Abstract
Background
We have previously shown that high salt stimulates the expression of miR-429 in the renal medulla, which induces mRNA decay of HIF prolyl-hydroxylase 2 (PHD2), an enzyme to promote the degradation of hypoxia-inducible factor (HIF)-1α, and increases the HIF-1α-mediated activation of antihypertensive genes in the renal medulla, consequently promoting extra sodium excretion. Our preliminary results showed that high salt-induced increase of miR-429 was not observed in Dahl S rats. This present study determined whether correction of this impairment in miR-429 would reduce PHD2 levels, increase antihypertensive gene expression in the renal medulla and attenuate salt-sensitive hypertension in Dahl S rats.
Methods
Lentiviruses encoding rat miR-429 were transfected into the renal medulla in uninephrectomized Dahl S rats. Sodium excretion and blood pressure were then measured.
Results
Transduction of lentiviruses expressing miR-429 into the renal medulla increased miR-429 levels, decreased PHD2 levels, and upregulated HIF-1α target gene NOS-2, which restored the adaptive mechanism to increase the antihypertensive gene after high-salt intake in Dahl S rats. Functionally, overexpression of miR-429 transgene in the renal medulla significantly improved pressure natriuretic response, enhanced urinary sodium excretion, and reduced sodium retention upon extra sodium loading, and consequently, attenuated the salt-sensitive hypertension in Dahl S rats.
Conclusions
Our results suggest that the impaired miR-429-mediated PHD2 inhibition in response to high salt in the renal medulla may represent a novel mechanism for salt-sensitive hypertension in Dahl S rats and that correction of this impairment in miR-429 pathway could be a therapeutic approach for salt-sensitive hypertension.
Keywords: blood pressure, hypertension, hypoxia-inducible factor, microRNA, nitric oxide synthase 2, sodium excretion
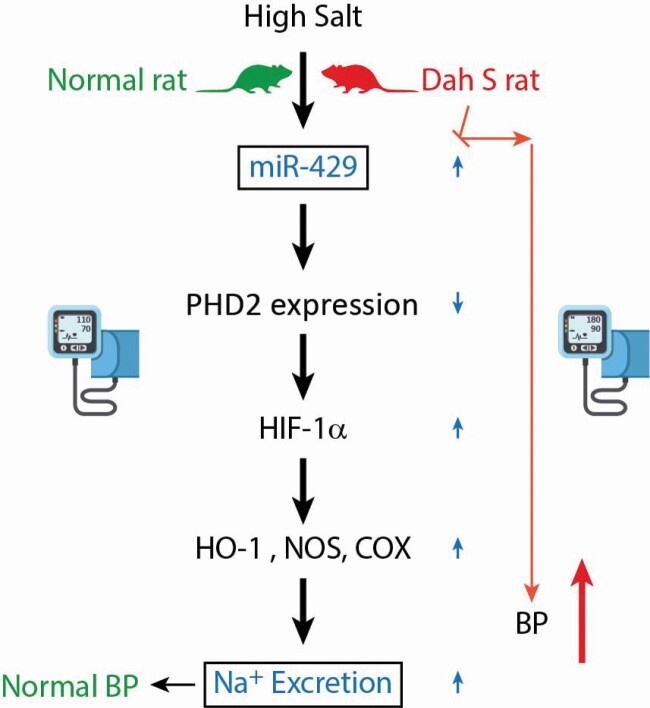
Graphical Abstract
Graphical Abstract.
Salt-sensitive hypertension is a significant health issue associated with enhanced risks of morbidity and mortality worldwide,1,2 while its mechanism remains to be fully elucidated. It is well established that the renal medulla plays a critical role in sodium homeostasis and the long-term control of arterial pressure, and that impaired renal medullary function underlies the development of salt-sensitive hypertension.3,4 We have previously found that high-salt intake inhibits the expression of HIF prolyl-hydroxylase 2 (PHD2), an enzyme promoting the degradation of hypoxia-inducible factor (HIF)-1α.5 This high salt-induced inhibition of PHD2 may function as an upstream signal to produce the HIF-1α-mediated activation of antihypertensive genes in the renal medulla and promote extra sodium excretion.5 Our recent serial studies have further demonstrated that PHD2 regulation of HIF-1α-mediated gene pathway in the renal medulla is one of the important adaptive mechanisms to high-salt challenge,6 and impaired PHD2 regulation of HIF-1α-mediated gene activation in the renal medulla may be responsible for the development of salt-sensitive hypertension in Dahl S rats,7,8 a widely used genetic model of human salt-sensitive hypertension.9
MicroRNAs (miRNAs) are small noncoding RNA molecules that downregulate gene expression at post-transcriptional level.10–12 Accumulating evidence has indicated that miRNAs are crucial players in the pathogenesis of hypertension.13,14 Our most recent study found that high-salt intake stimulated the expression of miR-429, which promoted the decay of PHD2 mRNA and consequently led to the accumulation of HIF-1α and the activation of many HIF-1α-regulated antihypertensive genes in the renal medulla in normal rats,15 and that inhibition of miR-429 in the renal medulla enhanced sodium retention and subsequently caused salt-sensitive hypertension.15 This study was performed in normotensive animals and the results suggest that miR-429-mediated regulation of PHD2/HIF-1α pathway may be an important molecular mechanism to promote extra sodium excretion and maintain blood pressure in response to high-salt challenge. However, it remains unknown whether the expression pattern of miR-429 is impaired in the renal medulla in Dahl S rats and whether renal medullary miR-429 pathway contributes to the pathogenesis of salt-sensitive hypertension.
Here, we first measured the levels of miR-429 in the renal medulla and found that the high salt-induced increase of miR-429 level was not observed in Dahl S rats. Then, we increased the miR-429 level in the renal medulla by in vivo transduction of lentiviruses expressing miR-429 in Dahl S rats and detected its effects on the expression of PHD2 and HIF-1α target gene, the pressure natriuresis response, the renal sodium excretion upon sodium overload, and the arterial blood pressure after high-salt challenge. Our data demonstrated that correction of the impairment in miR-429-mediated regulation of PHD2/HIF-1α pathway in response to high-salt intake attenuated salt-sensitive hypertension in Dahl S rats.
METHODS
Animals
Male Dahl salt-sensitive (Dahl S) and SS-13BN rats (Charles River), weighting 250–350 g were used. The SS-13BN rat is a subcolony of Dahl S rat with substitution of chromosome 13 from BN rat. The SS-13BN rat showed salt-resistant, had minimal genotypic difference from Dahl S rat compared with other control strains,16 and thus served as the control strain for Dahl S rat in the present study. Rats were kept on a low-salt diet (0.4% NaCl, Dyets), with some fed a high-salt diet (8% NaCl) as indicated in the Results section. All the animal protocols were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Transduction of lentivirus encoding rat miR-429 into the renal medulla
Dahl S rats were uninephrectomized 1 week before. Then, 2 × 107 TU lentiviruses encoding rat miR-429 (catalog#LP-RmiR6207-MR03-0205-S, GeneCopoeia) were injected into the renal medulla of the remaining left kidney at several sites. Lentiviruses expressing the scramble control miRNA (catalog#LP-CmiR0001-MR03-0205, GeneCopoeia) were used in control animals. In preliminary experiment, we confirmed that transduction of the miR-429 lentivirus into cultured rat cells remarkably increased the expression of miR-429.
Measurement of pressure natriuresis in response to the elevations of renal perfusion pressure
The rats were uninephrectomized and transducted with miR-429 or scramble miRNA lentiviruses as described above and kept on low-salt diet. Ten days after transduction, pressure natriuresis response was measured as described previously.7
Measurement of urinary sodium excretion in response to acute sodium loading
Additional groups of animals transducted with miR-429 or scramble miRNA lentiviruses as above were surgically prepared for the measurement of urinary sodium excretion as we described before.6 Briefly, after surgery and equilibration for 1 hour followed by 2 10-minute control period urine sample collections, a 5% body weight isotonic saline load was administered intravenously and 3 10-minute samples were collected over 30 minutes,6,17 and then 3 more 10-minute post-control samples were taken. Urinary volume and sodium excretion were measured and factored per gram kidney weight.
Measurement of chronic sodium balance
Additional group of Dahl S rats were treated the same as above and then housed in metabolic cages. Daily sodium balance was calculated by subtracting sodium excretion from sodium intake. After 1 day of control measurements, rats were fed with 2% NaCl water, and daily sodium balance was measured for 3 more days.17
Measurement of chronic arterial blood pressure
Mean arterial pressure (MAP) was measured daily for 3 hours using a telemetry transmitter (Data Sciences International) as we described previously.17 After baseline MAP was recorded for 2 consecutive control days while the rats remained on a low-salt diet, rats were switched to the high-salt diet (Dyets) and MAP was monitored for 12 more days. The groups of animals included rats treated with scramble miRNA lentivirus (scramble) + low-salt diet (LS), scramble + high-salt diet (HS), and miR-429 lentivirus + HS. At the study endpoint, renal tissues were collected for protein and RNA isolation later.
RNA extraction and quantitative RT-PCR analysis of miR-429, PHD2, and NOS-2 mRNA
Total RNA from the renal medulla was extracted with TRIzol solution. The miR-429 level was quantified by quantitative reverse transcription-PCR (qRT-PCR) using TaqMan MicroRNA Assays kits (Applied Biosystems) as per the manufacturer’s instruction, with U6 small nuclear RNA as internal normalizer. For PHD2 and NOS-2 mRNA quantification, cDNA was synthesized by using cDNA Synthesis Kit (Bio-Rad) and then the cDNA products were amplified using TaqMan Gene Expression Assays kits (Applied Biosystems) with 18S ribosomal RNA as internal control. The data were measured using a Stratagene Mx3000P Real-Time PCR Detection System (Agilent Technologies), and gene expression was calculated using cycle threshold (Ct) values in accordance with the ΔΔCt method.15
Western blot analysis of PHD2 in the renal medulla
Renal tissue homogenates from the renal medulla were extracted as previously described18 and then subjected (50 µg) to western blot analysis. Primary antibody was from Novus Biologicals: anti-PHD2 (rabbit polyclonal, 1:500). The intensities of the blots were analyzed using ImageJ software. The β-actin was used as internal control.
Statistical analysis
Data are presented as means ± SEM. Significant differences among and within multiple groups were examined using analysis of variance for repeated measures, followed by Tukey’s range test. Student’s t-test was used to evaluate the significance of differences between 2 groups of experiments. A value of P < 0.05 was considered statistically significant.
RESULTS
Comparison of high salt-induced changes in miR-429 expression in the renal medulla between Dahl S and SS-13BN rats
As summarized in Figure 1, there is no difference in the basal expression levels of renal medullary miR-429 between these 2 animal strains. However after high-salt intake the expression of miR-429 in the renal medulla was significantly increased in SS-13BN rats and this high salt-induced upregulation of miR-429 was not observed in Dahl S rats, indicating the impaired response of miR-429 in the renal medulla of Dahl S rats upon high-salt intake.
Figure 1.
Comparison of high salt-induced miR-429 expression in the renal medulla between Dahl salt-sensitive and SS-13BN rats. Total RNA from the renal medulla was extracted from Dahl S or SS-13BN rats fed with a low salt or high salt. The miR-429 expression was determined by quantitative reverse transcription-PCR (qRT-PCR) using TaqMan MicroRNA Assays kits. The relative levels of miR-429 were normalized to the value of SS-13BN LS group. *P < 0.05 vs. the rest (n = 6). Abbreviations: HS, high salt; LS, low salt; SS, Dahl salt-sensitive rats.
Effects of overexpression of miR-429 transgene on the levels of miR-429, PHD2, and NOS-2 in the renal medulla in Dahl S rats
Intrarenal medullary transduction of miR-429 lentivirus dramatically increased the miR-429 levels in Dahl S rats (Figure 2a), which was accompanied by a notable knockdown of PHD2 expression in the renal medulla after high-salt treatment (Figure 2b,c). Consequently, the expression of NOS-2, which served as a prototype of HIF-1α target genes and one of the important HIF-1α-mediated activations of antihypertensive genes, was significantly upregulated in the renal medulla in rats treated with miR-429 lentiviruses (Figure 2d). Taken together, these results confirmed the successful overexpression of miR-429 transgene and consequent inhibition of PHD2 in the renal medulla, which stimulated the activation of antihypertensive gene in response to high-salt intake.
Figure 2.
Effects of overexpression of miR-429 transgene by lentivirus transduction on the levels of miR-429, PHD2, and nitric oxide synthase 2 (NOS-2) in the renal medulla in Dahl S rats fed with low salt or high salt. Renal medullary samples were harvested at the end of blood pressure recording after 12 days high-salt intake. (a) Expression of medullary miR-429. (b) mRNA levels of PHD2. (c) Representative ECL gel documents of Western blot analyses depicting the protein levels of PHD2 and summarized intensities. (d) mRNA levels of NOS-2. The relative levels were normalized to the values of LS group. *P < 0.05 vs. the scramble (n = 6). Abbreviations: HS, high salt; LS, low salt; miR-429, transduction of lentiviruses expressing the miR-429; Scramble, transduction of lentiviruses expressing the scramble control miRNA.
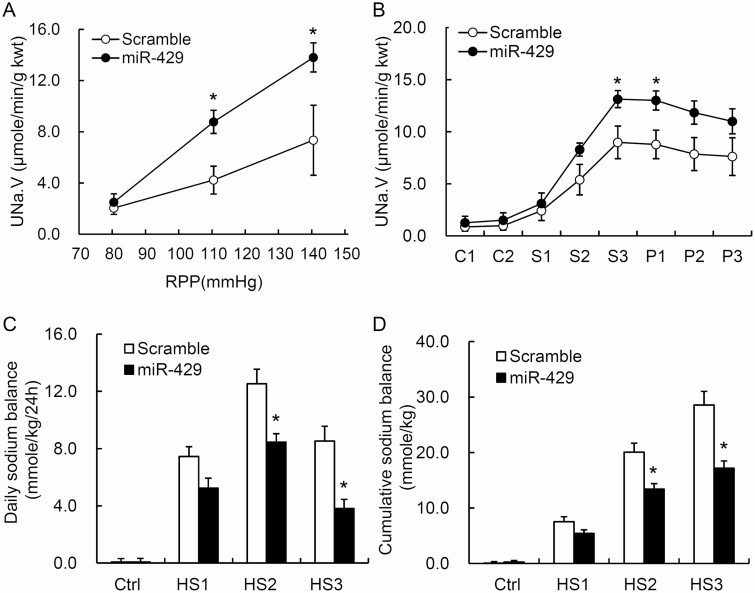
Effects of overexpression of miR-429 transgene in the renal medulla on pressure natriuresis in response to the elevation of renal perfusion pressure in Dahl S rats
The urinary sodium excretion rates were increased in response to the elevation of renal perfusion pressure. However, these pressure natriuretic responses were significantly enhanced in miR-429 lentivirus-treated rats compared with the scramble control group (Figure 3a). The urine volume and pressure diuretic responses showed the similar patterns (data not shown). These data indicated that overexpression of miR-429 transgene improved renal medullary function in Dahl S rats.
Figure 3.
Effect of overexpression of miR-429 transgene in the renal medulla on pressure natriuresis, urinary sodium excretion in response to acute sodium loading and salt balances after chronic sodium loading. (a) Urinary sodium excretion (UNa·V) in response to the elevations of renal perfusion pressure (RPP). kwt, kidney weight. (b) UNa·V in response to acute Na+ loading (labels for X axis: C, samples collected during control condition; S, samples collected during sodium loading; P, samples collected during post-control condition following sodium loading). (c and d) Daily and cumulative sodium balance. *P < 0.05 vs. the scramble (n = 6). Abbreviations: Ctrl, day on low-salt diet; HS, day on high-salt diet; kwt, kidney weight; miR-429, rats that transduced with lentiviruses expressing the miR-429; Scramble, rats that were transduced with lentiviruses expressing the scramble control miRNA.
Effects of overexpression of miR-429 transgene in the renal medulla on urinary sodium excretion in response to acute sodium loading in Dahl S rats
The acute sodium loading increased urinary sodium excretion (UNa·V). These increases in UNa·V were considerably enhanced in rats treated with miR-429 lentivirus compared with scramble control (Figure 3b). The responses of urine volume showed the similar patterns (data not shown). These results demonstrated that overexpression of miR-429 transgene in the renal medulla remarkably improved the capability of the kidneys to remove extra sodium load in Dahl S rats.
Effect of overexpression of miR-429 in the renal medulla on salt balance in Dahl S rats
A chronic high-salt intake induced a positive daily and cumulative salt balance. The daily positive salt balances were progressively increased in the first 2 days and decreased on the third day of high-salt diet. The positive salt balances were remarkably attenuated in miR-429-treated rats compared with the scramble control group (Figure 3c,d). These results suggested that overexpression of miR-429 transgene in the renal medulla dramatically reduces the sodium retention in response to chronic sodium overloading in Dahl S rats.
Effect of overexpression of miR-429 in the renal medulla on arterial blood pressure after high-salt challenge in Dahl S rats
The MAP changes are summarized in Figure 4. There was no difference in baseline MAP among rats treated with the scramble or miR-429 lentivirus when the animals were maintained on a low-salt diet. After the rats were challenged with a high-salt diet, the MAP was progressively increased from 111 ± 2 to 144 ± 4 mm Hg in the scramble group. However, this high salt-induced increase in MAP was significantly blocked and the MAP was only reached to 129 ± 2 mm Hg in miR-429 lentivirus-treated rats by the end of experiment (Figure 4). These results suggested that overexpression of miR-429 transgene in the renal medulla significantly improves the sodium excretion and attenuates salt-sensitive hypertension in Dahl S rats.
Figure 4.
Effect of overexpression of miR-429 transgene in the renal medulla on mean arterial pressure (MAP) after high-salt (HS) challenge in Dahl S rats. MAP was measured daily for 3 hours using a telemetry transmitter. After baseline MAP was recorded for 2 consecutive control days while the rats remained on a low-salt diet, rats were switched to the high-salt diet and MAP was monitored for 12 more days. *P < 0.05 vs. other groups (n = 7). Abbreviations: LS, low salt; miR-429, transduction of lentiviruses expressing the miR-429; Scramble, transduction of lentiviruses expressing the scramble control miRNA.
Discussion
The present study demonstrated that the high salt-induced increase of miR-429 level was not observed in the renal medulla of Dahl S rats, and that renal medullary overexpression of miR-429 transgene successfully increased the expression of miR-429 in the renal medulla, which remarkably corrected the impairment in PHD2 regulation of downstream antihypertensive gene activation upon high-salt intake, functionally enhanced the urinary sodium excretion in response to the elevations of renal perfusion pressure and extra sodium loading, dramatically decreased the sodium retention, and consequently attenuated the development of salt-sensitive hypertension in Dahl S rats.
Our present study found that the response of miR-429 to high-salt challenge was impaired in the renal medulla of Dahl S rats. Given the important role of miR-429 in the regulation of PHD2/HIF-1α signaling pathway,15 the present finding was consistent with our previous reports showing the impairment in PHD2/HIF-1α-mediated renal adaptation to high-salt challenge in this animal model.6–8 In addition, we have previously found that the high salt-induced PHD2 inhibition,5 and consequent HIF-1α-mediated activation of the antihypertensive genes are impaired in the renal medulla of Dahl S rats.8,19,20 The deficient miR-429 response to the high salt may be the upstream mediator for the abnormality in PHD2/HIF-1α-mediated renal adaptation to high-salt challenge in the renal medulla of Dahl S rats. Our current findings were consistent with previous reports by others showing that miR-429 bond the mRNA untranslated 3′ region of PHD2 and that upregulation of miR-429 was responsible for the downregulation of PHD2 mRNA and subsequent increase of HIF-1α during cerebral ischemia.21,22
Considering the functional importance of PHD2/HIF-1α-mediated gene activation in the management of renal sodium handling during high-salt challenge,8 correction of the impairment in miR-429-mediated regulation of PHD2/HIF-1α pathway in the renal medulla allowed us to determine the contribution of impaired miR-429 signaling to the deficient sodium handling and pathogenesis of salt-sensitive hypertension. We therefore evaluated the effects of miR-429 overexpression on pressure natriuresis response, a crucial mechanism for the stabilization of long-term blood pressure,23,24 which has been shown to be impaired in Dahl S rats. Our data showed that overexpression of miR-429 transgene decreased PHD2 levels and subsequently stimulated the activation of a prototype of antihypertensive genes in the renal medulla, which would be expected to improve the pressure natriuresis response in this model. Indeed, our data showed that transduction of miR-429 lentiviral particles into the renal medulla dramatically enhanced the pressure natriuresis, indicating that the impaired miR-429-mediated regulation of PHD2/HIF-1α pathway may be responsible for the renal medullary dysfunction in Dahl S rats.
To further determine the effects of impaired miR-429 regulation by high salt on the capacity of renal sodium handling, we measured the urinary sodium excretion after acute sodium overload and the sodium balance in response to chronic high-salt intake. Our previous studies have demonstrated that high salt-induced inhibition of PHD2 levels may serve as a signal to trigger downstream HIF-1α-mediated renal adaptive mechanism to maintain salt balance,5–7 which is impaired in Dahl S rats. Therefore, inhibition of PHD2 levels by overexpressing miR-429 transgene to correct the deficiency of PHD2-mediated regulation of downstream antihypertensive genes in the renal medulla would be expected to improve renal salt handling in Dahl S rats. The results from the acute and chronic sodium overloading experiments in the present study demonstrated that recovery of miR-429 levels in the renal medulla significantly restored the renal capability to promote extra sodium excretion, which reduced sodium retention. In addition, these results support a notion that the impairment of renal medullary miR-429 response to high salt may account for the dysregulation of renal sodium excretion in Dahl S rats.
Given the key role of pressure natriuresis and normal renal medullary function in the long-term management of blood pressure,4,23,25,26 overexpression of miR-429 transgene in the renal medulla to ameliorate renal sodium excretions might be beneficial for blood pressure control in responses to high-salt intake in Dahl S rats. To test this hypothesis, we compared MAPs between rats treated with miR-429 transgene and scramble miRNA in the renal medulla. The results showed that the MAP was progressively increased by high-salt intake in the scramble miRNA group and this high salt-induced hypertension was remarkably attenuated in miR-429-treated rats. These results suggested that induction of miR-429 in the renal medulla may restore the impaired renal sodium handling and attenuate salt-sensitive hypertension induced in Dahl S rats. Therefore, miR-429 may serve as an important upstream mediator in the regulation of salt sensitivity of blood pressure, through governing the renal adaptation mechanism associated with PHD2/HIF-1α pathway in response to high-salt challenge.
The present study did not attempt to explore the underlying mechanisms that cause the failed elevation of miR-429 levels after high-salt intake in the renal medulla of Dahl S rats. The transcription of miRNA genes is a major regulation level for specific miRNA expression.27 Therefore, transcription factors serve as major factors for the regulation of miRNA levels.27 Several transcriptional factors including Krüppel-like factor 8 (KLF8),28 p63 and/or p7329 have been shown to bind the miR-429 promoter region and regulate its expression transcriptionally. Future studies are required to detect the response of these transcriptional factors to high-salt intake and determine their roles in miR-429 expression.
Our studies have suggested that miR-429/PHD2/HIF-1α pathway could be potential targets for the management of salt-sensitive hypertension. Because high salt-induced inhibition of PHD and activation of HIF-1α in the renal medulla is not observed in Dahl S rats,7 inhibition of PHD to activate HIF-1α would be a potential therapeutic strategy. Notably, PHD inhibitors have been tested in clinic.30 These PHD inhibitors would be promising candidates for salt-sensitive hypertension. Additionally, miRNA-based therapies have emerged as a new frontier in the treatment of diseases and several miRNA therapeutics have reached clinical trials.31,32 Interestingly, approaches for kidney-targeted gene delivery are also under development, such as different viral vectors and DNA tetrahedrons,31,33 which may make it possible to manipulate miRNA in kidneys in the future. Although establishment of a mature miR-429 therapy is far beyond the main scope of the current study, our results would likely pave the way for the future of miR-429-based therapeutics for salt-sensitive hypertension.
Another limitation in the present study is whether renal medullary miR-429 influences circadian rhythm of blood pressure remains undetected. Salt-sensitive hypertension is characterized by the nondipper or riser pattern nocturnal hypertension, which associates with poor prognosis.32,34 Accumulating evidence has shown that miRNAs may play crucial roles in the mammalian circadian rhythm of different diseases,35,36 including nocturnal hypertension.37 However, the current study was not able to tell whether miR-429-mediated antihypertensive effects influence blood pressure rhythm. Further studies to monitor 24-hour both diurnal and nocturnal blood pressure are warranted to determine the effect of miR-429 on circadian variability.
In summary, the present study for the first time demonstrated that high salt-induced upregulation of miR-429 was impaired in the renal medulla of Dahl S rats, and that correction of the impaired miR-429 response successfully restored the PHD2-associated adaptive activation of antihypertensive gene in response to high-salt challenge in the renal medulla, which consequently improved pressure natriuresis, promoted sodium excretion and reduced sodium retention after extra sodium loading, thereby attenuating a salt-sensitive hypertension. These data indicate that the impairment in miR-429-mediated PHD2 inhibition in the renal medulla during high-salt challenge may represent a novel pathogenic mechanism for salt-sensitive hypertension in Dahl S rats. The miR-429 may serve as an important therapeutic target for better prevention and/or treatment of salt-sensitive hypertension.
FUNDING
This work was supported by grants from the National Institutes of Health (HL145163 and DK107991).
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension 1994; 23:531–550. [DOI] [PubMed] [Google Scholar]
- 2. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001; 37:429–432. [DOI] [PubMed] [Google Scholar]
- 3. Cowley AW Jr, Mattson DL, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension 1995; 25:663–673. [DOI] [PubMed] [Google Scholar]
- 4. Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 2003; 284:R13–R27. [DOI] [PubMed] [Google Scholar]
- 5. Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ, Li N. Hypoxia-inducible factor prolyl-hydroxylase 2 senses high-salt intake to increase hypoxia inducible factor 1alpha levels in the renal medulla. Hypertension 2010; 55:1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu Q, Liu M, Han WQ, Li PL, Wang Z, Li N. Overexpression of HIF prolyl-hydoxylase-2 transgene in the renal medulla induced a salt sensitive hypertension. J Cell Mol Med 2012; 16:2701–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu Q, Hu J, Han WQ, Zhang F, Li PL, Wang Z, Li N. Silencing of HIF prolyl-hydroxylase 2 gene in the renal medulla attenuates salt-sensitive hypertension in Dahl S rats. Am J Hypertens 2014; 27:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li N. Hypoxia inducible factor-1α-mediated gene activation in the regulation of renal medullary function and salt sensitivity of blood pressure. Am J Cardiovasc Dis 2012; 2:208–215. [PMC free article] [PubMed] [Google Scholar]
- 9. Tobian L. Salt and hypertension. Lessons from animal models that relate to human hypertension. Hypertension 1991; 17:I52–I58. [DOI] [PubMed] [Google Scholar]
- 10. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet 2011; 12:861–874. [DOI] [PubMed] [Google Scholar]
- 11. Bhatt K, Mi QS, Dong Z. microRNAs in kidneys: biogenesis, regulation, and pathophysiological roles. Am J Physiol Renal Physiol 2011; 300:F602–F610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Rooij E. The art of microRNA research. Circ Res 2011; 108:219–234. [DOI] [PubMed] [Google Scholar]
- 13. Liang M, Liu Y, Mladinov D, Cowley AW Jr, Trivedi H, Fang Y, Xu X, Ding X, Tian Z. MicroRNA: a new frontier in kidney and blood pressure research. Am J Physiol Renal Physiol 2009; 297:F553–F558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morris BJ. Renin, genes, microRNAs, and renal mechanisms involved in hypertension. Hypertension 2015; 65:956–962. [DOI] [PubMed] [Google Scholar]
- 15. Zhu Q, Hu J, Wang L, Wang W, Wang Z, Li PL, Boini KM, Li N. Inhibition of microRNA-429 in the renal medulla increased salt sensitivity of blood pressure in Sprague Dawley rats. J Hypertens 2017; 35:1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cowley AW Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 2001; 37:456–461. [DOI] [PubMed] [Google Scholar]
- 17. Li N, Chen L, Yi F, Xia M, Li PL. Salt-sensitive hypertension induced by decoy of transcription factor hypoxia-inducible factor-1alpha in the renal medulla. Circ Res 2008; 102:1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li PL. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol 2007; 292:F207–F216. [DOI] [PubMed] [Google Scholar]
- 19. Ikeda Y, Saito K, Kim JI, Yokoyama M. Nitric oxide synthase isoform activities in kidney of Dahl salt-sensitive rats. Hypertension 1995; 26:1030–1034. [DOI] [PubMed] [Google Scholar]
- 20. Szentiványi M Jr, Zou AP, Mattson DL, Soares P, Moreno C, Roman RJ, Cowley AW Jr. Renal medullary nitric oxide deficit of Dahl S rats enhances hypertensive actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol 2002; 283:R266–R272. [DOI] [PubMed] [Google Scholar]
- 21. Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics 2011; 43:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK, Kim M, Lee SK, Roh JK. MicroRNAs induced during ischemic preconditioning. Stroke 2010; 41:1646–1651. [DOI] [PubMed] [Google Scholar]
- 23. Granger JP, Alexander BT, Llinas M. Mechanisms of pressure natriuresis. Curr Hypertens Rep 2002; 4:152–159. [DOI] [PubMed] [Google Scholar]
- 24. Ivy JR, Bailey MA. Pressure natriuresis and the renal control of arterial blood pressure. J Physiol 2014; 592:3955–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergström G, Evans RG. Mechanisms underlying the antihypertensive functions of the renal medulla. Acta Physiol Scand 2004; 181:475–486. [DOI] [PubMed] [Google Scholar]
- 26. Hall JE. The kidney, hypertension, and obesity. Hypertension 2003; 41:625–633. [DOI] [PubMed] [Google Scholar]
- 27. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010; 11:597–610. [DOI] [PubMed] [Google Scholar]
- 28. Zhang L, Yang P, Liu Q, Wang J, Yan F, Duan L, Lin F. KLF8 promotes cancer stem cell-like phenotypes in osteosarcoma through miR-429-SOX2 signaling. Neoplasma 2020; 67:519–527. [DOI] [PubMed] [Google Scholar]
- 29. Knouf EC, Garg K, Arroyo JD, Correa Y, Sarkar D, Parkin RK, Wurz K, O’Briant KC, Godwin AK, Urban ND, Ruzzo WL, Gentleman R, Drescher CW, Swisher EM, Tewari M. An integrative genomic approach identifies p73 and p63 as activators of miR-200 microRNA family transcription. Nucleic Acids Res 2012; 40:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dhillon S. Daprodustat: first approval. Drugs 2020; 80:1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thai HBD, Kim KR, Hong KT, Voitsitskyi T, Lee JS, Mao C, Ahn DR. Kidney-targeted cytosolic delivery of siRNA using a small-sized mirror DNA tetrahedron for enhanced potency. ACS Cent Sci 2020; 6:2250–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Redon J, Lurbe E. Nocturnal blood pressure versus nondipping pattern: what do they mean? Hypertension 2008; 51:41–42. [DOI] [PubMed] [Google Scholar]
- 33. Rubin JD, Nguyen TV, Allen KL, Ayasoufi K, Barry MA. Comparison of gene delivery to the kidney by adenovirus, adeno-associated virus, and lentiviral vectors after intravenous and direct kidney injections. Hum Gene Ther 2019; 30:1559–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kario K. Nocturnal hypertension: new technology and evidence. Hypertension 2018; 71:997–1009. [DOI] [PubMed] [Google Scholar]
- 35. Liu K, Wang R. MicroRNA-mediated regulation in the mammalian circadian rhythm. J Theor Biol 2012; 304:103–110. [DOI] [PubMed] [Google Scholar]
- 36. Kinoshita C, Okamoto Y, Aoyama K, Nakaki T. MicroRNA: a key player for the interplay of circadian rhythm abnormalities, sleep disorders and neurodegenerative diseases. Clocks Sleep 2020; 2:282–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klimczak D, Kuch M, Pilecki T, Żochowska D, Wirkowska A, Pączek L. Plasma microRNA-155-5p is increased among patients with chronic kidney disease and nocturnal hypertension. J Am Soc Hypertens 2017; 11:831–841.e4. [DOI] [PubMed] [Google Scholar]