Abstract
The Wnt/β-catenin pathway is upregulated in uterine leiomyomas, the most common benign tumors in the female reproductive tract. Simvastatin is an antihyperlipidemic drug, and previous in vitro and in vivo reports showed that it may have therapeutic effects in treating leiomyomas. The objective of this study was to examine the effects of simvastatin on the Wnt/β-catenin signaling pathway in leiomyoma. We treated primary and immortalized human leiomyoma cells with simvastatin and examined its effects using quantitative real-time polymerase chain reaction, Western blotting, and immunocytochemistry. We also examined the effects using human leiomyoma tissues from an ongoing randomized controlled trial in which women with symptomatic leiomyoma received simvastatin (40 mg) or placebo for 3 months prior to their surgery. The results of this study revealed that simvastatin significantly reduced the expression of Wnt4 and its co-receptor LRP5. After simvastatin treatment, levels of total β-catenin and its active form, nonphosphorylated β-catenin, were reduced in both cell types. Additionally, simvastatin reduced the expression of Wnt4 and total β-catenin, as well as nonphosphorylated β-catenin protein expression in response to estrogen and progesterone. Simvastatin also inhibited the expression of c-Myc, a downstream target of the Wnt/β-catenin pathway. The effect of simvastatin on nonphosphorylated-β-catenin, the key regulator of the Wnt/β-catenin pathway, was recapitulated in human leiomyoma tissue. These results suggest that simvastatin may have a beneficial effect on uterine leiomyoma through suppressing the overactive Wnt/β-catenin pathway.
Keywords: uterine leiomyoma, Wnt/β-catenin pathway, simvastatin, therapeutics
The Wnt/β-catenin pathway is one of the most evolutionarily conserved signaling pathways, orchestrating several biological functions, including cell proliferation, apoptosis, and stem cell maintenance in adults (1). On the cell surface, the Wnt ligand binds to the Frizzled (FZD) receptor, promoting the release of β-catenin, allowing it to accumulate and translocate to the nucleus where it binds to the transcription activators and activates the expression of related genes that belong to the T-cell factor/lymphoid enhancer factor (TCF/LEF) family (1). In the absence of a Wnt ligand, β-catenin is targeted for degradation. The degradation complex is a multiprotein assembly that includes glycogen synthase kinase 3 (GSK-3), casein kinase 1 (CK1), the scaffolding protein Axin, and the adenomatous polyposis coli (APC) protein (2). Dysregulation of several components of this pathway has been implicated in several human pathologies, including uterine leiomyoma.
Uterine leiomyoma is the most common tumor of the female reproductive tract, affecting an estimated 70% of women (3). Symptoms include heavy menstrual bleeding, infertility, pelvic pain, dyspareunia, and urinary incontinence (4). Despite its heavy burden, the exact pathogenesis of these prevalent tumors is still unknown, and treatment options are limited. Several studies have described aberrations in several components of the Wnt/β-catenin pathways in uterine leiomyoma (5). Uterine leiomyoma cells have increased expression of Wnt5a (6), and constitutive activation of β-catenin in uterine stroma resulted in myometrial hyperplasia and the development in tumors resembling leiomyomas (7). Moreover, the inhibition of the Wnt/β-catenin pathway resulted in attenuation of leiomyoma growth (8).
Simvastatin is an FDA-approved drug used to treat hypercholesterolemia through inhibiting the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase enzyme. It has pleiotropic effects and has shown promising results in uterine leiomyoma. Simvastatin was shown to reduce the growth of leiomyoma in a patient-derived leiomyoma xenograft model and inhibit mitogen-activated protein kinase signaling, as well as to induce calcium-dependent and caspase-dependent apoptosis (9, 10). It was also shown to reduce the expression of extracellular matrix proteins as well as alter the mechanotransduction in human leiomyoma cells (11, 12), and it was shown to alter estrogen signaling in uterine leiomyoma (13). Large data set analysis has shown the benefit of simvastatin in several tumors, including uterine leiomyoma (14). Our ongoing, double-blind, phase 2 clinical trial is testing the effect of daily simvastatin treatment (40 mg) on patients who have at least one 3-cm leiomyoma. The purpose of this study is to investigate simvastatin’s effects on the Wnt/β-catenin pathway and its downstream signaling effects in uterine leiomyoma.
Methods
Primary Human Leiomyoma Cells Isolation and Maintenance
Primary leiomyoma cells were isolated from leiomyoma tissue from patients who underwent myomectomy or hysterectomy at the Johns Hopkins University Hospital. The institutional review board of Johns Hopkins University reviewed and approved the study (IRB00196175), and informed consents were attained prior to the surgery. Primary leiomyoma cells were isolated using a previously published protocol (11). In brief, tissues were washed several times with a Hanks’ Balanced Salt Solution (HBSS, Thermo Fisher Scientific, Waltham, MA) without calcium or magnesium and were manually cut into small pieces (1-2 mm3). They were incubated in the digestion buffer: sterile HBSS (without phenol, calcium, or magnesium) with collagenase (Worthington, Lakewood, NJ), deoxyribonuclease (DNase, Sigma-Aldrich, St. Louis, MO), antibiotic-antimycotic mixture (Thermo Fisher Scientific), and HEPES buffer solution (Thermo Fisher Scientific) and kept at 37 ºC on a shaker for 4 to 6 hours. The digest was filtered through a 40-mm filter and placed in a T75 flask where they were cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F12 (DMEM/F-12) (Thermo Fisher Scientific) medium supplemented with HEPES, L-glutamine, 10% fetal bovine serum, 0.1% gentamycin sulfate (50 mg/mL, Millipore) and 0.2% normocin (500 mg, InvivoGen). The media was changed after 24 hours. Depending on the cellular content of the digested tissue, the cells would be confluent in 2 to 7 days and could be used for experiments.
Immortalized Human Leiomyoma Cells
Immortalized human leiomyoma cells (HuLM) cells were immortalized and characterized by Dr. Darlene Dixon’s team (15), and we obtained them as a generous gift. They have been used in several publications (13, 16, 17). In brief, leiomyoma cells were obtained from a patient and were immortalized using a retroviral vector carrying human telomerase reverse transcriptase (15). In brief, hTERT, the catalytic subunit of telomerase gene, was cloned into the pLXIN retroviral vector, which contains the neo gene, conferring resistance to the antibiotic G-418 (15). The cloned retrovirus was transfected into the RetroPack PT67 Packaging Cell Line, where the vector was packaged into replication-incompetent retroviral particles. The packaged retrovirus was added to the media of the target cells at 50% to 60% confluency. After 24 hours, the medium was changed, and the infected cells were selected by treatment with G-418 antibiotic (15). Cells were confirmed to maintain the expression of molecular markers of human leiomyoma cells, including estrogen and progesterone receptors and smooth muscle actin (15). HuLM cells were maintained in DMEM/F-12 (Thermo Fisher Scientific) supplemented with HEPES, L-glutamine, 10% fetal bovine serum, and 0.2% normocin (500 mg, InvivoGen) in 5% CO2 at 37 °C.
Cell Culture and Treatment
Primary cells were used between passages 2 and 5, and HuLM cells were used between passages 9 and 15. For RNA extraction, we used a 6-well plate and seeded 60 000 cells per plate. For protein extraction, we used a 9-cm plate and seeded 600 000 cells per plate. For immunocytochemistry, we used 8-well chamber slides and seeded 10 000 cells per chamber. After 24 hours, the cells were 50% confluent and were treated with either DMSO 1μM (control) or simvastatin 1μM for 48 hours before RNA extraction, protein isolation, or fixation for immunocytochemistry.
Reagents
Simvastatin (Cayman Chemical, #10010344) stock solution (10mM) was prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich, #D2650) and stored at −20 °C until use. Estrogen was purchased from (Sigma-Aldrich, #E8875), and the stock solution (10mM) was prepared in ethanol. Progesterone was purchased from Sigma-Aldrich. Lithium chloride (Sigma-Aldrich, #203637) stock solution was prepared in water. Wnt agonist 1 was purchased from Selleck Chemicals (#S8178). The rest of the chemicals and reagents used in the experiments were purchased from Thermo Fisher Scientific and Sigma-Aldrich.
Real-Time Polymerase Chain Reaction
HuLM cells were plated onto a 6-well plate and maintained in DMEM/F-12 media until reaching 60% confluence. Cells were treated with 1µM simvastatin or DMSO (control) for 48 hours. Total RNA was extracted using an RNeasy Mini Kit (QIAGEN, #74104) and reverse-transcribed into cDNA using an iScript cDNA Synthesis Kit (Bio-Rad, #1708890) in a Bio-Rad Thermocycler according to the manufacturer’s instructions. The cDNA was then used for a quantitative real-time polymerase chain reaction (RT-qPCR) using a LightCycler 96 System (Roche Diagnostics, Mannheim, Germany) and FastStart Essential DNA Green Master (Roche Diagnostics). The human primer sequences were as follows: hRPLP0, sense, 5′-GCGACCTGGAAGTCCAACT-3′, and antisense, 5′-GGTCCTCCTTGGTGAACAC-3′; hWNT4, sense, 5′-ACCTGGAAGTCATGGACTCG-3′, and antisense, 5′-TCAGAGCATCCTGACCACTG-3′; hLRP5, sense, 5′- GCTGTAGATGTCGATGCTGAG-3′, and antisense, 5′- AGAACATCAAGCGAGCCAA-3′; hβ-Catenin, sense, 5′- AAAATGGCAGTGCGTTTAG-3′, and antisense, 5′- TTTGAAGGCAGTCTGTCGTA-3′. The relative mRNA expression was expressed as fold change and calculated using the 2-∆∆CT method.
Preparation of Subcellular Fractions
After cell treatment, the nuclear fractions were obtained using a NE-PER Nuclear and Cytoplasmic Extraction Reagents kit for cultured cells (Thermo Fisher Scientific, #78833) following the manufacturer’s instructions. The purity of the fraction in the input was confirmed by immunoblot analysis of markers β-actin (cytoplasm) and lamin B1 (nucleus).
Western Blotting
Cells were harvested after treatment with simvastatin (1µM) and estradiol (10nM), separately or in combination, at the specified time points. Cells were lysed in a lysis buffer (RIPA, radioimmunoprecipitation assay buffer, Sigma-Aldrich, #R0278) containing a protease and phosphatase inhibitor cocktail (Sigma-Aldrich, #PPC1010). An equal amount of protein lysates was resolved using 4% to 12% Bis-Tris protein gradient gels (Thermo Fisher Scientific) and transferred to a nitrocellulose membrane (Thermo Fisher Scientific). Membranes were blocked using 5% nonfat milk or bovine serum albumin (BSA) in Tris-buffered saline with 0.1% Tween-20 (TBST, Thermo Fisher Scientific) for 1 hour at room temperature and incubated with anti-WNT4 (Thermo Scientific, #9H2L10, AB_2608445), anti-total β-catenin (Cell Signaling, #8480S, AB_11127855), anti-nonphosphorylated- β-catenin (Cell Signaling, #4270S, AB_1903918), anti-β-actin (Sigma, #A3854, AB_262011), or anti-lamin B1 (Invitrogen, #PA5-19468, AB_10985414) antibodies diluted in 5% BSA (1:1000) at 4 °C overnight on a rocker. Membranes were washed with TBST, co-incubated with appropriate horseradish peroxidase–conjugated secondary antibodies (GE Healthcare) for 1 hour at room temperature (1:10 000) and visualized using an Azure Imager c300 (Azure Biosystems, Dublin, CA). Band signals were quantified using the NIH ImageJ software.
Immunocytochemistry
After 48 hours of treatment, cells were fixed in 4% formaldehyde, and incubated in a blocking solution (1× PBS, 5% normal goat serum [Cell Signaling, #5425] and 0.3% Triton X-100 [Sigma-Aldrich]) for 1 hour at room temperature. The cells were then incubated overnight at 4 °C with primary antibodies against anti-WNT4 (Thermo Scientific, #9H2L10, AB_2608445), anti-total-β catenin (Cell Signaling, #8480S, AB_11127855), anti-nonphosphorylated-β-catenin (Cell Signaling, #4270S, AB_1903918), anti-c-Myc (Cell Signaling, #D84C12, AB_1903938), diluted in antibody dilution buffer (1× PBS/1% BSA/0.3% Triton X-100) in 1:100 ratio. This was followed by anti-rabbit Alexa 488 (Invitrogen, #A11034, AB_2576217) and anti-mouse Alexa 546 (Invitrogen, #A11030, AB_2534089) conjugated secondary antibody for 1 hour at room temperature in the dark. The slide was fixed with a ProLong Gold Antifade Mountant (Thermo Fisher Scientific, #P10144) overnight at room temperature. The samples were examined using a Leica SP8 Microsystems (Wetzlar, Germany) confocal microscope. The time of acquaintance was 1.9 microseconds per pixel, and each image had 4 megapixels; therefore, the total scanning time was 7.6 seconds. The time of acquaintance was analogous for each collection. All images were taken at 20× magnification.
Simvastatin Leiomyoma Clinical Trial
Human leiomyoma tissue samples were obtained from an ongoing double-blind, phase 2, randomized control trial (NCT03400826) to evaluate the effects of simvastatin in patients with uterine leiomyoma. The trial was reviewed and approved by the institutional review board of Johns Hopkins University (IRB00149869). During a 12-week period, patients with uterine leiomyomas were treated with simvastatin (40 mg daily) or a placebo (Starch 1500 encapsulated). The study recruited patients from 18 to 55 years of age, with a body mass index of less than 45, and who had at least one fibroid larger than 3 cm. Exclusion criteria included any patient with malignancy, fibroids, or who was pregnant. Patients who were taking estrogen, progesterone, or any hormonal contraceptives had to be off the medication for 1 to 3 months prior to enrollment. Patients underwent a hysterectomy/myomectomy at the end of the treatment, and the leiomyoma samples were collected after surgery to evaluate the effects of simvastatin on leiomyoma tissue. The tissues were fixed with a 10% buffered formalin solution for 24 hours and kept in 70% ethanol at 4 °C until immunohistochemistry.
Immunohistochemical Staining
Immunohistochemical staining of human leiomyoma tissue was performed at the Oncology Tissue Services Core facility of the Johns Hopkins University School of Medicine. Immunolabeling for nonphosphorylated β-catenin was performed on formalin-fixed, paraffin-embedded sections on a Ventana Discovery Ultra autostainer (Roche Diagnostics). Following dewaxing and rehydration on board, epitope retrieval was performed using Ventana Ultra CC1 buffer (Roche Diagnostics, #6414575001) at 96 °C for 64 minutes. The primary antibody, anti-nonphosphorylated β-catenin (Cell Signaling, #8814), was applied at 36 °C for 20 minutes with a 1:500 dilution and was detected using an anti-rabbit HQ detection system (Roche Diagnostics, #7017936001 and 7017812001) and a Chromomap DAB IHC detection kit (Roche Diagnostics, #5266645001), counterstaining with Mayer’s hematoxylin, dehydration, and mounting. The optical density (OD) of nonphosphorylated β-catenin was analyzed via automated macroinstructions written in ImageJ software. The area of nonphosphorylated β-catenin coverage was determined by the ratio between DAB-labeled and total areas for each sample with the use of automated macro instruction on ImageJ.
Statistics
Statistical analyses were carried out using unpaired Student t test in GraphPad Prism 5.0. Differences at P < 0.05 (2-tailed) were considered statistically significant. All experiments were repeated 3 independent times, and the results were expressed as the mean ± standard error of the mean (SEM). The mRNA expression was calculated using the ΔΔCT method and is presented as fold changes. Protein expression was quantified by measuring the area and intensity of specific antibody staining using Image J software (National Institutes of Health). For immunohistochemistry, analysis was performed using automated macro instruction written in Image J and Image-Pro Plus software (Media Cybernetics, Rockville, MD).
Results
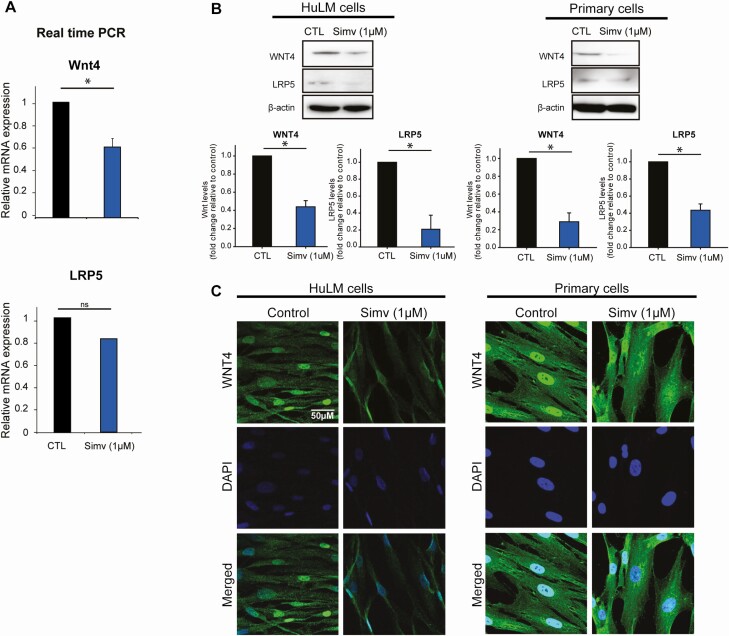
Simvastatin Reduced the Expression of Wnt4 and its Receptor in HuLM and Primary Cells
Our results showed that simvastatin treatment reduces Wnt4 mRNA levels by 80% in HuLM (Fig. 1A). Simvastatin also reduced Wnt4 protein levels by 56% in HuLM cells and by 78% in primary cells (Fig. 1B). These results were confirmed by immunocytochemistry (Fig. 1C). Wnt binds to the frizzled (FZD) receptor and its co-receptor, low-density lipoprotein receptor-related protein (LRP5). Simvastatin had no effect on FZD receptor protein levels, but it reduced the protein levels of LRP5 in HuLM cells by 79% and in primary leiomyoma cells by 57% (Fig. 1B).
Figure 1.
Simvastatin reduced WNT4 and LRP5 expression in human leiomyoma cells. Human leiomyoma cells (HuLM) and primary cells were treated with simvastatin (1uM) for 48 hours. A, Simvastatin significantly reduced the mRNA expression of Wnt4 but not LRP5 in HuLM cells. B-C, Simvastatin reduced the protein expression of WNT4 and LRP5 in both HuLM and primary cells as seen in Western blotting and immunohistochemistry. Abbreviation: LRP, low-density lipoprotein receptor-related protein.
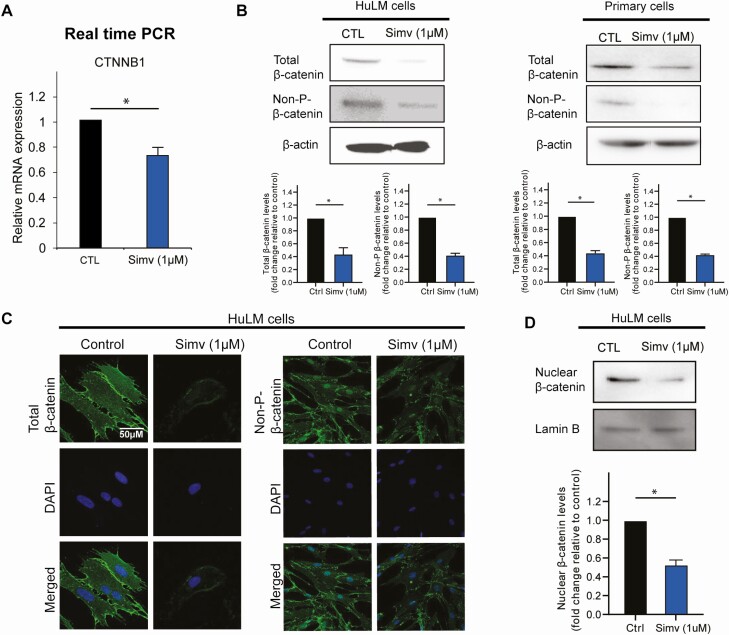
Simvastatin Reduced Total β-Catenin, Activated β-Catenin, and Nuclear β-Catenin Levels
In the presence of Wnt, β-catenin escapes degradation by the destruction complex. This escape allows β-catenin to translocate to the nucleus where it acts as a transcriptional coactivator. To examine the effect of simvastatin on β-catenin, we measured its mRNA levels in simvastatin-treated cells. We found that β-catenin mRNA levels decreased with simvastatin treatment (Fig. 2A). We then measured protein levels of total β-catenin and nonphosphorylated β-catenin, which is the active form of β-catenin. Simvastatin treatment reduced total β-catenin and nonphosphorylated β-catenin in HuLM cells, by 57% and 44% respectively, and in primary leiomyoma cells, by 55% and 42%, respectively, as measured by Western blot (Fig. 2B). Similarly, total β-catenin and nonphosphorylated β-catenin protein levels were lower as measured by immunocytochemistry in HuLM cells (Fig. 2C). Since β-catenin exerts its activity in the nucleus, we performed subcellular protein fractionation to isolate nuclear protein. Simvastatin treatment significantly reduced the nuclear β-catenin protein levels in HuLM cells by 47% (Fig. 2D).
Figure 2.
Simvastatin reduced β-catenin expression in human leiomyoma cells. Human leiomyoma cells (HuLM) and primary cells were treated with simvastatin (1uM) for 48 hours. A, Simvastatin significantly reduced the mRNA expression of CNTTB in HuLM cells. B-C, Simvastatin reduced the protein expression of total and nonphosphorylated β-catenin in both HuLM and primary cells as seen in Western blotting and immunohistochemistry. D, Nuclear protein isolation was performed to check nuclear β-catenin level, which is the site of action of the active form of β-catenin. Simvastatin reduced nuclear levels of β-catenin.
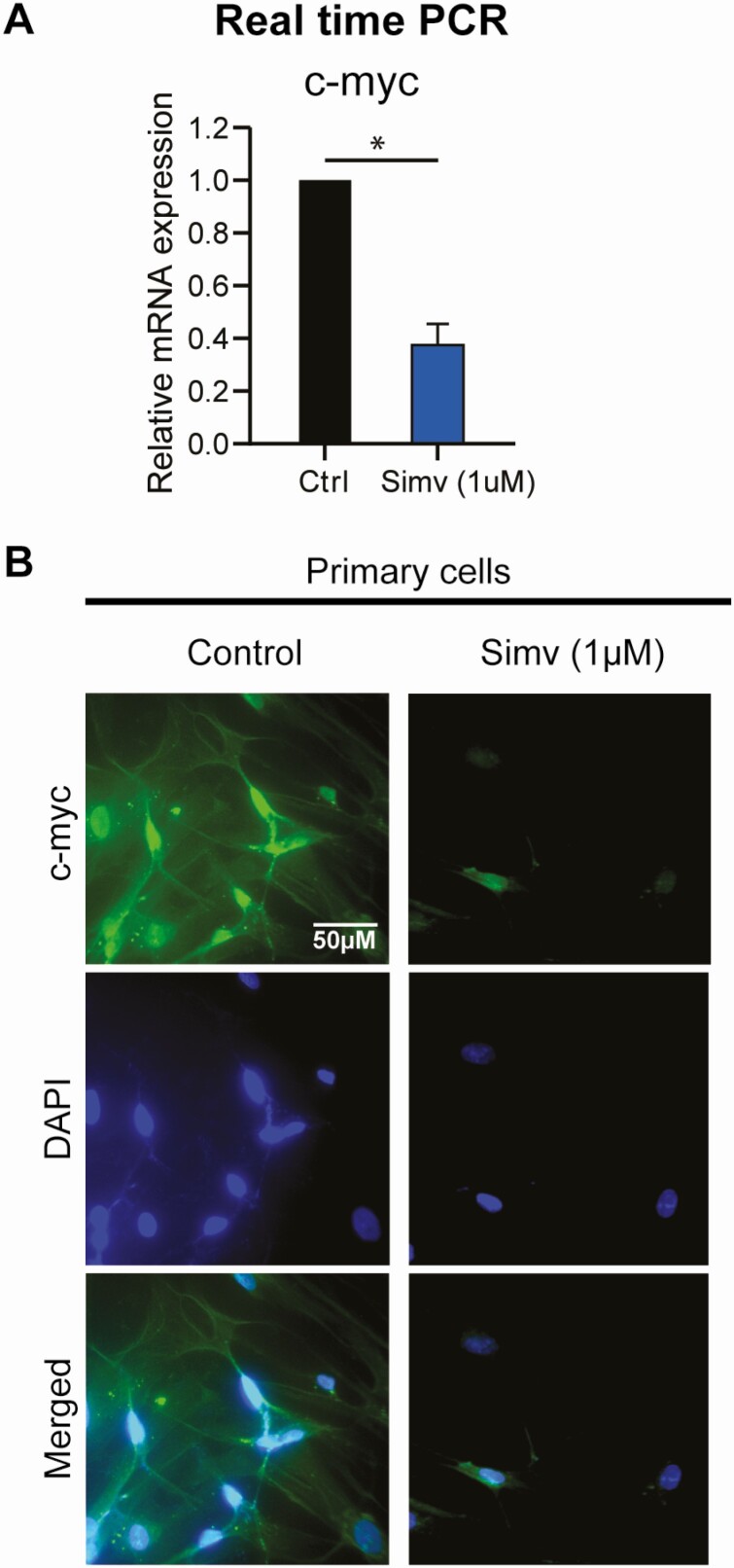
Simvastatin Reduced Downstream Wnt/β-Catenin Effectors
c-Myc and cyclin D1 are known downstream targets for the Wnt/β-catenin pathway (18, 19). Our group previously showed that simvastatin treatment reduced cyclin D1 mRNA and protein levels in human leiomyoma cells (11). Here, we assessed the effect of simvastatin on c-Myc levels. Simvastatin reduced mRNA levels of c-Myc by 71% and reduced the protein levels of c-Myc as measured by immunocytochemistry (Fig. 3).
Figure 3.
Simvastatin reduced c-Myc expression, a downstream signaling target of β-catenin, in human leiomyoma cells. Human leiomyoma cells (HuLM) and primary cells were treated with simvastatin (1uM) for 48 hours. A, Simvastatin significantly reduced the mRNA expression of c-Myc in HuLM cells. B, Simvastatin reduced the protein expression of c-Myc in primary cells as seen in immunohistochemistry. The band was not seen when Western blotting was attempted.
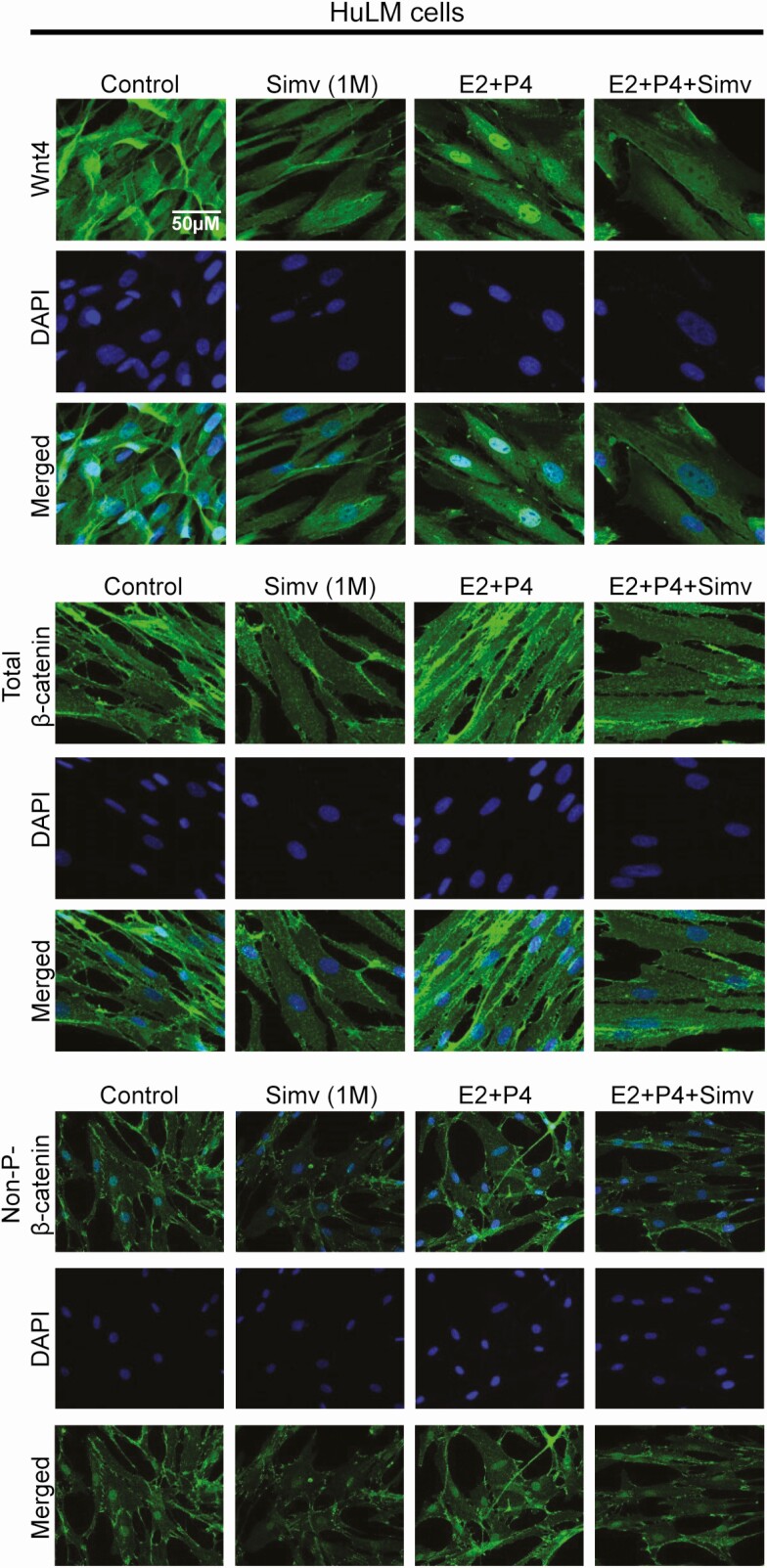
Simvastatin Reduced Estrogen and Progesterone-Induced Wnt Pathway Activation
Estrogen is known to modulate the Wnt/β-catenin pathway. Estrogen was shown to promote nuclear localization of active β-catenin in an estrogen receptor (ER)-independent manner (20). Moreover, treatment of leiomyoma stem cells with a β-catenin inhibitor inhibited estrogen- and progesterone-induced leiomyoma growth in vivo (21). In our study, as expected, estrogen and progesterone treatment resulted in a notable increase in the expression of WNT4, total β-catenin, and nonphosphorylated β-catenin (Fig. 4) in HuLM cells. This increase was reduced by simvastatin treatment.
Figure 4.
Simvastatin reduced estrogen- and progesterone-induced increase of Wnt/β-catenin activation in human leiomyoma cells. Immortalized human leiomyoma cells (HuLM) and primary cells were treated with simvastatin (1uM) for 48 hours. Estrogen (100nM) and progesterone (100nM) were added 24 hours prior to fixation. Estrogen and progesterone treatment increased the expression of Wnt4, total β-catenin, and nonphosphorylated β-catenin in HuLM and primary cells. Simvastatin reduced the protein-induced increase in expression of these proteins.
Simvastatin Reduced Wnt Agonist–Induced Wnt/β-Catenin Activation
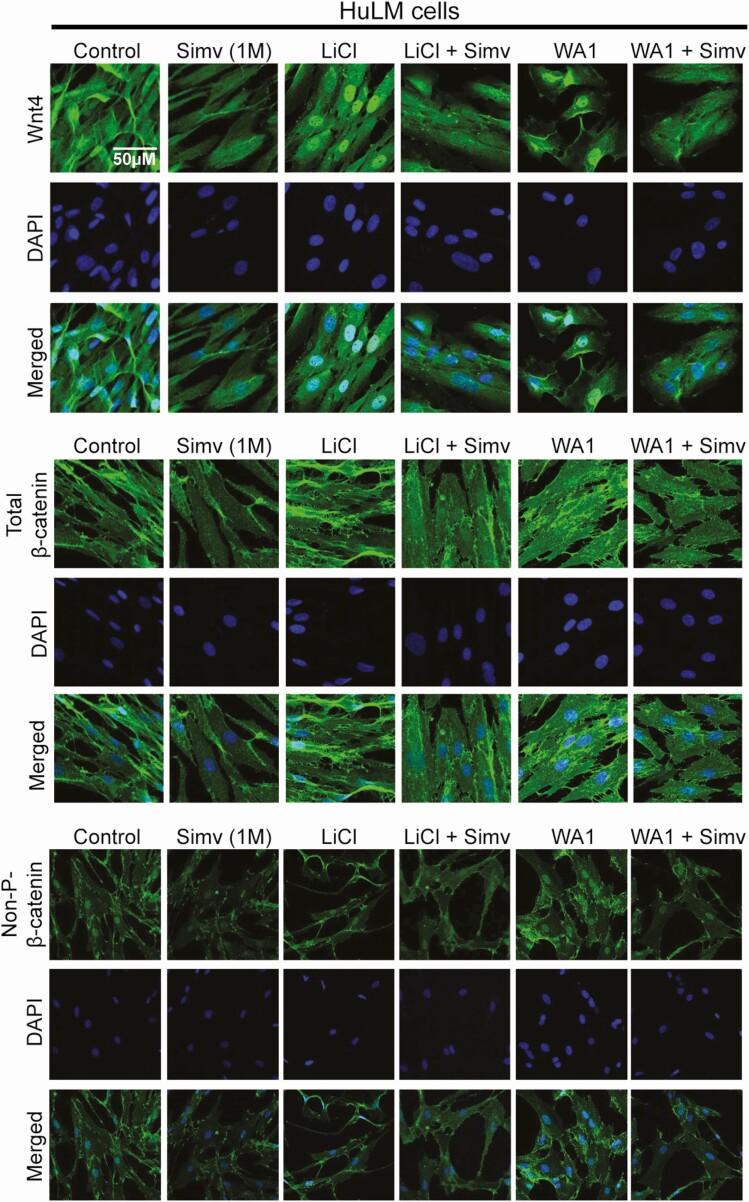
Lithium chloride (LiCl) and Wnt agonist 1 (WA1) are known agonists of Wnt/β-catenin pathway. LiCl activates this pathway by inhibiting glycogen synthase kinase-3β and consequently stabilizes the free cytosolic β-catenin (22). WA1 activates the pathway by inducing the nuclear accumulation of β-catenin thus driving its transcriptional activity (23). As expected, treatment with LiCl (20mM) and WA1 (1μM) resulted in a notable increase in the expression of Wnt4, total β-catenin, and nonphosphorylated β-catenin protein levels (Fig. 5) in HuLM cells. Simvastatin remarkably reduced the agonists’ induced increase of Wnt4, total β-catenin, and nonphosphorylated β-catenin protein levels.
Figure 5.
Simvastatin reduced Wnt agonist–induced Wnt/β-catenin activation in human leiomyoma cells. Immortalized human leiomyoma cells (HuLM) were treated with simvastatin (1uM) for 48 hours. Lithium chloride (LiCl, 20mM) or Wnt agonist (WA1, 1uM) were added 6 hours prior to fixation. As expected, LiCl and WA1 increased the expression of Wnt4, total β-catenin, and nonphosphorylated β-catenin in HuLM cells. Simvastatin reduced the protein-induced increase in expression of these proteins.
Simvastatin Reduced Nonphosphorylated β-Catenin in Human Tissue
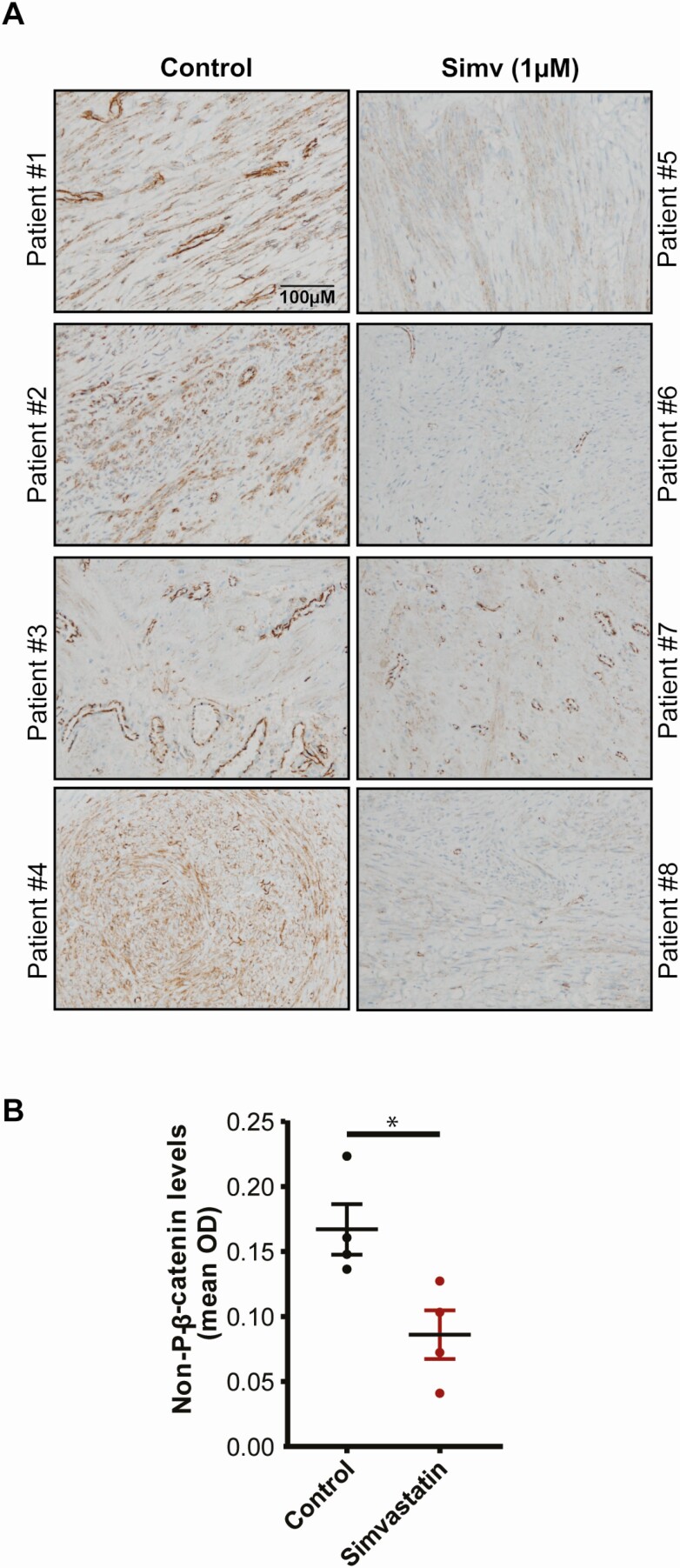
To check if the effect of simvastatin on nonphosphorylated β-catenin expression is recapitulated in humans, we used leiomyoma tissues from a randomized control trial where patients were randomized to receive placebo or simvastatin (40 mg) daily for 3 months before underdoing surgical removal of the tumors. Leiomyoma tissues were stained with nonphosphorylated β-catenin antibody. Immunohistochemical analysis (Fig. 6) showed that leiomyoma tissue from patients treated with simvastatin showed significantly lower nonphosphorylated β-catenin levels compared with placebo (P = 0.02).
Figure 6.
Simvastatin reduced nonphosphorylated β-catenin, the active form of β-catenin, in patients who received simvastatin for 3 months as part of a phase 2 randomized clinical trial. Human leiomyoma tissue samples were obtained from an ongoing, double-blind, phase 2 randomized control trial (NCT03400826). Patients with uterine leiomyomas were recruited for treatment with simvastatin (40 mg daily) or a placebo (starch) for a total of 12 weeks. Patients underwent a hysterectomy/myomectomy at the end of the treatment, and the leiomyoma samples were collected after surgery to evaluate the effects of simvastatin on leiomyoma tissue. The tissues were fixed with a 10% buffered formalin solution for 24 hours and kept in 70% ethanol at 4 °C until immunohistochemistry. Simvastatin significantly reduced the expression of nonphosphorylated β-catenin, the active form of β-catenin, in the patients who received the treatment.
Discussion
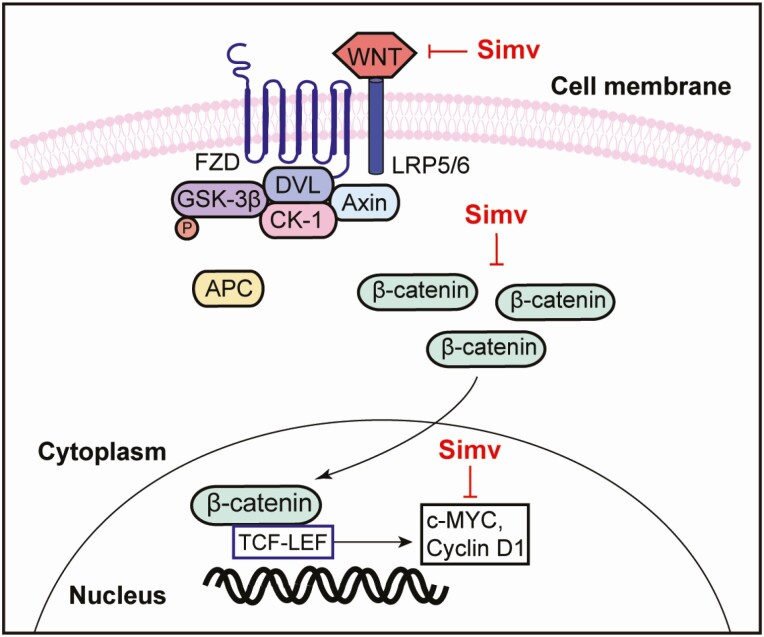
In this study, we demonstrated that simvastatin inhibits the Wnt/β-catenin pathway in human uterine leiomyoma cells and tissue (Fig. 7). Simvastatin reduced the expression of Wnt, its co-receptor LRP5, as well as total, nonphosphorylated, and nuclear β-catenin in HuLM and primary cells. Simvastatin also reduced estrogen- and progesterone-induced Wnt and β-catenin expression. Simvastatin treatment reduced the expression of a key downstream mediator, c-Myc. The effect of simvastatin on nonphosphorylated-β-catenin, the key regulator of the Wnt/β-catenin pathway, was recapitulated using human leiomyoma tissue from patients who received simvastatin.
Figure 7.
Summary illustration highlighting the effect of simvastatin on the Wnt/β-catenin pathway in human uterine leiomyoma. Simvastatin treatment reduced the expression of Wnt4 and its co-receptor LRP5. It also reduced the levels of total β-catenin and its translocation to the nucleus where it acts as a transcriptional activator. Treatment also reduced the levels of downstream mediators c-Myc and cyclin D1. Abbreviations: FZD, frizzled; LRP, low-density lipoprotein receptor-related protein; Dvl, disheveled; GSK3, glycogen synthase kinase 3; CK1, casein kinase 1; TCF/LEF, T-cell factor/lymphoid enhancer factor.
There is strong evidence that Wnt/β-catenin aberrations play a critical role in uterine leiomyomas. The simultaneous activation of β-catenin in uterine stroma mice leads to myometrial hyperplasia and the formation of leiomyomas (7), while the blocking of this pathway retarded leiomyoma growth (8). Ono et al showed that leiomyoma stem cells, which do not express estrogen and progesterone receptors, interact with neighboring cells through the Wnt/β-catenin pathway (21). Surrounding mature cells respond to estrogen and progesterone through releasing WNT11 and WNT16, which results in β-catenin translocation into the nucleus and an increase in its transcriptional activity in leiomyoma stem cells, leading to increased proliferation in these stem cells (21). This study confirms the role of Wnt/β-catenin in the initial pathogenesis of leiomyoma from leiomyoma stem cells (24). A recent study showed that WNT4 was overexpressed in intermediate stem cells, and its receptor FZD6 was primarily expressed in leiomyoma stem cells (25). Treatment with Wnt4 resulted in increased Akt phosphorylation, activation of β-catenin, and primary leiomyoma cell proliferation. Wnt4 treatment in these cells also upregulated the expression of the genes c-Myc and cyclin D1 (25). Cotreatment with an Akt inhibitor inhibited these effects (25). We previously showed that simvastatin treatment reduced Akt phosphorylation (10). We also previously showed that simvastatin reduced the mRNA and protein expression of cyclin D1, another downstream signaling target of Wnt/β-catenin in human leiomyoma cells (11).
The Wnt/β-catenin pathway is implicated in several key signaling pathways that are known to be upregulated in uterine leiomyoma. One example is its crosstalk with TGF-β3, which has been established to be overexpressed in leiomyoma compared with the corresponding myometrium. TGF-β is considered one of the key factors in the pathophysiology of leiomyomas and has been implicated in fibrosis and increased extracellular matrix deposition, a vital feature of leiomyoma development (26). Tanwar et al previously showed that constitutive activation of β-catenin in mice uteri resulted in higher levels of TGF-β3 expression in the endometrium and in central areas of leiomyoma-like lesions when compared with the myometrium (7). Moreover, fucoidan, an antifibrotic polysaccharide, which inhibited TGF-β3-induced cell growth, also reduced β-catenin translocation into the nucleus. These 2 studies establish a 2-way interaction between TGFβ3 and the Wnt/β-catenin pathway, highlighting the important role that Wnt/β-catenin plays in leiomyoma development, and the importance of targeting this pathway as a therapeutic option for this tumor.
Despite the significant burden and high prevalence of these common tumors, treatment options are limited, and most symptomatic patients undergo surgical removal (myomectomy or hysterectomy). There is a critical need for effective, nonhormonal treatment option for patients with uterine leiomyoma. Simvastatin’s safety profile and pharmacokinetics are well known, and it is inexpensive and already available in a generic form. Repurposing simvastatin as a treatment option for leiomyoma has several potential benefits, including shorter development time and reduced costs. Simvastatin can only be considered a therapeutic option for uterine leiomyoma after clinical trials confirm its efficacy in reducing tumor volume and improving the quality of life of patients, and our ongoing, double-blind, phase 2 clinical trial is testing this effect on patients who have at least one 3-cm leiomyoma.
This study has several strengths. We used both immortalized uterine leiomyoma and primary uterine leiomyoma cells isolated from fibroid tissue in several patients and showed consistent results in both cell types. Primary cells behave very close to in vivo biology, but they divide slowly and can only be used for few passages before they exhibit morphologic changes. Immortalized cells can be maintained for several passages without changes, but the immortalization process may have introduced some changes in biology. We conducted most experiments using both cell types to capitalize on the advantages of both cell types and showed consistent results in both cell types. Another major strength is validating the in vitro results using human tissue taken from patients who participated in the ongoing double-blind, phase 2 randomized control trial. One of the limitations of the study is that our experiments were limited to simvastatin, and other statins were not examined. This decision was based on our previous unpublished work using different statins, in which simvastatin showed the most pronounced effects on leiomyoma. We have also not explored the exact mechanism by which simvastatin reduced the expression level of the Wnt/β-catenin pathway, which we plan to investigate in our future work.
Conclusion
Our results suggest a possible mechanism by which simvastatin can prevent the development and growth of leiomyomas through inhibiting several components of the Wnt/β-catenin, a pathway necessary for leiomyoma growth and development. In order to assess the exact mechanism of simvastatin’s action, more research is needed.
Acknowledgments
Barbara Smith, Electron Microscopy Specialist, Cell Bio Imaging Facility, Johns Hopkins School of Medicine
Financial Support: This work was supported by NIH grant 1R01HD094380 to Mostafa A. Borahay.
Author Contributions: M.E., S.K., S.A., and M.B. contributed to the project’s intellectual development. M.E., S.K., and S.A. performed experimental work. M.E. wrote the manuscript. M.B. supervised the project.
Glossary
Abbreviations
- BSA
bovine serum albumin
- DMEM/F-12
Dulbecco’s Modified Eagle Medium/Nutrient Mixture F12
- DMSO
dimethyl sulfoxide
- FZD
frizzled
- LRP5
low-density lipoprotein receptor-related protein
- TGF-β3
transforming growth factor β3
- WA1
Wnt agonist 1
Additional Information
Disclosures: The authors declare no conflict of interest.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25(57):7482-7491. [DOI] [PubMed] [Google Scholar]
- 3. Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501-1512. [DOI] [PubMed] [Google Scholar]
- 4. Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21 746 women. BMC Womens Health. 2012;12:6. doi:10.1186/1472-6874-12-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El Sabeh M, Saha SK, Afrin S, Islam MS, Borahay MA. Wnt/β-catenin signaling pathway in uterine leiomyoma: role in tumor biology and targeting opportunities. Mol Cell Biochem. 2021;476(9):3513-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mangioni S, Viganò P, Lattuada D, Abbiati A, Vignali M, Di Blasio AM. Overexpression of the Wnt5b gene in leiomyoma cells: implications for a role of the Wnt signaling pathway in the uterine benign tumor. J Clin Endocrinol Metab. 2005;90(9):5349-5355. [DOI] [PubMed] [Google Scholar]
- 7. Tanwar PS, Lee HJ, Zhang L, et al. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81(3):545-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ono M, Yin P, Navarro A, et al. Inhibition of canonical WNT signaling attenuates human leiomyoma cell growth. Fertil Steril. 2014;101(5):1441-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borahay MA, Kilic GS, Yallampalli C, et al. Simvastatin potently induces calcium-dependent apoptosis of human leiomyoma cells. J Biol Chem. 2014;289(51):35075-35086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borahay MA, Vincent K, Motamedi M, et al. Novel effects of simvastatin on uterine fibroid tumors: in vitro and patient-derived xenograft mouse model study. Am J Obstet Gynecol. 2015;213(2):196.e1-196.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Afrin S, Islam MS, Patzkowsky K, et al. Simvastatin ameliorates altered mechanotransduction in uterine leiomyoma cells. Am J Obstet Gynecol. 2020;223(5):733.e1-733.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malik M, Britten J, Borahay M, Segars J, Catherino WH. Simvastatin, at clinically relevant concentrations, affects human uterine leiomyoma growth and extracellular matrix production. Fertil Steril. 2018;110(7):1398-1407.e1. [DOI] [PubMed] [Google Scholar]
- 13. Afrin S, El Sabeh M, Islam MS, et al. Simvastatin modulates estrogen signaling in uterine leiomyoma via regulating receptor palmitoylation, trafficking and degradation. Pharmacol Res. 2021;172:105856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borahay MA, Fang X, Baillargeon JG, Kilic GS, Boehning DF, Kuo YF. Statin use and uterine fibroid risk in hyperlipidemia patients: a nested case-control study. Am J Obstet Gynecol. 2016;215(6):750.e1-750.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carney SA, Tahara H, Swartz CD, et al. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest. 2002;82(6):719-728. [DOI] [PubMed] [Google Scholar]
- 16. Salama SA, Kamel MW, Botting S, et al. Catechol-o-methyltransferase expression and 2-methoxyestradiol affect microtubule dynamics and modify steroid receptor signaling in leiomyoma cells. Plos One. 2009;4(10):e7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ali M, Shahin SM, Sabri NA, Al-Hendy A, Yang Q. Activation of β-catenin signaling and its crosstalk with estrogen and histone deacetylases in human uterine fibroids. J Clin Endocrinol Metab. 2020;105(4):dgz227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509-1512. [DOI] [PubMed] [Google Scholar]
- 19. Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96(10):5522-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18(12):3035-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ono M, Yin P, Navarro A, et al. Paracrine activation of WNT/β-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc Natl Acad Sci U S A. 2013;110(42):17053-17058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Wu X, Mitchell B, Kintner C, Ding S, Schultz PG. A small-molecule agonist of the Wnt signaling pathway. Angew Chem Int Ed Engl. 2005;44(13):1987-1990. [DOI] [PubMed] [Google Scholar]
- 24. El Sabeh M, Afrin S, Singh B, Miyashita-Ishiwata M, Borahay M. Uterine stem cells and benign gynecological disorders: role in pathobiology and therapeutic implications. Stem Cell Rev Rep. 2021;17(3):803-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu S, Yin P, Dotts AJ, et al. Activation of protein kinase B by WNT4 as a regulator of uterine leiomyoma stem cell function. Fertil Steril. 2020;114(6):1339-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ciebiera M, Wlodarczyk M, Wrzosek M, et al. Role of transforming growth factor beta in uterine fibroid biology. Int J Mol Sci. 2017;18(11):2435. doi:10.3390/ijms18112435 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.