Abstract
Background
It is necessary to systematically evaluate the efficacy and adverse reactions of pirfenidone in the treatment of patients with idiopathic pulmonary fibrosis (IPF).
Methods
Pubmed et al. databases were searched up to March 15, 2021 for randomized controlled trials (RCT) of pirfenidone in the treatment of IPF. Two authors collected and compared the indicators including progression-free survival (PFS), vital capacity (VC), forced vital capacity (FVC), and adverse reactions. RevMan 5.3 software and Stata 15.0 software were used for meta-analysis.
Results
A total of 8 reports with 9 RCTs involving 1824 IPF patients were included. Meta-analysis results showed that compared with the control group, pirfenidone could prolong the PFS phase of IPF patients (HR = 0.65, 95% CI 0.55 ~ 0.76, P < 0.001), slow down the VC of IPF patients (SMD = 0.43, 95% CI 0.21 ~ 0.66, P < 0.001), and decrease FVC (SMD = 0.31, 95% CI 0.14 ~ 0.48, P < 0.001). The main adverse reactions of pirfenidone were gastrointestinal reactions, photosensitivity and skin rashes.
Conclusion
Pirfenidone is beneficial to prolong the PFS of IPF patients, improve lung function, and it is safe for clinical use. However, more high-quality RCTs are still needed to provide reliable evidence for the treatment of IPF.
Keywords: Pirfenidone, Idiopathic pulmonary fibrosis, Effect, Safety, Treatment
Background
Idiopathic pulmonary fibrosis (IPF) is a kind of unexplained, chronic progressive fibrotic interstitial pneumonia, and it is more likely to occur in middle-aged and elderly people [1]. The global annual incidence of IPF is 0.2 per 100,000 to 93.7 per 100,000, and it is increasing over time [2]. It has been reported that the annual incidence of IPF in Taiwan from 2000 to 2007 was 2.8 per 100,000 to 6.4 per 100,000, with the highest incidence in men over 75 years of age [3, 4]. Most patients have rapid disease progression and short survival time after being diagnosed with IPF [5]. Once fibrotic alterations occur, it is difficult to reverse, and early diagnosis and treatment are particularly important to the prognosis of IPF patients [6].
The previous treatment of IPF often included hormones and immunosuppressive agents, with the goal of reducing inflammation as much as possible and delaying the progression of inflammation to fibrosis [7, 8]. With the deepening of research, the pathogenesis has changed from “initiating factors of inflammation” to “fibrosis in the repair of alveolar epithelial injury”, and drug treatment has also gradually changed from "anti-inflammatory" to "anti-fibrosis", but there is still no drug that can completely cure IPF [9, 10]. New anti-fibrosis drugs seem to delay the progression of the disease and are recommended by domestic and foreign guidelines for the treatment of IPF [11]. Pirfenidone was approved for use in China in December 2013. In view of the current limited clinical application report data of pirfenidone and the inconsistent results of related studies for technical limitations of randomized controlled trials (RCTs), this present meta-analysis aimed to systematically evaluate the efficacy and safety of pirfenidone in the treatment of IPF, to provide evidence to the IPF treatment and clinical drug use.
Methods
This meta-analysis and systematic review was conducted and reported in comply with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [12].
Search strategy
Two investigators conducted computer search of Cochrane Library, PubMed, EMBase, China national knowledge infrastructure (CNKI), Chinese Biomedical Literature Database and Wanfang Database for RCTs on the applications of pirfenidone in the treatment of IPF, the search time limit was from the establishment of each database to March 15, 2021. Following search terms were applied: "Pirfenidone"," idiopathic pulmonary fibrosis ", “IPF”, “pulmonary fibrosis”, “randomized controlled trial”, “RCT”.
Inclusion and exclusion criteria
The inclusion criteria for RCTs in this meta-analysis were: ① RCT design on the pirfenidone use in the treatment of patients with IPF; ② all patients met the diagnostic criteria for IPF, and the experimental group was treated with pirfenidone and other clinical treatments were the same as the control group, the control group adopted conventional treatment methods using placebo or blank control, and the treatment duration was at least 3 months; ③ evaluation indicators were reported such as disease progression-free survival (PFS), vital capacity (VC), forced vital capacity (FVC), adverse reactions, etc.
The exclusion criteria in this meta-analyses were: ① duplicate publication; ② studies with failure to provide sufficient original data.
Literature screening and data extraction
Two investigators independently screened and cross-checked the literature according to the inclusion and exclusion criteria. In case of disagreement, they discussed or consulted a third party to assist in the judgment. Then two authors extracted the following information independently according to the designed data extraction table: author, publication year, basic information of the research population, sample size, treatment plan, outcome indicators and related data. The original authors were contacted as much as possible for the lack of related outcome data.
Quality evaluation
The Cochrane Collaborations risk of bias tool [13] was used to evaluate the methodological quality and risk of bias of included RCTs, any disagreements in the quality evaluation were resolved by discussion and consensus. The Cochrane risk of bias tool includes seven specific domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other issues. Every domain could be classified as low risk of bias, high risk of bias or unclear risk of bias according to the judgment criteria.
Statistical analysis
RevMan 5.3 software and Stata 15.0 software were used for meta-analysis. Firstly, a heterogeneity test on the synthesized data was conducted. If P > 0. 1, I2 < 50%, it was considered as homogeneous among multiple studies, and a fixed effects model was used for meta-analysis; if P < 0. 1. If I2 ≥ 50%, it was considered that there was heterogeneity in the effect size, then the source of heterogeneity was analyzed by conducting subgroup analysis, sensitivity analysis and applied random effects model. The effect size of each study was calculated according to the method of Parmar et al., and the combined effect size, combined weight and 95% CI were calculated. Publication bias was evaluated by using funnel plots, and asymmetry was assessed by conducting Egger regression test. In this meta-analysis, the difference was statistically significant with P < 0.05.
Results
Study inclusion
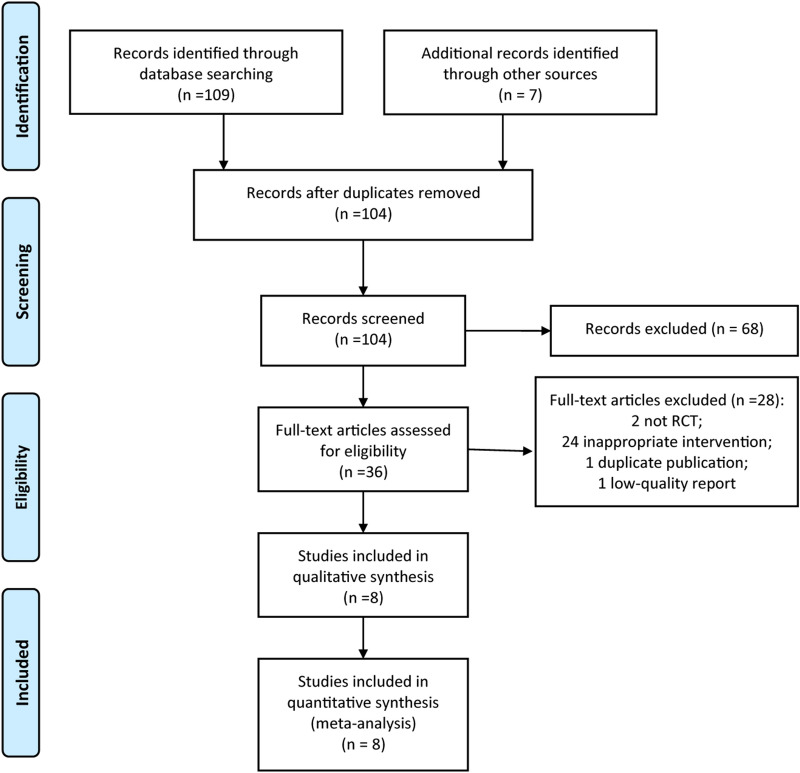
As shown in Fig. 1, 104 related reports were retrieved for the first time. After reading the title and abstract, 68 studies were excluded. Based on the inclusion and exclusion criteria, the documents that did not meet the inclusion criteria were removed, and 8 reports [14–21] with 9 RCTs were finally included. The conditions of all reported cases were in a stable phase. The total number of patients was 1824, with 930 cases in the experimental group, and 894 cases in the control group. The basic information of the included studies is shown in Table 1.
Fig. 1.
PRISMA flow diagram
Table 1.
The characteristics of included RCTs
| Study ID | Sample size | Interventions | Durations | Outcomes | |||
|---|---|---|---|---|---|---|---|
| Experimental group | Control group | Experimental group | Control group | ||||
| Azuma 2005 | 72 | 35 | Pirfenidone 1800 mg/d | Placebo | 9 months | Pirfenidone is not yet able to increase the lowest SpO2 in 6MWT, but it can increase the VC of IPF patients | |
| CAPACITY 004 2011 | 174 | 174 | Pirfenidone 2403 mg/d | Placebo | 72 weeks | Pirfenidone slowed the decline of FVC and prolonged the PFS period, but did not significantly increase the lowest SpO 2 of 6MWD and 6MWT | |
| CAPACITY 006 2011 | 171 | 173 | Pirfenidone 2403 mg/d | Placebo | 72 weeks | Pirfenidone did not increase the lowest SpO 2 in FVC, PFS phase and 6MWT, but pirfenidone could reduce the decline of 6MWD | |
| Huang 2015 | 38 | 38 | Pirfenidone 1800 mg/d | Placebo | 48 weeks | The pirfenidone group can significantly prolong the PFS period, without significantly delaying the decline of FVC, and not increasing the lowest SpO2 of 6MWD and 6MWT | |
| King 2014 (ASCEND) | 278 | 277 | Pirfenidone 1800 mg/d | Placebo | 52 weeks | Pirfenidone can slow down the decline of FVC and 6MWD, and significantly prolong the PFS | |
| Lei 2018 | 20 | 20 | Pirfenidone 1200 mg/d | Blank control | 48 weeks | Pirfenidone can delay the decline of FVC | |
| Li 2015 | 43 | 44 | Pirfenidone 1200 mg/d | Placebo | 48 weeks | Pirfenidone can improve FVC and slow down the decline of 6MWD | |
| Li 2016 | 24 | 24 | Pirfenidone 1200 ~ 1800 mg/d | Blank control | 6 months | Pirfenidone can improve FVC and increase 6MWD | |
| Taniguchi 2010 | 110 | 109 | Pirfenidone 1800 mg/d | Placebo | 52 weeks | Pirfenidone can slow down the decline of VC in IPF patients and prolong the PFS | |
PFS progression-free survival, VC vital capacity, FVC forced vital capacity
Quality of included studies
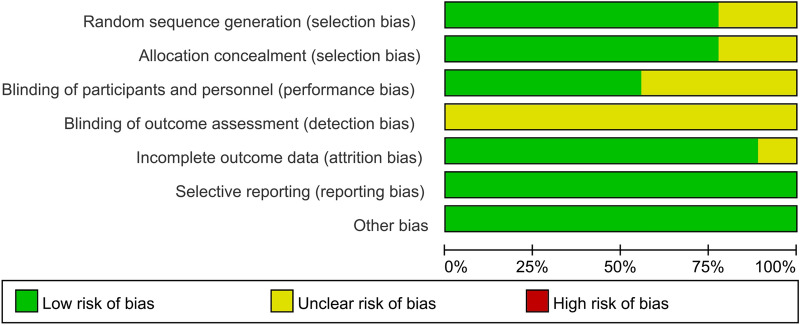
We used the bias risk assessment tool recommended by Cochrane Network for quality evaluation. Among the 9 included RCTs, 7 of the included studies [14, 16–20] had clear randomization schemes, the patients were randomized according to the random number table method or computer randomization method. The randomization plan of two RCTs is not clear. 7 studies [14, 16, 17, 19–21] reported allocation concealments, and 5 RCTs [14, 17, 19, 20] reported the blinding design on the participants and personnel. All the included studies did not report the blinding design on the outcome assessment. No other significant biases in the incomplete outcome data, selective reporting and other biases were found (Figs. 2, 3).
Fig. 2.
Risk of bias graph
Fig. 3.
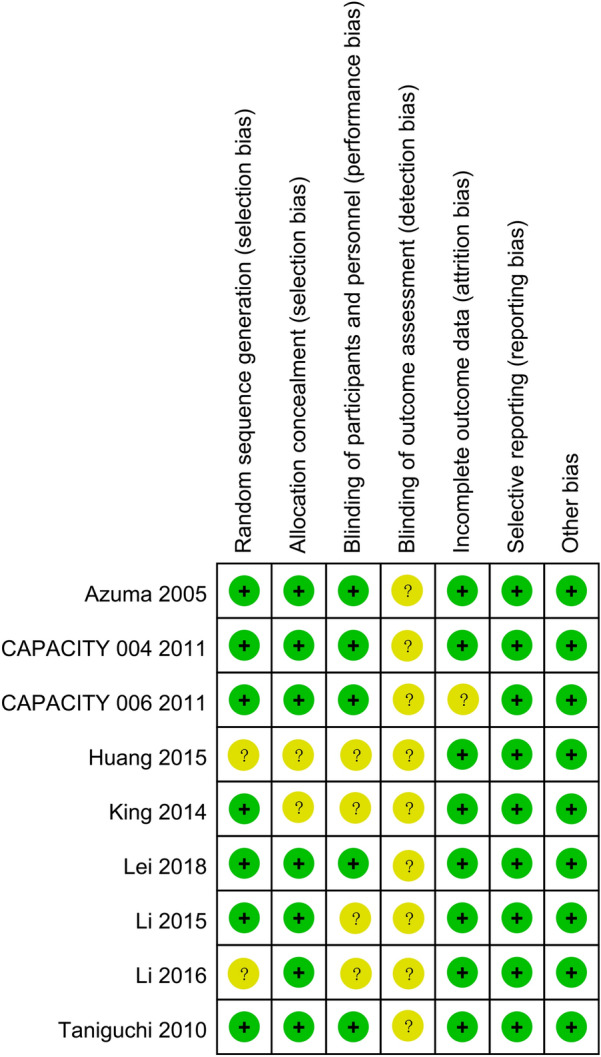
Risk of bias summary
Synthesized outcomes
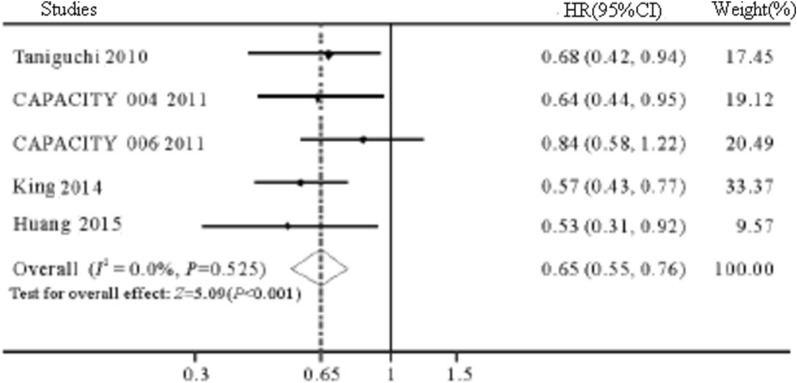
PFS 5 RCTs [15, 18–20] reported the effect of pirfenidone on PFS in patients with IPF. The results of meta-analysis showed that there was no heterogeneity among the studies (I2 = 0%, P = 0.525), and the fixed effects model was selected. Compared with control group, pirfenidone significantly prolonged the PFS of IPF patients (HR = 0.65, 95% CI 0.55 ~ 0.76, P < 001, Fig. 4).
Fig. 4.
The plot forest of PFS
VC 2 studies [14, 20] reported the changes in VC of IPF patients. Meta-analysis results showed that there was no heterogeneity between studies (I2 = 0%, P = 0.818), and the fixed effects model was selected. Compared with control group, pirfenidone could slow down the decline of VC in IPF patients (SMD = 0.43, 95% CI 0.21 ~ 0.66, P < 0.001, Fig. 5).
Fig. 5.
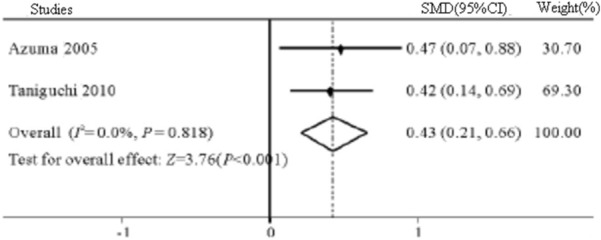
The plot forest of VC
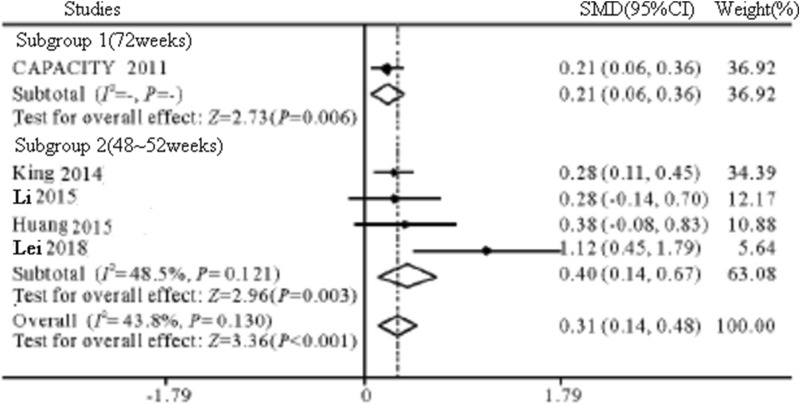
FVC 6 studies [15–19] reported the change of FVC from the baseline value. The results of meta-analysis showed that there was heterogeneity among the studies (I2 = 72. 6%, P = 0.003), and the random effects model was selected. Compared with control group, pirfenidone could delay the decline of FVC in IPF patients (SMD = 0.31, 95% CI 0.14 ~ 0.48, P < 001, Fig. 6). Due to different follow-up times, some studies reported changes in FVC values at multiple time points. Meta-analysis showed that the reported studies had homogeneity (all I2 < 50%), and fixed-effect models were selected. Compared with the control group, at 48–52 weeks and 72 weeks, Pirfenidone slowed down the decline of FVC in IPF patients (SMD = 0.40, 95% CI 0.14 ~ 0.67, P = 0.003; SMD = 0.21, 95% CI 0.06 ~ 0.36, P = 0.006).
Fig. 6.
The plot forest of FVC
Adverse complications The included reports had reported the adverse reactions of pirfenidone in the treatment of IPF, but there is a large heterogeneity among the studies (I2 = 88.15%, P < 0.001), because it is impossible to determine the source of heterogeneity, descriptive integration is used. As shown in Table 2, the incidence of adverse events in the two groups of patients was relatively high. Most of the adverse events were mild to moderate, and symptoms disappeared after dose reduction or discontinuation of pirfenidone and symptomatic treatment. In the studies of Azuma [14], CAPACITY [19], Huang15, and Lei [17], the differences in the results of the adverse reactions between the groups were statistically significant, while the results of the studies by Taniguchi20 and Li21 were not statistically significant. In several studies, there were no interruption of treatment due to serious adverse events, which might be related to the small sample size and the small oral dose.
Table 2.
Adverse reactions of pirfenidone treatment
| Study ID | Main adverse reactions | Major adverse reactions leading to interruption of treatment |
|---|---|---|
| Azuma 2005 | Photosensitivity (43.8%), gastrointestinal discomfort (30.1%), anorexia (31.5%) | Photosensitivity (5 cases), vomiting, fever, abnormal liver function, dizziness, facial paralysis, hepatocellular tumor (1 case each) |
| CAPACITY 2011 | Nausea (36.0%), skin rash (32.0%) | Nausea (36.0%), rash (28.1%), headache (25.9%), cough (25.2%), diarrhea (22.3%) |
| Huang 2015 | Skin rash (39.5%), nausea (5.26%), diarrhea (7.89%) | NA |
| King 2014 | Nausea (36.0%), rash (28.1%), headache (25.9%), cough (25.2%), diarrhea (22.3%) | Acute exacerbation of IPF, elevated liver enzymes, pneumonia (both 1.1%) |
| Lei 2018 | Photosensitivity, loss of appetite, fatigue | NA |
| Li 2015 | NA | NA |
| Li 2016 | Gastrointestinal reaction, abnormal liver function | NA |
| Taniguchi 2010 | Photosensitivity (51.4%), nasopharyngitis (49.5%), anorexia (16.5%) | Photosensitivity (2.8%), lung cancer (1.8%), fever (1.8%), respiratory failure (1.8%) |
Publications of bias
We attempted to evaluate publication bias by using a funnel plot if ten or more RCTs were included in outcome meta-analysis. Limited by the number of included RCTs, we could not perform funnel plot. Egger regression tests indicated that there was no significant publication of biases (all P > 0.05).
Sensitivity analyses, which investigate the influence of 1 study on the overall risk estimate by removing one study in each turn, suggested that the overall risk estimates were not substantially changed by any single study.
Discussion
The pathogenesis of IPF is complex, and it is not yet fully clear. There are fewer effective drugs for the treatment of IPF in the clinic, so although the incidence of the disease is low, the prognosis is extremely poor [22, 23]. Several studies [24–26] have proved that pirfenidone has anti-fibrosis, anti-oxidation and anti-inflammatory effects. It mainly inhibits transforming growth factor (TGF)-β, tumor necrosis factor (TNF)-α and platelet-derived growth factor (PDGF) to produce an anti-fibrosis effect. Pirfenidone is a pleiotropic pyridine compound that reduces the extracellular matrix by inhibiting the synthesis of collagen stimulated by transforming growth factor β, and prevents the proliferation of fibroblasts to achieve anti-fibrosis, anti-inflammatory and anti-oxidant effects [27, 28]. It has been reported that antioxidant activity mediates pirfenidone antifibrotic effects in human pulmonary vascular smooth muscle cells exposed to sera of idiopathic pulmonary fibrosis patients [29]. Besides, pirfenidone exerts beneficial effects on specific markers of oxidative stress and inflammation in IPF patients [30]. The results of this meta-analysis have showed that compared with the control group, pirfenidone reduces the risk of IPF progression or death by 35%, and it can significantly improve lung function, including delay the decline of VC and FVC. Although almost all patients have different degrees of adverse reactions, mainly including gastrointestinal reactions such as nausea and diarrhea, photosensitivity and skin rashes, most of them are mild to moderate. After drug reduction, discontinuation and symptomatic treatment, the symptoms can disappear. Although serious adverse reactions such as tumors, abnormal liver function, and respiratory failure have occurred, they are rare. Therefore, pirfenidone can effectively improve the quality of life of IPF patients and is safe for clinical use.
A number of studies [31–33] have investigated the efficacy of bosentan, imatinib, etanercept, interferon gamma, and prednisone, azathioprine and N-beta cysteine in the treatment of IPF. However, the results of the related studies show that the efficacy of each drug in the treatment of IPF is unsatisfactory. Therefore, finding effective drugs for the treatment of IPF is always one of the hot spots in this research field. Pirfenidone is an anti-fibrosis drug with anti-fibrosis, anti-inflammatory and antioxidant effects [34, 35]. However, existing guidelines recommend pirfenidone for the treatment of mild and moderate IPF, but its mechanism of action is still unclear and needs to be further explored.
Studies [36] have reported that the overall incidence of adverse reactions during the use of pirfenidone in China post-marketing patients is 8.90%. Most of the adverse reactions that occurred are mild to moderate and can be tolerated. There was no discontinuation of treatment due to adverse reactions happened so far, there have been no reports of serious adverse events related to drugs. The most common adverse reactions of pirfenidone are gastrointestinal reactions, skin allergic reactions, and elevated transaminases [37]. The other common side effects of nintedanib, another IPF treatment drug, are diarrhea, elevated transaminases, bleeding, etc. The side effects of those two drugs have similar symptoms. Studies [38–40] have reported that patients who are older than 60 years old and whose dosage of pirfenidone is greater than 1.2 g/day have a high probability of adverse reactions. Therefore, patients should be cautious when using high-dose pirfenidone. Meanwhile, more clinical studies are needed to further explore the effectiveness and safety of different doses of pirfenidone in patients with IPF.
This meta-analysis has certain limitations. First of all, among the 8 included reports, most of the studies have scientific and rigorous experimental design, with high patient compliance, low loss to follow-up, and high quality. However, there are still several RCTs did not explained the specific random allocation methods and blinding design. The above factors are the main reasons for the high-risk bias, and it may be the important reasons for the large heterogeneity of the results. Additionally, the included RCTs did not report in detail whether pirfenidone can reduce the mortality of IPF patients. Therefore, more large samples and high-quality RCTs are needed to further confirm the efficacy and safety of pirfenidone on IPF.
Conclusions
In summary, pirfenidone is beneficial for prolonging the PFS phase of patients with IPF, improve lung function with good safety. It is recommended for clinical IPF treatment, but clinicians still need to make a reasonable choice based on the patient's condition. With the development of medicine, it is still necessary to design larger sample size and more scientific RCTs to verify the therapeutic effect and safety of pirfenidone in patients with IPF, to provide more reliable medical evidence for the treatment of IPF.
Acknowledgements
None.
Abbreviations
- IPF
Idiopathic pulmonary fibrosis
- RCT
Randomized controlled trials
- PFS
Progression-free survival
- VC
Vital capacity
- FVC
Forced vital capacity
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- CNKI
China national knowledge infrastructure
- TGF
Transforming growth factor
- TNF
Tumor necrosis factor
- PDGF
Platelet-derived growth factor
Authors’ contributions
LL designed research; CZ, YZ, YW, LL conducted research; CZ, LL analyzed data; CZ, YZ wrote the first draft of manuscript; LL had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
In this study, all methods were performed in accordance with the relevant guidelines and regulations. Ethics approval and consent to participate were not applicable since our study is a meta-analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chenchen Zang and Yan Zheng are equal contributors
References
- 1.Phan THG, Paliogiannis P, Nasrallah GK, Giordo R, Eid AH, Fois AG, Zinellu A, Mangoni AA, Pintus G. Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis. Cell Mol Life Sci. 2021;78(5):2031–2057. doi: 10.1007/s00018-020-03693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 3.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 4.Werderman DS. Idiopathic pulmonary fibrosis. Radiol Technol. 2020;91(4):361–376. [PubMed] [Google Scholar]
- 5.Glass DS, Grossfeld D, Renna HA, Agarwala P, Spiegler P, Kasselman LJ, Glass AD, DeLeon J, Reiss AB. Idiopathic pulmonary fibrosis: molecular mechanisms and potential treatment approaches. Respir Investig. 2020;58(5):320–335. doi: 10.1016/j.resinv.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Wuyts WA, Wijsenbeek M, Bondue B, Bouros D, Bresser P, Robalo Cordeiro C, Hilberg O, Magnusson J, Manali ED, Morais A, et al. Idiopathic pulmonary fibrosis: best practice in monitoring and managing a relentless fibrotic disease. Respiration. 2020;99(1):73–82. doi: 10.1159/000504763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torrisi SE, Kahn N, Vancheri C, Kreuter M. Evolution and treatment of idiopathic pulmonary fibrosis. Presse Med. 2020;49(2):104025. doi: 10.1016/j.lpm.2020.104025. [DOI] [PubMed] [Google Scholar]
- 8.Hadjicharalambous MR, Lindsay MA. Idiopathic pulmonary fibrosis: pathogenesis and the emerging role of long non-coding RNAs. Int J Mol Sci. 2020;21(2):524. doi: 10.3390/ijms21020524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu H, Yu Y, Huang H, Hu Y, Fu S, Wang Z, Shi M, Zhao X, Yuan J, Li J, et al. Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell. 2020;180(1):107–121 e117. doi: 10.1016/j.cell.2019.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Trachalaki A, Irfan M, Wells AU. Pharmacological management of idiopathic pulmonary fibrosis: current and emerging options. Expert Opin Pharmacother. 2021;22(2):191–204. doi: 10.1080/14656566.2020.1822326. [DOI] [PubMed] [Google Scholar]
- 11.Yanqing Y. A systematic review of the efficacy of pirfenidone in the treatment of idiopathic pulmonary fibrosis. J Clin Intern Med. 2020;37(7):489–493. [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Dai HP, Kang J, Chen BY, Sun TY, Xu ZJ. Double-blind randomized trial of pirfenidone in Chinese idiopathic pulmonary fibrosis patients. Medicine (Baltimore) 2015;94(42):e1600. doi: 10.1097/MD.0000000000001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huiping L, Siqin W, Jianying Z. Phase II clinical study of domestic pirfenidone in the treatment of idiopathic pulmonary fibrosis. Chin J Respir Crit Care. 2015;14(3):229–235. [Google Scholar]
- 17.Kaichun L, Hongmei Y, Tingting Z. The clinical efficacy and adverse reactions of pirfenidone in the treatment of idiopathic pulmonary fibrosis. Chin J N Drugs Clin Med. 2018;37(3):160–163. [Google Scholar]
- 18.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 19.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE, Jr, Lancaster L, Sahn SA, Szwarcberg J, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 21.Yunxia L, Qiong L, Hui J. Clinical study of pirfenidone in the treatment of idiopathic pulmonary fibrosis. J Aerosp Med. 2016;27(11):1365–1367. [Google Scholar]
- 22.Raghu G, Ley B, Brown KK, Cottin V, Gibson KF, Kaner RJ, Lederer DJ, Noble PW, Song JW, Wells AU, et al. Risk factors for disease progression in idiopathic pulmonary fibrosis. Thorax. 2020;75(1):78–80. doi: 10.1136/thoraxjnl-2019-213620. [DOI] [PubMed] [Google Scholar]
- 23.Richeldi L, Fernandez Perez ER, Costabel U, Albera C, Lederer DJ, Flaherty KR, Ettinger N, Perez R, Scholand MB, Goldin J, et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2020;8(1):25–33. doi: 10.1016/S2213-2600(19)30262-0. [DOI] [PubMed] [Google Scholar]
- 24.Behr J, Bendstrup E, Crestani B, Gunther A, Olschewski H, Skold CM, Wells A, Wuyts W, Koschel D, Kreuter M, et al. Safety and tolerability of acetylcysteine and pirfenidone combination therapy in idiopathic pulmonary fibrosis: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2016;4(6):445–453. doi: 10.1016/S2213-2600(16)30044-3. [DOI] [PubMed] [Google Scholar]
- 25.Nathan SD, Albera C, Bradford WZ, Costabel U, du Bois RM, Fagan EA, Fishman RS, Glaspole I, Glassberg MK, Glasscock KF, et al. Effect of continued treatment with pirfenidone following clinically meaningful declines in forced vital capacity: analysis of data from three phase 3 trials in patients with idiopathic pulmonary fibrosis. Thorax. 2016;71(5):429–435. doi: 10.1136/thoraxjnl-2015-207011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goos T, De Sadeleer LJ, Yserbyt J, Verleden GM, Vermant M, Verleden SE, Wuyts WA. Progression in the management of non-idiopathic pulmonary fibrosis interstitial lung diseases, where are we now and where we would like to be. J Clin Med. 2021;10(6):1330. doi: 10.3390/jcm10061330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Rodriguez E, Gay-Jordi G, Knudsen L, Ochs M, Serrano-Mollar A. Improved alveolar dynamics and structure after alveolar epithelial type ii cell transplantation in bleomycin induced lung fibrosis. Front Med (Lausanne) 2021;8:640020. doi: 10.3389/fmed.2021.640020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lancaster LH, de Andrade JA, Zibrak JD, Padilla ML, Albera C, Nathan SD, Wijsenbeek MS, Stauffer JL, Kirchgaessler KU, Costabel U. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. 2017;26(146):170057. doi: 10.1183/16000617.0057-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fois AG, Posadino AM, Giordo R, Cossu A, Agouni A, Rizk NM, Pirina P, Carru C, Zinellu A, Pintus G. Antioxidant activity mediates pirfenidone antifibrotic effects in human pulmonary vascular smooth muscle cells exposed to sera of idiopathic pulmonary fibrosis patients. Oxid Med Cell Longev. 2018;2018:2639081. doi: 10.1155/2018/2639081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fois AG, Sotgiu E, Scano V, Negri S, Mellino S, Zinellu E, Pirina P, Pintus G, Carru C, Mangoni AA, et al. Effects of pirfenidone and nintedanib on markers of systemic oxidative stress and inflammation in patients with idiopathic pulmonary fibrosis: a preliminary report. Antioxidants (Basel) 2020;9(11):1064. doi: 10.3390/antiox9111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. Drug Treatment of idiopathic pulmonary fibrosis: systematic review and network meta-analysis. Chest. 2016;149(3):756–766. doi: 10.1016/j.chest.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Trawinska MA, Rupesinghe RD, Hart SP. Patient considerations and drug selection in the treatment of idiopathic pulmonary fibrosis. Ther Clin Risk Manag. 2016;12:563–574. doi: 10.2147/TCRM.S81144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valenzuela C, Ancochea J. Treatment switching in idiopathic pulmonary fibrosis: from triple therapy to enrollment into a clinical investigational drug trial. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30(Suppl 1):44–47. [PubMed] [Google Scholar]
- 34.Sgalla G, Iovene B, Calvello M, Ori M, Varone F, Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res. 2018;19(1):32. doi: 10.1186/s12931-018-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogliani P, Calzetta L, Cavalli F, Matera MG, Cazzola M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2016;40:95–103. doi: 10.1016/j.pupt.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Sathiyamoorthy G, Sehgal S, Ashton RW. Pirfenidone and nintedanib for treatment of idiopathic pulmonary fibrosis. South Med J. 2017;110(6):393–398. doi: 10.14423/SMJ.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 37.Vancheri C, Kreuter M, Richeldi L, Ryerson CJ, Valeyre D, Grutters JC, Wiebe S, Stansen W, Quaresma M, Stowasser S, et al. Nintedanib with add-on pirfenidone in idiopathic pulmonary fibrosis results of the INJOURNEY trial. Am J Respir Crit Care Med. 2018;197(3):356–363. doi: 10.1164/rccm.201706-1301OC. [DOI] [PubMed] [Google Scholar]
- 38.Nathan SD, Costabel U, Albera C, Behr J, Wuyts WA, Kirchgaessler KU, Stauffer JL, Morgenthien E, Chou W, Limb SL, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis and more advanced lung function impairment. Respir Med. 2019;153:44–51. doi: 10.1016/j.rmed.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Vianello A, Molena B, Turato C, Braccioni F, Arcaro G, Paladini L, Andretta M, Saetta M. Pirfenidone improves the survival of patients with idiopathic pulmonary fibrosis hospitalized for acute exacerbation. Curr Med Res Opin. 2019;35(7):1187–1190. doi: 10.1080/03007995.2019.1565530. [DOI] [PubMed] [Google Scholar]
- 40.Flaherty KR, Fell CD, Huggins JT, Nunes H, Sussman R, Valenzuela C, Petzinger U, Stauffer JL, Gilberg F, Bengus M, et al. Safety of nintedanib added to pirfenidone treatment for idiopathic pulmonary fibrosis. Eur Respir J. 2018;52(2):1800230. doi: 10.1183/13993003.00230-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.