Abstract
BACKGROUND
Blood pressure variability (BPV) is associated with adverse events (AEs) independently of hypertension. It has been suggested that calcium channel blockers (CCBs) may reduce BPV, and thus be particularly valuable in hypertensives with high BPV. We sought to investigate how CCB affect BPV progression and whether long-term adverse effects of BPV differ after CCB treatment than after treatment with other antihypertensives.
METHODS
We retrospectively analyzed 25,268 US veterans who had been followed for 3 years without hypertensive therapy, started on a single class of antihypertensive agents (thiazides, CCBs, ACE inhibitors, or beta blockers [BBs]), treated for 6 years, and then followed for 3 additional years. BPV was calculated as SD of systolic or diastolic blood pressures from at least 10 measurements over each 3-year period. A combined AE endpoint included hospitalization, coronary artery bypass grafting, carotid endarterectomy, angioplasty, amputation, arteriovenous fistula creation, and mortality was assessed in years 9–12.
RESULTS
Post-medication high BPV and BB or thiazide use were associated with increased AE risk. Medication type also affected mean post-medication BPV. The effects of medications except for BBs on AE and mortality was independent of the patient BPV.
CONCLUSIONS
The possible deleterious effects of thiazides should be considered within the context of the study population, who were mostly male and received only a single class of hypertensives. While CCB may ameliorate BPV over time, this study does not support choosing CCB over other agents specifically to lessen BPV-associated risk.
Keywords: ACE inhibitors, beta blockers, blood pressure, blood pressure variability, calcium channel blockers, hypertension, thiazide diuretics
Graphical Abstract
Graphical Abstract.

Blood pressure variability (BPV) is the SD or other measure of variability of a series of consecutive outpatient ambulatory blood pressure (BP) readings over prolonged time. High BPV, independent of hypertension, poses increased risk of adverse events (AEs) over time, including myocardial infarction, cerebrovascular accident, diabetic foot ulcer, amputation, worsening renal disease, hospitalization, and death.1–6 The conundrum has been what to do about high BPV. No treatment reduces BPV beyond controlling the hypertension itself, which may reduce BPV simply by reducing the range over which BP can fluctuate. Moreover, it remains unknown whether reducing BPV would actually reduce risk, since it is unclear whether BPV itself increases risk or whether BPV simply marks unfavorable patient biology.
Although BPV is an AE risk factor with or without hypertension, BPV is often studied in retrospective analyses of antihypertensive trials that track BP and outcomes over time. There have been suggestions that calcium channel blockers (CCBs) might reduce BPV7–11 and thus reduce AE risk, but this has been difficult to determine from selected groups of patients on specific drug protocols. We sought to compare the outcomes of a cohort of patients with high BPV over time who were started on CCBs to parallel cohorts of patients who received either ACE inhibitors (ACE), beta blockers (BBs), or thiazides. We used the US Department of Veterans’ Affairs VINCI network to identify a cohort of patients with high BPV who were not receiving antihypertensive medication over a 3-year period, and were then started on a single class of medication and followed for 9 years without receiving another class of antihypertensive. We calculated BPV from recordings at outpatient clinic visits over this 12-year period. We identified as AE procedural events unambiguous in the database: hospitalization, coronary artery bypass grafting, carotid endarterectomy, angioplasty, amputation, arteriovenous fistula creation, or death. We studied associations of BPV and antihypertensive class with subsequent AE in this large cohort in the final 3 years to allow ample time for effects of the antihypertensive treatment to occur.
We compared the deleterious long-term relationships of 4 classes of monotherapy antihypertensive medications to AEs, of BPV to AEs, and of 4 medications to BPVs. We sought to understand whether the choice of monotherapeutic antihypertensive drug and its relationships to AEs is affected by BPV.
METHODS
Data
Approved by the UND and Fargo VAMC IRB’s, we examined 25,268 records from 2007 to 2019. We selected patients who had been prescribed 1 of 4 medications (ACE inhibitor, BB, CCB, or thiazide) and for whom at least 10 BP readings were available in the 3 years prior to medication (pre-med time span), and in each of 3 post-medication 3-year time spans. Patients were excluded if they had if they received more than one class of antihypertensive medication simultaneously or were changed from one class to another during the study, if they were diagnosed with congestive heart failure, if they were older than 50 at first medication. 8,814 patients with AEs at unknown times were also excluded, leaving 16,454 patients for study who either had no AE during any study period or had an AE within a known time span. 14,620 (88.85%) had no AE, 1,834 (11.15%) had AE 7–9 years after medication, and 707 (4.30%) died 7–9 years after medication. Gender, age, body mass index, and systolic and diastolic BPV measures did not differ significantly between patients with known and unknown AE times. Those 8,814 patients removed were slightly more likely to have diabetes (35% vs. 33%), more likely to receive CCBs (19% vs. 16%) and less likely to receive thiazides (0.2% vs. 11%).
Within each time period, patients averaged 28–40 BP. Systolic and diastolic SD during each period yielded BPV measures (comprising 8 BPV measures). High BPVs and low BPVs were defined as the highest and lowest 25% within each time span.
Hospitalization for any reason, coronary artery bypass grafting, arteriovenous fistula creation, amputation, angioplasty, carotid endarterectomy, and death were considered events. Having at least 1 in the final 3 years of the study (7–9 years post-medication) was considered an AE and a primary outcome. Having 1 of the first 6 events (excluding death) pre-medication or in the first 1–6 years following medication was noted as a potential covariate. A secondary endpoint was the number of days between start of medication and death. Number of days from start of medication to nonmortal AE was not available for study.
Medication was considered a potential confounder of AE and mortality. Patients received 1 of 4 medications: CCB (17.74%), ACE (43.07%), BB (34.96%), and thiazide (4.23%). Covariates controlled for in the models included having an AE either before medication began or before the final time span, sex, age, body mass index, heart rate, and diabetic status at the start of the pre-medication time. Average systolic and diastolic as well as hypertensive status (normal systolic under 120, elevated systolic 120–129, hypertension I systolic 130–139 or diastolic 80–89, hypertension II systolic 140 or higher or diastolic 90 or higher) and the number of BPs per year were used for each time period.
Statistics
Chi-square and independent t-tests described patient characteristics, medication use, and BPV with AE. Multicollinearity of BP variables (hypertension, number of readings per year, systolic and diastolic BPV) from 4 time periods was tested using tolerance and variance inflation from multiple linear regression adjusted by weight matrices derived from maximum likelihood estimations. All variable sets had tolerances from 0.603 to 0.927 suggesting little multicollinearity and that all 4 time periods of the variables could be together in further models.
The separate relationships of BPV and medication to AE and mortality were estimated using logistic regressions controlling for patient characteristics. To further test for potential confounding, General Linear Model Repeated Measures Design tested the relationship of mean BPV over time between different medications (using Bonferroni adjustments for paired comparisons between medications) while controlling for patient characteristics.
To address confounding suggested by previous results, full logistic models predicting AE with both medication and BPV (including covariates) were developed. As date of death was available (AE date was not), the potential confounding of medication from BPV in prediction of time to death was described using Cox proportional hazard models (including patient characteristics as covariates). Changes in odds or hazard ratios between models with and without specific medications of less than 10% suggested that the effect medication had on the outcome was independent of BPV.
RESULTS
Description of variables to AEs and mortality
Table 1 compares patients without AE (N = 14,620) to those with AE (N = 1,834), or who died (N = 707) in the fourth time span (7–9 years post-medication). Most were male with an average age of 62. 66% had hypertension I or II. There was a relationship between diagnoses of hypertension I or II and medication type. 69% (62%–70% range over time) of ACE-using patients were hypertensive, vs. 52% (49%–51%) on BB, 77% (73%–77%) on thiazides, and 77% (72%–79%) on CCB (P < 0.001). In unadjusted analysis, having an AE or mortality 7–9 years post-medication was more likely for males, older patients, lower body mass index, higher heart rate, nondiabetics, and a post-medication AE. Pre-medication AE was only related to mortality. Patients with an AE or mortality were more likely to have more BP readings per year. Interestingly, average BP (as opposed to BPV) was significantly lower for patients with AE or mortality at any time cohort.
Table 1.
Descriptive characteristics of 16,454 patients by adverse event
| All patients (N = 16,434) | No adverse event (N = 14,620) | Adverse event (N = 1,834) | Mortality only (N = 707) | |
|---|---|---|---|---|
| Mean ± SD or N (%) | Mean ± SD or N (%) | Mean ± SD or N (%) | Mean ± SD or N (%) | |
| Male | 14,912 (90.62%) | 13,216 (90.39%) | *1,696 (92.47%) | *680 (96.18%) |
| Mean age at start | 62.04 ± 7.4 | 61.82 ± 7.16 | *63.75 ± 8.87 | *67.37 ± 9.75 |
| Mean BMI | 28.92 ± 5.17 | 29.13 ± 5.11 | *27.32 ± 5.41 | *25.81 ± 4.99 |
| Mean heart rate | 75.36 ± 9.36 | 75.08 ± 9.32 | *77.61 ± 9.43 | *79.22 ± 9.71 |
| Has diabetes | 6,353 (38.61%) | 5,768 (39.45%) | * 585 (31.89%) | *224 (31.68%) |
| Had AE pre-years 1–3 | 1,370 (8.32%) | 1,236 (8.45%) | 134 (7.30%) | *128 (18.10%) |
| Had AE post-years 1–6 | 2,390 (14.52%) | 2,158 (14.76%) | *232 (12.64%) | *226 (31.96%) |
| BP readings | ||||
| Systolic: pre-years 1–3 | 129.94 ± 9.72 | 130.04 ± 9.66 | *129.16 ± 10.17 | 129.73 ± 10.72 |
| Diastolic: pre-years 1–3 | 77.74 ± 6.92 | 77.86 ± 6.88 | *76.82 ± 7.16 | *75.62 ± 7.49 |
| Hypertension I or II | 9,953 (60.48%) | 8,922 (61.02%) | *1,031 (56.21%) | *392 (55.44%) |
| Number per year | 8.35 ± 11.00 | 8.02 ± 9.96 | *10.97 ± 16.94 | *11.65 ± 21.45 |
| Systolic: post-years 1–3 | 130.93 ± 9.96 | 131.03 ± 9.88 | *130.12 ± 10.56 | 130.29 ± 11.11 |
| Diastolic: post-years 1–3 | 77.37 ± 7.09 | 77.50 ± 7.01 | *76.37 ± 7.62 | *75.01 ± 8.35 |
| Hypertension I or II | 10,296 (62.57%) | 9,224 (63.09%) | *1,072 (58.45%) | *407 (57.56%) |
| Number per year | 8.95 ± 16.30 | 8.25 ± 12.96 | *14.49 ± 31.77 | *15.78 ± 41.76 |
| Systolic: post-years 4–6 | 132.84 ± 10.67 | 133.06 ± 10.54 | *131.12 ± 11.5 | *131.06 ± 12.13 |
| Diastolic: post-years 4–6 | 77.14 ± 6.84 | 77.32 ± 6.78 | *75.76 ± 7.12 | *74.38 ± 7.07 |
| Hypertension I or II | 10,933 (66.81%) | 9,890 (67.64%) | *1,103 (60.14%) | *410 (57.99%) |
| Number per year | 9.84 ± 21.31 | 8.87 ± 15.91 | *17.57 ± 44.62 | *20.00 ± 47.97 |
| Systolic: post-years 7–9 | 133.54 ± 11.33 | 133.93 ± 11.14 | *130.50 ± 12.32 | *128.25 ± 13.79 |
| Diastolic: post-years 7–9 | 76.25 ± 6.77 | 76.46 ± 6.68 | *74.50 ± 7.22 | *72.41 ± 7.42 |
| Hypertension I or II | 10,964 (66.63%) | 9,949 (68.05%) | *1,015 (55.34%) | *319 (45.12%) |
| Number per year | 10.38 ± 21.88 | 9.40 ± 8.06 | *18.22 ± 40.33 | *20.07 ± 41.57 |
| Medications | ||||
| Calcium channel blocker | 2,785 (16.92%) | 2,488 (17.01%) | 297 (16.19%) | 126 (17.82%) |
| ACE inhibitors | 7,278 (44.23%) | 6,631 (43.35%) | *647 (35.27%) | *240 (33.94%) |
| Beta blockers | 5,337 (32.43%) | 4,625 (31.63%) | *712 (38.82%) | *297 (42.00%) |
| Thiazide | 1,054 (6.40%) | 876 (5.99%) | *178 (9.70%) | 44 (6.22%) |
| BPV readings | ||||
| Systolic: pre-years 1–3 | 11.95 ± 3.52 | 11.87 ± 3.49 | *12.59 ± 3.7 | *12.97 ± 3.75 |
| SD ≥13.848 | 4,113 (24.99%) | 3,540 (24.21%) | *573 (31.24%) | *259 (36.63%) |
| SD ≤9.509 | 4,114 (25.00%) | 3,763 (25.73%) | *351 (19.13%) | *121 (17.11%) |
| Systolic: post-years 1–3 | 12.29 ± 3.6 | 12.14 ± 3.55 | *13.46 ± 3.8 | *13.90 ± 3.84 |
| SD ≥11.5 | 4,112 (24.99%) | 3,429 (23.45%) | *683 (37.24%) | *300 (42.43%) |
| SD ≤9.509 | 4,113 (24.99%) | 3,843 (26.28%) | *270 (14.72%) | *79 (11.17%) |
| Systolic: post-years 4–6 | 13.01 ± 3.72 | 12.86 ± 3.68 | *14.14 ± 3.88 | *14.74 ± 4.19 |
| SD ≥11.5 | 4,114 (25.00%) | 3,470 (23.73%) | *644 (35.11%) | *285 (40.31%) |
| SD ≤9.509 | 4,114 (25.00%) | 3,845 (26.29%) | *269 (14.66%) | *86 (12.16%) |
| Systolic: post-years 7–9 | 13.67 ± 3.96 | 13.52 ± 3.89 | *14.87 ± 4.29 | *15.41 ± 4.82 |
| SD ≥11.5 | 4,112 (24.99%) | 3,466 (23.70%) | *647 (35.27%) | *293 (41.44%) |
| SD ≤9.509 | 4,113 (24.99%) | 3,821 (26.13%) | *291 (15.86%) | 109 (15.41%) |
| Diastolic: pre-years 1–3 | 7.89 ± 2.26 | 7.85 ± 2.25 | *8.18 ± 2.31 | *8.18 ± 2.36 |
| SD ≥7.5 | 4,113 (24.99%) | 3,579 (24.48%) | *534 (29.11%) | *211 (29.84%) |
| SD ≤9.509 | 4,113 (24.99%) | 3,717 (25.42%) | *396 (21.59%) | *153 (21.64%) |
| Diastolic: post-years 1–3 | 7.84 ± 2.27 | 7.77 ± 2.25 | *8.41 ± 2.31 | *8.43 ± 2.37 |
| SD ≥7.5 | 4,112 (24.99%) | 3,494 (23.89%) | *618 (33.69%) | *235 (33.23%) |
| SD ≤9.509 | 4,114 (25.00%) | 3,794 (25.95%) | *320 (17.44%) | *122 (17.25%) |
| Diastolic: post-years 4–6 | 7.73 ± 2.22 | 7.65 ± 2.19 | *8.33 ± 2.34 | *8.36 ± 2.56 |
| SD ≥7.5 | 4,115 (25.00%) | 3,478 (23.78%) | *637 (34.73%) | *239 (33.80%) |
| SD ≤9.509 | 4,112 (24.99%) | 3,826 (26.16%) | *286 (15.59%) | *110 (15.55%) |
| Diastolic: post-years 7–9 | 7.73 ± 2.26 | 7.64 ± 2.23 | *8.43 ± 2.41 | *8.70 ± 2.65 |
| SD ≥7.5 | 4,114 (25.00%) | 3,449 (23.59%) | *665 (36.25%) | *288 (40.73%) |
| SD ≤9.509 | 4,112 (24.99%) | 3,829 (26.19%) | *283 (15.43%) | *102 (14.42%) |
Comparisons between AE and no AE or mortality and no AE were made using independent t-tests for continuous variables and chi-square was used for categorical variables. Abbreviations: ACE, ACE inhibitors; AE, adverse event; BMI, body mass index; BP, blood pressure; BPV, blood pressure variability.
*P < 0.05.
Of the 4 medications, CCB was not associated with AE or mortality in time 7–9 and thiazide was only associated with increased AE (Table 1). ACE-using patients were less likely to have AE or mortality while BB users were more likely. Average systolic and diastolic BPVs over each time span were higher for patients with AE and mortality. High BPVs were more prevalent in patients with AEs or mortality by 10%–15%.
Associations with AEs and mortality controlling for covariates
Logistic regression tested the multivariate relationships of medication or BPV to having an outcome of AE or mortality while controlling for covariates. Figure 1a shows odds ratios (ORs) of usage of each medication predicting AE (lower half) and death (upper half). BB and thiazide use were associated with increased AE risk (OR 1.2 and 2.0, respectively) relative to other medications, while ACE and CCB use were associated with decreased risk (OR 0.73 and 0.85, respectively). ACE use was the only medication associated with mortality (a decreased risk, OR 0.82).
Figure 1.
Odds ratios from logistic regressions of medication type (a), systolic BPV (b), and diastolic BPV (c) predicting AE and death 7–9 years after medication while controlling for age, sex, BMI, average heart rate, diabetes, hypertension, number of BP readings, and having an AE pre- or post-medication. Each medication (yes or no) was used as a separate regression with not using that specific medication the reference value. Abbreviations: ACE, ACE inhibitors; AE, adverse event; BB, beta blocker; BMI, body mass index; BP, blood pressure; BPV, blood pressure variability; CCB, calcium channel blocker.
Figure 1b,c shows BPV predicting AE and mortality for systolic and diastolic values, respectively, when logistic regression controlled for covariates. Systolic BPV at any of the 3 time spans post-medication significantly predicted AE and mortality (ORs 1.0–1.8). Systolic BPV in the pre-medication time cohort was not associated with any outcome. Post-medication diastolic BPV was associated with AE (ORs 2.0–2.6) but only BPVs 7–9 years post-medication were associated with mortality (OR = 3.0). Appendix A online presents these 12 logistic regressions.
Association of BPV with medication use
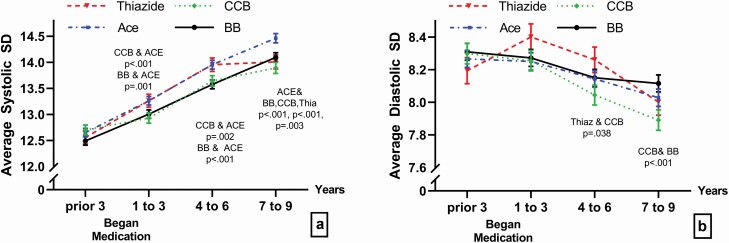
As both medication and BPV were associated separately with AE and mortality, we next investigated relationships between those variables. Figure 2 shows average BPV at each time interval for each medication (adjusted for covariates). Appendix B online presents full results of the Repeated Measures Design.
Figure 2.
Change in mean systolic (a) and diastolic (b) BPV (±SE) over time for 16,434 patients on thiazide, ACE inhibitors (ACE), beta blockers (BBs), and calcium channel blockers (CCBs) while controlling for age, sex, BMI, heart rate, diabetes, hypertension, number of BP readings, and having an AE pre- or post-medication. BB is the solid black line, thiazide is the dashed red line, CCB is the green dotted line, and ACE is the dot-dash blue line. Means with SEs are shown for each drug in each time cohort. Abbreviations: AE, adverse event; BMI, body mass index; BP, blood pressure; BPV, blood pressure variability.
All patients increased systolic BPV (P < 0.001, Figure 2a) and decreased diastolic BPV over time (P < 0.001, Figure 2b). Although patients in each group had similar systolic and diastolic BPV before starting medication, thiazide and ACE users had the highest systolic BPV from 1 to 6 years post-medication and CCB and BB users had the lowest systolic BPV post-medication. Bonferroni adjusted means were tested between each of the 4 medications within each time cohort. ACE users’ systolic BPV differed from BB and CCBs at all post-medication times (times 1–3: CCB P < 0.001 and BB P = 0.001; times 4–6: CCB P = 0.002 and BB P < 0.001; times 7–9: CCB P < 0.001 and BB P < 0.001). Also ACE systolic BPVs differed from thiazides in times 7–9 (P = 0.003). For diastolic BPV, medications differences were found only in times 3–6 and 7–9 where CCB was lower than thiazide (P = 0.038) and BB (P < 0.001).
Association of medication with AE and mortality controlling for BPV
To control for the relationship of medication and BPV, the association of BPV and medication together with AE (using logistic regression) and time to mortality (using proportional hazard models) was examined. Unadjusted ORs of systolic and diastolic BPV predicting AE (see Figure 1) were compared with ORs taken from logistic regression where the use of each drug class was adjusted for (Table 2). The percent that the adjusted ORs changed from the unadjusted ORs was estimated. Any change over 10% was considered indicative that the medication in that adjustment contributed to AE through BPV. The systolic BPV ORs changed 13%–68% when BBs were in the model, suggesting BBs need to be with BPV when predicting AE. No other medication changed the systolic or diastolic ORs more than 10%.
Table 2.
Unadjusted ORs and HRs compared with medication adjusted for systolic and diastolic BPV
| Unadjusted | CCB Adj | % Change | ACE Adj | % Change | BB Adj | % Change | Thiazide Adj | % Change | |
|---|---|---|---|---|---|---|---|---|---|
| Adverse event | |||||||||
| Systolic | |||||||||
| Pre-years 1–3 | 1.037 | 1.043 | −0.6% | 1.037 | 0% | 1.216 | −14.7% | 1.048 | −1% |
| Post-years 1–3 | 1.755 | 1.751 | 0.2% | 1.768 | −0.7% | 1.043 | 68.3% | 1.743 | 0.7% |
| Post-years 1–3 | 1.476 | 1.476 | 0% | 1.489 | −0.9% | 1.76 | −16.1% | 1.468 | 0.5% |
| Post-years 1–3 | 1.676 | 1.669 | 0.4% | 1.706 | −1.8% | 1.485 | 12.9% | 1.701 | −1.5% |
| Diastolic | |||||||||
| Pre-years 1–3 | 1.105 | 1.108 | −0.3% | 1.104 | 0.1% | 1.103 | 0.2% | 1.128 | −2% |
| Post-years 1–3 | 1.957 | 1.962 | −0.3% | 1.947 | 0.5% | 1.96 | −0.2% | 1.944 | 0.7% |
| Post-years 1–3 | 2.116 | 2.107 | 0.4% | 2.132 | −0.8% | 2.118 | −0.1% | 2.093 | 1.1% |
| Post-years 1–3 | 2.57 | 2.558 | 0.5% | 2.577 | −0.3% | 2.552 | 0.7% | 2.615 | −1.7% |
| Time to death | |||||||||
| Systolic | |||||||||
| Pre-years 1–3 | 1.002 | 1.002 | 0% | 1.002 | 0% | 1.003 | −0.1% | 1.002 | 0% |
| Post-years 1–3 | 1.040 | 1.04 | 0% | 1.040 | 0% | 1.040 | 0% | 1.039 | 0.1% |
| Post-years 1–3 | 1.034 | 1.034 | 0% | 1.033 | 0.1% | 1.034 | 0% | 1.034 | 0% |
| Post-years 1–3 | 1.047 | 1.047 | 0% | 1.048 | −0.1% | 1.047 | 0% | 1.047 | 0% |
| Diastolic | |||||||||
| Pre-years 1–3 | 0.992 | 0.992 | 0% | 0.992 | 0% | 0.991 | 0.1% | 0.992 | 0% |
| Post-years 1–3 | 1.032 | 1.032 | 0% | 1.031 | 0.1% | 1.032 | 0% | 1.032 | 0% |
| Post-years 1–3 | 1.038 | 1.038 | 0% | 1.038 | 0% | 1.038 | 0% | 1.038 | 0% |
| Post-years 1–3 | 1.094 | 1.094 | 0% | 1.094 | 0% | 1.093 | 0.1% | 1.094 | 0% |
Logistic regressions used to estimate adverse events and proportional hazard model used to estimate time to death were controlled for sex, age, BMI, heart rate, diabetic status at the start of the pre-medication time, having an adverse event pre-medication or in the fist 6 years post-medication, hypertension I or II and number of BP readings at any of the 4 time cohorts. Unadjusted has BPV without medications, adjusted add in binary medication use to the model with BPV. Abbreviations: ACE, ACE inhibitors; BB, beta blocker; BMI, body mass index; BP, blood pressure; BPV, blood pressure variability; CCB, calcium channel blocker; HRs, hazard ratios; ORs, odds ratio.
Days to death from beginning of medication for patients who died in the final cohort were used to create survival curves for each drug class adjusting for systolic and diastolic BPV at each time span (Figure 3). A separate curve was generated for each medication and BB users appeared to live longer while Ace users appeared to die soonest. These unadjusted hazard ratios without medication for systolic and diastolic BPVs are shown in Table 2 along with the adjusted HRs from adding a medication to the model. No medication changed the ORs more than 10% when added to the model suggesting a lack of confounding of medication and BPV (complete results in Appendix C online).
Figure 3.
Survival curves adjusted for age, sex, BMI, heart rate, diabetes, hypertension, number of BP readings, having an AE pre- or post-medication, and BPV (systolic in a, diastolic in b) for each separate medication predicting death. BB is the highest solid green line and ACE is the lowest solid black line. Abbreviations: ACE, ACE inhibitors; AE, adverse event; BB, beta blocker; BMI, body mass index; BP, blood pressure; BPV, blood pressure variability.
DISCUSSION
Previous retrospective analyses5,12–15 suggested that CCB may protect against at least some effects of high BPV in hypertensives. BPV can be calculated in many ways, including SD, coefficient of variation, or greatest sequential change, but results are generally similar.3 This retrospective analysis of a large veteran cohort each treated with only a single class of antihypertensive confirms that BPV and medication class are each associated with increased risk of adverse outcomes. We observed substantial differences in subsequent rates of adverse outcomes among patients receiving different classes of antihypertensives. In particular, outcomes seemed worse among patients receiving thiazide monotherapy. Different classes of medication also appeared to impact BPV differently over time, whether because of effects of the different medications or because of differences among the patients prescribed each drug class. However, high BPV was associated with a subsequently higher risk of adverse outcomes in patients taking each class of antihypertensives. When medication and BPV were considered together predicting AEs, the effect of BPV on outcomes of AEs or death, accounting for most of the risk of AE or death, did not change the observed drug effect. Thus, we could not demonstrate a protective effect of CCBs against the negative effects of BPV on adverse outcomes.
BPV, distinct from hypertension, is associated with long-term risk of adverse outcomes including mortality, myocardial infarction, cerebrovascular accident and hospitalization among medical patients,1–5,16,17 and a shorter term risk of adverse outcomes after invasive surgical procedures.18,19 Whether BPV itself contributes directly to the risk of adverse outcomes or is simply a marker of poor physiology has not been clear. Our results suggest the latter. While the patient cohort treated with CCBs tended to not increase their BPV as greatly over time as the other patients studied, patients with originally high BPV taking CCBs did not seem to have ameliorated the increased risk specific to their BPV compared with patients with originally low BPV taking CCBs. This suggests that the BPV in these patients was simply a marker of their pathophysiology. Slowing BPV progression by CCBs did not lessen the correlative risk for AEs.
Conversely, patient outcomes differed among treatment groups after treatment initiation. Adverse outcomes were more frequent among patients receiving thiazides. Because this was a retrospective analysis, we cannot determine whether this represents a direct effect of the medication or more subtle differences among patients allocated to each medication. It seems unlikely to reflect differences in hypertensive control, since mean systolic pressures for each group were similar over time. Moreover, in our multivariate analysis, the drug that they were taking and the degree of their BPV dominated the model predicting the likelihood of adverse outcomes. A diagnosis of hypertension at different time cohorts was included in all multivariate models. The degree of systolic or diastolic hypertension did not add further predictive value to the model.
The ALLHAT trial also compared multiple antihypertensives.20 Unlike ALLHAT, we found thiazides inferior. However, our subjects were predominantly male (87%), pharmacologically naive at outset with only single agent use throughout, took hydrochlorothiazide as their thiazide, were followed longer, and were excluded if diagnosed with congestive heart failure. Our data compare favorably with the inferior thiazide cardiovascular reduction noted in the ANBP2 and the ACCOMPLISH trials.21,22
One potential concern about this study is that the BP readings used to calculate BPV were derived from charts, not specific sphygmomanometric protocols. It is recommended that more than 1 BP be measured and an average taken and that posture, rest, and other issues be considered, while 24-hour BP ambulatory readings may be averaged. While such precise practices may be prescribed in clinical trials, they are not always present in the “real world.” BPV calculated from charted BPs in just this fashion predict long-term adverse outcomes in many patient cohorts, including veterans,3,6 and may be more representative of how physicians assess individual patients in clinic. Similarly, although VA physicians are encouraged to follow evidence-based guidelines, we cannot know why individual patients were placed on one medication or another, or even whether it was for hypertension or some other indication such as arrhythmia or post-MI secondary prevention. This nevertheless allows us to investigate in this real world dataset the interaction of these medications with BPV and adverse outcomes. We chose to study patients who had at least 10 BP readings recorded in each 3-year period because previous analysis of a different veteran dataset suggested that increasing the required number of BP readings to 15 did not substantially change results while reducing the number of BP readings to 5 decreased the mathematical reliability of the calculations.3 One might also question whether preexisting conditions not analyzed here might have biased the results. Indeed, there were surely some reasons why physicians chose to start some patients on CCB and some on other classes of antihypertensives. Detailed analysis of particular hypotheses in this regard awaits further exploration outside the scope of the current analysis. Finally, we assessed prescription but not compliance, which could have also affected results.
Choosing an antihypertensive medication for a patient is complex, and must balance concerns about renal protection, erectile dysfunction, cost, orthostatic hypotension, medication interactions, and patient priorities and preferences among potential side-effects. Such differences cannot be captured from a database. This certainly may have invisibly biased patient allocation to the different drug therapy cohorts. In turn, this could have worsened outcomes not because the therapy was less effective but because the patients were in some fashion different and therefore destined for worse outcomes regardless of their therapy. A long-term prospective randomized trial would be required to address this fully. In addition, these results only apply to patients receiving monotherapy. For the moment, given the intricacies of antihypertensive choices, these results do not suggest that BPV should further complicate that choice. However, these results also confirm that high BPV is a risk factor regardless of antihypertensive treatment, and suggest that at least some of the adversity may reflect underlying biology rather than the BPV itself, since CCBs did appear to slow patients’ further increase in BPV with aging compared with other therapies without preventing the underlying risk that patients who originally had high BPV prior to therapy continued to experience. Clinicians should be aware of this risk, and patients should be counseled about it. Patients confronted with an increased but unmodifiable risk might be more motivated to address controllable risk factors such as obesity, smoking, and inactivity.
FUNDING
This work was supported in part by National Institutes of Health 1 U54 GM128729.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
REFERENCES
- 1. Dai L, Cheng A, Hao X, Xu J, Zuo Y, Wang A, Meng X, Li H, Wang Y, Zhao X, Wang Y. Different contribution of SBP and DBP variability to vascular events in patients with stroke. Stroke Vasc Neurol 2020; 5:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jhee JH, Seo J, Lee CJ, Park JT, Han SH, Kang SW, Park S, Yoo TH. Ambulatory blood pressure variability and risk of cardiovascular events, all-cause mortality, and progression of kidney disease. J Hypertens 2020; 38:1712–1721. [DOI] [PubMed] [Google Scholar]
- 3. Basson MD, Klug MG, Hostetter JE, Wynne J. Visit-to-visit variability of blood pressure is associated with hospitalization and mortality in an unselected adult population. Am J Hypertens 2018; 31:1113–1119. [DOI] [PubMed] [Google Scholar]
- 4. Budiman-Mak E, Epstein N, Brennan M, Stuck R, Guihan M, Huo Z, Emanuele N, Sohn MW. Systolic blood pressure variability and lower extremity amputation in a non-elderly population with diabetes. Diabetes Res Clin Pract 2016; 114:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brennan MB, Guihan M, Budiman-Mak E, Kang H, Lobo JM, Sutherland BL, Emanuele N, Huang ES, Sohn MW. Increasing SBP variability is associated with an increased risk of developing incident diabetic foot ulcers. J Hypertens 2018; 36:2177–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, Kovesdy CP. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol 2016; 68:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang L, Yang J, Li L, Liu D, Xie X, Dong P, Lin Y. Comparison of amlodipine versus other calcium channel blockers on blood pressure variability in hypertensive patients in China: a retrospective propensity score-matched analysis. J Comp Eff Res 2018; 7:651–660. [DOI] [PubMed] [Google Scholar]
- 8. Lee JW, Choi E, Son JW, Youn YJ, Ahn SG, Ahn MS, Kim JY, Lee SH, Yoon J, Ryu DR, Park SM, Hong KS, Yoo BS. Comparison of blood pressure variability between losartan and amlodipine in essential hypertension (COMPAS-BPV). Am J Hypertens 2020; 33:748–755. [DOI] [PubMed] [Google Scholar]
- 9. Nagai M, Dote K, Kato M, Oda N, Kunita E. Visit-to-visit blood pressure variability and a risk of diabetic foot ulcers: how calcium channel blockers moderate? J Hypertens 2019; 37:860–861. [DOI] [PubMed] [Google Scholar]
- 10. Eguchi K. Effects of antihypertensive therapy on blood pressure variability. Curr Hypertens Rep 2016; 18:75. [DOI] [PubMed] [Google Scholar]
- 11. Kollias A, Stergiou GS, Kyriakoulis KG, Bilo G, Parati G. Treating visit-to-visit blood pressure variability to improve prognosis: is amlodipine the drug of choice? Hypertension 2017; 70:862–866. [DOI] [PubMed] [Google Scholar]
- 12. Aalbers J. Reduced blood pressure variability in ASCOT-BPLA trial favours use of amlodipine/perindopril combination to reduce stroke risk. Cardiovasc J Afr 2010; 21:115. [PMC free article] [PubMed] [Google Scholar]
- 13. Nagai M, Dote K, Kato M, Sasaki S, Oda N, Kagawa E, Nakano Y, Yamane A, Kubo Y, Higashihara T, Miyauchi S, Harada W. Visit-to-visit blood pressure variability and classes of antihypertensive agents; associations with artery remodeling and the risk of stroke. Curr Pharm Des 2016; 22:383–389. [DOI] [PubMed] [Google Scholar]
- 14. Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Poulter NR, Sever PS; ASCOT-BPLA and MRC Trial Investigators . Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol 2010; 9:469–480. [DOI] [PubMed] [Google Scholar]
- 15. Sato N, Saijo Y, Sasagawa Y, Morimoto H, Takeuchi T, Sano H, Koyama S, Takehara N, Morita K, Sumitomo K, Maruyama J, Kikuchi K, Hasebe N; CAMUI Investigators . Visit-to-visit variability and seasonal variation in blood pressure: Combination of Antihypertensive Therapy in the Elderly, Multicenter Investigation (CAMUI) Trial subanalysis. Clin Exp Hypertens 2015; 37:411–419. [DOI] [PubMed] [Google Scholar]
- 16. Nagai M, Dote K, Kato M, Sasaki S, Oda N, Kagawa E, Nakano Y, Yamane A, Kubo Y, Higashihara T, Miyauchi S, Harada W, Masuda H. Visit-to-visit blood pressure variability, average BP level and carotid arterial stiffness in the elderly: a prospective study. J Hum Hypertens 2017; 31:292–298. [DOI] [PubMed] [Google Scholar]
- 17. Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016; 354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Basson MD, Klug MG, Newman WE, Dyke C. Preoperative outpatient blood pressure variability predicts postoperative mortality, readmission and morbidity after surgery. Am J Surg 2020; 220:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dyke CM, Benz CL, Taggart CM, Klug MG, Basson MD. Systolic and diastolic blood pressure variability as risk factors for adverse events after coronary artery bypass grafting. JAMA Surg 2019; 154:92–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group, The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 21. Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Marley JE, Morgan TO, West MJ; Second Australian National Blood Pressure Study Group . A comparison of outcomes with angiotensin-converting—enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med 2003; 348:583–592. [DOI] [PubMed] [Google Scholar]
- 22. Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ; ACCOMPLISH Trial Investigators . Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359:2417–2428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.