Abstract
Objective
A community-based cross-sectional study was done to assess Plasmodium falciparum exposure in areas with different malaria endemicity in north-eastern Tanzania using serological markers; PfAMA-1 and PfMSP-119.
Results
Bondo had a higher seroprevalence 36.6% (188) for PfAMA-1 as compared to Hai 13.8% (33), χ2 = 34.66, p < 0.01. Likewise, Bondo had a higher seroprevalence 201(36.6%) for PfMSP-1 as compared to Hai 41 (17.2%), χ2 = 29.62, p < 0.01. Anti-PfAMA-1 titters were higher in malaria positive individuals (n = 47) than in malaria negative individuals (n = 741) (p = 0.07). Anti-PfMSP-1 antibody concentrations were significantly higher in malaria-positive individuals (n = 47) than in malaria-negative individuals (n = 741) (p = 0.003). Antibody response against PfAMA-1 was significantly different between the three age groups; < 5 years, 5 to 15 years and > 15 years in both sites of Bondo and Hai. Likewise, antibody response against PfMSP-119 was significantly different between the three age groups in the two sites (p < 0.001). We also found significant differences in the anti-PfAMA-1and anti-PfMSP-119 antibody concentrations among the three age groups in the two sites (p = 0.004 and 0.005) respectively. Immunological indicators of P. falciparum exposure have proven to be useful in explaining long-term changes in the transmission dynamics, especially in low transmission settings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13104-021-05818-y.
Keywords: Malaria, Plasmodium falciparum, Seroprevalence, Transmission, Tanzania
Introduction
Africa carries the highest burden of malaria with more than 70% of all malaria cases and deaths [1]. Each year, 10 to 12 million people contract malaria and more than 80,000 dies [2, 3]. Plasmodium falciparum is mainly responsible for 99.7% of estimated malaria cases [4].
In many countries, local malaria transmission has decreased due to the extensive efforts being devoted to malaria control and elimination [5]. P. falciparum accounts for 96 percent of cases [6], malaria prevalence varies from < 1 percent in the highlands of Arusha to as high as 15 percent in the Southern Zone and 24 percent along the Lake and Western Zones. Immunity to P. falciparum malaria is poorly understood, however, evidence shows that antibody-dependent cellular mechanisms play a key role in immunity against P. falciparum malaria parasite [7, 8]. The rate of its development is believed to be associated with transmission intensity which is stage-specific and is rarely sterile [6]. In many epidemiological studies, the determination of malaria transmission has been based on the antibody levels against P. falciparum antigens [9]. Recent immunological studies revealed that antibodies against merozoite antigens act as biomarkers of malaria exposure and that, with increasing exposure and responses of higher levels, antibodies may act as biomarkers of protective immunity [10].
Apical membrane antigen 1 (AMA-1) is expressed on merozoites and sporozoites of P. falciparum as a type I integral membrane protein [11] while Merozoite surface protein1 (MSP-1), is a highly conserved protein among Plasmodium species as well as the most abundant protein expressed on the surface of merozoites [12]. Antibodies against MSP-1 and AMA-1 antigens are potential markers of both exposure to P. falciparum and protection against the disease [7, 13] and have proven to be informative, in areas where transmission has dropped to low sustained levels, for monitoring the timing and magnitude of transmission reduction [13] as well as in obtaining epidemiological information in malaria control programmes [14].
In areas with low malaria transmission, it has become extremely difficult to detect changes in transmission intensity using conventional methods such as the entomologic inoculation rate (EIR) or malaria prevalence rates. Low transmission areas (low endemicity) sometimes have low mosquito density, below the detection limits of common mosquito trapping methods [15, 16] and the parasite prevalence also becomes less reliable [17–19]. Malaria serological markers may aid in estimating malaria transmission intensity [20–22]. Seroconversion rates may provide insight into recent changes in malaria transmission [23]. Due to the fact antibodies can persist for months or years after infection, seroconversion rates are less affected by the effects of unstable or seasonal transmission [20, 21]. We investigated the antibody response to recombinant AMA-1 and MSP-1 in individuals living in two regionally distinct malaria-endemic zones.
Main text
Materials and methods
Study area
The study was conducted during April and December 2014 in two different areas of the Tanzanian mainland. The first site was Bondo in the Tanga region, inhabited by 7970 people [24]. The second study site was Hai in Kilimanjaro region [14]. Participant recruitment procedures and study design have been previously described [25], (Additional file 1: Fig. S1).
Participant enrolment and sample collection
Participants 2 years of age and above who reside in the study areas were enrolled in the study. A blood sample was obtained by finger prick, a portion of blood was used for malaria rapid test, which was performed on-site. A blood spot was prepared for each participant, then dried and stored for further analysis. A 3.0 mm diameter circle of dried blood spot (equivalent to 2 µl whole blood/1 µl serum) was reconstituted in 200 µl of sodium azide-phosphate buffered saline-tween (0.05%) (PBST/0.1% Azide).
Enzyme-linked immuno-sorbent assay (ELISA)
Indirect immunosorbent Assay (ELISA)was performed using two P. falciparum surface antigens, P. falciparum MSP 119 (PfMSP 119) and P. falciparum AMA-1(PfAMA-1) [21].
Malaria parasite detection by polymerase chain reaction (PCR)
Parasite DNA was extracted using the simple Chelex–Saponin method, Plasmodium nucleic acid amplification was conducted using genus-specific reverse and forward primers (rPLU6-5′TTAAAATTGTTGCAGTTAAAACG3′ and rPLU5-5′CCTGTTGTTGCCTTAAACTCC3′) targeting small sub-unit ribosomal RNA (ssurRNA) of the parasite. A reaction mix of 20 µl per sample was used, 5 µl of template DNA extracted from participants whole blood plus 15 µl of nuclease-free water, dNTPs, Taq enzyme, buffers and salts. Amplification conditions were, 95 °C for 5 min followed by 30 cycles of 94 °C for 1 min, 58 °C for 2 min and 72 °C for 5 min then one final extension cycle at 72 °C for 10 min. Amplification products were run in Ethidium bromide agarose gel (2%) electrophoresis at 120 V, 50 watts and 120 mA. The amplified bands were visualized under ultra-violet light trans-illuminator [26, 27].
Data analysis
All data were analyzed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism8 software (San Diego, CA). After verifying that Optical density (OD) values were not normally distributed (p < 0.0001; Anderson–Darling test), non-parametric tests were performed to compare the OD. The Mann–Whitney test was used for the comparison of Antibody levels of two independent groups. The non-parametric Kruskal–Wallis test was used for the comparison of more than two groups. Pearson’s Chi-squared (χ2) test was used to compare two proportions.
Results
Population characteristics and malaria prevalence
The study enrolled a total of 788 participants, 239 (30.3%) from Hai and 549 (69.7%) from Bondo. Males were 283 (35.9%) and females were 505 (64.1%). About 405 (51.4%) participants had more than 15 years of age, 212 (26.9%) were between 5 and 15 years and 171 (21.7%) were below 5 years. The malaria prevalence by mRDT was 8.6% (47) in Bondo and 0% in Hai (Fisher exact test *p < 0.001). By PCR, malaria prevalence was 20.4% (161), with Bondo having a higher prevalence 28.1% (n = 154) than Hai 2.9%, (n = 7), χ2 = 64.64, p < 0.01 (Additional file 2: Table S1).
Seroprevalence of anti-PfAMA-1 and PfMSP-119 antibodies
Bondo had a higher seroprevalence 36.6% (188) for PfAMA-1 as compared to Hai 13.8% (33), χ2 = 34.66, p < 0.01. Likewise, Bondo had a higher seroprevalence 201(36.6%) for PfMSP-1 as compared to Hai 41 (17.2%) (χ2 = 29.62, p < 0.01). In Bondo, participants with more than 15 years had a significantly higher seroprevalence of PfAMA-1 61.7% (116) (χ2 = 58.69, p < 0.001) and PfMSP-119 63.7 (128) (χ2 = 65.36, p < 0.001) as compared to other age groups. Likewise, participants with 5–15 years and < 5 years had a higher prevalence of malaria as measured by mDRT (χ2 = 30.76, p < 0.001) (Table 1).
Table 1.
Age-specific prevalence of malaria by serology, mRDT, microscopy and PCR
| Study site | Age group | PfAMA-1 % (n) |
PfMSP-119 % (n) |
mRDT % (n) |
PCR % (n) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | ||
| Bondo | < 5 years | 9.0 (17) | 32.7 (118) | 14.4 (29) | 30.5 (106) | 46.8 (22) | 22.5 (113) | 18.8 (29) | 26.8 (106) |
| 5–15 years | 29.3 (55) | 36.6 (132) | 21.9 (44) | 41.1 (143) | 48.9 (23) | 32.7 (164) | 36.4 (56) | 33.2 (131) | |
| > 15 years | 61.7 (116) | 30.7 (111) | 63.7 (128) | 28.4 (99) | 4.3 (2) | 44.8 (225) | 44.8 (69) | 40.0 (158) | |
| χ2 = 58.69, p < 0.001 | χ2 = 65.36, p < 0.001 | χ2 = 30.76, p < 0.001 | χ2 = 3.8, p = 0.1 | ||||||
| Hai | < 5 years | 18.2 (6) | 14.6 (30) | 9.8 (4) | 16.2 (32) | 0.0 (0) | 15.1 (36) | 14.3 (1) | 15.1 (35) |
| 5–15 years | 6.1 (2) | 11.2 (23) | 4.9 (2) | 11.6 (23) | 0.0 (0) | 10.5 (25) | 28.6 (2) | 9.9 (23) | |
| > 15 years | 75.8 (25) | 74.3 (153) | 85.4 (35) | 72.2 (143) | 0.0 (0) | 74.5 (178) | 57.1 (4) | 75.0 (174) | |
| *p = 0.6 | *p = 0.2 | – | *p = 0.2 | ||||||
*Computed by Fisher exact test
Anti-PfAMA-1 and PfMSP-119 antibody concentrations
Anti-PfAMA-1 titters were higher in malaria positive individuals (n = 47) than in malaria negative individuals (n = 741) (Mann–Whitney U test, p = 0.07) (Additional file 3: Fig. S2A). Anti-PfMSP-1 antibody concentrations were significantly higher in malaria-positive individuals (n = 47) than in malaria-negative individuals (n = 741) (Mann–Whitney U test, p = 0.003) (Additional file 3: Fig. S2B).
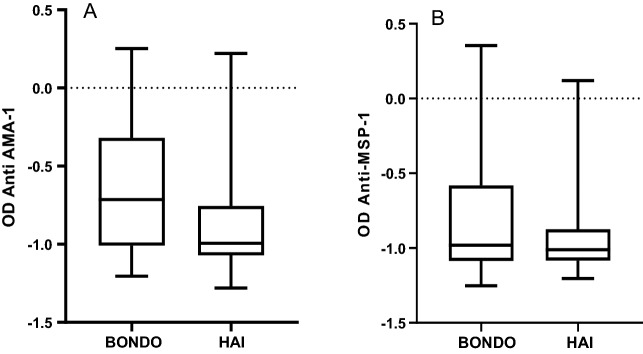
We determined whether the two sites differed in antibody concentration and found that anti-PfAMA-1 antibody concentrations, were higher among participants in Bondo (n = 549) as compared in Hai (n = 239), (Mann–Whitney U test, p < 0.001) (Fig. 1A). Anti-PfMSP-1 antibody concentrations were higher among participants in Bondo (n = 549) than those of Hai (n = 239), (Mann–Whitney U test, p = 0.01) (Fig. 1B).
Fig. 1.
A graph showing mean OD values for PfAMA-1 (A) and PfMSP-119 (B) at Bondo and Hai sites. Presented in the Y-axis is the Log10 transformed OD values in two sites (X-axis)
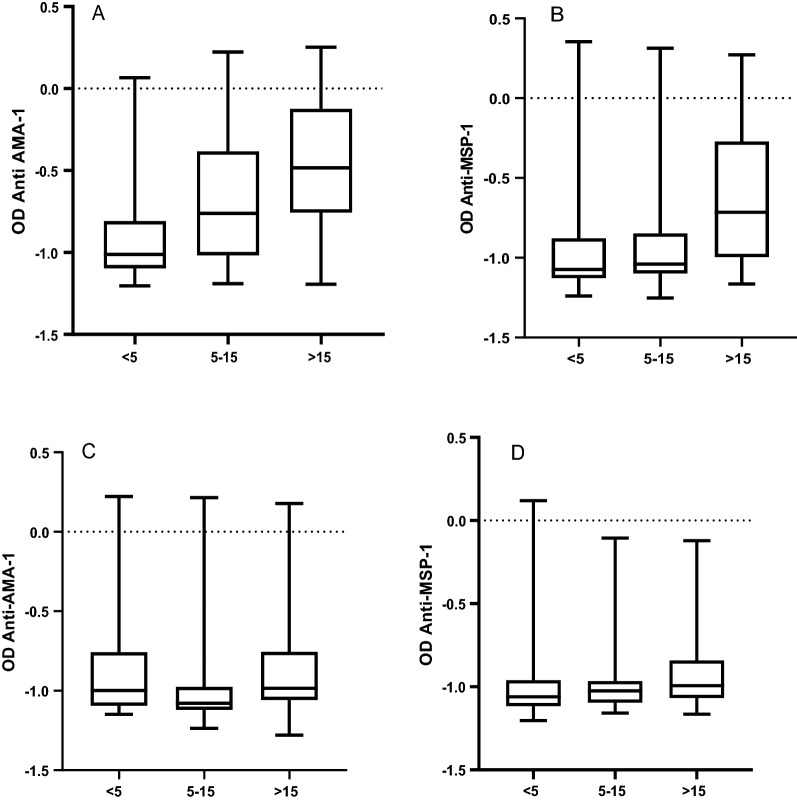
In assessing whether these differences were influenced by age, we calculated the differences among < 5 years, 5 to 15 years and > 15 years per site. Antibody response against PfAMA-1 was significantly different between the three age groups in both sites. (Kruskal–Wallis test, p < 0.001) (Table 1). Likewise, antibody response against PfMSP-119 was significantly different between the three-age groups in the two sites (Kruskal–Wallis test, p < 0.001) (Table 1). We also found significant differences in the anti-PfAMA-1antibody concentrations among the groups (Kruskal–Wallis test, p = 0.004), as indicated in Fig. 2A, B. Lastly, we also noted significant differences in the anti-PfMSP-119 antibody concentrations among the age groups (Kruskal–Wallis test, p = 0.005) (Fig. 2C, D).
Fig. 2.
A graph showing mean OD values for anti-PfAMA-1 antibodies and anti-PfMSP-119 antibodies. A, B Show OD values for anti-PfAMA-1 and anti-PfMSP-119 in Bondo respectively. C, D Show OD values for anti-PfAMA-1 ad anti-PfMSP-119 antibodies in Hai respectively. Presented in the Y-axis is the log10 transformed mean OD values in different age groups (X-axis)
Discussion
The purpose of this study was to use immunological markers to investigate malaria transmission patterns in areas with diverse malaria endemicities.
In this study, malaria prevalence by PCR in Bondo was 28.1%. Since Bondo is a malaria-endemic area, malaria transmission occurs nearly all year long with a peak period from April to June. No significant difference was observed in malaria prevalence among age groups in the present study. This is contrary to the study conducted in 2011 which suggested a widening of the age group at risk for malaria infection to older children of 5–15 years [28]. A previous study conducted in two villages in the same region about 70 km from the current study found a re-emergence of malaria despite previous reports of a decline in malaria [29]. It is estimated that parasite prevalence at that time was 25% and it stayed there throughout 2016 [30]. Hai site had a very low malaria prevalence, thus remaining an area of low transmission and The mRDT tests did not detect any active infections, which suggests low-density parasite circulating in the population, similar to earlier findings [31]. There is, however, some evidence that individuals harbouring sub-microscopic parasites could be sources of new infections since mosquitoes can carry parasites with very low density (< 5 parasites/µl) [26, 32, 33], and hence, the use of a more sensitive diagnostic tool like PCR in clinical malaria diagnosis is necessary. Consequently, scientific evidence from these findings is consistent with the notion of mass drug therapy for individuals with microscopic parasites considering efforts to eliminate malaria.
In our study, Interestingly, when the age-dependent analysis was done, older children (5–15 years) had a relatively low seroprevalence to PfAMA-1 antigens as compared to younger children and Adults. Antibodies to malaria antigens can explain long-term changes in malaria transmission dynamics [21]. In 2009 a survey conducted in Moshi district, an area bordering Hai found a low seroprevalence in younger children suggesting very low exposure to malaria parasites [34]. In populations with low immunity, such as young children, antibodies to MSP-1 act as a significant biomarker of malaria exposure and with increasing exposure the antibodies may contribute to protective immunity [10].
Seroprevalence in moderate malaria transmission setting such as Bondo can play a small role in determining malaria transmission patterns although seroprevalence is almost two folds higher than Hai. A slight decline in seroprevalence was observed in the study area when compared with previous studies [21, 31], indicating a long-term reduction in malaria parasite exposure, which may be attributed to intense malaria interventions in Tanzania [35, 36].
Study results showed that the overall concentration of PfMSP-119 was significantly higher in participants with positive malaria tests than in non-positive participants. As expected Bondo had higher antibody concentrations against both antigens as compared to Hai. Children with < 5 years present with low antibody titters suggesting a lack of recent malaria exposure and this makes the group vulnerable to the symptomatic manifestation of the disease. Earlier findings revealed that more than half of the participants reported being symptomatic and were malaria positive by mRDT [21]. There is evidence of malaria transmission in low malaria-endemic areas, where traditional malaria indicators like prevalence and sporozoite levels may underestimate the burden of the disease.
Conclusion
In this study, immunological markers were found to be a useful indicator of ongoing malaria transmission, especially in low-endemic areas. Routine malaria surveillance can be made more effective by using these immunological markers to highlight the importance of customized and targeted control interventions.
Study limitation
This study might not explain the recent changes in malaria transmission since it was a cross-sectional survey. A longitudinal study would have been appropriate in explaining seasonal variations in malaria infection rates across the study areas.
Supplementary Information
Additional file 1: Figure S1. Map of Tanzania showing the study sites, the map was produced using ArcGIS version 10.3 software.
Additional file 2: Table S1. Prevalence of Malaria by serology, mRDT, Microscopy and PCR.
Additional file 3: Figure S2. A graph showing mean OD values for PfAMA-1 (A) and PfMSP-119 (B) among malaria positive and negative individuals. Presented in the Y-axis is the Log10 transformed OD values among malaria positives and negatives (X-axis).
Acknowledgements
The authors would like to thank all participants and Community leaders in Bondo and Hai for their cooperation. We also like to thank KCMUCo-PAMVERC for the research facilities and space to conduct our laboratory experiments.
Patients and public involvement
Participants were not involved in the design of this study. Community leaders were involved during participant’s recruitment. There is a plan to disseminate results to the participating sites.
Abbreviations
- CRERC
College Research and Ethics Review Committee
- OD
Optical density
- AMA-1
Apical membrane antigen1
- PCR
Polymerase chain reaction
- ELISA
Enzyme-Linked Immuno-Sorbent Assay
- ssurRNA
Small sub-unit ribosomal RNA
- PfMSP 119
Plasmodium falciparum Merozoite Surface Protein 1
- PfAMA-1
Plasmodium falciparum Apical Membrane Antigen 1
Authors’ contributions
RDK: conceptualization of the study, data analysis, and writing the original draft of the manuscript; DCK: funding acquisition, investigation, data analysis and review of the manuscript; JJM, AJN, FWM and JOC: Interpretation of data and critical review of the manuscript; RAK: overall study design and review of the manuscript. All authors read and approved the final manuscript.
Funding
This work was partly supported by DANIDA through DFC in the Building strong Universities (BSU) project. Also, RDK is supported by DELTAS Africa Initiative grant #DEL-15-011 to THRiVE-2. The funding sources had no role in the study design, data collection, analysis, and interpretation of results or in the decision to submit the manuscript for publication.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Kilimanjaro Christian Medical University College Research and Ethics Review Committee (CRERC) with certificate number 658. Permission to conduct the study was sought from Handeni/Bondo and Hai district authorities. Written informed consent was obtained from all participants and from parents or guardians for children under 18 years of age who agreed to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Robert D. Kaaya, Email: robert.kaaya@kcmuco.ac.tz, http://www.pamverc.or.tz
Johnson J. Matowo, Email: johnson.matowo@kcmuco.ac.tz, http://www.pamverc.or.tz
Arnold J. Ndaro, Email: a.ndaro@kcri.ac.tz
Franklin W. Mosha, Email: fmosha@pamverc.or.tz, http://www.pamverc.or.tz
Jaffu O. Chilongola, Email: jaffu.chilongola@kcmuco.ac.tz
Reginald A. Kavishe, Email: reginald.kavishe@kcmuco.ac.tz
References
- 1.WHO. World Malaria Report-2019. Vol. WHO/HTM/GM, World Health Organisation. 2019. p. 238. https://www.who.int/malaria/publications/world-malaria-report-2019/World-Malaria-Report-2019-briefing-kit-eng.pdf?ua=1. Accessed 20 May 2020.
- 2.MalariaSpot. Malaria in Tanzania. MalariaSpot. 2016. https://malariaspot.org/en/eduspot/malaria-in-tanzania/. Accessed 25 May 2020.
- 3.Kilonzi M, Minzi O, Mutagonda R, Sasi P, Kamuhabwa A, Aklillu E. Comparison of malaria treatment outcome of generic and innovator’s anti-malarial drugs containing artemether–lumefantrine combination in the management of uncomplicated malaria amongst Tanzanian children. Malar J. 2019;18(1):1–8. doi: 10.1186/s12936-019-2769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Malaria Fact Sheet. World Health Organisation. 2020. https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed 20 May 2020.
- 5.Dhiman S. Are malaria elimination efforts on right track? An analysis of gains achieved and challenges ahead. Infect Dis Poverty. 2019;8(14):1–19. doi: 10.1186/s40249-019-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MoHCDGEC (Tanzania Mainland), MoH (Zanzibar), NBS, OCGS, and ICF.2017. Tanzania Malaria Indicator Survey (TMIS) 2017. Dar es Salaam, Tanzania, and Rockville, Maryland, USA
- 7.Greenhouse B, Ho B, Hubbard A, Njama-Meya D, Narum DL, Lanar DE, et al. Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic. J Infect Dis. 2011;204(1):19–26. doi: 10.1093/infdis/jir223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 9.Roy A, Adak T. Evaluation of malaria endemicity by peptide ELISA. J Commun Dis. 2005;37(3):183–189. [PubMed] [Google Scholar]
- 10.Stanisic DI, Fowkes FJI, Koinari M, Javati S, Lin E, Kiniboro B, et al. Acquisition of antibodies against Plasmodium falciparum merozoites and malaria immunity in young children and the influence of age, force of infection, and magnitude of response. Infect Immun. 2015;83(2):646–660. doi: 10.1128/IAI.02398-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yap A, Azevedo MF, Gilson PR, Weiss GE, O’Neill MT, Wilson DW, et al. Conditional expression of apical membrane antigen 1 in Plasmodium falciparum shows it is required for erythrocyte invasion by merozoites. Cell Microbiol. 2014;16(5):642–656. doi: 10.1111/cmi.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kale S, Yadav CP, Rao PN, Shalini S, Eapen A, Srivasatava HC, et al. Antibody responses within two leading Plasmodium vivax vaccine candidate antigens in three geographically diverse malaria-endemic regions of India. Malar J. 2019;18(1):1–13. doi: 10.1186/s12936-019-3066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong J, Hamel MJ, Drakeley CJ, Kariuki S, Shi YP, Lal AA, et al. Serological markers for monitoring historical changes in malaria transmission intensity in a highly endemic region of Western Kenya, 1994–2009. Malar J. 2014;13(1):1–14. doi: 10.1186/1475-2875-13-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerkhof K, Sluydts V, Willen L, Kim S, Canier L, Heng S, et al. Serological markers to measure recent changes in malaria at population level in Cambodia. Malar J. 2016;15(1):1–18. doi: 10.1186/s12936-015-1044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oesterholt MJ, Bousema JT, Mwerinde OK, Harris C, Lushino P, Masokoto A, et al. Spatial and temporal variation in malaria transmission in a low endemicity area in northern Tanzania. Malar J. 2006;5:98. doi: 10.1186/1475-2875-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith T, Charlwood JD, Takken W, Tanner M, Spiegelhalter DJ. Mapping the densities of malaria vectors within a single village. Acta Trop. 1995;59:1–18. doi: 10.1016/0001-706X(94)00082-C. [DOI] [PubMed] [Google Scholar]
- 17.Hay SI, Smith DL, Snow RW. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis. 2008;8:369–378. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beier JC, Killeen GF, Githure JI. Short report: Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- 19.Yekutiel P. Problems of epidemiology in malaria eradication. Bull World Health Organ. 1960;22:669–683. [PMC free article] [PubMed] [Google Scholar]
- 20.Coran P, Coleman P, Riley E, Drakeley C. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol. 2007;23:575–582. doi: 10.1016/j.pt.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SL, Carneiro I, et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–5113. doi: 10.1073/PNAS.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mwanziva C, Shekalaghe S, Ndaro A, Mengerink B, Megiroo S, Mosha F, et al. Overuse of artemisinin-combination therapy in Mto wa Mbu (river of mosquitoes), an area misinterpreted as high endemic for malaria. Malar J. 2008;7:232. doi: 10.1186/1475-2875-7-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart L, Gosling R, Griffin J, Gesase S, Campo J, Hashim R, et al. Rapid assessment of malaria transmission using age-specific seroconversion rates. PLoS ONE. 2009;4:e6083. doi: 10.1371/journal.pone.0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.PHC. Population and Housing Census-Tanzania (2012). National Bureau Of Statistics: United Republic of Tanzania. 2013. www.nbs.go.tz/. Accessed 20 June 2018.
- 25.Kajeguka DC, Kaaya RD, Mwakalinga S, Ndossi R, Ndaro A, Chilongola JO, et al. Prevalence of dengue and chikungunya virus infections in north-eastern Tanzania: a cross sectional study among participants presenting with malaria-like symptoms. BMC Infect Dis. 2016;16(183):1–9. doi: 10.1186/s12879-016-1511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miguel RB, Coura JR, Samudio F, Suárez-Mutis MC. Evaluation of three different DNA extraction methods from blood sample colected in dried blood filter papers in Plasmodium subpatent infection from the Amazon Region Brazil. Rev Inst Med Trop Sao Paulo. 2013;55(3):205–208. doi: 10.1590/S0036-46652013000300012. [DOI] [PubMed] [Google Scholar]
- 27.Haanshuus CG, Mohn SC, Mørch K, Langeland N, Blomberg B, Hanevik K. A novel, single-amplification PCR targeting mitochondrial genome highly sensitive and specific in diagnosing malaria among returned travellers in Bergen, Norway. Malar J. 2013;12(26):1–8. doi: 10.1186/1475-2875-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winskill P, Rowland M, Mtove G, Malima RC, Kirby MJ. Malaria risk factors in north-east Tanzania. Malar J. 2011;10(98):1–7. doi: 10.1186/1475-2875-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishengoma DS, Mmbando BP, Segeja MD, Alifrangis M, Lemnge MM, Bygbjerg IC. Declining burden of malaria over two decades in a rural community of Muheza district, north-eastern Tanzania. Malar J. 2013;12(1):338. doi: 10.1186/1475-2875-12-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishengoma DS, Mmbando BP, Mandara CI, Chiduo MG, Francis F, Timiza W, et al. Trends of Plasmodium falciparum prevalence in two communities of Muheza district North-eastern Tanzania: correlation between parasite prevalence, malaria interventions and rainfall in the context of re-emergence of malaria after two decades of progressive. Malar J. 2018;17(252):1–10. doi: 10.1186/s12936-018-2395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shekalaghe SA, Bousema JT, Kunei KK, Lushino P, Masokoto A, Wolters LR, et al. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop Med Int Health. 2007;12:547–553. doi: 10.1111/j.1365-3156.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 32.Schneider P, Bousema JT, Gouagna LC, Otieno S, Van De Vegte-Bolmer M, Omar SA, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. 2007;76:470–474. doi: 10.4269/ajtmh.2007.76.470. [DOI] [PubMed] [Google Scholar]
- 33.Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3(1237):1–9. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart L, Gosling R, Griffin J, Gesase S, Campo J, Hashim R, et al. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS ONE. 2009;4(6):e6083. doi: 10.1371/journal.pone.0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO. World Malaria Report 2012. World Health Organization. 2013. p. 1–288. https://www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_full_report.pdf. Accessed 12 June 2019.
- 36.PMI. President’s Malaria Initiative: fighting malaria and saving lives: Tanzania country profile. USAID. 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Map of Tanzania showing the study sites, the map was produced using ArcGIS version 10.3 software.
Additional file 2: Table S1. Prevalence of Malaria by serology, mRDT, Microscopy and PCR.
Additional file 3: Figure S2. A graph showing mean OD values for PfAMA-1 (A) and PfMSP-119 (B) among malaria positive and negative individuals. Presented in the Y-axis is the Log10 transformed OD values among malaria positives and negatives (X-axis).
Data Availability Statement
All data generated or analysed during this study are included in this published article.