that of Norway spruce. The equivalence factors adjust these species-specific differences. Supplementary Data Table S4 gives an overview of the equivalence factors that were applied in this study.
Before calculating the local SDI values and mixing proportions for neighbourhood analysis, we established a toroidal shift of the plot to all eight directions of the plot periphery for edge bias compensation (Radtke and Burkhart, 1998; Pommerening and Stoyan, 2006; Pretzsch, 2009). We use the plot SON 814/2 at the survey in autumn 2011 for visualization of this method in Supplementary Data Fig. S2. Using the toroidal shift, we extended the same mixing patterns and distances between trees in all eight directions and avoided any overestimation of density, which could result from other techniques (Radtke and Burkhart, 1998).
Structure and growth on the sample circles
In order to compare structure, growth and yield characteristics between mono-specific and mixed parts of our study plots we used the sample circles as described in the section Analysing tree competition and neighbourhood and in Fig. 2B. For all of the ~92 000 sample circles (one circle for each tree and survey) we evaluated the standing volume, mixing proportion, stand density, LAI, above-ground stem mass and mass growth and scaled it up to a hectare [see variables in Table 2, section (ii)]. The SDIc on each circle was calculated analogously to the calculation of SDIci introduced in the section Analysing tree competition and neighbourhood. SDIc was based on all trees on the circle, whereas SDIci was based on all trees on the circle except the central tree. SDIc was calculated for all trees on each circle with and without the removed trees. The LAI for each circle (LAIc) was based on the functions introduced in the section Auxiliary relationships. We further calculated the standing stem volume and above-ground biomass per hectare based on all trees on each circle using the biomass functions in the section Auxiliary relationships. For some rare tree species, we used the generalized functions for conifers and broad-leaved species, respectively. We used the functions by Forrester et al., (2017) for estimating leaf area and biomass; the functions are provided in Supplementary Data Tables S2 and S3. To explore the effects of the codetermining variables on the mixing effects, we introduced dummy variables that indicated the respective species assemblage. Circles with an admixture <10 % of other species based on the SDIc were classified as mono-specific (m = 0), and those with an admixture of ≥10 % were classified as mixed (m = 1).
This separation was used as it is in line with common definitions (Bravo-Oviedo et al., 2014) of mixed species stands and it split our data in two subgroups with similar sample size. An increase of the threshold from 10 to 15 or 20 % hardly changed the results, however, and increase beyond 20 % obscured the differences between mono- and mixed-species stands. The calculations at the sample circle level resulted in the variables stand density (SDIc), above-ground mass growth (IMc), quadratic mean tree diameter (dqc), diameter growth (id), leaf area index (LAIc) and the categorical variable (m). These variables were calculated for both species in each mixture separately but also as sums (e.g. in the case of LAIc) or means (e.g. in the case of dgc) for both species. In order to address the fact that these variables were derived at the sample circle level we attached a ‘c’ to the variable names [see section (2) in Table 2].
Stand level characteristics
To give an overview of the included age series and their plots, we also evaluated them at the stand level. The stand level characteristics were derived from the successive inventories of the tree diameters and heights, and records of the removal trees. We used standard evaluation methods according to the DESER-norm recommended by the German Association of Forest Research Institutes (in German ‘Deutscher Verband Forstlicher Forschungsanstalten’) (Biber, 2013; Johann, 1993). For estimating the merchantable stem volume in dependence on tree diameter, tree height and form factor, we used the approach by Franz et al. (1973) with the stem form equations and coefficients published by Pretzsch (2002, p. 170). The results encompassed the quadratic mean tree diameter, stand volume and volume growth. By applying auxiliary functions (section Auxiliary relationships), we also calculated the LAI and carbon stock for each stand and survey, and the overarching mean, minimum and maximum values for the six species mixtures (see stand variables in Table 2 and Overview of tree and stand characteristics in the Results section).
Statistical models
To test hypotheses H I to H III, we applied the linear mixed Models 4–10 shown below. The dependent variables were the IMc, LAIc and mean and species-specific id values. The independent variables were individual tree diameter, d, quadratic mean diameter within the respective circle (dqc), quadratic mean stem diameter of the stand (dq), the leaf area index LAIc and SDIc on the circle, and the categorical variable m, which indicates mono-specific (m = 0) and mixed-species conditions (for explanation of variables see Table 2). The diameter d indicates the size of the central tree, dqc is the mean tree size in the local environment of the individual tree, dq is the development stage and age of the stand, and the categorical variable m indicates whether the subset was mono-specific or mixed.
In all equations, indexes i and k represent the kth observation of the ith tree. The fixed effects were covered by parameters a0 to an. With the random effect , we cover the correlation between the single observations at the tree level. In preliminary model formulations, we also worked with random effects at the plot level; that is, one additional nesting level. As this caused confounding effects with the fixed effects, we constrained ourselves to the simpler random effect structure of eqns (4)–(10). With εik, we denoted independently and identically distributed errors.
| (4.1) |
| (4.2) |
| (5) |
| (6) |
| (7) |
| (8) |
The de-logarithmic version of the models, for example , shows that the dummy variable m becomes in the case of monoculture and in the case of mixed stands. This means that directly reveals any multiplicative effects of mixing on the dependent variables. Assuming , the mixing effect on the target variable would be , and the effect would be 28.4 %. This helps to easily interpret the biological meaning of the respective coefficients of m in Models 1–8.
To analyse whether the trade-off between stem size growth and stand productivity is modified by tree species mixing, we first fitted the relationships between stem diameter growth and stand density for mono- and mixed-species stands, , and in the same way the relationships between stand growth and stand density for mono- and mixed-species stands, .
| (9) |
| (10) |
Both equations were equalized in terms of ln(SDIc), rearranged, and solved so that IMc was on the left and id on the right side, . By inserting m = 0 and m = 1, respectively, this resulted in productivity–stem growth relationships for mono-specific and mixed-species stands, respectively. For the step-by-step derivation of eqn (11), see Supplementary Data Derivation A.
| (11) |
The 95 % confidence intervals displayed with the model predictions in the Results section were derived from bootstrapped model predictions for each combination of input variable values used in the diagrams. For the bootstrapping procedure, we used the function bootMer from the R-Package lme4 (Bates et al., 2015). Even though the confidence bands in Figs 3–7 partly overlapped, the visualized differences between curves for mixed and mono-specific stands resulted from effects that were clearly identified as significant in the mixed model regression. The confidence bands for mixed and mono-specific stands in the same diagram must not be seen independently; that is, an upward deviation relative to the mono-specific curve would mean an upward deviation relative to the mixed stand curve. In other words, one such diagram does not show two independently fitted regression models, but a differentiated view of the same model. For all calculations, we used the statistical software R 3.6.3, and we used the libraries nlme (Pinheiro et al., 2021) and lme4 (Bates et al., 2015).
Fig. 3.
Development of the stand stem mass growth of mixed stands compared with mono-specific stands with increasing quadratic mean stand diameter, dq, as proxy of stand development, shown for (A) Norway spruce and European beech, (B) Norway spruce, silver fir and European beech, (C) Scots pine and European beech, (D) Scots pine and Norway spruce, (E) sessile oak and European beech, and (F) European ash and sycamore maple. The vertical dashed lines and the values (ratios) shown to their right indicate the relationship between mixed and mono-specific stand productivity at a development stage of dq = 20 and 40 cm, respectively. The graphs are based on Model 4 (statistical characteristics are shown in Table 5).
Fig. 7.
Species-specific dependency of stem diameter increment on stand density in mixed compared with mono-specific stands, shown for (A, B) Norway spruce and European beech, (C, D) Norway spruce, silver fir and European beech, (E, F) Scots pine and European beech, (G, H) Scots pine and Norway spruce, (I, J) sessile oak and European beech, and (K, L) European ash and sycamore maple. The vertical dashed lines and the values (ratios) shown to their right indicate the relationship between stem increment in mixed to mono-specific stands for local SDI values of 500 and 1500 trees per hectare. The visualization was based on Model 8, and the mean diameters were set to 25 cm. N. spruce (E. beech) addresses the behaviour of Norway spruce in mixture with European beech. (N. spruce), E. beech addresses the growth of European beech in mixture with Norway spruce. This nomenclature was used for the other mixtures analogously.
RESULTS
Overview of tree and stand characteristics
Table 4 shows the mean stand characteristics over all surveys and substantiates that all mixtures are represented by young to old stands. For further evaluation, we used the mean tree diameter as a substitute for stand age, as in practice it is easier for access. The stand volume was the highest (1774 m3 ha−1) in the mature stands of Norway spruce and European beech. The LAI was higher in mixtures with shade-tolerant species than in those with light-demanding species. The carbon storage in the above-ground stem mass was the highest (388 mg ha−1) in old Norway spruce/European beech stands. Annual stem volume growth was the highest in Scots pine/European beech, Norway spruce/European beech and Scots pine/Norway spruce stands.
Table 4.
Overview of some characteristics of the study stands shown separately for the six tree species mixtures. The table shows the mean, standard deviation, minimum and maximum of the stand age, quadratic mean stem diameter (dq), stand stem volume (V), LAI, above-ground carbon content (C) and mean annual stem volume increment (IV) of the stands of all surveys
| Variable | Unit | Mean | s.d. | Minimum | Maximum |
|---|---|---|---|---|---|
| Norway spruce and European beech | |||||
| Stand age | years | 79·9 | 28·3 | 36·0 | 138·0 |
| dq | cm | 32·1 | 10·4 | 14·3 | 51·5 |
| V | m3 ha−1 | 684·5 | 268·5 | 172·0 | 1774·0 |
| LAI | m m−1 | 10·2 | 2·6 | 5·6 | 24·2 |
| C | mg ha−1 | 160·9 | 48·0 | 63·5 | 388·2 |
| IV | m3 ha−1 yr−1 | 17·7 | 4·7 | 7·3 | 27·7 |
| Sessile oak and European beech | |||||
| Stand age | years | 81·2 | 42·3 | 17·0 | 238 |
| dq | cm | 23·2 | 9·2 | 7·8 | 47·6 |
| V | m3 ha−1 | 443·4 | 177·7 | 72·0 | 774·0 |
| LAI | m m−1 | 10·1 | 2·0 | 6·2 | 13·7 |
| C | mg ha−1 | 163·8 | 63·2 | 46·6 | 295·5 |
| IV | m3 ha−1 yr−1 | 13·6 | 2·6 | 7·9 | 18·8 |
| Scots pine and European beech | |||||
| Stand age | years | 77·7 | 51·5 | 15·0 | 214·0 |
| dq | cm | 22·2 | 7·4 | 10·2 | 36·7 |
| V | m3 ha−1 | 490·4 | 255·6 | 120·0 | 1063·0 |
| LAI | m m−1 | 6·5 | 2·4 | 2·6 | 12·8 |
| C | mg ha−1 | 115·8 | 43·6 | 43·7 | 209·3 |
| IV | m3 ha−1 yr−1 | 18·4 | 6·1 | 10·0 | 36·2 |
| Norway spruce, silver fir and European beech | |||||
| Stand age | years | 147·3 | 64·9 | 43 | 218 |
| dq | cm | 26·3 | 6·5 | 15·2 | 37·2 |
| V | m3 ha−1 | 457·3 | 129·3 | 279·0 | 672·0 |
| LAI | m m−1 | 8·5 | 2·5 | 5·4 | 12·6 |
| C | mg ha−1 | 117·5 | 27·9 | 75·4 | 167·2 |
| IV | m3 ha−1 yr-1 | 11·9 | 1·0 | 11·2 | 13·1 |
| European maple and European ash | |||||
| Stand age | years | 56·44 | 21·9 | 20 | 116 |
| dq | cm | 20·5 | 7·2 | 8·1 | 34·2 |
| V | m3 ha−1 | 356·0 | 153·6 | 76·0 | 656·0 |
| LAI | m m−1 | 5·7 | 1·4 | 3·3 | 9·0 |
| C | mg ha-1 | 104·1 | 33·9 | 39·7 | 188·5 |
| IV | m3 ha−1 yr−1 | 15·9 | 2·1 | 10·4 | 18·6 |
| Scots pine and Norway spruce | |||||
| Stand age | years | 74·7 | 32·1 | 22·0 | 118·0 |
| dq | cm | 23·3 | 8·1 | 11·4 | 36·7 |
| V | m3 ha−1 | 625·7 | 205·4 | 256·0 | 918·0 |
| LAI | m m−1 | 7·2 | 1·1 | 4·9 | 8·6 |
| C | mg ha−1 | 128·7 | 29·5 | 79·2 | 171·3 |
| IV | m3 ha−1 yr−1 | 19·8 | 3·9 | 14·4 | 25·8 |
Stand and tree growth in mixed versus mono-specific stands throughout the rotation (H I)
For all mixtures, we found a decrease in stand stem mass growth, IMc, with increasing quadratic mean tree diameter, which we used as a proxy for stand age. Regarding H I, we found that, in all cases, mixed stands were more productive in terms of stand stem mass growth than mono-specific stands throughout the entire rotation. In the stand development phase of dq = 20 cm, we found an overyielding in terms of stand stem mass of 15–53 %, and at the stand phase of dq = 40 cm an overyielding of 7–53 % (Fig. 3). In only one case, the mixture Norway spruce/European beech, overyielding decreased with age (Fig. 3A), and in all other cases we found a constancy or even an increase in overyielding (Fig. 3B–F). The superiority continued until the advanced stand development phases. Interestingly, we also found high overyielding for species combinations with rather similar physiological traits, for example in stands with shade-tolerant mixtures (Norway spruce, silver fir, European beech; 30 %) or light-demanding mixtures (European ash, sycamore maple, 53 %).
Except for the mixture of Norway spruce, silver fir and European beech, the mean stem diameter growth of both species was always higher in mixed stands than in mono-specific stands (Fig. 4) throughout the whole rotation. We found no significant change in this effect with progressing stand development, i.e. there were no significant interactions between factors m and dq in Model 5 (Table 5). Referring to the index diameters of dq = 20 and dq = 40 cm (vertical dashed lines in Fig. 4), the superiority remained the same. The superiority ranged from 6 % in Norway spruce and European beech to 31 % in European ash and sycamore maple. Note that this analysis only distinguished between mono-specific (mixing portion < 10%) and mixed-species conditions (mixing portion ≥10 %); future studies may analyse the impact of different mixing proportions.
Fig. 4.
Development of the mean stem diameter increment, id, in mixed compared with mono-specific stands with increasing quadratic mean stand diameter, dq, as proxy of stand development, shown for (A) Norway spruce and European beech, (B) Norway spruce, silver fir, European beech, (C) Scots pine and European beech, (D) Scots pine and Norway spruce, (E) sessile oak and European beech, and (F) European ash and sycamore maple. The id value reflects the mean of both tree species. The vertical dashed lines and the values (ratios) shown to their right indicate the relationship between mixed and mono-specific stand diameter increment at a development state of dq = 20 and 40 cm, respectively. The graphs are based on Model 5 (statistical characteristics are shown in Table 5).
Table 5.
Statistical characteristics of the main models used in this study. The equation numbers refer to the models introduced in the Statistical models section. For limitations of space, the table reports only the fixed-effect variables of the respective models. In column “Variables IMc~ dq, m addresses the relationship between IMc and and m. This nomenclature applies for the other relationship analogously. For explanation of variables see footnote of Table 1
| Equation | Group | Variables | n | a 0 | s.d. (a0) | P-value | a 1 | s.d. (a1) | P-value | a 2 | s.d. (a2) | P-value | a 3 | s.d. (a3) | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.1 | N. sp., E. be. | IMc~ dq, m | 20755 | 1·6273 | 0·1079 | <0·001 | −0·0290 | 0·0329 | <0·3781 | 0·8599 | 0·1449 | <0·001 | −0·2149 | 0·0439 | <0·001 |
| 4.2 | N. sp., s. fir, E. be. | IMc~ dq, m | 1521 | 1·9347 | 0·1166 | <0·001 | −0·1148 | 0·0420 | <0·001 | 0·2645 | 0·0683 | <0·001 | |||
| 4.2 | N. sp., S. pi. | IMc~ dq, m | 12256 | 2·6578 | 0·0905 | <0·001 | −0·5624 | 0·0296 | <0·001 | 0·3609 | 0·0230 | <0·001 | |||
| 4.2 | S. pi., E. be. | IMc~ dq, m | 11517 | 1·7428 | 0·0778 | <0·001 | −0·0865 | 0·0255 | <0·001 | 0·1402 | 0·0196 | <0·001 | |||
| 4.1 | S. oak, E. be. | IMc~ dq, m | 24824 | 2·3473 | 0·0349 | <0·001 | −0·4570 | 0·0117 | <0·001 | −0·4104 | 0·0602 | <0·001 | 0·2054 | 0·0197 | <0·001 |
| 4.2 | E. ash, syc. map. | IMc~ dq, m | 3279 | 1·7640 | 0·1522 | <0·001 | −0·1489 | 0·0491 | <0·001 | 0·4270 | 0·0420 | <0·001 | |||
| 5 | N. sp., E. be. | id~ dqc, dq, m | 16611 | −2·2701 | 0·0891 | <0·001 | 0·1813 | 0·0266 | <0·001 | 0·05827 | 0·0156 | <0·001 | |||
| 5 | N. sp., s. fir, E. be. | id~dqc, dq, m | 1420 | −1·2868 | 0·1306 | <0·001 | 0·0819 | 0·0467 | <0·001 | 0·0068 | 0·0751 | <0·001 | |||
| 5 | N. sp., S. pi. | id~dqc, dq, m | 9398 | −2·5987 | 0·0928 | <0·001 | 0·0968 | 0·0296 | <0·001 | 0·1679 | 0·0244 | <0·001 | |||
| 5 | S. pi., E. be. | id~dqc, dq, m | 9617 | −2·2357 | 0·0913 | <0·001 | 0·1263 | 0·0296 | <0·001 | 0·0955 | 0·0214 | <0·001 | |||
| 5 | S. oak, E. be | id~dqc, dq, m | 20205 | −2·3588 | 0·0442 | <0·001 | 0·0880 | 0·0145 | <0·001 | 0·2052 | 0·0140 | <0·001 | |||
| 5 | E. ash, syc. map. | id~dqc, dq, m | 2731 | −3·9440 | 0·1718 | <0·001 | 0·6937 | 0·0543 | <0·001 | 0·2684 | 0·0442 | <0·001 | |||
| 6 | N. sp., E. be. | LAIc~dqc, dq, m | 20755 | 0·9845 | 0·0294 | <0·001 | 1·1332 | 0·0180 | <0·001 | −0·6494 | 0·0200 | <0·001 | 0·0471 | 0·0052 | <0·001 |
| 6 | N. sp., s. fir, E. be. | LAIc~dqc, dq, m | 1521 | 0·2393 | 0·0642 | <0·001 | 0·6562 | 0·0227 | <0·001 | 0·1599 | 0·0352 | <0·001 | |||
| 6 | N. sp., S. pi. | LAIc~dqc, dq, m | 12256 | 0·9290 | 0·0272 | <0·001 | 1·5342 | 0·0168 | <0·001 | −1·3499 | 0·0176 | <0·001 | 0·0820 | 0·0072 | <0·001 |
| 6 | S. pi., E. be. | LAIc~dqc, dq, m | 11517 | −1·4635 | 0·0311 | <0·001 | 1·5742 | 0·0191 | <0·001 | −0·5711 | 0·0196 | <0·001 | 0·0329 | 0·0065 | <0·001 |
| 6 | S. oak, E. be | LAIc~dqc, dq, m | 24824 | 1·2427 | 0·0160 | <0·001 | 1·2016 | 0·0105 | <0·001 | −0·8581 | 0·0102 | <0·001 | 0·3050 | 0·0048 | <0·001 |
| 6 | E. ash, syc. map. | LAIc~dqc, dq, m | 3279 | −0·8704 | 0·0627 | <0·001 | 2·0059 | 0·0364 | <0·001 | −0·7368 | 0·0338 | <0·001 | 0·0729 | 0·0167 | <0·001 |
| 7 | N. sp., E. be. | IMc~LAIc, m | 20755 | −1·2060 | 0·0470 | <0·001 | 1·1026 | 0·0182 | <0·001 | 0·0497 | 0·0155 | <0·001 | |||
| 7 | N. sp., s. fir, E. be. | IMc~LAIc, m | 1521 | 0·2407 | 0·0739 | <0·001 | 0·6973 | 0·0352 | <0·001 | −0·0497 | 0·0565 | <0·379 | |||
| 7 | N. sp., S. pi. | IMc~LAIc, m | 12256 | 0·7381 | 0·0280 | <0·001 | 1·5081 | 0·0189 | <0·001 | 0·0025 | 0·0196 | <0·001 | |||
| 7 | S. pi., E. be. | IMc~LAIc, m | 11517 | 0·4374 | 0·0249 | <0·001 | 0·8446 | 0·0170 | <0·001 | 0·0834 | 0·0171 | <0·001 | |||
| 7 | S. oak, E. be | IMc~LAIc, m | 24824 | −1·0045 | 0·0166 | <0·001 | 0·9444 | 0·0073 | <0·001 | −0·1282 | 0·0072 | <0·001 | |||
| 7 | E. ash, syc. map. | IMc~LAIc, m | 3279 | −0·8101 | 0·0714 | <0·001 | 0·8759 | 0·0280 | <0·001 | 0·2702 | 0·0356 | <0·001 | |||
| 8 | N. sp., (E. be.) | id~d, SDIci, m | 9936 | −2·7460 | 0·1686 | <0·001 | 0·7589 | 0·0254 | <0·001 | −0·1869 | 0·0213 | <0·001 | 0·0114 | 0·0195 | <0·560 |
| 8 | (N. sp.), E. be. | id~d, SDIci, m | 6206 | −3·7709 | 0·1780 | <0·001 | 0·9980 | 0·0296 | <0·001 | −0·1652 | 0·0217 | <0·001 | 0·0464 | 0·0242 | <0·050 |
| 8 | N. sp., (s. fir, E. be.) | id~d, SDIci, m | 1123 | −0·8046 | 0·2092 | <0·001 | 0·4199 | 0·0422 | <0·001 | −0·2185 | 0·0298 | <0·001 | −0·0975 | 0·0884 | <0·270 |
| 8 | N. sp., s. fir, E. be. | id~d, SDIci, m | 206 | −2·5526 | 0·7979 | <0·001 | 0·5208 | 0·1487 | <0·001 | −0·0689 | 0·0843 | <0·415 | −0·0500 | 0·1575 | <0·751 |
| 8 | N. sp., S. pi. | id~d, SDIci, m | 3576 | −4·8825 | 0·3307 | <0·001 | 0·5192 | 0·0397 | <0·001 | 0·2007 | 0·0418 | <0·001 | 0·0611 | 0·0537 | <0·226 |
| 8 | N. sp., S. pi. | id~d, SDIci, m | 5788 | −4·5425 | 0·1751 | <0·001 | 1·3464 | 0·0290 | <0·001 | −0·2142 | 0·0231 | <0·001 | 0·0658 | 0·0296 | <0·027 |
| 8 | S. pi., E. be. | id~d, SDIci, m | 3762 | −1·2316 | 0·2196 | <0·001 | 0·5162 | 0·0373 | <0·001 | −0·2860 | 0·0297 | <0·001 | 0·0966 | 0·0337 | <0·004 |
| 8 | S. pi., E. be. | id~d, SDIci, m | 3554 | −2·9990 | 0·2011 | <0·001 | 1·0253 | 0·0367 | <0·001 | −0·2582 | 0·0269 | <0·001 | 0·1260 | 0·0332 | <0·001 |
| 8 | S. oak, E. be | id~d, SDIci, m | 8173 | −1·9319 | 0·1628 | <0·001 | 0·5833 | 0·0178 | <0·001 | −0·2219 | 0·0214 | <·001 | −0·2037 | 0·0235 | <0·001 |
| 8 | s. oak, E. be | id~d, SDIci, m | 11098 | −5·0467 | 0·0907 | <0·001 | 1·0927 | 0·0144 | <0·001 | −0·0258 | 0·0125 | <0·038 | 0·1740 | 0·0157 | <0·001 |
| 8 | E. ash, syc. map. | id~d, SDIci, m | 709 | −3·6485 | 0·3790 | <0·001 | 1·1182 | 0·0564 | <0·001 | −0·1739 | 0·0572 | <0·002 | 0·1322 | 0·0594 | <0·027 |
| 8 | E. ash, syc. map. | id~d, SDIci, m | 1290 | −5·7095 | 0·4062 | <0·001 | 1·5194 | 0·0483 | <0·001 | −0·0388 | 0·0576 | <0·500 | 0·0398 | 0·0582 | <0·495 |
| 9 | N. sp., E. be. | id~SDIc, m | 16611 | −0·5153 | 0·1078 | <0·001 | −0·1690 | 0·0156 | <0·001 | 0·0920 | 0·0158 | <0·001 | |||
| 9 | N. sp., s. fir, E. be. | id~SDIc, m | 1420 | −0·0578 | 0·1772 | <0·001 | −0·1663 | 0·0287 | <0·001 | 0·1294 | 0·0722 | <0·071 | |||
| 9 | N. sp., S. pi. | id~SDIc, m | 9398 | −1·2340 | 0·1392 | <0·001 | −0·1618 | 0·0202 | <0·001 | 0·2209 | 0·0254 | <0·001 | |||
| 9 | S. pi., E. be. | id~SDIc, m | 9617 | −0·2529 | 0·1213 | <0·037 | −0·2440 | 0·0182 | <0·001 | 0·1463 | 0·0215 | <0·001 | |||
| 9 | S. oak, E. be. | id~SDIc, m | 20205 | −0·9649 | 0·0744 | <0·001 | −0·1832 | 0·0117 | <0·001 | 0·2492 | 0·0138 | <0·001 | |||
| 9 | E. ash, syc. map. | id~SDIc, m | 2731 | −0·8018 | 0·2556 | <0·002 | −0·1615 | 0·0411 | <0·001 | 0·2705 | 0·0460 | <0·001 | |||
| 10 | N. sp., E. be. | IM~SDIc, m | 20755 | −3·1854 | 0·1057 | <0·001 | 0·6883 | 0·0153 | <0·001 | 0·0563 | 0·0160 | <0·001 | |||
| 10 | N. sp., s. fir, E. be. | IM~SDIc, m | 1521 | −1·0519 | 0·1391 | <0·001 | 0·4409 | 0·0225 | <0·001 | 0·0329 | 0·0565 | <0·561 | |||
| 10 | N. sp., S. pi. | IM~SDIc, m | 12256 | −5·0963 | 0·1268 | <0·001 | 0·9000 | 0·0187 | <0·001 | 0·0788 | 0·0227 | <0·001 | |||
| 10 | S. pi., E. be. | IM~SDIc, m | 11517 | −2·2157 | 0·1041 | <0·001 | 0·5567 | 0·0156 | <0·001 | 0·0703 | 0·0182 | <0·001 | |||
| 10 | S. oak, E. be. | IM~SDIc, m | 24824 | −3·6260 | 0·0353 | <0·001 | 0·7481 | 0·0056 | <0·001 | 0·0251 | 0·0069 | <0·001 | |||
| 10 | E. ash, syc. map. | IM~SDIc, m | 3279 | −4·2795 | 0·2086 | <0·001 | 0·8999 | 0·0334 | <0·001 | 0·3350 | 0·0369 | <0·001 |
Modified stand density and growth efficiency of mixed versus mono-specific stands throughout the rotation (H II)
H II refers to two different potential causes of overyielding, i.e. an increased stand density that may increase productivity by higher packing density of trees and leaf area, and an increased growth efficiency of a given density or leaf area. Figure 5 shows, for all six considered species combinations, a higher stand leaf area in mixed compared with the mean of the mono-specific stands. The visualization of the results is based on Model 6 and the respective coefficients are shown in Table 5. In order to demonstrate the magnitude of overdensity and any changes with progressing stand development, the figures indicate the ratios for stands with dq = 20 and 40 cm (dashed vertical lines with added ratios). In all mixtures the overdensity of mixed stands is maintained throughout the whole rotation (). The overdensity ranged between 3 % in case of Scots pine/European beech and 36% in mixed stands of sessile oak and European beech. We found no decrease or increase of the overdensity with progressing stand development.
Fig. 5.
Development of the local leaf area index, LAIc, with increasing quadratic mean stand diameter, dq, as proxy of stand development phase shown for mixed (blue line) compared with mono-specific stands (red line), shown for (A) Norway spruce and European beech, (B) Norway spruce, silver fir, European beech, (C) Scots pine and European beech, (D) Scots pine and Norway spruce, (E) sessile oak and European beech, and (F) European ash and sycamore maple. The vertical dashed lines and the values (ratios) shown to their right indicate the relationship between mixed and mono-specific stand productivity at a development state of dq = 20 and 40 cm, respectively. The graphs are based on Model 6 (statistical characteristics are shown in Table 5).
Regarding the growth efficiency per given stand density and leaf area (Fig. 6), we found a less general effect of mixing. In half of the considered mixtures, leaf area efficiency hardly increased (Scots pine and Norway spruce) or even reduced (sessile oak and European beech). In the other cases, the growth efficiency of the leaf area increased by 5–31 %. In summary, we found that the increased productivity was mainly a density effect in the case of sessile oak and European beech, mainly an efficiency effect in the case of European ash and sycamore maple, and a combination of both in the other species assemblages.
Fig. 6.
Development of the stand stem mass growth of mixed compared with mono-specific stands depending on stand density in terms of stand leaf area, LA, shown for (A) Norway spruce and European beech, (B) Norway spruce, silver fir, European beech, (C) Scots pine and European beech, (D) Scots pine and Norway spruce, (E) sessile oak and European beech, and (F) European ash and sycamore maple. The vertical dashed lines and the values (ratios) shown to their right indicate the relationship between mixed and mono-specific stand productivity LAI = 5 and 15 m2 m2, respectively. The graphs are based on Model 7 (statistical characteristics are shown in Table 5).
Reduction of competition and attenuation of the relationship between stand density and stem growth (H III)
Under the same competition index, SDIci tree diameter increment was higher in mixed stands than in mono-specific stands in 9 out of 12 cases (Fig. 7A–L). Figure 7 visualizes for each of the six analysed species combinations and for both species of the respective mixtures how they grow in mixed (blue) compared with mono-specific (red) neighbourhood. For example, Fig. 7A shows that in the combination Norway spruce/European beech, the growth of Norway spruce in mixture (+ 1%) hardly differs from its growth in mono-specific stands. The superiority of the growth in mixture ranged from 1 to 19 %. N. spruce (E. beech) addresses the behaviour of Norway spruce in mixture with European beech. (N. spruce), E. beech addresses the growth of European beech in mixture with Norway spruce. This nomenclature was used for the other mixtures analogously. Norway spruce and European beech in a mixture of Norway spruce, silver fir and European beech (Fig. 7C, D) and sessile oak in mixture with European beech (Fig. 7I) grew less under similar competition. With the exception of Scots pine when mixed with Norway spruce (Fig. 7G), the stem diameter growth, id, always decreased with increasing stand density.
In the majority of cases, the growth efficiency was increased by tree species mixing. This again indicates that there were no overall species-specific patterns and that both density and efficiency effects needed to be considered for understanding, modelling or silvicultural steering.
Figure 8 shows the trade-off between stand productivity and stem growth in mono- and mixed-species stands. For this purpose, we equalized in terms of ln(SDIc) the relationships between mean stem diameter increment and SDIc (Table 5, Model 9) and the relationship between stand mass growth and SDIc (Table 5, Model 10) [eqn (11) and Supplementary Data Derivation A].
Fig. 8.
Trade-off between stand mass growth and stem diameter increment in mixed compared with mono-specific stands, shown by example for the mixture of (A) Norway spruce and European beech, (B) sessile oak and European beech, and (C) European ash and sycamore maple. Analogous relationships for the other considered species mixture are shown in Supplementary Data Fig. S3.
Figure 8 shows how much of stand productivity IMc is required to increase the mean tree diameter by stand density reduction in mono-specific stands (red lines), and how the relationship changes in mixed stands (blue lines). As we found similar patterns in all six species assemblages we restricted the visualization of the results to three mixtures (see Supplementary Data Fig. S3 for the other three mixtures). It is known from other studies of mono-specific stands that stem diameter increment decreases with increasing stand density. The red hyperbolas in Fig. 8 reflect this well-known trade-off. Interestingly, tree species mixing attenuates this relationship; similar tree diameter growth can be achieved under higher density and higher stand productivity. This means that a higher density level comes along with higher size growth and taller trees. The ratios shown in Fig. 8 indicate that a stem diameter increment of 2 mm, for example, can be achieved with much higher stand density in mixed compared with mono-specific stands, with the ratios ranging between 1·5 and 6·31 (Fig. 8 and Supplementary Data Fig. S3).
DISCUSSION
Increased stand productivity and stem diameter growth throughout the whole rotation (H I)
As our study was based on 11 age series and 63 plots in stands with mixed and mono-specific parts with an age range of 22–238 years, we arrived at substantial information about the continuation of overyielding and diameter growth increase with progressing stand development. Whereas most studies so far have dealt with young- or medium-aged stands, our results provide evidence that overyielding in terms of stem mass growth of 7–53 % can continue over the whole rotation (Fig. 3). Overyielding slightly abated when plotted over quadratic mean tree diameter (species combination of Norway spruce and European beech; Fig. 3A), staying constant (in most cases; Fig. 3–D, F) or slightly increasing (sessile oak/European beech; Fig. 3E). A mean overyielding of 1–2 mg ha−1 year−1 throughout the whole rotation accumulates to a plus of 100–200 mg ha−1, which is a plus of ~200–400 m3 ha−1 in the case of a rotation time of 100 years. Longer rotation times, as usual for sessile oak/European beech or Scots pine/European beech, result in even higher accumulated amounts of overyielding.
We found overyielding similar to the magnitude reported by Pretzsch et al. (2010, 2015, 2020), Thurm and Pretzsch (2016), Jactel et al. (2018), Steckel et al. (2019) and Ruiz-Peinado et al. (2021). Similar to Kelty (1992) and Jactel et al. (2018), we found that mixtures within conifers and broad-leaved species and between these two groups achieved significant overyielding. The superiority of stem diameter growth (Fig. 4) ranged from 6 % in Norway spruce and European beech to 31 % in European ash and sycamore maple, and continued throughout the rotation.
We based the analyses on stem mass growth as the biomass yield is most informative for production ecology (Landsberg, 1986; Kelty, 1992) and in order to eliminate any overestimation of overyielding due to differences in the species’ wood densities (Knigge and Schulz, 1966; Zeller et al., 2017). Especially in mixtures with conifers of relatively low wood densities (e.g. Norway spruce, Scots pine; 0.3–0.4 g cm−3), the overyielding in terms of volume growth can be 1–5 % higher when mixed with species with higher wood density (e.g. European beech, European beech; 0.4–0.6 g cm−3).
When comparing the stand and tree traits between mixed and mono-specific stands, we used the quadratic mean tree diameter of the stand as a proxy for the stand development phase. The results were analogous when stand age (Table 4) was used as a predictor. However, stem diameter is more feasible for silvicultural guidelines and forest management, as they commonly use the mean stem dimensions (e.g. stem diameter, height or volume) as measures and criteria for scheduling silvicultural interventions (Schober, 1987; Abetz, 1974, 1988; Newton, 1997; Bégin et al., 2001).
Increased stand density and growth efficiency determine mixing effects (H II)
In this study, we did not analyse the mechanisms of species interactions; however, the reaction pattern in terms of stand density and the increase in growth efficiency as a result of mixing hypotheses about the underlying causes. The strong increase in stand density that resulted in significant overyielding of all mixtures was probably promoted by species complementarity in space occupation.
Species complementarity in structure and function has been repeatedly discussed as relevant for competition reduction, density increase and overyielding (Barbeito et al., 2017; Juchheim et al., 2017; Zeller et al., 2021). Other studies have found that stand density was higher in terms of basal area and SDI (Williams et al., 2017; Thurm and Pretzsch, 2021), crown projection area (Pretzsch, 2014) or LAI (Peng et al., 2017) in mixed compared with mono-specific stands. The different species-specific crown allometry may enable a higher packing density of crowns (Jucker et al., 2015). In addition, mixing may modify crown shyness and mechanical abrasion (Fish et al., 2006; Hajek et al., 2015). Meng et al. (2006) showed that the prevention of crown collisions by fixing trees with ropes can increase the crown cover and leaf area of mature stands. Crown shyness may be reduced and leaf area increased if the mechanical abrasion is reduced as species occupy different layers and have their maximal lateral extension (Barbeito et al., 2017). Differences in light ecology may enable a richer vertical layering and structuring; for example, European beech may easily survive under Scots pine because of its higher shade tolerance and lower light compensation point (Ellenberg and Leuschner, 2010). All this may contribute to overyielding via increased packing density in the inter-specific neighbourhood.
We used leaf area equations (la = f(d) relationships) that were mainly based on mono-specific stands (Forrester et al., 2017). As crowns of a given stem diameter can be larger and more plastic in mixed compared with mono-specific stands (Barbeito et al., 2017; Pretzsch, 2019) and can have higher crown projection and leaf area (Thurm and Pretzsch, 2016), the reported relative superiority may even be underestimated. As we always chose tree positions as the midpoint of the constructed sampling circles (Fig. 2), the absolute leaf area estimation may have a positive bias. This may be caused by the fact that the centre of the plot is certainly always covered by the central tree, so canopy coverage and leaf area are over-represented. By a selection of random positions as circle midpoints, also locations without trees and shading leaf area would have been included. This would probably have resulted in lower values of the stand characteristics. However, we applied the same sampling procedure (concentric circles around the tree positions) in the mixed and mono-specific parts of the plots. This means that the absolute values might be overestimated; however, the comparison between mixed and mono-specific sample circles, both equally biased, should result in reliable relationships between the two groups. Potential sources of errors by using auxiliary functions were kept as small as possible. For this purpose, we only applied functions for height and leaf area estimation and allometric exponents and equivalence factors that were derived for the studied tree species, the included stands or the study region.
We used generalized equivalence factors for calculating appropriate area-related mixing proportions for the species with different growing space requirements. Alternative approaches would have been the use of yield tables (Kramer und Akça, 1987, p. 187), wood dry mass relationships between species (Assmann, 1970, pp. 360–361) or leaf area measurements on the plots (Dirnberger et al., 2017). However, the common yield tables seemed to be too outdated for this purpose, the use of wood dry mass is questionable due to dependency of the specific gravity on the respective stand density management, and measurements of the leaf area in the stands were not available. Therefore, we applied the density equivalence factors that were derived for this purpose from stands in the actual study region (Pretzsch and Biber, 2016) and that have already been successfully applied in previous studies (del Río et al., 2016; Pretzsch and del Río, 2020). For analysing the effect of mixing on the stand density and growth efficiency (Figs 4 and 5) we used the LAI as the density measure in order to keep the link to forest ecology, tree eco-physiology and remote sensing. Stand basal area, standing stem volume and SDI are proven dendrometric measures for stand density but hardly known beyond forest growth and yield science. However, the SDI as density measure was available for all trees and sample circles and yielded similar effects of tree species mixing on stand density and growth. The increase in growth efficiency suggests a facilitation effect in inter-specific neighbourhoods as repeatedly reported, especially on harsh sites and in dry years (Callaway, 1998; Pretzsch et al., 2013; del Río et al., 2014). Trees with the same diameter were more efficient in mixed than in mono-specific stands despite the higher stand density, which suggests an efficiency increase of the leaf area and resources use. An increase in efficiency means that at lower densities an inter-specific neighbour may increase the efficiency of the crown, similar to a fertilization effect (Khanna, 1997). In this case, benefits may emerge independently of stand density, but also under wide spacing and strong thinning. The facilitation effects on our plots may be caused by hydraulic lift (Zapater et al., 2011; Steckel et al., 2019), improvement of nutrients (Rothe and Binkley, 2001; Augusto et al., 2002; Jonard et al., 2008) and light supply (Forrester et al., 2018, 2019).
Reduction of competition and attenuation of stand density–stem growth trade-off (H III)
The acceleration of stem diameter growth to achieve taller trees in a shorter time or to reduce rotation requires stand density reduction by thinning (Zeide, 2001; Pretzsch, 2020). Density reductions stronger than moderate thinning cost stand growth (Assmann, 1970). These relationships are well known from numerous thinning trials in mono-specific stands (O’Hara, 1988; Juodvalkis et al., 2005), well integrated into growth models (Courbaud et al., 2001; Franklin et al., 2009) and reflected by silvicultural guidelines (Pelletier and Pitt, 2008). This study showed that mixture can significantly attenuate the trade-off between individual stem diameter growth and stand growth (Fig. 8). Similar growth rates may be achieved under higher density and higher stand productivity; ceteris paribus, they require lower losses of stand productivity. On the other hand, a higher density level resulted in higher size growth and taller trees. These findings indicate that the mitigation potential of forests by production of highly dimensioned and long-lived forest products becomes possible with lower expense on stand stock and mass productivity at the stand level. In this way, mixed stands may increase both the adaptation to climate change risks and the mitigation effect of higher carbon storage. To implement such findings, they should be integrated into silvicultural guidelines for mixed species stands, as claimed by Coll et al. (2018) and proposed by Pretzsch and Zenner (2017) and Mason et al. (2018).
Consequences for measurement and modelling mixed-species stands
This study showed that species-specific structure and composition strongly co-determine the density and productivity of the stands and the growth of individual trees. In the past, the structural properties of the stands have been measured mainly manually (e.g. using measuring tape, crown mirror, theodolite), and the structural information of mixed stand analyses can be currently obtained more efficiently by terrestrial LiDAR (T-LiDAR) (Olivier et al., 2016; Juchheim et al., 2017). T-LiDAR, especially, can provide key information such as tree size and mass (Puletti et al., 2020), crown characteristics (Barbeito et al., 2017; Jacobs et al., 2020), spatial stand structure (Bayer et al., 2013, Bayer and Pretzsch, 2017; Beyer et al., 2017, del Río et al., 2017), species identity (Terryn et al., 2020) and leaf area (Soma et al., 2020) and crown transparency (Jacobs et al., 2021) more easily than in the past. Methods for measuring leaf area, stand structure and species identification are occasionally used in forest and ecological science (Dassot et al., 2011) are in preparation for standard application and will get much easier access to exactly those tree and stand characteristics that make the difference between mixed and mono-specific stand dynamics and performance (Lintunen et al., 2011).
The finding that the species combination within a tree neighbourhood strongly determines tree and stand growth underlines the need for spatially dependent model approaches and species-specific parameterization. Most of these models are based on a potential growth rate that is continuously reduced with increasing competition (potential modifier approach; Pretzsch et al., 2002, 2015). The finding that mixing can increase the stand density, reduce the competition and significantly raise the growth beyond the growth level in mono-specific stands suggests that the commonly used inverse J-shaped potential modifier function should be reconsidered. Maybe it should be replaced by a unimodal-shaped function with a maximum growth rate not under solitary and mono-specific conditions but under low density and in an inter-specific neighbourhood. We suggest that the level and shape of the potential modifier functions in individual tree models need to be adapted in order to take into consideration the beneficial effects of competition reduction and facilitation. In addition, competition and mortality models need to be adapted to consider the mixing effects on competition and density. Tree-level-based models have the advantage that they can deduce the reaction patterns for the whole continuum of mono-specific and mixed species stands mechanistically from tree–tree interactions (Cole and Lorimer, 1994; Maguire et al., 1998; Pretzsch et al., 2002) and can consider both density and efficiency effects (Thorpe et al., 2010; Bravo et al., 2019).
Consequences for forest management
This study addressed several of the ten highest-ranked questions regarding mixed-forest functioning and management, which Coll et al. (2018) identified by interviewing 168 managers from European countries. We considered which species combinations may be most beneficial (question 4), how is the productivity of mixtures different compared with that of mono-specific stands (question 5), which positive and negative effects mixtures can have and what is the balance or trade-off (questions 6 and 7); and finally, we reveal whether mixed stands are only denser or more efficient in resource use (question 10) (Coll et al., 2018). Our results represent the mixing effects for medium- and high-quality site conditions. The range of site conditions of the used age series was not wide enough for exploring the dependency of the mixing effects on site quality. For the revelation of such dependencies transects of mono-specific and mixed-species stands along productivity gradients across Europe are more suitable (del Rio et al., 2014; Heym et al., 2017).
We found that mixing can be beneficial for trees and stands throughout the entire rotation. The finding that mixing increases leaf area efficiency means that the benefit of the mixture can also be exploited under lower stand densities. Any changes in maximum stand density are relevant for silvicultural stand assessment and density regulation. The natural maximum stand density is commonly used as a reference for defining density reductions in silvicultural guidelines. In this case, the site-specific maximum density was used as the ceiling density, and desired density trajectories were formulated in relation to the maximum. Density may increase the size growth and stability of remaining trees, but should be carefully assessed and regulated, as density reduction can also cause a decrease in stand growth. Thus, any neglect or underestimation of the maximum stand density may cause growth losses.
CONCLUSIONS
The strong beneficial effects of species mixture and stand structure on tree and stand growth suggest potential research for the future. This study provides various new starting points for better understanding, design, and silvicultural steering, and exploits the benefits of mixed compared with mono-specific stands. The essential 3-D structure may be better and less costly, as measured by T-LiDAR in existing and newly established experiments. The species-specific behaviour suggests the avoidance of premature species-overarching generalization. The differentiation between density and efficiency effects offers promising starting points for further causal analyses and modelling mixing effects depending on site conditions. The attenuated trade-off between stand productivity and stem size growth enables increased stem diameter growth, even with similar or higher stand productivity and density compared with non-specific stands. The benefits of the mixture come on top of the other well-known superiority of provisioning and regulation services.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: visualizes by the example of Schongau 814 the setup of an age series by the crown maps of its corresponding plots SON 814/1–8 in 2011. Figure S2: demonstrates the method of plot edge correction by toroidal shift shown by example for SON 814/2 at the survey in autumn 2011. Figure S3: trade-off between stand mass growth and stem diameter increment in mixed compared with mono-specific stands for the mixtures Norway spruce/silver fir/European beech, Scots pine/Norway spruce and Scots pine/European beech. Tables S1–S4: parameters of the auxiliary relationships introduced in the Materials and methods section. Supplementary Derivation A: step-by-step derivation of eqn (11) introduced in the Statistical models section .
ACKNOWLEDGEMENTS
Thanks are due to Risto Sievänen for initiating the 3DFDYN Workshop 2020 and the corresponding Special Issue of Annals of Botany. We also thank Peter Biber for revising the statistical parts of this manuscript and the anonymous reviewers for their constructive criticism. The authors declare that they have no conflicts of interest. H.P. initiated and conceptualized the study; G.S. repeatedly measured the plots; H.P. and G.S. evaluated the data; H.P. wrote and revised the manuscript.
FUNDING
This publication is part of the CARE4C project that has received funding from the European Union’s HORIZON 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 778322. The authors also thank the German Science Foundation [Deutsche Forschungsgesellschaft (DFG)] for funding the projects ‘Structure and dynamics of mixed-species stands of Scots pine and European beech compared with mono-specific stands; analysis along an ecological gradient through Europe’ (no. DFG PR 292/15-1). The study was funded through the 2017–2018 Belmont Forum and BiodivERsA joint call for research proposals, under the BiodivScen ERA-Net COFUND programme, with the national funding organization German Science Foundation (grant no. 16LC1805B).
LITERATURE CITED
- Abetz P. 1974. Zur Standraumregulierung in Mischbeständen und Auswahl von Zukunftsbäumen. Allgemeine Forst- und Jagdzeitung 29: 871–873. [Google Scholar]
- Abetz P. 1988. Erwiderung zu Schober: Durchforstung nach Zahlen. Allgemeine Forst- und Jagdzeitung 10: 174–183. [Google Scholar]
- Amoroso MM, Turnblom EC. 2006. Comparing the productivity of pure and mixed Douglas-fir and western hemlock plantations in the Pacific Northwest. Canadian Journal of Forest Research 36: 1484–1496. [Google Scholar]
- Assmann E. 1970. The principles of forest yield study. Oxford: Pergamon Press. [Google Scholar]
- Augusto L, Ranger J, Binkley D, Rothe A. 2002. Impact of several common tree species in European temperate forests on soil fertility. Annals of Forest Science 59: 233–253. [Google Scholar]
- Barbeito I, Dassot M, Bayer D, et al. 2017. Terrestrial laser scanning revealed differences in the crown structure of Fagus sylvatica in mixed vs. pure European forests. Forest Ecology and Management 405: 381–390. [Google Scholar]
- Bates D, Mäechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using the lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Bauhus J, Khanna PK, Menden N. 2000. Aboveground and belowground interactions in mixed plantations of Eucalyptus globulus and Acacia mearnsii. Canadian Journal of Forest Research 30: 1886–1894. [Google Scholar]
- Bayer D, Pretzsch H. 2017. Reactions to gap emergence: Norway spruce increases growth, while European beech features horizontal space occupation – evidence from repeated 3D TLS measurements. Silva Fennica 51. doi: 10.14214/sf.7748. [DOI] [Google Scholar]
- Bayer D, Seifert S, Pretzsch H. 2013. Structural crown properties of Norway spruce (Picea abies [L.] Karst.) and European beech (Fagus sylvatica [L.]) in mixed versus pure stands revealed by terrestrial laser scanning. Trees 27: 1035–1047. [Google Scholar]
- Bégin E, Bégin J, Bélanger L, Rivest LP, Tremblay S. 2001. Balsam fir self-thinning relationship and its constancy among different ecological regions. Canadian Journal of Forest Research 31: 950–959. [Google Scholar]
- Beyer R, Bayer D, Letort V, Pretzsch H, Cournède PH. 2017. Validation of a functional structural tree model using terrestrial Lidar data. Ecological Modeling 357: 55–57. [Google Scholar]
- Biber P. 2013. Kontinuität durch Flexibilität – Standardisierte Datenauswertung im Rahmen eines waldwachstumskundlichen information systems. Allgemeine Forst- und Jagdzeitung 184: 167–177. [Google Scholar]
- Bravo F, Fabrika M, Ammer C, et al. 2019. Modelling approaches for mixed forests dynamics prognosis. Research gaps and opportunities. Forest Systems 28: eR002. [Google Scholar]
- Bravo-Oviedo A, Pretzsch H, Ammer C, et al. 2014. European mixed forests: definition and research perspectives. Forest Systems 23: 518–533. [Google Scholar]
- Brooks JR, Meinzer FC, Coulombe R, Gregg J. 2002. Hydraulic redistribution of soil water during summer drought in two contrasting Pacific Northwest coniferous forests. Tree Physiology 22: 1107–1117. [DOI] [PubMed] [Google Scholar]
- Brunner A, Forrester DI. 2020. Effects of tree species mixture on stem growth vary with stand density – an analysis based on individual tree responses. Forest Ecology and Management 473: 118334. [Google Scholar]
- Callaway RM. 1998. Competition and facilitation of elevation gradients in subalpine forests of the northern Rocky Mountains, USA. Oikos 82: 561–573. [Google Scholar]
- Cole WG, Lorimer CG. 1994. Predicting tree growth from crown variables in managed northern hardwood stands. Forest Ecology and Management 67: 159–175. [Google Scholar]
- Coll L, Ameztegui A, Collet C, et al. 2018. Knowledge gaps about mixed forests: what do European forest managers want to know and what answers can science provide? Forest Ecology and Management 407: 106–115. [Google Scholar]
- Courbaud B, Goreaud F, Dreyfus P, Bonnet FR. 2001. Evaluating thinning strategies using a tree distance-dependent growth model: some examples based on the CAPSIS software ‘uneven-aged spruce forests’ module. Forest Ecology and Management 145: 15–28. [Google Scholar]
- Dănescu A, Kohnle U, Bauhus J, Sohn J, Albrecht AT. 2018. Stability of tree increment in relation to episodic drought in uneven-structured mixed stands in southwestern Germany. Forest Ecology and Management 415–416: 148–159. [Google Scholar]
- Dassot M, Constant T, Fournier M. 2011. The use of terrestrial LiDAR technology in forest science: application fields, benefits, and challenges. Annals of Forest Science 68: 959–974. [Google Scholar]
- Dieler J, Uhl E, Biber P, Müller J, Rötzer T, Pretzsch H. 2017. Effect of forest stand management on species composition, structural diversity, and productivity in the temperate zone of Europe. European Journal of Forest Research 136: 739–766. [Google Scholar]
- Dirnberger G, Sterba H, Condés S, et al. 2017. Species proportions by area in mixtures of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.). European Journal of Forest Research 136: 171–183. [Google Scholar]
- Ellenberg H, Leuschner, C. 2010. Vegetation Mitteleuropas mit den Alpen: in ökologischer, dynamischer und historischer Sicht . Stuttgart: UTB. [Google Scholar]
- Fish H, Lieffers VJ, Silins U, Hall RJ. 2006. Crown shyness in lodgepole pine stands of varying stand height, density, and site index in the upper foothills of Alberta. Canadian Journal of Forest Research 36: 2104–2111. [Google Scholar]
- Forrester DI. 2014. Spatial and temporal dynamics of species interactions in mixed-species forests: from pattern to process. Forest Ecology and Management 312: 282–292. [Google Scholar]
- Forrester DI, Bauhus J, Cowie, AL, Vanclay JK. 2006. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: a review. Forest Ecology and Management 233: 211–230. [Google Scholar]
- Forrester DI, Kohnle U, Albrecht AT, Bauhus J. 2013. Complementarity in mixed-species stands of Abies alba and Picea abies varies with climate, site quality, and stand density. Forest Ecology and Management 304: 233–242. [Google Scholar]
- Forrester DI, Tachauer IHH, Annighoefer P, et al. 2017. Generalized biomass and leaf area allometric equations for European tree species incorporating stand structure, tree age, and climate. Forest Ecology and Management 396: 160–175. [Google Scholar]
- Forrester DI, Ammer C, Annighöfer PJ, et al. 2018. Effects of crown architecture and stand structure on light absorption in mixed and monospecific Fagus sylvatica and Pinus sylvestris forests along a productivity and climate gradient throughout Europe. Journal of Ecology 106: 746–760. [Google Scholar]
- Forrester DI, Rodenfels P, Haase J, et al. 2019. Tree-species interactions increase light absorption and growth in Chinese subtropical mixed-species plantations. Oecologia 191: 421–432. [DOI] [PubMed] [Google Scholar]
- Franklin O, Aoki K, Seidl R. 2009. A generic model of thinning and stand density affects forest growth, mortality and net increment. Annals of Forest Science 66: 815. [Google Scholar]
- Franz F, Bachler J, Deckelmann B, et al. 1973. Bayerische Waldinventur 1970/71, Inventurabschnitt I: Großrauminventur Aufnahme- und Auswertungsverfahren. Forstliche Forschungsberichte München 11: 143. [Google Scholar]
- Gillerot L, Forrester DI, Bottero A, Rigling A, Lévesque M. 2020. Tree neighbourhood diversity has negligible effects on the drought resilience of European beech, silver fir, and Norway spruce. Ecosystems 24: 20–36. [Google Scholar]
- Goelz JCG. 2001. Systematic experimental designs for mixed-species plantings. Native Plants Journal 2: 90–96. [Google Scholar]
- Griess VC, Acevedo R, Härtl F, Staupendahl K, Knoke T. 2012. Does mixing tree species enhance the resistance to natural hazards? Case study for spruce. Forest Ecology and Management 267: 284–296. [Google Scholar]
- Grossiord C. 2020. Having the right neighbors: how tree species diversity modulates drought impacts on forests. New Phytologist 228: 42–49. [DOI] [PubMed] [Google Scholar]
- Hajek P, Seidel D, Leuschner C. 2015. Mechanical abrasion, and not competition for light, is the dominant canopy interaction in a temperate mixed forest. Forest Ecology and Management 348: 108–116. [Google Scholar]
- Heym M, Ruíz-Peinado R, Del Río M, et al. 2017. EuMIXFOR empirical forest mensuration and ring width data from pure and mixed stands of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) through Europe. Annals of Forest Science 74: 1–9. [Google Scholar]
- Hilmers T, Avdagić A, Bartkowicz L, et al. 2019. The productivity of mixed mountain forests comprised of Fagus sylvatica, Picea abies, and Abies alba across Europe. Forestry 92: 512–522. [Google Scholar]
- Jacobs M, Rais A, Pretzsch H. 2020. Analysis of stand density effects on the stem form of Norway spruce trees and volume miscalculation by traditional form factor equations using terrestrial laser scanning (TLS). Canadian Journal of Forest Research 50: 51–64. [Google Scholar]
- Jacobs M, Rais A, Pretzsch H. 2021. How drought stress becomes visible upon detecting tree shapes using terrestrial laser scanning (TLS). Forest Ecology and Management 489: 118975. [Google Scholar]
- Jactel H, Brockerhoff EG. 2007. Tree diversity reduces herbivory by forest insects. Ecology Letters 10: 835–848. [DOI] [PubMed] [Google Scholar]
- Jactel H, Gritti ES, Drössler L, et al. 2018. Positive biodiversity–productivity relationships in forests: climate matters. Biology Letters 14: 20170747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jactel H, Moreira X, Castagneyrol B. 2021. Tree diversity and forest resistance to insect pests: patterns, mechanisms, and prospects. Annual Review of Entomology 66: 277–296. [DOI] [PubMed] [Google Scholar]
- Johann K. 1993. DESER-Norm 1993. Normen der Sektion Ertragskunde im Deutschen Verband Forstlicher Forschungsanstalten zur Aufbereitung von waldwachstumskundlichen Dauerversuchen. In: Proceedings Deutscher Verband Forstlicher Forschungsanstalten, Sektion Ertragskunde, Tagung in Unterreichenbach-Kapfenhardt, 96–104. [Google Scholar]
- Jonard M, Andre F, Ponette Q. 2008. Tree species-mediated effects on leaf litter dynamics in pure and mixed stands of oak and beech. Canadian Journal of Forest Research 38: 528–538. [Google Scholar]
- Juchheim J, Annighöfer P, Ammer C, Calders K, Raumonen P, Seidel D. 2017. How management intensity and neighborhood composition affect the structure of beech (Fagus sylvatica L.) trees. Trees - Structure and Function 31: 1723–1735. [Google Scholar]
- Jucker T, Bouriaud O, Coomes DA. 2015. Crown plasticity enables trees to optimize canopy packing in mixed-species forests. Functional Ecology 29: 1078–1086. [Google Scholar]
- Juodvalkis A, Kairiukstis L, Vasiliauskas R. 2005. Effects of thinning on growth of six tree species in the northern temperate forests of Lithuania. European Journal of Forest Research 124: 187–192. [Google Scholar]
- Kelty MJ. 1992. Comparative productivity of monoculture and mixed-species stands. In: Kelty MJ, Larson BC, Oliver CD, eds. The ecology and silviculture of mixed-species forests. Dordrecht: Springer, 125–141. [Google Scholar]
- Khanna PK. 1997. Comparison of growth and nutrition of young monocultures and mixed stands of Eucalyptus globulus and Acacia mearnsii. Forest Ecology and Management 94: 105–113. [Google Scholar]
- Knigge W, Schulz H. 1966. Grundriss der Forstbenutzung. Hamburg: Paul Parey. [Google Scholar]
- Knoke T, Ammer C, Stimm B, Mosandl R. 2008. Admixing broadleaved trees to coniferous tree species: a review on yield, ecological stability and economics. European Journal of Forest Research 127: 89–101. [Google Scholar]
- Körner C. 2002. Ökologie. In: Sitte P, Weiler EW, Kadereit JW, Bresinsky A, Körner C, eds. Strasburger Lehrbuch für Botanik, 35th edn. Heidelberg: Spektrum Akademischer, 886–1043. [Google Scholar]
- Kozlowski TT. 1962. Tree growth. New York: Ronald Press. [Google Scholar]
- Kramer H, Akça A. 1987. Leitfaden für Dendrometrie und Bestandesinventur. Frankfurt am Main: J.D. Sauerländer. [Google Scholar]
- Lamlom SH, Savidge RA. 2003. A reassessment of carbon content in wood: variation within and between 41 North American species. Biomass Bioenergy 25: 381–388. [Google Scholar]
- Landsberg JJ. 1986. Physiological ecology of forest production. Cambridge: Academic Press. [Google Scholar]
- Lebourgeois F, Gomez N, Pinto P, Mérian P. 2013. Mixed stands reduce Abies alba tree-ring sensitivity to summer drought in the Vosges Mountains, Western Europe. Forest Ecology and Management 303: 61–71. [Google Scholar]
- Liang J, Crowther TW, Picard N, et al. 2016. Positive biodiversity-productivity relationships are predominant in global forests. Science 354. [DOI] [PubMed] [Google Scholar]
- Lintunen A, Sievänen R, Kaitaniemi P, Perttunen J. 2011. Models of 3D crown structure for Scots pine (Pinus sylvestris) and silver birch (Betula pendula) grown in mixed forests. Canadian Journal of Forest Research 41: 1779–1794. [Google Scholar]
- Magin R. 1959. Struktur und Leistung mehrschichtiger Mischwälder in den Bayerischen Alpen. Mitteilungen aus der Staatsforstverwaltung Bayerns 30: 161. [Google Scholar]
- Maguire DA, Brissette JC, Gu L. 1998. Crown structure and growth efficiency of red spruce in uneven-aged, mixed-species stands in Maine. Canadian Journal of Forest Research 28: 1233–1240. [Google Scholar]
- Mäkinen H, Isomäki A. 2004a. Thinning intensity and growth of Scots pine stands in Finland. Forest Ecology and Management 201: 311–325. [Google Scholar]
- Mäkinen H, Isomäki A. 2004b. Thinning intensity and long-term changes in increment and stem form of Norway spruce trees. Forest Ecology and Management 201: 295–309. [Google Scholar]
- Mason WL, Connolly T. 2014. Mixtures with spruce species can be more productive than monocultures, as evidenced by the Gisburn experiment in Britain. Forestry 87: 209–217. [Google Scholar]
- Mason WL, Löf M, Pach M, Spathelf P. 2018. Development of silvicultural guidelines for creating mixed forests. In: Bravo-Oviedo A, Pretzsch H, del RÃo M. eds. Dynamics, silviculture and management of mixed forests. Cham: Springer, 255–270. [Google Scholar]
- Meng SX, Rudnicki M, Lieffers VJ, Reid DE, Silins U. 2006. Preventing crown collisions increases the crown cover and leaf area of maturing lodgepole pine. Journal of Ecology 94: 681–686. [Google Scholar]
- Mitscherlich G. 1970. Wald, Wachstum und Umwelt. Band 1: Form und Wachstum von Baum und Bestand. Frankfurt am Main: J.D. Sauerländer. [Google Scholar]
- Neuner S, Albrecht A, Cullmann D, et al. 2015. Survival of Norway spruce remains higher in mixed stands under a dryer and warmer climate. Global Change Biology 21: 935–946. [DOI] [PubMed] [Google Scholar]
- Newton PF. 1997. Stand density management diagrams: review of their development and utility in stand-level management planning. Forest Ecology and Management 98: 251–265. [Google Scholar]
- Nichols JD, Bristow M, Vanclay JK. 2006. Mixed-species plantations: prospects and challenges. Forest Ecology and Management 233: 383–390. [Google Scholar]
- O’Hara KL. 1988. Stand structure and growing space efficiency following thinning in an even-aged Douglas-fir stand. Canadian Journal of Forest Research 18: 859–866. [Google Scholar]
- Olivier MD, Robert S, Fournier RA. 2016. Response of sugar maple (Acer saccharum, Marsh.) tree crown structure to compete with pure versus mixed stands. Forest Ecology and Management 374: 20–32. [Google Scholar]
- Pardos M, del Río M, Pretzsch H, et al. 2021. The greater resilience of mixed forests to drought mainly depends on their composition: analysis along a climate gradient across Europe. Forest Ecology and Management 481: 118687. [Google Scholar]
- Pelletier G, Pitt DG. 2008. Silvicultural responses of two spruce plantations to midrotation commercial thinning in New Brunswick. Canadian Journal of Forest Research 38: 851–867. [Google Scholar]
- Peng S, Schmid B, Haase J, Niklaus PA. 2017. Leaf area increased with species richness in young experimental stands of subtropical trees. Journal of Plant Ecology 10: 128–135. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team . 2021. nlme: linear and nonlinear mixed effects models. R package version 3.1–152. https://CRAN.R-project.org/package=nlme (19 July 2018, date last accessed).
- Piotto D. 2008. A meta-analysis comparing tree growth in monoculture and mixed plantations. Forest Ecology and Management 255: 781–786. [Google Scholar]
- Pommerening A, Stoyan D. 2006. Edge-correction needs to estimate the indices of spatial forest structure. Canadian Journal of Forest Research 36: 1723–1739. [Google Scholar]
- Pretzsch H. 2002. Grundlagen der Waldwachstumsforschung. Berlin: Blackwell Wissenschafts. [Google Scholar]
- Pretzsch H. 2009. Forest dynamics. Growth and yield. Berlin: Springer. [Google Scholar]
- Pretzsch H. 2014. Canopy space filling and tree crown morphology in mixed-species stands compared to monocultures. Forest Ecology and Management 327: 251–264. [Google Scholar]
- Pretzsch H. 2019. The effect of tree crown allometry on community dynamics in mixed-species stands versus monocultures. A review and perspectives for modeling and silvicultural regulation. Forests 10: 810. [Google Scholar]
- Pretzsch H. 2020. Density and growth of forest stands revisited. Effect of temporal scale of observation, site quality, and thinning. Forest Ecology and Management 460: 117879. [Google Scholar]
- Pretzsch H, Biber P. 2005. A re-evaluation of Reineke’s rule and stand density index. Forest Science 51: 304–320. [Google Scholar]
- Pretzsch H, Biber P. 2016. Tree species mixing can increase maximum stand density. Canadian Journal of Forest Research 46: 1179–1193. [Google Scholar]
- Pretzsch H, del Río M. 2020. Density regulation of mixed and mono-specific forest stands as a continuum: a new concept based on species-specific coefficients for density equivalence and density modification. Forestry 93: 1–15. [Google Scholar]
- Pretzsch H, Schütze G. 2009. Transgressive overyielding in mixed stands compared with pure stands of Norway spruce and European beech in Central Europe: evidence on stand level and explanation on individual tree level. European Journal of Forest Research 128: 183–204 [Google Scholar]
- Pretzsch H, Zenner EK. 2017. Toward managing mixed-species stands: from parameterization to prescription. Forest Ecosystems 4: 1–17. [Google Scholar]
- Pretzsch H, Biber P, Durský J. 2002. The single tree-based stand simulator SILVA: construction, application and evaluation. Forest Ecology and Management 162: 3–21. [Google Scholar]
- Pretzsch H, Block J, Dieler J, et al. 2010. Comparison between the productivity of pure and mixed stands of Norway spruce and European beech along an ecological gradient. Annals of Forest Science 67: 712. [Google Scholar]
- Pretzsch H, Bielak K, Bruchwald A, et al. 2013. Mischung und Produktivität von Waldbeständen Ergebnisse langfristiger ertragskundlicher Versuche. Allgemeine Forst- und Jagdzeitung 184: 177–196. [Google Scholar]
- Pretzsch H, del Río M, Ammer C. 2015. Growth and yield of mixed versus pure stands of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) analyzed along a productivity gradient throughout Europe. European Journal of Forest Research 134: 927–947. [Google Scholar]
- Pretzsch H, Forrester DI, Bauhus J. 2017. Mixed-species forests. Ecology and management. Heidelberg: Springer. [Google Scholar]
- Pretzsch H, Steckel M, Heym M, et al. 2020. Stand growth and structure of mixed-species and monospecific stands of Scots pine (Pinus sylvestris L.) and oak (Q. robur L., Quercus petraea (Matt.) Liebl.) analyzed along a productivity gradient throughout Europe. European Journal of Forest Research 139: 349–367. [Google Scholar]
- Pretzsch H, Hilmers T, Uhl E, et al. 2021. European beech stem diameter grows better in mixed stands than in mono-specific stands at the edge of its distribution in mountain forests. European Journal of Forest Research 140: 127–145. [Google Scholar]
- Prodan M. 1968a. Einzelbaum, Stichprobe, und Versuchsfläche. Allgemeine Forst- und Jagdzeitung 139: 239–248. [Google Scholar]
- Prodan M. 1968b. Zur Gesetzmäßigkeit der Flächenverteilung von Bäumen. Allgemeine Forst- und Jagdzeitung 139: 214–217. [Google Scholar]
- Puletti N, Grotti M, Ferrara C, Chianucci F. 2020. Lidar-based estimates of aboveground biomass through ground, aerial, and satellite observations: a case study in a Mediterranean forest. Journal of Applied Remote Sensing 14: 044501. [Google Scholar]
- Radtke PJ, Burkhart HE. 1998. Comparison of methods for edge-bias compensation. Canadian Journal of Forest Research 28: 942–945. [Google Scholar]
- Reineke LH. 1933. Perfecting a stand-density index for even-aged forests. Journal of Agricultural Research 46: 627–638. [Google Scholar]
- del Río M, Schütze G, Pretzsch H. 2014. Temporal variation of competition and facilitation in mixed-species forests in Central Europe. Plant Biology 16: 166–176. [DOI] [PubMed] [Google Scholar]
- del Río M, Pretzsch H, Alberdi I, et al. 2016. Characterization of the structure, dynamics, and productivity of mixed-species stands: review and perspectives. European Journal of Forest Research 135: 23–49. [Google Scholar]
- del Río MD, Bravo-Oviedo A, Pretzsch H, Löf M, Ruiz-Peinado R. 2017. A review of thinning effects on Scots pine stands: from growth and yield to new challenges under global change. Forest Systems 26. doi: 10.5424/fs/2017262-11325 [DOI] [Google Scholar]
- Rothe A, Binkley D. 2001. Nutritional interactions in mixed species forests: a synthesis. Canadian Journal of Forest Research 31: 1855–1870. [Google Scholar]
- Ruiz-Peinado R, Pretzsch H, Löf M, et al. 2021. Mixing effects on Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) Karst.) productivity along a climatic gradient across Europe. Forest Ecology and Management 482: 118834. [Google Scholar]
- Schober R. 1987. Durchforstung nach Zahlen? Allgemeine Forst- und Jagdzeitung 158: 174–183. [Google Scholar]
- Snowdon P. 1991. Ratio estimator for bias correction in the logarithmic regressions. Canadian Journal of Forest Research 21: 720–724. [Google Scholar]
- Soma M, Pimont F, Allard D, Fournier R, Dupuy JL. 2020. Mitigating occlusion effects in leaf area density estimates from terrestrial LiDAR using a specific kriging method. Remote Sensing of Environment 245: 111836. [Google Scholar]
- Steckel M, Heym M, Wolff B, Reventlow DOJ, Pretzsch H. 2019. Transgressive overyielding in mixed compared with monospecific Scots pine (Pinus sylvestris L.) and oak (Quercus robur L., Quercus petraea (Matt.) Liebl.) stands – productivity gains increase with annual water supply. Forest Ecology and Management 439: 81–96. [Google Scholar]
- Terryn L, Calders K, Disney M, et al. 2020. Tree species classification using structural features derived from terrestrial laser scanning. ISPRS Journal of Photogrammetry and Remote Sensing 168: 170–181. [Google Scholar]
- Thorpe HC, Astrup R, Trowbridge A, Coates KD. 2010. Competition and tree crowns: a neighborhood analysis of three boreal tree species. Forest Ecology and Management 259: 1586–1596. [Google Scholar]
- Thurm EA, Pretzsch H. 2016. Improved productivity and modified tree morphology of mixed versus pure stands of European beech (Fagus sylvatica) and Douglas-fir (Pseudotsuga menziesii) with increasing precipitation and age. Annals of Forest Science 73: 1047–1061. [Google Scholar]
- Thurm EA, Pretzsch H. 2021. Growth–density relationship in mixed stands – results from long-term experimental plots. Forest Ecology and Management 483: 118909. [Google Scholar]
- Thurm EA, Biber P, Pretzsch H. 2017. Stem growth is favored at expenses of root growth in mixed stands and humid conditions for Douglas-fir (Pseudotsuga menziesii) and European beech (Fagus sylvatica). Trees 31: 349–365. [Google Scholar]
- Vanclay JK, Lamb D, Erskine PD, Cameron DM. 2013. Spatially explicit competition in a mixed planting of Araucaria cunninghamii and Flindersia brayleyana. Annals of Forest Science 70: 611–619. [Google Scholar]
- VDFV . 1902. Verein Deutscher Forstlicher Versuchsanstalten, Beratungen der vom Vereine Deutscher Forstlicher Versuchsanstalten eingesetzten Kommission zur Feststellung des neuen Arbeitsplanes für Durchforstungs- und Lichtungsversuche. Allgemeine Forst- und Jagdzeitung 78: 180–184. [Google Scholar]
- Williams LJ, Paquette A, Cavender-Bares J, Messier C, Reich PB. 2017. Spatial complementarity in tree crowns explains overyielding in species mixtures. Nature Ecology & Evolution 1: 63. [DOI] [PubMed] [Google Scholar]
- Zapater M, Hossann C, Bréda N, Bréchet C, Bonal D, Granier A. 2011. Evidence of hydraulic lift in a young beech and oak mixed forest using 18O soil water labeling. Trees 25: 885–894. [Google Scholar]
- Zeide B. 1993. Analysis of the growth equations. Forest Science 39: 594–616. [Google Scholar]
- Zeide B. 2001. Thinning and growth: a full turnaround. Journal of Forestry 99: 20–25. [Google Scholar]
- Zeller L, Pretzsch H. 2019. The effect of forest structure on stand productivity in Central European forests depends on the developmental stage and tree species diversity. Forest Ecology and Management 434: 193–204. [Google Scholar]
- Zeller L, Ammer C, Annighöfer P, et al. 2017. Tree ring wood density of Scots pine and European beech was lower in mixed-species stands than in monocultures. Forest Ecology and Management 400: 363–374. [Google Scholar]
- Zeller L, Caicoya AT, Pretzsch H. 2021. Analyzing the effect of silvicultural management on the trade-off between stand structural heterogeneity and productivity over time. European Journal of Forest Research, 140: 615–634. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.