Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in people with cystic fibrosis (pwCF) can lead to severe outcomes.
Methods
In this observational study, the European Cystic Fibrosis Society Patient Registry collected data on pwCF and SARS-CoV-2 infection to estimate incidence, describe clinical presentation and investigate factors associated with severe outcomes using multivariable analysis.
Results
Up to December 31, 2020, 26 countries reported information on 828 pwCF and SARS-CoV-2 infection. Incidence was 17.2 per 1000 pwCF (95% CI: 16.0–18.4). Median age was 24 years, 48.4% were male and 9.4% had lung transplants. SARS-CoV-2 incidence was higher in lung-transplanted (28.6; 95% CI: 22.7–35.5) versus non-lung-transplanted pwCF (16.6; 95% CI: 15.4–17.8) (p≤0.001).
SARS-CoV-2 infection caused symptomatic illness in 75.7%. Factors associated with symptomatic SARS-CoV-2 infection were age >40 years, at least one F508del mutation and pancreatic insufficiency.
Overall, 23.7% of pwCF were admitted to hospital, 2.5% of those to intensive care, and regretfully 11 (1.4%) died. Hospitalisation, oxygen therapy, intensive care, respiratory support and death were 2- to 6-fold more frequent in lung-transplanted versus non-lung-transplanted pwCF.
Factors associated with hospitalisation and oxygen therapy were lung transplantation, cystic fibrosis-related diabetes (CFRD), moderate or severe lung disease and azithromycin use (often considered a surrogate marker for Pseudomonas aeruginosa infection and poorer lung function).
Conclusion
SARS-CoV-2 infection yielded high morbidity and hospitalisation in pwCF. PwCF with forced expiratory volume in 1 s <70% predicted, CFRD and those with lung transplants are at particular risk of more severe outcomes.
Short abstract
In a European study of #SARSCoV2 infection in 828 people with #cysticfibrosis, those with moderate–severe lung disease, CF-related diabetes and lung transplant had poorer outcomes. People with CF, especially these groups, should shield in priority. https://bit.ly/3vPjD2f
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected over 79 million people worldwide in 2020, causing 1.7 million deaths [1].
Given that viral infection can cause pulmonary exacerbations and hasten lung function decline [2–4], people with cystic fibrosis (CF) (pwCF) took early steps to protect themselves from infection by shielding [5, 6]. Nonetheless, adult and paediatric pwCF have been infected [7–9].
We recently assessed the incidence of SARS-CoV-2 infection in a cohort of 130 pwCF in Europe up to June 30, 2020 [7]. Other national and global studies have also assessed incidence and outcomes of SARS-CoV-2 infection in pwCF during the first wave of the pandemic [8, 10–12]. Lung-transplanted pwCF appear to have worse outcomes than those without lung transplant. However robust multivariable data are still lacking regarding risk factors, as well as up-to-date incidence estimates.
Here we expand our previously described cohort [7] to include European pwCF who were diagnosed with SARS-CoV-2 infection up to December 31, 2020. In this cohort of 828 pwCF, we update SARS-CoV-2 incidence, and provide the first large, detailed analysis of clinical presentation (including individual symptoms) and identification of risk factors associated with poorer outcomes.
Methods
Study design
The methodology of this prospective observational study has been previously described in a paper presenting data collected between February 1, 2020 and June 30, 2020 [7]. Briefly, data regarding pwCF with PCR-confirmed SARS-CoV-2 infection were collected from CF centres participating in the European Cystic Fibrosis Society Patient Registry (ECFSPR). Cases diagnosed by computed tomography scan, serology or antigen test without PCR confirmation were excluded. Data were reported directly to ECFSPR using a standardised case report form, except for Belgium, France, Germany and the UK who contributed data via their national registries. Two data sources were reported for Italy (national registry and the Italian CF society), with no double cases reported.
We collected data about demographics, pre-infection CF characteristics (latest data available, collected within 12 to 18 months before infection depending on the national data collection strategy) and information about SARS-CoV-2 infection regarding diagnosis, symptoms, complications, treatments and outcomes. Where appropriate, variables were defined according to ECFSPR standards (www.ecfs.eu/projects/ecfs-patient-registry/Variables-Definitions). Per cent predicted forced expiratory volume in 1 s (ppFEV1) is referred to as mild (>70), moderate (>40–70) or severe (≤40) lung disease [13].
Each participating centre or national registry has ethical approval and patients’ informed consent for data collection and ECFSPR participation, including consent that data may be used for future research.
Definitions of symptoms and outcomes
A pwCF was defined as symptomatic if they reported at least one symptom of SARS-CoV-2 infection. Symptoms were categorised as general, pulmonary, gastrointestinal or ear, nose and throat (ENT) and eye (see table 1). Outcomes were hospitalisation, intensive care, oxygen therapy, respiratory support and death (see table 1).
TABLE 1.
Symptoms and outcomes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in people with cystic fibrosis
| Total | Non-lung transplant | Lung transplant | ||||
| n (%) | Missing | n (%) | Missing | n (%) | Missing | |
| Subjects n | 828 | 750 | 78 | |||
| Symptoms | ||||||
| Presence of symptoms | 586 (75.7) | 54 | 528 (75.1) | 47 | 58 (81.7) | 7 |
| General symptoms | 467 (64.8) | 107 | 418 (64.2) | 99 | 49 (70.0) | 8 |
| Fever | 353 (43.9) | 23 | 311 (42.6) | 20 | 42 (56.0) | 3 |
| Fatigue | 228 (34.2) | 162 | 200 (33.3) | 150 | 28 (42.4) | 12 |
| Myalgia or arthralgia | 149 (22.4) | 163 | 128 (21.5) | 154 | 21 (30.4) | 9 |
| Headache | 114 (13.9) | 10 | 108 (14.6) | 10 | 6 (7.7) | 0 |
| Pulmonary symptoms | 405 (54.0) | 78 | 366 (53.9) | 71 | 39 (54.9) | 7 |
| Increased cough | 341 (43.2) | 39 | 317 (43.8) | 26 | 24 (36.9) | 13 |
| Increased dyspnoea | 146 (18.6) | 43 | 122 (16.9) | 30 | 24 (36.9) | 13 |
| Chest tightness | 45 (5.5) | 8 | 42 (5.7) | 8 | 3 (3.8) | 0 |
| Wheezing | 14 (1.7) | 7 | 13 (1.7) | 7 | 1 (1.3) | 0 |
| Increased sputum | 96 (13.9) | 136 | 93 (15.0) | 131 | 3 (4.1) | 5 |
| Haemoptysis | 10 (1.2) | 4 | 10 (1.3) | 4 | 0 (0.0) | 0 |
| Pulmonary exacerbation | 124 (21.2) | 242 | 120 (22.2) | 210 | 4 (8.7) | 32 |
| Respiratory failure | 15 (2.7) | 271 | 11 (2.1) | 236 | 4 (9.3) | 35 |
| Gastrointestinal symptoms | 70 (8.5) | 7 | 63 (8.5) | 6 | 7 (9.1) | 1 |
| Diarrhoea | 37 (4.5) | 5 | 33 (4.4) | 4 | 4 (5.2) | 1 |
| Vomiting/nausea | 26 (3.2) | 3 | 24 (3.2) | 3 | 2 (2.6) | 0 |
| Abdominal pain | 29 (3.5) | 5 | 26 (3.5) | 5 | 3 (3.8) | 0 |
| ENT and eye symptoms | 198 (34.9) | 261 | 184 (34.7) | 220 | 14 (37.8) | 41 |
| Pharyngitis | 95 (11.6) | 7 | 90 (12.1) | 6 | 5 (6.5) | 1 |
| Conjunctivitis | 8 (1.0) | 5 | 8 (1.1) | 3 | 0 (0.0) | 2 |
| Acute rhinitis | 83 (13.9) | 230 | 76 (13.6) | 192 | 7 (17.5) | 38 |
| Acute anosmia | 52 (9.0) | 247 | 49 (9.1) | 211 | 3 (7.1) | 36 |
| Acute ageusia | 39 (6.7) | 249 | 38 (7.1) | 213 | 1 (2.4) | 36 |
| Outcomes | ||||||
| Hospitalisation | 195 (23.7) | 4 | 156 (20.9) | 3 | 39 (50.6) | 1 |
| Oxygen therapy | 96 (11.7) | 5 | 76 (10.2) | 5 | 20 (25.6) | 0 |
| Respiratory support | 32 (3.9) | 7 | 23 (3.1) | 7 | 9 (11.5) | 0 |
| Noninvasive ventilation (BIPAP, CPAP) | 16 (1.9) | 7 | 13 (1.7) | 7 | 3 (3.8) | 0 |
| High-flow nasal canula oxygen therapy | 5 (1.4) | 475 | 5 (1.5) | 416 | 0 (0.0) | 59 |
| Invasive ventilation | 12 (1.5) | 8 | 6 (0.8) | 8 | 6 (7.7) | 0 |
| ECMO | 4 (0.5) | 71 | 2 (0.3) | 67 | 2 (2.7) | 4 |
| Intensive care unit | 21 (2.5) | 2 | 13 (1.7) | 2 | 8 (10.3) | 0 |
| Death | 11 (1.4) | 16 | 7 (0.9) | 12 | 4 (5.4) | 4 |
Percentages were calculated on total numbers in each group (not on number of symptomatic patients/group). ENT: ear, nose and throat; BIPAP: bilevel positive airway pressure; CPAP: continuous positive airway pressure; ECMO: extracorporeal membrane oxygenation.
Statistics
Results are presented for all pwCF and by lung transplant status. Demographics and pre-infection CF characteristics and treatments are presented using descriptive statistics. Categorical variables are described as counts and percentages and continuous variables as median and interquartile range. Fisher exact test was used to compare the percentage of categorical variables between groups and Wilcoxon test was used to compare the median on continuous variables between groups.
The denominator for incidence was the ECFSPR population from 2018 [14] (2017 for France [15]). We evaluated the association of demographic and pre-infection clinical characteristics of pwCF with the symptoms and outcome of SARS-CoV-2 infection. Mixed effects univariable logistic regression analyses considered SARS-CoV-2 symptoms and outcomes as response variable and the characteristics of pwCF as explanatory variable (retaining variables with <30% missing data). A country random effect accounted for the effect of health systems. Odds ratios with 95% confidence intervals and p-values were calculated.
Variables with <5% missing data were included in multivariable logistic regression models to identify independent predictors of symptoms and outcomes. Moreover, models were only fitted when the number of events in the response variable was ≥5 times the number of predictor variables [16]. Adjusted OR with 95% CI and p-values were calculated. Data analysis was performed by ECFSPR statisticians, using SAS 9.4 and R 4.0.3 with the additional package geepack.
Results
Incidence
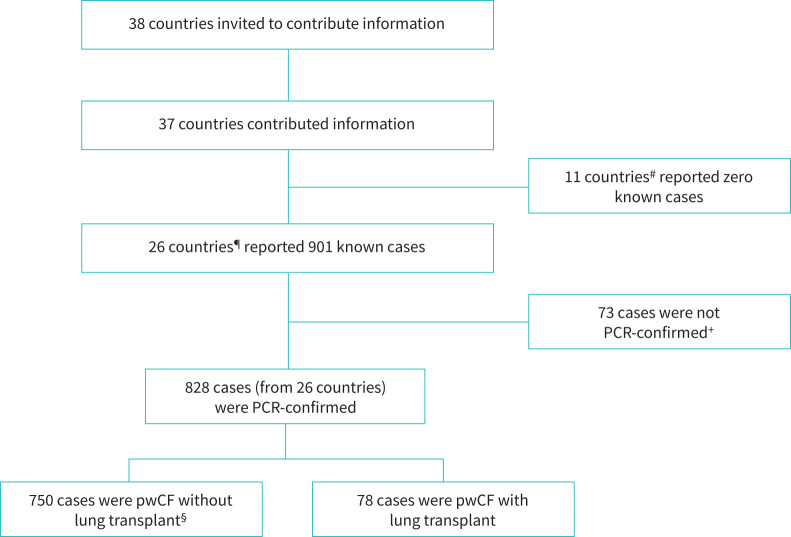
Of the 38 ECFSPR countries, 37 contributed information about SARS-CoV-2 infection in pwCF (figure 1).
FIGURE 1.
Data collection for people with cystic fibrosis (pwCF) and SARS-CoV-2 infection. #: Albania, Belarus, Bulgaria, Cyprus, Georgia, Lithuania, Luxembourg, Republic of Moldova, Romania, Serbia and Ukraine. Hungary did not report information to ECFSPR. ¶: Armenia, Austria, Belgium, Croatia, Czech Republic, Denmark, France, Germany, Greece, Israel, Ireland, Italy, Latvia, Netherlands, Norway, North Macedonia, Portugal, Poland, Russia, Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Turkey and UK. +: these cases were diagnosed by antibody test, antigen test, CT scan or medical team opinion without PCR confirmation. §: this group included 10 people with non-lung solid organ transplants (seven liver, two kidney, one unspecified).
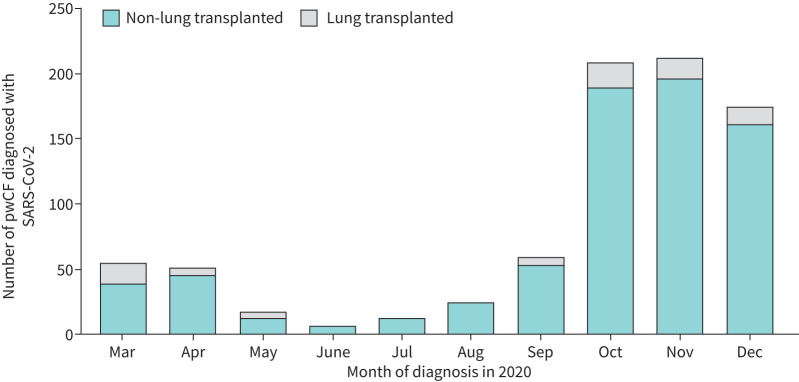
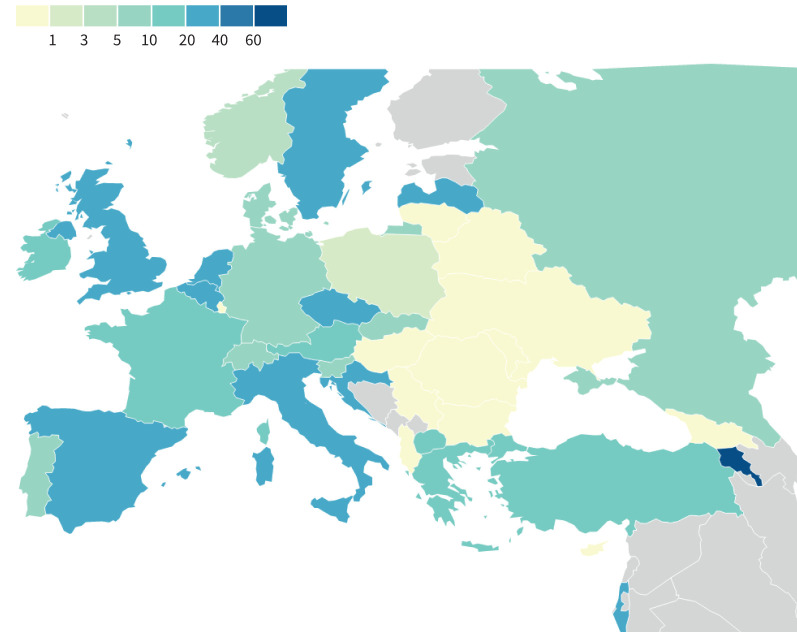
SARS-CoV-2 infections occurred in two distinct waves, the first in March and April 2020 with a second larger wave from October to December 2020. The second wave was ongoing at the time of data cut-off (figure 2). As per our previous report, incidence varied widely by country (figure 3, supplementary Table 1).
FIGURE 2.
Diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in people with cystic fibrosis (pwCF) (n=828) in 2020, by month.
FIGURE 3.
SARS-CoV-2 incidence per 1000 people with cystic fibrosis in 2020, by country.
Overall, 828 PCR-confirmed cases were reported from 26 countries, yielding an incidence of 17.2 per 1000 pwCF (95% CI: 16.0–18.4) (table 2). Incidence was significantly higher in lung-transplanted pwCF (28.6; 95% CI: 22.7–35.5) versus non-lung-transplanted pwCF (16.6; 95% CI: 15.4–17.8) (p<0.001).
TABLE 2.
Incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection up to 31 December 2020 in people with cystic fibrosis (pwCF) by lung transplant status and by age group
| Age group years | All | Non-lung transplant | Lung transplant | ||||||
| Cases | CF population |
Incidence per 1000
(95% CI) |
Cases | CF population |
Incidence per 1000
(95% CI) |
Cases | CF population |
Incidence per 1000
(95% CI) |
|
| Total | 828 | 48 211 | 17.2 (16.0–18.4) | 750 | 45 266 | 16.6 (15.4–17.8) | 78 | 2729 | 28.6 (22.7–35.5) |
| 0–11 | 134 | 17 179 | 7.8 (6.5–9.2) | 134 | 17 100 | 7.8 (6.6–9.3) | 0 | 13 | 0.0 (0.0–247.1) |
| 12–17 | 113 | 7396 | 15.3 (12.6–18.3) | 111 | 7278 | 15.3 (12.6–18.3) | 2 | 84 | 23.8 (2.9–83.4) |
| 18–29 | 291 | 12 162 | 23.9 (21.3–26.8) | 268 | 11 286 | 23.7 (21.0–26.7) | 23 | 816 | 28.2 (17.9–42) |
| 30–39 | 164 | 6493 | 25.3 (21.6–29.4) | 135 | 5445 | 24.8 (20.8–29.3) | 29 | 1014 | 28.6 (19.2–40.8) |
| 40–49 | 87 | 3280 | 26.5 (21.3–32.6) | 67 | 2679 | 25.0 (19.4–31.7) | 20 | 583 | 34.3 (21.1–52.5) |
| ≥50+ | 39 | 1701 | 22.9 (16.4–31.2) | 35 | 1478 | 23.7 (16.5–32.8) | 4 | 219 | 18.3 (5.0–46.1) |
All cases of SARS-CoV-2 in pwCF and the general population were PCR-confirmed. Incidence was calculated as (SARS-CoV-2 cases/number of people in the population) × 1000. CF population size was from the 2018 European Cystic Fibrosis Society Patient Registry report (2017 for France).
Incidence increased along with age group (Fisher exact test; p<0.001) and was notably higher in all adult age groups compared to paediatric age groups. Similar trends were observed for non-lung-transplanted pwCF. In lung-transplanted pwCF, incidence did not vary notably between the age groups spanning 18–49 years; younger and older age groups had too few cases (<5) to allow comparison.
Demographics and CF characteristics
Of the 828 cases, 48.4% were male with a median age of 24 years (table 3). Most pwCF had normal body mass index (90.6%), pancreatic insufficiency (80.6%) and mild lung disease (59.9%). 26.1% had CF-related diabetes (CFRD) and 26.6% had chronic liver disease. Pre-infection medication use was common, and as expected for pwCF (table 3). The most frequent pulmonary infections were Staphylococcus aureus (57.7%) and Pseudomonas aeruginosa (43.4%).
TABLE 3.
Demographics and pre-infection characteristics of people with cystic fibrosis (CF)
| Total | Non-lung transplant | Lung transplanted# | ||||
| n (%) ¶ | Missing | n (%) ¶ | Missing | n (%) ¶ | Missing | |
| Subjects n | 828 | 750 | 78 | |||
| Sex | 0 | 0 | 0 | |||
| Female | 427 (51.6) | 384 (51.2) | 43 (55.1) | |||
| Male | 401 (48.4) | 366 (48.8) | 35 (44.9) | |||
| Median age years | 24.0 | 0 | 23.0 | 0 | 34.5 | 0 |
| 0–11 years | 134 (16.2) | 134 (17.9) | 0 (0) | |||
| 12–17 years | 113 (13.6) | 111 (14.8) | 2 (2.6) | |||
| 18–29 years | 291 (35.1) | 268 (35.7) | 23 (29.5) | |||
| 30–39 years | 164 (19.8) | 135 (18.0) | 29 (37.2) | |||
| 40–49 years | 87 (10.5) | 67 (8.9) | 20 (25.6) | |||
| ≥50 years | 39 (4.7) | 35 (4.7) | 4 (5.1) | |||
| CFTR genotype | 0 | 0 | 0 | |||
| F508del/F508del | 218 (26.3) | 180 (24.0) | 38 (48.7) | |||
| F508del/other | 262 (31.6) | 236 (31.5) | 26 (33.3) | |||
| Other/Other | 348 (42) | 334 (44.5) | 14 (17.9) | |||
| BMI, z-score+ | 39 | 36 | 3 | |||
| < −2 | 54 (7.1) | 40 (5.8) | 14 (18.7) | |||
| −2–2 | 692 (90.6) | 631 (91.6) | 61 (81.3) | |||
| >2 | 18 (2.4) | 18 (2.6) | 0 (0) | |||
| Lung disease FEV1 % pred§ | 28 | 26 | 2 | |||
| Severe (≤40) | 76 (10.3) | 65 (9.8) | 11 (14.5) | |||
| Moderate (>40–70) | 221 (29.9) | 206 (31.0) | 15 (19.7) | |||
| Mild (>70) | 443 (59.9) | 393 (59.2) | 50 (65.8) | |||
| Pancreatic insufficiency | 660 (80.6) | 9 | 584 (78.8) | 9 | 76 (97.4) | 0 |
| CF-related diabetes | 206 (26.1) | 39 | 153 (21.4) | 34 | 53 (72.6) | 5 |
| ABPA | 47 (7.3) | 188 | 41 (6.9) | 158 | 6 (12.5) | 30 |
| Chronic liver GI disease | 163 (26.6) | 215 | 148 (26.7) | 196 | 15 (25.4) | 19 |
| Systemic arterial hypertension | 32 (5.1) | 199 | 20 (3.4) | 156 | 12 (34.3) | 43 |
| Treatment | ||||||
| CFTR modulator therapy | 260 (31.5) | 2 | 260 (34.8) | 2 | 0 (0.0) | 0 |
| Iva | 43 (5.2) | 43 (5.7) | 0 (0.0) | |||
| Lum/Iva | 72 (8.7) | 72 (9.6) | 0 (0.0) | |||
| Tez/Iva | 75 (9.1) | 75 (10.0) | 0 (0.0) | |||
| Elexa/Tez/Iva | 63 (7.6) | 63 (8.4) | 0 (0.0) | |||
| Yes, type unknown | 4 (0.5) | 4 (0.5) | 0 (0.0) | |||
| Yes, other | 3 (0.4) | 3 (0.4) | 0 (0.0) | |||
| Inhaled antibiotics | 332 (50.7) | 173 | 313 (50.6) | 131 | 19 (52.8) | 42 |
| Oral antibiotics | 234 (38.5) | 220 | 215 (37.3) | 174 | 19 (59.4) | 46 |
| Inhaled steroid | 318 (42.0) | 71 | 302 (43.7) | 59 | 16 (24.2) | 12 |
| Azithromycin | 307 (38.1) | 22 | 253 (34.7) | 21 | 54 (70.1) | 1 |
| DNase | 382 (58.3) | 173 | 377 (60.9) | 131 | 5 (13.9) | 42 |
| Hypertonic saline | 338 (51.4) | 171 | 334 (53.8) | 129 | 4 (11.1) | 42 |
| Flu vaccine | 207 (57.8) | 470 | 180 (55.6) | 426 | 27 (79.4) | 44 |
| Microbiology | ||||||
| Pseudomonas aeruginosa | 346 (43.4) | 31 | 313 (42.6) | 15 | 33 (53.2) | 16 |
| Staphylococcus aureus | 420 (57.7) | 100 | 403 (59.0) | 67 | 17 (37.8) | 33 |
| Burkholderia cepacia complex | 29 (4.4) | 168 | 28 (4.5) | 122 | 1 (3.1) | 46 |
| MRSA | 65 (9.3) | 126 | 63 (9.5) | 84 | 2 (5.6) | 42 |
| Non-tuberculous mycobacteria | 28 (5.2) | 292 | 28 (5.5) | 242 | 0 (0.0) | 50 |
| Stenotrophomonas maltophilia | 65 (8.8) | 90 | 63 (9.1) | 61 | 2 (4.1) | 29 |
| Achromobacter species | 60 (8.1) | 89 | 54 (7.8) | 61 | 6 (12.0) | 28 |
| Aspergillus colonisation | 102 (14.0) | 99 | 94 (13.8) | 71 | 8 (16.0) | 28 |
CFTR: cystic fibrosis transmembrane conductance regulator; BMI: body mass index; FEV1 % pred: per cent predicted forced expiratory volume in 1 s; ABPA: allergic bronchopulmonary aspergillosis; GI: gastrointestinal; Iva: ivacaftor; Lum: lumacaftor; Tez: tezacaftor; Elexa: elexacaftor; MRSA: methicillin-resistant Staphylococcus aureus. #: 10 recipients of other solid organ transplants were included in this group (7 liver, 2 kidney, 1 unspecified). ¶: percentages are computed excluding missing data. +: BMI z-score was only calculated for patients aged 2 years and over, using Centers for Disease Control and Prevention reference values [40]. §: FEV1 % pred was only calculated for patients aged 6 years and over.
Compared to non-lung-transplanted pwCF (n=750), lung-transplanted pwCF (n=78) were older and more frequently F508del homozygous. They had higher rates of pancreatic insufficiency, CFRD and systemic arterial hypertension. Concomitant medications also differed, due to different indications and medical needs.
Symptoms and outcomes of SARS-CoV-2 infection
SARS-CoV-2 infection gave rise to symptomatic illness in 75.7% of pwCF (81.7% in lung-transplanted pwCF versus 75.1% in non-lung-transplanted). Symptoms were most commonly general (64.8%), pulmonary (54.0%) and ENT and eyes (34.9%). The most common individual symptoms were fever (43.6%), increased cough (43.2%), fatigue (34.2%), myalgia/arthralgia (22.4%) and pulmonary exacerbation (21.2%) (table 1).
Lung-transplanted pwCF had notably different rates of specific symptoms, with more frequent dyspnoea and respiratory failure and less frequent increased sputum and pulmonary exacerbation.
Of the 828 cases, 11.7% needed extra oxygen and 3.9% needed respiratory support, 23.7% were admitted to hospital and 2.5% to intensive care. Regretfully, 11 pwCF (1.4%) died. The case fatality rate was 1.4% (95% CI: 0.7–2.4). Demographic and baseline CF characteristics for the 11 pwCF who died are presented in supplementary Table 2.
Oxygen therapy, respiratory support and hospitalisation were >2-fold more common in lung-transplanted pwCF versus non-lung-transplanted; similarly, intensive care admission and death were 6-fold more common. In hospitalised patients, intensive care and death were around 2-fold more frequent in lung-transplanted pwCF versus non-lung-transplanted pwCF.
Factors associated with symptoms and worse outcomes
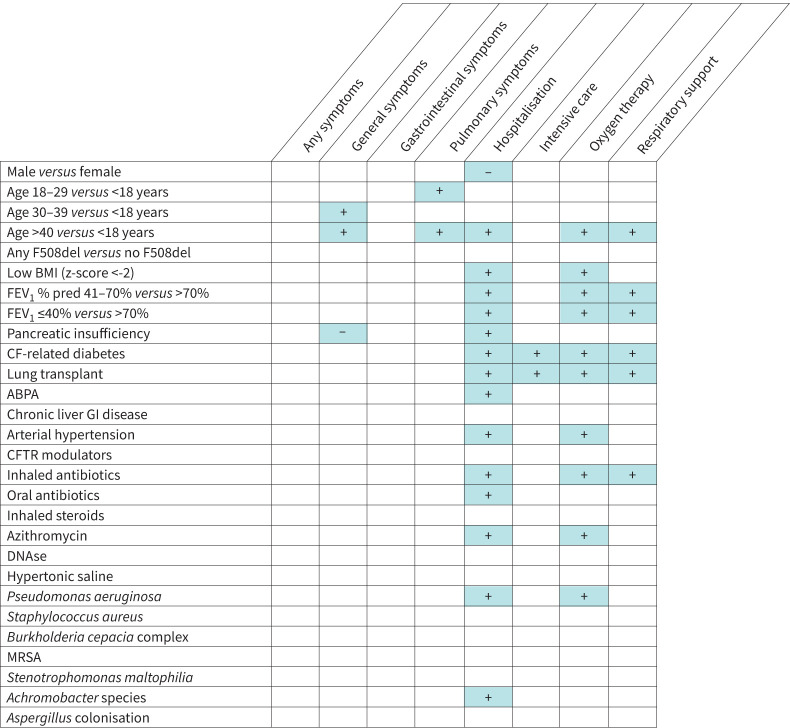
Univariable analyses are summarised in figure 4, with full results in supplementary Tables 3 and 4.
FIGURE 4.
Factors positively (+) and negatively (−) associated with SARS-CoV-2 infection symptoms and outcomes. ABPA: allergic bronchopulmonary aspergillosis; BMI: body mass index; CFTR: cystic fibrosis transmembrane conductance regulator; GI: gastrointestinal; MRSA: methicillin-resistant Staphylococcus aureus; FEV1 % pred: per cent predicted forced expiratory volume in 1 s. A person was considered underweight if their BMI z-score was ≤2, using Centers for Disease Control and Prevention reference values [40].
Multivariable models were fitted including only variables with <10% missing data and for response variables with sufficient events (any symptoms, pulmonary symptoms, general symptoms, hospitalisation and oxygen therapy). No significant interactions existed between predictor variables and lung transplant in any of the multivariable models, meaning that risk factors have similar effects in non-lung-transplanted and lung-transplanted pwCF. Therefore, we present multivariable analyses for all 828 pwCF with SARS-CoV-2 infection.
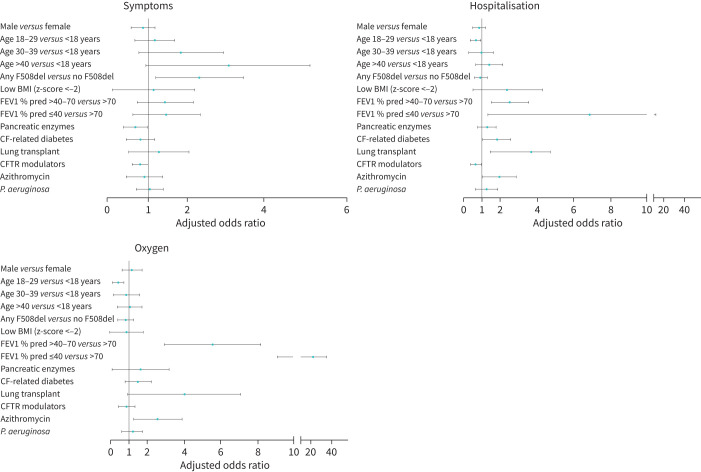
Factors associated with symptoms of SARS-CoV-2 infection were age >40 years, any F508del mutation and taking pancreatic enzymes (figure 5). General symptoms and pulmonary symptoms were associated with any F508del mutation. Pulmonary symptoms were also associated with age ≥18 years. Additionally, use of cystic fibrosis transmembrane conductance regulator (CFTR) modulators tended towards protecting against general symptoms (p=0.058) (supplementary Table 5).
FIGURE 5.
Multivariable analysis of factors associated with symptoms and outcomes of SARS-CoV-2 infection in people with cystic fibrosis (CF). BMI: body mass index; CFTR: cystic fibrosis transmembrane conductance regulator; FEV1 % pred: per cent predicted forced expiratory volume in 1 s; P. aeruginosa: Pseudomonas aeruginosa.
Regarding outcomes, lung transplant, CFRD, moderate and severe lung function as well as azithromycin use (often considered surrogate marker for P. aeruginosa infection and worse lung function) were significantly associated with hospitalisation and oxygen therapy (figure 5 and supplementary Table 6). Age 18–29 years versus <18 years was negatively associated with oxygen therapy, and CFTR modulator use was negatively associated with hospitalisation. Although multivariable models could not be fitted for the outcome death, nine out of 11 pwCF who died and had complete information available had at least one risk factor for hospitalisation and/or oxygen therapy (information was incomplete for two adult pwCF).
Discussion
In this report we estimate the incidence of SARS-CoV-2 infection in pwCF in Europe to be 17.2/1000 pwCF in the year up to December 31, 2020. This is markedly higher than previous estimates of 0.7 to 4.1/1000 pwCF from earlier publications covering the first wave of the pandemic (data cut-offs before July 2020) [7, 8, 10, 11], although it is similar to an Italian estimate of 15.8/1000 pwCF up to November 2020 [12]. The data collected covers the 38 countries reporting to the ECFSPR and involves a cohort of 828 pwCF who were PCR positive for SARS-CoV-2. We also present risk factors for symptoms and worse outcomes of SARS-CoV-2 infection.
Infections between February and June 2020 (wave 1) were concentrated in Western Europe. The second wave (July to December 2020) extended towards the east and south, with higher peaks of infections. The much higher incidence in pwCF after summer 2020 reflects increased incidence in the general European population after summer 2020, which is only partly explained by different testing strategies and public restrictions [17]. Nevertheless, we probably underestimate incidence due to the voluntary nature of case reporting, burdened healthcare staff and low ECFSPR coverage (including <80% of patients) in some countries (Armenia, Belarus, Bulgaria, Lithuania, Poland, Romania, Spain, Turkey and Ukraine). Selection bias towards voluntary reporting of more severe cases cannot be excluded.
Incidence was notably higher in lung-transplanted versus non-lung-transplanted pwCF (28.6 versus 16.6/1000 pwCF). Interestingly, the fold increase in incidence between the first and second waves was considerably lower for lung-transplanted pwCF compared to non-transplanted pwCF (1.4-fold versus 3.8-fold, respectively). This could be due to different testing rates in the two populations, or sustained guidance that transplanted people continue highly vigilant shielding and hygiene, while non-transplanted pwCF might have resumed more activities after June [18].
Confirming our earlier report [7], around three-quarters of pwCF and SARS-CoV-2 infection had symptomatic illness, lower than earlier reports from smaller CF studies (82–100%) [8, 10, 11] but similar to rates in the general population [19]. Again, this may reflect differing availability and strategy of testing different patient groups and the general population over time and between countries. The true rates of incidence, as well as asymptomatic infection, can only be determined by systematic wide-scale testing of all pwCF, either in a trial or as part of routine care.
We found that pwCF mostly had general and pulmonary symptoms, as also reported in a French study [11]. Some of the most frequent symptoms of SARS-CoV-2 infection reported here are common features of CF (increased cough and pulmonary exacerbation), some less so (fever, myalgia/arthralgia). Ageusia and anosmia were uncommon symptoms in pwCF in this report (<10%) and previous CF reports [9, 11], compared to the general population (38% and 41%, respectively [20]). These surprisingly low rates may be due to high levels of missing data for these symptoms, under-reporting or concomitant sinus disease, a regular feature in CF. Of note, 71.5% of pwCF demonstrated impaired smell in a small 2012 study [21].
Factors associated with symptomatic SARS-CoV-2 infection in pwCF were age >40 years, any F508del mutation and pancreatic insufficiency, indicating that older individuals with “classic” CF might be more prone to become symptomatic than younger pwCF with milder CFTR mutations.
Lung-transplanted pwCF had slightly higher rates of SARS-CoV-2 symptoms compared to other pwCF, confirming previous observations [8]. Transplanted individuals more often had increased dyspnoea and respiratory failure, but lower rates of increased sputum and pulmonary exacerbation, which is in line with differing lung disease phenotypes transplanted and non-transplanted pwCF.
The case fatality rate of SARS-CoV-2 infection in pwCF dropped from 3.85% up to June 30, 2020 [7] to 1.4% up to December 31, 2020, despite the higher numbers of infections during the second wave. Likewise, markedly fewer pwCF and SARS-CoV-2 infection required oxygen therapy, respiratory support, hospitalisation and intensive care in wave 2 versus wave 1. This mirrors decreased rates of intensive care and death in the general population [22] and could reflect improved management of severe cases of SARS-CoV-2 infection based on clinical experience and trials such as RECOVERY [23]. In CF, clinicians may have reduced precautionary hospitalisations and even intensive care admissions in favour of a more “watch and wait” approach to care, reassured by the observations that SARS-CoV-2 has a less severe impact on pwCF than initially expected. The fascinating but currently theoretical hypothesis that CFTR dysfunction may protect against SARS-CoV-2 replication in pwCF needs further investigation [24].
Solid organ transplant recipients are at increased risk of severe outcomes upon SARS-CoV-2 infection, including hospitalisation and intensive care [25–28]. In our cohort, lung transplant was associated with hospitalisation and oxygen therapy. In previous studies, lung-transplanted pwCF were more frequently treated and hospitalised [7, 8, 11]; our multivariable analysis confirms these descriptive findings in a substantial cohort of 828 pwCF. This supports recommendations that solid organ transplant recipients are vaccinated against SARS-Co-V-2. Reduced antibody response to the first mRNA vaccine dose in people after lung transplant was reported recently; however, a final conclusion on vaccination success cannot be drawn from these preliminary data and vaccination against SARS-CoV-2 continues to be strongly recommended for transplanted individuals [29].
Moderate and severe lung disease and long-term azithromycin (often considered a surrogate for worse lung disease) were also associated with hospitalisation and additional oxygen use. Moderate–severe lung disease (ppFEV1<70) was also associated with hospitalisation in univariable analyses in a previous global study in pwCF [8].
Azithromycin was proposed as a possible therapy for coronavirus disease 2019 (COVID-19) but did not improve outcomes in the RECOVERY trial [30]. Our finding suggesting an adverse effect of long-term azithromycin use on SARS-CoV-2 outcome should be interpreted cautiously. Azithromycin has different indications in non-transplanted and transplanted pwCF, and results cannot be compared for these groups. Also, azithromycin is often considered as a surrogate for chronic P. aeruginosa infection and severe lung disease [31, 32], and therefore cannot be counted as an independent variable in our multivariable analysis. This contributes to a strong indication bias for azithromycin, where pwCF treated with azithromycin appear to have worse outcomes. Analysing matched groups of azithromycin users and non-users could overcome this bias [33]; however, this is unfeasible in our analysis. Protopathic bias could also exist for azithromycin, whereby preferential treatment of sicker patients seems to reverse cause and effect, suggesting that the treatment is associated with worsening disease. Overall, we must be cautious not to over-interpret azithromycin treatment as a risk factor for a more severe SARS-CoV-2 outcome. The identification of more advanced lung disease as a risk factor for worse outcomes supports our previous advice that pwCF need to protect their lung health by adhering to medication and physiotherapy regimens and exercise.
CFRD, reported for 26.1% pwCF in our cohort, was associated with hospitalisation and oxygen therapy, although not with symptoms. In an earlier study, hospitalisation was more frequent in pwCF with CFRD, although oxygen use was less frequent [8]. Diabetes Type 1 and 2 is an established risk factor for severe outcomes with SARS-CoV-2 infection [34], but CFRD differs in mechanism and clinical impact [35]. Indeed, CFRD prevalence increases with age and could be considered as a proxy for advanced CF (creating the same potential bias as azithromycin, discussed above). Nonetheless, good control of CFRD is essential for overall health, and telehealth clinics can help pwCF and CFRD to maintain good glycaemic control during the pandemic [36].
Male sex is a risk factor for severe outcomes and death in SARS-CoV-2 [37, 38]. In our cohort, male sex was slightly underrepresented (48.4%) and not associated with symptoms or adverse outcomes. Female pwCF have a more severe clinical course of CF, culminating in younger median age at death [39]. It is possible that in our cohort the risk of worse SARS-CoV-2 outcomes in males is offset by a worse outcome for female pwCF. Further studies need to confirm this hypothesis.
Multivariable analyses in non-transplanted pwCF yielded similar risk factors. ppFEV1 <70 and long-term azithromycin were associated with hospitalisation and additional oxygen use, and CFRD was associated with hospitalisation only. Altogether, these results indicate that the relevant risk factors for severe SARS-CoV-2 disease in pwCF are CFRD, lung transplantation and more advanced lung disease.
We discussed the limits of our registry-based multinational data collection in depth previously [7]. Limitations specific to the multivariable analysis include lack of context around some demographic and baseline CF characteristics. For example, the exact duration of comorbidities and concomitant medications are unknown. Some variables had high rates of missing data, due to differences in data available from national registries. Importantly, the demographic and pre-infection CF characteristics could have dated from the registry collection of the previous calendar year, depending on when SARS-CoV-2 infection occurred. Finally, SARS-CoV-2 incidence may be underestimated due to incomplete surveillance and voluntary reporting bias towards severe cases and because many mild and asymptomatic cases probably went undiagnosed. Thus, we may have overestimated severity. Similarly, surveillance for SARS-CoV-2 infection may have been more complete in certain groups than others, based on previous reports of risk factors (e.g., male sex, transplant, etc. in the general and CF populations). Without a good understanding of surveillance rates, comparisons of incidence between different groups should be interpreted with caution. Prospective data collection on SARS-CoV-2 infection in pwCF in Europe is ongoing, and aims to enhance understanding, prevention and treatment of SARS-CoV-2 infection in pwCF. Future work includes long-term follow-up of lung function in patients with SARS-CoV-2 versus the wider CF population, and follow-up of incidence and severity following vaccination. In future, we may need to include cases diagnosed by antigen lateral flow test only, as many countries now accept a positive result as definitive, without confirmatory PCR. In addition, ECFSPR works closely together with a large global CF registry group to further improve our knowledge on SARS-CoV-2 in pwCF worldwide.
In summary, we report the first prospective study in a large cohort of pwCF infected with SARS-CoV-2 in Europe during the pandemic until the end of 2020. Clinical symptoms in pwCF are highly variable, and pulmonary symptoms resemble those from a CF exacerbation. We identified lung transplantation, CFRD and moderate to severe lung disease as independent risk factors for severe outcome after SARS-CoV-2 infection. All pwCF should maintain protective measures to prevent SARS-CoV-2 infection and be vaccinated against SARS-CoV-2. In particular, we strongly recommend that pwCF with lung transplants, ppFEV1 <70% predicted and/or CFRD shield more vigorously and be prioritised for vaccination.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00411-2021.supplement (154.6KB, pdf)
Acknowledgements
We thank the people with CF, and their families, for consenting to their data being included in the ECFSPR. We thank the centres and individual country representatives for allowing the use of the anonymised patient data.
Footnotes
Provenance: Submitted article, peer reviewed.
Collaborators: We would like to thank the people who provided the data and the members of the ECFSPR Scientific Committee. Austria: Sabine Burghart and Andrea Lakatos-Krepcic, Abteilung für Atmungs- und Lungenerkrankungen, Krankenhaus Hietzing, Vienna, Austria; Johannes Eder, Zertifiziertes CF-Zentrum für Kinder und Erwachsene, Medizinische Universität Innsbruck, Innsbruck, Austria; Katharina Kainz, Abteilung für Kinder- und Jugendheilkunde mit Ambulanz, Wilhelminenspital, Vienna, Austria; Margit Kallinger and Monika Pell, Abteilung für Kinder- und Jugendheilkunde und Abteilung für Lungenheilkunde, Landeskrankenhaus Steyr, Austria; Marta Mozdzen, Abteilung für Lungenkrankheiten, Klinikum Wels-Grieskirchen, Wels, Austria; Sabine Renner, Klinik für Kinder- und Jugendheilkunde, Cystische Fibrose Ambulanz, Medizinische Universität Wien, Vienna, Austria; Martin Stadlinger, Klinik für Lungenheilkunde/ Pneumologie, Kepler Universitats Klinikum, Linz, Austria; Christina Thir, Univ. Klinik für Kinder- und Jugendheilkunde, Kepler Universitätsklinikum, Linz, Austria. Belarus: Svetalana Keegan, Belarusian Republic Children's Center of Pulmonology and Cystic Fibrosis, Pulmonary Department, 3rd City Children's Clinical Hospital, Minsk, Belarus. Belgium: Hedwige Boboli, Department of Pediatrics, Pediatric Pulmonology, University Hospital Liège, Liège, Belgium; Elke De Wachter, Department of Paediatric Pulmonology, Universitair ziekenhuis Brussel, Brussels, Belgium; Lieven Dupont, Department of Pneumology, University Hospitals Leuven, Leuven, Belgium; Sophie Gohy, Department of Pulmonology Cliniques Universitaires Saint-Luc UCL, and Cystic Fibrosis Reference Center Cliniques Universitaires Saint-Luc UCL, Brussels, Belgium; Laurence Hanssens, CF Centre, Hôpital Universitaire des Enfants Reine Fabiola (HUDERF), and Institut de mucoviscidose de l'université libre de Bruxelles (ULB), hôpital universitaire des enfants Reine Fabiola – ULB, Brussels, Belgium; Christiane Knoop, Institut de mucoviscidose de l'université libre de Bruxelles (ULB), hôpital universitaire Erasme – ULB, Brussels, Belgium; Elise Lammertijn (on behalf of the scientific committee), Cystic Fibrosis Europe, and Association Muco A.S.B.L. – Mucovereniging V.Z.W., Brussels, Belgium; Vicky Nowé, Dept of Pulmonology, GZA Sint-Vincentius Hospital, Antwerp, Belgium; Jessica Pirson, Service de Pneumologie, CHR Citadelle, Liège, Belgium; Matthieu Thimmesch, Service de Pédiatrie, CHC Clinique du MontLégia, Liège, Belgium; Eva Van Braeckel, Cystic Fibrosis Reference Centre, Dept of Respiratory Medicine, Ghent University Hospital, and Dept of Internal Medicine and Pediatrics, Ghent University, Ghent, Belgium; Kim Van Hoorenbeeck, Dept of Pediatrics, Antwerp University Hospital, Edegem, Belgium; Eef Vanderhelst, Respiratory Division, University Hospital UZ Brussel, Brussels, Belgium. Croatia: Duška Tješić-Drinković and Ivan Bambir, University Hospital Centre Zagreb, Cystic Fibrosis Centre – Paediatrics and Adults, Zagreb, Croatia. Czech Republic: Alena Bilkova, Cystic Fibrosis Centre, Dept of Pneumology, Dept of Preventive Care, Second Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic; Macek Milan Jr (on behalf of the scientific committee), Dept of Biology and Medical Genetics, Second Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic. Denmark: Tania Pressler, Copenhagen Cystic Fibrosis Centre, Rigshospitalet, Copenhagen, Denmark. France: Pierre-Régis Burgel, Respiratory Medicine and National Cystic Fibrosis Reference Center, Cochin Hospital, Assistance Publique-Hôpitaux de Paris, Université de Paris, Institut Cochin, INSERM U1016, Paris, France; Lydie Lemonnier-Videau, Vaincre la Mucoviscidose, Paris, France. French Cystic Fibrosis Reference Network study group: Michel Abely, Centre Hospitalier Universitaire de Reims, Reims, France; Carole Bailly Piccini, Centre Hospitalier Universitaire de Nice, Nice, France; Chantal Belleguic, Centre Hospitalier Universitaire de Rennes – Hôpital Pontchaillou, Rennes, France; Tiphaine Bihouee, Centre Hospitalier Universitaire de Nantes, Nantes, France; Yves Billon, Centre Hospitalier Régional Universitaire de Nancy, Nancy, France; Stéphanie Bui, Centre Hospitalier Universitaire Bordeaux, Bordeaux, France; Boubou Camara, Centre Hospitalier Universitaire de Grenoble, La Tronche, France; Marie-Christine Cheraud, Centre Hospitalier Universitaire de Clermont-Ferrand, Clermont-Ferrand France; Raphael Chiron, Centre Hospitalier Universitaire de Montpellier, Montpellier, France; Emmanuelle Coirier Duet, Centre Hospitalier de Versailles, Le Chesnay-Rocquencourt, France; Laure Cosson, Centre Hospitalier Régional Universitaire de Tours – Clocheville Hospital, Tours, France; Marie-Laure Dalphin, Centre hospitalier universitaire de Besançon, Besançon, France; Isabelle Danner Boucher, Centre Hospitalier Universitaire de Nantes, Nantes, France; Sandra De Miranda, Hôpital Foch, Suresnes, France; Eric Deneuville, Centre Hospitalier Universitaire de Rennes – Hôpital Sud, Rennes, France; Jean-Christophe Dubus, Hôpitaux Universitaires de Marseille, Marseille, France; Isabelle Durieu, Centre Hospitalier Lyon-Sud, Pierre-Bénite, France; Ralph Epaud, Hôpital Intercommunal de Créteil, Créteil, France; Michèle Gerardin, AP-HP - Hôpital Robert-Debré, Paris, France; Dominique Grenet, Hôpital Foch, Suresnes, France; Véronique Houdouin, AP-HP - Hôpital Robert-Debré, Paris, France; Frédéric Huet, Centre Hospitalier Universitaire de Dijon Bourgogne, Dijon, France; Kanaan Reem, AP-HP Hôpital Cochin, Paris, France; Romain Kessler, Hôpitaux Universitaires de Strasbourg, Strasbourg, France; Jeanne Languepin, Centre Hospitalier Universitaire de Limoges, Limoges, France; Muriel Laurans, Centre Hospitalier Universitaire de Caen, Caen, France; Sylvie Leroy, Centre Hospitalier Universitaire de Nice, Nice, France; Cathie Llerena, Centre Hospitalier Universitaire de Grenoble, La Tronche, France; Julie Macey, Centre Hospitalier Universitaire de Bordeaux – Hôpital Haut-Lévêque, Pessac, France; Julie Mankikian, Centre Hospitalier Régional Universitaire de Tours – Bretonneau, Tours, France; Christophe Marguet, Centre Hospitalier Universitaire de Rouen, Rouen, France; Clémence Martin, AP-HP Hôpital Cochin, Paris, France; Laurent Mely, Hospices Civils de Lyon – Hôpital Renée Sabran, Giens-Hyères, France; Marie Mittaine, Centre Hospitalier Universitaire de Toulouse – Hôpital des Enfants, Toulouse, France; Marlène Murris-Espin, Centre Hospitalier Universitaire de Toulouse – Hôpital Larrey, Toulouse, France; Caroline Perisson, Centre Hospitalier Universitaire de La Réunion – sites Sud, Saint-Pierre, France; Anne Prevotat, Centre Hospitalier Universitaire de Lille, Lille, France; Sophie Ramel, Fondation ILYDS, Roscoff, France; Cinthia Rames, Centre Hospitalier Universitaire Amiens-Picardie, Amiens, France; Philippe Reix, Hospices Civils de Lyon, Hôpital Femme Mère Enfant, Bron, France; Marine Revillon, Centre Hospitalier Universitaire de Lille, Lille, France; Martine Reynaud-Gaubert, Hôpitaux Universitaires de Marseille, Marseille, France; Bénédicte Richaud-Thiriez, Centre hospitalier universitaire de Besançon, Besançon, France; Jean-Luc Rittie, Centre Hospitalier Universitaire de La Réunion – site Felix Guyon, Saint-Denis, France; Manuëla Scalbert-Dujardin, Centre Hospitalier Dunkerque, Dunkerque, France; Isabelle Sermet-Gaudelus, AP-HP - Hôpital Necker Enfants malades, Paris, France; Véronique Storni, Centre hospitalier Bretagne-Atlantique, Vannes, France; Aurélie Tatopoulos, Centre Hospitalier Régional Universitaire de Nancy, Nancy, France; Guillaume Thouvenin, AP-HP Hôpital Armand-Trousseau, Paris, France; Françoise Troussier, Centre hospitalier universitaire d'Angers, Angers, France; Laurence Weiss, Hôpitaux Universitaires de Strasbourg, Strasbourg, France; Nathalie Wizla, Centre Hospitalier Universitaire de Lille, Lille, France. Germany: Eva-Susanne Behl, Klinikum Westbrandenburg, Klinik für Kinder- und Jugendmedizin, Potsdam, Germany; Folke Brinkmann, Universitätsklinikum der Ruhr-Universität Bochum, St. Josef-Hospital am Katholischen Klinikum Bochum, Klinik für Kinder- und Jugendmedizin, Christiane Herzog Centrum Ruhr, Bochum, Germany; Martin Claßen, Klinikverbund Bremen gGmbH, Klinikum Links der Weser, Christiane Herzog-Ambulanz für Mukoviszidose, Bremen, Germany; Ute Graepler-Mainka, Department of General Pediatrics, Hematology and Oncology, Children's Hospital, Eberhard-Karls-University, Tübingen, Germany; Matthias Griese, Ludwig-Maximillian Klinikum der Universität München, Kinderklinik und Kinderpoliklinik im Dr. von Haunerschen Kinderspital, Christiane-Herzog-Ambulanz, München, Germany; Armin Grübl, Klinik für Kinder- und Jugendmedizin München, Klinik Schwabing und Harlaching, München, Germany; Jutta Hammermann, Universitätsklinikum Carl-Gustav Carus, Klinik und Poliklinik für Kinder- und Jugendmedizin, Universitäts Mukoviszidose-Centrum “Christiane Herzog”, Dresden, Germany; Helge Hebestreit, Universitäts-Kinderklinik, Christiane–Herzog-Anmbulanz für Mukoviszidose, Würzburg, Germany; Andrea Heinzmann, Universitätsklinikum Freiburg, Klinik für Allgemeine Kinder- und Jugendmedizin, Ambulanz und Arbeitsgruppe Pneumologie, Allergologie und Mukoviszidose, Freiburg, Germany; Alexander Herz, Universitätsklinikum Schleswig-Holstein, Campus Lübeck, Klinik für Kinder- und Jugendmedizin, Pädiatrische Pneumologie, Lübeck, Germany; Alexander Kiefer, KUNO Klinik St. Hedwig, Regensburg, Germany; Birte Kinder, Dietrich Bonhoeffer Klinikum Neubrandenbrurg, Klinik für Kinder- und Jugendmedizin, Neubrandenburg, Germany; Holger Köster, Klinikum Oldenburg, Oldenburg, Germany; Stefan Kuhnert, Universitätsklinikum Gießen und Marburg GmbH, Medizinische Klinik und Poliklinik II, Giessen, Germany; Jochen Mainz, Brandenburg Medical School (MHB), University, Klinikum Westbrandenburg, Brandenburg an der Havel, Germany; Angelika Mayer, Robert-Bosch-Krankenhaus, Klinik Schillerhöhe, Pneumologie, Gerlingen, Germany; Susanne Naehrig, Medizinische Klinik V (Pneumology), LMU University of Munich, Pneumology, Medizinische Klinik Innenstadt, University of Munich, Munich, Germany; Tim Niehues, Helios Klinikum Krefeld, Zentrum für kinder- und Jugendmedizin, Mukoviszidose-Zentrum, Krefeld, Germany; Thomas Nüßlein, Gemeinschaftsklinikum Mittelrhein, Klinik für Kinder- und Jugendmedizin, Koblenz, Koblenz, Germany; Krystyna Poplawska, Universitätskinderklinik Mainz, Pädiatrische Pneumologie und Allergologie, Mukoviszidose, Mainz, Germany; Felix Ringshausen, Medizinische Hochschule Hannover, Klinik für Innere Medizin, Pneumologische Ambulanz, Hannover, Germany; Markus Rose, Klinikum Stuttgart, Olgahospital- Pediatric Pulmonology, Stuttgart, Germany; Josef Rosenecker, Fachkliniken Wangen, Wangen, Germany; Renate Ruppel, Kinderklinik des Universitätsklinikums Erlangen, Erlangen, Germany; Anette Scharschinger, Paediatric and Adolescent Medicine, University Medical Center Augsburg, Augsburg, Germany; Christian Schropp, Children's Hospital Dritter Orden, Passau, Germany; Carsten Schwarz, Dept of Pediatric Pneumology, Immunology and Intensive Care Medicine, Cystic Fibrosis Center, Charité – Universitätsmedizin Berlin, Berlin, Germany; Christina Smaczny, Universitätsklinikum Frankfurt, Goethe-Universität, Christiane Herzog CF-Zentrum für Kinder, Jugendliche und Erwachsene, Frankfurt, Germany; Olaf Sommerburg, Universitätsklinikum Heidelberg, Sektion Pädiatrische Pneumologie, Allergologie und Mukoviszidose-Zentrum, Heidelberg, Germany; Sivagurunathan Sutharsan, Ruhrlandklinik, Pneumologie, Essen, Germany; Simone Stolz, Klinik für Kinder- und Jugendmedizin, Carl-Thiem-Klinikum gGmbH, Cottbus, Germany; Wolfgang Thomas, Klinikum Mutterhaus der Borromäerinnen, Kinder- und Jugendmedizin, Trier, Germany; Sabine Wege, Department of Pneumology and Critical Care Medicine, Thoraxklinik at the University Hospital Heidelberg, Heidelberg, Germany; Britta Welzenbach, Josefinum hospital for children and adolescents, Augsburg, Germany; Bettina Wollschläger, Martin-Luther-University Halle, Clinic for Internal Medicine, Halle, Germany. Greece: Filia Diamantea, Adult Cystic Fibrosis Unit, Sismanoglio General Hospital of Attica, Athens, Greece; Katerina Manika, Pulmonary Dept, Aristotle University of Thessaloniki, G Papanikolaou Hospital, Thessaloniki, Greece. Hungary: Andrea Párniczky, Heim Pál National Pediatric Institute, Budapest, Hungary. Ireland: Des Cox, Children's Health Ireland, Crumlin, Ireland; Basil Elnazir, Children's Health Ireland, Tallaght University Hospital, Dublin, Ireland; Godfrey Fletcher, The Cystic Fibrosis Registry of Ireland, Dublin, Ireland; Cedric Gunaratnam, Beaumont Hospital, Dublin, Ireland; Barry J. Plant, University College Cork, Cork, Ireland. Israel: Michal Gur, Pediatric Pulmonary Institute and CF Center, Rappaport Children's Hospital, Rambam Health Care Campus, Haifa, Israel; Malena Cohen-Cymberknoh, Pediatric Pulmonology Unit and Cystic Fibrosis Center, Hadassah Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Israel; Galit Livnat, Carmel CF Center, Technion-Israel Institute of Technology, Haifa, Israel. Italy: Annalisa Amato and Gianluca Ferrari, technical board of ICFR, Italian Cystic Fibrosis Ligue, Rome, Italy; Raffaele Badolato and Piercarlo Poli, Department of Pediatrics, Regional support Centre for Cystic Fibrosis, Children's Hospital – ASST Spedali Civili Pz. le Spedali Civili, University of Brescia, Brescia, Italy; Fiorella Battistini and Valentina Donati, CF Referral Center Emilia-Romagna Region, Cesena, Italy; Elisabetta Bignamini and Anna Folino, CF Referral Center Piemonte and Valle D'Aosta Regions, Ospedale Infantile Regina Margherita – Sant’ Anna, Turin, Italy; Vincenzo Carnovale, Adult CF Referral Center Campania Region, Naples, Italy; Carlo Castellani and Rosaria Casciaro, CF Referral Center Liguria Region, IRCCS Istituto Giannina Gaslini Genova, Genoa, Italy; Giuseppe Cimino, CF Referral Center Lazio Region, Rome, Italy; Marco Cipolli and Francesca Lucca, CF Referral Center Veneto Region, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy; Mirella Collura and Francesca Ficili, CF Referral Center Sicily Region, Palermo, Italy; Valeria Daccò, Vanessa Gagliano and Giovanna Pizzamiglio, CF Referral Center Lombardia Region, Fondazione IRCCS Cà Granda - Ospedale Maggiore Policlinico, Milan, Italy; Valeria Mencarini and Nicola Palladino, CF Referral Center Umbria Region, Gubbio, Italy; Salvatore Leonardi and Novella Rotolo, CF Support Center Sicily Region, Catania, Italy; Maria Cristina Lucanto and Ester Quattromano, CF Referral Center Sicily Region, Messina, Italy; Vincenzina Lucidi, Fabio Majo, Federico Alghisi and Fabiana Ciciriello, CF UOC, Paediatric Hospital “Bambino Gesù”, Rome, Italy; Antonio Manca and Giuseppina Leonetti, CF Referral Center Puglia Region, Bari, Italy; Massimo Maschio, CF Referral Center Friuli-Venezia Giulia Region, IRCCS Materno Infantile Burlo Garofolo, Trieste, Italy; Barbara Messore, Adult CF Centre Torino, Pulmonolgy Dept, Azienda Ospedaliero Universitaria San Luigi Gonzaga, Orbassano, Italy; Stefano Pantano, CF Referral Center Abruzzi and Molise Region, Teramo, Italy; Giovanna Pisi and Cinzia Spaggiari, CF Referral Center Emilia-Romagna Region, Parma, Italy; Valeria Raia and Caterina Laezza, CF Pediatric Referral Center Campania Region, Naples, Italy; Mirco Ros, Veneto Region CF Support Center of Treviso, Ospedale Ca' Foncello, Treviso, Italy; Donatello Salvatore, Cystic Fibrosis Center, Hospital San Carlo, Potenza, Italy; Marco Salvatore, Undiagnosed Rare Diseases Interdepartmental Unit, National Center Rare Diseases, Istituto Superiore di Sanità, Rome, Italy; Giovanni Taccetti and Michela Francalanci, Cystic Fibrosis Center, Toscana Region, Florence, Italy; Pamela Vitullo, CF Support Center Puglia Region, Cerignola, Italy. Luxembourg: Hélène De la Barrière, Department of Pulmonology, Hôpitaux Robert Schuman, Luxembourg, Luxembourg. The Netherlands: Josje Altenburg, Dept of Pulmonology, Amsterdam University Medical Center, The Netherlands; Michiel Bannier, Dept of Pediatric Pulmonology, University Medical Center Maastricht, The Netherlands; Harry Heijerman, Dept of Pulmonology, University Medical Center Utrecht, The Netherlands; Hettie Janssens, Dept of Pediatric Pulmonology, Erasmus Medical Center Rotterdam, The Netherlands; Gerard Koppelman, Dept of Pediatric Pulmonology, University Medical Center Groningen, The Netherlands; Renske van der Meer, Dept of Pulmonology, Haga Ziekenhuis Den Haag, The Hague, The Netherlands; Peter Merkus, Dept of Pediatric Pulmonology, Radboud University Medical Center Nijmegen, The Netherlands; Jacquelien Noordhoek, NCFS, Baarn, The Netherlands; Marianne Nuijsink, Department of Pediatric Pulmonology, Haga Ziekenhuis Den Haag, The Hague, The Netherlands; Suzanne Terheggen, Dept of Pediatric Pulmonology, Amsterdam University Medical Center, The Netherlands; Hester van der Vaart, Dept of Pulmonology, University Medical Center Groningen,The Netherlands; Geert-Jan Wesseling, Dept of Pulmonology, University Medical Center Maastricht, The Netherlands; Karin de Winter, Dept of Pediatric Pulmonology, University Medical Center Utrecht, The Netherlands; Domenique Zomer-van Ommen, NCFS, Baarn, The Netherlands. North Macedonia: Tatjana Jakovska Maretti, Center for Cystic Fibrosis, Children and Adults, Institute for Respiratory Diseases in Children, Kozle, North Macedonia. Norway: Anita Senstad Wathne, Norwegian Cystic Fibrosis Registry, Oslo, Norway. Portugal: Adelina Amorim, Centro Hospitalar S. João, Pulmonology Dept, Porto, Portugal; Fabienne Gonçalves, Centro Hospitalar do Porto Materno-Infantil, Porto, Portugal; Sónia Silva, Dept of Pediatrics, Centro Hospitalar Universitário de São João, Porto, Portugal. Russia: Elena Amelina, Cystic Fibrosis Dept, Scientific Research Institute of Pulmonology of the Federal Medical and Biological Agency of Russia, Moscow, Russian Federation; Evgeniya Boitsova, Department of Propaedeutics of Children's Diseases, Federal State Budgetary Scientific Institution of Higher Education “Saint Petersburg state pediatric medical University” of the Russian Federation Ministry of Health, Saint Petersburg, Russia; Yuliya Gorinova, National Medical Research Center for Children's Health, Moscow, Russia; Stanislav Krasovskiy, Laboratory of Cystic Fibrosis, Scientific Research Institute of Pulmonology of the Federal Medical and Biological Agency of Russia, Moscow, Russia; Maria Mukhina, Medical and Genetic Dept, Cystic Fibrosis Office of the State Budgetary Healthcare Institution “Morozovskaya Children's Municipal Clinical Hospital” Moscow, Russia; Victoria Sherman, The Scientific and Clinical Dept of Cystic Fibrosis, Research Centre for Medical Genetics, Moscow, Russia; Olga Simonova, National Medical Research Center for Children's Health, Morozov State Pediatric Teaching Hospital, Moscow Healthcare Dept, I.M. Sechenov First Moscow State Medical University (Sechenov University), Healthcare Ministry of Russia, Moscow, Russian Federation; Nataliya Kashirskaya, Laboratory of Genetic Epidemiology, “Research Centre for Medical Genetics”, Moscow, Russian Federation; Elena Zhekaite, Dept of Cystic Fibrosis, “Research Centre for Medical Genetics”, Moscow, Russian Federation. Slovakia: Eva Bérešová, Centrum cystickej fibrozy pre dospelych FNSP FDR, Banská Bystrica, Slovakia; Nina Bližnáková, pracovisko Podunajské Biskupice, Klinika detskej pneumologie SZU UN Bratislava, Bratislava, Slovakia. Slovenia: Julij Šelb, University Clinic of Pulmonary and Allergic Diseases, Golnik, Slovenia. Spain: Antonio Alvarez Fernàndez, Adult Cystic Fibrosis unit, Hospital Universitario Vall d'Hebron, Barcelona, Spain; Oscar Asensio de la Cruz, Unitat de Pneumologia Pediátrica i Unitat de Fibrosi Quística, Parc Taulí Hospital Universitario, Hospital de Sabadell, Barcelona, Spain; Félix Baranda García and Ainhoa García Bonilla, Servicio de Neumología y Fibrosis Quística, Osakidetza, Hospital Universitario Cruces, Bizkaia, Spain; Marina Blanco Aparicio, Servicio de Neumología, Hospital Universitario A Coruña, A Coruña, Spain; Silvia Castillo Corullón, Unidad de Fibrosis Quística Pediátrica, Hospital Clínico Universitario de Valencia, Valencia, Spain; Isidoro Cortell-Aznar and Inés Pérez, Unidad de Trasplante Pulmonar y Fibrosis Quística, Hospital Universitario y Politécnico La Fe, Valencia, Spain; Jordi Costa i Colomer and María Cols Roig, Unitat de Pneumologia Pediátrica i Fibrosi Quística, Hospital Sant Joan de Déu, Barcelona, Spain; Isabel Delgado Pecellín and Esther Quintana, Unidad de Fibrosis Quística, Hospital Universitario Virgen del Rocío, Seville, Spain; Layla Diab Cáceres and Carmen Luna Paredes, Unidad de Fibrosis Quística, Hospital 12 de Octubre, Madrid, Spain; Silvia Gartner, Unidad Fibrosis Quística y Neumología Pediátrica, Hospital Vall d'Hebron, Barcelona, Spain; Estela González Castro, Servicio de Neumología, Hospital Universitario Torrecárdenas, Almería, Spain; José Ramón Gutiérrez Martínez, Unidad de Fibrosis Quística, Hospital Universitario Central de Asturias, Oviedo, Spain; Inés Herrero Labarga, Unidad de Neumología y Fibrosis Quística (Adultos), Hospital Universitario Miguel Servet, Zaragoza, Zaragoza, Spain; Rosa Maria Girón-Moreno, Neumología Adultos, Hospital Universitario La Princesa, Madrid, Spain; Esperanza Jiménez Nogueira, Neumología Pediatrica, Hospital Universitario Torrecárdenas, Almería, Spain; Adelaida Lamas Ferreiro, Alejandro López Neyra and Enrique Blitz Castro, Unidad de Fibrosis Quística Neumología Pediátrica, Servicio de Pediatría Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS) Hospital Universitario Ramón y Cajal, Madrid, Spain; Laura Moreno Galarraga, Navarra Institute for Health Research (IdisNa); Department of Pediatrics, Complejo Hospital de Navarra, Pamplona, Spain; Carlos Martin de Vincente, Unidad de Neumología Pediátrica y Fibrosis Quística, Hospital Universitario Miguel Servet, Zaragoza, Spain; Silvia Merlos Navarro, Servicio de Neumología, Hospital Universitario Virgen de las Nieves, Granada, Spain; Pedro Mondejar Lopez, Pediatric Pulmonology and Cystic Fibrosis Unit, Virgen de la Arrixaca Clinic University Hospital, Murcia, Spain; Rosa Nieto-Royo, Unidad de Fibrosis Quística, Hospital Universitario de Ramón y Cajal, Madrid, Spain; Casilda Olveira Fuster, Unidad Fibrosis Quística Adultos, Hospital Regional Universitario de Málaga, Málaga, Spain; Carlos Peñalver Mellado, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain; Estela Pérez-Ruiz and Pilar Caro-Aguilera, Unidad de Fibrosis Quística Pediátrica, Hospital Regional Universitario de Málaga, Málaga, Spain; Concepción Prados-Sánchez, Unidad de Fibrosis Quistica Adultos, Servicio de Neumología, Hospital Universitario La Paz, Madrid, Spain; Isabel Ramos Cancelo, Hospital Clínico Universitario de Valladolid, Vallalodid, Spain; Marta Ruiz de Valbuena, Sección de Neumología Pediátrica, Unidad de Fibrosis Quística Pediátrica, Hospital Infantil La Paz, Madrid, Spain; José R. Villa Asensi, Veronica Sanz Santiago and Patricia Fernández García, Sección de Neumología Pediátrica, Unidad de Fibrosis Quística, Hospital Niño Jesús, Madrid, Spain. Sweden: Adrienn Banki, Stockholm CF centre, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden; Stefanie Diemer and Christine Hansen, Lunds University Hospital, Lund, Sweden; Marita Gilljam, Gothenburg CF center, Sahlgrenska University Hospital, Sweden; Christina Krantz, Dept of Women's and Children's Health, Research Group, Paediatric Inflammation, Metabolism and Child Health Research, Uppsala University, Uppsala, Sweden; Ulrika Lindberg, Dept of Respiratory Medicine and Allergology, Lund CF Center, Skane University Hospital, Lund, Sweden; Anders Lindblad (on behalf of the scientific committee), Gothenburg CF Centre, Queen Silvia Children's Hospital, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden. Switzerland: Christian Clarenbach, Klinik für Pneumologie, Adultes CF Zentrum, Universitätsspital Zürich, Zürich, Switzerland; Reta Fischer, Lindenhofspital Quartier Bleu, Bern, Switzerland; Dominik Mueller, Kantonsspital Aarau AG, Klinik für Kinder und Jugendliche, Abteilung pädiatrische Pneumologie, Allergologie und Immunologie, Aarau, Switzerland; Isabelle Rochat, Département femme-mère-enfant, Service de pédiatrie, Unité de pneumologie et mucoviscidose pédiatrique, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland; Macé Schuurmans, Klinik für Pneumologie, Adultes CF Zentrum, Universitätsspital Zürich, Zürich, Switzerland; Renate Spinas, Dept of Paediatric Pulmonology, University Children`s Hospital Zurich, Zurich, Switzerland; Anna-Lena Walter, Lungenzentrum, Zentrum für Cystische Fibrose für Erwachsene, Kantonsspital St. Gallen, St Gallen, Switzerland. Turkey: Dilber Ademhan Tural and Ugur Ozcelik, Dept of Pediatric Pulmonology, Hacettepe University Faculty of Medicine, Ankara, Turkey; Pelin Asfuroğlu and Ayse Tana Aslan, Dept of Pediatric Pulmonology, Gazi University Faculty of Medicine, Ankara, Turkey; Ayşen Bingöl, Division of Pediatric Pulmonology, Allergy and Immunology, Faculty of Medicine, Akdeniz University, Antalya, Turkey; Nazan Çobanoğlu, Division of Pediatric Pulmonology, Faculty of Medicine, Ankara University, Ankara, Turkey; Yasemin Gökdemir, Division of Paediatric Pulmonology, Marmara University Faculty of Medicine, Istanbul, Turkey; Mehmet KÖSE, Department of Pediatrics, Division of Pediatric Pulmonology, Erciyes University, Kayseri, Turkey; Sevgi Pekcan, Division of Pediatric Pulmonology, Meram Faculty of Medicine, Necmettin Erbakan University, Konya, Turkey; Tuğba Şişmanlar Eyüboğlu, Department of Pediatric Pulmonology, Gazi University School of Medicine, Ankara, Turkey. Ukraine: Lyudmyla Bober, Western Ukrainian Specialised Children's Medical Centre, Lviv, Ukraine. UK: Elliot McClenaghan, Cystic Fibrosis Trust, London, UK; Elaine Gunn, Cystic Fibrosis Trust, London, UK; Keith Brownlee, Cystic Fibrosis Trust, London, UK.
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: A. Jung has nothing to disclose.
Conflict of interest: A. Orenti has nothing to disclose.
Conflict of interest: F. Dunlevy reports support for the present manuscript from Chiesi Farmaceutici SpA.
Conflict of interest: E. Aleksejeva has nothing to disclose.
Conflict of interest: E. Bakkeheim has nothing to disclose.
Conflict of interest: V. Bobrovnichy has nothing to disclose.
Conflict of interest: S.B. Carr reports receiving speaker honoraria from Vertex and Chiesi, outside the submitted work; participation on a Data Safety Monitoring Board or Advisory Board for Vertex, Profile Pharma and Chiesi, outside the submitted work; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from UK CF Trust; PI for Pharmacovigilance study, payment to institution from Pharmaxis, outside the submitted work.
Conflict of interest: C. Colombo has nothing to disclose.
Conflict of interest: H. Corvol has nothing to disclose.
Conflict of interest: R. Cosgrif declares outside the submitted work to be the director of the Cystic Fibrosis Trust.
Conflict of interest: G. Daneau has nothing to disclose.
Conflict of interest: D. Dogru has nothing to disclose.
Conflict of interest: P. Drevinek reports grants or contracts from Ministry of Health, Czech Republic, outside the submitted work; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Vertex Pharmaceuticals and Actelion Pharmaceuticals, outside the submitted work; participation on a Data Safety Monitoring Board or Advisory Board for Vertex Pharmaceuticals, outside the submitted work; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from Society for Medical Microbiology, outside the submitted work.
Conflict of interest: A.D. Vukic has nothing to disclose.
Conflict of interest: I. Fajac reports grants or contracts from Boehringer Ingelheim, Celtaxsys, Corbus Pharmaceuticals, and Vertex Pharmaceuticals, outside the submitted work; consulting fees from Boehringer Ingelheim and Vertex Pharmaceuticals, outside the submitted work; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Vertex Pharmaceuticals and Boehringer Ingelheim, outside the submitted work; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for European Cystic Fibrosis Society.
Conflict of interest: A. Fox support for the present manuscript from Chiesi Farmaceutici SpA.
Conflict of interest: S. Fustik has nothing to disclose.
Conflict of interest: V. Gulmans has nothing to disclose.
Conflict of interest: S. Harutyunyan has nothing to disclose.
Conflict of interest: E. Hatziagorou has nothing to disclose.
Conflict of interest: I. Kasmi has nothing to disclose.
Conflict of interest: H. Kayserová has nothing to disclose.
Conflict of interest: E. Kondratyeva reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from All-Russian online school with international participation, outside the submitted work.
Conflict of interest: U. Krivec has nothing to disclose.
Conflict of interest: H. Makukh has nothing to disclose.
Conflict of interest: K. Malakauskas has nothing to disclose.
Conflict of interest: E.F. McKone reports grants or contracts from vertex, outside the submitted work; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Vertex Pharmaceuticals and Roche Pharmaceuticals, outside the submitted work; support for attending meetings and/or travel from A Menarini, outside the submitted work; participation on a Data Safety Monitoring Board or Advisory Board for Insmed and Janssen Pharmaceuticals, outside the submitted work.
Conflict of interest: M. Mei-Zahav has nothing to disclose.
Conflict of interest: I. de Monestrol reports grant from Vertex for an academic research study regarding gastrointestinal outcome in CF patients taking Orkambi medication, outside the submitted work; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from Swedish CF Registry and the Swedish Society of Medicine's CF working group.
Conflict of interest: H.V. Olesen has nothing to disclose.
Conflict of interest: R. Padoan has nothing to disclose.
Conflict of interest: T. Parulava has nothing to disclose.
Conflict of interest: M.D. Pastor-Vivero has nothing to disclose.
Conflict of interest: L. Pereira has nothing to disclose.
Conflict of interest: G. Petrova reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from TEVA Pharmaceuticals, Mylan and Alvogen, outside the submitted work.
Conflict of interest: A. Pfleger has nothing to disclose.
Conflict of interest: L. Pop has nothing to disclose.
Conflict of interest: J.G. van Rens has nothing to disclose.
Conflict of interest: M. Rodić has nothing to disclose.
Conflict of interest: M. Schlesser has nothing to disclose.
Conflict of interest: V. Storms has nothing to disclose.
Conflict of interest: O. Turcu has nothing to disclose.
Conflict of interest: L. Woźniacki has nothing to disclose.
Conflict of interest: P. Yiallouros has nothing to disclose.
Conflict of interest: A. Zolin has nothing to disclose.
Conflict of interest: D.G. Downey has nothing to disclose.
Conflict of interest: L. Naehrlich reports support for the present manuscript from Chiesi Farmaceutici SpA; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from ArticulateScience LLC, outside the submitted work; participation on a Data Safety Monitoring Board or Advisory Board for Trial Steering committee of CF Storm, outside the submitted work; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from Society for German CF Registry European CF Patient Registry, outside the submitted work; other financial or nonfinancial interests from Vertex Pharmaceuticals and Boehringer Ingelheim; Institutional fees for site participation (PI) in clinical trials from Vertex Pharmaceuticals and Boehringer Ingelheim.
Support statement: This paper was supported by an unrestricted grant from Chiesi Pharmaceuticals, Italy. The funder had no role in the planning, conduct, analysis or reporting of the study, nor did they review the draft paper before submission. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.World Health Organisation . Weekly epidemiological update – 29 December 2020. www.who.int/publications/m/item/weekly-epidemiological-update---29-december-2020 Date last updated: 29 December 2020. Date last accessed: 23 March 2021.
- 2.Viviani L, Assael BM, Kerem E, et al. . Impact of the A (H1N1) pandemic influenza (season 2009–2010) on patients with cystic fibrosis. J Cyst Fibros 2011; 10: 370–376. doi: 10.1016/j.jcf.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 3.Kiedrowski MR, Bomberger JM. Viral-bacterial co-infections in the cystic fibrosis respiratory tract. Front Immunol 2018; 9: 3067. doi: 10.3389/fimmu.2018.03067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis JB, Jones AM, Davies EA, et al. . Influenza B outbreak at an adult cystic fibrosis centre: clinical impact and factors influencing spread. J Cyst Fibros 2020; 19: 808–814. doi: 10.1016/j.jcf.2020.04.011 [DOI] [PubMed] [Google Scholar]
- 5.Colombo C, Burgel PR, Gartner S, et al. . Impact of COVID-19 on people with cystic fibrosis. Lancet Respir Med 2020; 8: e35–e36. doi: 10.1016/S2213-2600(20)30177-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Koningsbruggen-Rietschel S, Dunlevy F, Bulteel V, et al. . SARS-CoV-2 disrupts clinical research: the role of a rare disease-specific trial network. Eur Respir J 2020; 56: 2002114. doi: 10.1183/13993003.02114-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naerlich L, Orenti A, Dunlevy F, et al. Incidence of SARS-CoV-2 in people with cystic fibrosis in Europe between February and June 2020. J Cyst Fibros 2021; 20: 566–577. doi: 10.1016/j.jcf.2021.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClenaghan E, Cosgriff R, Brownlee K, et al. . The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J Cyst Fibros 2020; 19: 868–871. doi: 10.1016/j.jcf.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bain R, Cosgriff R, Zampoli M, et al. . Clinical characteristics of SARS-CoV-2 infection in children with cystic fibrosis: an international observational study. J Cyst Fibros 2021; 20: 25–30. doi: 10.1016/j.jcf.2020.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondejar-Lopez P, Quintana-Gallego E, Giron-Moreno RM, et al. . Impact of SARS-CoV-2 infection in patients with cystic fibrosis in Spain: incidence and results of the national CF-COVID19-Spain survey. Respir Med 2020; 170: 106062. doi: 10.1016/j.rmed.2020.106062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corvol H, de Miranda S, Lemonnier L, et al. . First wave of COVID-19 in French patients with cystic fibrosis. J Clin Med 2020; 9: 3624. doi: 10.3390/jcm9113624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padoan R, Carnovale V, Salvatore D, et al. . First and second wave of SARS-CoV2 in Italian cystic fibrosis patients: data from Italian cystic fibrosis registry. J Cyst Fibros 2021; 26: S1569–S1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellegrino R, Viegi G, Brusasco V, et al. . Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 14.Zolin A, Orenti A, Naehrlich L, et al. . ECFS Patient Registry Annual Report 2018. www.ecfs.eu/projects/ecfs-patient-registry/annual-reports Date last updated: 2020. Date last accessed: 26 November 2021.
- 15.Zolin A, Orenti A, Naehrlich L, et al. . ECFS Patient Registry Annual Report 2017. www.ecfs.eu/projects/ecfs-patient-registry/annual-reports Date last updated: 2019. Date last accessed: 26 November 2021.
- 16.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007; 165: 710–718. doi: 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
- 17.European Centre for Disease Control . Data on testing for COVID-19 by week and country. https://www.ecdc.europa.eu/en/publications-data/covid-19-testing Date last updated: 25 November 2021. Date last accessed: 26 November 2021.
- 18.International Society of Heart and Lung Transplantation (ISHLT) . Guidance from the International Society of Heart and Lung Transplantation regarding the SARS CoV-2 pandemic. https://ishlt.org/ishlt/media/documents/SARS-CoV-2_Guidance-for-Cardiothoracic-Transplant-and-VAD-center.pdf Date last updated: 1 February 2021. Date last accessed: 31 March 2021.
- 19.Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. . Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med 2020; 17: e1003346. doi: 10.1371/journal.pmed.1003346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agyeman AA, Chin KL, Landersdorfer CB, et al. . Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc 2020; 95: 1621–1631. doi: 10.1016/j.mayocp.2020.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindig J, Steger C, Beiersdorf N, et al. . Smell in cystic fibrosis. Eur Arch Otorhinolaryngol 2013; 270: 915–921. doi: 10.1007/s00405-012-2124-2 [DOI] [PubMed] [Google Scholar]
- 22.Karagiannidis C, Windisch W, McAuley DF, et al. . Major differences in ICU admissions during the first and second COVID-19 wave in Germany. Lancet Respir Med 2021; 9: e47–e48. doi: 10.1016/S2213-2600(21)00101-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalmers JD, Crichton ML, Goeminne PC, et al. . Management of hospitalised adults with coronavirus disease-19 (COVID-19): A European Respiratory Society living guideline. Eur Respir J 2021; 57: 2100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peckham D, McDermott MF, Savic S, et al. . COVID-19 meets Cystic Fibrosis: for better or worse? Genes Immun 2020; 21: 260–262. doi: 10.1038/s41435-020-0103-y [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) . Evidence used to update the list of underlying medical conditions that increase a person's risk of severe illness from COVID-19. www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html Date last updated: 29 March 2021. Date last accessed: 22 December 2020. [PubMed]
- 26.Raja MA, Mendoza MA, Villavicencio A, et al. . COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando) 2021; 35: 100588. doi: 10.1016/j.trre.2020.100588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saez-Gimenez B, Berastegui C, Barrecheguren M, et al. . COVID-19 in lung transplant recipients: a multicenter study. Am J Transplant 2020; 21: 1816–1824. doi: 10.1111/ajt.16364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapriniotis K, Giannis D, Geropoulos G, et al. . Heart and lung transplantation in the Era of COVID-19: early recommendations and outcomes. Exp Clin Transplant 2021; in press [ 10.6002/ect.2020.0289]. [DOI] [PubMed] [Google Scholar]
- 29.International Society of Heart and Lung Transplantation (ISHLT) . SARS-CoV-2 Vaccination in Heart and Lung Transplantation: Recommendations from the ISHLT COVID-19 Task Force. https://ishlt.org/ishlt/media/Documents/COVID19_Vaccine-Recommendations_3-15-2021.pdf Date last updated: 15 March 2021. Date last accessed: 31 March 2021.
- 30.Recovery Collaborative Group . Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397: 605–612. doi: 10.1016/S0140-6736(21)00149-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saiman L, Siegel J. Infection control in cystic fibrosis. Clin Microbiol Rev 2004; 17: 57–71. doi: 10.1128/CMR.17.1.57-71.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellani C, Duff AJA, Bell SC, et al. . ECFS best practice guidelines: the 2018 revision. J Cyst Fibros 2018; 17: 153–178. doi: 10.1016/j.jcf.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 33.Nichols DP, Odem-Davis K, Cogen JD, et al. . Pulmonary outcomes associated with long-term azithromycin therapy in cystic fibrosis. Am J Respir Crit Care Med 2020; 201: 430–437. doi: 10.1164/rccm.201906-1206OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGurnaghan SJ, Weir A, Bishop J, et al. . Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol 2021; 9: 82–93. doi: 10.1016/S2213-8587(20)30405-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bridges N, Rowe R, Holt RIG. Unique challenges of cystic fibrosis-related diabetes. Diabet Med 2018; 35: 1181–1188. doi: 10.1111/dme.13652 [DOI] [PubMed] [Google Scholar]
- 36.Hasan S, Cecilia Lansang M, Salman Khan M, et al. . Managing Cystic Fibrosis related diabetes via telehealth during COVID-19 pandemic. J Clin Transl Endocrinol 2021; 23: 100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Booth A, Reed AB, Ponzo S, et al. . Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS ONE 2021; 16: e0247461. doi: 10.1371/journal.pone.0247461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peckham H, de Gruijter NM, Raine C, et al. . Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020; 11: 6317. doi: 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam GY, Goodwin J, Wilcox PG, et al. . Sex disparities in cystic fibrosis: review on the effect of female sex hormones on lung pathophysiology and outcomes. ERJ Open Res 2021; 7: 00475-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuczmarski RJ, Ogden CL, Guo SS, et al. . 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002; 246: 1–190. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00411-2021.supplement (154.6KB, pdf)