Abstract
Infantile spasms (IS) syndrome is a catastrophic, epileptic encephalopathy of infancy that is often refractory to current antiepileptic therapies. The ketogenic diet (KD) has emerged as an alternative treatment for patients with medically intractable epilepsy, though the prospective validity and mechanism of action for IS remains largely unexplored. We investigated the KD’s efficacy as well as its mechanism of action in a rodent model of intractable IS. The spasms were induced using the triple-hit paradigm and the animals were then artificially reared and put on either the KD (4:1 fats: carbohydrate + protein) or a control milk diet (CM; 1.7:1). 31Phosphorus magnetic resonance spectroscopy (31P MRS) and head-out plethysmography were examined in conjunction with continuous video-EEG behavioural recordings in lesioned animals and sham-operated controls. The KD resulted in a peripheral ketosis observed both in the blood and urine. The KD led to a robust reduction in the frequency of spasms observed, with approximately a 1.5-fold increase in the rate of survival. Intriguingly, the KD resulted in an intracerebral acidosis as measured with 31P MRS. In addition, the respiratory profile of the lesioned rats on the KD was significantly altered with slower, deeper and longer breathing, resulting in decreased levels of expired CO2. Sodium bicarbonate supplementation, acting as a pH buffer, partially reversed the KD’s protective effects on spasm frequency. There were no differences in the mitochondrial respiratory profiles in the liver and brain frontal cortex measured between the groups, supporting the notion that the effects of the KD on breathing are not entirely due to changes in intermediary metabolism. Together, our results indicate that the KD produces its anticonvulsant effects through changes in respiration leading to intracerebral acidosis. These findings provide a novel understanding of the mechanisms underlying the anti-seizure effects of the KD in IS. Further research is required to determine whether the effects of the KD on breathing and intracerebral acid-base balance are seen in other paediatric models of epilepsy.
Keywords: acidosis, respiration, epilepsy, plethysmography, spectroscopy
See Auvin (https://doi.org/10.1093/braincomms/fcab234) for a scientific commentary on this article.
Infantile spasms is a debilitating epileptic disorder for which treatments are often ineffective. Choudhary et al. show strong pre-clinical evidence that the ketogenic diet is effective in the treatment of intractable infantile spasms and the mechanism of action for the ketogenic diet is mediated by breathing induced brain acidosis.
Graphical Abstract
Graphical Abstract.

Introduction
Infantile spasms (IS) syndrome is a devastating developmental epileptic encephalopathy of infancy that is characterized by frequent epileptic spasms and a disorganized interictal EEG pattern termed hypsarrhythmia, which collectively lead to developmental arrest or regression1–3 and mortality in up to 30% of cases.2–4 There is strong evidence indicating that prompt institution of successful treatments for the spasms and hypsarrhythmia improves outcome.5,6 Unfortunately, the two first-line treatments, which are either hormonal [adrenocorticotropic hormone (ACTH) or prednisolone] and/or vigabatrin are effective in only 50% of patients.7 A marginally greater response rate can be achieved in the short-term if ACTH and vigabatrin are given in combination,8 but with greater side effects.9 Aetiology is an important prognostic factor and patients with IS due to brain injury (previously termed symptomatic IS) have the worst prognosis.1,10,11
There is mounting clinical evidence that the ketogenic diet (KD), a high-fat, low-carbohydrate, normal protein diet, is an effective treatment for IS refractory to ACTH and vigabatrin. Several retrospective studies show that in intractable cases, control of IS (i.e. complete resolution of spasms and the hypsarrhythmic EEG) can be achieved in 40–50% of patients.12–18 One prospective randomized control study reported that the KD was equally as effective as ACTH in IS.19 The classical KD is often used with a ratio 3–4:1 (fats: carbohydrate + protein) which can be adjusted to improve seizure control and tolerability. Additionally, children with IS tend to tolerate the diet well with minimal side effects.18 Clinical improvements can be seen within days of starting the diet, although an effect on hypsarrhythmia can lag for several months.16 To our knowledge, there is no high-quality clinical evidence supporting the effectiveness of the KD in the treatment of IS.20 Thus, there is an urgent need for carefully designed pre-clinical studies to validate the effectiveness of the KD in the treatment of IS, hence providing the scientific rationale for future prospective clinical trials.
The mechanism(s) of action responsible for the KD’s effects on spasms and hypsarrhythmia in some patients with intractable IS is(are) unknown. Studies conducted mainly in adult rodent models of epilepsy suggest that there are multiple synergistic mechanisms that include the direct anticonvulsant effects of ketones bodies, GABAergic mechanisms, glycolytic restriction, mitochondrial ATP-sensitive potassium (KATP) channels, anti-seizure effects of adenosine, anti-oxidant activity, KD-induced changes in noradrenergic and serotonergic tone and anti-inflammatory effects of the diet.21 Maturational differences between the adult and immature brain that may impact the mechanism of action of the KD include the precocious development of excitatory neurotransmission, the delayed development of inhibition and the late maturation of endogenous systems involved in seizure control.22–24 The immature brain is also particularly susceptible to fluctuations in acid-base states and it is well recognized that acidosis has anticonvulsant effects whereas alkalosis has the opposite effect.25–30
Although acidosis is a common finding in patients on the KD31–33 and in rodents,34 it arguably does not lead to an intracerebral acidosis as one study conducted in normal adult rats using ex vivo spectrophotometric methods did not find evidence of intracerebral acidosis after 6 weeks on the diet.35 There are no additional studies looking at the effect of the KD on brain pH using modern in vivo methods such as 31Phosphorus magnetic resonance spectroscopy (31P MRS) in rodents or humans. Whether the KD results in an intracerebral acidosis in the immature epileptic and/or injured brain also has not been studied. Additionally, the observations that (i) some patients on the KD develop acidosis and others do not despite being on similar formulations and (ii) acidosis can fluctuate over time despite strict adherence to the KD suggest that other factors may be involved in the mechanism of action of KD-induced acidosis beyond primary metabolic effects. Acid-base regulation in mammals is controlled by the kidneys and the lungs36 and granted that the acidosis induced by the KD is likely primarily due to the metabolism of fats to acidic ketone bodies [β-hydroxybutyrate (BHB), acetoacetate and acetone],37 the possibility of a KD-induced effect on respiration as a contributing factor has not been determined.
In this study, we validated the effectiveness of the KD in the control of seizures and inter-ictal EEG abnormalities in the triple-hit model of IS.38 This model is a brain injury model of IS with seizures that do not respond to ACTH but partially to vigabatrin, therefore paralleling the population of patients with intractable IS we aim to address. By employing brain MRS and neonatal plethysmography, we show the mechanism of action of the KD in the injured epileptic brain involves KD-induced changes in respiration leading to intracerebral acidosis.
Materials and methods
Animals
Sprague Dawley females were bred in house, in full compliance with the institutional guidelines of the University of Calgary’s Health Sciences Animal Care Committee. The animals were housed at a constant temperature (20–22°C) on a 12-h light/dark cycle at the Health Sciences Animal Resource Centre, University of Calgary, Canada. The day of birth was considered as postnatal day (P) 0, and all experimental protocols were started at P4.
Induction of infantile spasms
The spasms were induced in the triple-hit model as previously described.38 Intracerebral injections of doxorubicin (DOX) and lipopolysaccharide (LPS) were administered stereotaxically at P4 (Fig. 1A). Animals were anaesthetized using whole-body hypothermia. The pups were given a subcutaneous injection of buprenorphine analgesic (0.03 mg/kg; CDMV Inc., Quebec, Canada) prior to surgery. A midline incision was made on the skin overlying the skull, and the pup was positioned between the ear bars of the stereotaxic frame (Leica Biosystems, Ontario, Canada). A Hamilton syringe was used to inject DOX (Sigma, MO, USA) into the right lateral ventricle (5 μg/2.5 μl) and LPS (Sigma, MO, USA) into the right parietal cortex (2 μg/1.5 μl) using the following coordinates: DOX = 2.68 mm anterior to lambda, 1.10 mm lateral to the sagittal suture, 3.30 mm deep; LPS = 2.55 mm anterior to lambda, 1.00 mm lateral to the sagittal suture, 1.70 mm deep. The skin incision was closed with VetBond (3M, MN, USA). The pups recovered under a heat lamp until the anaesthetic effect wore off and then were allowed to fully recover at room temperature. At P5, p-chlorophenylalanine (PCPA; 200 mg/kg; Sigma, MO, USA) was injected subcutaneously. Sham-operated control animals received the same volume of intracerebral injections of saline.
Figure 1.
The rat pups in the model of infantile spasms (IS) were successfully reared on the control milk diet (CM) and ketogenic diet (KD), using the pup-in-a-cup setup. (A) A representative schematic of the experimental protocols. (B) The growth trajectories of all groups were similar until P10, where control-KD (C-KD; n = 9) and lesioned-KD (L-KD; n = 9) animals had lower body weights compared to untreated controls (control-CM; C-CM; n = 8). Body weights were not different between lesioned-CM (L-CM; n = 8) and C-CM or between L-KD and C-KD. (C, D) The KD resulted in elevated BHB levels both in the blood (C) and urine (D) at P7 and P12. The data were analysed with two-way repeated-measures ANOVA followed by Holm-Sidak comparison and are presented as (B) mean ± SEM and (C, D) box and whisker plots (median with 5th and 95th percentile). *P < 0.05, **P < 0.01, ***P < 0.001. DOX, doxorubicin; LPS, lipopolysaccharide; MRS, magnetic resonance spectroscopy; PCPA, p-chlorophenylalanine.
Artificial rearing
The pups were artificially reared using the ‘pup-in-cup’ setup as described previously.39,40 A cheek cannula was inserted on P4 immediately after the intracerebral injections, while the pups were still under hypothermic anaesthesia. A lead metal wire covered with polyethylene 10 tubing was used to pierce the cheek and was reinforced with washers and glued into place. The pups were then transferred individually to large styrofoam cups filled with bedding, and the cups placed in a heated water bath maintained at 43–45°C. The artificial rearing protocol was started immediately after surgery on P4. The pups were connected through the cheek cannulas to a time-controlled infusion pump (KD Scientific Inc., MA, USA), and the milk diets were delivered continuously. The pups were fed 336 kcal/100 ml of either a KD (4:1, fats: carbohydrate + protein) or an isocaloric control milk diet (CM) (1.7:1, Table 1).40 The KD was created based on the KetoCal© formulation used in humans. The diet’s infusion rate was calculated daily based on the average body weight of the groups. At P4, animals fed the CM were started at a volume 35% of their mean body weight and the KD animals were fed a volume 41% of their mean body weight over 24 h. This was done to aim for comparable growth between the groups. The volume of the milk was then increased by 2% each day. The pups were housed in the cups without access to their mothers for the entirety of the experiment.
Table 1.
Artificial rearing milk diet compositions
| Control milk diet |
Ketogenic diet |
||
|---|---|---|---|
| Calorie: 336 kcal/100 ml; Ratio: 1.7:1 | Calorie: 336 kcal/100 ml; Ratio: 4:1 | ||
| 47 g | Evaporated milk, whole milk—carnation | 10.1 g | Milk, evaporated, whole milk—carnation |
| 3.9 g | Whey protein—progressive organics | 6.6 g | Whey protein—progressive organics |
| 4 g | KetoCal 4:1 powder—CANDADIAN VIEW | 3.6 g | KetoCal 4:1 powder—CANDADIAN VIEW |
| 23 g | Corn oil—Mazola | 30.2 g | Corn oil—Mazola |
| 5.6 g | SolCarb | ||
The pups were subsequently divided into four experimental groups as follows: sham-operated controls treated with CM or the KD (C-CM and C-KD, respectively) and lesioned rats given either the control milk (L-CM) or ketogenic diet (L-KD). Blood and urine BHB levels were measured using Freestyle Precision β-ketone strips (Abbott Laboratories, Quebec, Canada) before the start of the artificial rearing protocol at P4, P7 and P12.
To test the effect of sodium bicarbonate supplementation on the spasm suppressing effect of the KD we gave a single-dose oral gavage NaHCO3 6 mEq per kilogram in 100 µl of saline at P5 in the AM. For this part of the study, behavioural analysis was performed at P7.
Behavioural assessment of spasm frequency
The behaviour of the pups was video-recorded continuously from P4 (post-surgery) until the end of the experiments at P12, as spasms do not occur beyond this time-point in this model. The videos were analysed for evidence of epileptic spasms, and the spasms were scored for hourly frequency for 2 h every 4 h. Epileptic spasms included flexor and extensor types.38 A flexor spasm was recognized as the sudden and simultaneous flexion of the head and the trunk, while the limbs were extended. An extensor spasm was characterized as exaggerated extension of the animal’s body. The spasms were analysed by two separate reviewers and the rate of inter-rater reliability was determined.41
Neurodevelopmental assessment
Tracked open field activity (OFA) was conducted on P10 to assess locomotion. To analyse OFA, the animal was placed in the centre of a box above a camera equipped with a tracking system (TopScan tracking, VA, USA) and was allowed to move freely for 5 min, and animal movements were quantified as previously described.42 Ultrasonic vocalizations (USV; Avisoft Bioacoustics, Glienicke/Nordbahn, Germany) were recorded on P7 and P11 to assess the effects of the treatments on communication. The animals were placed in a chamber for 5 min and rate of calls were recorded and quantified as previously described.43 We also analysed the effects of the KD on mortality using Kaplan-Meier Survival analysis.
Assessment of inter-ictal EEG abnormalities with continuous EEG and EMG
In a separate study, continuous video-EEG was conducted for 8 days to quantify and compare inter-ictal epileptiform activity. The EEG recordings were not used for the quantification of behavioural-electrographic spasms. P4 animals were anaesthetized using whole-body hypothermia and placed within a stereotaxic frame for neonatal rat surgery. A circuit headmount configured to record both electroencephalogram (EEG) and electromyogram (EMG) signals were affixed onto the animal’s skull using VetBond and four stainless steel screws (#8021 3EEG/1EMG Headmounts, Pinnacle Technology, KS, USA). Epoxy resin was applied to the top of the EEG screws to enhance electrical conductivity. Once in place, dental cement was applied to the ensure the screws and electrodes were held firmly in situ. The two EMG wire electrodes were inserted into the trapezius muscles. The animals were given 24 h to recover post-operatively, before the headmounts were connected, and continuous EEG and EMG recordings initiated. The EEG recordings were obtained with a sampling rate of 2000 Hz. EEG recordings were analysed using an in-house MATLAB code for the analysis of inter-ictal spikes. Spikes were detected if the signal was 3 SD from the background noise, with no activity in the EMG channels—thus accounting for any movement artefact. The spikes were then manually assessed by a blinded reviewer to account for false positives.
Magnetic resonance spectroscopy
To assess for intracerebral acidosis, non-localized 31P MRS was performed at P7. The animals were anaesthetized with 2% isoflurane in 100% oxygen and placed onto the MRI cradle and secured to a plexiglass place, ear bar and nose clamp. MRS was performed using a 9.4 T Oxford magnet with Bruker AM400 spectrometer, a single 13 mm surface coil tuned to P (162.12 MHz), spectral width = 12 500 Hz, pulse width = 6 µs, inter-pulse delay = 2 s and the number of scans = 2048; modified from Tracey et al.44 pH was calculated using the equation pH = 6.75 + log[(S-3.27)/(5.69-S)], where S is the chemical shift between Pi and PCr.44 The signal processing was done using the AMARES quantification algorithm in jMRUI (http://www.jmrui.eu/ Accessed 26 August 2021).45
Head-out plethysmography
To examine the effects of KD on respiration, head-out plethysmography was conducted.25 The pups were placed in the plethysmograph and allowed to acclimate for 20 min at 30°C, while room air was pulled through the head chamber at a rate of ∼400 ml/min by a downstream pump (PP-2, Sable Systems International Inc., NV, USA) and was sampled by a CO2 analyzer (Oxzilla II, CA-2A; Sable Systems International Inc., NV, USA). Following acclimatization, the pup’s respiration was recorded for 5 min. Respiratory signals were amplified (Amplifier 440, Brownlee Precision Co., CA, USA) and recorded using the Axon data acquisition system (Digidata 1322A, Axon Instruments Inc., CA, USA), and the data analysed with LabChart8 Reader (AD Instruments Inc., Colorado Springs, CO, USA). The respiratory rate (RR; breaths/min), tidal volume (VT; ml/g), minute ventilation (VE•; ml/min/g), rate of expired CO2 (V•CO2; ml/min/g), duration of breath (TTot) and VE•/V•CO2 were calculated.
Mitochondrial respirometry
Mitochondrial respirometry46 was performed on tissue obtained from the liver and prefrontal cortex using an Oroboros Oxygraph-2K (Oroboros Instruments, Innsbruck, Austria). On P7, the animals were sacrificed, and liver and frontal cortex tissue was dissected and weighed to 2 mg. The tissue was then transferred to the oxygraph chambers containing Miro5 as a respiratory medium, and the oxygen concentrations were recorded using DatLab.46 The tissues were permeabilized with 50 µg ml−1 saponin. The substrate-inhibitor-uncoupled titration protocols were employed as follow: 5 mM Pyruvate, 0.5 mM malate, 10 mM glutamate, 2.5 mM ADP (complex I respiration), 5 μM oligomycin (ATP synthase inhibitor), 0.5 mM FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone), 0.05 µM rotenone (complex I inhibitor, to prevent electron backflow) and 2.5 µM antimycin A (complex III inhibitor). FCCP was used as a respiration uncoupler to obtain maximal respiratory activity. The ratios of basal respiration, maximal mitochondrial respiration, ATP-linked mitochondrial respiration and total mitochondrial respiration were calculated as previously described.46 Oxygen concentration (μM) as well as oxygen flux per tissue mass (pmol O2.s−1·mg−1) were recorded in real-time using DatLab software (Oroboros Instruments, Innsbruck, Austria).
Histology
The pups were euthanized with ketamine/xylazine (100 mg/kg, i.m.; CDMV Inc., Quebec, Canada) at P12 and perfused transcardially using 1× phosphate buffered saline followed by 4% paraformaldehyde. The brains were harvested, cyrosectioned (20 µm) and stained using the haematoxylin and eosin staining protocol to evaluate the extent of lesions induced in the model. Using the ImageJ programme, the brain hemispheres were traced and separately outlined using a drawing pad. The cross-sectional areas of the two regions were then calculated based on the tracings and magnification factor as previously described and the inter-hemispheric ratios (injected/non-injected hemispheres) were compared between the groups.47
Statistical analysis
As we did not observe any sex-specific differences between the groups the results of male and female animals were pooled. The data are expressed as mean ± standard error of the mean or as box and whisker plots (median with 5th and 95th percentile). Two-way repeated measures ANOVA followed by Holm-Sidak posthoc comparisons were made when evaluating differences in body weight, blood and urine BHB levels, spasm frequency, interictal spikes and ultrasonic vocalizations. One-way ANOVA was conducted to assess the extent of brain lesions, OFA, mitochondrial respiration, head-out plethysmography data and MRS results. The inter-rate reliability of spasm frequency was calculated using the Kappa statistic. Kaplan-Meier survival analysis was conducted to analyse the animals’ survival rates. Differences between groups were considered significant at P < 0.05 and a power of 95%. All statistical analyses were completed using SigmaPlot v14.0 (Systat Software Inc., CA, USA).
Data availability
The data that support the findings of this study are available on request from the corresponding author, upon reasonable request.
Results
The KD reduces growth rate and causes sustained ketosis
There were no differences in the weight between groups at baseline (P4) (Fig. 1B). All animals tolerated the diet showing significant increases in body weight over time. Growth rates were comparable between the groups until P10, where the growth trajectory changed, with KD-treated animals growing at a slower rate compared to animals fed the CM diet (P < 0.001; Fig. 1B). Brain lesions did not impair growth in either CM- or KD-treated animals (Fig. 1B). The BHB levels were similar between all groups at baseline (Fig. 1C and D). The KD induced a significant ketosis, as indicated by the higher BHB levels both in the blood (P < 0.001 and P = 0.014; Fig. 1C) and urine (P = 0.011 and P < 0.001; Fig. 1D) of KD-treated animals at P7 and P12 compared to CM-treated animals.
The KD reduces spasm frequency, interictal EEG abnormalities, mortality and lesion size
Behavioural spasms were not observed in control animals (C-CM and C-KD) at any age (Fig. 2A). In L-CM and L-KD animals, behavioural spasms were manifested promptly after inducing the lesions at P4. Spasm frequency was highest in L-CM animals at P5 and decreased gradually to control levels by P12. The KD significantly reduced the spasm frequency in the lesioned animals between P5 and P10, with a maximal 56% reduction at P6 compared to L-CM (P < 0.001; Fig. 2A). Behavioural spasms were abolished two days earlier in L-KD animals at P10 compared to L-CM animals (P = 0.026; Fig. 2A). The spasm frequency counts were standardized between two reviewers with an inter-rater reliability rate of approximately 94%.
Figure 2.
The ketogenic diet (KD) reduced behavioural spasms and interictal spikes in lesioned rats. (A) Lesioned-KD (L-KD; n = 9) rats had significantly lower levels of behavioural spasms from P5 to P12 compared to lesioned rats fed the control milk diet (L-CM; n = 9). Behavioural spasms in L-KD rats were abolished by P10, while spasms in L-CM rats were not abolished until P12. Behavioural spasms were not observed in control-CM (C-CM; n = 9) or control-KD (C-KD; n = 9) rats. (B) EEG activity was recorded in a subset of animals. Interictal spike frequency was highest in L-CM rats (n = 5) throughout the recording period, but the KD significantly reduced this lesion-induced increase in spike frequency in L-KD rats (n = 5) from P5 to P12. The spike frequency decreased sharply from P5 to P6 in both C-KD (n = 4) and C-CM (n = 4) rats and was sustained at this low level for the remainder of the experimental period with a transient increase in spiking activity in C-CM rats (n = 4) at P9 and P10. (C) A representative example from the spike detection software showing 3 SD threshold from the background signal for spike detection. Each circle indicates the identification of one spike on the raw EEG signal. (A, B) The data were analysed with two-way repeated measures ANOVA followed by Holm-Sidak comparison. The data are presented as mean ± SEM. *P < 0.05, ***P < 0.001.
Interictal EEG was recorded in a subset of animals from all groups. Inter-ictal spikes were evident from the first day of these recordings in all animals; however, the interictal spike frequency was highest in L-CM (P < 0.001) and L-KD (P < 0.001) animals compared to controls (Fig. 2B). The spiking activity in L-CM animals was sustained at this higher frequency throughout the entire recording period from P5-P12 (P < 0.001). The KD was effective in gradually reducing this interictal spiking abnormality in lesioned animals such that the spike frequency in L-KD animals was comparable to that of controls by P12 (Fig. 2B). Elevated spike frequency observed in the C-CM and C-KD control rats at P5 dropped sharply at P6 (both P < 0.001) and was maintained for the remainder of the duration of the experiment. In the C-CM controls, there was a transient increase in spike frequency at P9 and P10 (P < 0.001) that was quickly resolved (Fig. 2B). A representative example of the interictal EEG recording from an L-CM animal is shown in Fig. 2C.
Intracerebral injections of the drugs used to induce IS in the model resulted in a diffuse right brain hemisphere lesion, ipsilateral to the injection site (Fig. 3A) that led to a reduction in the area of the right hemisphere, such that the inter-hemispheric ratio in lesioned animals was significantly lower than that in sham-operated controls (P < 0.001; Fig. 3B). The KD was effective in reducing the severity of the lesion, as indicated by the larger inter-hemispheric ratio in L-KD animals compared to L-CM (P < 0.001; Fig. 3B). KD’s neuroprotective effects were similarly observed in L-KD animals supplemented with sodium bicarbonate (L-KD-HCO3), as both L-KD and L-KD-HCO3 animals displayed a similar inter-hemispheric ratio. L-KD-HCO3 animals had a greater inter-hemispheric ratio compared to L-CM animals (P < 0.001; Fig 3B). Intracerebral injections of the vehicle did not impact the size of the brain hemispheres in sham-operated control animals such that no significant difference in the inter-hemispheric ratio was observed between these control animals (Fig. 3B). These findings indicate the neuroprotective effect of the KD in reducing lesion size.
Figure 3.
The ketogenic diet (KD) has neuroprotective effects and is associated with increased survival. (A) A representative image of a 20 µm coronal section of the brain from a lesioned rat fed the control milk diet- (L-CM; upper image) and a lesioned rat fed the KD (L-KD; lower image) rat depicting the lesion induced on the right hemisphere of the brain in the model of IS. (R) = right brain hemisphere; (L) = left brain hemisphere. Scale bar = 1500 µm. (B) The right/left brain hemispheric ratio was not different between the control-CM (C-CM; n = 7) and control-KD (C-KD; n = 8) rats but was significantly reduced in the lesioned rats. The KD significantly increased the interhemispheric ratio in L-KD (n = 9) compared to L-CM (n = 9) rats. There was no difference in the interhemispheric ratio between L-KD and lesioned rats on the KD supplemented with sodium bicarbonate (L-KD-HCO3; n = 9). L-KD-HCO3 rats also displayed an increased interhemispheric ratio compared to L-CM. (C) The survival rate was lowest in lesioned rats, but KD treatment significantly prolonged survival in these animals. The data were analysed using one-way ANOVA followed by Holm-Sidak comparison (B), and Kaplan-Meier survival analysis (C). (B) The data are presented as box and whisker plots (median with 5th and 95th percentile). *P < 0.05, ***P < 0.001.
Mortality of L-CM animals was 43% by the endpoint of the experiment, with animals expiring as early as 2 days following induction of IS (P < 0.001; Fig. 3C). The KD significantly improved survival in these lesioned animals from 57% in L-CM rats to 82% in L-KD animals (P < 0.001). Furthermore, the onset of mortality was delayed in L-KD animals, as the first death was not observed until P10 versus P6 in L-CM.
The KD improves neurodevelopmental outcomes
The neurodevelopmental profiles of the lesioned and sham-operated control animals were determined by assessing tracked OFA at P10 and by quantifying ultrasonic vocalizations at P7 and P11. There were no differences in the distance travelled in the tracked OFA between sham-operated control animals (C-CM and C-KD) (Fig. 4A and B). There was a significant decrease in the distance travelled by lesioned animals fed the control diet (L-CM; P < 0.001) which was completely reversed by the KD (P < 0.001; Fig. 4A and B). Similarly, analysis of ultrasonic vocalizations as depicted in the representative tracing (Fig. 4C) showed no differences in the number of vocalizations recorded at P7 and P11 in the sham-operated control groups (Fig. 4D). However, in L-CM animals, there was a significant reduction in the number of calls both at P7 and P11 (P < 0.001), which was also completely reversed by the KD (P = 0.007 at P7 and P < 0.001 at P11; Fig. 4D). Note that ultrasonic vocalizations decreased with age in all groups, but the magnitude of this decrease was smaller in lesioned animals fed the CM (P < 0.001; Fig. 4D).
Figure 4.
The ketogenic diet (KD) improved the neurodevelopmental profile in lesioned rats. (A) Representative tracings of the tracked open field activity of lesioned and sham-operated control rats at postnatal day (P) 10. (B) The total distance travelled in the tracked open field activity was lowest in lesioned rats given the control milk-diet (L-CM; n = 8). The KD treatment significantly improved motor activity in lesioned rats (L-KD; n = 8) compared to control-CM (C-CM; n = 6) and control-KD (C-KD). Motor activity was not different between C-CM and C-KD (n = 6). (C) An ultrasonic vocalization (USV) schematic illustrating the detection of ultrasonic syllables (I) and (II) based on a set detection threshold. X-axis = time; Y-axis = frequency. (D) USV calls were significantly reduced in L-CM rats compared to all other groups at P7 and P11. The data were analysed using one-way ANOVA (B) and two-way repeated measures ANOVA (D) followed by Holm-Sidak comparison. The data are presented as box and whisker plots (median with 5th and 95th percentile). **P < 0.01, ***P < 0.001.
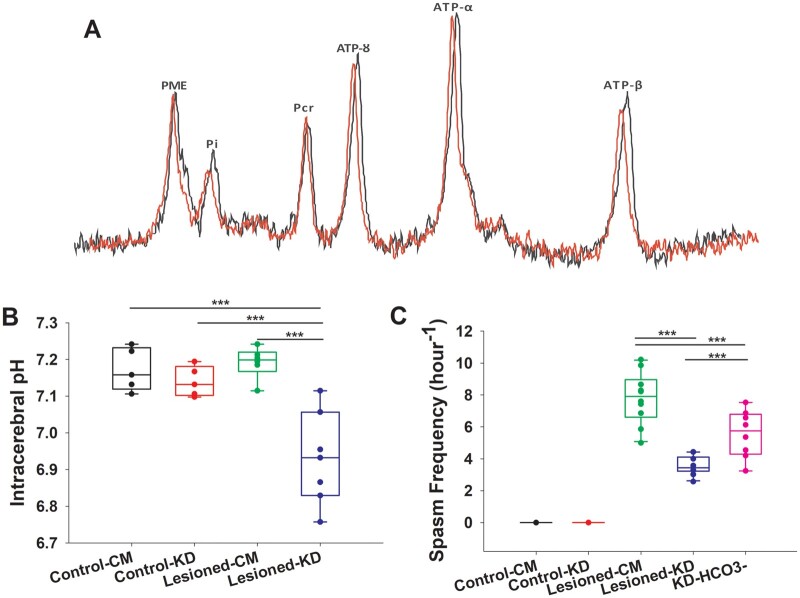
The KD in lesioned rats resulted in changes in breathing leading to an intracerebral acidosis
Intracerebral pH was measured using 31P MRS (Fig. 5A). There were no differences in the brain pH between sham-operated controls and L-CM animals (Fig. 5B). The KD significantly reduced the intracerebral pH from 7.2 ± 0.02 in L-CM animals to 6.9 ± 0.04 in L-KD animals (P < 0.001; Fig. 5B). Interestingly, treating L-KD animals with bicarbonate significantly attenuated the spasm-suppressing effects of the KD (P < 0.001; Fig. 5C).
Figure 5.
The ketogenic diet (KD) induced intracerebral acidosis which had anticonvulsant effects. (A) Representative image of the 31P magnetic resonance spectroscopy (MRS) peaks obtained from postnatal day 7 lesioned rats on the control milk diet (L-CM; red) and lesioned rats on the KD (L-KD; black) rats. Note the chemical shift in inorganic phosphate (Pi) in the L-KD animal. (B) Intracerebral pH, as determined from the chemical shift in (Pi) and phosphocreatine (PCr) peaks on the 31P MRS, was lower in L-KD (n = 7) rats compared to L-CM (n = 6), control-CM (n = 5) and control-KD (n = 5) animals. (C) Pre-treatment of lesioned rats exposed to the KD (L-KD) with sodium bicarbonate (n = 8) to reverse intracerebral acidosis, resulted in a higher spasm frequency compared to L-KD, but lower spasm frequency compared to L-CM. The data were analysed using one-way ANOVA followed by Holm-Sidak comparison. The data are presented as box and whisker plots (median with 5th and 95th percentile). ***P < 0.001.
Plethysmography was conducted to determine whether the KD-induced breathing abnormalities in lesioned animals may have contributed to the intracerebral acidosis observed with 31P MRS. There were no differences between the sham-operated control groups and lesioned animals with respect to any respiratory variables (Fig. 6A–G), excepting a lower TTot in L-CM animals (Fig. 6D). Compared to lesioned animals fed the CM, L-KD animals had a slower RR (P = 0.012; Fig. 6B), but higher tidal volume (P = 0.021; Fig. 6C) and TTot (P < 0.001; Fig. 6D), which resulted in no differences in minute ventilation (Fig. 6E). However, the V•CO2 was significantly reduced in L-KD animals (P < 0.001; Fig. 6F) which resulted in a higher VE•/V•CO2 (P = 0.014; Fig. 6G), indicating a mismatch between ventilation and metabolism in these animals. Bioenergetic studies in the liver and the brain frontal cortex revealed no differences between the groups, supporting our hypothesis that KD-induced changes in respiration is not directly related to changes in mitochondrial metabolism (Fig. 7A–I).
Figure 6.
The ketogenic diet (KD) altered the respiratory profile of lesioned rats. (A) Representative breathing tracings for lesioned rats fed the control milk diet- (L-CM) and KD (L-KD) at postnatal day 7. Compared L-CM (n = 8), L-KD (n = 9) rats displayed a (B) lower respiration rate (RR), (C) higher tidal volume (VT), (D) an increase in total duration of breath (TTot), (E) no changes in minute ventilation (F) a decreased level of expired CO2 (VCO2), (G) increase in ventilatory efficiency slope (VE/VCO2). The increase in TTot (D) and decrease in VCO2 (F) in L-KD rats was also observed compared to the controls. No ventilatory changes were observed in control-CM (n = 8) and control-KD (n = 7) animals. The data were analysed using one-way ANOVA followed by Holm-Sidak comparison. The data are presented as box and whisker plots (median with 5th and 95th percentile). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 7.
The ketogenic diet (KD) had no effect on mitochondrial respiration in the liver and the frontal cortex. (A) A schematic of the mitochondrial respiration protocol. No differences were observed in basal respiration, total mitochondrial respiration, maximal respiration or ATP-linked mitochondrial respiration in the liver (B-E) or frontal cortex (F-I) between all control and treatment groups. Control-Control milk diet (C-CM; n = 6), control-KD (C-KD; n = 6); lesioned-CM (L-CM; n = 7); lesioned-KD (L-KD; n = 7). The data were analysed using one-way ANOVA followed by Holm-Sidak comparison. The data are presented as box and whisker plots (median with 5th and 95th percentile).
Discussion
The major findings of this study are that, in the triple-hit model of IS, the KD significantly reduced the frequency of epileptic spasms and interictal epileptiform abnormalities that were associated with improved neurodevelopmental outcomes and decreased lesion size and mortality. Mechanistically, the KD in lesioned rats resulted in changes in breathing which in turn led to an intracerebral acidosis, and sodium bicarbonate which was given to reverse the acidosis significantly attenuated the KD-induced anti-seizure effects. To our knowledge, this is the first study to demonstrate that the anti-seizure and neuroprotective effects of the KD in a paediatric model of epilepsy are linked to an increased intracerebral acidosis.
This study was performed using the novel ‘pup-in-a-cup’ setup allowing for 24-h behavioural and video-EEG recordings in neonatal animals. In support of the retrospective clinical literature, we observed a strong effect of the KD in reducing spasm frequency and interictal spike-wave activity in the model of IS that does not respond to ACTH and is only partially responsive to vigabatrin. The interictal spikes observed in non-lesioned rats receiving either the KD or CM may be due to the placement of EEG electrodes48 or immaturity of the brain.49 The improvements in neurodevelopmental outcomes and mortality are most likely due to the KD-induced reduction in seizures, interictal spiking activity, and lesion size. Of note, KD’s anti-seizure effects were attenuated in lesioned rats supplemented with sodium bicarbonate, a pH buffer, without a reduction in lesion severity. This supports that the beneficial effects of the KD on seizures, interictal spiking activity, behaviour and mortality are not exclusively due to the neuroprotective effects of the KD; but rather due to KD’s ability to induce an intracerebral acidosis in the model (discussed below). These findings provide strong support for the use of the KD in the population of patients resistant to first-line treatments.
Interestingly, natural rat breast milk has a high concentration of fats early in the neonatal period and therefore should theoretically induce anti-seizure effects. However, a comparable seizure frequency and rate of mortality are seen between lesioned animals receiving the control diet in the pup-in-a-cup setup and rats with spasms who are returned to the dam as reported in prior studies.38,50 The reason for this is because in actuality, the fat content in rats milk is highly variable early in life, declining for a period as lactation progresses.39,51,52 For example, a detailed study has shown that between P0-P6 a ratio of 4–5:1 (crudely calculated) is seen which falls rapidly, reaching a nadir of 1–2:1 by P10–12.51 The ratio then slowly rises to 2–3:1 around P18 but never achieves the height of the early postnatal period. Plasma ketone levels in naturally-reared neonatal rats are similarly elevated at birth (2.5 mM) but rapidly fall to between 1 and 1.5 mM at P7 (which is the time of a high expression of spasms in the present model) with a more gradual decline to 0.5 mM by the time of weaning.53 In our study, we observed higher sustained BHB levels in the KD-treated rats with an average of 2.5 mmol/L at P7. The elevated BHB levels were successfully sustained throughout the entirety of the experiment because of the tightly controlled diet provided through the pup-in-a-cup setup. We did not compare the frequency of spasms between naturally-reared lesioned pups and those reared artificially as the results would be confounded by factors inherent to the pup-in-a-cup setup such as stress.54
As previously mentioned, the mechanism(s) of action of the KD to control spasms and improve outcomes in the model is(are) likely multifactorial.21 The novelty of this study is our observation of intracerebral acidosis, as measured with 31P MRS, in lesioned pups treated with the KD and that treating these pups with bicarbonate reversed the KD-induced anti-seizure effects. 31P MRS was not conducted in L-KD pups treated with bicarbonate and remains a limitation of the study. Nevertheless, these results strongly implicate intracerebral acidosis as a central mechanism of action for the KD, specifically in the immature epileptic brain. We did not observe an intracerebral acidosis in control pups treated with the KD which is in line with previously reported studies in adult animals using ex vivo spectrophotometric methods.35 The reason for the finding of intracerebral acidosis in the L-KD group is likely because these animals had an immature and injured epileptic brain. Another possible reason for the differences may be related to the duration of exposure (6 weeks versus 3 days) and the short duration of exposure to the KD in the triple-hit model may provide insufficient time for potential compensatory mechanisms to take effect. Further studies are required in older animals in the triple-hit model to determine the longevity of the effect of the KD on brain pH. Nevertheless, the short-term effects of the KD to potentially lead to an intracerebral acidosis is clinically important for patients with IS. Studies indicate that if the spasms and hypsarrhythmia are not controlled in 2–3 weeks the chances of a poor outcome increases.5,6 Spasms in IS are often controlled within 2 weeks of starting the diet–which may be related to intracerebral acidosis as suggested by our findings.14 Studies in normal juvenile mice indicate that the minimum duration of treatment to produce an anticonvulsant effect is as little as 3 days.55 It also must be emphasized that in the clinical setting KD-induced acidosis, is often aggressively treated with bicarbonate and based on our findings, treating the acidosis may potentially reverse the anti-seizure effects of the KD which would result in a worse prognosis.
The mechanisms that underlie the anticonvulsant effects of acidosis are incompletely understood. The two principal mechanisms are believed to entail the modulation of acid sensing ion channels (ASICs) and/or gap junctions. ASICs are pH-sensitive, sodium-selective, ion channels that are widely expressed in the brain in both neurons and astrocytes and have been implicated in multiple pathologic disease states including epilepsy.56 In one study, acidosis-induced activation of ASICs in inhibitory interneurons, has been shown to be an important mechanism for the endogenous control of seizures.57 ASICs expressed in inhibitory interneurons have higher current densities than in excitatory glutamatergic neurons which may explain the anti-seizure effects induced by low pH.58 One study has shown that inhibitory interneurons in the triple-hit model are significantly reduced in the cerebral cortex.59,60 We speculate that the KD may increase their expression through the multiple mechanisms accounting for its neuroprotective effects. In contrast, activation of ASICs in astrocytes leads to proconvulsant effects,60 which potentially can be mitigated by the anti-inflammatory effects of the KD.21 The immature brain also has a high expression of gap junctions which when coupled allows for the direct electrical communication between neurons.61 Acidosis is a potent uncoupler of gap junctions which may be another mechanism of action of the KD.62
Little is known regarding the effect of the KD on respiration in rodents or humans under basal conditions. In one study, a high-fat diet fed to naïve adult rats for 3 weeks led to an increased RR, and VE•but not VT.63 However, a high-fat diet fed to adult rats for 12 weeks induced no changes in respiratory parameters measured at the end of the study.64 Note that although these diets are high in fat, the ratios ranged from 0.21 to 0.71:1 (fats: carbohydrates + protein) and therefore are likely not ketogenic per se. Notwithstanding these considerations, the results of these studies indicate a short-term effect of a high-fat diet on respiration. A report in prepubescent rats using calorimetry has shown that the KD results in an increase in total body energy expenditure that is associated with an increase V˙o2 and decreased V•CO2 and heat.65 The authors concluded that the KD induced a moderate ‘uncoupling state’ and less oxidative efficiency compared to glucose oxidation. However, the full spectrum of the effects of the KD on respiratory parameters was not reported in this study. To our knowledge, there are no published studies looking at the effect of the KD on respiration in neonatal animals.
In humans, one study in adults has shown that a 20-day exposure to the KD resulted in a decrease in (i) patient carbon dioxide end-tidal partial pressure and (ii) respiratory exchange ratio, indicating hypoventilation and/or the metabolism of fats as opposed to glucose as an energy source.66 In another study, patients with chronic obstructive pulmonary disease treated with the KD for 3 weeks showed a decreased VE• and consequent V•CO2indicating KD-induced changes in respiration and that these changes were independent of metabolism.67 Adult patients exposed to low glycogen states, as would occur in patients on the KD, showed a decreased VE• and V•CO2accompanied by increased VE•/V•CO2, the latter indicating a mismatch between ventilation and metabolism.68 We have also observed the same respiratory pattern in spasms lesioned rats exposed to the KD. Together, these results strongly suggest a direct effect of the KD on respiration independent of its effect on metabolism, thereby leading to an intracerebral acidosis. The breathing pattern observed in lesioned animals treated with the KD is reminiscent of Kussmaul breathing which is thought to occur in association with severe metabolic acidosis. However, given that sham-operated control animals treated with the KD did not have an intracerebral acidosis, it is unlikely that animals treated with the KD had a primary metabolic acidosis accounting for the changes in breathing. The question arises as to whether the changes in breathing would increase the risk of sudden unexpected death in epilepsy. This is unlikely as there is no differences in VE• between the groups indicating that ventilation in animals with spasms exposed to the KD is adequate though different from controls and untreated lesioned animals. The improved mortality in rats exposed to the KD is supportive of this argument.
The cause of the breathing changes seen in lesioned rats exposed to the KD is currently unknown. It is appealing to speculate that the effect of the KD in our study was due to low whole-body glycogen stores but this is unlikely given that the KD had no effect on the weight at P7 (age of maximal effectiveness of the KD) and breathing in control animals. This was similarly concluded in the adult study by Sabapathy et al.68 mentioned above. Although several studies in older animals have shown that the KD improves mitochondrial respiration in the hippocampus,69 we did not observe an effect of the KD on mitochondrial respiration in the liver and frontal cortex between the groups. The reason for these differences is unknown but the effects of the KD on mitochondrial respiration may have differential regional and possibly age-specific effects. Nevertheless, this finding further supports a direct effect of the KD on breathing.
How then does the KD act to induce changes in breathing in the model? Leptin, a hormone produced by white adipocytes, has been shown to stimulate breathing in rats through activation of its receptor localized within the hypothalamus and hindbrain structures.70 Interestingly, functional and structural hypothalamic and hindbrain abnormalities are a prominent feature in patients with IS71 and in the triple-hit model.38 The KD has been shown to reduce leptin levels,72 which we hypothesize, in the presence of hind-brain abnormalities, may result in changes in breathing leading to an intracerebral acidosis (at least in the short-term) in the presence of keto-acids produced by the metabolism of the KD due to the inability to activate respiratory compensatory mechanisms. Our hypothesis may also explain why some patients develop acidosis following treatment with the KD and others do not although receiving similar formulations. However, whether patients with IS on the KD exhibit respiratory abnormalities and consequently are at an increased risk of acidosis stratified by aetiology has not been studied. Finally, as the KD has recently been shown to alter the gut microbiome, the effect on breathing may be an indirect consequence of either metabolic and/or inflammatory changes modulated by the gut flora.73
Conclusions
Our findings indicate that the anti-seizure effects of the KD in the triple-hit model of IS is in part mediated through intracerebral acidosis, which may arise via KD-induced effects on respiration. This finding takes us one step closer to understanding the mechanism of action of the KD in the paediatric population with intractable epilepsy. A more detailed mechanistic understanding of the KD is a prerequisite for optimizing treatments and dietary formulations for the control of spasms in clinical practice. Further research is required to determine whether this effect of the KD on breathing and intracerebral acid-base balance is seen in other paediatric models of epilepsy, especially those due to brain injury and humans with symptomatic IS treated with the diet.
Acknowledgements
We would like to thank Mr Mitchell Kesler for granting access to his custom proprietary inter-ictal spike detection software. We would also like to thank Mr Tadeusz Foniok, Mr David Rushforth and Dr Jeffery Dunn from the Experimental imaging centre, University of Calgary for their assistance with data acquisition for the 31P MRS study. We would also like to thank Nutricia for the generous gift of the Ketocal formula.
Funding
This work was supported by the Alberta Innovates Health Solutions (to A.C.), Alberta Children’s Hospital Research Institute for Child and Maternal Health (to .M.H.S.), Branch Out Neurological Foundation (to M.H.S.) and University of Calgary Eyes High Post-Doctoral Fellowship (to W.N.M.).
Competing interests
The authors report no competing interests.
Glossary
- 31P MRS =
31phosphorus magnetic resonance spectroscopy
- ACTH =
adrenocorticotropic hormone
- ASICs =
acid sensing ion channels
- BHB =
β-hydroxybutyrate
- C-CM =
sham-operated controls fed CM
- C-KD =
sham-operated controls treated with KD
- CM =
control milk diet
- DOX =
doxorubicin
- FCCP =
carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- IS =
infantile spasms
- KATP =
ATP-sensitive potassium channels
- KD =
ketogenic diet
- L-CM =
lesioned rats fed CM
- L-KD =
lesioned rats treated with KD
- L-KD-HCO3 =
lesioned rats treated with KD and supplemented with sodium bicarbonate
- LPS =
lipopolysaccharide
- OFA =
open field activity
- P =
postnatal
- PCPA =
p-chlorophenylalanine
- RR =
respiratory rate
- TTot =
duration of breath
- USV =
ultrasonic vocalizations
- VCO2 =
rate of expired CO2
- VE =
minute ventilation
- VT =
tidal volume
See Auvin (https://doi.org/10.1093/braincomms/fcab234) for a scientific commentary on this article.
References
- 1. Karvelas G, Lortie A, Scantlebury MH, Duy PT, Cossette P, Carmant L.. A retrospective study on aetiology based outcome of infantile spasms. Seizure. 2009;18(3):197–201. [DOI] [PubMed] [Google Scholar]
- 2. Riikonen R. A long-term follow-up study of 214 children with the syndrome of infantile spasms. Neuropediatrics. 1982;13(1):14–23. [DOI] [PubMed] [Google Scholar]
- 3. Riikonen R. Infantile spasms: Outcome in clinical studies. Pediatr Neurol. 2020;108:54–64. [DOI] [PubMed] [Google Scholar]
- 4. Krijgh EJC, Catsman-Berrevoets CE, Neuteboom RF.. Early seizure freedom is a prognostic factor for survival in patients with West syndrome. Neuropediatrics. 2018;49(4):279–282. [DOI] [PubMed] [Google Scholar]
- 5. Darke K, Edwards SW, Hancock E, et al. Developmental and epilepsy outcomes at age 4 years in the UKISS trial comparing hormonal treatments to vigabatrin for infantile spasms: A multi-centre randomised trial. Arch Dis Child. 2010;95(5):382–386. [DOI] [PubMed] [Google Scholar]
- 6. O'Callaghan FJK, Lux AL, Darke K, et al. The effect of lead time to treatment and of age of onset on developmental outcome at 4 years in infantile spasms: Evidence from the United Kingdom Infantile Spasms Study. Epilepsia. 2011;52(7):1359–1364. [DOI] [PubMed] [Google Scholar]
- 7. Lux AL, Edwards SW, Hancock E, et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: A multicentre randomised trial. Lancet Neurol. 2005;4(11):712–717. [DOI] [PubMed] [Google Scholar]
- 8. O'Callaghan FJK, Edwards SW, Alber FD, et al. Vigabatrin with hormonal treatment versus hormonal treatment alone (ICISS) for infantile spasms: 18-month outcomes of an open-label, randomised controlled trial. Lancet Child Adolesc Health. 2018;2(10):715–725. [DOI] [PubMed] [Google Scholar]
- 9. Bhalla S, Skjei K.. Fulminant vigabatrin toxicity during combination therapy with adrenocorticotropic hormone for infantile spasms: Three cases and review of the literature. Epilepsia. 2020;61(10):e159–e164. [DOI] [PubMed] [Google Scholar]
- 10. Dulac O, Plouin P, Schlumberger E.. Infantile spasms. In: Wyllie E, ed. The treatment of epilepsy: Principals and practice, 2nd ed. Baltimore, MD: Williams & Wilkins; 1997:540–572. [Google Scholar]
- 11. Wolf PS, Moshe SL.. Treatment of infantile spasms. In: Johnson RT, Griffin JW, McArthur JC, eds. Current therapy in neurological disease. St. Louis, MO: Mosby; 2002:30–34. [Google Scholar]
- 12. Eun SH, Kang HC, Kim DW, Kim HD.. Ketogenic diet for treatment of infantile spasms. Brain Dev. 2006;28(9):566–571. [DOI] [PubMed] [Google Scholar]
- 13. Freeman JM, Vining EP, Pillas DJ, Pyzik PL, Casey JC, Kelly LM.. The efficacy of the ketogenic diet-1998: A prospective evaluation of intervention in 150 children. Pediatrics. 1998;102(6):1358–1363. [DOI] [PubMed] [Google Scholar]
- 14. Hong AM, Turner Z, Hamdy RF, Kossoff EH.. Infantile spasms treated with the ketogenic diet: Prospective single-center experience in 104 consecutive infants. Epilepsia. 2010;51(8):1403–1407. [DOI] [PubMed] [Google Scholar]
- 15. Kang HC, Lee YJ, Lee JS, et al. Comparison of short- versus long-term ketogenic diet for intractable infantile spasms. Epilepsia. 2011;52(4):781–787. [DOI] [PubMed] [Google Scholar]
- 16. Kossoff EH, Hedderick EF, Turner Z, Freeman JM.. A case-control evaluation of the ketogenic diet versus ACTH for new-onset infantile spasms. Epilepsia. 2008;49(9):1504–1509. [DOI] [PubMed] [Google Scholar]
- 17. Kossoff EH, Pyzik PL, McGrogan JR, Vining EP, Freeman JM.. Efficacy of the ketogenic diet for infantile spasms. Pediatrics. 2002;109(5):780–783. [DOI] [PubMed] [Google Scholar]
- 18. Numis AL, Yellen MB, Chu-Shore CJ, Pfeifer HH, Thiele EA.. The relationship of ketosis and growth to the efficacy of the ketogenic diet in infantile spasms. Epilepsy Res. 2011;96(1-2):172–175. [DOI] [PubMed] [Google Scholar]
- 19. Dressler A, Benninger F, Trimmel-Schwahofer P, et al. Efficacy and tolerability of the ketogenic diet versus high-dose adrenocorticotropic hormone for infantile spasms: A single-center parallel-cohort randomized controlled trial. Epilepsia. 2019;60(3):441–451. [DOI] [PubMed] [Google Scholar]
- 20. Prezioso G, Carlone G, Zaccara G, Verrotti A.. Efficacy of ketogenic diet for infantile spasms: A systematic review. Acta Neurol Scand. 2018;137(1):4–11. [DOI] [PubMed] [Google Scholar]
- 21. Rho JM. How does the ketogenic diet induce anti-seizure effects? Neurosci Lett. 2017;637:4–10. [DOI] [PubMed] [Google Scholar]
- 22. Rakhade SN, Jensen FE.. Epileptogenesis in the immature brain: Emerging mechanisms. Nat Rev Neurol. 2009;5(7):380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scantlebury M, Galanopoulou A, Veliskova J, Moshe S.. The substantia nigra in the control of seizures. In: Philip A. Schwartzkroin (ed.) Encyclopedia of basic epilepsy research. Oxford: Academic Press; 2009:846–854. [Google Scholar]
- 24. Scantlebury MH, Heida JG, Hasson HJ, et al. Age-dependent consequences of status epilepticus: Animal models. Epilepsia. 2007;48(Suppl 2):75–82. [DOI] [PubMed] [Google Scholar]
- 25. Barrett KT, Roy A, Rivard KB, Wilson RJA, Scantlebury MH.. Vagal TRPV1 activation exacerbates thermal hyperpnea and increases susceptibility to experimental febrile seizures in immature rats. Neurobiol Dis. 2018;119:172–189. [DOI] [PubMed] [Google Scholar]
- 26. Helmy MM, Ruusuvuori E, Watkins PV, Voipio J, Kanold PO, Kaila K.. Acid extrusion via blood-brain barrier causes brain alkalosis and seizures after neonatal asphyxia. Brain. 2012;135(Pt 11):3311–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Helmy MM, Tolner EA, Vanhatalo S, Voipio J, Kaila K.. Brain alkalosis causes birth asphyxia seizures, suggesting therapeutic strategy. Ann Neurol. 2011;69(3):493–500. [DOI] [PubMed] [Google Scholar]
- 28. Ruusuvuori E, Kaila K.. Carbonic anhydrases and brain pH in the control of neuronal excitability. Subcell Biochem. 2014;75:271–290. [DOI] [PubMed] [Google Scholar]
- 29. Ruusuvuori E, Kirilkin I, Pandya N, Kaila K.. Spontaneous network events driven by depolarizing GABA action in neonatal hippocampal slices are not attributable to deficient mitochondrial energy metabolism. J Neurosci. 2010;30(46):15638–15642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schuchmann S, Schmitz D, Rivera C, et al. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat Med. 2006;12(7):817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bjurulf B, Magnus P, Hallböök T, Strømme P.. Potassium citrate and metabolic acidosis in children with epilepsy on the ketogenic diet: A prospective controlled study. Dev Med Child Neurol. 2020;62(1):57–61. [DOI] [PubMed] [Google Scholar]
- 32. Lyczkowski DA, Pfeifer HH, Ghosh S, Thiele EA.. Safety and tolerability of the ketogenic diet in pediatric epilepsy: Effects of valproate combination therapy. Epilepsia. 2005;46(9):1533–1538. [DOI] [PubMed] [Google Scholar]
- 33. Yancy WS Jr, Olsen MK, Dudley T, Westman EC.. Acid-base analysis of individuals following two weight loss diets. Eur J Clin Nutr. 2007;61(12):1416–1422. [DOI] [PubMed] [Google Scholar]
- 34. Arsyad A, Idris I, Rasyid AA, et al. Long-term ketogenic diet induces metabolic acidosis, anemia, and oxidative stress in healthy wistar rats. J Nutr Metab. 2020;2020:3642035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Al-Mudallal AS, LaManna JC, Lust WD, Harik SI.. Diet-induced ketosis does not cause cerebral acidosis. Epilepsia. 1996;37(3):258–261. [DOI] [PubMed] [Google Scholar]
- 36. Hamm LL, Nakhoul N, Hering-Smith KS.. Acid-base homeostasis. Clin J Am Soc Nephrol. 2015;10(12):2232–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carnauba RA, Baptistella AB, Paschoal V, Hübscher GH.. Diet-induced low-grade metabolic acidosis and clinical outcomes: A review. Nutrients. 2017;9(6):538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scantlebury MH, Galanopoulou AS, Chudomelova L, Raffo E, Betancourth D, Moshé SL.. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37(3):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Medeiros CB, Fleming AS, Johnston CC, Walker CD.. Artificial rearing of rat pups reveals the beneficial effects of mother care on neonatal inflammation and adult sensitivity to pain. Pediatr Res. 2009;66(3):272–277. [DOI] [PubMed] [Google Scholar]
- 40. Messer M, Thoman EB, Galofre A, Dallman T, Dallman PR.. Artificial feeding of infant rats by continuous gastric infusion. J Nutr. 1969;98(4):404–410. [DOI] [PubMed] [Google Scholar]
- 41. McHugh ML. Interrater reliability: The kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–282. [PMC free article] [PubMed] [Google Scholar]
- 42. Sestakova N, Puzserova A, Kluknavsky M, Bernatova I.. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip Toxicol. 2013;6(3):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scattoni ML, Ricceri L, Crawley JN.. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10(1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tracey I, Dunn JF, Radda GK.. Brain metabolism is abnormal in the mdx model of Duchenne muscular dystrophy. Brain. 1996;119(Pt 3):1039–1044. [DOI] [PubMed] [Google Scholar]
- 45. von Elverfeldt D, Niekisch M, Quaschning T, et al. Kinetics of PME/Pi in pig kidneys during cold ischemia. NMR Biomed. 2007;20(7):652–657. [DOI] [PubMed] [Google Scholar]
- 46. Herbst EA, Holloway GP.. Permeabilization of brain tissue in situ enables multiregion analysis of mitochondrial function in a single mouse brain. J Physiol. 2015;593(4):787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gibbs SA, Scantlebury MH, Awad P, et al. Hippocampal atrophy and abnormal brain development following a prolonged hyperthermic seizure in the immature rat with a focal neocortical lesion. Neurobiol Dis. 2008;32(1):176–182. [DOI] [PubMed] [Google Scholar]
- 48. Burman RJ, Parrish RR.. The widespread network effects of focal epilepsy. J Neurosci. 2018;38(38):8107–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grant AC, Chau L, Arya K, Schneider M.. Prevalence of epileptiform discharges in healthy 11- and 12-year-old children. Epilepsy Behav. 2016;62:53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raffo E, Coppola A, Ono T, Briggs SW, Galanopoulou AS.. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43(2):322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Keen CL, Lönnerdal B, Clegg M, Hurley LS.. Developmental changes in composition of rat milk: Trace elements, minerals, protein, carbohydrate and fat. J Nutr. 1981;111(2):226–236. [DOI] [PubMed] [Google Scholar]
- 52. Patel MS, Vadlamudi SP, Johanning GL.. Overview of pup in a cup model: Hepatic lipogenesis in rats artificially reared on a high-carbohydrate formula. J Nutr. 1993;123(2 Suppl):373–377. [DOI] [PubMed] [Google Scholar]
- 53. Robles-Valdes C, McGarry JD, Foster DW.. Maternal-fetal carnitine relationship and neonatal ketosis in the rat. J Biol Chem. 1976;251(19):6007–6012. [PubMed] [Google Scholar]
- 54. Lomanowska AM, Chatterjee-Chakraborty M, Steiner M, Kraemer GW.. Effects of motherless rearing on basal and stress-induced corticosterone secretion in rat pups. Stress. 2011;14(6):685–696. [DOI] [PubMed] [Google Scholar]
- 55. Rho JM, Kim DW, Robbins CA, Anderson GD, Schwartzkroin PA.. Age-dependent differences in flurothyl seizure sensitivity in mice treated with a ketogenic diet. Epilepsy Res. 1999;37(3):233–240. [DOI] [PubMed] [Google Scholar]
- 56. Kweon HJ, Suh BC.. Acid-sensing ion channels (ASICs): Therapeutic targets for neurological diseases and their regulation. BMB Rep. 2013;46(6):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ziemann AE, Schnizler MK, Albert GW, et al. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11(7):816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weng JY, Lin YC, Lien CC.. Cell type-specific expression of acid-sensing ion channels in hippocampal interneurons. J Neurosci. 2010;30(19):6548–6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Katsarou AM, Li Q, Liu W, Moshé SL, Galanopoulou AS.. Acquired parvalbumin-selective interneuronopathy in the multiple-hit model of infantile spasms: A putative basis for the partial responsiveness to vigabatrin analogs? Epilepsia Open. 2018;3(Suppl 2):155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang F, Sun X, Ding Y, et al. Astrocytic acid-sensing ion channel 1a contributes to the development of chronic epileptogenesis. Sci Rep. 2016;6:31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peracchia C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim Biophys Acta. 2004;1662(1-2):61–80. [DOI] [PubMed] [Google Scholar]
- 62. Mylvaganam S, Ramani M, Krawczyk M, Carlen PL.. Roles of gap junctions, connexins, and pannexins in epilepsy. Front Physiol. 2014;5:172- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ribeiro MJ, Sacramento JF, Gallego-Martin T, et al. High fat diet blunts the effects of leptin on ventilation and on carotid body activity. J Physiol. 2018;596(15):3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Speretta GF, Lemes EV, Vendramini RC, et al. High-fat diet increases respiratory frequency and abdominal expiratory motor activity during hypercapnia. Respir Physiol Neurobiol. 2018;258:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Prince A, Zhang Y, Croniger C, Puchowicz M.. Oxidative metabolism: Glucose versus ketones. Adv Exp Med Biol. 2013;789:323–328. [DOI] [PubMed] [Google Scholar]
- 66. Rubini A, Bosco G, Lodi A, et al. Effects of twenty days of the ketogenic diet on metabolic and respiratory parameters in healthy subjects. Lung. 2015;193(6):939–945. [DOI] [PubMed] [Google Scholar]
- 67. Brede J, Linares M, Kuck S, et al. Dynamics of molecular self-ordering in tetraphenyl porphyrin monolayers on metallic substrates. Nanotechnology. 2009;20(27):275602- [DOI] [PubMed] [Google Scholar]
- 68. Sabapathy S, Morris NR, Schneider DA.. Ventilatory and gas-exchange responses to incremental exercise performed with reduced muscle glycogen content. J Sci Med Sport. 2006;9(3):267–273. [DOI] [PubMed] [Google Scholar]
- 69. Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM.. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55(4):576–580. [DOI] [PubMed] [Google Scholar]
- 70. Malli F, Papaioannou AI, Gourgoulianis KI, Daniil Z.. The role of leptin in the respiratory system: An overview. Respir Res. 2010;11(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lado FA, Moshé SL.. Role of subcortical structures in the pathogenesis of infantile spasms: What are possible subcortical mediators? Int Rev Neurobiol. 2002;49:115–140. [DOI] [PubMed] [Google Scholar]
- 72. Ainslie DA, Proietto J, Fam BC, Thorburn AW.. Short-term, high-fat diets lower circulating leptin concentrations in rats. Am J Clin Nutr. 2000;71(2):438–442. [DOI] [PubMed] [Google Scholar]
- 73. O'Connor KM, Lucking EF, Golubeva AV, et al. Manipulation of gut microbiota blunts the ventilatory response to hypercapnia in adult rats. EBioMedicine. 2019;44:618–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, upon reasonable request.