Abstract
Interest in understanding the environmental distribution of the alkane monooxygenase (AlkB) enzyme led to the identification of over 100 distinct alkane monooxygenase (AlkB) enzymes containing a covalently bound, or fused, rubredoxin. The rubredoxin-fused AlkB from Dietzia cinnamea was cloned as a full-length protein and as a truncated protein with the rubredoxin domain deleted. A point mutation (V91W) was introduced into the full-length protein, with the goal of assessing how steric bulk in the putative substrate channel might affect selectivity. Based on activity studies with alkane and alkene substrates, the rubredoxin-fused AlkB oxidizes a similar range of alkane substrates relative to its rubredoxin domain-deletion counterpart. Oxidation of terminal alkenes generated both an epoxide and a terminal aldehyde. The products of V91W-mutant-catalyzed oxidation of alkenes had a higher aldehyde-to-epoxide ratio than the products formed in the presence of the wild type protein. These results are consistent with this mutation causing a structural change impacting substrate positioning.
Keywords: alkane oxidation, monooxygenase, diiron enzymes, AlkB, rubredoxin, biocatalysis
Graphical abstract

1. INTRODUCTION
Alkane monooxygenase (AlkB) catalyzes the conversion of medium- to long-chain n-alkanes to primary alcohols.1 Its ability to selectively activate a C-H bond and oxidize energetically rich -- although frequently toxic -- alkanes to ecologically less harmful alcohols has made AlkB an attractive candidate for biocatalysis and bioremediation of oil in the environment.2–5 In addition to its function as an alkane monooxygenase, AlkB has been shown to catalyze the further oxidation of primary alcohols to aldehydes and carboxylic acids.6, 7 These overoxidation reactions have been investigated for their utility in biocatalysis.8, 9 In addition to alkanes, AlkB is active on terminal alkenes, producing epoxides and aldehydes.10–12
Obtaining a high-resolution structure of AlkB has been a long-coveted goal, as it would enable a greater understanding of the molecular basis of its reactivity. Although no such structure has been obtained, analyses of the protein function and sequence have been performed through comparing the activity of mutants to wildtype proteins. These analyses have revealed clues to the protein’s overall structure. AlkB is an integral membrane protein that contains a non-heme dinuclear iron active site coordinated by three regions of conserved histidines.13–16 It is related to a diverse group of enzymes that includes fatty acid desaturases, fatty acid hydroxylases, and decarbonylases.17 Members of this group contribute not only to bioremediation of oil, but also to human pathologies such as diabetes,18 obesity,19 cancer,19 development problems,20 and attention-deficit/hyperactivity disorder.21 AlkB itself may also be relevant to human health. It is expressed in pathogenic bacteria such as Mycobacterium tuberculosis, Legionella pneumophila, and Pseudomonas aeruginosa.17, 22, 23 Its role in these bacteria is poorly understood.
The catalytic activity of AlkB depends on the presence of two essential electron transfer partners, a rubredoxin (AlkG) and a rubredoxin reductase (AlkT).24–27 However, an alternative domain structure of AlkB has a fused rubredoxin domain located at the C-terminus of the protein.28 Previous studies have identified fewer than 30 proteins with this fused domain structure.17, 28–30 Functional analyses have indicated that these rubredoxin-fused AlkBs enable cells to grow on long-chain alkanes.17, 29, 31 Previous functional studies have been conducted in vivo and have been limited in scope to proteins from only two genera, although genes encoding rubredoxin-fused AlkBs exist in at least 28 genera. To our knowledge, the function of rubredoxin-fused AlkBs has not been explored in vitro. Consequently, little is known about their mechanism or the products they generate.
This study describes the bioinformatic analysis of rubredoxin-fused AlkBs and the subsequent investigation of one homologue from D. cinnamea through analysis of its activity on both alkane and alkene substrates. The activity of the full-length protein was examined, as was the activity of its truncated form without the fused rubredoxin domain and the activity of the full-length protein with a point mutation of a residue hypothesized to be in the substrate channel.
2. EXPERIMENTAL
2.1. Materials and methods
Chemicals and solvents were purchased from Millipore Sigma, Fisher Scientific, and Anatrase. Genes were synthesized by Integrated DNA Technologies (IDT). PCR was performed using a C1000 Series Touch Thermal Cycler (Bio-Rad), and Sanger sequencing was performed by Genewiz.
Low-speed spins were done using a Beckman Coulter Avanti J-26S XPI centrifuge with a JLA-8.1000 rotor, using a Beckman-Coulter Allegra X-30 centrifuge with a C0650 rotor, or using a Fisher Scientific AccuSpin Micro 17 centrifuge. High-speed spins were done with a Thermo Scientific Sorvall wX + Ultra Series centrifuge with an F50L-8 × 39 rotor. Cells were lysed using a French Press G-M.
Gas chromatography mass spectrometry (GCMS) analyses were performed on an Agilent 7820A GC with an Agilent HP-5 column and an Agilent 5977E MSD.
2.2. Bioinformatics.
The NCBI database was searched for genes coding for rubredoxin-fused AlkBs. These were filtered by cross-referencing with the SILVA database for “Type” strains to remove any redundancy.32 The programs RAxML, Clustal Omega, and ETE 3were used to generate a cladrogram.33–35 All codes used have been deposited in a public repository available at https://github.com/ajlopatkin.
2.3. Genetic transformation of E. coli
The bacterial strains, plasmids, and oligonucleotide primers used in this study are described in the supplemental material (Supplemental Table 1). G-blocks encoding the desired AlkB proteins were codon optimized for E. coli expression and ordered from IDT (Supplemental Table 2). Genes were inserted into the pET-15b plasmid using a modified fast-cloning method.36 Correct gene insertion was confirm using Sanger sequencing of the entire open reading frame (Genewiz). The miniprepped DNA was used to transform chemically competent BL21-DE3-star cells. These were plated on Luria-Bertani (LB)/agar plates with ampicillin.
2.4. Cell growth
For all cell cultures, a single colony was selected from the LB/agar plate and used to inoculate 50 mL sterile liquid LB media with 100 μg/mL ampicillin and grown overnight while shaking at 37 °C. The overnight inoculum was then transferred to 500 mL sterile liquid LB media with 100 μg/mL ampicillin and incubated at 37 °C until OD600 reached 0.5. At this point, isopropyl β- D-1-thiogalactopyranoside (IPTG) was added to a final concentration of 200 μM to induce alkB expression. FeCl3 (50 μM) was also added, and the cells were incubated for an additional 6–8 h. Cells were harvested by centrifugation at 7500 ×g for 15 minutes and stored at –80 °C.
2.5. Collection of AlkB
Frozen cell pellets were thawed and resuspended in 1 mL lysis buffer (20 mM Tris-HCl, 150 mM NaCl, pH 7.8) per 1 g cell pellet. DNase (1.6 units/mL) and phenylmethylsulfonyl fluoride (PMSF, 0.4 mM) were added. Cells were lysed by French Press at a pressure of 10,000–12,000 psi. Unbroken cells in the crude lysate were removed by centrifugation at 7000 ×g for 20 minutes. The supernatant was saved as cell free extract.
2.6. Measuring AlkB activity
AlkB-containing cell free extract (0.5 mL) was diluted in lysis buffer to a final volume of 1 mL. It was incubated in the presence of ferrous ammonium sulfate hexahydrate (1 mM), sodium hydrosulfite (1 mM), NADH (12.7 mM), and purified substrate (2 μL) for 30 min at 37 °C.37, 38 The reaction products were extracted with methylene chloride (100 μL) and quantified by GCMS. For AlkB-containing cells expressing the rubredoxin-deletion mutant, 125 uL of a buffered solution containing AlkG and AlkT from P. putida GPo1 was added.39 Control experiments in which all reagents were added, including cell free extract from E. coli not expressing AlkB, were done.
3. RESULTS AND DISCUSSION
3.1. Diversity and prevalence of rubredoxin-fused AlkBs
A search of the NCBIdatabase revealed 345 genes coding for rubredoxin-fused AlkBs. After filtering remove redundancy,32 110 results from high-quality genomes of distinct species were found to encode rubredoxin-fused AlkBs. These results came from 28 genera in the phyla proteobacteria and actinobacteria and included both gram-positive and gram-negative species. A cladogram was constructed to compare these rubredoxin-fused AlkB genes.33–35.
The cladogram (Figure 1) demonstrates a genetic diversity that marks a significant increase from the fewer than 30 rubredoxin-fused AlkBs from only two genera that had been previously identified, several of which came from the same species.30 It was found that of the 110 species identified to contain genes for rubredoxin-fused AlkBs, over 70 of them were from genera with organisms known to produce biosurfactants (highlighted in Figure 1).40–47 Given the prevalence of rubredoxin-fused AlkBs and the observation that many organisms encoding them produce biosurfactants, we hypothesize that AlkB may play a role in biosynthetic pathways in addition to pathways that breakdown alkanes as an energy source. This hypothesis is supported by an analysis of the gene environment of 24 rubredoxin-fused AlkBs, showing that approximately ¾th are near genes associated with cell wall biosynthesis (see SI). In order to better understand the biochemical capabilities of rubredoxin-fused AlkBs, the catalytic activity of AlkB from Dietzia cinnamea (Dc AlkB) was studied.48, 49 It was cloned both to encode the full-length protein and to encode the rubredoxin-deletion mutant (Dc nulRd AlkB).
Figure 1.

A cladogram prepared with RAxML, Clustal Omega, and ETE 3 comparing the similarity of rubredoxin-fused AlkB genes from 110 distinct bacterial species. Color-coded by genus. Labels indicate unique species, and the names in boxes indicate species known to produce surfactants.
3.2. Fused rubredoxin does not affect reactivity on alkane substrates
AlkBs can oxidize a variety of alkanes from C3 to C30, although each individual AlkB that has been examined shows a much narrower substrate range. The first AlkB isolated (and still the most widely studied), the AlkB from P. putida GPo1, oxidizes alkanes from propane to dodecane with a marked preference for nonane and octane.26, 50–53 Previous work has found that the presence of a rubredoxin-fused AlkB enables organisms to consume as their sole carbon source longer-chain alkanes than can be consumed by organisms bearing AlkBs that lack the rubredoxin fusion.30 This work suggests that the rubredoxin-fused AlkB plays a role in long-chain alkane degradation. This role was investigated by examining the activity of Dc AlkB and comparing it to the activity of the rubredoxin deletion mutantDc nulRd AlkB. The activity was studied in cell lysate to which NADH (the electron source) and purified alkane substates were added. (Ferrous iron and dithionite were also added to ensure that the protein was fully metallated.37) In experiments with the rubredoxin deletion mutant, the electron transfer proteins AlkT and AlkG from P. putida Gpo1 were also added. Prior work has shown that rubredoxins from one AlkB-expressing organism can complement AlkBs from other organisms.17, 54–56 Control experiments showed no transformation of any alkane in the absence of cell lysate from cells expressing alkB genes.
Contrary to the initial hypothesis, the presence of the fused rubredoxin did not yield increased overall activity on longer substrates (Figure 2). This result indicates that the previously reported in vivo results are likely influenced by factors other than the biochemical constraints of the AlkB protein.29
Figure 2.
The relative substrate preference of Dc AlkB compared to that of Dc nulRd AlkB. Error bars +/− 1 se for 4 replicates.
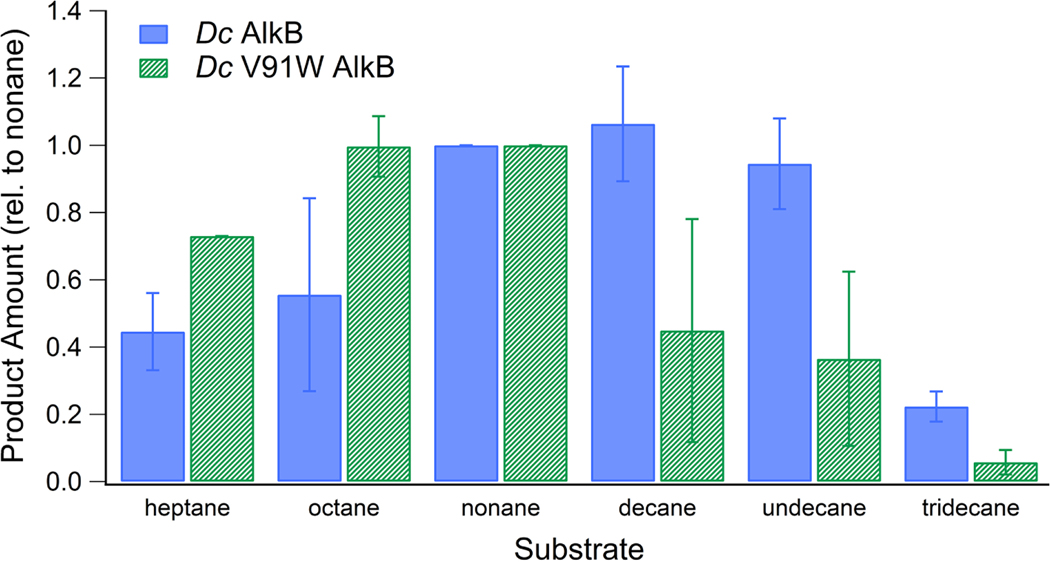
3.3. Point mutation V91W in Dc modestly affects substrate preference
Previous studies have also identified the amino acid residue at location 55 in AlkB from P. putida as a determinant of substrate preference.57 This position is occupied by a bulky tryptophan in AlkBs that oxidize smaller substrates but is replaced by a smaller residue in systems that oxidize larger substrates (Fig. 3).56, 57 A sequence alignment of AlkB from P. putida and D. cinnamea reveals that this residue lies at position 91 in the hydroxylase portion of Dc AlkB (Fig 3). Because most rubredoxin-fused AlkBs, including Dc AlkB, contain a smaller residue such as valine at this position, its relevance for substrate preference in rubredoxin-fused AlkBs was tested. The residue V91 in Dc AlkB was mutated to a tryptophan in Dc V91W AlkB, and the activity of the protein was examined.
Figure 3.
Alignment of region linked to substrate specificity for the AlkB from D. cinnamea and P. putida GPo1, along with two other AlkBs: one (the AlkB from A. borkumensis56) that oxidizes shorter chain alkanes and one (the AlkB from P. aeruginosa22) that oxidizes longer chain alkanes.The red block (corresponding to residue 91 in D. cinnamea) marks the position of interest. Created with Geneious version 2021.0 (created by Biomatters. Available from https://www.geneious.com
The results (Figure 4) demonstrate that in the case of the rubredoxin-fused AlkB, Dc AlkB, the point mutation V91W had a modest shift in substrate preference. The mutant, which contained a bulky tryptophan residue at the location 91, retained activity over all surveyed substrates on which the wildtype protein was active, but with slightly more activity on shorter substrates and slightly less activity on longer substrates, consistent with the hypothesis that this residue points into the substrate channel at a position that can impact substrate positioning.
Figure 4.
The relative substrate preference of Dc AlkB compared to that of Dc V91W AlkB. Error bars +/− 1 se for 3 replicates.
3.4. Point mutation V91W in Dc AlkB changes rubredoxin-fused AlkB reactivity on alkenes
The point mutation V91W also changed the activity of the rubredoxin-fused AlkB on alkenes. AlkB yields two products when catalyzing the oxidation of alkenes: a terminal epoxide and a terminal aldehyde.12 Extensive work has determined that these products stem from two unique reaction pathways (Scheme 1).17, 58
Scheme 1.
Proposed mechanism for the AlkB-catalyzed oxidation of alkenes to form A. epoxide and B. aldehyde products.
In the epoxidation pathway, one electron from the terminal carbon of the alkene attacks the oxygen in the iron-oxo moiety, reducing one of the irons in the di-iron site. Published work showing the loss of olefin configuration during epoxidation led May and coworkers to propose a step-wise mechanism for AlkB-catalyzed epoxidation.59 The attack at the terminal carbon generates a radical at the C2 position, which is then quenched by the oxygen after rotation of the incipient bond to facilitate ring closure. May and coworkers demonstrated that the AlkB-catalyzed epoxidation of a cis-alkene (deuterated at the terminal position to create the cis-configuration) produced 70% trans epoxide while the same reaction done with the trans-alkene produced 70% cis epoxide. This result is consistent with preferential attack of the metal oxo from the re face and then ring closure from the si face of C-2 to avoid steric clashes with the methylene chain binding region as depicted in the mechanism above.12 The aldehyde forms by the creation of a C-O bond on the terminal carbon as well; however this occurs when both electrons from the alkene form a bond with the oxygen in the iron-oxo intermediate, reducing the iron and creating a carbocation on C2. Hydrogen migration to C2, previously demonstrated with deuterium-labeling studies12, displaces the carbocation to C1 which can then be quenched by collapse of the iron-oxygen bond to make an anti-Markovnikov aldehyde.
Under our experimental conditions, in which an alcohol dehydrogenase is present in the cell lysates, the aldehyde was immediately and completely reduced to the primary alcohol, which is observed by GC-MS, consistent with prior work.8,41
The Dc AlkB-catalyzed oxidation of octadiene yieldsa ratio of aldehyde to epoxide of approximately 1:2. When Dc V91W AlkB catalyzes the oxidation of octadiene, however, the ratio of the aldehyde to epoxide is approximately 1:1. This represents a major shift in the distribution of products in favor of the production of the aldehyde. The shift towards the aldehyde product is also observed when Dc V91W AlkB oxidizes the longer substrate decadiene (Figure 5, with more detail provided in SI). To our knowledge, this is the first report of any AlkB homologue that produces a comparable amount of aldehyde relative to epoxide when oxidizing an alkene and provides an enzymatic route to the production of anti-Markovnikov products.
Figure 5.
Product distribution of Dc AlkB and Dc V91W AlkB with diene substrates. Alcohol concentration plotted as a proxy for aldehyde produced. Error bars +/− 1 standard deviation for three experimental replicates.
These results lead us to propose a model in which the tryptophan mutation at position 91 orients the alkene substrate in the active site. In the wild-type Dc AlkB, the absence of this amino acid gate allows for more lateral movement of the substrate, which enables the substrate to move closer to the diiron active site and facilitates the ring closure necessary for epoxide formation. In contrast, a tryptophan side chain at this position prevents the lateral movement of the substrate intermediate towards the active site and thereby inhibits the formation of the epoxide. Favorable interactions between the aromatic side chain and the substrate π bond may also play a role in limiting substrate motion.60 Because the aldehyde formation mechanism does not require the active site to bridge both carbons, it is preferred when the substrate has less mobility the active site (as illustrated in Figure 6). This model reflects the knowledge that subtle structural changes that impact substrate position relative to high valent metal oxo intermediates can alter reactivity as has been seen with Ole-T61 and the OG-dependent Fe enzymes62 and is also informed by an intriguing early report that when AlkB oxidizes a diene twice to produce a diepoxide, the stereochemistry of the epoxide formed first impacts the stereochemical outcome of the second epoxide63. More structural information is required to build a more precise model, information we hope in later work to provide.
Figure 6.
Illustration of how the bulky aromatic amino acid at position 91 could influence the reaction trajectory by making the diene substrate less mobile in the active site, which as illustrated in scheme 1, would favor the formation of the aldehyde.
4. CONCLUSION
Rubredoxin-fused AlkBs are far more ubiquitous in nature than previously appreciated. This study reveals the presence of rubredoxin-fused AlkB-encoding genes in at least 110 distinct species across 28 genera from both gram-positive and gram-negative bacteria. It is likely that these genes have been conserved because their encoded proteins provide useful and distinct reactivity. One such protein, Dc AlkB was expressed as the full-length protein, the rubredoxin-deletion mutant, and with a point mutation V91W. The rubredoxin-deletion mutant did not shift the substrate range towards shorter substrates for the range of alkanes tested. The point mutation V91W had a modest effect on alkane substrate preference and changed the protein’s reactivity on alkene substrates, leading to the increased generation of an anti-Markovnikov aldehyde product. Because they do not require an exogenous rubredoxin, these rubredoxin-fused AlkBs are good candidates for biocatalysis. Further studies will investigate the evolutionary selection for AlkBs, including the extent to which horizontal gene transfer may have spurred the dispersion of rubredoxin-fused AlkBs throughout so many bacterial species and the potential role of these proteins in biosynthetic processes in vivo.
Supplementary Material
Highlights.
Alkane monooxygenases with fused rubredoxin widely distributed in nature
Rubredoxin deletion mutant has similar substrate range to wild type
Single point mutation in presumed substrate domain impacts reactivity and leads to an enzyme with substantial anti-Markovnikov activity on alkenes
ACKNOWLEDGEMENTS
RNA acknowledges NIH (R01 GM130989) and an award from the Presidential Research Fund at Barnard College. Additional support has come from the Office of the Provost at Barnard. Shoshana Williams and Juliet Lee were the grateful recipients of additional funding from the Arnold and Mabel Beckman Foundation. Allison Forsberg received additional funding through the Evan B. Segal Grant Fund. We thank Lucy Zorzano for her contributions to the experiments described in this paper.
Footnotes
Author statement
SCW was involved in conceptualization, data collection and analysis, and manuscript preparation
APW was involved in data collection and analysis, and manuscript preparation
JL was involved in discussion and manuscript preparation
CV was involved in conceptualization and manuscript preparation
AJL was involved in all aspects of the bioinformatics work and manuscript preparation
RNA was involved in conceptualization, data collection and analysis, and manuscript preparation
conflicts of interest
We have no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Austin RN; Groves JT, Alkane-oxidizing metalloenzymes in the carbon cycle. Metallomics 2011,3, 775–787. [DOI] [PubMed] [Google Scholar]
- 2.Koch DJ; Chen MM; van Beilen JB; Arnold F, In vivo Evolution of Butane Oxidation by Terminal Alkane Hydroxylases AlkB and CYP153A6. Appl. Environ. Microbiol 2009,75, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie M; Alonso H; Roujeinikova A, An Improved Procedure for the Purification of Catalytically Active Alkane Hydroxylase from Pseudonomas putida. Appl. Biochem. Biotechnol. 2011. [DOI] [PubMed] [Google Scholar]
- 4.Ramua R; Chang C-W; Chou H-H; Wua L-L; Chiang C-H; Yu SS-F, Regio-selective hydroxylation of gem-difluorinated octanes by alkane hydroxylase (AlkB). Tetrahedron Letters 2011,52, 2950–2953. [Google Scholar]
- 5.Grant C; Deszcz D; Wei Y-C; Martinez-Torres RJ; Morris P; Folliard T; Sreenivasan R; Ward J; Dalby P; Woodley JM; Baganz F, Identification and use of an alkane transporter plug-in for applications in biocatalysis and whole-cell biosensing of alkanes. Scientific Reports 2014,4, srep05844(1–9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Julsing MK; Schrewe M; Cornelissen S; Hermann I; Schmid A; Bühler B, Outer Membrane Protein AlkL Boosts Biocatalytic Oxyfunctionalization of Hydrophobic Substrates in E coli. Applied and Environmental Microbiology 2012,78 (16), 5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrewe M; Ladkau N; Bühler B; Schmid A, Direct Terminal Alkylamino-Functionalization via Multistep Biocatalysis in One Recombinant Whole-Cell Catalyst. Advanced Synthesis & Catalysis 2013,355 (9), 1693–1697. [Google Scholar]
- 8.van Nuland YM; de Vogel FA; Eggink G; Weusthuis RA, Expansion of the ω-oxidation system AlkBGTL of Pseudomonas putida GPo1 with AlkJ and AlkH results in exclusive mono-esterified dicarboxylic acid production in E. coli. Microb Biotechnol 2017,10 (3), 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrewe M; Julsing MK; Lange K; Czarnotta E; Schmid A; Bühler B, Reaction and catalyst engineering to exploit kinetically controlled whole-cell multistep biocatalysis for terminal FAME oxyfunctionalization. Biotechnology and Bioengineering 2014,111 (9), 1820–1830. [DOI] [PubMed] [Google Scholar]
- 10.Huybregtse R; van der Linden AC, The oxidation of Olefins by a Pseudomonas Reactions Involving the Double Bond. Antonie Van Leeuwenhoek 1964,30, 185–196. [DOI] [PubMed] [Google Scholar]
- 11.May SW; Abbott BJ, Mechanistic Studies on Non-Heme Iron Monooxygenase Systems of Pseudomonas oleovorans. Biochem. Biophys. Res. Commun. 1972,48, 1230–1234. [DOI] [PubMed] [Google Scholar]
- 12.Katopodis AG; Wimalasena K; Lee J; May SW, Mechanistic Studies on Non-Heme Iron monooxygenase Catalysis: Epoxidation, Aldehyde Formation, and Demethylation by the omega-Hydroxylation system of Pseudomonas oleovorans. J. Am. Chem. Soc. 1984,106, 7928–7935. [Google Scholar]
- 13.Shanklin J; Achim C; Schmidt H; Fox BG; Münck E, Mossbauer studies of alkane omega-hydroxylase: Evidence for a diiron cluster in an integral-membrane enzyme. Proc. Natl. Acad. Sci. 1997,94, 2981–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanklin J; Whittle E, Evidence linking the Pseudomonas oleovorans alkane omega-hydroxylase, an integral membrane diiron enzyme, and the fatty acid desaturase family. FEBS Lett. 2003,545, 188–192. [DOI] [PubMed] [Google Scholar]
- 15.Shanklin J; Whittle E; Fox BG, Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoryl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 1994,33, 12787–12794. [DOI] [PubMed] [Google Scholar]
- 16.Alonso H; Kleifeld O; Yeheskel A; Ong PC; Liu YC; Stok JE; De Voss JJ; Roujeinikova A, Structural and mechanistic insight into alkane hydroxylation by Pseudomonas putida AlkB. Biochemical Journal 2014. [DOI] [PubMed] [Google Scholar]
- 17.Smits THH; Balada SB; Witholt B; van Beilen JB, Functional analysis of alkane hydroxylases from gram-negative and gram-positive bacteria. Journal of Bacteriology 2002,184, 1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner RR, Hormonal modulation of delta6 and delta5 desaturases: case of diabetes. Prostaglandins Leukot Essent Fatty Acids 2003,68 (2), 151–62. [DOI] [PubMed] [Google Scholar]
- 19.Aguilar PS; de Mendoza D, Control of fatty acid desaturation: a mechanism conserved from bacteria to humans. Molecular Microbiology 2006,62, 1507–1514. [DOI] [PubMed] [Google Scholar]
- 20.Williard DE; Nwankwo JO; Kaduce TL; Harmon SD; Irons M; Moser H; Raymond GV; Spector A, Identification of a fatty acid delta-6-desaturase deficiency in human skin fibroflasts. Journal of Lipid Research 2001,42, 501–508. [PubMed] [Google Scholar]
- 21.Brookes KJ; Chen W; Xu X; Taylor E; Asherson P, Association of fatty acid desaturase genes with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2006,60, 1053–1061. [DOI] [PubMed] [Google Scholar]
- 22.Belhaj A; Desnoues N; Elmerich C, Alkane biodegradation in Pseudomonas aeruginosa strains isolated from a polluted zone: identification of alkB and alkB-related genes. Research in Microbiology 2002,153 (6), 339–344. [DOI] [PubMed] [Google Scholar]
- 23.van Ravenswaay Claasen JC; van Der Linden AC, Substrate specificity of the paraffin hydroxylase of Pseudomonas aeruginosa. Antonie van Leeuwenhoek 1971,37, 339–352. [DOI] [PubMed] [Google Scholar]
- 24.Peterson JA; Kusunose M; Kusunose E; Coon MJ, Function of rubredoxin as the electron carrier in omega hydroxylation. J. Biol. Chem. 1967,242, 4334–4340. [PubMed] [Google Scholar]
- 25.Peterson JA; Coon MJ, Enzymatic ω-Oxidation: III Purification and Properties of Rubredoxin, a Component of the ω-hydroxylation system of Pseudomonas oleovorans. Journal of Biological Chemistry 1968,243, 329–334. [PubMed] [Google Scholar]
- 26.Tsai Y-F; Luo W-I; Chang J-L; Chang C-W; Chuang H-C; Ramua R; Wei G-T; Zen J-M; Yu SS-F, Electrochemical Hydroxylation of C3-C12 n-Alkanes by Recombinant Alkane Hydroxylase (AlkB) and Rubredoxin-2 (AlkG) from Pseudomonas putida GPo1. Scientific Reports 2017,7, 8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Beilen JB; Neuenschwander M; Smits THM; Roth C; Balada SB; Witholt B, Rubredoxins Involved in Alkane Oxidation. Journal of Bacteriology 2002,184 (6), 1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamamura N; Yeager CM; Arp DJ, Two Distinct Monooxygenases for Alkane Oxidation in<em>Nocardioides</em> sp. Strain CF8. Applied and Environmental Microbiology 2001,67 (11), 4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nie Y; Liang J; Fang H; Tang Y-Q; Wu X-L, Two novel alkane hydroxylase-rubredoxin fusion genes isolated from a Dietzia bacterium and the function of fused rubredoxin domains in long-chain n alkane degradation. Appl. Environ. Microbiol 2011,77, 7279–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie Y; Chi C-Q; Fang H; Liang J-L; Lu S-L; Lai G-L; Tang Y-Q; Wu X-L, Diverse alkane hydroxylase genes in microorganisms and environments. Scientific Reports 2014,4 (1), 4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bihari Z; Szvetnik A; Szabó Z; Blastyák A; Zombori Z; Balázs M; Kiss I, Functional analysis of long-chain n-alkane degradation by Dietzia spp. FEMS Microbiology Letters 2011,316 (2), 100–107. [DOI] [PubMed] [Google Scholar]
- 32.Glöckner FO; Yilmaz P; Quast C; Gerken J; Beccati A; Ciuprina A; Bruns G; Yarza P; Peplies J; Westram R; Ludwig W, 25 years of serving the community with ribosomal RNA gene reference databases and tools. J Biotechnol 2017,261, 169–176. [DOI] [PubMed] [Google Scholar]
- 33.Stamatakis A, RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. . Bioinformatics 2014,30, 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huerta-Cepas J. S. F; Bork P, ETE 3: Reconstruction, Analysis, and Visualization of Phylogenomic Data. . Mol. Biol. Evol. 2016,33, 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sievers F. W. A; Dineen D; Gibson TJ; Karplus K; Li W; Lopez R; McWilliam H; Remmert M; Söding J; Thompson JD; Higgins DG, Fast, Scalable Generation of High-Quality Protein Multiple Sequence Alignments Using Clustal Omega. Mol. Syst. Biol. 2011,7, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C; Wen A; Shen B; Lu J; Huang Y; Chang Y, FastCloning: a highly simplified, purification-free, sequence- and ligation-independent PCR cloning method. BMC Biotechnology 2011,11 (1), 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katopodis AG, Mechanistic studies on the epoxidation system of P. oleovorans. Ph.D. thesis, Georgia Institute of Technology 1982. [Google Scholar]
- 38.Austin RN; Born D; Lawton TJ; Hamilton GE, Protocols for purifying and characterizing integral membrane AlkB enzymes. In Hydrocarbon and Lipid Microbiology Protocols, , 2015. [Google Scholar]
- 39.Austin RN; Chang H-K; Zylstra G; Groves JT, The Non-Heme Diiron Alkane Monooxygenase of Pseudomonas oleovorans (AlkB) Hydroxylates via a Substrate Radical Intermediate J. Am. Chem. Soc. 2000,122, 11747–8. [Google Scholar]
- 40.Oso S; Walters M; Schlechter RO; Remus-Emsermann MNP, Utilisation of hydrocarbons and production of surfactants by bacteria isolated from plant leaf surfaces. FEMS Microbiology Letters 2019,366 (6). [DOI] [PubMed] [Google Scholar]
- 41.Santos DK; Rufino RD; Luna JM; Santos VA; Sarubbo LA, Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int J Mol Sci 2016,17 (3), 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colin VL; Castro MF; Amoroso MJ; Villegas LB, Production of bioemulsifiers by Amycolatopsis tucumanensis DSM 45259 and their potential application in remediation technologies for soils contaminated with hexavalent chromium. J Hazard Mater 2013,261, 577–83. [DOI] [PubMed] [Google Scholar]
- 43.Wang W; Cai B; Shao Z, Oil degradation and biosurfactant production by the deep sea bacterium Dietzia maris As-13–3. Frontiers in Microbiology 2014,5, 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saeki H; Sasaki M; Komatsu K; Miura A; Matsuda H, Oil spill remediation by using the remediation agent JE1058BS that contains a biosurfactant produced by Gordonia sp. strain JE-1058. Bioresour Technol 2009,100 (2), 572–7. [DOI] [PubMed] [Google Scholar]
- 45.Ettoumi B; Chouchane H; Guesmi A; Mahjoubi M; Brusetti L; Neifar M; Borin S; Daffonchio D; Cherif A, Diversity, ecological distribution and biotechnological potential of Actinobacteria inhabiting seamounts and non-seamounts in the Tyrrhenian Sea. Microbiological Research 2016,186–187, 71–80. [DOI] [PubMed] [Google Scholar]
- 46.Chooklin CS; Phertmean S; Cheirsilp B; Maneerat S; Saimmai A, Utilization of Palm Oil Mill Effluent as a Novel and Promising Substrate for Biosurfactant Production by Nevskia Ramosa NA3. Songklanakarin Journal of Science and Technology 2013,35, 167–176. [Google Scholar]
- 47.Le Roes-Hill M; Durrell KA; Kügler JH, Biosurfactants from Actinobacteria: State of the Art and Future Perspectives. In Microibial Biosurfactants and their Environmnetal and Industrial Applications, 2019; pp 174–208. [Google Scholar]
- 48.Yassin AF; Humpfer H; Schaal KP, Dietzia cinnamea sp. nov., a novel species isoalted frm a perianal swab of a patient with a bond marrow transplant. Int. J. Syst. Evol. Microbiol. 2006,56, 641–645. [DOI] [PubMed] [Google Scholar]
- 49.Procopio L; de Cassia Pereira e Silva M; Dirk van Elsad J; Seldin L, Transcriptional profiling of genes involved in n-hexadecane compounds assimilation in the hydrocarbon degrading Dietzia cinnamea P4 strain. Brazilian Journal of Microbiology 2013,44, 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKenna EJ; Coon MJ, Enzymatic ω-Oxidation: IV Purification and Properties of the ω-Hydroxylase of Pseudomonas oleovorans. Journal of Biological Chemistry 1970,225, 3882–3890. [PubMed] [Google Scholar]
- 51.Lode ET; Coon MJ, Enzymatic omega-oxidation V. Forms of Pseudomonas oleovorans rubredoxin containing one or two iron atoms: structure and function in omega hydroxylation. Journal of Biological Chemistry 1971,246, 791–802. [PubMed] [Google Scholar]
- 52.van Beilen JB; Kingma J; Witholt B, Substrate specificity of the alkane hydroxylase system of Pseudomonas oleovorans GPo1. Enzyme Microb. Technol. 1994,16, 904–911. [Google Scholar]
- 53.Johnson EL; Hyman MR, Propane and n-butane oxidation by psuedomonas putida GPo1. Appl. Environ. Microbiol. 2006,72 (1), 950–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smits THM; Seeger MA; Witholt B; van Beilen JB, New Alkane-Responsive Expression Vectors for Escherichia coli and Pseudonomas. Plasmid 2001,46, 16–24. [DOI] [PubMed] [Google Scholar]
- 55.Whyte LG; Smits TH; Labbe D; Witholt B; Greer CW; van Beilen JB, Gene cloning and characterization of multiple alkane hydroxylase systems in Rhodococcus strains Q15 and NRRL B-16531. Appl. Environ. Microbiol. 2002,68, 5933–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naing S-H; Parvez S; Pender-Cudlip M; Groves JT; Austin RN, Substrate specificity and reaction mechanism of purified alkane hydroxylase (AlkB) from the hydrocarbonoclastus bacterium Alcanivorax borkumensis. Journal of Inorganic Biochemistry 2013,121, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Beilen JB; Smits TH; Roos F; Brunner T; Balada SB; Rothlisberger M; Witholt B, Identification of an Amino Acid Position that Determines the Substrate Range of Integral Membrane Alkane Hydroxylases. J. Bacteriol. 2005,187, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammer SC; Kubik G; Watkins E; Huang S; Hannah M; Arnold FH, Anti-Markovnikov alkene oxidation by metal-oxo-mediated enzyme catalysis. Science 2017,358, 215–218. [DOI] [PubMed] [Google Scholar]
- 59.May SW; Gordon SL; Steltenkamp MS, Enzymic epoxidation of trans,trans-1,8-dideuterio-1,7-octadiene. Analysis using partially relaxed proton Fourier transform NMR. Journal of the American Chemical Society 1977,99 (7), 2017–2024. [DOI] [PubMed] [Google Scholar]
- 60.Corne V; Sarotti AM; Ramirez de Arellano C; Spanevello RA; Suárez AG, Experimental and theoretical insights in the alkene-arene intramolecular π-stacking interaction. Beilstein J Org Chem 2016,12, 1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsieh CH; Huang X; Amaya JA; Rutland CD; Keys CL; Groves JT; Austin RN; Makris TM, The Enigmatic P450 Decarboxylase OleT Is Capable of, but Evolved To Frustrate, Oxygen Rebound Chemistry. Biochemistry 2017,56 (26), 3347–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinie RJ L. J; Chang W.-c.; Green MT; Krebs C; Bollinger JM Jr.; Silakov A, Experimental Correlation of Substrate Position with Reaction Outcome in the Aliphatic Halogenase, SyrB2. J. Am. Chem. Soc. 2015,137, 6912–6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.May SW; Steltenkamp MS; Schwartz RD; McCoy CJ, Stereoselective Formation of Diepoxides by an Enzyme System of Pseudomonas oleovorans. J. Am. Chem. Soc. 1976,98, 7856–7858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.