Abstract
In higher plants, whole-genome duplication (WGD) is thought to facilitate the evolution of C4 photosynthesis from C3 photosynthesis. To understand this issue, we used new and existing leaf-development transcriptomes to construct two coding sequence databases for C4Gynandropsis gynandra and C3Tarenaya hassleriana, which shared a WGD before their divergence. We compared duplicated genes in the two species and found that the WGD contributed to four aspects of the evolution of C4 photosynthesis in G. gynandra. First, G. gynandra has retained the duplicates of ALAAT (alanine aminotransferase) and GOGAT (glutamine oxoglutarate aminotransferase) for nitrogen recycling to establish a photorespiratory CO2 pump in bundle sheath (BS) cells for increasing photosynthesis efficiency, suggesting that G. gynandra experienced a C3–C4 intermediate stage during the C4 evolution. Second, G. gynandra has retained almost all known vein-development-related paralogous genes derived from the WGD event, likely contributing to the high vein complexity of G. gynandra. Third, the WGD facilitated the evolution of C4 enzyme genes and their recruitment into the C4 pathway. Fourth, several genes encoding photosystem I proteins were derived from the WGD and are upregulated in G. gynandra, likely enabling the NADH dehydrogenase-like complex to produce extra ATPs for the C4 CO2 concentration mechanism. Thus, the WGD apparently played an enabler role in the evolution of C4 photosynthesis in G. gynandra. Importantly, an ALAAT duplicate became highly expressed in BS cells in G. gynandra, facilitating nitrogen recycling and transition to the C4 cycle. This study revealed how WDG may facilitate C4 photosynthesis evolution.
Keywords: C4 photosynthesis, C4 evolution, whole-genome duplication, comparative genomics
Introduction
Kranz anatomy is a distinctive structure of C4 leaves in which the vein is wrapped around by one inner layer of bundle sheath (BS) cells and then one outer layer of mesophyll (M) cells (Hatch 1987). This structure has allowed the evolution of a CO2 concentration mechanism (CCM) that transports CO2 from M to BS cells for its final fixation through the Calvin cycle in BS cells (Hatch 1987). Compared with C3 plants, this unique structure coupled with a high vein density in leaves confers C4 plants a superior photosynthesis efficiency with increased tolerance to high light, heat, and drought. The Kranz anatomy with the special CCM is a well-known example of convergent evolution in plant evolution, which has occurred in over 60 plant lineages (Sage et al. 2011).
Increased leaf vein density and development of Kranz leaf anatomy have been considered an early primary step in C4 evolution (Sinha and Kellogg 1996; Gowik and Westhoff 2011; Christin et al. 2013). From a comparative study of the transcriptomes of developing leaves in C3Tarenaya hassleriana and C4Gynandropsis gynandra, we proposed that elevated auxin biosynthesis and transport are responsible for the development of high vein density in C4 leaves (Huang et al. 2017).
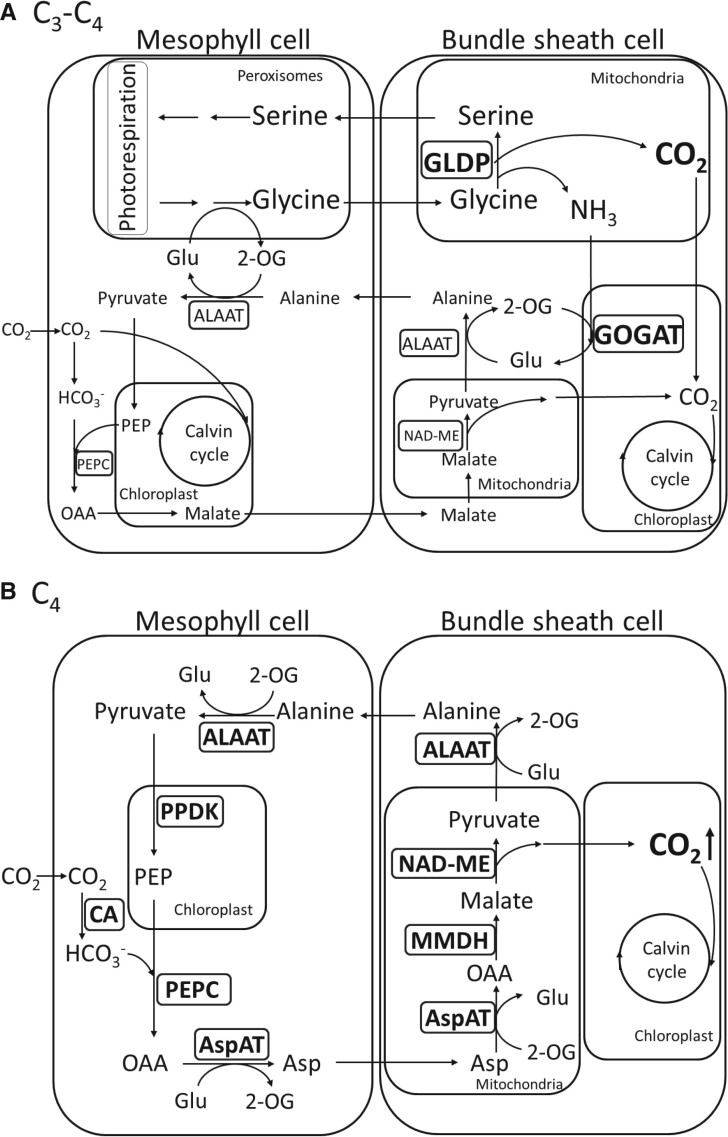
Various models, including anatomical, physiological, phylogenetic, and computational modeling, predicted that confinement of photorespiration in BS cells, to raise CO2 concentration at the site of Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase) in BS cells, provides an important intermediate step in C3 to C4 evolution (Monson 1999; Bauwe 2011; Heckmann et al. 2013; Williams et al. 2013; Mallmann et al. 2014). Rubisco is the key enzyme of photosynthetic carboxylation reaction via the Calvin–Benson or C3 cycle. However, photorespiration, also known as the oxidative photosynthetic carbon cycle, may occur simultaneously with carboxylation on Rubisco, leading to CO2 release in mitochondria and thus causing loss of fixed carbon (Sharkey 1988). The key enzyme for releasing fixed CO2 in mitochondria is glycine decarboxylase (GDC) (see supplementary table S1, Supplementary Material online for gene name abbreviations and functions) comprising the P-, L-, T-, and H-proteins in mitochondria. The GDC P-protein (GLDP) is the decarboxylase that catalyzes the decarboxylation of glycine to release CO2 (Oliver and Raman 1995). In C3 species, CO2 released from photorespiration in M cells diffuses out of the leaf, thus reducing net photosynthesis with a high CO2 compensation point. In C3–C4 intermediate species, glycine derived from photorespiration serves as a CO2 carrier and is shuttled from M to BS cells where GLDP decarboxylates glycine to release CO2 for refixation, which is called the photorespiratory CO2 pump in C3–C4 plants (fig. 1) (Hylton et al. 1988). Due to the elevated CO2 concentration in BS cells, carboxylation by BS Rubisco is favored over oxidation, leading to reduced photorespiratory CO2 loss and an overall increase of photosynthesis efficiency. At the same time, a basic C4 cycle is recruited to prevent nitrogen imbalance created by the photorespiratory CO2 pump in C3–C4 plants (fig. 1) (Mallmann et al. 2014).
Fig. 1.
Comparison of the photosynthesis pathways at the C3–C4 intermediate stage and the C4 stage. (A) At the C3–C4 intermediate stage, a photorespiratory CO2 pump is established for transferring CO2 from M cells to BS cells. At this stage, C4 enzymes may be recruited to establish a basic C4 cycle to balance nitrogen metabolism. Glycine derived from photorespiration is shuttled from M cells to BS cells. Then, the BS-restricted GLDP decarboxylates glycine to release CO2 and ammonia in BS cells. The increased CO2 concentration enhances the carboxylation of BS-Rubisco and reduces photorespiratory CO2 loss. BS-ammonia is refixed to alanine by GOGAT (glutamine oxoglutarate aminotransferase) and ALAAT (alanine aminotransferase) and alanine is shuttled from BS cells to M cells, which sustains the photorespiratory activity in peroxisome. (B) At the C4 stage, C4 enzymes involved in the NAD-ME subtype C4 photosynthesis are upregulated in expression to enhance the concentration of CO2 in BS cells. Because Rubisco is restricted in BS cells at the C4 stage, the increased CO2 concentration limits photorespiration activity, making the photorespiratory CO2 pump obsolete. The pathway in (A) was modified from the model of Mallmann et al. (2014). Glu, glutamate; 2-OG, 2-oxoglutarate; PEP, phosphoenolpyruvate; OAA, oxaloacetate; Asp, aspartic acid; AspAT, aspartate aminotransferase.
At the final step of C4 evolution, C4 enzyme genes are upregulated and recruited to participate in the operation of C4 cycle between well differentiated M and BS cells (fig. 1). After the establishment of the C4 cycle, the C4-type CCM replaces the photorespiratory CO2 pump to concentrate CO2 in the BS cells of C4 plants (fig. 1).
Compared with C3 photosynthesis, C4 photosynthesis requires two extra ATPs to drive the CCM for each CO2 molecule fixed, and the cyclic electron flow (CEF) around photosystem I (PSI) was predicted to contribute the additional ATPs required (Munekage et al. 2004). Two distinct CEF pathways, NADH dehydrogenase-like (NDH) complex- and ferredoxin: plastoquinone oxidoreductase (FQR)-dependent flows, have been identified in C3 plants (Munekage et al. 2002; Ifuku et al. 2011). Both pathways transfer excited electrons to the cytochrome b6f (Cyt b6f) complex, which pumps protons into the thylakoid space (Wikstrom et al. 1981). The pumped protons contribute to the electrochemical proton gradient across the thylakoid membrane of chloroplasts, which is then used to drive ATP synthesis.
Gene duplication, either single or whole-genome duplication (WGD), has been proposed to be a prerequisite for C4 evolution (Monson 2003) because it provides extra gene copies to reduce selective constraint and to acquire beneficial morphological or biochemical modifications (Panchy et al. 2016). A previous study showed that a photorespiratory GLDP experienced duplication in ancestral C3Flaveria species. One copy of the GLDP duplicates became preferentially expressed in BS cells and helped to establish the photorespiratory CO2 pump in Flaveria C3–C4 intermediate species (Schulze et al. 2013). In addition, several C4 enzyme genes, including PEPC, PPDK, NADP-ME, NADP-MDH, and CA, have undergone duplication in the ancestral C3Flaveria plants. One copy of each C4 gene duplicate pair was subsequently upregulated and modified for organelle-, cell-, and organ-specific expression to support the CCM of the NADP-ME subtype C4 photosynthesis in Flaveria (Monson 2003).
Cleomaceae, a sister family of Brassicaceae, has undergone the evolution of C4 photosynthesis at least three times (Bayat et al. 2018). Cleomaceae and Brassicaceae shared two common ancient WGD events, namely the β and γ WGD events (Barker et al. 2009). In addition, both lineages underwent an independent WGD event after their divergence, called Th-α in the Cleomaceae lineage and At-α in the Arabidopsis lineage (Barker et al. 2009). The At-α and Th-α WGD events were estimated to occur ∼34 and ∼20 Ma (million years ago), respectively (Bayat et al. 2018), whereas At-β occurred ∼170–235 Ma (Bowers et al. 2003). Because C4 photosynthesis in G. gynandra evolved after the Th-α WGD event (van den Bergh et al. 2014), it provides a good model for studying the role of a WGD event in the evolution of C4 photosynthesis. Also, it may help explore why C4 photosynthesis did not evolve in T. hassleriana, although it shared the Th-α WGD event.
In this study, we first used PacBio Iso-Seq to obtain long transcripts of G. gynandra genes to assemble a CDS (coding sequence) data set and we then added the de novo CDSs assembled from the Illumina RNA-seq reads (Huang et al. 2017) to complement the PacBio Iso-Seq data. We also constructed a T. hassleriana CDS data set from the previously predicted CDSs of T. hassleriana (Cheng et al. 2013) and the de novo CDSs assembled from Illumina RNA-seq reads (Huang et al. 2017). After constructing two CDS databases for the two species, we used the RNA-seq reads of six leaf-development stages (S0–S5) deposited at NCBI (Külahoglu et al. 2014) to calculate expression levels of the CDSs. (S0 was the stage with leaves ∼2 mm in length. Then, every two consecutive stages were separated by 2 days. The leaves initiated secondary vein formation at S1 and fully developed by S4 and S5.) The data were then used to identify paralogs, date the duplication time, and calculate the ratio of the nonsynonymous substitution rate to the synonymous substitution rate (i.e., the KA/KS ratio). Using these data, we explored the relationship between the Th-α WGD event and the evolution of C4 photosynthesis in G. gynandra. This study revealed that the Th-α WGD event played a crucial role in the evolution of C4 photosynthesis in G. gynandra, including anatomical and biochemical modifications. Also, this study provides insights into why C4 photosynthesis failed to evolve in T. hassleriana, although it shared the Th-α event with G. gynandra.
Results
cDNA Assembly
The PacBio sequencing of the G. gynandra cDNA library produced 662,111 raw reads, with an average length of 1,854 bp (supplementary table S2, Supplementary Material online). Our CDS assembly procedure is illustrated in supplementary figure S1, Supplementary Material online, and explained in detail in Materials and Methods. After the pipeline processing involving circular consensus sequencing (CCS), read classification, transcript clustering and polishing using IsoSeq3, we obtained 39,766 polished high-quality (HQ) isoforms (supplementary table S2, Supplementary Material online). Then, we combined the PacBio and Illumina RNA-seq transcript data set to obtain a G. gynandra CDS data set containing 21,345 ORFs, including 15,431 full-length CDSs (supplementary data set S1a, Supplementary Material online). The number of CDS sequences inferred from PacBio reads alone was 1,454, that from Illumina reads alone was 9,325 and that from both PacBio and Illumina reads was 10,566; the total number was 21,345. In T. hassleriana only Illumina reads were available, and we obtained a CDS database that contains 27,500 CDSs including 21,162 full-length CDSs (supplementary data set S1b, Supplementary Material online). Fewer CDSs and full-length CDSs were inferred in G. gynandra than in T. hassleriana, because the genome of G. gynandra has not yet been sequenced, making it more difficult to infer coding sequences in G. gynandra.
Identification of Duplicate Genes Derived from the Th-α WGD Event
To identify the duplicate (paralogous) genes in a species, we used reciprocal BLASTp searches with E ≦ 1.0e-05. In T. hassleriana and G. gynandra, the set of duplicate genes thus obtained includes duplicate genes derived from not only the Th-α WGD event but also the Th-β WGD event. These two groups of duplicate genes may be separated by using the KS values between homologous genes, where KS is the number of synonymous substitutions per synonymous site. Barker et al. (2009) estimated that in T. hassleriana, the median KS value for duplicate genes derived from the Th-α WGD (denoted as “Th-α median KS”) is 0.41 (0 < KS < 1.1) and Th-β median KS = 1.68 (1.1 ≤ KS < 2.1). Thus, we used KS = 1.1 to divide the paralogs of T. hassleriana (G. gynandra) into a group of Th-α paralogs with KS < 1.1 and a group of Th-β paralogs with 1.1 ≤ KS < 2.1. The criterion of KS < 1.1 assumes that there is no Th-α paralog pair in T. hassleriana and G. gynandra that has KS >1.1. It will be seen that this assumption holds well when we consider the distribution of KS values in the next subsection. By this separation criterion, we obtained for T. hassleriana 6,787 duplicate-gene pairs derived from the Th-α WGD and 295 pairs derived from the Th-β WGD (supplementary data set S2, Supplementary Material online). The corresponding values for G. gynandra are 3,454 and 224 pairs (supplementary data set S2, Supplementary Material online). Note that in both species, the Th-α duplicate-gene pairs also include duplicate-gene pairs derived from non-WGD duplications. This issue will be discussed in the next subsection.
Distribution of KS Values between Paralogous Genes in a Genome
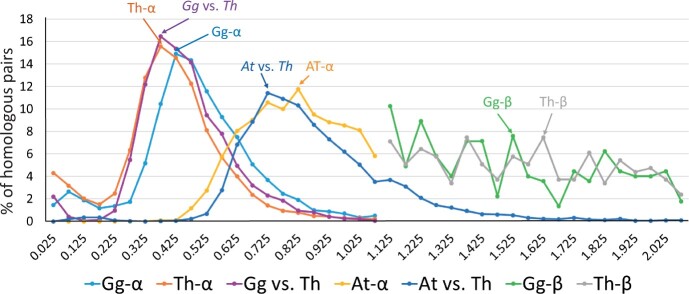
The KS value between two homologous genes is usually not strongly affected by natural selection and thus can give a sense of the divergence time between the two genes. We therefore computed the KS values between paralogous genes in G. gynandra and in T. hassleriana. For this purpose, we used the KaKsAnalysis tool of PlantTribes 2 (produced by the AssemblyPostProcessor) in PAML (Yang 2007), which gives not only KS but also KA (number of nonsynonymous substitutions per nonsynonymous site). As our CDSs for G. gynandra were constructed solely from leaf transcriptomes, for both G. gynandra and T. hassleriana, we used only genes that are expressed in leaves. Here, a gene is said to be expressed if its RPKM (reads per kilobase per million mapped reads) is ≧1 in the RNA-seq data (Külahoglu et al. 2014); we identified 3,745 and 7,197 sets of expressed paralogous pairs in G. gynandra and T. hassleriana, respectively (supplementary data sets S1 and S2, Supplementary Material online). Figure 2 shows the distribution of KS values for the paralog pairs in T. hassleriana and also the distribution in G. gynandra; the KS values are given in supplementary data set S2, Supplementary Material online. Note that the two distributions are very similar to each other with a slight shift of the former distribution to the left; a two-sample Kolmogorov–Smirnov test found no significant difference between the two KS distributions (P value = 0.28). Note further that the two distribution curves decrease to almost 0 when KS = 1.025. Thus, our assumption that there is no Th-α paralog pair with KS >1.1 in G. gynandra and T. hassleriana holds approximately. From the two distributions we obtained Th-α median KS =0.41 and Gg-α median KS = 0.48, which agree well with the estimate of Th-α median KS = 0.41 in T. hassleriana by Barker et al. (2009).
Fig. 2.
Distributions of KS values between paralogs or between orthologs. Th-α (Gg-α) refers to the distribution of KS values between paralogs in Tarenaya hassleriana (Gynandropsis gynandra) derived from the Th-α WGD event; the paralogs actually also include non-WGD duplicates. At-α refers to the distribution of KS values between paralogs in Arabidopsis thaliana derived from the At-α WGD event. “Gg versus Th” refers to the distribution of KS values between G. gynandra and T. hassleriana single copy genes. “At versus Th” refers to the distribution of KS values between A. thaliana and T. hassleriana single copy genes. Th-β (Gg-β) refers to the distribution of KS values between paralogs in T. hassleriana (G. gynandra) derived from the Th-β WGD event. The number (n) of gene pairs used: Gg-α: n = 3,454 paralogous pairs; Th-α: n = 6,787 paralogous pairs; Gg versus Th: n = 5,840 orthologous pairs of single-copy genes; At-α: n = 2,113 paralogous pairs; Gg-β: n = 224 paralogous pairs; Th-β: n = 295 paralogous pairs; At versus Th: n = 3,064 orthologous pairs of single copy genes.
We also computed the KS values of orthologous genes between T. hassleriana and G. gynandra. For simplicity, we considered only single-copy orthologs in the two species (supplementary data set S2, Supplementary Material online), which were identified using the OrthoFinder tool (Emms and Kelly 2019). Figure 2 shows that the distribution of KS values between G. gynandra and T. hassleriana single-copy genes is very close to the distributions of KS values for the Th-α paralog pairs in G. gynandra and T. hassleriana. This implies that the speciation between G. gynandra and T. hassleriana occurred soon after the Th-α WGD event.
Figure 2 also includes the distributions of KS values between paralogs in T. hassleriana and in G. gynandra derived from the Th-β WGD event. We estimated Th-β median KS = 1.54 and Gg-β median KS = 1.48, which are somewhat smaller than Barker et al.’s estimate of Th-β median KS = 1.68.
The groups of Th-α and Gg-α paralogs likely included a substantial number of non-WGD duplicates as indicated by a bump in the distribution curve in the end region with KS < 0.2 (fig. 2). In Arabidopsis thaliana, we identified 3,009 duplicate pairs with Ks ≦ 1.1, using the KaKsAnalysis tool of PlantTribes 2 in PAML (Yang 2007). Among them we found 896 pairs in the set of non-WGD (tandem) duplicate pairs and the remaining 2,113 pairs in the set of At-α WGD duplicate pairs in Wang et al. (2013). A bump in the end region with KS <0.55 is seen in the distribution of KS values for the pool of non-WGD and At-α WGD duplicate pairs (3,009 pairs), when compared with the distribution of KS values for the At-α WGD duplicate pairs (2,113 pairs) alone (supplementary fig. S2, Supplementary Material online). As mentioned above, the Th-α WGD and the At-α WGD were estimated to occur 20 and 34 Ma, respectively. If we assume that non-WGD occurs at a constant rate in T. hassleriana, then the number of non-WGD duplicates that occurred after the Th-α WGD is estimated to be (20/34) × 896 = 527. In T. hassleriana, the pool of non-WDG and Th-α WGD duplicate pairs is found to be 6,787. Thus, the proportion of non-WGD duplicate pairs in the pool of non-WGD and Th-α WGD duplicate pairs (527/6,787 = 0.08) in T. hassleriana is considerably smaller than that in A. thaliana (896/3,009 = 0.30). For this reason, in T. hassleriana the contribution of non-WGD duplicate pairs to the pool of non-WGD and Th-α WGD duplicate pairs is likely less than 10%. This may explain why the distribution curve denoted by Th-α in figure 2, which includes both non-WGD and Th-α WGD duplicate pairs, is much sharper than that for At-α, although the latter includes only At-α WGD duplicate pairs. However, in principle, in the absence of recent non-WGD duplicates, the two distribution curves for Th-α and Gg-α should decrease to almost 0 when KS becomes smaller than 0.2 as in the distribution of KS values between G. gynandra and T. hassleriana single-copy genes (fig. 2). Therefore, we may assume that most or nearly all paralogs with KS < 0.2 in G. gynandra and T. hassleriana were derived from non-WGD duplications.
Figure 2 also includes the distribution of KS values between duplicate genes in A. thaliana derived from the At-α WGD event. This distribution is some distance from the left of the distribution of KS values for the paralog pairs in G. gynandra, suggesting that the At-α WGD occurred considerably earlier than the Th-α WGD. It is, however, close to the distribution of KS values between A. thaliana and T. hassleriana single-copy genes, suggesting that the At-α WGD event occurred soon after the divergence between the A. thaliana and the T. hassleriana lineage.
Duplicates of C4-Related Genes in G. gynandra and T. hassleriana
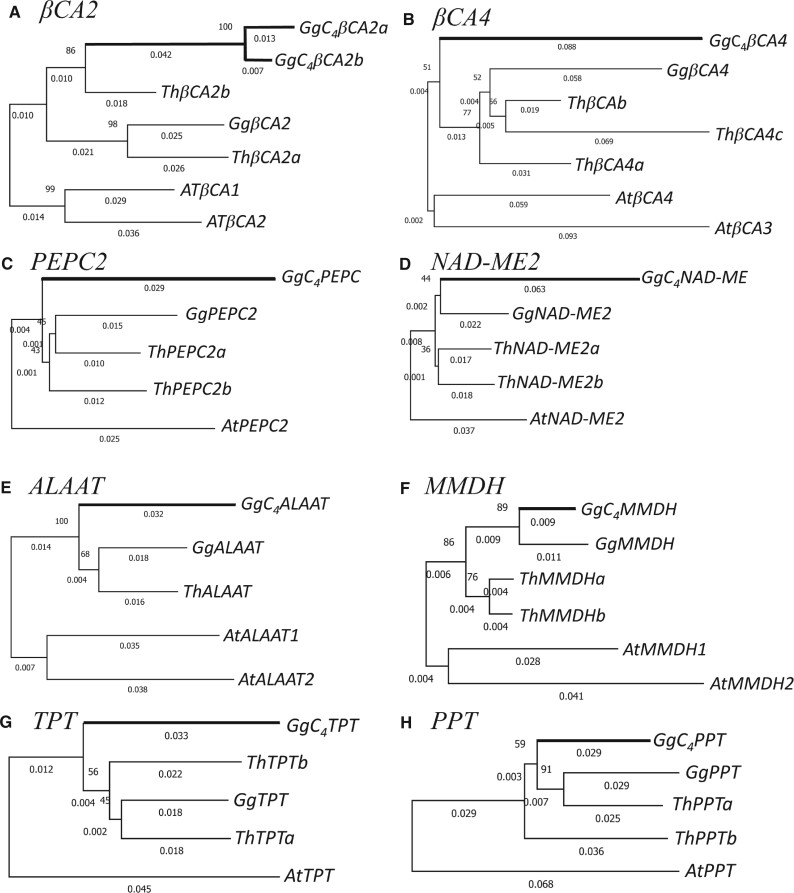
In Flaveria (yellowtops), several C4 enzyme genes, including PEPC, PPDK, NADP-ME, NADP-MDH, and CA, have undergone duplication in the ancestral C3 plants and subsequent neofunctionalization in C4 photosynthesis of Flaveria species (NADP-ME subtype) with higher expression levels (Monson 2003). In G. gynandra, a C4 plant belonging to the NAD-ME subtype C4 plants, we found eight genes (βCA2, βCA4, PEPC2, NAD-ME2, ALAAT, MMDH, TPT, and PPT) in the C4 photosynthesis pathway that have at least two copies in G. gynandra (fig. 3); the first six genes encode enzymes whereas the last two encode triophospate and PEP transporters. For all eight genes, the paralog pairs in both G. gynandra and T. hassleriana have KS values smaller than 0.85 (supplementary table S3, Supplementary Material online), which is considerably smaller than the median value (1.48) for the paralog pairs derived from the Th-β WGD (fig. 2). Thus, none of these paralog pairs was derived from the Th-β WGD. Below, we investigate whether these paralogs were derived from the Th-α WGD.
Fig. 3.
NJ trees of C4 enzyme and transporter genes in Gynandropsis gynandra and their homologs in Tarenaya hassleriana constructed using nonsynonymous substitutions and using Arabidopsis thaliana orthologous genes as the outgroup. (A) βCA2, (B) βCA4, (C) PEPC2, (D) NAD-ME2, (E) ALAAT2, (E) MMDH1, (G) TPT, and (H) PPT. Thick branches indicate C4 enzyme or transporter genes. Bootstrap percentage values are shown as integers on the left sides of branching nodes. The length of a branch is shown as the number of nonsynonymous substitutions per nonsynonymous site below the branch.
Figure 3 shows the neighbor-joining (NJ) trees based on nonsynonymous substitutions for the eight genes in G. gynandra and T. hassleriana, with the homologous genes in A. thaliana as the outgroup. Figure 3A shows the NJ tree for the βCA2 genes. Note that GgC4βCA2a and GgC4βCA2b are closely related with a KS value of only 0.10 (supplementary table S3, Supplementary Material online), which is much smaller than 0.48, the Gg-α median KS, suggesting that these two genes were derived from a recent non-WGD duplication. Their common ancestor and ThβCA2b form an ortholog pair supported by a bootstrap value of 86% (fig. 3A). Moreover, GgβCA2 and ThβCA2a form another ortholog pair supported by a bootstrap value of 98%. Thus, GgβCA2 and the common ancestor of GgC4βCA2a and GgC4βCA2b were apparently derived from the Th-α WGD and so were ThβCA2a and ThβCA2b. This conclusion is supported by the maximum-likelihood (ML) trees based on nucleotide and amino acid sequences (supplementary fig. S3, Supplementary Material online). The KS value between GgC4βCA2a (Gg C4βCA2b) and GgβCA2 is 0.57 (0.58), which is larger than the Gg-α median KS (0.48). The KS value between ThβCA2a and ThβCA2b is 0.42, which is almost the same as the Th-α median KS (0.41). Thus, the KS values support the view that the above paralog pairs were derived from the Th-α WGD.
Figure 3E shows the NJ tree for the ALAAT genes. It is identical in topology to both of the ML trees in supplementary fig. S3, Supplementary Material online, though it is different from the NJ tree based on synonymous substitutions. The single ALAAT gene in T. hassleriana is clustered with GgALAAT in three of the four trees (fig. 2 and supplementary fig. S3, Supplementary Material online), so it is likely the ortholog of GgALAAT. The KS value between GgC4ALAAT and GgALAAT is 0.68, which is larger than the Th-α median of 0.41, so it is reasonable to assume that they were derived from the Th-α WGD rather than from a non-WGD duplication.
Figure 3B shows the NJ tree for the CA4 genes. The phylogenetic positions of the three βCA4 genes in T. hassleriana differ among the four trees in figure 3B and supplementary figure S3, Supplementary Material online. However, as the KA (0.05) and KS (0.45) values between ThβCA4a and ThβCA4b are smaller than those between ThβCA4a and ThβCA4c (0.11 and 0.55) and between ThβCA4b and ThβCA4c (0.09 and 0.60), we may assume that ThβCA4a and ThβCA4b were derived from a more recent duplication than the duplication that produced their common ancestor and ThβCA4c, which was likely derived from the Th-α WGD because the KS values between ThβCA4a and ThβCA4b (0.55 and 0.60) are larger than the Th-α median (0.41). Similarly, as the KS value between GgβCA4 and GgC4βCA4 (0.67) is larger than the Gg-α median (0.48), we assume that the two genes were derived from the Th-α WGD.
Like the case of CA4 genes, although the phylogenetic trees for the other five genes in figure 3 give no clear evidence that the two paralogs in G. gynandra were derived from the Th-α WGD, this view is supported by the KS values (supplementary table S3, Supplementary Material online). However, the case of MMDH genes is less certain. In this case, we obtained only a partial CDS assembly of 540 bp (the first 180 codons) for one of the two GgMMDH paralogs of G. gynandra. For this reason, the tree in figure 3F was constructed using partial sequences. In this tree, GgC4MMDH and GgMMDH form a pair and so do MMDHa and ThMMDHa, providing no support for the assumption of being derived from the Th-α WGD. However, the KS value between ThMMDHa and ThMMDHa is 0.33, so they were likely derived from the Th-α WGD. In comparison, the KS value between GgC4MMDH and GgMMDH is only 0.22, substantially smaller than the Gg-α median (0.48). Thus, it is uncertain whether they were derived from the Th-α WGD. However, note that the assumption of “being derived from the Th-α WGD” is more parsimonious than that of “being derived from a non-WGD duplication” because the latter requires two additional events: 1) loss of a Th-α WGD duplicate and 2) gain of a non-WGD duplicate.
In summary, we propose that all of the eight C4-related genes studied, with the possible exception of MMDH, have retained the Th-α WGD duplicates in G. gynandra and all of them, except for ALAAT, have retained the Th-α WGD duplicates in T. hassleriana.
Upregulation of C4-Related Genes in G. gynandra
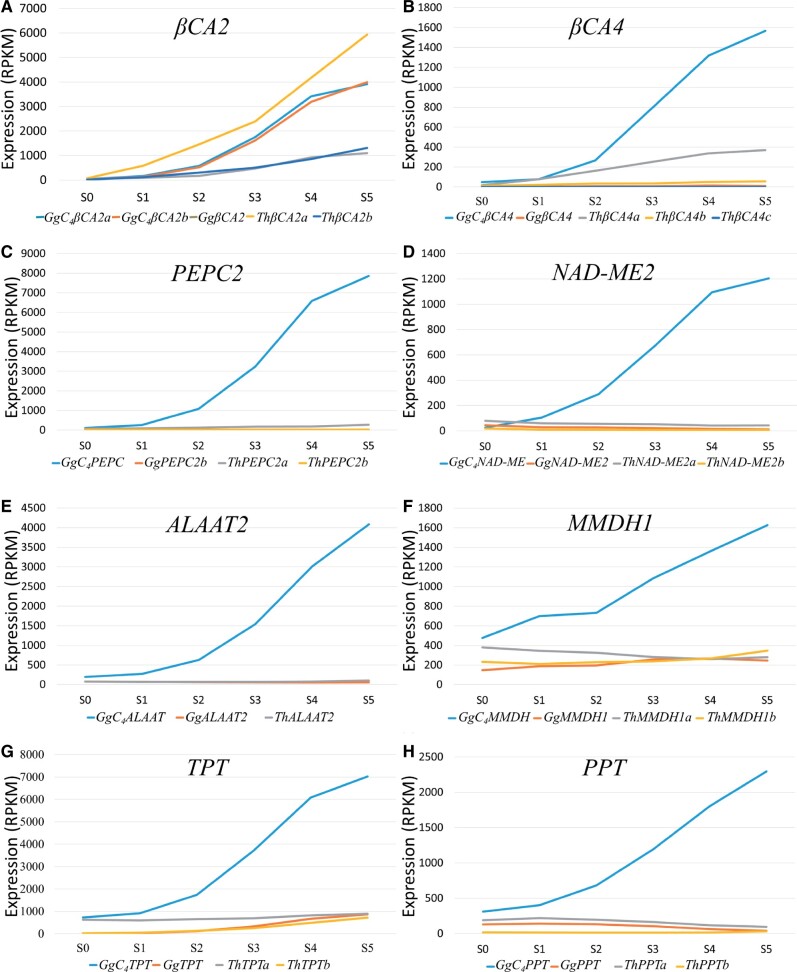
The first critical step of the C4 photosynthesis pathway is the conversion of gaseous CO2 to HCO3- in the cytosol of M cells. This reaction is catalyzed by CA (carbonic anhydrase), and HCO3- serves as the substrate for PEPC (phosphoenolpyruvate carboxylase). In G. gynandra, βCA2 and βCA4 are expressed at a much higher level in M cells than in BS cells, suggesting that βCA2 and βCA4 were recruited in C4 photosynthesis (Williams et al. 2016). We predict that AtβCA2, GgβCA2a and ThβCA2a contain a chloroplast transit peptide of 30 amino acids at the first N-terminal by LOCALIZER (Sperschneider et al. 2017), iPSORT (Bannai et al. 2002), and ProtComp 9.0 (a commercial program from Softberry Inc.). This prediction suggests that these proteins are transported to the chloroplast. In contrast, GgC4βCA2a, GgC4βCA2b, and ThβCA2b do not possess the chloroplast transit peptide, suggesting that these βCA2s are expressed in the cytoplasm, and GgC4βCA2a and GgC4βCA2b are much upregulated compared with ThβCA2b (fig. 4A). Note that the C4 form of βCA in C4Flaveria bidentis was also found to have lost the chloroplast transit peptide and showed an increased expression in the cytosol (Tanz et al. 2009). Therefore, we suggest that GgC4βCA2a and GgC4βCA2b were involved in the evolution of C4 photosynthesis in G. gynandra.
Fig. 4.
The expression levels (RPKMs) of C4 enzyme and transporter genes and their paralogs at six leaf developmental stages (S0–S5). (A) βCA2, (B) βCA4, (C) PEPC2, (D) NAD-ME2, (E) ALAAT2, (E) MMDH1, (G) TPT, and (H) PPT. The C4 enzyme and transporter genes are shown in blue lines.
In the case of PEPC paralogs, we found that the upregulated PEPC2, called GgC4PEPC, in G. gynandra has the C4-type-specific alanine-to-serine change at codon 780 (Christin et al. 2007). In contrast, the other paralogous PEPC is without the alanine-to-serine change and maintains low expression levels in both species (fig. 4C). These data suggest that the upregulated GgC4PEPC was involved in the C4 evolution in G. gynandra.
Note that GgC4βCA4a, GgC4βCA4b, GgC4NAD-ME, GgC4ALAAT, and GgC4MMDH are also upregulated in G. gynandra (fig. 4).
To test whether the above C4 genes underwent positive selection after duplication, we compared the KA/KS ratios between paralogous and orthologous genes (supplementary table S3, Supplementary Material online). Although the KA/KS values of the C4 paralogs and orthologs were all smaller than 1, the C4 candidates βCA2, βCA4, PEPC2, NAD-ME2, and ALAAT showed higher nonsynonymous rates than the other paralogous and orthologous genes (supplementary table S3, Supplementary Material online). So, the above C4 enzymes have evolved faster and are expressed dramatically higher in C4G. gynandra than the corresponding homologous genes in C3T. hassleriana (fig. 4). Compared with the other Th-α paralogous genes in both species, the C4 enzyme genes have evolved faster than other paralogous non-C4 genes (fig. 3 and supplementary table S3, Supplementary Material online). These observations suggest that the C4 enzyme genes underwent positive selection in C4G. gynandra.
Unlike the enzymes, the C4 cycle transporters, including TPT, PPT, BASS2, and DIC1, have similar nonsynonymous substitution rates in the two species (supplementary table S3, Supplementary Material online). Importantly, however, one TPT paralog and one PPT paralog are dramatically upregulated in G. gynandra (fig. 4G and H). This observation suggests that these newly evolved transporters are specifically recruited and upregulated for supporting the rapid metabolite shuttles required for the C4 pathway.
Duplicates of Photorespiration Genes in G. gynandra and T. hassleriana
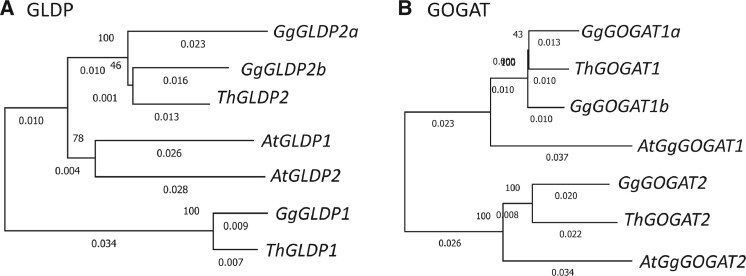
The GLDP catalyzes the decarboxylation of glycine to release CO2 in mitochondria (Oliver and Raman 1995). A previous study inferred that the GLDP gene duplicates facilitated the establishment of a photorespiratory CO2 pump in C3–C4 intermediate Flaveria species (Schulze et al. 2013). In G. gynandra, there are three GLDP genes: GLDP1, GLDP2a, and GLDP2b (fig. 5A). The KS value between GLDP2a and GLDP2b is 0.46 (supplementary table S3, Supplementary Material online), so they were appparently derived from the Th-α WGD event. The KS values between the GLDP1 gene and the GLDP2a and GLDP2b genes are 1.54 and 1.57, respectively, so GLDP1 and the common ancestor of GLDP2a and GLDP2b were apparently derived from the Th-β WGD event. In T. hassleriana, there are only two GLDP genes (GLDP1 and GLDP2) (fig. 5A) and as the KS value between them is 1.48, they were apparently derived from the Th-β WGD event. Both of these two genes should have been duplicated at the Th-α WGD event, but only one copy of both genes is now found in T. hassleriana, suggesting loss of a duplicate copy for both genes after the Th-α WGD. In both G. gynandra and T. hassleriana, the RPKM value of GLDP1 is over 8-fold higher than that of GLDP2 (supplementary fig. S4, Supplementary Material online). This suggests that GLDP1 plays a major role in photorespiration by decarboxylation of glycine in the BS cells of G. gynandra.
Fig. 5.
NJ trees of of GLDPs and GOGATs. The trees were constructed using synonymous substitutions. The length of a branch is shown as the number of synonymous substitutions per synonymous site below the branch.
ALAAT and GOGAT are involved in critical nitrogen balance for establishing a photorespiratory CO2 pump at the C3–C4 intermediate stage (Mallmann et al. 2014). As mentioned above, ALAAT has retained the two Th-α duplicates in G. gynandra, though only one copy is retained in T. hassleriana (fig. 3E). There are three GOGAT genes in G. gynandra: GgGOGAT1a, GgGOGAT1b, and GgGOGAT2 (fig. 5B). The KS value between GgGOGAT1a and GgGOGAT1b is 0.42, so they were likely derived from the Th-α WGD. The KS values between GgGOGAT2 and GgGOGAT1a (GgGOGAT1b) is 1.30 (1.37) (supplementary table S3, Supplementary Material online), so GgGOGAT2 and the common ancestor of GgGOGAT1a and GgGOGAT1b were apparently derived from the Th-β WGD. In T. hassleriana, only two GOGAT genes are found (fig. 5B) and the KS value between them is 1.41 (supplementary table S3, Supplementary Material online), so they were likely derived from the Th-β WGD. Thus, T. hassleriana has apparently lost one of two Th-α duplicates.
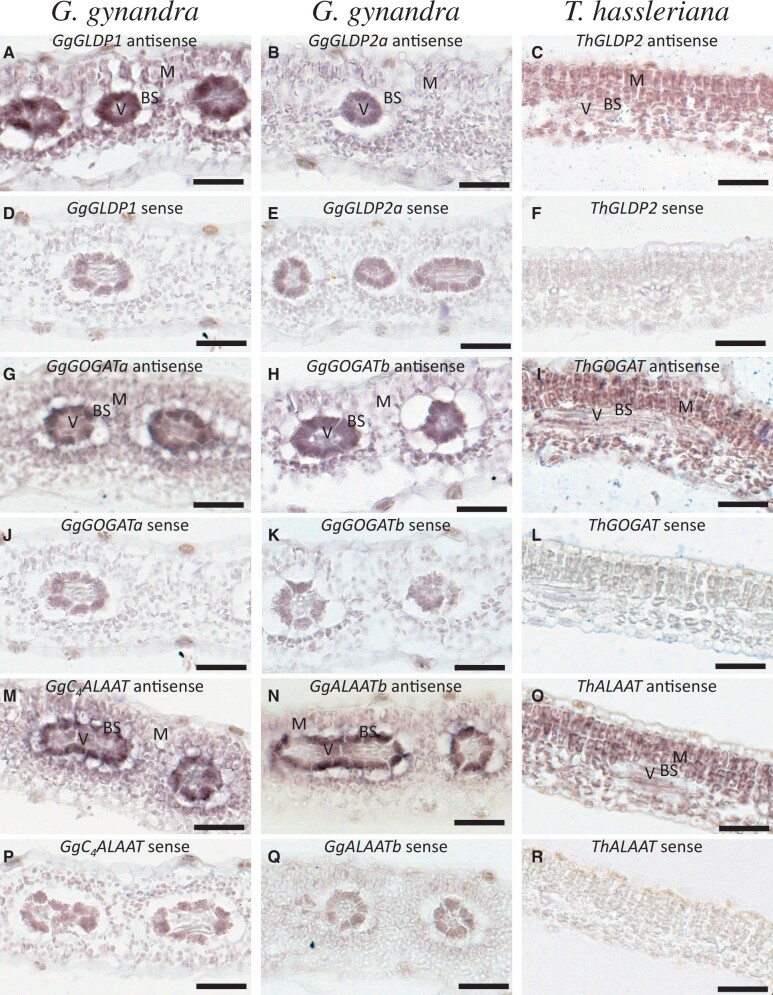
Our RNA in situ hybridization experiments showed that the GLDP and GOGAT in T. hassleriana, called ThGLDP2 (fig. 6C) and ThGOGAT (fig. 6I), are expressed in BS and M cells, respectively. ThALAAT in T. hassleriana is specifically expressed in M cells (fig. 6O). In G. gynandra, one GLDP paralog, GgGLDP1 (fig. 6A), is mainly expressed in BS cells whereas the other GLDP paralogs, called GgGLDP2a (fig. 6B) and GgGLDP2b (data not shown), are expressed at very low levels in mature leaves. Both GOGAT paralogs in G. gynandra, named GgGOGATa (fig. 6G) and GgGOGATb (fig. 6H), are restricted to express in BS cells. Different from GgGLDP1 and the two GgGOGAT paralogs, the C4-type ALAAT in G. gynandra, called GgC4ALAAT, is expressed in both BS and M cells (fig. 5M). This is not surprising because GgC4ALAAT catalyzes the reversible transfer of an amino group from glutamate to pyruvate, forming alanine and 2-oxoglutarate (2OG) in C4 cycle, to shuttle alanine from BS to M cells in the NAD-ME subtype C4G. gynandra (fig. 1). Consistent with this specific C4 mechanism, the other ALAAT paralog, called GgALAATb (fig. 6N), is mainly expressed in the BS cells in G. gynandra for conversion of pyruvate, a product of MAD-ME, to alanine. Alanine shuttled back to M cells is, in turn, converted back to pyruvate in the cytosol and then to PEP by PPDK in the chloroplast.
Fig. 6.
In situ hybridization of GLDP2, GOGAT, and ALAAT in Gynandropsis gynandra and Tarenaya hassleriana mature leaves. (A) GgGLDP1antisense, (B) GgGLDP2a antisense, (C) ThGLDP1 antisense, (D) GgGLDP1 sense, (E) GgGLDP2a sense, (F) ThGLDP1 sense, (G) GgGOGATa antisense, (H) GgGOGATb antisense, (I) ThGOGAT antisense, (J) GgGOGATa sense, (K) GgGOGATb sense, (L) ThGOGAT sense, (M) GgC4ALAAT antisense, (N) GgALAATb antisense, (O) ThALAAT antisense, (P) GgC4ALAAT sense, (Q) GgALAATb sense, and (R) ThALAAT sense. mRNA expression indicated by dark intensity. Bars: 50 μm.
From these observations, we suggest that GgGLDP1, GgGOGATa, GgGOGATb, and GgC4ALAAT were recruited to establish a photorespiratory CO2 pump in the BS cells at the C3–C4 intermediate stage during the C4 photosynthesis evolution of G. gynandra. Later, the upregulated C4CA, PEPC, NAD-MDH, and NAD-ME replaced the photorespiratory CO2 pump mechanism to concentrate CO2 in the BS cells, leading to downregulation of the photorespiration genes in G. gynandra after it evolved C4 photosynthesis. Notably, because GgC4ALAAT is also involved in pyruvate-alanine shuttling in the NAD-ME subtype C4 photosynthesis, its expression level, compared with ThALAAT, is dramatically upregulated not only in BS but also in M cells. We suggest that the GgALAAT duplication played a key role for G. gynandra to enter the C3–C4 intermediate stage during the C4 photosynthesis evolution from C3 photosynthesis.
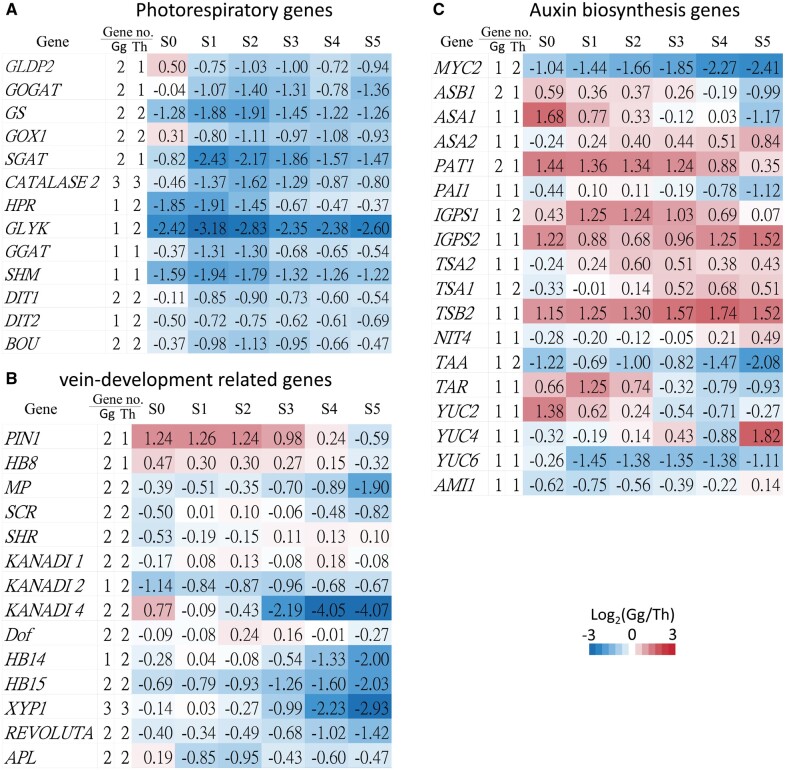
Not only GLDP and GOGAT but also several other photorespiration genes underwent duplication and retained paralogs in both species. Because the elevated CO2 concentration in BS cells of C4 plants minimizes photorespiration (Sage 2001), those photorespiration genes are repressed in C4G. gynandra (fig. 7A).
Fig. 7.
Expression ratios of genes and numbers of paralogous genes involved in (A) photorespiration, (B) vein development, and (C) auxin biosynthesis. The first column shows gene names. The second and third columns show paralogous gene numbers in Gynandropsis gynandra and Tarenaya hassleriana. The fourth to ninth columns show gene expression ratios between G. gynandra and T. hassleriana. S0–S5 denote the six leaf developmental stages. The color bar indicates the fold differences (log2 ratios) in gene expression between G. gynandra and T. hassleriana. Gene no.: gene number.
Duplicates of Other Photosynthesis Genes
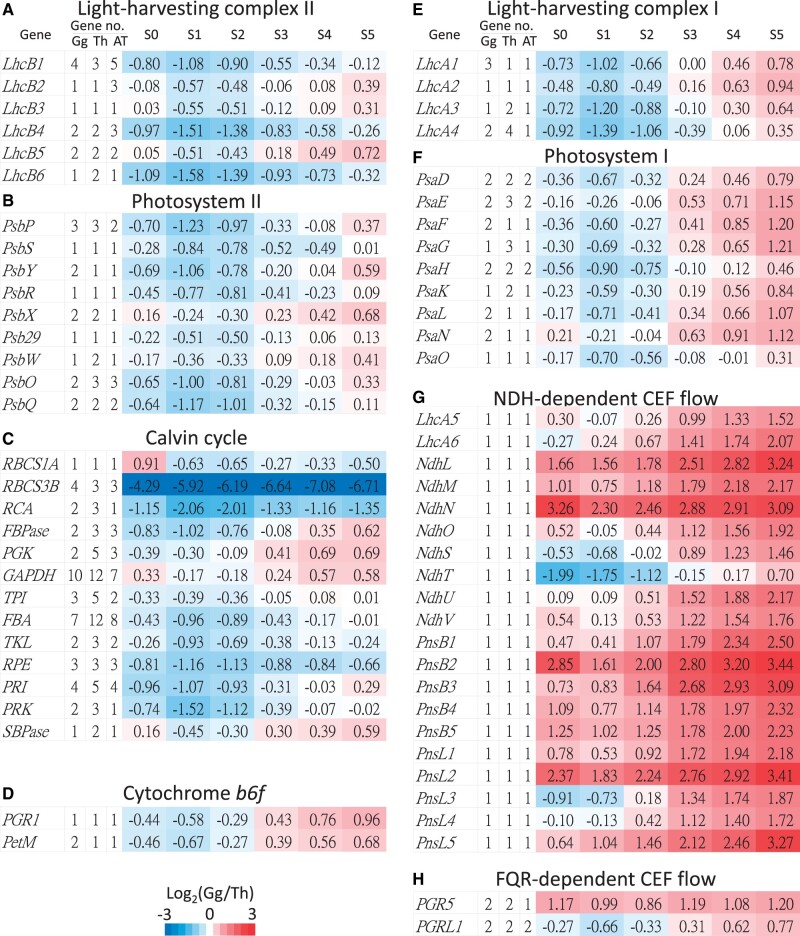
In plant photosynthesis, the CEF in PSI can create a proton gradient by the cytochrome b6f (Cyt b6f) complex to produce ATPs without production of NADPH (Munekage et al. 2004). Thus, CEF probably contributes to the additional ATPs required for the CCM in C4 photosynthesis. The two CEF pathways in C3 plants are the NDH- and FQR-dependent flows, and the NDH-dependent flow has been suggested to play a central role in C4 photosynthesis (Kubicki et al. 1996; Takabayashi et al. 2005; Ishikawa et al. 2016). Consistent with this notion, our transcriptome analysis revealed that the genes encoding the subunits of the NDH complex are also dramatically upregulated in G. gynandra (fig. 8G). Interestingly, these genes have retained only one copy in G. gynandra, T. hassleriana, and A. thaliana. The reason is unclear, but it could be to maintain a balanced gene dosage. In addition, the expression levels of genes participating in the Cyt b6f complex and FQR-dependent CEF flow are also up-regulated in G. gynandra (fig. 8D and H), presumably to boost ATP production. Especially, PetM in the Cyt b6f complex and PGR5 in the FQR-dependent CEF flow exhibit upregulation of the Th-α paralogs in G. gynandra.
Fig. 8.
Expression ratios of genes and paralogous gene numbers involved in photosynthesis. (A) Light-harvesting complex II. (B) PSII. (C) Calvin cycle. (D) Cytochrome b6f complex. (E) Light-harvesting complex I. (F) PSI. (G) NDH-dependent CEF flow. (H) FQR-dependent CEF flow. In each panel, the first column shows the gene names. The second to fourth columns show paralogous gene numbers in Gynandropsis gynandra, Tarenaya hassleriana, and Arabidopsis thaliana. The fifth to tenth columns show the expression ratios of genes between G. gynandra and T. hassleriana. The color bar indicates the fold differences (log2 ratios) in gene expression between G. gynandra and T. hassleriana at the same stage. Gene no.: gene number.
The NDH complex is associated with PSI by two linker proteins, Lhca5 and Lhca6, to form the NDH–PSI supercomplex for stabilizing the NDH complex (Peng et al. 2009; Peng and Shikanai 2011). Our transcriptome data showed that the PSI and PSI light-harvesting complex (LHCI) genes in G. gynandra display 2 and 1.5 times higher expression levels than in T. hassleriana at the mature leaf stage (S5) (fig. 8E and F). On the other hand, most of the genes encoding photosystem II (PSII), PSII light-harvesting complex (LHCII), and Calvin cycle are expressed at the same or only slightly higher levels in G. gynandra than in T. hassleriana (fig. 8A–C).
A previous study on the evolution of photosynthesis genes in Glycine max, Medicago truncatula, and A. thaliana found that PSI, PSII, and LHC genes retain more duplicates derived from WGD but not from single gene duplication because single gene duplication may cause dosage imbalance (Coate et al. 2011). Gynandropsis gynandra and T. hassleriana have retained several Th-α paralogs in PSI, PSII, Cyt b6f, LHCI, LHCII, and Calvin cycle, but these genes show different expression patterns (fig. 8). Several genes in Cyt b6f, PSI, and LHCI derived from the Th-α WGD show increased expression in G. gynandra compared with T. hassleriana. Although several Th-α paralogs in PSII, LHCII, and Calvin cycle are retained, most paralogs still maintain similar expression levels in both of these C3 and C4 species. Therefore, the observation of Th-α paralogs showing increased expression levels in PSI is consistent with the view that upregulated subunits of the NDH complex is to stabilize the NDH complex. Additionally, LHCI subunits play a crucial role for light harvesting in PSI–LHCI supercomplex (Bressan et al. 2016). We suggest that the Th-α paralogs with upregulation in LHCI contribute to increased absorption of photons by the PSI–LHCI supercomplex to transfer more excitation energy to CEF. Then, the upregulated Cyt b6f complex could transfer more electrons to increase proton pumping by enhanced CEF due to upregulated NDH- and FQR-dependent flows, which produce extra ATPs for CCM in the C4G. gynandra.
Duplicates of Leaf Vein Development-Related Genes
Examining the Th-α duplicate gene pairs in auxin biosynthesis pathways, we found that IGPS and TSA1 have retained duplicates in T. hassleriana, whereas ASB1 and PAT1 have retained duplicates in G. gynandra (fig. 7C). In G. gynandra, both ASB1and PAT1 are upregulated over 1.5 and 2.5 times during early leaf development (S0), compared with T. hassleriana (fig. 7C). We also found that MYC2, a negative regulator of auxin biosynthesis (Dombrecht et al. 2007), has retained two duplicated genes in T. hassleriana but only one copy in G. gynandra, leading to a higher expression of MYC2 in T. hassleriana than G. gynandra. In our previous study, a lower MYC2 expression in G. gynandra resulted in higher auxin biosynthesis than in T. hassleriana (Huang et al. 2017), supporting our hypothesis that increased auxin level and transport is required for developing high vein density, an important feature of Kranz leaf anatomy in C4 plants.
We also investigated genes involved in vein development (Huang et al. 2017) in the two species. In both species, almost all known vein-development-related genes have retained their duplicates, including MONOPTEROS, Homeobox gene 15 (HB15), HB14, HB8, SHR, SCR, PIN1, REVOLUTA, XYP1, APL, Dof-type zinc finger (AT2G28510), KANADI1 (KAN1), KAN2, and KAN4 (fig. 7B). Most of these genes are expressed at similar levels in the two species during early leaf development (S0–S2, fig. 7B). The prevailing retention of the vein-development-related duplicated genes probably has contributed to vein complexity in the two species, that is, septenary order venation in G. gynandra and senary order venation in T. hassleriana whereas only quinary order venation in A. thaliana (Huang et al. 2017). Additionally, the early vein-development-related genes, HB8 and PIN1, have retained duplicated copies and are expressed at higher levels in G. gynandra (fig. 7B). Thus, it might be the additional copies and the increased expression level of PIN1 and HB8, combined with low expression of MYC2, that have led to the higher vein density in G. gynandra than in T. hassleriana.
Discussion
Gene duplication, either whole genome or single gene duplication, is considered a precondition for C4 evolution (Sage 2004). Several C4 enzyme genes, including PEPC, PPDK, NADP-ME, NADP-MDH, and CA have been duplicated and then became involved in the evolution of C4 photosynthesis in the genus Flavera (Monson 2003). Although gene duplication has long been thought to be important in C4 photosynthesis evolution, the focus so far has been on the modification of C4 enzyme genes. Our study suggests that the most recent WGD, Th-α, in the Cleomaceae played an important role not only in C4 cycle formation but also in vein patterning and the establishment of a photorespiratory CO2 pump at the C3–C4 intermediate stage.
During C4 evolution at the C3–C4 intermediate stage, restriction of the GLDP expression to BS cells was an important step to establish a photorespiratory CO2 pump after GLDP duplication in Flaveria (Hylton et al. 1988; Schulze et al. 2016). Our study showed that not only GLDP but also GOGAT and ALAAT have retained Th-α WGD paralogs in G. gynandra, a C4 plant with NAD-ME subtype pathway. Importantly, RNA in situ hybridization experiments showed that GgGLDP1, GgGOGATa, and GgGOGATb are restricted to express in BS cells. Moreover, GgC4ALAAT is also restricted to express in BS cells. Therefore, we suggest that the Th-α WGD facilitated the establishment of a photorespiratory CO2 pump at the C3–C4 intermediate stage and the maintenance of nitrogen balance during C4 photosynthesis formation in G. gynandra.
Tarenaya hassleriana has retained ThGLDP paralogs but lost ThGOGAT and ThALAAT duplicates. RNA in situ hybridization experiments showed that ThGLDP1 and ThGOGAT are expressed in both BS and M cells, and ThALAAT is expressed only in M cells. Thus, T. hassleriana failed to establish a photorespiratory CO2 pump. We suggest that losses of ThALAAT and ThGOGAT paralogs were the reason why T. hassleriana failed to evolve into the C3–C4 intermediate stage.
A previous analysis of the sorghum (the NADP-ME subtype C4 plant) genome concluded that the WGD duplicated copies of PEPC and NADP-ME have been preserved, whereas other C4 enzyme gene paralogs were probably lost (Wang et al. 2009). In this study, we found six or five genes in G. gynandra and five genes in T. hassleriana encoding C4 cycle enzymes have retained duplicated copies after the Th-α WGD (fig. 3). Although the C4 cycle genes that were duplicated, including βCA2, βCA4, PEPC2, NAD-ME2, and ALAAT, showed no case of KA/KS>1, they showed higher nonsynonymous rates than their paralogous and orthologous genes. Thus, it is possible that these C4 cycle enzymes only need to change some important amino acids, such as the specific alanine-to-serine transition in C4 PEPC (Bailey and Elkan 1994), to alter their catalytic properties for functioning in the C4 cycle after gene duplication. However, the duplicates of the four C4 cycle transport genes, BASS2, DIC1, PTP, and PPT, have similar nonsynonymous substitution rates between their homologs (supplementary table S3, Supplementary Material online), probably because the transporters only function in moving C4 cycle intermediates but not in enzyme reaction. Importantly, the predicted C4 cycle genes are dramatically upregulated in G. gynandra (fig. 4). Thus, it is possible that these C4 cycle genes underwent a short period of positive selection and increased their expression levels for recruitment into the C4 cycle after the Th-α WGD event.
CEF has been suggested to generate additional ATPs for the C4 CCM (Munekage et al. 2004). Two distinct CEF pathways, NDH- and FQR-dependent flows, have been identified in C3 plants. Both pathways transfer electrons to the Cyt b6f complex, creating a greater proton gradient across the thylakoid membrane of chloroplasts, which is then used to drive ATP synthesis. The NDH-dependent flow was shown to play a role in C4 photosynthesis (Takabayashi et al. 2005; Ishikawa et al. 2016), and in Flaveria both pathways showed higher activities in C4 species than in C3 species (Nakamura et al. 2013). Our study also finds that both pathways, especially the NDH-dependent flow, are upregulated in C4G. gynandra (fig. 8G and H). In addition, the genes encoding Cyt b6f complex subunits are upregulated in G. gynandra (fig. 8D), which may allow the Cyt b6f complex to accept more electrons to create additional ATPs for the C4 cycle. The association of the NDH complex with PSI through Lhca5 and Lhca6 to form the NDH–PSI supercomplex (Peng et al. 2009; Peng and Shikanai 2011) may enhance the CEF function. We also found that the genes encoding PSI and LHCI are upregulated in G. gynandra (fig. 8E and F), which may enhance CEF in G. gynandra. Compared with the expression levels of photosynthesis genes in both species, the genes encoding the Cyt b6f complex, LHCI, PSI, and CEF proteins are upregulated in G. gynandra (fig. 8D–H), whereas the genes encoding PSII, LHCII, and Calvin cycle proteins are not. Therefore, the CEF-associated complex might play an important role in generating extra ATPs in C4G. gynandra.
The chloroplast NDH complex is encoded by 11 subunit genes in the plastid genome and 20 subunit genes in the nuclear genome (Shikanai 2016). Interestingly, there are no Th-α paralogs for any of the 20 nuclear-encoded subunits in both species and A. thaliana, suggesting that there was no advantage for retaining the duplicates for these genes. In contrast, several genes participating in photosynthesis have retained their Th-α duplicates. Moreover, those paralogous genes encoding the Cyt b6f complex subunits, LHCI, PSI, and FQR-dependent flow exhibit higher expression levels in G. gynandra than in T. hassleriana (fig. 8D–F and H). It seems that the Th-α WGD facilitated the upregulation of CEF to capture more protons and produce additional ATPs for the C4 photosynthesis CCM.
Finally, we found that almost all known vein-development-related genes have retained their duplicates after the Th-α WGD (fig. 7B). We suggest that the vein-development-related gene duplicates have contributed to more complex leaf venation architecture and probably differentiation of M and BS cells in G. gynandra (septenary orders) and T. hassleriana (senary orders) than in A. thaliana (quinary orders) (Huang et al. 2017). Unlike the vein-development-related genes, only a few genes in auxin biosynthesis pathways have retained duplicates, but most of these genes are upregulated in G. gynandra (fig. 7C). It might be that only some steps in the auxin biosynthesis pathway may be rate limiting and require higher dosages of the biosynthetic enzymes to boost the formation of high vein density in C4 leaves. Interestingly, MYC2, a negative regulator of tryptophan biosynthesis, has lost its duplicate in G. gynandra, leading to a low expression level in G. gynandra and thus a higher level of auxin biosynthesis for vein development. In addition, an important auxin transport gene, PIN1, and an early vein-development related gene, HB8, have retained duplicates in G. gynandra but not in T. hassleriana, resulting in higher PIN1 and HB8 expression levels in G. gynandra. Thus, the low expression of the negative regulator MYC2 for auxin biosynthesis coupled with the additional copies and the increased expression levels of PIN1 and HB8 in G. gynandra together have likely contributed to the high vein density in the leaves of G. gynandra (Huang et al. 2017).
In summary, our study has provided evidence that the Th-α WGD facilitated the C4 photosynthesis evolution in G. gynandra, including the early step of increased vein density, the establishment of a photorespiratory CO2 pump at the C3–C4 intermediate stage, the recruitment of C4 cycle genes in C4 cycle and upregulated CEF-associated complex for production of extra energy for the C4 cycle. Although C3T. hassleriana shared the Th-α WGD with G. gynandra, it has stayed at the anatomical preconditioning stage of increased vein density (senary order venation) compared with quinary order venation in A. thaliana, likely because it did not undergo the C3–C4 intermediate stage for establishing the photorespiratory CO2 pump.
Materials and Methods
Plant Material and RNA Isolation
Gynandropsis gynandra was grown in growth chambers under the light-dark cycle: 12 h light (200–250 μmol m−2 s−1) at 27 °C and 12 h darkness at 25 °C. For isolation of total RNA, fifth leaves (∼0.5–10 mm long) of 20 plants (12–14 days old) were harvested at midday and immediately frozen in liquid nitrogen. Total RNA was isolated by TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, using a 1:2 ratio of sample: TRIZOL reagent. RNA samples were treated with DNase I at 37 °C for 30 min to eliminate contaminating genomic DNA.
Iso-Seq cDNA Library Preparation and SMRT Sequencing
The total RNA sample of G. gynandra leaves was used to construct a cDNA library by the High Throughput Genomics Core, Academia Sinica, Taiwan. Full-length cDNAs were synthesized from HQ total RNA with oligo-dT priming, and amplified using the Clontech SMARTer PCR cDNA Synthesis Kit with PrimeSTAR GXL DNA Polymerase (Takara Bio, USA). The PCR products were purified with AMPure PB magnetic beads (PacBio), end-repaired, and constructed into an Iso-Seq library by ligating to the blunt-end adaptor of the SMRTBell Template Prep Kit 1.0 (Pacific Biosciences, USA). The library profiles showed distribution from 300 bp to 6 kb, with major peaks at around 1.8 kb. To minimize loading bias, two size-fractionated libraries (≦2 kb and >2 kb) were isolated on 0.75% gel cassette using the BluePippin system (Sage Science, USA). The two fractions were polished by PreCR Repair Mix (New England Biolabs), and their concentrations and size profiles were determined using Qubit dsDNA HS Assay Kit with the Qubit® 3.0 Fluorometer (Thermo Fisher Scientific, USA) and Agilent BioAnalyzer 2100 High Sensitivity DNA Kit (Agilent Technologies, USA), respectively. Finally, the libraries were pooled and loaded into the SMRTcell and sequenced on the PacBio Sequel platform by the High Throughput Genomics Core, Academia Sinica, Taiwan.
PacBio Long-Read Processing
The PacBio raw reads were classified into CCS and non-CCS subreads by running the IsoSeq3 module in PacBio SMRT Analysis v6.0. IsoSeq v3 determines a CCS or subread sequence to be full length if the 5′- and 3′-primers and poly A tail signal were all present in the correct order. After clustering, the full-length transcripts were polished with predicted consensus accuracy ≥0.99, which were considered polished HQ transcripts. To correct the potential indel errors, we polished the full-length transcripts using the trimmed Illumina RNA-seq reads deposited at NCBI (Huang et al. 2017) as input to the Pilon software (Walker et al. 2014).
Assembly of RNA-Seq Reads and Construction of ORF Databases
The Illumina RNA-seq reads from G. gynandra and T. hassleriana deposited at NCBI (Huang et al. 2017) were trimmed using the Trimmomatic tool (Bolger et al. 2014). After the quality trimming at Q30, the trimmed reads were end merged to generate longer reads by FLASH (Magoc and Salzberg 2011). The merged and unmerged paired-end reads in each species were assembled de novo, using the CLC Genomics Workbench (QIAGEN, Germany) with default options.
To construct the ORF database of G. gynandra, we used the following procedure (supplementary fig. S1, Supplementary Material online): First, the PacBio polished HQ isoform transcripts were combined with the CLC assembled contigs for predicting CDSs by the orf-finder-py tool (Stewart et al. 2017). Redundant CDSs were removed by CD-HIT (Li and Godzik 2006; Fu et al. 2012) and the longest CDSs in the combined database were retained as the representative transcripts. To annotate the CDSs, we used BLASTp (Camacho et al. 2009) against the Araport11 gene database (Camacho et al. 2009). CDSs in the representative transcripts covering over 90% of the length of their target A. thaliana genes (Araport11) (Cheng et al. 2017) were collected to form a full-length CDS data set. Second, we mapped the Illumina RNA-seq reads against the full-length CDSs using Bowtie 2 (Langmead and Salzberg 2012). Then, we collected the unmapped reads for de novo assembly using CLC. The new full-length CDSs from the second assembly were added to the full-length CDS data set. Third, to obtain more full-length CDSs, the remaining transcripts (<90% coverage against the target A. thaliana genes) from PacBio Iso-seq and CLC assembled contigs were used with the assembled CDSs using CAP3 (Huang and Madan 1999). Finally, we added the CAP3 assembly to the full-length CDS data set and removed redundant CDSs. The final G. gynandra CDS data set contained 21,535 ORFs in which 16,535 ORFs were full-length CDSs.
The procedure for constructing the T. hassleriana CDS database was similar to the above. The previously constructed T. hassleriana CDSs (Cheng et al. 2013), which were inferred from the annotated genes of T. hassleriana, were combined with the CDSs from the CLC-assembled contigs. The remaining steps were the same as the procedure of the construction of the G. gynandra CDS database (supplementary fig. S1, Supplementary Material online). The final T. hassleriana CDS database contained 27,617 CDSs with 22,511 full-length CDSs.
Estimating Gene Expression Levels
To quantify the expression levels of the assembled CDSs in a species (G. gynandra or T. hassleriana), the Illumina reads of the six developmental stages (S0–S5) (Külahoglu et al. 2014) deposited at NCBI were subjected to quality trimming at Q30, and were mapped to the corresponding ORF database for that species. The single-end read data were then mapped to the ORFs using Bowtie 2 (Langmead and Salzberg 2012) with default settings. Finally, the eXpress software (Roberts and Pachter 2013) was used to resolve the multihit reads and calculate the relative measurements of RPKMs as the expression levels of the CDSs.
To have meaningful comparisons of gene expression levels for the six developmental stages between two species, the RPKM values were normalized using the upper quartile normalization procedure (Bullard et al. 2010), using the S0 stage in G. gynandra as the reference.
In Situ Hybridization
In situ hybridization experiments were carried out as described by Jackson (Jackson 1991). Plant material was fixed in 4% paraformaldehyde (Sigma) in 0.1 M phosphate buffer (pH 7.2) for 16 h at 4 °C and embedded in Paraplast Plus (Sigma-Aldrich). Sections (12 µm) were cut using a microtome (RM 2135; Leica), and collected in xylene-coated slides. Slides were deparaffinized and treated with 20 mg/ml proteinase K. In vitro transcription of the digoxigenin UTP-labeled (Roche) RNA sense and antisense probes were obtained using T7 and Sp6 polymerases. Primers used to generate the probe clones are listed in supplementary table S4, Supplementary Material online. Hybridization was performed in hybridization solution at 50–55 °C overnight. Digoxigenin detection and signal visualization were carried out using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Roche), following the manufacturer’s instructions. Slides were air dried and mounted with Kaiser’s glycerol gelatine mounting medium (Sigma-Aldrich).
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgments
This study was supported by Academia Sinica (AS-TP-109-L10). We thank the High‐Throughput Sequencing Core, Biodiversity Research Center, Academia Sinica for high-throughput sequencing and support.
Author Contributions
C.-F.H. and W.-H.L. designed the study. W.-H.L. supervised the study. C.-F.H. conducted bioinformatics analyses. C.-F.H. and W.-Y.L. performed in situ hybridization experiments. M.-Y.J.L., and Y.-H.C. performed PacBio sequencing. C.-F.H. and Y.-H.C. captured microscopic images. C.-F.H. and W.-H.L. wrote the first draft. C.-F.H., W.-Y.L., M.-Y.J.L., Y.-H.C., M.S.B.K., and W.-H.L wrote the article.
Data Availability
The sequence data have been deposited in the Sequence Read Archive, www.ncbi.nlm.nih.gov/sra (SRR13973106, SRR13973107, and SRR13973108) and the Transcriptome Shotgun Assemblies, www.ncbi.nlm.nih.gov/Traces/wgs/?view=TSA (GJBA00000000 and GFML02000000).
References
- Bailey TL, Elkan C.. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol. 2:28–36. [PubMed] [Google Scholar]
- Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S.. 2002. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics 18(2):298–305. [DOI] [PubMed] [Google Scholar]
- Barker MS, Vogel H, Schranz ME.. 2009. Paleopolyploidy in the Brassicales: analyses of the Cleome transcriptome elucidate the history of genome duplications in Arabidopsis and other brassicales. Genome Biol Evol. 1:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwe H. 2011. Photorespiration: the bridge to C4 photosynthesis. In: Raghavendra AS, Sage RF, editors. C4 photosynthesis and related CO2 concentrating mechanisms. Dordrecht: Springer Netherlands. p. 81–108. [Google Scholar]
- Bayat S, Schranz ME, Roalson EH, Hall JC.. 2018. Lessons from Cleomaceae, the sister of Crucifers. Trends Plant Sci. 23(9):808–821. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B.. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JE, Chapman BA, Rong J, Paterson AH.. 2003. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 422(6930):433–438. [DOI] [PubMed] [Google Scholar]
- Bressan M, Dall’Osto L, Bargigia I, Alcocer MJP, Viola D, Cerullo G, D’Andrea C, Bassi R, Ballottari M.. 2016. LHCII can substitute for LHCI as an antenna for photosystem I but with reduced light-harvesting capacity. Nat Plants. 2:16131. [DOI] [PubMed] [Google Scholar]
- Bullard JH, Purdom E, Hansen KD, Dudoit S.. 2010. Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinformatics. 11:94–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL.. 2009. BLAST+: architecture and applications. BMC Bioinformatics. 10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Krishnakumar V, Chan AP, Thibaud-Nissen F, Schobel S, Town CD.. 2017. Araport11: a complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 89(4):789–804. [DOI] [PubMed] [Google Scholar]
- Cheng S, van den Bergh E, Zeng P, Zhong X, Xu J, Liu X, Hofberger J, de Bruijn S, Bhide AS, Kuelahoglu C, et al. 2013. The Tarenaya hassleriana genome provides insight into reproductive trait and genome evolution of crucifers. Plant Cell. 25(8):2813–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ.. 2013. Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proc Natl Acad Sci U S A. 110(4):1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Salamin N, Savolainen V, Duvall MR, Besnard G.. 2007. C4 Photosynthesis evolved in grasses via parallel adaptive genetic changes. Curr Biol. 17(14):1241–1247. [DOI] [PubMed] [Google Scholar]
- Coate JE, Schlueter JA, Whaley AM, Doyle JJ.. 2011. Comparative evolution of photosynthetic genes in response to polyploid and nonpolyploid duplication. Plant Physiol. 155(4):2081–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, Fitt GP, Sewelam N, Schenk PM, Manners JM, et al. 2007. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell. 19(7):2225–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms DM, Kelly S.. 2019. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Niu B, Zhu Z, Wu S, Li W.. 2012. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28(23):3150–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowik U, Westhoff P.. 2011. The path from C3 to C4 photosynthesis. Plant Physiol. 155(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis: a unique belend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta. 895(2):81–106. [Google Scholar]
- Heckmann D, Schulze S, Denton A, Gowik U, Westhoff P, Weber AP, Lercher MJ.. 2013. Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153(7):1579–1588. [DOI] [PubMed] [Google Scholar]
- Huang CF, Yu CP, Wu YH, Lu MJ, Tu SL, Wu SH, Shiu SH, Ku MSB, Li WH.. 2017. Elevated auxin biosynthesis and transport underlie high vein density in C4 leaves. Proc Natl Acad Sci U S A. 114(33):E6884–e6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Madan A.. 1999. CAP3: a DNA sequence assembly program. Genome Res. 9(9):868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylton CM, Rawsthorne S, Smith AM, Jones DA, Woolhouse HW.. 1988. Glycine decarboxylase is confined to the bundle-sheath cells of leaves of C3-C4 intermediate species. Planta 175(4):452–459. [DOI] [PubMed] [Google Scholar]
- Ifuku K, Endo T, Shikanai T, Aro EM.. 2011. Structure of the chloroplast NADH dehydrogenase-like complex: nomenclature for nuclear-encoded subunits. Plant Cell Physiol. 52(9):1560–1568. [DOI] [PubMed] [Google Scholar]
- Ishikawa N, Takabayashi A, Noguchi K, Tazoe Y, Yamamoto H, von Caemmerer S, Sato F, Endo T.. 2016. NDH-mediated cyclic electron flow around photosystem I is crucial for C4 photosynthesis. Plant Cell Physiol. 57(10):2020–2028. [DOI] [PubMed] [Google Scholar]
- Jackson D. 1991. In situ hybridization in plants. Molecular plant pathology: a practical approach. In: Gurr SJ, McPherson MJ, Bowles DJ, editors. London, UK: Oxford University Press. p. 163–174.
- Kubicki A, Funk E, Westhoff P, Steinmüller K.. 1996. Differential expression of plastome-encoded ndh genes in mesophyll and bundle-sheath chloroplasts of the C4 plant Sorghum bicolor indicates that the complex I-homologous NAD(P)H-plastoquinone oxidoreductase is involved in cyclic electron transport. Planta 199(2):276–281. [Google Scholar]
- Külahoglu C, Denton AK, Sommer M, Maß J, Schliesky S, Wrobel TJ, Berckmans B, Gongora-Castillo E, Buell CR, Simon R, et al. 2014. Comparative transcriptome atlases reveal altered gene expression modules between two Cleomaceae C3 and C4 plant species. Plant Cell. 26(8):3243–3260.,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL.. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods. 9(4):357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Godzik A.. 2006. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22(13):1658–1659. [DOI] [PubMed] [Google Scholar]
- Magoc T, Salzberg SL.. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21):2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallmann J, Heckmann D, Brautigam A, Lercher MJ, Weber AP, Westhoff P, Gowik U.. 2014. The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria. Elife 3:e02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK. 1999. The origins of C4 genes and evolutionary pattern in the C4 metabolic phenotype. In: Sage RF, Monson RK, editors. C4 plant biology San Diego (CA: ): Academic Press. p. 377–410. [Google Scholar]
- Monson RK. 2003. Gene duplication, neofunctionalization, and the evolution of C4 photosynthesis. Int J Plant Sci. 164(S3):S43–S54. [Google Scholar]
- Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T.. 2004. Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429(6991):579–582. [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T.. 2002. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110(3):361–371. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Iwano M, Havaux M, Yokota A, Munekage YN.. 2013. Promotion of cyclic electron transport around photosystem I during the evolution of NADP-malic enzyme-type C4 photosynthesis in the genus Flaveria. New Phytol. 199(3):832–842. [DOI] [PubMed] [Google Scholar]
- Oliver DJ, Raman R.. 1995. Glycine decarboxylase: protein chemistry and molecular biology of the major protein in leaf mitochondria. J Bioenerg Biomembr. 27(4):407–414. [DOI] [PubMed] [Google Scholar]
- Panchy N, Lehti-Shiu M, Shiu S-H.. 2016. Evolution of gene duplication in plants. Plant Physiol. 171(4):2294–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Fukao Y, Fujiwara M, Takami T, Shikanai T.. 2009. Efficient operation of NAD(P)H dehydrogenase requires supercomplex formation with photosystem I via minor LHCI in Arabidopsis. Plant Cell. 21(11):3623–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Shikanai T.. 2011. Supercomplex formation with photosystem I is required for the stabilization of the chloroplast NADH dehydrogenase-like complex in Arabidopsis. Plant Physiol. 155(4):1629–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Pachter L.. 2013. Streaming fragment assignment for real-time analysis of sequencing experiments. Nat Methods. 10(1):71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF. 2001. Environmental and evolutionary preconditionsfor the origin and diversification of the C4 photosynthetic syndrome. Plant Biol. 3(3):202–213. [Google Scholar]
- Sage RF. 2004. The evolution of C4 photosynthesis. New Phytol. 161(2):341–370. [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin PA, Edwards EJ.. 2011. The C4 plant lineages of planet Earth. J Exp Bot. 62(9):3155–3169. [DOI] [PubMed] [Google Scholar]
- Schulze S, Mallmann J, Burscheidt J, Koczor M, Streubel M, Bauwe H, Gowik U, Westhoff P.. 2013. Evolution of C4 photosynthesis in the genus Flaveria: establishment of a photorespiratory CO2 pump. Plant Cell. 25(7):2522–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze S, Westhoff P, Gowik U.. 2016. Glycine decarboxylase in C3, C4 and C3-C4 intermediate species. Curr Opin Plant Biol. 31:29–35. [DOI] [PubMed] [Google Scholar]
- Sharkey TD. 1988. Estimating the rate of photorespiration in leaves. Physiol Plant. 73(1):147–152. [Google Scholar]
- Shikanai T. 2016. Chloroplast NDH: a different enzyme with a structure similar to that of respiratory NADH dehydrogenase. Biochim Biophys Acta. 1857(7):1015–1022. [DOI] [PubMed] [Google Scholar]
- Sinha NR, Kellogg EA.. 1996. Parallelism and diversity in multiple origins of C4 photosynthesis in the grass family. Am J Bot. 83(11):1458–1470. [Google Scholar]
- Sperschneider J, Catanzariti AM, DeBoer K, Petre B, Gardiner DM, Singh KB, Dodds PN, Taylor JM.. 2017. LOCALIZER: subcellular localization prediction of both plant and effector proteins in the plant cell. Sci Rep. 7:44598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart ZK, Pavasovic A, Hock DH, Prentis PJ.. 2017. Transcriptomic investigation of wound healing and regeneration in the cnidarian Calliactis polypus. Sci Rep. 7:41458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabayashi A, Kishine M, Asada K, Endo T, Sato F.. 2005. Differential use of two cyclic electron flows around photosystem I for driving CO2-concentration mechanism in C4 photosynthesis. Proc Natl Acad Sci U S A. 102(46):16898–16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanz SK, Tetu SG, Vella NGF, Ludwig M.. 2009. Loss of the transit peptide and an increase in gene expression of an ancestral chloroplastic carbonic anhydrase were instrumental in the evolution of the cytosolic C4 carbonic anhydrase in Flaveria. Plant Physiol. 150(3):1515–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bergh E, Külahoglu C, Bräutigam A, Hibberd JM, Weber APM, Zhu X-G, Eric Schranz M.. 2014. Gene and genome duplications and the origin of C4 photosynthesis: birth of a trait in the Cleomaceae. Curr Plant Biol. 1:2–9. [Google Scholar]
- Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, et al. 2014. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 9(11):e112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gowik U, Tang H, Bowers JE, Westhoff P, Paterson AH.. 2009. Comparative genomic analysis of C4 photosynthetic pathway evolution in grasses. Genome Biol. 10(6):R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tan X, Paterson AH.. 2013. Different patterns of gene structure divergence following gene duplication in Arabidopsis. BMC Genomics. 14:652–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom M, Krab K, Saraste M.. 1981. Proton-translocating cytochrome complexes. Annu Rev Biochem. 50:623–655. [DOI] [PubMed] [Google Scholar]
- Williams BP, Burgess SJ, Reyna-Llorens I, Knerova J, Aubry S, Stanley S, Hibberd JM.. 2016. An untranslated cis-element regulates the accumulation of multiple C4 enzymes in Gynandropsis gynandra mesophyll cells. Plant Cell. 28(2):454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BP, Johnston IG, Covshoff S, Hibberd JM.. 2013. Phenotypic landscape inference reveals multiple evolutionary paths to C4 photosynthesis. Elife 2:e00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 24(8):1586–1591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence data have been deposited in the Sequence Read Archive, www.ncbi.nlm.nih.gov/sra (SRR13973106, SRR13973107, and SRR13973108) and the Transcriptome Shotgun Assemblies, www.ncbi.nlm.nih.gov/Traces/wgs/?view=TSA (GJBA00000000 and GFML02000000).