Abstract
Aims
Shock index (SI), defined as the ratio of heart rate (HR) to systolic blood pressure (SBP), is easily obtained and predictive of mortality in patients with ST-segment elevation myocardial infarction. However, large-scale evaluations of SI in patients with non-ST-segment elevation myocardial infarction (NSTEMI) are lacking.
Methods and results
Hospitalizations for acute myocardial infarction were sampled from four US areas by the Atherosclerosis Risk in Communities (ARIC) study and classified by physician review. Shock index was derived from the HR and SBP at first presentation and considered high when ≥0.7. From 2000 to 2014, 18 301 weighted hospitalizations for NSTEMI were sampled and had vitals successfully obtained. Of these, 5753 (31%) had high SI (≥0.7). Patients with high SI were more often female (46% vs. 39%) and had more prevalent chronic kidney disease (40% vs. 32%). TIMI (Thrombolysis in Myocardial Infarction) risk scores were similar between the groups (4.3 vs. 4.2), but GRACE (Global Registry of Acute Coronary Syndrome) score was higher with high SI (140 vs. 118). Angiography, revascularization, and guideline-directed medications were less often administered to patients with high SI, and the 28-day mortality was higher (13% vs. 5%). Prediction of 28-day mortality by SI as a continuous measurement [area under the curve (AUC): 0.68] was intermediate to that of the GRACE score (AUC: 0.87) and the TIMI score (AUC: 0.54). After adjustments, patients with high SI had twice the odds of 28-day mortality (odds ratio = 2.02; 95% confidence interval: 1.46–2.80).
Conclusion
The SI is easily obtainable, performs moderately well as a predictor of short-term mortality in patients hospitalized with NSTEMI, and may be useful for risk stratification in emergency settings.
Keywords: NSTEMI, Acute myocardial infarction, Risk score, Mortality, Epidemiology
Graphical abstract
Introduction
Shock index (SI) is defined as the ratio of heart rate (HR) to systolic blood pressure (SBP), which is universally obtained on initial clinical presentation. Shock index has been previously utilized to predict adverse outcomes in patients with sepsis,1,2 pneumonia,3,4 and pulmonary embolism5,6 and is closely associated with early recognition of cardiogenic, septic, and hypovolaemic shock.5–9 Shock index is also reported to effectively predict short- and long-term mortality in patients with ST-segment elevation myocardial infarction (STEMI).10–13 Rapid risk stratification is crucial for clinical decision-making and management of patients presenting with acute myocardial infarction (AMI). However, large-scale evaluations of SI in patients admitted with non-ST-segment elevation myocardial infarction (NSTEMI) are lacking, and prior studies are limited to patients undergoing coronary angiography.14 Established risk scores such as TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Syndrome) have demonstrated effective risk stratification in patients with AMI15–22 and are recommended by the American Heart Association and the American College of Cardiology for assessing prognosis of patients with NSTEMI.23 However, the algorithms require extensive clinical inputs, such as laboratory tests, making them difficult to apply especially in resource limited settings. The present study, therefore, aims to evaluate the prognostic utility of the SI in a large-scale, unselected sample of patients admitted with NSTEMI.
Methods
ARIC study community surveillance
Since 1987, the Atherosclerosis Risk in Communities (ARIC) study has conducted ongoing surveillance of hospitalized AMI in four geographically defined regions of the USA: Forsyth County, North Carolina; Washington County, Maryland; Jackson, Mississippi; and eight northwest suburbs of Minneapolis, Minnesota. As previously described,24 eligible hospitalizations were selected on the basis of age, residence in the community, and discharge code [International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 402 (hypertensive heart disease); 410 to 414 [AMI, other acute, subacute ischaemic heart disease, old myocardial infarction (MI), angina pectoris, and other forms of chronic ischaemic heart disease]; 427 (cardiac dysrhythmias); 428 (heart failure); and 518.4 (acute oedema of the lung)], through random sampling within discharge code strata. The study sample for this analysis is limited to hospitalizations in the contemporary era with discharge dates between 1 January 2000 and 31 December 2014.
Electrocardiography, biomarkers, and chest pain
The first, third, and the last 12-lead electrocardiograms (ECGs) over the course of hospitalization were obtained from the medical record and coded electronically at the Minneapolis ECG Reading Center. Laboratory values for cardiac troponin were abstracted chronologically, recording up to three measurements per day and noting the laboratory-specified upper limit of normal (ULN) for each hospitalization. Presence of acute chest pain was abstracted from the medical record, with origin determined by review of the physician notes.
Acute myocardial infarction classification
As previously described,24 events were classified by a validated computer algorithm with a physician panel review, as definite, probable, suspected, or no AMI, based on ECG evidence (evolving diagnostic, diagnostic, evolving ST-segment/T-wave changes, equivocal, or absent/uncodable), presence of chest pain, and cardiac biomarkers (which were considered ‘abnormal’ if ≥2× ULN), and ‘equivocal’ if exceeding the ULN but <2× the ULN. Classification of an event as definite or probable AMI required the presence of at least one of the following: (i) evolving diagnostic ECG pattern, (ii) diagnostic ECG pattern and abnormal biomarkers (≥2× ULN), (iii) cardiac pain and abnormal biomarkers (≥2× ULN), (iv) cardiac pain and equivocal biomarkers (exceeding the ULN but <2× the ULN) with evolving ST-segment/T-wave pattern or diagnostic ECG pattern, or (v) abnormal biomarkers with evolving ST-segment/T-wave pattern.
Clinical data abstraction and covariate definitions
Clinical data were collected from the hospital record by trained abstractors following a standardized protocol, using the physician notes, laboratory reports, patient histories, and discharge summaries. Laboratory values were abstracted by recording the first and worst value during the course of hospitalization. The first recorded value was used for the purposes of this analysis. We estimated the glomerular filtration rate using the CKD-EPI(Chronic Kidney Disease - Epidemiology Collaboration) formula and abstracted serum creatinine. Chronic kidney disease was defined by an estimated glomerular filtration rate <45 mL/min/1.73 m2 or receipt of dialysis. Hypertension was defined by known history of hypertension, antihypertensive medication use, SBP ≥140, or diastolic blood pressure ≥90 mmHg. Diabetes mellitus was defined by documented history in the medical record or diabetic medication use. Current smoking and prior history of coronary revascularization were classified by documentation in the medical record.
Shock index
The SI (ratio of HR/SBP) was derived using the first recorded blood pressure and pulse rate, excluding any recorded during cardiopulmonary resuscitation. Based on availability, the ARIC study abstracted the first blood pressure and pulse rate from the ambulance record, the emergency department sheet, the clinical graph, or the nursing admission sheet, in that order. We considered an SI ≥0.7 to be high, based on cutpoints used in the previous literature.25
Thrombolysis in myocardial ischaemia score
TIMI risk scores for unstable angina/NSTEMI were derived from medical histories and presenting features at admission, using data abstracted from the hospital record. Clinical inputs for the TIMI risk score include age ≥65, presence of three or more coronary artery disease (CAD) risk factors (family history of CAD, hypertension, hypercholesterolaemia, diabetes, or tobacco use), known history of CAD (stenosis ≥ 50%), and aspirin use within the past 7 days, severe angina, ST-segment deviation on ECG, and elevated cardiac biomarkers.26 Diagnosis of hypercholesterolaemia was not abstracted by the ARIC surveillance but was inferred by lipid-lowering medication use. Likewise, family history of CAD was not abstracted from the medical record. Known history of CAD was inferred by history of prior angioplasty or coronary bypass graft. Severe angina was inferred by acute chest pain precipitating hospitalization. Routine aspirin use was abstracted from the medical record, and from this aspirin use within the past 7 days was inferred. A TIMI score of 2–4 was considered ‘low risk’, and a score of 5–7 was considered ‘high risk’.
Global Registry of Acute Coronary Syndrome score
The GRACE score was derived from the presenting features at admission and over the course of hospitalization, using clinical data abstracted from the medical record. Clinical inputs include patient age, HR, SBP, serum creatinine level, Killip class, cardiac arrest, ST-segment deviation, and abnormal cardiac enzymes. Clinical inputs for the Killip class include presence of jugular venous distension, s3 gallop, acute pulmonary oedema/congestive heart failure, and cardiogenic shock. The Killip class ranges from 1 to 4, with a value of ≥3 considered elevated. Cardiac arrest was abstracted by the ARIC study as a composite of ventricular arrhythmia/cardiac arrest/asystole. Because the ARIC study began routine abstraction of creatinine levels in 2004, derivation of the GRACE score was limited to 2004–2014. A GRACE score >140 was considered elevated.27
Medical therapies and cardiac procedures
Medications were recorded if administered during hospitalization or prescribed at hospital discharge. Aspirin required routine rather than pro re nata administration for abstraction. Non-aspirin antiplatelet therapy was recorded as a single category and included P2Y12 inhibitors (clopidogrel, prasugrel, ticagrelor, and ticlopidine), glycoprotein IIb/IIIa inhibitors (abciximab, eptifibatide, and tirofiban), phosphodiesterase 3 inhibitors (cilostazol), phosphodiesterase 5 inhibitors (dipyridamole), and protease-activated receptor-1 antagonists. Beta blockers included β1 adrenergic antagonists. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (ACEis/ARBs) were recorded as a single category. Lipid-lowering agents included statins, niacin, and fibrates. Coronary angiography and revascularization procedures were abstracted from the medical record. Revascularization included percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG).
Mortality outcomes
Mortality was ascertained within 28 days and 1 year of hospital admission by the ARIC Community Surveillance study, by linking hospitalizations with the National Death Index.
Statistical analysis
Statistical tests and models accounted for the stratified sampling design and were weighted by the inverse of the sampling probabilities.28 Baseline characteristics of the study population were compared with stratification by SI. Continuous variables were compared using the difference in least square means from weighted linear regression and categorical variables were compared using Rao–Scott χ2 tests. The association between decile of SI with 28-day mortality was first visualized by constructing a bar graph. The prognostic value of the SI (as a continuous value) for prediction of 28-day mortality was analysed by logistic regression, by calculating the area under the curve (AUC) from receiver operating characteristics. For comparison, the AUC was also calculated for the TIMI and GRACE risk scores. Calibration of the SI prediction model was assessed within subgroups based on demographics (sex, race, and age categories), half-decade of admission (2000–2004, 2005–2009, and 2010–2014), mode of arrival (ambulance vs. other transportation), and clinical management (medical vs. invasive strategy). Because antihypertensive and beta blocker medication use may influence the assessment of SI, we also examined model calibration among patients who were vs. were not managed by these therapies. As a sensitivity analysis, predictions of 28-day mortality by SI were compared to predictions by SBP or HR alone.
The association between high SI (dichotomized by the 0.7 partition value) and 28-day mortality was assessed by logistic regression, with adjustment for demographics (age, race, sex, and year of admission), comorbidities (smoking, diabetes mellitus, chronic kidney disease, prior coronary revascularization, and history of stroke), and medical therapies (aspirin, antiplatelets, ACEi/ARBs, lipid-lowering medications, and revascularization either by PCI or CABG). To examine the outcomes of invasive strategy among patients with high SI, we analysed the relationship between coronary revascularization (vs. medical management) and 28-day mortality in the subset of patients classified with high SI, using multivariable logistic regression models adjusted for demographics and comorbidities.
Results
A total of 52 641 eligible hospitalizations were sampled from 2000 to 2014. Of these, 11 507 were classified as definite or probable AMI upon physician review, and 8889 were classified as NSTEMI. After the omission of transfer patients, those with in-hospital onset of AMI, or those with unobtainable vitals, 7785 remained, corresponding to 18 301 weighted events. The study population selection flowchart is shown in Supplementary material online, FigureS1. All subsequent results are presented with weighting by the sampling fraction.
The overall distribution of SI values was approximately normal but skewed slightly right, with a median value of 0.59 (Supplementary material online, FigureS2). Nearly a third of the included patients (31%) had a high SI (≥0.7), Table 1. Compared to patients with SI <0.7, those with high SI were more often women (46% vs. 39%) and slightly older (65 vs. 63 years), but racial distributions were similar. Smoking and history of diabetes mellitus were comparable between the groups, but patients with high SI had a greater prevalence of chronic kidney disease (40% vs. 32%). NSTEMI hospitalizations with high SI were more often complicated by acute pulmonary oedema/heart failure (42% vs. 30%), ventricular fibrillation/cardiac arrest (11% vs. 4%), and cardiogenic shock (5% vs. 2%). The mean Killip class increased with high SI (2.0 vs. 1.6), and participants with high SI more often had an elevated Killip class (44% vs. 30%). Interestingly, the mean TIMI risk score was comparable between the groups (4.3 vs. 4.2), and a high TIMI score was slightly less prevalent among participants with high SI (37% vs. 40%). The mean GRACE score was substantially higher in patients with high SI (142 vs. 118), as was prevalence of a high GRACE score (50% vs. 29%).
Table 1.
Demographics and characteristics of patients admitted with non-ST-segment elevation myocardial infarction, stratified by shock index
| Characteristic | Shock index < 0.7 (N = 12 548) | Shock index ≥ 0.7 (N = 5753) | P-value |
|---|---|---|---|
| Demographics | |||
| Age | 63 ± 0.2 | 65 ± 0.2 | 0.0006 |
| Female | 4846 (39%) | 2624 (46%) | <0.0001 |
| White | 7499 (60%) | 3595 (62%) | 0.1 |
| Medical history | |||
| Smoker | 3951 (31%) | 1723 (30%) | 0.9 |
| Diabetes mellitus | 5261 (42%) | 2463 (43%) | 0.6 |
| Chronic kidney disease | 3200 (32%) | 1946 (40%) | <0.0001 |
| Prior coronary revascularization | 4317 (34%) | 1655 (29%) | 0.0007 |
| In-hospital clinical course | |||
| Acute pulmonary oedema/heart failure | 3712 (30%) | 2408 (42%) | <0.0001 |
| Ventricular fibrillation/arrest/asystole | 558 (4%) | 634 (11%) | <0.0001 |
| Cardiogenic shock | 200 (2%) | 311 (5%) | <0.0001 |
| Risk scores | |||
| Killip class | 1.6 ± 0.01 | 2.0 ± 0.02 | <0.0001 |
| Elevated Killip class (≥3) | 3775 (30%) | 2503 (44%) | <0.0001 |
| TIMI score | 4.3 ± 0.01 | 4.2 ± 0.02 | 0.0003 |
| Elevated TIMI score (≥5) | 5058 (40%) | 2111 (37%) | 0.04 |
| GRACE scorea | 118 ± 0.9 | 142 ± 1.3 | <0.0001 |
| Elevated GRACE score (>140) | 2914 (29%) | 2449 (50%) | <0.0001 |
| Hospital procedures | |||
| Cardiopulmonary resuscitation/cardioversion | 409 (3%) | 495 (9%) | <0.0001 |
| Angiography | 7304 (58%) | 2052 (36%) | <0.0001 |
| Coronary revascularization | 5162 (41%) | 1069 (19%) | <0.0001 |
| Hospital medications | |||
| Aspirin | 11253 (90%) | 4810 (84%) | <0.0001 |
| Antiplatelets | 7349 (59%) | 1978 (34%) | <0.0001 |
| Beta blockers | 11181 (89%) | 4662 (81%) | <0.0001 |
| Statins/lipid-lowering agents | 9588 (76%) | 3495 (61%) | <0.0001 |
| ACEi/ARB | 8123 (65%) | 3225 (56%) | <0.0001 |
The community surveillance component of the Atherosclerosis Risk in Communities Study, 2000–2014.
Values are presented as mean ± standard error or number (percentage).
ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; GRACE, Global Registry of Acute Coronary Syndrome; TIMI, thrombolysis in myocardial ischaemia.
Chronic kidney disease and GRACE score limited to hospitalizations from 2004 to 2014 with available serum creatinine abstractions (N = 14 911; 3390 missing).
Patients with high SI were differentially managed, compared to patients with SI <0.7. Those with high SI received less guideline-directed medications, such as aspirin (84% vs. 90%), antiplatelets (34% vs. 59%), lipid-lowering agents (61% vs. 76%), and ACEis/ARBs (56% vs. 65%); and were less frequently evaluated by angiography (36% vs. 58%). Coronary revascularization was less often performed in patients with high SI, both overall (19% vs. 41%) and among the subset undergoing coronary angiography (51% vs. 70%).
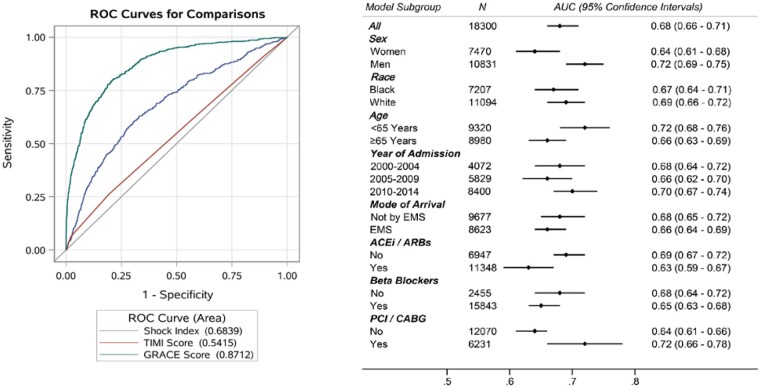
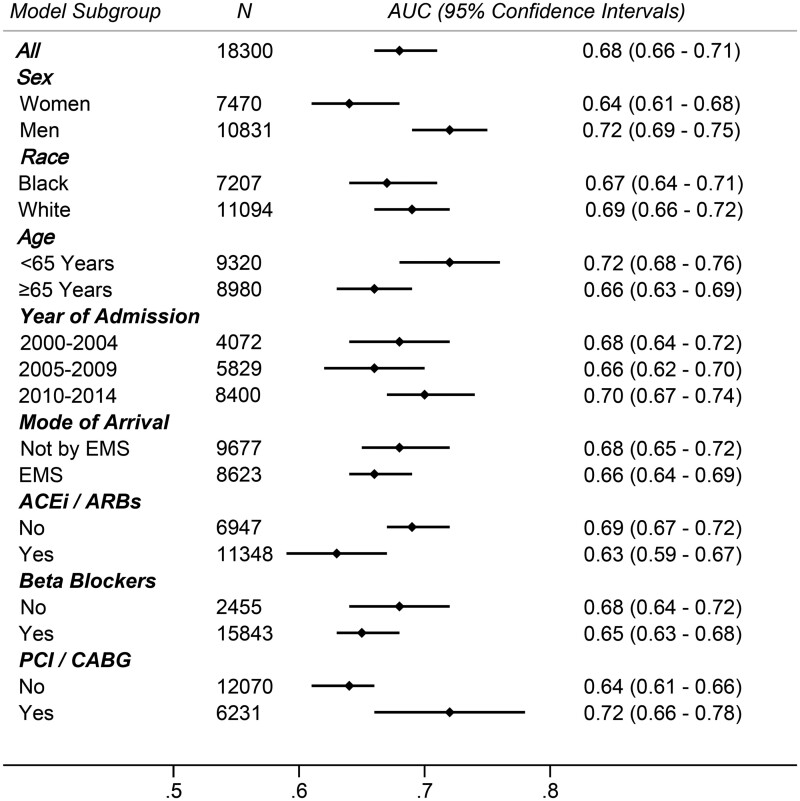
Within 28 days of hospital admission, there were 1331 deaths. The 28-day all-cause mortality was nearly three times higher (13% vs. 5%; P < 0.0001) with high SI. An increasing proportion of participants died within 28 days of hospitalization with increasing decile of SI (Supplementary material online, Figure S3). When examined as a continuous value, the SI performed moderately well as a predictor of 28-day mortality [AUC = 0.68; (95% confidence interval (CI): 0.66–0.71)], with predictive performance that was intermediate to the GRACE score [AUC = 0.87; (95% CI: 0.86–0.89)] and the TIMI score [AUC = 0.54; (95% CI: 0.52–0.57)], Figure 1. Model calibration was similar by race (Black vs. White), half-decade of admission (2000–2004, 2005–2009, and 2010–2014), and mode of arrival (by ambulance or by car). However, the SI was a slightly better predictor of 28-day mortality in men compared to women (AUC: 0.72 vs. 0.64), in younger patients compared to those ≥65 years (AUC: 0.72 vs. 0.66), in patients undergoing coronary revascularization (AUC: 0.72 vs. 0.64), Figure 2. In patients who were revascularized, the prediction of 28-day mortality by SI (AUC: 0.72; 95% CI: 0.66–0.78) was better than predictions by either SBP alone (AUC: 0.68; 95% CI: 0.63–0.74) or by HR alone (AUC: 0.63; 95% CI: 0.57–0.69). Predictions of 28-day mortality by SI value were comparable for patients managed by ACEi/ARBS or beta blockers compared to those not managed by these therapies.
Figure 1.
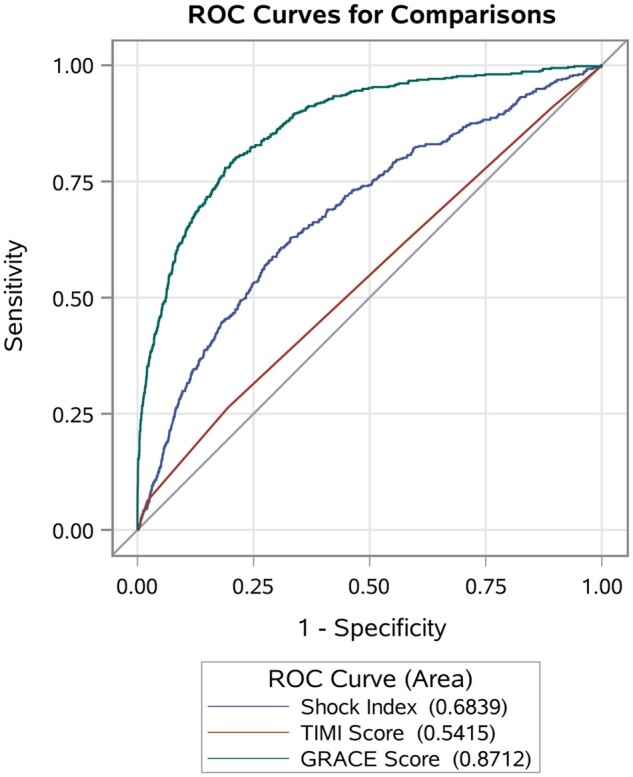
Predictive accuracy of shock index, GRACE score, and TIMI risk score in prognosis of 28-day all-cause mortality, among patients admitted with non-ST-segment elevation myocardial infarction. The community surveillance component of the Atherosclerosis Risk in Communities Study, 2000–2014. GRACE, Global Registry of Acute Coronary Syndrome; ROC, receiver operating characteristic curve; TIMI, thrombolysis in myocardial ischaemia.
Figure 2.
Predictive accuracy of shock index in prognosis of 28-day all-cause mortality, among various subgroups of patients admitted with non-ST-segment elevation myocardial infarction. The community surveillance component of the Atherosclerosis Risk in Communities Study, 2000–2014. ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; AUC, area under the curve from receiver operating characteristics; CABG, coronary artery bypass graft; EMS, emergency medical services; PCI, percutaneous coronary intervention.
When dichotomized by the partition value of 0.7, high SI was associated with nearly three times the odds of 28-day mortality, relative to SI <0.7 [odds ratio (OR) = 2.89; 95% CI: 2.24–3.6]. The association persisted after adjustment for demographics, year of admission, smoking, diabetes mellitus, chronic kidney disease, prior revascularization, history of stroke, and history of hypertension (OR = 2.46; 95% CI: 1.81–3.33), and upon further adjustment for aspirin, antiplatelets, ACEi/ARBs, lipid-lowering medications, and coronary revascularization (OR = 2.02; 95% CI: 1.46–2.80), Table 2.
Table 2.
Adjusted odds ratios of 28-day mortality associated with elevated shock index (≥0.7) relative to shock index <0.7, among patients admitted with non-ST-segment elevation myocardial infarction
| Model adjustmenta | OR (95% CI) |
|---|---|
| Crude | 2.89 (2.24–3.69) |
| Demographics | 2.77 (2.15–3.59) |
| Demographics, comorbidities | 2.46 (1.81–3.33) |
| Demographics, comorbidities, therapies | 2.02 (1.46–2.80) |
The community surveillance component of the Atherosclerosis Risk in Communities Study, 2000–2014.
Demographics = age, race, sex, and year of hospital admission. Comorbidities = smoking, diabetes mellitus, chronic kidney disease, prior coronary revascularization, and history of stroke. Therapies = aspirin, antiplatelets, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, lipid-lowering medications, and coronary revascularization either by percutaneous coronary intervention or by coronary artery bypass graft.
Among the subset of patients with high SI who underwent coronary revascularization, the 28-day mortality rate was 7%, which was nearly half the mortality rate of patients with high SI who were managed by medical therapy alone (14%). After adjustment for age, race, sex, and year of admission, the mortality OR for patients with high SI who received coronary revascularization was 0.49 (0.29–0.82). The mortality OR was largely unchanged after additional adjustments for smoking, diabetes, hypertension, history of stroke, and history of prior revascularization [OR = 0.49 (95% CI: 0.29–0.81)].
Discussion
In this large, multi-year surveillance of patients admitted with NSTEMI, we evaluate the predictive value SI and make the following observations: (i) the SI predicted short-term mortality moderately well in patients with NSTEMI, with predictive accuracy that was intermediate to the GRACE score and the TIMI risk score. (ii) Patients with high SI, as defined by the partition value of ≥0.7, were more often older, women, had a higher prevalence of chronic kidney disease, and a more severe clinical course during hospitalization. (iii) Patients with high SI less often received guideline-directed NSTEMI therapies and had three times the mortality within 28 days of hospitalization, compared to patients with an SI <0.7.
The SI is a simple metric for gauging the degree of hypovolaemia in haemorrhagic and infectious shock states29 and is widely used in critically ill patients to indicate severity of disease, response to treatment, and need for intensive care therapy. Prognostic value of the SI has been demonstrated in patients with trauma,30,31 with better predictions of adverse events than either SBP or HR alone.32 In the setting of acute coronary syndrome, the SI reflects sympathetic neural hyperactivity compensating left ventricular systolic dysfunction.33 Several prior studies have investigated the association of SI with mortality and major adverse cardiovascular events in patients with STEMI.11,12,34 A recent meta-analysis of patients hospitalized with STEMI reported an 11-fold higher risk of in-hospital mortality and twice the risk of longer-term adverse outcomes for patients with high SI.35 A high SI has also been shown to correspond to larger areas of myocardial injury in patients with STEMI and higher rates of adverse cardiovascular events.36 Both SBP and HR have been shown to predict mortality in patients with NSTEMI37,38; however, to date, there have been no large-scale evaluations of the SI in patients admitted with NSTEMI, and previous studies have been limited to patients undergoing cardiac catheterization.14
Evidence suggests that high-risk patients with NSTEMI benefit from early revascularization.39–41 The American Heart Association (AHA)/American College of Cardiology (ACC) guidelines for the management of NSTEMI recommend immediate revascularization within 2 h for patients with haemodynamic instability,23 and assign a Class 1A recommendation for assessing prognosis by risk scores. Our study suggests that SI may be an important tool for rapid identification of patients with impending haemodynamic instability, who may benefit from immediate or early invasive strategy. In our analysis of patients admitted with NSTEMI, prediction of 28-day mortality by SI was better than predictions by either SBP or HR alone. While the TIMI risk score for non-ST-elevation acute coronary syndrome is widely used as a risk stratification tool for predictions of all-cause mortality, urgent revascularization, and recurrent MI in patients with NSTEMI,26,42,43 in our study, SI was more predictive of 28-day mortality than the TIMI score, both overall and among the subset of patients who were revascularized. The GRACE score was the best overall predictor of 28-day mortality, but its use in emergency settings may be less convenient, owing to the time required to process blood for serum creatinine. In contrast, the SI can be rapidly calculated, and unlike the TIMI and GRACE scores does not require extensive clinical inputs for the algorithm. Unlike both the SI and GRACE score, the TIMI score for NSTEMI relies upon historical data from the medical record, such as history of cardiovascular risk factors (hypertension, hypercholesterolaemia, diabetes, or tobacco use), known history of coronary disease, and family history of coronary disease. The differences in clinical inputs for the three risk scores likely explains the closer alliance of the SI with the GRACE score, evidenced by the higher prevalence of elevated GRACE score among patients with an elevated SI.
Interestingly, a lower utilization of coronary angiography and revascularization was observed for patients in the highest risk category (SI ≥ 0.7). Similar results have been emphasized in the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines) trial, which showed an inverse relationship between cardiac catheterization and probability of severe CAD in patients with NSTEMI.44 The higher utilization of coronary catheterization for patients with SI <0.7 may have been influenced by hospital, geographic, or temporal factors, such as availability of cardiac catheterization facilities and cardiac catheterization volumes. Alternatively, treatment decisions may have been more influenced by clinical variables associated with lower complication rates, such as younger age and preserved renal function.45–48 This emphasizes the need for improvement in quality that could promote appropriate use of cardiac catheterization procedures among patients with the greatest potential benefit. Although our analysis from the ARIC study community surveillance encompassed a wide time interval, during which standard treatment evolved both in terms of revascularization strategy and medical treatment, predictions of 28-day mortality by a SI >0.7 were fairly consistent from 2000 to 2004, 2005 to 2009, and 2010 to 2014.
Our study has some limitations. This was an observational analysis, and data were limited to availability in the medical record. The ARIC study did not include classifications of type 1 or type 2 AMI, and we were unable to consider any differential predictions of short-term mortality by AMI type. However, increasing SI was more predictive of mortality among the subset of patients undergoing coronary revascularization, who presumably would have had type 1 MI. Although measurements of HR and SBP were unstandardized and may have been subject to interobserver variability, our analysis reflects clinical practice which increases generalizability of these findings. Our investigation also has several noteworthy strengths. The ARIC Study provides a large, multi-year surveillance of four diverse US communities, allowing an analysis of contemporary trends spanning 15 years. All hospitalizations for NSTEMI were validated by a standardized physician review of the medical record, minimizing misclassification of events, and mortality outcomes were verified by the National Death Index.
Conclusions
The SI is easily obtainable, performs moderately well as a predictor of short-term mortality in patients hospitalized with NSTEMI, and may be useful for risk stratification in emergency settings.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Data availability
The Atherosclerosis Risk in Communities (ARIC) study’s data are owned by the National Heart Lung and Blood Institute (NHLBI). The data are publicly available to qualified investigators with an approved manuscript proposal and data use agreement. Upon reasonable request, the ARIC coordinating center may make the data, analytic methods, and study materials available to other researchers for purposes of reproducing the results or replicating the procedure.
Supplementary Material
Acknowledgements
Z.S.C. and M.C.C. conceptualized the study and wrote the manuscript. M.C.C. performed the statistical analysis. M.E.H., S.A., X.D., V.M., S.C.S., and K.M. interpreted the data and revised the manuscript critically. The authors thank the staff and participants of the ARIC study for their important contributions.
Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract numbers (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I).
Conflict of interest: none declared.
References
- 1. Yussof SJ, Zakaria MI, Mohamed FL, Bujang MA, Lakshmanan S, Asaari AH.. Value of shock index in prognosticating the short-term outcome of death for patients presenting with severe sepsis and septic shock in the emergency department. Med J Malaysia 2012;67:406–411. [PubMed] [Google Scholar]
- 2. Rousseaux J, Grandbastien B, Dorkenoo A, Lampin ME, Leteurtre S, Leclerc F.. Prognostic value of shock index in children with septic shock. Pediatr Emerg Care 2013;29:1055–1059. [DOI] [PubMed] [Google Scholar]
- 3. Sankaran P, Kamath AV, Tariq SM, Ruffell H, Smith AC, Prentice P, Subramanian DN, Musonda P, Myint PK.. Are shock index and adjusted shock index useful in predicting mortality and length of stay in community-acquired pneumonia? Eur J Intern Med 2011;22:282–285. [DOI] [PubMed] [Google Scholar]
- 4. Myint PK, Bhaniani A, Bradshaw SM, Alobeidi F, Tariq SM.. Usefulness of shock index and adjusted shock index in the severity assessment of community-acquired pneumonia. Respiration 2009;77:468–469. [DOI] [PubMed] [Google Scholar]
- 5. Toosi MS, Merlino JD, Leeper KV.. Prognostic value of the shock index along with transthoracic echocardiography in risk stratification of patients with acute pulmonary embolism. Am J Cardiol 2008;101:700–705. [DOI] [PubMed] [Google Scholar]
- 6. Otero R, Trujillo-Santos J, Cayuela A, Rodriguez C, Barron M, Martin JJ, Monreal M; Registro Informatizado de la Enfermedad Tromboembolica (RIETE) Investigators. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 2007;30:1111–1116. [DOI] [PubMed] [Google Scholar]
- 7. Birkhahn RH, Gaeta TJ, Terry D, Bove JJ, Tloczkowski J.. Shock index in diagnosing early acute hypovolemia. Am J Emerg Med 2005;23:323–326. [DOI] [PubMed] [Google Scholar]
- 8. Berger T, Green J, Horeczko T, Hagar Y, Garg N, Suarez A, Panacek E, Shapiro N.. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med 2013;14:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cannon CM, Braxton CC, Kling-Smith M, Mahnken JD, Carlton E, Moncure M.. Utility of the shock index in predicting mortality in traumatically injured patients. J Trauma 2009;67:1426–1430. [DOI] [PubMed] [Google Scholar]
- 10. Shangguan Q, Xu JS, Su H, Li JX, Wang WY, Hong K, Cheng XS.. Modified shock index is a predictor for 7-day outcomes in patients with STEMI. Am J Emerg Med 2015;33:1072–1075. [DOI] [PubMed] [Google Scholar]
- 11. Bilkova D, Motovska Z, Widimsky P, Dvorak J, Lisa L, Budesinsky T.. Shock index: a simple clinical parameter for quick mortality risk assessment in acute myocardial infarction. Can J Cardiol 2011;27:739–742. [DOI] [PubMed] [Google Scholar]
- 12. Huang B, Yang Y, Zhu J, Liang Y, Tan H, Yu L, Gao X, Li J.. Usefulness of the admission shock index for predicting short-term outcomes in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2014;114:1315–1321. [DOI] [PubMed] [Google Scholar]
- 13. Spyridopoulos I, Noman A, Ahmed JM, Das R, Edwards R, Purcell I, Bagnall A, Zaman A, Egred M.. Shock-index as a novel predictor of long-term outcome following primary percutaneous coronary intervention. Eur Heart J Acute Cardiovasc Care 2015;4:270–277. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi A, Misumida N, Luger D, Kanei Y.. Shock index as a predictor for in-hospital mortality in patients with non-ST-segment elevation myocardial infarction. Cardiovasc Revasc Med 2016;17:225–228. [DOI] [PubMed] [Google Scholar]
- 15. Kozieradzka A, Kamiński KA, Maciorkowska D, Olszewska M, Dobrzycki S, Nowak K, Kralisz P, Prokopczuk P, Musial WJ.. GRACE, TIMI, Zwolle and CADILLAC risk scores—do they predict 5-year outcomes after ST-elevation myocardial infarction treated invasively? Int J Cardiol 2011;148:70–75. [DOI] [PubMed] [Google Scholar]
- 16. D'Ascenzo F, Biondi-Zoccai G, Moretti C, Bollati M, Omedè P, Sciuto F, Presutti DG, Modena MG, Gasparini M, Reed MJ, Sheiban I, Gaita F.. TIMI, GRACE and alternative risk scores in acute coronary syndromes: a meta-analysis of 40 derivation studies on 216,552 patients and of 42 validation studies on 31,625 patients. Contemp Clin Trials 2012;33:507–514. [DOI] [PubMed] [Google Scholar]
- 17. Lev EI, Kornowski R, Vaknin-Assa H, Porter A, Teplitsky I, Ben-Dor I, Brosh D, Fuchs S, Battler A, Assali A.. Comparison of the predictive value of four different risk scores for outcomes of patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2008;102:6–11. [DOI] [PubMed] [Google Scholar]
- 18. Aragam KG, Tamhane UU, Kline-Rogers E, Li J, Fox KA, Goodman SG, Eagle KA, Gurm HS.. Does simplicity compromise accuracy in ACS risk prediction? A retrospective analysis of the TIMI and GRACE risk scores. PLoS One 2009;4:e7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, Giugliano RP, McCabe CH, Braunwald E.. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000;102:2031–2037. [DOI] [PubMed] [Google Scholar]
- 20. Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA; Global Registry of Acute Coronary Events Investigators. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003;163:2345–2353. [DOI] [PubMed] [Google Scholar]
- 21. Addala S, Grines CL, Dixon SR, Stone GW, Boura JA, Ochoa AB, Pellizzon G, O'Neill WW, Kahn JK.. Predicting mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention (PAMI risk score). Am J Cardiol 2004;93:629–632. [DOI] [PubMed] [Google Scholar]
- 22. Halkin A, Singh M, Nikolsky E, Grines CL, Tcheng JE, Garcia E, Cox DA, Turco M, Stuckey TD, Na Y, Lansky AJ, Gersh BJ, O’Neill WW, Mehran R, Stone GW.. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol 2005;45:1397–1405. [DOI] [PubMed] [Google Scholar]
- 23. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ.. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 24. Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR.. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987-2008. Circulation 2012;125:1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shangguan Q, Xu JS, Su H, Li JX, Wang WY, Hong K, Cheng XS.. Modified shock index is a predictor for 7-day outcomes in patients with STEMI. Am J Emerg Med 2015;33:1072–1075. [DOI] [PubMed] [Google Scholar]
- 26. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E.. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284:835–842. [DOI] [PubMed] [Google Scholar]
- 27. Everett CC, Fox KA, Reynolds C, Fernandez C, Sharples L, Stocken DD, Carruthers K, Hemingway H, Yan AT, Goodman SG, Brieger D, Chew DP, Gale CP.. Evaluation of the impact of the GRACE risk score on the management and outcome of patients hospitalised with non-ST elevation acute coronary syndrome in the UK: protocol of the UKGRIS cluster-randomised registry-based trial. BMJ Open 2019;9:e032165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mansournia MA, Altman DG.. Inverse probability weighting. BMJ 2016;352:i189. [DOI] [PubMed] [Google Scholar]
- 29. Allgöwer M, Burri C.. [“Shock index”]. Dtsch Med Wochenschr 1967;92:1947–1950. [DOI] [PubMed] [Google Scholar]
- 30. Rady MY, Smithline HA, Blake H, Nowak R, Rivers E.. A comparison of the shock index and conventional vital signs to identify acute, critical illness in the emergency department. Ann Emerg Med 1994;24:685–690. [DOI] [PubMed] [Google Scholar]
- 31. Sloan EP, Koenigsberg M, Clark JM, Weir WB, Philbin N.. Shock index and prediction of traumatic hemorrhagic shock 28-day mortality: data from the DCLHb resuscitation clinical trials. West J Emerg Med 2014;15:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zarzaur BL, Croce MA, Fischer PE, Magnotti LJ, Fabian TC.. New vitals after injury: shock index for the young and age x shock index for the old. J Surg Res 2008;147:229–236. [DOI] [PubMed] [Google Scholar]
- 33. Graham LN, Smith PA, Stoker JB, Mackintosh AF, Mary DA.. Sympathetic neural hyperactivity and its normalization following unstable angina and acute myocardial infarction. Clin Sci (Lond) 2004;106:605–611. [DOI] [PubMed] [Google Scholar]
- 34. Abe N, Miura T, Miyashita Y, Hashizume N, Ebisawa S, Motoki H, Tsujimura T, Ishihara T, Uematsu M, Katagiri T, Ishihara R, Tosaka A, Ikeda U.. Long-term prognostic implications of the admission shock index in patients with acute myocardial infarction who received percutaneous coronary intervention. Angiology 2017;68:339–345. [DOI] [PubMed] [Google Scholar]
- 35. Zhang X, Wang Z, Wang Z, Fang M, Shu Z.. The prognostic value of shock index for the outcomes of acute myocardial infarction patients: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reinstadler SJ, Fuernau G, Eitel C, de Waha S, Desch S, Metzler B, Schuler G, Thiele H, Eitel I.. Shock index as a predictor of myocardial damage and clinical outcome in ST-elevation myocardial infarction. Circ J 2016;80:924–930. [DOI] [PubMed] [Google Scholar]
- 37. Bangalore S, Messerli FH, Ou FS, Tamis-Holland J, Palazzo A, Roe MT, Hong MK, Peterson ED; CRUSADE Investigators. Blood pressure paradox in patients with non-ST-segment elevation acute coronary syndromes: results from 139,194 patients in the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines (CRUSADE) quality improvement initiative. Am Heart J 2009;157:525–531. [DOI] [PubMed] [Google Scholar]
- 38. Bangalore S, Messerli FH, Ou FS, Tamis-Holland J, Palazzo A, Roe MT, Hong MK, Peterson ED; for the CRUSADE Investigators. The association of admission heart rate and in-hospital cardiovascular events in patients with non-ST-segment elevation acute coronary syndromes: results from 135 164 patients in the CRUSADE Quality Improvement Initiative. Eur Heart J 2010;31:552–560. [DOI] [PubMed] [Google Scholar]
- 39. Diderholm E, Andrén B, Frostfeldt G, Genberg M, Jernberg T, Lagerqvist B, Lindahl B, Venge P, Wallentin L.. The prognostic and therapeutic implications of increased troponin T levels and ST depression in unstable coronary artery disease: the FRISC II invasive troponin T electrocardiogram substudy. Am Heart J 2002;143:760–767. [DOI] [PubMed] [Google Scholar]
- 40. Neumann FJ, Kastrati A, Pogatsa-Murray G, Mehilli J, Bollwein H, Bestehorn HP, Schmitt C, Seyfarth M, Dirschinger J, Schömig A.. Evaluation of prolonged antithrombotic pretreatment ("cooling-off" strategy) before intervention in patients with unstable coronary syndromes: a randomized controlled trial. JAMA 2003;290:1593–1599. [DOI] [PubMed] [Google Scholar]
- 41. Arora S, Matsushita K, Qamar A, Stacey RB, Caughey MC.. Early versus late percutaneous revascularization in patients hospitalized with non ST-segment elevation myocardial infarction: The atherosclerosis risk in communities surveillance study. Catheter Cardiovasc Interv 2018;91:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sabatine MS, Antman EM.. The thrombolysis in myocardial infarction risk score in unstable angina/non-ST-segment elevation myocardial infarction. J Am Coll Cardiol 2003;41(4 Suppl S):89s–95s. [DOI] [PubMed] [Google Scholar]
- 43. Bekler A, Altun B, Gazi E, Temiz A, Barutçu A, Güngör Ö, Özkan MT, Özcan S, Gazi S, Kırılmaz B.. Comparison of the GRACE risk score and the TIMI risk index in predicting the extent and severity of coronary artery disease in patients with acute coronary syndrome. Anatol J Cardiol 2015;15:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cohen MG, Filby SJ, Roe MT, Chen AY, Menon V, Stouffer GA, Gibler WB, Smith SC, Pollack CV, Peterson ED, Ohman EM.. The paradoxical use of cardiac catheterization in patients with non-ST-elevation acute coronary syndromes: lessons from the can rapid stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J 2009;158:263–270. [DOI] [PubMed] [Google Scholar]
- 45. Roe MT, Chen AY, Mehta RH, Li Y, Brindis RG, Smith SC Jr, Rumsfeld JS, Gibler WB, Ohman EM, Peterson ED.. Influence of inpatient service specialty on care processes and outcomes for patients with non ST-segment elevation acute coronary syndromes. Circulation 2007;116:1153–1161. [DOI] [PubMed] [Google Scholar]
- 46. Lee CH, Tan M, Yan AT, Yan RT, Fitchett D, Grima EA, Langer A, Goodman SG.. Use of cardiac catheterization for non-ST-segment elevation acute coronary syndromes according to initial risk: reasons why physicians choose not to refer their patients. Arch Intern Med 2008;168:291–296. [DOI] [PubMed] [Google Scholar]
- 47. Bhatt DL, Roe MT, Peterson ED, Li Y, Chen AY, Harrington RA, Greenbaum AB, Berger PB, Cannon CP, Cohen DJ, Gibson CM, Saucedo JF, Kleiman NS, Hochman JS, Boden WE, Brindis RG, Peacock WF, Smith SC, Pollack CV, Gibler WB, Ohman EM, Crusade Investigators FT.. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA 2004;292:2096–2104. [DOI] [PubMed] [Google Scholar]
- 48. Pilote L, Miller DP, Califf RM, Rao JS, Weaver WD, Topol EJ.. Determinants of the use of coronary angiography and revascularization after thrombolysis for acute myocardial infarction. N Engl J Med 1996;335:1198–1205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Atherosclerosis Risk in Communities (ARIC) study’s data are owned by the National Heart Lung and Blood Institute (NHLBI). The data are publicly available to qualified investigators with an approved manuscript proposal and data use agreement. Upon reasonable request, the ARIC coordinating center may make the data, analytic methods, and study materials available to other researchers for purposes of reproducing the results or replicating the procedure.