Cardiac myosin-binding protein C (cMyBP-C, MYBPC3, cMyC; UniProtKB—Q14896) is a 140 kDa sarcomeric protein that is loosely associated with both myosin and actin. It was identified in the coronary effluent from ischaemic myocardium about 10 years ago and after systematic screening of monoclonal antibodies a sensitive sandwich immunoassay was formulated.1 Using this assay, cMyC has been measured in a variety of patient groups and directly compared to cardiac troponin T (cTnT) and cardiac troponin I (cTnI) measured in the same blood samples using high-sensitivity assays.1
Direct comparisons of cMyC with cTnI/T have established the following:
cMyC is more abundant than cTnT/I and consequently it is possible to measure smaller volumes of myocardium undergoing injury, based on spiking human heart into human blood.1
After myocardial injury cMyC can be detected in the blood earlier, and its concentration rises more rapidly, than cTnT/I or novel RNA biomarkers.1,2
Based on blood samples taken at presentation in patients with a suspected acute coronary syndrome, the diagnostic accuracy for acute myocardial infarction of cMyC, cTnI, and cTnT are similar, but cMyC is more efficient at rapid rule out.3
Despite cMyC having a sarcomeric location and kinetic profile that differs from cTnT/I, its concentration is similarly increased by chronic myocardial injury and acute (non-ischaemic) myocardial injury.3,4
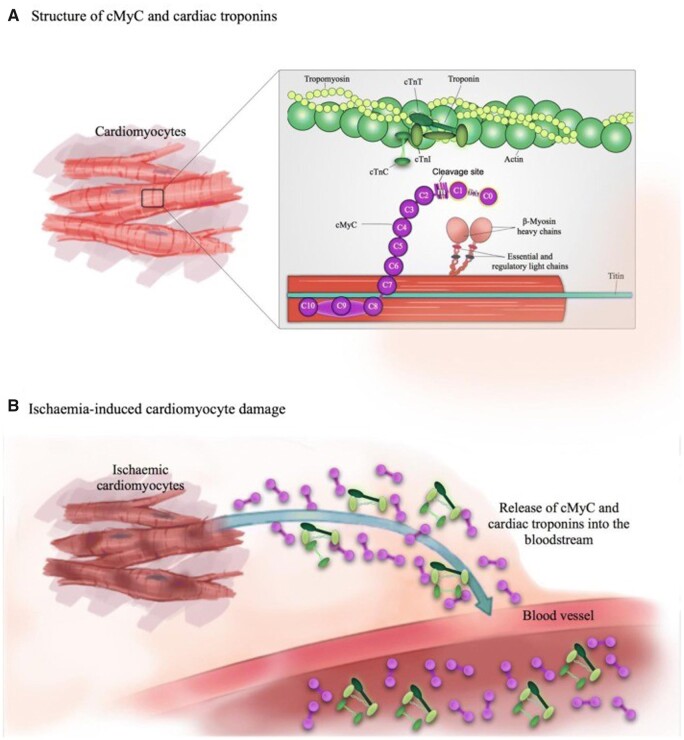
Based on these results the diagnostic performance of cMyC is similar to cTnT/I. Whilst this is a remarkable accomplishment, the question remains whether cMyC has sufficient distinctive advantage to possibly replace cTnT/I or add enough incremental value to be used in conjunction with cTnT/I? Figure 1 shows the location of cMyC and cTn within the cardiac sarcomere and their migration into the bloodstream. One of the difficulties with any new biomarker of myocardial injury is that cTnT/I is not just the comparator, but also to some extent the referee—since the final adjudicated diagnosis of acute myocardial infarction is heavily reliant on its use—creating strong confirmation bias. This is particularly problematic in real-world studies where treatment decisions, such as early discharge and lack of subsequent blood samples, are driven by the comparator. One possible way to overcome this bias is to perform randomized controlled diagnostic trials where the biomarker concentration drives treatment decisions and the endpoints are clinical events (not biomarker determined). Such trials require enormous resource and superior analytic sensitivity does not necessarily translate into a clinically meaningful advantage.5 For these reasons, current research efforts focus on further improving the specificity of the cMyC assay for acute myocardial infarction caused by sudden reductions in myocardial blood supply.
Figure 1.
Structure of cMyC and relationship with the cTn complex from ref.1 . Panel A depicts the location of the troponins (cTnC, cTnI and cTnT) and cardiac myosin binding protein C (cMyC) within the sarcomere of a cardiac myocyte. cMyC undergoes clevage to generate an amino-terminal fragment containing the C0 and C1 domains. The clevage of cMyC is a regulated process and is prevented by phosphorylation of key amino acids by stress-responsive kinases such as protein kinase A. Panel B depicts the forms of cTn and cMyC that enter the circulation from the damaged cardiomyocyte. The image is simplified since the biomarkers circulate as complexes of full-length proteins as well as various fragments.
In summary, cMyC is a myocardial injury biomarker that behaves similarly to cTnI/T but is more sensitive. However, this advantage is being challenged by the combination of confirmation bias, the evolving analytic sensitivity of the cTn assays and methodological difficulties in translating improvements in analytic sensitivity into reductions in hard clinical endpoints.
Other members of the Study Group on Biomarkers of the ESC Association for Acute Cardiovascular Care include:
Evangelos Giannitsis1, Allan S. Jaffe2, Kurt Huber3, Johannes Mair4, Louise Cullen5, Ola Hammarsten6, Martin Möckel7, Konstantin Krychtiuk8, Kristian Thygesen9, and Bertil Lindahl10
1Department of Cardiology, University Heidelberg, Heidelberg, Germany
2Mayo Clinic and Medical School, Rochester, MN, USA
3Department of Medicine, Cardiology and Intensive Care Medicine, Wilhelminenhospital, and Sigmund Freud University, Medical School, Vienna, Austria
4Department of Internal Medicine III—Cardiology and Angiology, Medical University Innsbruck, Innsbruck, Austria
5Emergency and Trauma Centre, Royal Brisbane and Women’s Hospital, University of Queensland, Herston, QLD, Australia
6Department of Clinical Chemistry and Transfusion Medicine, University of Gothenburg, Gothenburg, Sweden
7Division of Emergency Medicine, Charité-Universitätsmedizin Berlin, Berlin, Germany
8Department of Internal Medicine II, Division of Cardiology, Medical University of Vienna, Vienna, Austria
9Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark
10Department of Medical Sciences, Uppsala University, Uppsala, Sweden
Funding
M.S.M. is supported by the United Kingdom Department of Health through the National Institute for Health Research Biomedical Research Centre award to Guy’s and St Thomas’ National Health Service Foundation Trust.
N.L.M. is supported by a Research Excellence Award (RE/18/5/34216) and a Chair Award (CH/F/21/90010) from the British Heart Foundation.
Conflict of interest: M.S.M. is named as an inventor on a patent held by King’s College London for the detection of cardiac myosin-binding protein C as a biomarker of myocardial injury. C.M. has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the University Hospital Basel, the University of Basel; Abbott, Beckman Coulter, Brahms, Idorsia, Novartis, Ortho Diagnostics, Quidel, Roche, Siemens, Singulex, Sphingotec outside the submitted work, as well as speaker honoraria/consulting honoraria from Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, BMS, Novartis, Osler, Roche, and Sanofi, all paid to the institution. E.G. reports personal fees from Bayer Vital, grants and personal fees from Roche Diagnostics, personal fees from Brahms Deutschland, personal fees from Boehringer Ingelheim, grants and personal fees from Daiichi Sankyo, grants from Deutsche Herzstiftung, outside the submitted work. J.M. reports research collaboration on cardiac biomarker point of care diagnostics with Siemens Healthineers Netherlands. A.S.J. reports personal fees from Abbott, personal fees from Siemens, personal fees from Beckman-Coulter, personal fees from Roche, personal fees from ET Healthcare, personal fees from Sphingotec, personal fees from Quidel, personal fees from Brava, personal fees from Blade, personal fees from Novartis, outside the submitted work. N.L.M. reports research grants awarded to the University of Edinburgh from Abbott Diagnostics and Siemens Healthineers outside the submitted work, and honoraria from Abbott Diagnostics, Siemens Healthineers, Roche Diagnostics and LumiraDx. M.M. reports grants and personal fees from Roche Diagnostics, grants and personal fees from BRAHMS GmbH, grants from Innovationsfonds, grants from BMBF, grants from Berlin University Alliance, outside the submitted work. All other coauthors had no conflicts to report.
References
- 1. Kaier TE, Alaour B, Marber M.. Cardiac myosin-binding protein C-from bench to improved diagnosis of acute myocardial infarction. Cardiovasc Drugs Ther 2019;33:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schulte C, Barwari T, Joshi A, Theofilatos K, Zampetaki A, Barallobre-Barreiro J, Singh B, Sörensen NA, Neumann JT, Zeller T, Westermann D, Blankenberg S, Marber M, Liebetrau C, Mayr M.. Comparative analysis of circulating noncoding RNAs versus protein biomarkers in the detection of myocardial injury. Circ Res 2019;125:328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaier TE, Twerenbold R, Puelacher C, Marjot J, Imambaccus N, Boeddinghaus J, Nestelberger T, Badertscher P, Sabti Z, Giménez MR, Wildi K, Hillinger P, Grimm K, Loeffel S, Shrestha S, Widmer DF, Cupa J, Kozhuharov N, Miró Ò, Martín-Sánchez FJ, Morawiec B, Rentsch K, Lohrmann J, Kloos W, Osswald S, Reichlin T, Weber E, Marber M, Mueller C.. Direct comparison of cardiac myosin-binding protein C with cardiac troponins for the early diagnosis of acute myocardial infarction. Circulation 2017;136:1495–1508. Erratum in: Circulation 2017;136:e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kozhuharov N, Wussler D, Kaier T, Strebel I, Shrestha S, Flores D, Nowak A, Sabti Z, Nestelberger T, Zimmermann T, Walter J, Belkin M, Michou E, Lopez Ayala P, Gualandro DM, Keller DI, Goudev A, Breidthardt T, Mueller C, Marber M; BASEL V Investigators. Cardiac myosin-binding protein C in the diagnosis and risk stratification of acute heart failure. Eur J Heart Fail 2021;23:716–725. [DOI] [PubMed] [Google Scholar]
- 5. Shah ASV, Anand A, Strachan FE, Ferry AV, Lee KK, Chapman AR, Sandeman D, Stables CL, Adamson PD, Andrews JPM, Anwar MS, Hung J, Moss AJ, O'Brien R, Berry C, Findlay I, Walker S, Cruickshank A, Reid A, Gray A, Collinson PO, Apple FS, McAllister DA, Maguire D, Fox KAA, Newby DE, Tuck C, Harkess R, Parker RA, Keerie C, Weir CJ, Mills NL; High-STEACS Investigators. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet 2018;392:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]