Abstract
Background
This phase I/II trial in patients with recurrent glioblastoma (GBM) evaluates the safety and preliminary efficacy of marizomib, an irreversible pan-proteasome inhibitor that crosses the blood–brain barrier.
Methods
Part A assessed the safety and efficacy of marizomib monotherapy. In Part B, escalating doses of marizomib (0.5–0.8 mg/m2) in combination with bevacizumab were evaluated. Part C explored intra-patient dose escalation of marizomib (0.8–1.0 mg/m2) for the combination.
Results
In Part A, 30 patients received marizomib monotherapy. The most common AEs were fatigue (66.7%), headache (46.7%), hallucination (43.3%), and insomnia (43.3%). One patient (3.3%) achieved a partial response. In Part B, the recommended phase II dose of marizomib was 0.8 mg/m2 when combined with bevacizumab 10 mg/kg. In Part C, dose escalation to 1.0 mg/m2 was not tolerated. Pooled analysis of 67 patients treated with marizomib ≤0.8 mg/m2 and bevacizumab showed a nonoverlapping safety profile consistent with the known safety profile of each agent: the most common grade ≥3 AEs were hypertension (16.4%), confusion (13.4%), headache (10.4%), and fatigue (10.4%). The overall response rate was 34.3%, including 2 patients with complete response. Six-month progression-free survival was 29.8%; median overall survival was 9.1 months.
Conclusions
The safety profile of marizomib as monotherapy and in combination with bevacizumab was consistent with previous observations that marizomib crosses the blood–brain barrier. Preliminary efficacy did not demonstrate a meaningful benefit of the addition of marizomib to bevacizumab for the treatment of recurrent GBM.
Keywords: clinical trials, glioblastoma, marizomib
Key Points.
Marizomib is an irreversible, pan-proteasome inhibitor that crosses the blood–brain barrier.
The safety profile of marizomib indicated CNS penetration in patients with GBM.
Importance of the Study.
Marizomib is a pan-proteasome inhibitor with a distinct structure and unique irreversible mechanism of action. In preclinical studies, glioma cell lines and glioma stem cells exhibited preferential sensitivity to marizomib. Unlike other clinically available proteasome inhibitors, marizomib crosses the blood–brain barrier in mice, rats, and nonhuman primates, making it a potential treatment for central nervous system (CNS) malignancies such as glioblastoma. Increased vascular endothelial growth factor (VEGF) secretion from glioma cells exposed to proteasome inhibitors provided the rationale for combining marizomib with the VEGF inhibitor bevacizumab. Results of this phase I/II trial suggest that marizomib crosses the blood–brain barrier. The safety profile of marizomib monotherapy was consistent with previous experience with marizomib in advanced malignancies.
Despite advances in our understanding of the biology of glioblastoma (GBM), little has changed regarding treatment in the past decade.1,2 Nearly all patients with GBM relapse on first-line therapy with radiation and temozolomide within 9 months of the initial diagnosis.3,4 Treatment options for recurrent disease are limited, and median overall survival (OS) is approximately 6–9 months.5,6 The vascular endothelial growth factor (VEGF) inhibitor bevacizumab has been widely used in the USA for patients with recurrent GBM,6–9 although its use has not been shown to improve OS in recurrent GBM10 or when added to first-line therapy.11,12 Efforts to identify patients most likely to respond to treatment have met with limited success; however, unmethylated O6-methylguanine-DNA-methyltransferase (MGMT) promoter status has been shown to be a marker of poor prognosis in patients treated with temozolomide13–17 or bevacizumab.6,9
Marizomib (also known as NPI-0052) is an irreversible, pan-proteasome inhibitor with a unique structure and biological profile compared with other proteasome inhibitors.18–23 It has been shown to induce sustained inhibition of all 3 proteolytic subunits (the chymotrypsin-like [CT-L], trypsin-like [T-L], and caspase-like [C-L] subunits).24 Unlike bortezomib and carfilzomib, marizomib crosses the blood–brain barrier in rats and nonhuman primates,25–28 making it suitable for evaluation in patients with brain malignancies. In animal models, marizomib inhibits proteasome activity in the brain and demonstrates antitumor activity in an orthotopic xenograft model of human GBM.25 In previous clinical studies of marizomib in non-GBM indications, most dose-limiting toxicities (DLTs) were central nervous system (CNS) adverse events (AEs), providing further evidence that marizomib crosses the blood–brain barrier.29,30 Proteasome inhibition was also previously found to increase VEGF secretion from glioma cells in vitro,31 providing a rationale for combining marizomib with bevacizumab in the GBM setting. Based on these observations, a phase I/II clinical trial was conducted to evaluate marizomib alone or in combination with bevacizumab in patients with recurrent GBM.
Materials and Methods
Trial Design
MRZ-108 was a phase I/II multicenter, open-label, dose-escalation study evaluating marizomib with or without bevacizumab in patients with recurrent GBM (NCT02330562).
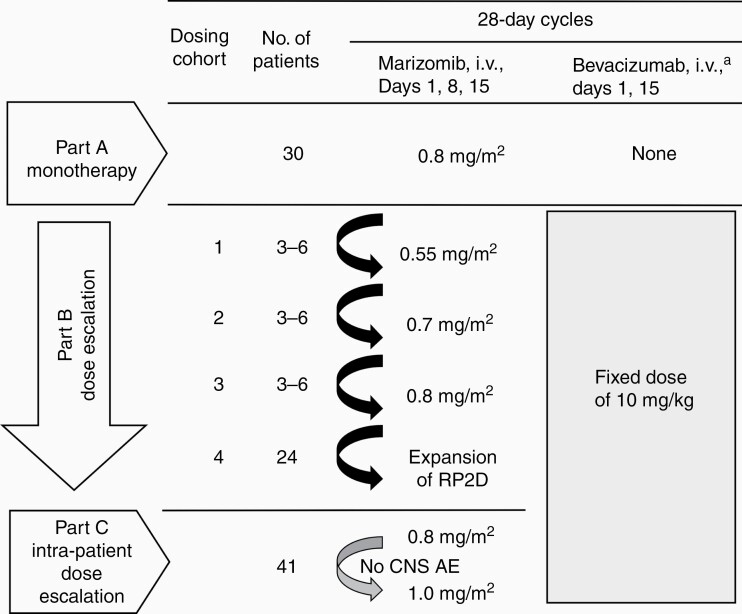
Part A was a phase II study to assess the efficacy of marizomib administered as monotherapy and was conducted as a modified 2-stage sequential design (Figure 1). Marizomib was administered as a 10-min i.v. infusion once weekly for 3 weeks in 28-day cycles at a dose of 0.8 mg/m2. This dose and infusion time were selected as the recommended phase II dose (RP2D) based on earlier studies in solid tumors and multiple myeloma.29,30 The protocol specified that the study would be terminated if there were no objective responses in the first 15 response-evaluable patients enrolled. If 1 or more responses were observed, enrollment would continue until an additional 15 response-evaluable patients were treated. If 5 or more responses were observed, marizomib was to be considered active as a single agent.
Figure 1.
Study design. a Bevacizumab infusion duration was 90 min for the first dose and, if tolerated, 60 min for the second dose and 30 min for subsequent doses. AE, adverse event; CNS, central nervous system; RP2D, recommended phase II dose.
Part B was a phase I, 3+3 dose-escalation study32 of marizomib in combination with bevacizumab, followed by a phase II expansion cohort. Marizomib was administered as a 10-min i.v. infusion in dose cohorts ranging from 0.55 to 0.8 mg/m2 on days 1, 8, and 15 of each 28-day cycle (Figure 1). Bevacizumab was administered at a fixed dose of 10 mg/kg i.v. on days 1 and 15 of each 28-day cycle in all patients.6 An expansion cohort at the RP2D was included to further assess the safety and efficacy of combination therapy.
Part C assessed the activity of marizomib and bevacizumab using intra-patient dose escalation of marizomib based on the presence of specific CNS AEs in a patient population similar to Part B. It was decided to initiate Part C after a longer median progression-free survival (PFS) was observed in Part B patients who experienced CNS AEs compared with patients who did not experience them. In Part C, marizomib was given once weekly for 3 weeks, and bevacizumab (10 mg/kg) was given every 2 weeks in 28-day cycles. The starting dose of marizomib was 0.8 mg/m2. Patients were assessed in cycle 1 for marizomib-related AEs related to disturbances in the cerebellum (ie, ataxia, dizziness, dysarthria, fall, gait disturbances), hallucinations of any grade, or any other AE grade ≥2. After 1 cycle without the presence of these toxicities, the marizomib dose was increased to 1.0 mg/m2. If the increased dose was tolerated, the dose was further increased to 1.2 mg/m2 in the next cycle (cycle 3).
Patients
Inclusion and exclusion criteria were the same for Parts A–C. Eligible patients were aged ≥18 years, had a KPS score ≥70, and grade IV malignant glioma (including GBM and gliosarcoma). All patients were experiencing a first or second relapse with radiographic evidence of disease progression or recurrence, defined according to Response Assessment in Neuro-Oncology (RANO) criteria.33 All patients had previously undergone standard radiation therapy and received temozolomide. Patients with prior antiangiogenic therapy or proteasome inhibition therapy were excluded. This study was approved by the local institutional review boards, and complied with good clinical practice, as described in the International Council for Harmonisation Guideline E6, and in accordance with the general ethical principles outlined in the Declaration of Helsinki. All patients provided written informed consent.
Study Endpoints and Statistical Analyses
The primary endpoint of Part A was best response of marizomib monotherapy in patients with recurrent GBM. For Part B, the primary endpoints were the maximum tolerated dose (MTD) or maximum administered dose (MAD) and the RP2D of marizomib when given in combination with a fixed dose and schedule of bevacizumab. The MTD was defined as the dose level below the cohort in which a DLT was observed in at least 2 patients during cycle 1. A DLT was defined as any of the following AEs occurring during cycle 1: grade ≥3 thrombocytopenia or grade 2 thrombocytopenia with bleeding; grade 4 neutropenia or anemia lasting more than 4 days; febrile neutropenia; any grade ≥2 neurologic event lasting more than 4 days; or grade 3 or 4 nonhematologic AEs (excluding alopecia) lasting more than 4 days despite adequate supportive care or preventing administration of the next scheduled dose. For grade ≥3 fatigue to be considered a DLT, it must have been present for more than 7 days.
Secondary endpoints of Part A and Part B were safety and efficacy (overall response rate [ORR], PFS, and OS). AEs were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. An interim analysis of the safety and efficacy of the marizomib and bevacizumab combination suggested that patients who experienced certain CNS AEs related to disturbances in the cerebellum (ataxia, balance disorder, dizziness, dysarthria, fall, and gait disturbance) and hallucination appeared to have better efficacy outcomes than patients without these CNS AEs of interest. Therefore, efficacy was also assessed in patients with and without these CNS AEs of interest. In addition, blood samples were collected for pharmacokinetic (PK) and pharmacodynamic (PD) analysis from patients in the Part B dose-escalation cohorts. For PK analysis of marizomib, samples were collected on days 1 and 15 of cycle 1 prior to marizomib infusion, just before the end of infusion, and 2, 5, 15, 30, 45, 60, 90, and 120 min after infusion. For PK analysis of bevacizumab, samples were collected on days 1 and 15 of cycle 1 prior to infusion and just before the end of infusion. PK parameters evaluated included maximum concentration (Cmax), time of maximum concentration (Tmax), elimination half-life (t1/2), area under the concentration–time curve (AUC) from time 0 to the last time point with a concentration level exceeding the limit of quantitation (AUC0–t), AUC extrapolated from time 0 to infinity (AUC0–inf), clearance, and volume of distribution (Vd). For PD analysis, samples were collected on days 1, 8, and 15 of cycle 1, and days 1 and 15 of each cycle thereafter. Analysis consisted of inhibition of proteasome activity (CT-L, T-L, and C-L) in packed whole blood samples.
The primary endpoint of Part C was OS. Secondary endpoints included safety (AEs and DLT) as well as efficacy (ORR and PFS).
Results
Part A (Marizomib Monotherapy)
Patients.
—Thirty patients were enrolled and received marizomib monotherapy (Table 1). The median age was 58.5 years (range 25–80 years), the median time since diagnosis was 10.7 months (range 1–20 months), and the median number of prior therapies was 1 (range 1‒3).
Table 1.
Patient Demographics and Baseline Characteristics
| Characteristic | Part A | Part B | Part C | Part B + Ca |
|---|---|---|---|---|
| Marizomib (N = 30) |
Marizomib + Bevacizumab (N = 36) |
Marizomib + Bevacizumab (N = 41) |
Marizomib ≤0.8 mg/m2 + Bevacizumab (N = 67) |
|
| Age, median (range), years | 58.5 (25–80) | 55 (27–76) | 56 (19–75) | 56 (27–76) |
| Female, n (%) | 13 (43.3) | 13 (36.1) | 21 (51.2) | 27 (40.3) |
| Baseline KPS score, n (%) | ||||
| 70 | 4 (13.3) | 5 (13.9) | 7 (17.1) | 12 (17.9) |
| 80 | 9 (30.0) | 14 (38.9) | 15 (36.6) | 25 (37.3) |
| 90 | 15 (50.0) | 11 (30.6) | 15 (36.6) | 21 (31.3) |
| 100 | 2 (6.7) | 6 (16.7) | 4 (9.8) | 9 (13.4) |
| Time since grade IV diagnosis, median (range), months | 10.7 (1–20) | 10.0 (2–41) | 11.2 (0.4–34.6) | 9.7 (0.4–40.7) |
| Disease status, n (%) | ||||
| Unifocal | 20 (66.7) | 31 (86.1) | 27 (65.9) | 50 (74.6) |
| Multifocal | 10 (33.3) | 5 (13.9) | 14 (34.1) | 17 (25.4) |
| EGFR variant III status, n (%) | ||||
| Positive | 2 (6.7) | 4 (11.1) | 4 (9.8) | 7 (10.4) |
| Negative | 18 (60.0) | 21 (58.3) | 29 (70.7) | 44 (65.7) |
| Missing/unknown | 10 (33.3) | 11 (30.6) | 8 (19.5) | 16 (23.9) |
| MGMT promoter methylation status, n (%) | ||||
| Methylated | 6 (20.0) | 10 (27.8) | 14 (34.1) | 21 (31.3) |
| Unmethylated | 18 (60.0) | 22 (61.1) | 21 (51.2) | 38 (56.7) |
| Missing/unknown | 6 (20.0) | 4 (11.1) | 6 (14.6) | 8 (11.9) |
| Prior therapeutic regimens, median (range), n | 1 (1‒3) | 2 (1–4) | 1 (1–4) | 1 (1–4) |
a Excluding dose-escalated patients.
EGFR, epidermal growth factor receptor; MGMT, O6-methylguanine-DNA-methyltransferase.
Safety.
—Patients received a median of 2 cycles of marizomib (range 1‒12). The most common AEs (any grade) were fatigue (66.7%), headache (46.7%), hallucination (43.3%), and insomnia (43.3%) (Table 2). Common CNS AEs included ataxia (26.7%), aphasia (23.3%), and dysarthria (23.3%). CNS AEs of interest (ataxia, balance disorder, dizziness, dysarthria, fall, gait disturbance, and/or hallucination) were reported in 25 patients (83.3%); the median time to onset was 19 days (range 1–243 days), and the median duration was 6 days (range 1–587 days). These CNS AEs of interest were managed with dexamethasone in 5 patients (20.0%), antipsychotics in 6 patients (24.0%), dose reduction in 2 patients (8.0%), and dose delay in 1 patient (4%). The most common grade ≥3 AEs were fatigue, hallucination, hypertension, nausea, and vomiting (6.7% each). Infusion-site reactions were reported in 20.0% of patients, all of which were grade 1 or 2. Three patients (10.0%) had at least 1 serious treatment-emergent adverse event (TEAE) suspected to be related to marizomib (1 patient had ataxia; 1 patient had delusion and hallucination; and 1 patient had mental status changes, hypertension, and lethargy), all of which resolved after marizomib dose delays. Overall, 3 patients (10.0%) had at least 1 dose delay, and 5 patients (16.7%) had at least 1 dose reduction due to an AE. No discontinuations due to AEs occurred.
Table 2.
Incidence of TEAEs (Any Grade) Reported in >30% of Patients and Grade ≥3 TEAEs Reported in >5% of Patients
| TEAE, n (%) | Part A | Part B | Part C | Part B + Ca | ||||
|---|---|---|---|---|---|---|---|---|
| Marizomib (N = 30) |
Marizomib + Bevacizumab (N = 36) |
Marizomib + Bevacizumab (N = 41) |
Marizomib ≤0.8 mg/m2 + Bevacizumab (N = 67) |
|||||
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |
| Fatigue | 20 (66.7) | 2 (6.7) | 25 (69.4) | 3 (8.3) | 33 (80.5) | 7 (17.1) | 49 (73.1) | 7 (10.4) |
| Nausea | 11 (36.7) | 2 (6.7) | 23 (63.9) | 0 | 20 (48.8) | 3 (7.3) | 36 (53.7) | 2 (3.0) |
| Hallucination | 13 (43.3) | 2 (6.7) | 13 (36.1) | 2 (5.6) | 26 (63.4) | 1 (2.4) | 32 (47.8) | 3 (4.5) |
| Confusion | 7 (23.3) | 0 | 10 (27.8) | 3 (8.3) | 24 (58.5) | 8 (19.5) | 27 (40.3) | 9 (13.4) |
| Vomiting | 10 (33.3) | 2 (6.7) | 19 (52.8) | 0 | 22 (53.7) | 2 (4.9) | 35 (52.2) | 1 (1.5) |
| Headache | 14 (46.7) | 1 (3.3) | 19 (52.8) | 5 (13.9) | 18 (43.9) | 3 (7.3) | 32 (47.8) | 7 (10.4) |
| Gait disturbance | 5 (16.7) | 0 | 5 (13.9) | 0 | 20 (48.8) | 1 (2.4) | 20 (29.9) | 0 |
| Hypertension | 4 (13.3) | 2 (6.7) | 16 (44.4) | 6 (16.7) | 11 (26.8) | 8 (19.5) | 24 (35.8) | 11 (16.4) |
| Insomnia | 13 (43.3) | 1 (3.3) | 4 (11.1) | 1 (2.8) | 11 (26.8) | 1 (2.4) | 13 (19.4) | 2 (3.0) |
| Ataxia | 8 (26.7) | 0 | 8 (22.2) | 1 (2.8) | 16 (39.0) | 7 (17.1) | 20 (29.9) | 5 (7.5) |
| Dizziness | 5 (16.7) | 0 | 10 (27.8) | 0 | 16 (39.0) | 1 (2.4) | 22 (32.8) | 1 (1.5) |
| Fall | 4 (13.3) | 0 | 10 (27.8) | 1 (2.8) | 15 (36.6) | 0 | 22 (32.8) | 0 |
| Muscular weakness | 6 (20.0) | 1 (3.3) | 7 (19.4) | 1 (2.8) | 15 (36.6) | 4 (9.8) | 19 (28.4) | 5 (7.5) |
| Constipation | 10 (33.3) | 0 | 9 (25.0) | 0 | 9 (22.0) | 0 | 15 (22.4) | 0 |
| Dysarthria | 7 (23.3) | 1 (3.3) | 6 (16.7) | 1 (2.8) | 13 (31.7) | 0 | 15 (22.4) | 0 |
| Dysphonia | 1 (3.3) | 0 | 11 (30.6) | 0 | 10 (24.4) | 0 | 20 (29.9) | 0 |
| Hyperglycemia | 8 (26.7) | 1 (3.3) | 8 (22.2) | 2 (5.6) | 8 (19.5) | 1 (2.4) | 15 (22.4) | 2 (5.6) |
| Aphasia | 7 (23.3) | 1 (3.3) | 6 (16.7) | 1 (2.8) | 9 (22.0) | 3 (7.3) | 12 (17.9) | 3 (4.5) |
| Convulsion | 5 (16.7) | 1 (3.3) | 7 (19.4) | 1 (2.8) | 9 (22.0) | 3 (7.3) | 13 (19.4) | 3 (4.5) |
| Hemiparesis | 5 (16.7) | 0 | 4 (11.1) | 3 (8.3) | 7 (17.1) | 2 (4.9) | 8 (11.9) | 5 (7.5) |
| Urinary tract infection | 1 (3.3) | 0 | 4 (11.1) | 0 | 7 (17.1) | 3 (7.3) | 11 (16.4) | 3 (4.5) |
| Decreased lymphocyte count | 5 (16.7) | 1 (3.3) | 3 (8.3) | 3 (8.3) | 3 (7.3) | 1 (2.4) | 6 (9.0) | 4 (6.0) |
| Proteinuria | 2 (6.7) | 0 | 4 (11.1) | 2 (5.6) | 4 (9.8) | 0 | 8 (11.9) | 2 (3.0) |
| Disease progression | 1 (3.3) | 1 (3.3) | 2 (5.6) | 2 (5.6) | 0 | 0 | 2 (3.0) | 2 (3.0) |
a Excluding dose-escalated patients.
TEAE, treatment-emergent adverse event.
Efficacy.
—One partial response (PR; duration 9.6 months) occurred in the first 15 patients enrolled, allowing enrollment of an additional 15 patients. No other responses by RANO criteria were observed, resulting in an ORR of 3.3% (Table 3). Eight patients (26.7%) had stable disease (duration ranging from 0.7 to 9.5 months), and 3 patients had stable disease for more than 6 cycles. Two patients categorized as having progressive disease per RANO criteria prior to undergoing subsequent surgery were found to have little or no tumor present upon histopathologic assessment of the resected area, suggesting the possibility of pseudoprogression with marizomib monotherapy. Overall, the PFS rate at 6 months was 16%, median OS was 11.4 months, and the OS rates at 6 and 12 months were 62% and 44%, respectively, with no patients alive at 18 months.
Table 3.
Efficacy Outcomes
| Outcome | Part A | Part B | Part C | Part B + Ca |
|---|---|---|---|---|
| Marizomib (N = 30) |
Marizomib + Bevacizumab (N = 36) |
Marizomib + Bevacizumab (N = 41) |
Marizomib ≤0.8 mg/m2 + Bevacizumab (N = 67) |
|
| ORR, n (%) | 1 (3.3) | 16 (44.4) | 9 (22.0) | 23 (34.3) |
| Complete response | 0 (0) | 1 (2.8) | 1 (2.4) | 2 (3.0) |
| Partial response | 1 (3.3) | 15 (41.7) | 8 (19.5) | 21 (31.3) |
| Stable disease | 8 (26.7) | 11 (30.6) | 18 (43.9) | 25 (37.3) |
| Progressive disease | 20 (66.7) | 6 (16.7) | 11 (26.8) | 13 (19.4) |
| Not evaluable | 1 (3.3) | 3 (8.3) | 3 (7.3) | 6 (9.0) |
| Duration of response, median (95% CI) | 9.6 (NA–NA) | 6.6 (3.1–8.8) | 4.7 (3.5–7.6) | 5.2 (3.7–7.6) |
| PFS | ||||
| At 6 months, % | 16 | 34 | 22 | 30 |
| OS | ||||
| Median, months (95% CI) | 11.4 (5.5–13.0) | 9.4 (6.3–12.6) | 8.3 (4.8–10.5) | 9.1 (6.3–10.9) |
| At 9 months, % | 55 | 60 | 41 | 52 |
a Excluding dose-escalated patients.
CI, confidence interval; NA, not available; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
Part B (Combination Therapy with Fixed-Dose Bevacizumab, Marizomib Dose Escalation)
Patients.
—Of the 36 patients enrolled in Part B, the median age was 55 years (range 27–76 years), the median time since diagnosis of GBM was 10 months (range 2–41 months), the median number of prior regimens was 2 (range 1–4 regimens) (Table 1), and all patients were documented to have progressed on or after their last prior therapy.
Safety.
—One patient in Cohort 1 (0.55 mg/m2 marizomib and 10 mg/kg bevacizumab) experienced a DLT of grade 3 fatigue; therefore, a total of 6 patients were enrolled at this dose level. No DLTs were observed in Cohort 2 (0.7 mg/m2, n = 3) or Cohort 3 (0.8 mg/m2, n = 3). The MTD of marizomib in combination with bevacizumab was not formally reached, and the MAD of marizomib of 0.8 mg/m2 administered intravenously once weekly for 3 weeks on days 1, 8, and 15 every 28 days was considered the RP2D. Twenty-four patients were treated with this dose of marizomib in combination with bevacizumab in the expansion cohort.
Patients received a median of 4 treatment cycles (range 1–17) of marizomib in combination with bevacizumab. The most common AEs (any grade) among patients receiving combination therapy were fatigue (69.4%), nausea (63.9%), vomiting (52.8%), and headache (52.8%) (Table 2). Common CNS AEs included hallucination (36.1%), dysphonia (30.6%), confusion (27.8%), dizziness (27.8%), fall (27.8%), and ataxia (22.2%). The most frequently reported grade ≥3 AEs were hypertension (16.7%); headache (13.9%); fatigue, confusion, hemiparesis, and decreased lymphocyte count (8.3% each); hallucination, hyperglycemia, proteinuria, and disease progression (5.6% each; Table 2). Infusion-site reactions were reported in 36.1% of patients; all were grade 1 or 2 and did not require treatment modifications. The most common grade ≥3 AEs attributed to marizomib were headache (8.3%), confusional state (8.3%), fatigue (5.6%), and hallucination (5.6%), whereas the most common grade ≥3 AEs attributed to bevacizumab were hypertension (16.7%), headache (5.6%), fatigue (5.6%), and proteinuria (5.6%) (Supplementary Table S1).
During combination treatment, 14 patients (38.9%) required dose delays of marizomib and 14 patients (38.9%) required dose delays of bevacizumab. Thirteen patients (36.1%) required a dose reduction of marizomib; no dose reductions of bevacizumab occurred. The most commonly reported (in more than 2 patients) AEs leading to dose reductions or delays of 1 or both agents were hallucination, confusional state, fatigue, vomiting, muscular weakness, nausea, and ataxia. Overall, 6 patients discontinued marizomib and bevacizumab, and 1 additional patient discontinued bevacizumab treatment only due to a TEAE; events leading to discontinuation in more than 1 patient were headache and hemiparesis (2 patients each). Three patients died during the study (2 due to disease progression, and 1 due to intracranial hemorrhage with suspected relationship to bevacizumab only).
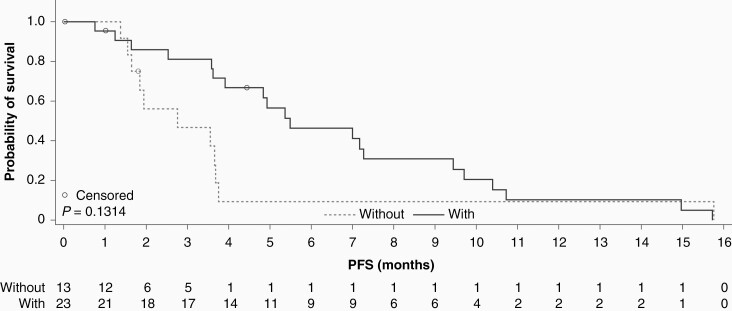
Twenty-three of the 36 patients (63.9%) experienced at least 1 of the following CNS AEs of interest: ataxia, balance disorder, dizziness, dysarthria, fall, gait disturbance, hallucination. The median time to onset of these events was 43.5 days (range 1–388 days), and the median duration was 13 days (range 1–570 days); 5 patients (21.7%) received dexamethasone, 5 patients (21.7%) received antipsychotics, 4 patients (17.4%) had a dose reduction and 1 patient (4.4%) had a dose delay to manage a CNS AE of interest. In patients who experienced these CNS AEs, median PFS was numerically longer than in patients who did not experience them (5.5 vs 2.8 months; P = .13; Figure 2). Median OS was also numerically longer in patients with these CNS AEs compared with patients without them (10.9 vs 6.3 months; P = .28).
Figure 2.
PFS in Part B based on the presence (with) or absence (without) of the following CNS AEs: hallucination, ataxia, balance disorder, dizziness, dysarthria, fall, gait disturbance, and/or hallucination. AE, adverse event; CNS, central nervous system; PFS, progression-free survival.
Efficacy.
—Sixteen of 36 patients responded to treatment, including 1 patient with a CR, resulting in an ORR of 44.4% (Table 3). The median duration of response was 6.6 months. The PFS rate at 6 months was 34% and the median PFS was 3.9 months. The median OS was 9.4 months, and the survival rates at 6, 12, and 18 months were 75%, 37%, and 21%, respectively.
PK/PD analysis.
—PK data for marizomib are summarized in Supplementary Table S2. For patients in the 0.8 mg/m2 dose cohort, the mean t1/2 was 7.27 min, and mean clearance was 304 L/h. Values for t1/2, Vd, and clearance appeared to be dose-independent; overall PK were variable, and AUC was not clearly related to dose. There was no evidence that marizomib affected the PK of concomitant bevacizumab.
Blood PD data demonstrated that after the first dose of marizomib in cycle 1, mean CT-L activity was inhibited by 69.9%, 72.4%, and 76.2% relative to preinfusion levels in patients receiving 0.55, 0.7, and 0.8 mg/m2 marizomib, respectively. (Supplementary Fig. S1). Subsequent postinfusion samples revealed complete (100%) inhibition of CT-L at all dose levels, indicating a cumulative effect on proteasome inhibition. Increases in T-L and C-L activity were observed after the first infusion in all dose cohorts, after which inhibition of T-L (50–60% in Cohorts 1 and 2, up to 70% in Cohort 3) and C-L (10–30% in Cohort 1, 25–50% in Cohorts 2 and 3) was observed at the remainder of time points examined. Reductions in proteasome inhibition in samples collected prior to infusion were observed with up to 2 weeks between marizomib administrations. Once proteasome subunit activity was inhibited by marizomib (CT-L and T-L activity at all dose levels, C-L activity at the 0.7 and 0.8 mg/m2 dose levels), it remained inhibited relative to pretreatment levels at each subsequent preinfusion sample, demonstrating the sustained inhibition of proteasome activity with this schedule of marizomib.
Part C (Combination Therapy, Marizomib Intra-Patient Dose Escalation)
Patients.
—A total of 41 patients were enrolled in Part C. The median age was 56 years (range 19–75 years), the median time since diagnosis was 11.2 months (range 0.4–34.6 months), and the median number of prior therapies was 1 (range 1‒4) (Table 1).
Safety.
—Of the 41 patients, 10 did not experience a CNS AE of interest in cycle 1 and were dose-escalated to 1.0 mg/m2 of marizomib in cycle 2, whereas 31 were not dose-escalated. Eight of the 10 dose-escalated patients (80.0%) experienced DLTs at the 1.0 mg/m2 dose of marizomib and bevacizumab and required dose reductions. Of the other 2 patients, 1 completed cycle 2 but then discontinued due to disease progression, the other discontinued during cycle 2 due to AEs.
Among the 41 patients, the most frequently reported TEAEs were fatigue (80.5%), hallucination (63.4%), confusional state (58.5%), and vomiting (53.7%). These TEAEs were more frequent (>5% difference between groups) in patients who were dose-escalated to 1.0 mg/m2 of marizomib in cycle 2 than those not dose-escalated. Overall, 95.1% had CNS AEs of interest (ataxia, balance disorder, dizziness, dysarthria, fall, gait disturbance, or hallucination); the median time to onset was 37 days (range 1–289 days) and the median duration was 4 days (range 1–405 days). These CNS AEs of interest were managed with dexamethasone in 4 patients (10.3%), antipsychotics in 10 patients (25.6%), and dose reduction in 3 patients (7.7%).
Of the 41 patients, 80.5% reported grade ≥3 TEAEs including confusional state, hypertension, ataxia, fatigue, muscular weakness, aphasia, convulsion, headache, nausea, and urinary tract infection. One grade 5 AE of intracranial hemorrhage, attributed to bevacizumab, was reported in a patient who received 0.8 mg/m2 marizomib and bevacizumab.
Efficacy.
—The ORR in the safety population was 22.0%, including 1 CR and 8 PRs (Table 3); median time to response was 1.9 months (range 1.7–2.7 months) and median duration of response was 4.7 months. Median PFS and OS were 3.1 and 8.3 months, respectively. Outcomes were nominally more favorable in patients not dose-escalated compared with those who were dose-escalated: PFS (3.5 vs 2.1 months) and OS (8.3 vs 7.5 months). Subgroup analyses by CNS AEs were not possible due to the small number of patients without CNS AEs (n = 2).
Parts B + C (Pooled Combination Data)
Safety findings in the combined cohort of 67 patients treated with marizomib ≤0.8 mg/m2 and bevacizumab were generally similar to those observed in the individual parts. The most frequently reported TEAEs were fatigue (73.1%), nausea (53.7%), and vomiting (52.2%). Grade ≥3 TEAEs included hypertension (16.4%), confusional state (13.4%), headache and fatigue (10.4% each), ataxia (7.5%), muscle weakness (7.5%), hemiparesis (7.5%), and decreased lymphocyte count (6.0%) (Table 2).
The ORR in the pooled cohort of 67 patients given combination therapy was 34.3%, including 2 CRs and 21 PRs. Median PFS was 3.6 months, and median OS was 9.1 months (Table 3).
MGMT Methylation Status
In Part A, median PFS was longer among those with methylated vs unmethylated MGMT promoter status (4.9 vs 1.8 months); median OS was similar in both groups (13.0 vs 11.8 months) (Supplementary Table S3).
In the pooled group of 67 patients from Parts B and C who received marizomib ≤0.8 mg/m2 in combination with bevacizumab, the ORR was 33.3% in patients with methylated vs 32.4% in patients with unmethylated MGMT promoter status. The median PFS was the same regardless of MGMT methylation status (both 3.6 months). Median OS was numerically longer in patients with methylated vs unmethylated MGMT promoter status (11.3 vs 6.8 months) (Supplementary Table S3). Separate results for Parts B and C were similar to the combined results (Supplementary Table S3). It should be noted that no comparative statistics were conducted for any of the MGMT status comparisons.
Discussion
Results of this multi-part, phase I/II study provide insight into the safety of marizomib, when given alone or in combination with bevacizumab, in patients with recurrent GBM. In Part A, the toxicity profile of marizomib monotherapy was shown to be consistent with previous experience in advanced malignancies.29 The primary TEAEs were fatigue, headache, hallucination, and insomnia. The presence of CNS AEs suggests that marizomib crosses the blood–brain barrier, which is consistent with preclinical models.25 Overall, CNS AEs were manageable with dose delays/reductions, dexamethasone, and/or antipsychotics. Based on these findings, current recommendations for the management of marizomib-related CNS toxicities include the management of grade 2 or 3 CNS AEs with adequate dose delays and/or dose modifications and appropriate antipsychotic or dexamethasone treatment if needed.
The ORR of 3.3% observed with marizomib monotherapy may be due to a stimulatory effect on VEGF production and angiogenesis, which has been documented in vitro with other proteasome inhibitors.31 This potential effect provided the rationale for combining marizomib with the VEGF inhibitor bevacizumab, which was explored in Parts B and C of the study.
In Part B, the maximum dose of marizomib was set at 0.8 mg/m2 based on the safety profile, which had been observed in previous studies, particularly when a short duration of administration of marizomib was used.29,30 No DLTs were reported at the highest dose evaluated; however, several patients required dose modifications due to CNS AEs. The RP2D for marizomib was established at 0.8 mg/m2 when combined with bevacizumab 10 mg/kg. This was supported by findings from Part C, which showed that escalating doses of marizomib from 0.8 to 1.0 mg/m2 (among patients without dose-limiting AEs on 0.8 mg/m2) was not tolerated. Notable CNS AEs associated with marizomib included hallucination, confusion, ataxia, and dizziness. These AEs were reported in approximately 15–40% of patients treated with marizomib monotherapy and 25–40% of patients treated with marizomib in combination with bevacizumab; grade ≥3 AEs were rare at doses up to 0.8 mg/m2. This finding is consistent with results from earlier studies, particularly where marizomib infusion times were ≤10 min,30 and further suggests that marizomib crosses the blood–brain barrier.25,26
Pooling data from Parts B and C indicated that marizomib (≤0.8 mg/m2) and bevacizumab have a nonoverlapping safety profile when used concomitantly. The most common grade ≥3 TEAEs were hypertension, confusional state, headache, and fatigue. The safety profile reflects AEs typically seen with each agent (eg, CNS AEs for marizomib, hypertension for bevacizumab), and concomitant use of both agents did not appear to increase the incidence or severity of these events, based on previous experience with marizomib29,34 and bevacizumab.6–9 Most TEAEs were manageable with dose delays and/or dose reductions.
An ORR of 34.3% was found in the pooled analysis of 67 patients treated with the combination of marizomib and bevacizumab. Median PFS was 3.6 months, 6-month PFS was 29.8%, and the median OS was 9.1 months. Overall, the results are comparable to historical data from uncontrolled trials evaluating bevacizumab in recurrent GBM (response rates of 6–38% and 6-month PFS rates of 16–43%),7–9,35 particularly when trials used RANO imaging response criteria.33 However, caution is warranted when comparing results from small, uncontrolled trials. In a phase III trial comparing the combination of bevacizumab and lomustine with lomustine alone, there was no significant difference between the treatment groups in median OS (9.1 vs 8.6 months, respectively), although PFS was modestly improved with the combination (median 4.2 vs 1.5 months, respectively; P < .001).6 Preliminary efficacy in this study did not demonstrate a meaningful benefit of the addition of marizomib to bevacizumab for the treatment of recurrent GBM, compared with historical data.
In Part A, 2 patients with progressive disease by RANO criteria on study were found to have little or no tumor at poststudy subsequent surgery, suggesting pseudoprogression. Both patients had surgery prior to enrollment; one patient had a single surgery, over a year prior to enrollment, and the other patient had 2 surgeries, the last one being just over 3 months prior to enrollment. Pseudoprogression has been reported to occur after radiotherapy, and the pathophysiology of pseudoprogression remains unclear.36 It may be beneficial to continue treatment in patients in whom pseudoprogression is suspected and the agent is tolerated. The potential issue of pseudoresponse is also important, as this has been estimated to occur in 20–60% of patients treated with bevacizumab.37 This is thought to occur via effects on the blood–brain barrier affecting vascular leakage and contrast extravasation, which can lead to overestimation of response and PFS without affecting OS.36–40 In the current study, PFS was longer among those who received marizomib and bevacizumab (Parts B and C) versus marizomib monotherapy (Part A), whereas OS was not (Table 3). Although cross-cohort comparisons of this sample size should be undertaken with caution, pseudoresponse as reported in the literature could contribute to the response rate and PFS reported in patients who received marizomib with bevacizumab.
The whole-blood proteasome inhibition data suggest that complete inhibition of CT-L, the catalytically active β5 subunit, and the rate-limiting step of proteolysis, is achieved within the first dosing cycle with once-weekly infusions of marizomib at all dose levels assessed. C-L and T-L activities were unchanged or increased in the first cycle of marizomib dosing, suggesting compensatory hyperactivation in response to effective blockade of CT-L activity. This response was overcome by further treatment with marizomib, with inhibition of T-L and C-L activity observed across dose levels with repeated dosing.
With respect to PK, marizomib exhibited a very short half-life which ranged across the study from 7.27 to 16.00 min. The short systemic half-life of marizomib could be due to extensive extrahepatic metabolism, instability at physiological pH, irreversible binding to the proteasomes, and/or partitioning to blood cells. Marizomib also exhibited a very high volume of distribution, indicating extensive distribution into peripheral tissues and/or binding to blood components, and very high clearance, much higher than the human liver blood flow (21 mL/min/kg or 1470 mL/min), indicating extensive extrahepatic metabolism. Taken together, these attributes obscure any dose-related exposure of marizomib over the relatively narrow dose range studied.
In summary, based on preclinical data, marizomib crosses the blood–brain barrier. The safety profile of marizomib, both as monotherapy and when combined with bevacizumab in patients with GBM, was consistent with the known safety profile of marizomib, indicating CNS penetration. PD data demonstrate sustained inhibition of proteolytic activity of all 3 subunits at all doses administered once weekly for 3 weeks in 28-day cycles. In this study in patients with recurrent GBM, neither marizomib monotherapy nor combination treatment with bevacizumab demonstrated notably increased efficacy compared with existing therapies.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and their families and caregivers for their participation in these studies, as well as the clinical research staff at the investigational sites for their expertise. The leadership of the Triphase Accelerator clinical research team is gratefully acknowledged, and in particular Amanda Brown for expert management of these clinical studies. Kaijun Di and Naomi Lomeli are acknowledged for providing their critical review of the manuscript.
The authors received editorial and writing support, provided by Miriam de Boeck, from Excerpta Medica, supported by Bristol Myers Squibb. The authors are fully responsible for all content and editorial decisions.
Conflict of interest statement. D.A.B: received grants from Triphase Accelerator; speaker’s bureau for Novocure. W.M.: consulting role for AbbVie; speaker’s bureau for Zai Lab. S.K.: institutional Principal Investigator for Triphase Accelerator. R.M.: no conflicts of interest to disclose. B.W.: former employee of Celgene Corporation and has Bristol Myers Squibb equity ownership. I.E.: employee of Bristol Myers Squibb and has equity ownership. S.D.R.: contractor for Triphase Accelerator. N.L., M.T.: employees of Triphase Accelerator. A.D.: received research funding from and serves on an Advisory Committee for Orbus Therapeutics; received research funding from Orbus Therapeutics, Symphogen A/S, Triphase Accelerator; holds stock or has other equity ownership in, holds a patent from, and serves on an Advisory Committee for Istari Oncology.
Authorship Statement. Conception and design: D.A.B., W.M., S.D.R., N.L., A.D.; collection and assembly of data: D.A.B., W.M., S.K., R.M., I.E., S.D.R., N.L., M.T., A.D.; data analysis and interpretation: all authors; manuscript writing: all authors; final approval of manuscript: all authors
Funding
This study was conducted by Triphase Accelerator and sponsored by Celgene, a Bristol-Myers Squibb Company, Summit, NJ, USA.
References
- 1. Geraldo LHM, Garcia C, da Fonseca ACC, et al. . Glioblastoma therapy in the age of molecular medicine. Trends Cancer. 2019; 5(1):46–65. [DOI] [PubMed] [Google Scholar]
- 2. Zanders ED, Svensson F, Bailey DS. Therapy for glioblastoma: is it working? Drug Discov Today. 2019; 24(5):1193–1201. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Wong ET, Kanner AA, et al. . NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012; 48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 5. Gallego O. Nonsurgical treatment of recurrent glioblastoma. Curr Oncol. 2015; 22(4):e273–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wick W, Gorlia T, Bendszus M, et al. . Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017; 377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 7. Field KM, Simes J, Nowak AK, et al. . Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015; 17(11):1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friedman HS, Prados MD, Wen PY, et al. . Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009; 27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 9. Taal W, Oosterkamp HM, Walenkamp AM, et al. . Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014; 15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 10. Gramatzki D, Roth P, Rushing EJ, et al. . Bevacizumab may improve quality of life, but not overall survival in glioblastoma: an epidemiological study. Ann Oncol. 2018; 29(6):1431–1436. [DOI] [PubMed] [Google Scholar]
- 11. Gilbert MR, Dignam JJ, Armstrong TS, et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014; 370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chinot OL, Wick W, Mason W, et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014; 370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 13. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 14. Malmström A, Grønberg BH, Marosi C, et al. . Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012; 13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 15. Stupp R, Hegi ME, Mason WP, et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 16. Weller M, Tabatabai G, Kästner B, et al. . MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res. 2015; 21(9):2057–2064. [DOI] [PubMed] [Google Scholar]
- 17. Wick W, Platten M, Meisner C, et al. . Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012; 13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 18. Chauhan D, Hideshima T, Anderson KC. A novel proteasome inhibitor NPI-0052 as an anticancer therapy. Br J Cancer. 2006; 95(8):961–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinospora. Angew Chem Int Ed Engl. 2003; 42(3):355–357. [DOI] [PubMed] [Google Scholar]
- 20. Kubiczkova L, Pour L, Sedlarikova L, Hajek R, Sevcikova S. Proteasome inhibitors - molecular basis and current perspectives in multiple myeloma. J Cell Mol Med. 2014; 18(6):947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Obaidat A, Weiss J, Wahlgren B, et al. . Proteasome regulator marizomib (NPI-0052) exhibits prolonged inhibition, attenuated efflux, and greater cytotoxicity than its reversible analogs. J Pharmacol Exp Ther. 2011; 337(2):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Potts BC, Albitar MX, Anderson KC, et al. . Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011; 11(3):254–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teicher BA, Tomaszewski JE. Proteasome inhibitors. Biochem Pharmacol. 2015; 96(1):1–9. [DOI] [PubMed] [Google Scholar]
- 24. Levin N, Spencer A, Harrison SJ, et al. . Marizomib irreversibly inhibits proteasome to overcome compensatory hyperactivation in multiple myeloma and solid tumour patients. Br J Haematol. 2016; 174(5):711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Di K, Lloyd GK, Abraham V, et al. . Marizomib activity as a single agent in malignant gliomas: ability to cross the blood-brain barrier. Neuro Oncol. 2016; 18(6):840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williamson MJ, Blank JL, Bruzzese FJ, et al. . Comparison of biochemical and biological effects of ML858 (salinosporamide A) and bortezomib. Mol Cancer Ther. 2006; 5(12):3052–3061. [DOI] [PubMed] [Google Scholar]
- 27. Yu LJ, Riordan B, Hatsis P, et al. . Study of brain and whole blood PK/PD of bortezomib in rat models [abstract]. J Clin Oncol. 2006; 24(suppl.):12036. [Google Scholar]
- 28. Demo SD, Kirk CJ, Aujay MA, et al. . Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007; 67(13):6383–6391. [DOI] [PubMed] [Google Scholar]
- 29. Harrison SJ, Mainwaring P, Price T, et al. . Phase I clinical trial of marizomib (NPI-0052) in patients with advanced malignancies including multiple myeloma: study NPI-0052-102 final results. Clin Cancer Res. 2016; 22(18):4559–4566. [DOI] [PubMed] [Google Scholar]
- 30. Richardson PG, Zimmerman TM, Hofmeister CC, et al. . Phase 1 study of marizomib in relapsed or relapsed and refractory multiple myeloma: NPI-0052-101 part 1. Blood. 2016; 127(22):2693–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bota DA, Alexandru D, Keir ST, Bigner D, Vredenburgh J, Friedman HS. Proteasome inhibition with bortezomib induces cell death in GBM stem-like cells and temozolomide-resistant glioma cell lines, but stimulates GBM stem-like cells’ VEGF production and angiogenesis. J Neurosurg. 2013; 119(6):1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989; 45(3):925–937. [PubMed] [Google Scholar]
- 33. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 34. Millward M, Price T, Townsend A, et al. . Phase 1 clinical trial of the novel proteasome inhibitor marizomib with the histone deacetylase inhibitor vorinostat in patients with melanoma, pancreatic and lung cancer based on in vitro assessments of the combination. Invest New Drugs. 2012; 30(6):2303–2317. [DOI] [PubMed] [Google Scholar]
- 35. Brandes AA, Finocchiaro G, Zagonel V, et al. . AVAREG: a phase II, randomized, noncomparative study of fotemustine or bevacizumab for patients with recurrent glioblastoma. Neuro Oncol. 2016; 18(9):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zikou A, Sioka C, Alexiou GA, Fotopoulos A, Voulgaris S, Argyropoulou MI. Radiation necrosis, pseudoprogression, pseudoresponse, and tumor recurrence: imaging challenges for the evaluation of treated gliomas. Contrast Media Mol Imaging. 2018; 2018:6828396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arevalo OD, Soto C, Rabiei P, et al. . Assessment of glioblastoma response in the era of bevacizumab: longstanding and emergent challenges in the imaging evaluation of pseudoresponse. Front Neurol. 2019; 10:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Auer TA, Breit HC, Marini F, et al. . Evaluation of the apparent diffusion coefficient in patients with recurrent glioblastoma under treatment with bevacizumab with radiographic pseudoresponse. J Neuroradiol. 2019; 46(1):36–43. [DOI] [PubMed] [Google Scholar]
- 39. Brandes AA, Finocchiaro G, Zagonel V, et al. . Early tumour shrinkage as a survival predictor in patients with recurrent glioblastoma treated with bevacizumab in the AVAREG randomized phase II study. Oncotarget. 2017; 8(33):55575–55581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. George E, Kijewski MF, Dubey S, et al. . Voxel-wise analysis of fluoroethyltyrosine PET and MRI in the assessment of recurrent glioblastoma during antiangiogenic therapy. AJR Am J Roentgenol. 2018; 211(6):1342–1347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.