Abstract
Background
To evaluate the effectiveness of the nurse-led alcohol guidance to control home blood pressure (HBP) in the morning among male patients with hypertension during outpatient visits.
Methods
We enrolled 53 male patients with an HBP of ≥135/85 mm Hg with excessive drinking (alcohol ≥210 g/week or ≥60 g/day habitually) among outpatients in a randomized trial. Patients were assigned to a nurse-led alcohol guidance intervention or to the control. The primary outcomes were the mean HBP of 5 consecutive days at 6 months and alcohol consumption.
Results
Twenty-eight and 25 patients were randomized to intervention and control groups, respectively (mean age; 62.7 years old and 64.5, respectively). At baseline, the groups were well balanced across most characteristics. At 6 months, the mean HBP was 131/82 mm Hg in the intervention group vs. 145/87 mm Hg in the control group (SBP <0.001, DBP = 0.09). An HBP level of less than 135/85 mm Hg was achieved among 55.6% of the participants in the intervention group vs. 16.7% in the control group (P = 0.004). The alcohol consumption at 6 months was 256 ± 206 g/w vs. 413 ± 260 g/w, respectively (P = 0.020).
Conclusions
We confirmed the effectiveness of the nurse-led alcohol guidance to control the HBP in male patients with hypertension during outpatient visits.
Public trials registry number
UMIN000017454 (UMIN Clinical Trials Registry).
Keywords: alcohol guidance, blood pressure, home morning blood pressure, hypertension, outpatient
Although the treatment of hypertension is improving, the presence of poorly controlled patients remains an important issue. In the guidelines for treating hypertension (JSH 2019),1 lifestyle modification is advocated as the first step of treatment. In Japan, the National Health Promotion Movement in the 21st Century (Healthy Japan 21), nutrition (such as reduction of salt), physical activity, avoidance of excessive alcohol intake, and 10% increase in antihypertensive medication are mentioned as the 4 pillars of lifestyle improvement for hypertension as a national policy. However, in terms of alcohol drinking, there are many Japanese male patients who drink heavily due to their cultural background, such as business communication, and which often leads to poor blood pressure (BP) management. Daily alcohol intake is associated with increased BP levels, especially in the morning.2–5 A high morning BP is associated with a higher incidence of stroke independent of the mean 24-hour systolic BP level.6 Furthermore, 50% of those with a daily intake of alcohol greater than 66 g have hypertension.7,8
However, the current medical system is insufficient to provide guidance on avoidance of excessive alcohol intake to outpatients with hypertension. Physicians provide advice as part of their busy medical practice, but further intervention by health professionals is limited in Japan. One of the reasons is that health guidance by health professionals other than physicians for outpatients is not covered by the Japanese medical insurance system. The specific health guidance introduced in Japan in 2008 is effective at improving metabolic syndrome and it has become common in the preventive health-checkup system.9 However, this unique Japanese system is not applicable to those receiving medical treatment in the outpatient setting because lifestyle modification is expected as part of medical treatment, which is an issue.
The aim of this study was to investigate the effectiveness of nurse-led alcohol guidance during the outpatient visits for male hypertensive patients with drinking habits at reducing home blood pressure (HBP) in the morning and alcohol consumption.
METHODS
Recruitment
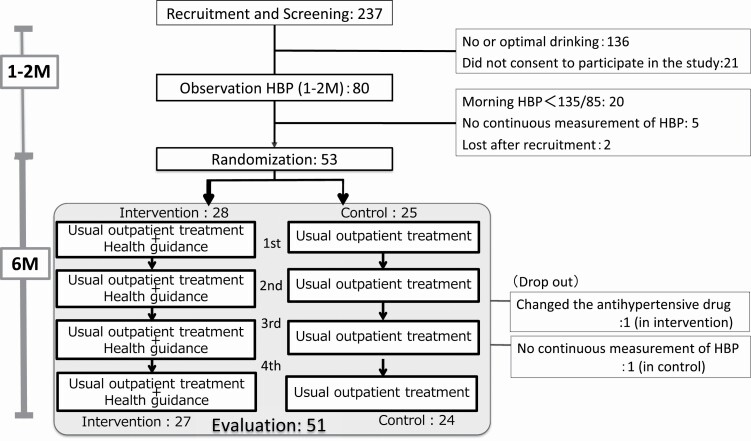
This study was an open-label, prospective, randomized controlled trial. The study flow is shown in Figure 1.
Figure 1.
Study flow and the number of participants. The medications affecting blood pressure were unchanged from recruitment to evaluation. All participants measured the HBP from recruitment until evaluation. Abbreviation: HBP, home blood pressure.
The inclusion criteria for recruitment were male patients aged 20–74 who were undergoing regular medical treatment (≥every 2 months) and had essential hypertension with habitual excessive alcohol consumption. In the recruitment process, habitual alcohol consumption was first screened among hypertensive patients who were visiting the outpatient clinic. To patients with drinking habits that met the inclusion criteria and who consented to participate in the study, we lent an electronic sphygmomanometer (OMRON EM7251G) to measure the HBP. We asked them to measure the HBP for approximately 1–2 months until the next outpatient visit. Consumption of ≥210 g/week or ≥60 g/day of alcohol at least 3–4 times a month was considered excessive.10 The final determination of eligibility for the study was based on the mean HBP of 5 consecutive days before the outpatient visit, which was required to be >135 mm Hg systolic and/or >85 mm Hg diastolic BP with or without antihypertensive medication. The HBP criteria were based on the Japanese Guidelines for the management of Hypertension (JSH 2014).11 Medications related to reducing BP were unchanged until the evaluation date. The exclusion criteria were severe BP (Stage 3 hypertension: home SBP ≥175 mm Hg or home DBP ≥105 mm Hg) and clinically diagnosed alcoholism.
Randomization
Randomization was performed by central allocation using the Research Electronic Data Capture (Red cap) system.12 Stratification criteria were age (20–59 years old or 60–74 years old), affiliation (4 medical institutions), and baseline HBP (<160/90 or ≥160/90 mm Hg).
Ethics and study registration
The present study was approved by the ethics review board of Osaka University Graduate School of Medicine (approval number 14299-2). Written informed consent was received from all participants. The study was registered to the UMIN Clinical Trials Registry as “A Randomized Controlled Trial of Saving Alcohol Amount Program for Keeping Optimal Blood Pressure Control in Patients with Hypertension (OSAKE study: UMIN000017454).”
Intervention and comparison groups
First, eligible patients were selected during the observation period, and at the next outpatient visit, they were randomly assigned to the intervention group and the observation group by central allocation using the Red Cap system. For the observation group, the patients continued to receive the usual outpatient treatment until 6 months. Regarding the intervention group, patients received health guidance aiming at optimal alcohol habits every 2 months (4 times total) for half a year by trained nurses in addition to the usual treatment. The guidance was provided face-to-face during outpatient visits. The alcohol guidance prototype is based on the short term intervention program10,13 to promote changes in drinking habits in combination with self-monitoring using a “drinking diary.” In their diaries patients recorded the type and the amount of alcohol consumed every day during the intervention period.
Outcome evaluation
HBP and office BP
The primary outcome was the HBP measured by patients using electronic sphygmomanometer (OMRON EM7251G) and recorded on the Medical Link website. Medical Link is an online BP management system of OMRON HEALTHCARE and the data measured by this sphygmomanometer are automatically transferred and stored on a dedicated server managed by OMRON HEALTHCARE using a 3G universal mobile telecommunications system. We accessed the server through the OMRON HEALTHCARE website and downloaded the data for analysis. We used the average HBP value for 5 consecutive days before the outpatient visit. Patients measured the HBP within 1 hour after waking up, after urination, before taking medicine in the morning, and after resting for 1–2 minutes in the sitting posture according to JSH 2014.11 We asked them to measure twice consecutively (1–2 minutes apart). Two measurements (the first 2 if multiple measurements were taken) were averaged and used as the HBP of that day. Office BP was measured at the outpatient visit by medical professionals.
Alcohol consumption
Alcohol consumption was measured by self-reporting using the Timeline Followback Method.14 We asked patients about alcohol consumption (situation of drinking, the type of beverage containing alcohol, and total number of drinks) for each day within the latest week of the outpatient visit using the calendar. We calculated pure alcohol from amount and concentration with the following formula: pure alcohol (g) = amount (ml) × 0.8 (specif ic gravity of alcohol) × concentration (%). The reference list we used to calculate the amount of alcohol intake at the survey venue is shown in Supplementary Table S1 online.
Other outcomes (metabolic indicators and salt intake)
We measured the following metabolic indicators and salt intake as other outcomes: body mass index, abdominal circumference, blood testing (aspartate aminotransferase [AST], alanine aminotransferase, γ-glutamyl transpeptidase [γ-GTP], fasting blood sugar, HbA1c, triglycerides, LDL-cholesterol, HDL-cholesterol, and uric acid), and salt intake by the salt check sheet.15 The other outcomes were collected at baseline and at the evaluation during the outpatient visit.
RESULTS
We screened 237 hypertensive outpatients for alcohol consumption by the interview during the outpatient visit. As a result, 101 met the inclusion criteria for excessive alcohol consumption. Among them, 80 who consented to the study were asked to measure their HBP until the next outpatient day (about 1–2 months) to assess their eligibility in the baseline period. As a result of measurement during the baseline period, 20 patients were normotensive, 5 patients did not measure the HBP continuously, and 2 patients were unable to be contacted after consenting. Thus, 53 patients were assigned.
Twenty-eight and 25 patients were randomized to intervention and control groups, respectively (mean age; 62.7 ± 8.8 years old and 64.5 ± 5.9, respectively), and 67.8% were 65–74 years old. The demographic and baseline data are listed in Table 1. The groups were well balanced at baseline across most characteristics. Of the 53 patients, 51 (96.2%) completed the trial (27 intervention and 24 control). The mean home SBP ± SD/home DBP ± SD at baseline was 142.9 ± 10.3/90.1 ± 8.3 mm Hg in the intervention group and 144.0 ± 10.2/88.2 ± 7.8 mm Hg in the control group.
Table 1.
Baseline characteristics
| Intervention group | Control group | P value | |
|---|---|---|---|
| Number of participants | 27 | 24 | |
| Age-years old, mean (SD) | 63.4 (8.9) | 64.6 (5.9) | n.s. |
| Office BP hypertension_Stage 1, n (%) | 15 (55.6) | 13 (54.2) | n.s. |
| Office BP hypertension_Stage 2, n (%) | 2 (7.4) | 2 (8.3) | n.s. |
| Home BP hypertension_Stage 1, n (%) | 17 (63.0) | 16 (66.7) | n.s. |
| Home BP hypertension_Stage 2, n (%) | 10 (37.0) | 8 (33.3) | n.s. |
| With hypertension medication, n (%) | 24 (88.9) | 24 (100.0) | n.s. |
| BMI (SD) | 25.4 (2.8) | 24.6 (3.3) | n.s. |
| Abdominal circumference (SD) | 91.6 (8.3) | 90.7 (8.3) | n.s. |
| Current smoker (%) | 5 (18.5) | 5 (20.8) | n.s. |
| Diabetes (%) | 6 (22.2) | 4 (16.7) | n.s. |
Abbreviations: BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure. Office BP hypertension_Stage 1: an office BP of 140 to 159 and/or DBP ranging from 90 to 99 mm Hg. Office BP hypertension_Stage 2: a home morning SBP of 160 to 179 mm Hg and/or DBP of 100 to 109 mm Hg. Home BP hypertension_Stage 1: a home morning SBP of 135 to 154 mm Hg and/or DBP ranging from 85 to 94 mm Hg. Home BP hypertension_Stage 2: a home morning SBP of 155 to 175 mm Hg and/or DBP of 95 to 104 mm Hg. The P values were calculated by the t-test for the continuous variables and the chi-square test for the categorical variables.
Primary outcomes
The primary outcome at month 6 (Table 2), the amount of alcohol consumed per week (mean ± SD) in the intervention group was significantly lower than that the control group; 256.3 ± 206.2 vs. 413 ± 260.1 g/w, respectively (P = 0.020). The intervention effect (the difference in mean at 6 months and 95% confidence interval) was −157.0 (−288.4 to −25.6) g/w. The number of patients with reduced alcohol consumption was 24 (88.9%) in the intervention group and 10 (44.4%) in the control group (P < 0.001).
Table 2.
Alcohol consumption outcomes
| Outcome | Intervention group | Control group | Intervention effect | P valued |
|---|---|---|---|---|
| Alcohol consumption per week: means (SD), g | ||||
| At baseline | 479.1 (352.7) | 360.4 (181.5) | n.s. | |
| At 6 months | 256.3 (206.2) | 413.3 (260.1) | −157.0 (−288.4 to −25.6)a | 0.020 |
| Change | −222.8 (344.9) | 52.9 (174.1) | −275.7 (−432.5 to −118.9)b | 0.001 |
| Patients with reduced alcohol consumption per week at 6 months, % | ||||
| Reduced from baseline | 88.9 | 37.5 | 2.4 (1.4 to 4.0)c | <0.001 |
| Optimal alcohol consumption (<210 g/w), no. (%) | 44.4 | 12.5 | 3.6 (1.1 to 11.1)c | 0.013 |
aShown is the difference in mean at 6 months and 95% confidence interval.
bShown is the difference in mean change and 95% confidence interval.
cShown is the relative risk and 95% confidence interval.
dFor blood pressure, P values were calculated by the t-test. For hypertension control at 6 months, P values were calculated by the chi-square test.
As shown in Table 3, the mean home systolic BP (SBP) ± SD in the intervention group was significantly lower than that in the control group 131.1 ± 12.4 vs. 144.8 ± 12.9 mm Hg (P < 0.001). The intervention effect was −13.7 (−20.8 to −6.6) mm Hg. The mean home DBP in the intervention group was also significantly lower than that in the control group 82.3 ± 10.1 vs. 87.3 ± 10.3 mm Hg (P < 0.001). The intervention effect was −5.0 (−10.7 to 0.8) mm Hg. The office BP at 6 months in the intervention group was 131.8 ± 12.2 vs. 139.8 ± 15.8 mm Hg in the control group (P = 0.058). The intervention effect was −8.0 (−15.9 to −0.1) mm Hg. The office diastolic BP (DBP) in the intervention group was 80.3 ± 10.0 vs. 80.8 ± 11.0 mm Hg in the control group (n.s.). The intervention effect was −0.5 (−6.4 to 5.5) mm Hg. An HBP level of less than 130/80 mm Hg was achieved in 55.6% of the participants in the intervention group vs. 16.7% in the control group (P = 0.004).
Table 3.
Blood-pressure outcomes
| Outcome | Intervention group | Control group | Intervention effect | P valued |
|---|---|---|---|---|
| Home SBP: means (SD), mm Hg | ||||
| At baseline | 142.9 (10.3) | 144.0 (10.2) | n.s. | |
| At 6 months | 131.1 (12.4) | 144.8 (12.9) | −13.7 (−20.8 to −6.6)a | <0.001 |
| Change | −11.8 (10.0) | 0.8 (12.8) | −12.6 (−19.1 to −6.2)b | <0.001 |
| Home DBP: means (SD), mm Hg | ||||
| At baseline | 90.1 (8.3) | 88.2 (7.8) | n.s. | |
| At 6 months | 82.3 (10.1) | 87.3 (10.3) | −5.0 (−10.7 to 0.8)a | 0.088 |
| Change | −7.8 (7.1) | −0.9 (7.3) | −6.9 (−11.0 to −2.9)b | 0.001 |
| Office SBP: means (SD), mm Hg | ||||
| At baseline | 140.4 (10.5) | 141.6 (14.7) | n.s. | |
| At 6 months | 131.8 (12.2) | 139.8 (15.8) | −8.0 (−15.9 to −0.1)a | 0.058 |
| Change | −8.6 (10.7) | −1.8 (16.6) | −6.8 (−14.5 to 1.0)b | 0.087 |
| Office DBP: means (SD), mm Hg | ||||
| At baseline | 84.2 (9.8) | 82.1 (6.7) | n.s. | |
| At 6 months | 80.3 (10.0) | 80.8 (11.0) | −0.5 (−6.4 to 5.5)a | n.s. |
| Change | −3.9 (9.6) | <0.001 (10.5) | −3.9 (−9.6 to 1.8)b | n.s. |
| Hypertension control at 6 months, % | ||||
| Home BP <130/80 mm Hg | 55.6 | 16.7 | 3.3 (1.3 to 8.7)c | 0.004 |
| Office BP <135/85 mm Hg | 63 | 25 | 2.5 (1.2 to 5.3)c | 0.007 |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
aShown is the difference in mean at 6 months and 95% confidence interval.
bShown is the difference in mean change and 95% confidence interval.
cShown is the relative risk and 95% confidence interval.
dFor blood pressure, P values were calculated by the t-test. For hypertension control at 6 months, P values were calculated by the chi-square test.
Metabolic indicators and salt intake
Metabolic indicators and salt intake (abdominal circumference, body mass index, γ-GTP, AST, alanine aminotransferase, uric acid, fasting blood sugar, HbA1c, triglycerides, LDL-cholesterol, HDL-cholesterol, and salt check sheet score) are listed in Table 4. There was no significant difference from baseline at 6 months. However, the mean (±SD) γ-GTP at 6 months was 63.41 ± 49.39 IU/l in the intervention group, which was within the normal range, whereas that in the control group was 81.50 ± 69.33 IU/l. The AST was 24.56 ± 10.32 in the intervention group and 29.83 ± 11.45 in the control group (P = 0.09). The intervention group had a slightly lower γ-GTP and AST (Table 4).
Table 4.
Metabolic indicators and salt intake outcomes
| Outcome: means (SD) | Intervention group | Control group | P value |
|---|---|---|---|
| BMI | |||
| At baseline | 25.46 (2.78) | 24.64 (3.28) | n.s. |
| At 6 months | 25.26 (3.01) | 24.40 (3.21) | n.s. |
| Abdominal circumference | |||
| At baseline | 91.64 (8.27) | 90.65 (8.30) | n.s. |
| At 6 months | 92.03 (8.65) | 91.88 (8.88) | n.s. |
| γ-GTP (IU) | |||
| At baseline | 86.88 (82.66) | 85.38 (71.49) | n.s. |
| At 6 months | 63.41 (49.39) | 81.50 (69.33) | n.s. |
| AST (U/l) | |||
| At baseline | 27.81 (12.69) | 31.00 (16.10) | n.s. |
| At 6 months | 24.56 (10.32) | 29.83 (11.45) | 0.09 |
| ALT (U/l) | |||
| At baseline | 25.69 (13.48) | 28.25 (16.94) | n.s. |
| At 6 months | 21.07 (8.70) | 25.75 (13.45) | n.s. |
| UA (mg/dl) | |||
| At baseline | 6.77 (1.35) | 6.60 (1.37) | n.s. |
| At 6 months | 6.76 (1.36) | 6.70 (1.26) | n.s. |
| FBS (mm Hg/dl) | |||
| At baseline | 116.50 (31.10) | 105.74 (25.76) | n.s. |
| At 6 months | 121.19 (41.94) | 111.33 (27.35) | n.s. |
| HbA1c (%) | |||
| At baseline | 5.81 (0.46) | 5.80 (0.71) | n.s. |
| At 6 months | 5.87 (0.53) | 5.83 (0.75) | n.s. |
| TG (mm Hg/dl) | |||
| At baseline | 206.08 (39.99) | 201.96 (30.60) | n.s. |
| At 6 months | 196.12 (40.19) | 204.55 (33.74) | n.s. |
| LDL-C (mm Hg/dl) | |||
| At baseline | 107.96 (31.81) | 110.51 (32.77) | n.s. |
| At 6 months | 105.00 (26.47) | 108.81 (35.70) | n.s. |
| HDL-C (mm Hg/dl) | |||
| At baseline | 62.35 (14.85) | 61.68 (13.45) | n.s. |
| At 6 months | 58.12 (13.39) | 59.08 (17.47) | n.s. |
| Salt intake (questionnaire score) | |||
| At baseline | 13.30 (4.63) | 13.00 (3.93) | n.s. |
| At 6 months | 13.41 (4.70) | 11.88 (4.14) | n.s. |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; FBS, fasting blood sugar; γ-GTP, γ-glutamyl transpeptidase; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; TG, triglycerides; UA, uric acid.
Discussion
The present study revealed that health guidance to promote alcohol reduction by nurses during outpatient treatment of male hypertensive subjects is effective for reducing the HBP and alcohol consumption after 6 months. To the best of our knowledge, the effectiveness of alcohol guidance on HBP control has not been evaluated in a randomized trial. The effects of reduced alcohol consumption on BP were clarified in several previous studies,16–21 but no studies have evaluated the effectiveness of alcohol guidance by randomized controlled trial in the outpatient setting using the HBP as an outcome. Evaluating the HBP enables the assessment of masked hypertension or white coat hypertension; therefore, we were able to clarify the effectiveness of the intervention more accurately. In particular, the morning BP, especially the morning surge, was reported to be more strongly associated with hypertensive organ damage and cardiovascular prognosis than office BP.22–25 However, HBP levels are not sufficiently controlled among approximately 60% of the patients receiving hypertensive treatment.22,26,27 As the risk of morning surge increases with increased alcohol consumption,28 it is better to assess the HBP rather than the office BP in terms of evaluation of alcohol guidance.
The HBP (±SD) decreased by SBP 11.8 ± 10.0/DBP 7.8 ± 7.1 mm Hg, the office BP decreased by SBP 8.6 ± 10.7/DBP 3.9 ± 9.6 mm Hg, and alcohol (mean ± SD) decreased by 222.8 ± 344.9 g/w in the intervention group. In a previous study among Japanese, the SBP decreased to 3.6 and DBP decreased to 1.9 mm Hg by reducing the consumption of pure alcohol by 30 ml every day for 3 weeks.29 The present study demonstrated a greater effect. Our study may have been more effective because the intervention period lasted for half a year, being longer than previous studies (mostly 1–8 weeks). Thus, our study revealed the effectiveness of the modified lifestyle of drinking, not the temporary effects of reduced alcohol consumption. In addition, we observed a greater reduction of BP because subjects had hypertension and were well motivated to change their drinking habits. Furthermore, as the baseline value was higher than normal, the reduction was more notable.
Our study results are consistent with those of Lang’s study,18 which confirmed the effectiveness of counseling by trained medical doctors on reduction of BP and alcohol consumption. However, our study had a low drop-out rate. Compared with previous studies that had a drop-out rate of 25% or skipped planned interventions, our study had a low drop-out rate (3.8%) with no absences because we utilized the outpatient visits. Thus, the present study clarified the effects of alcohol intervention for patients, including those with low motivation.
The Japanese nationwide life modification intervention system, which is for those with metabolic syndrome or borderline patients, was confirmed to be effective for the improvement of abdominal obesity and BP.30,31 Although, the specific health guidance system targets the prevention of metabolic syndrome: those who do not aware of any symptoms, that patients already being treated in the hospital or clinic are not included.9 Thus, a low intervention ratio and high drop-out ratio in Japanese health guidance system are issues because of the hassle and low motivation undergo consultation during busy daily life.32 Therefore, if the health guidance system utilizing outpatient visits at a hospital or clinic is established, it may solve those problems. Furthermore, nondrug therapy may improve the quality of life of patients and help reduce the increase in medical costs. As interventions related to alcohol consumption have been mainly performed as a part of addiction treatment in psychiatry,33 there is limited systematic health guidance in the internal medical outpatient setting focusing on alcohol reduction in terms of preventing the aggravation of lifestyle-related diseases.
There are many hypotheses regarding the mechanism of home morning antihypertensive effects by alcohol guidance. The major factors were considered to be the improvement of sympathetic nerves, sleep apnea, excretion of calcium in urine, and decrease in sodium excretion from the kidney by the decrease in BP at night achieved by reducing the alcohol amount.5 We also predicted that weight loss and reduction of salt intake accompanied by the reduced alcohol consumption will influence BP. In our study, there was no change in the salt intake in the intervention group (Table 4), but the guidance was effective at reducing BP regardless of weight loss.
Our study has several limitations. First, the actual amount of alcohol was unclear because it was self-reported. However, the credibility was high because γ-GTP, which is considered to be a direct reflection of the ingested alcohol amount, decreased to the normal in the intervention group, although not significantly. Furthermore, there was no large difference in the control group after 6 months. In contrast, regarding the HBP, we minimized the bias in self-reported data by using the online recording system for BP (Medical Link), which was a strength of our study. The second limitation was that we were unable to consider the effects of lifestyle habits other than drinking, thus there is a possibility that factors, such as exercise habits, dietary habits, stress, and working conditions, influenced the BP. Third, patients with low motivation may have declined to participate in the study, resulting in selection bias. However, the present study had a low drop-out rate, which enabled assessment of the effects of intervention for the patients regardless of their motivation levels.
Taken together, we confirmed the effectiveness of the nurse-led life modification intervention focused on reducing alcohol intake to control the HBP in male patients with hypertension as a method of nonpharmacological treatment during outpatient visits. Future investigations are needed to clarify the life-long effects of the intervention for reducing BP. This study suggested the need to develop a medical system for implementing effective health guidance intervention in the outpatient setting by health professionals.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all study participants and the medical staff at the study site. We would like to thank Dr Kayo Godai, Dr Eri Kiyoshige, Ms Eriko Kojiya, Ms Yasuka Sawayama, Mr Atusto Hirose, and the members of the Health Science Division, Osaka University Graduate School of Medicine for their assistance in conducting this study. The valuable help of Ms Yumiko Aoshima and Ms Tae Matsue for their secretarial work are gratefully acknowledged. We are grateful to Dr Akira Okayama and Prof. Haruo Sugiyama for the special advice and supports, Dr Takefumi Yuzuriha and Dr Hitoshi Maesato for their technical advice about alcohol guidance.
FUNDING
This study was supported by a research grant from the Japan Heart Foundation, the Japanese Society of Cardiovascular Disease Prevention and AstraZeneca K.K. (K.M.), and research aid from the blood pressure management research group supported by Omron Healthcare (K.M.).
DISCLOSURE
The authors declared no conflict of interest.
References
- 1. Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, Ito M, Ito S, Iwashima Y, Kai H, Kamide K, Kanno Y, Kashihara N, Kawano Y, Kikuchi T, Kitamura K, Kitazono T, Kohara K, Kudo M, Kumagai H, Matsumura K, Matsuura H, Miura K, Mukoyama M, Nakamura S, Ohkubo T, Ohya Y, Okura T, Rakugi H, Saitoh S, Shibata H, Shimosawa T, Suzuki H, Takahashi S, Tamura K, Tomiyama H, Tsuchihashi T, Ueda S, Uehara Y, Urata H, Hirawa N. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res 2019; 42:1235–1481. [DOI] [PubMed] [Google Scholar]
- 2. Ishikawa J, Kario K, Eguchi K, Morinari M, Hoshide S, Ishikawa S, Shimada K; J-MORE Group . Regular alcohol drinking is a determinant of masked morning hypertension detected by home blood pressure monitoring in medicated hypertensive patients with well-controlled clinic blood pressure: the Jichi Morning Hypertension Research (J-MORE) study. Hypertens Res 2006; 29:679–686. [DOI] [PubMed] [Google Scholar]
- 3. Ueshima H, Ozawa H, Baba S, Nakamoto Y, Omae T, Shimamoto T, Komachi Y. Alcohol drinking and high blood pressure: data from a 1980 national cardiovascular survey of Japan. J Clin Epidemiol 1992; 45:667–673. [DOI] [PubMed] [Google Scholar]
- 4. Ohira T, Tanigawa T, Tabata M, Imano H, Kitamura A, Kiyama M, Sato S, Okamura T, Cui R, Koike KA, Shimamoto T, Iso H. Effects of habitual alcohol intake on ambulatory blood pressure, heart rate, and its variability among Japanese men. Hypertension 2009; 53:13–19. [DOI] [PubMed] [Google Scholar]
- 5. Husain K, Ansari RA, Ferder L. Alcohol-induced hypertension: mechanism and prevention. World J Cardiol 2014; 6:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 7. Dyer AR, Stamler J, Paul O, Berkson DM, Shekelle RB, Lepper MH, McKean H, Lindberg HA, Garside D, Tokich T. Alcohol, cardiovascular risk factors and mortality: the Chicago experience. Circulation 1981; 64:III20–III27. [PubMed] [Google Scholar]
- 8. Curtis AB, James SA, Strogatz DS, Raghunathan TE, Harlow S. Alcohol consumption and changes in blood pressure among African Americans. The Pitt County Study. Am J Epidemiol 1997; 146:727–733. [DOI] [PubMed] [Google Scholar]
- 9. Matsuzawa Y. Specific health guidance, the nationwide lifestyle intervention program targeting metabolic syndrome, seems to be successful in Japan. J Atheroscler Thromb 2018; 25:304–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito C, Yuzuriha T, Noda T, Ojima T, Hiro H, Higuchi S. Brief intervention in the workplace for heavy drinkers: a randomized clinical trial in Japan. Alcohol Alcohol 2015; 50:157–163. [DOI] [PubMed] [Google Scholar]
- 11. Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S; Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension . The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014; 37:253–390. [DOI] [PubMed] [Google Scholar]
- 12. Patridge EF, Bardyn TP. Research Electronic Data Capture (REDCap). J Med Libr Assoc 2018; 106:142–144. [Google Scholar]
- 13. Bien TH, Miller WR, Tonigan JS. Brief interventions for alcohol problems: a review. Addiction 1993; 88:315–335. [DOI] [PubMed] [Google Scholar]
- 14. Sobell L, Sobell M. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In Litten RZ, Allen JP (eds), Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press: Totowa, NJ, 1992, pp. 41–72. [Google Scholar]
- 15. Yasutake K, Miyoshi E, Kajiyama T, Umeki Y, Misumi Y, Horita N, Murata Y, Ohe K, Enjoji M, Tsuchihashi T. Comparison of a salt check sheet with 24-h urinary salt excretion measurement in local residents. Hypertens Res 2016; 39:879–885. [DOI] [PubMed] [Google Scholar]
- 16. Kawano Y, Abe H, Takishita S, Omae T. Effects of alcohol restriction on 24-hour ambulatory blood pressure in Japanese men with hypertension. Am J Med 1998; 105:307–311. [DOI] [PubMed] [Google Scholar]
- 17. Klatsky AL, Friedman GD, Siegelaub AB, Gérard MJ. Alcohol consumption and blood pressure. N Engl J Med 1977; 296:1194–1200. [DOI] [PubMed] [Google Scholar]
- 18. Lang T, Nicaud V, Darné B, Rueff B. Improving hypertension control among excessive alcohol drinkers: a randomised controlled trial in France. The WALPA Group. J Epidemiol Community Health 1995; 49:610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maheswaran R, Beevers M, Beevers DG. Effectiveness of advice to reduce alcohol consumption in hypertensive patients. Hypertension 1992; 19:79–84. [DOI] [PubMed] [Google Scholar]
- 20. Roerecke M, Kaczorowski J, Tobe SW, Gmel G, Hasan OSM, Rehm J. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta-analysis. Lancet Public Health 2017; 2:e108–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soardo G, Donnini D, Varutti R, Milocco C, Basan L, Esposito W, Casaccio D, Isola M, Soldano F, Sechi LA. Effects of alcohol withdrawal on blood pressure in hypertensive heavy drinkers. J Hypertens 2006; 24:1493–1498. [DOI] [PubMed] [Google Scholar]
- 22. Ohkubo T, Imai Y, Tsuji I, Nagai K, Kato J, Kikuchi N, Nishiyama A, Aihara A, Sekino M, Kikuya M, Ito S, Satoh H, Hisamichi S. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens 1998; 16:971–975. [DOI] [PubMed] [Google Scholar]
- 23. Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet 2014; 384:45–52. [DOI] [PubMed] [Google Scholar]
- 24. Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension 2006; 47:149–154. [DOI] [PubMed] [Google Scholar]
- 25. Tsunoda S, Kawano Y, Horio T, Okuda N, Takishita S. Relationship between home blood pressure and longitudinal changes in target organ damage in treated hypertensive patients. Hypertens Res 2002; 25:167–173. [DOI] [PubMed] [Google Scholar]
- 26. Mallick S, Kanthety R, Rahman M. Home blood pressure monitoring in clinical practice: a review. Am J Med 2009; 122:803–810. [DOI] [PubMed] [Google Scholar]
- 27. Ohkubo T, Obara T, Funahashi J, Kikuya M, Asayama K, Metoki H, Oikawa T, Takahashi H, Hashimoto J, Totsune K, Imai Y; J-HOME Study Group . Control of blood pressure as measured at home and office, and comparison with physicians’ assessment of control among treated hypertensive patients in Japan: first report of the Japan Home versus Office Blood Pressure Measurement Evaluation (J-HOME) study. Hypertens Res 2004; 27:755–763. [DOI] [PubMed] [Google Scholar]
- 28. Nakashita M, Ohkubo T, Hara A, Metoki H, Kikuya M, Hirose T, Tsubota-Utsugi M, Asayama K, Inoue R, Kanno A, Obara T, Hoshi H, Totsune K, Satoh H, Imai Y. Influence of alcohol intake on circadian blood pressure variation in Japanese men: the Ohasama study. Am J Hypertens 2009; 22:1171–1176. [DOI] [PubMed] [Google Scholar]
- 29. Ueshima H, Mikawa K, Baba S, Sasaki S, Ozawa H, Tsushima M, Kawaguchi A, Omae T, Katayama Y, Kayamori Y. Effect of reduced alcohol consumption on blood pressure in untreated hypertensive men. Hypertension 1993; 21:248–252. [DOI] [PubMed] [Google Scholar]
- 30. Nakao YM, Miyamoto Y, Ueshima K, Nakao K, Nakai M, Nishimura K, Yasuno S, Hosoda K, Ogawa Y, Itoh H, Ogawa H, Kangawa K, Nakao K. Effectiveness of nationwide screening and lifestyle intervention for abdominal obesity and cardiometabolic risks in Japan: the metabolic syndrome and comprehensive lifestyle intervention study on nationwide database in Japan (MetS ACTION-J study). PLoS One 2018; 13:e0190862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muramoto A, Matsushita M, Kato A, Yamamoto N, Koike G, Nakamura M, Numata T, Tamakoshi A, Tsushita K. Three percent weight reduction is the minimum requirement to improve health hazards in obese and overweight people in Japan. Obes Res Clin Pract 2014; 8:e466–e475. [DOI] [PubMed] [Google Scholar]
- 32. Egawa Ki, Haruyama Y. Specific health checkups and counseling guidance in Japan: their progress and challenges. Jpn J Health Educ Promot 2016; 24:43–51. [Google Scholar]
- 33. Osaki Y, Kinjo A, Higuchi S, Matsumoto H, Yuzuriha T, Horie Y, Kimura M, Kanda H, Yoshimoto H. Prevalence and trends in alcohol dependence and alcohol use disorders in Japanese adults; results from periodical nationwide surveys. Alcohol Alcohol 2016; 51:465–473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.