Abstract
The relationship between resistance to antibiotics on the part of Streptococcus pneumoniae and Streptococcus pyogenes was studied by comparing different prevalences of resistance among hospitals obtained from a recent microbiological surveillance of community-acquired respiratory tract infections. A high correlation for erythromycin resistance was found between S. pneumoniae isolates from lower respiratory tract infections and S. pyogenes isolates collected from pharyngeal swabs.
Although the reason for the link between drug prescription practices and antimicrobial resistance has not been established (2), this phenomenon has been studied in the context of the geographical association of macrolide resistance in Streptococcus pneumoniae (3) and longtime antibiotic consumption and the increase in macrolide resistance in Streptococcus pyogenes (8). This link was confirmed by the decrease in macrolide resistance by S. pyogenes following restrictions on macrolide prescriptions in Finland (12).
In Spain, the consumption of antibiotics has increased in areas where high resistance to S. pneumoniae has been reported (9). The increased resistance of S. pyogenes to macrolides in Spain may be explained in part by the confirmed increase in macrolide use (1, 4). As the association between consumption and resistance is ecologically related, it seems logical to study the relationship between the antimicrobial resistances of two species of the genus Streptococcus (S. pneumoniae and S. pyogenes) that are respiratory pathogens and members of respiratory flora. If the rise in the consumption of antimicrobial agents increases resistance patterns for both S. pyogenes and S. pneumoniae, the prevalence of resistance in both species may be related, despite the different macrolide resistance phenotypes, constitutive for S. pneumoniae (5) and phenotype M (presumed efflux) for S. pyogenes (4).
This article explores the relationship of the prevalence of erythromycin resistance between S. pneumoniae and S. pyogenes by means of data obtained from a survey carried out with S. pneumoniae and S. pyogenes isolates between May 1996 and April 1997 in 11 Spanish hospitals (4, 5).
A total of 914 beta-hemolytic streptococci and 1,113 S. pneumoniae isolates were isolated from community-acquired respiratory tract infections in 14 Spanish hospitals (4, 5). Only 11 hospitals which obtained more than 10 S. pyogenes and 10 S. pneumoniae strains were included in this study, so 787 S. pneumoniae and 786 S. pyogenes isolates were evaluated (Table 1). In accordance with National Committee for Clinical Laboratory Standards (NCCLS) breakpoints, S. pyogenes and S. pneumoniae penicillin resistances were defined as MICs of ≥4 μg/ml and ≥2 μg/ml, respectively (9a). Resistance to erythromycin was defined as a MIC of ≥1 μg/ml for both species (9a). Antibiotic susceptibility was determined by using a semiautomated microdilution method according to the NCCLS guidelines, and the mechanism of resistance to erythromycin was evaluated by using a double diffusion disk test with erythromycin (15 μg) and clindamycin (2 μg) disks placed 20 mm apart as described elsewhere (11, 13).
TABLE 1.
Susceptibility to macrolides of 786 S. pyogenes isolates and 787 S. pneumoniae isolates by Spanish hospital
| Hospital and city where located |
S. pyogenes isolates
|
S. pneumoniae isolates
|
||
|---|---|---|---|---|
| No. of isolates | Prevalence of resistance to erythromycin (%)a | No. of isolates | Adjusted prevalence of resistance to erythromycin (%)a | |
| Gregorio Marañón H., Madrid | 158 | 20.9 | 197 | 30.5 |
| La Fe H., Valencia | 222 | 30.7 | 81 | 41.5 |
| La Paz H., Madrid | 145 | 18.6 | 73 | 52.9 |
| Virgen de las Nieves H., Granada | 40 | 35.0 | 73 | 40.9 |
| Clínico H., Zaragoza | 19 | 5.3 | 93 | 29.9 |
| Basurto H., Bilbao | 73 | 31.5 | 27 | 35.2 |
| Clínico Universitario H., Salamanca | 23 | 17.4 | 75 | 34.0 |
| Virgen Macarena H., Sevilla | 45 | 33.3 | 46 | 31.6 |
| Xeral de Galicia H., Santiago | 12 | 8.3 | 75 | 22.6 |
| Reina Sofía H., Córdoba | 37 | 70.3 | 35 | 58.6 |
| Infanta Cristina H., Badajoz | 12 | 8.3 | 12 | 23.2 |
| Total | 786 | 26.7 | 787 | 34.1 |
The breakpoint used was ≥1 μg/ml (NCCLS M100-S9) (9a).
Statistical analysis was carried out by using SPPS for Windows version 7.5. The Spearman nonparametric correlation coefficient between the prevalences of resistance to erythromycin on the part of S. pneumoniae and S. pyogenes was calculated for each hospital.
As erythromycin resistance is strongly associated with pediatric samples in S. pneumoniae (5), and some hospitals do not have pediatric wards, the crude prevalence was adjusted. For this purpose, prevalences were adjusted by a direct method (10). First, a hypothetical standard hospital was designed, with the total S. pneumoniae population studied used as a standard, and was distributed into four wards as follows: internal medicine, intensive care unit, pediatrics, and other (mainly emergencies). Secondly, crude specific prevalences (ps) of erythromycin resistance were calculated for each hospital ward. A specific weight (ws) was calculated for each hospital ward by means of the proportion of isolates belonging to each ward. For each hospital ward, adjusted specific prevalence was calculated by using the formula ws × ps. Finally, the adjusted prevalence for each hospital was defined as the sum of the four adjusted specific prevalences.
Table 1 shows the number of strains and the prevalence of erythromycin resistance corresponding to each hospital center. Phenotype M is the most prevalent phenotype in S. pyogenes (93%), with 6 and 1% of constitutive and inducible MLSB phenotype, respectively, whereas for S. pneumoniae the constitutive mechanism was the most common (99.6%), with only 0.4% presenting inducible MLSB phenotype and no strains with M phenotype. All S. pyogenes isolates included in this study were from pharyngeal swabs, and 61.8% of samples were collected in pediatric wards. With respect to S. pneumoniae, 84.8% of strains were collected from adult patients, and 91.9% were collected from lower respiratory tract samples. Overall resistance to erythromycin was 34.1% for S. pneumoniae and 26.7% for S. pyogenes, with maximal erythromycin resistance prevalences for S. pneumoniae and S. pyogenes of 59 and 70%, respectively, whereas minimal prevalences were 23 and 5%, respectively.
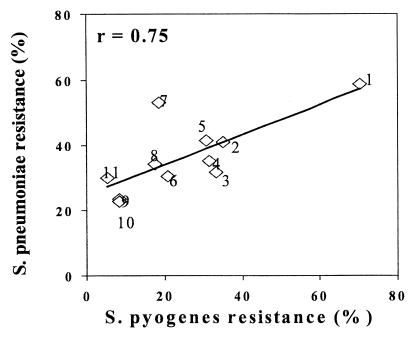
No relation was found between penicillin resistance in either species (0% for S. pyogenes and 37.2% for S. pneumoniae), but hospitals exhibiting a high prevalence of S. pneumoniae resistance to erythromycin also exhibited a high prevalence of S. pyogenes resistance to erythromycin. The relationship between both species’ erythromycin is shown by hospital in Fig. 1. A high correlation coefficient was found (r = 0.75, P < 0.01).
FIG. 1.
Relationship between erythromycin resistance in S. pneumoniae and S. pyogenes by Spanish hospital. Hospitals: 1, Reina Sofía (Córdoba); 2, Virgen de las Nieves (Granada); 3, Virgen de la Macarena (Sevilla); 4, Basurto (Bilbao); 5, La Fe (Valencia); 6, Gregorio Marañón (Madrid); 7, La Paz (Madrid); 8, Clínico (Salamanca); 9, Xeral (Santiago de Compostela); 10, Infanta Cristina (Badajoz); 11, Clínico (Zaragoza).
The problem of increased resistance to antibiotics on the part of community respiratory pathogens is well known. In Spain, penicillin resistance in S. pneumoniae has increased since 1989 from 44% (7) to 60% in 1996 to 1997 (5). Similarly, erythromycin resistance increased from 10% in 1985 (7) to 33.7% in 1996 to 1997 (5). Data for S. pyogenes show an increase in erythromycin resistance from 3% in 1991 to 1992 (6) to 27% in 1996 to 1997 (4), due in part to the increase of highly resistant strains (MIC, ≥8 μg/ml). This increase was attributed to the spread of the M phenotype (4), and an ecological relation with macrolide consumption has been described previously (8). The existence of this type of relationship was also suggested for S. pneumoniae (9). Based on this phenomenon, a relationship between macrolide resistance in S. pyogenes and S. pneumoniae is suspected.
Despite the ecological nature of the present study, a high correlation between macrolide resistance in S. pyogenes and S. pneumoniae both was found. S. pyogenes isolates are mainly of pediatric origin, from upper respiratory tract infections (pharyngeal swabs), and from a self-limited disease (streptococcal pharyngitis), and exhibit a high prevalence of M phenotype resistance to macrolides. In contrast, S. pneumoniae strains are of adult origin, from lower respiratory tract infections (i.e., sputum and bronchial aspirate) and lung infections (pneumonia and acute exacerbation of chronic bronchitis), and show a high prevalence of constitutive phenotype. Thus, the ecological relation between consumption and resistance seems to warrant further study.
Acknowledgments
This study was supported by a grant from SmithKline Beecham S.A. Spain.
We thank Francisco Soriano and César García-Rey for critically reviewing the manuscript.
REFERENCES
- 1.Baquero F the Task Force of the General Direction for Health Planning of the Spanish Ministry of Health. Antibiotic resistance in Spain: what can be done? Clin Infect Dis. 1996;23:819–823. doi: 10.1093/clinids/23.4.819. [DOI] [PubMed] [Google Scholar]
- 2.Baquero F. Trends in antibiotic resistance of respiratory pathogens: an analysis and commentary on a collaborative surveillance study. J Antimicrob Chemother. 1998;38(Suppl. A):117–132. doi: 10.1093/jac/38.suppl_a.117. [DOI] [PubMed] [Google Scholar]
- 3.Baquero F. Evolving resistance patterns of Streptococcus pneumoniae: a link with long-acting macrolide consumption? J Chemother. 1999;11(Suppl. 1):35–43. doi: 10.1179/joc.1999.11.Supplement-2.35. [DOI] [PubMed] [Google Scholar]
- 4.Baquero F, García-Rodríguez J A, García-de-Lomas J, Aguilar L the Spanish Surveillance Group for Respiratory Pathogens. Antimicrobial resistance of 914 beta-hemolytic streptococci isolated from pharyngeal swabs in Spain: results of a 1-year (1996–1997) multicenter surveillance study. Antimicrob Agents Chemother. 1999;43:178–180. doi: 10.1128/aac.43.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baquero F, García-Rodríguez J A, García-de-Lomas J, Aguilar L the Spanish Surveillance Group for Respiratory Pathogens. Antimicrobial resistance of 1,113 Streptococcus pneumoniae isolates from patients with respiratory tract infections in Spain: results of a 1-year (1996–1997) multicenter surveillance study. Antimicrob Agents Chemother. 1999;43:357–359. doi: 10.1128/aac.43.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betriu C, Sánchez A, Gómez M, Cruceyra A, Picazo J J. Antibiotic susceptibility of group A streptococci: a 6-year follow up study. Antimicrob Agents Chemother. 1993;37:1717–1719. doi: 10.1128/aac.37.8.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenoll A, Burgon C M, Muñoz R, Vicioso D, Casal T. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing systemic infections in Spain, 1979–1989. Rev Infect Dis. 1991;13:56–60. doi: 10.1093/clinids/13.1.56. [DOI] [PubMed] [Google Scholar]
- 8.Granizo J, Aguilar L, Dal-Ré R. Proceedings of the Second International Meeting on the Therapy of the Infections, Florence, Italy, 18–21 November. 1998. Ten years Streptococcus pyogenes resistance to macrolides in Spain, in relation to macrolide consumption; p. 99. [Google Scholar]
- 9.Liñares J, Pallarés R, Alonso T, Pérez J L, Ayats J, Gudiol F, Villadrich P F, Martín R. Trends in antimicrobial resistance of clinical isolates of Streptococcus pneumoniae in Bellvitge hospital, Barcelona, Spain (1979–1990) Clin Infect Dis. 1992;15:99–105. doi: 10.1093/clinids/15.1.99. [DOI] [PubMed] [Google Scholar]
- 9a.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; ninth informational supplement. M100-S9. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 10.Rothman K, Greenland S. Measures of effect and measures of association. In: Rothman K, Greenland S, editors. Modern epidemiology. 2nd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1998. pp. 47–66. [Google Scholar]
- 11.Seppälä H, Nissinen A, Yu Q, Huovinen P. Three different phenotypes of erythromycin-resistant Streptococcus pyogenes in Finland. J Antimicrob Chemother. 1993;32:885–891. doi: 10.1093/jac/32.6.885. [DOI] [PubMed] [Google Scholar]
- 12.Seppälä H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, Huovinen P. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 13.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]