Abstract
The influence of excess iodine on human health has been paid more and more attention. Although numerous studies have reported that excess iodine may cause deleterious effects, the mental damage and its mechanism is yet to be identified. Using Sprague-Dawley rats exposed to excess iodine from pregnancy to 6 months post-delivery as in vivo model, this study explored the impacts of long-term repetitive excess iodine administration on the hippocampus of offspring rats, focusing on mitochondrial apoptosis pathway, with changes in monoamine neurotransmitters. The results showed that excess iodine could increase urinary iodine and brain organ coefficient in offspring of both genders, change the hippocampal cell structure, and damage the spatial learning and memory capacities. Poly ADP-ribose polymerase (PARP), P53, Cleaved Caspase-3, and cytochrome C proteins expression increased and Bcl2 protein expression decreased in hippocampus of excess iodine-treated offspring, indicating that excess iodine could activate the mitochondrial apoptosis pathway. Besides, excess iodine showed different effects on monoamine neurotransmitter in different gender. Collectively, our experimental data indicated that the learning and memory impairment induced by excess iodine may be mediated via mitochondrial apoptotic pathway. Long-term repetitive excess iodine exposure affected monoamine neurotransmitters in hippocampus of offspring rats.
Keywords: excess iodine, rat, mitochondrial apoptosis, monoamine neurotransmitters, hippocampus
Introduction
Iodine—the main component of thyroid hormone—is an essential trace element of human body, and plays an important role in the growth, development, and metabolism of mammals. The effect of iodine on the body is U-shaped. Both iodine deficiency and excess can damage the body [1, 2]. As a worldwide public health problem, iodine deficiency has been gradually controlled with the implementation of the policy of iodized salt and bread. However, the health problems caused by excess iodine have gradually become the focus. The human body can ingest excess iodine through medical drug, eating iodine rich food, and drinking high iodine water [3], whereas the high iodine water is the main cause in many countries such as China [4]. Excess iodine has been proved to induce goiter, autoimmune diseases and hypothyroidism, and so on [2]. However, the influence of excess iodine on the human intelligence is still controversial. We believe that excess iodine has the theoretical basis of impairing intelligence: excess iodine can induce hypothyroidism [2], which can cause mental retardation [5]; in addition, more and more experiments in vitro and in vivo have found that excess iodine damaged nerve cells. Therefore, we are interested in whether long-term repeated excess iodine exposure (at least 6 months) could damage hippocampus in rats of different genders.
The mechanism of the damage induced by excess iodine to intelligence also needs further study. Apoptosis is one of the mechanisms of excess iodine-induced target organ damage. Moreover, mitochondrial apoptosis is one of the three major pathways of apoptosis. It is known that iodine could activate human breast cancer cells apoptosis [6] and neutrophil apoptosis [7]. We also have found that excess iodine can cause apoptosis of SH-SY5Y nerve cells in vitro with cytochrome C (Cyt C) increasing in the cytosol [8]. In hence, whether mitochondrial apoptosis pathway is involved in the process of excess iodine-induced nerve injury in rats has become our focus.
Monoamine neurotransmitters also involved in the effects of other chemicals on intelligence. For example, fluoride-induced brain damage in rats is associated with decreased 5-HT [9]. Therefore, it is also worth exploring whether iodine, which is the same halogen element as fluorine, can also change the concentration of 5-HT or other monoamine neurotransmitters and further affect the learning and memory ability.
In this study, we intended to simulate the process of human excess iodine exposure, establish a long-term excess iodine exposure model of Sprague-Dawley (SD) rats through drinking-water, and study the learning and memory ability, hippocampal cell damage, neurotransmitter, and mitochondrial apoptosis pathway related factors ( Poly ADP-ribose polymerase (PARP), P53, Bcl2, Cleaved Caspase-3, and Cyt C) of rats of different genders, in order to provide practical insights on the neurotoxic effect and damage mechanism after long-term repeated excess iodine exposure.
Materials and Methods
Chemicals
Potassium iodate (KIO3, CAS NO. 7758-05-6) was purchased from sinopharm Chemical Reagent Corp (Shanghai, China). Test kits of norepinephrine (NE), epinephrine (E), and 5-HT were purchased from Nanjing Jiancheng Bioengineering institute (Nanjing, China). Urinary iodine test kit was obtained from Wuhan ZhongSheng Biochemical Technique Co, Ltd (Wuhan, China). Primary antibodies against Cleaved Caspase-3 and PARP were obtained from Cell Signaling Technology (Beverly, MA). Antibodies against CytC and GAPDH were obtained from Bioworld Technology, Inc. (USA). Antibody against Bcl2 was obtained from Proteintech Group, Inc. (USA), and antibody against P53 was purchased from ABclonal (ABclonal Biotech Co., Ltd). All other chemical reagents were of analytical grade from standard commercial suppliers unless otherwise indicated in the specified methods.
Animals and treatments
In total, 24 adult female and 12 male SD rats were purchased from the Laboratory Animal Center of Hubei Provincial Center for Disease Control and Prevention [SCXK 2015-0018]. The temperature of the animal room is controlled at about 23°C, and the relative humidity is ~55%. After 1 week of adaptive feeding, the rats were randomly divided into four groups (nine rats in each group, male to female ratio 1:2): the control group (tap water, water iodine concentration not detected) and three excess iodine groups (tap water containing 500, 2500, and 5000 μg/l KIO3, respectively). The male and female rats in each group were mated, and the date of observation of vaginal plug was regarded as the starting date of pregnancy. The pregnant rats were fed in single cage. On the fourth day after birth, 10 male and 10 female pups were kept in each group. On the 21st day after birth, the pups were weaned, fed separately according to the sex and dosage, and continued to be treated according to the same exposure concentration as the maternal rats. During the experiment, rats ate and drank freely. The schematic representation of the experimental procedure was presented in Fig. 1. The experiment was approved by the ethics committee of Tianjin Centers for Disease Control and Prevention (TJCDC2014002).
Figure 1.

The schematic representation of the experimental procedure.
The offspring were sacrificed 6 months later. Food was forbidden the night before the sacrifice. The hippocampus of three rats in each group were randomly selected, fixed with 4% paraformaldehyde, dehydrated, and routinely embedded in paraffin for immunofluorescence analysis. The remaining samples was frozen with liquid nitrogen for a short time and then transferred to −80°C for subsequent experiments.
Urinary iodine content test
One week before sacrifice, urine of five rats in each group was collected for 24 h. The concentration of urinary iodine was detected by the urinary iodine test kit, and the procedure was carried out according to the instructions of the kit. The detection principle and steps of the kit were referred to the Chinese national standard recommended method for urinary iodine detection (WST 107.1-2016).
Morris water maze test
One week before sacrifice, the learning and memory ability of rats were tested by Morris water maze (MWM) experiment [10]. The specific steps were described in details in our earlier articles [11, 12]. Briefly, after 3 days of training, the place navigation test (PNT) was carried out on the fourth day to record the latency and distance of rats to find a fixed platform. On the fifth day, the spatial probe test (SPT) was carried out. After the withdrawal of the platform, the proportion of swimming time (distance) in the quadrant where the platform was located in the total swimming time (distance) was detected within 60 s.
Morphological observation of hippocampal tissue
The hippocampal tissue fixed with paraformaldehyde was dehydrated, paraffin-embedded, and sliced into 5-μm-thick sections. After hematoxylin and eosin staining, the section specimens were examined under an optical microscope (Olympus, Japan) to observe the morphological changes in hippocampal tissue.
TUNEL assay of apoptotic cells in hippocampal tissue
Apoptotic cells in the CA3 region in the hippocampus were detected using a TUNEL apoptosis detection kit (Roche Diagnostics, China) following the manufacturer’s instructions. The specific steps were reported in our earlier articles [11, 12]. Finally, the TUNEL-positive cells in sections were observed under the light microscopy (Olympus, Japan).
Western blot analysis
Hippocampal tissue samples were collected and added 1 ml RIPA buffer (Beyotime, Shanghai, China) with 1% protease inhibitor PMSF for 30 min to every 100 mg of hippocampal tissue. After grinding and mixing, the suspension was centrifuged (14 000 g, 15 min, 4°C). The supernatant was quantitated with bicinchoninic acid protein assay kit (Beyotime, Shanghai, China) and then denatured. After the protein was added to 3.9% stacking gel, it was electrophoresed at a constant voltage (60 volts) for about 30 min and when the protein entered the 12% resolving gel, the voltage was adjusted to 110 volts for 55 min. Finally, the protein was transferred to PVDF membrane (Roche, Inc., USA) with a constant current 350 milliampere for 35 min. The membranes were blocked with 5% nonfat milk in TBST at room temperature for 1 h, and then incubated with primary antibodies against PARP (1:500), P53 (1:1000), Bcl2 (1:1000), Cleaved Caspase-3 (1:500), Cyt C (1:500), and GAPDH (1:8000) at 4°C for 24 h. Then the membrane was incubated with HRP-conjugated secondary antibodies (1:2000) at room temperature for 1.5 h. The signal was detected using chemiluminescence reagents (Western Lightning-ECL, Millipore). The density of the bands was detected with Quantity One software version 4.6.2 (Bio-Rad Laboratories, Inc., USA).
Detection of monoamine neurotransmitters (NE, E, and 5-HT) in hippocampus
Hundred milligrams hippocampal tissue was weighed, and PBS buffer (4°C) was added according to 1:9 (M/V) to make tissue homogenate. The homogenate was centrifuged at 3500 r/min (the centrifugation radius was 14 cm) for 20 min, the supernatant was taken, NE, E, and 5-HT were detected by ELISA kit, according to the instructions.
Statistical methods
All data expressed as mean ± standard deviation. One-way ANOVA analysis of variance was performed for overall significance and the Student–Newman–Keuls comparison was used to compare the means of independent groups, with P value of <0.05 was served as the criterion for statistical significance. Data were analyzed using SPSS 24.0 Statistics (IBM SPSS, Inc., USA).
Results
Effects of excess iodine on urinary iodine in offspring rats
As shown in Fig. 2, the urinary iodine levels of male and female excess iodine groups were higher than those of the control group (P < 0.05, all).
Figure 3.
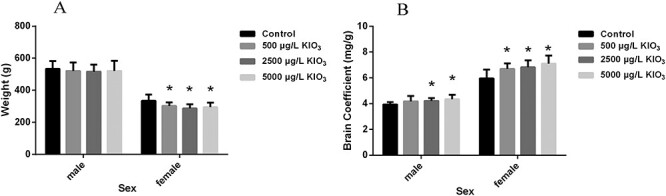
Effects of excess iodine on body weight, and brain coefficient (N = 10). (A) The body weight of male and female rats in different groups. (B) The brain coefficient of male and female rats in different groups.*P < 0.05 as compared with the control group.
Figure 2.
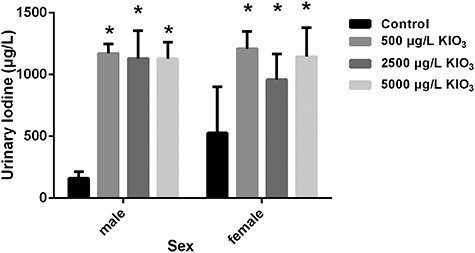
Effects of excess iodine on urinary iodine (N = 5). Quantitative analyses of the levels on urinary iodine in rats. Each bar denotes as the mean ± SD. *P < .05 as compared with the control group.
Effects of excess iodine on body weight and brain coefficient of offspring rats
As shown in Fig. 3, excess iodine did not change the body weight of male rats, but the brain coefficient of 2500 and 5000 μg/l KIO3 male rats were significantly higher than those of the control group (P < 0.05, all). Compared with the control group, the body weight of female rats in the 500, 2500, and 5000 μg/l KIO3 groups were decreased significantly (P < 0.05, all), and the brain coefficient was significantly higher than that in the control group (P < 0.05, all).
Figure 5.

Effects of excess iodine on the hippocampal morphology (×400). The hippocampus was fixed with paraformaldehyde and stained with hematoxylin and eosin.
Effects of excess iodine on learning and memory of offspring rats
PNT and SPT in MWM were used to test the effect of excess iodine on learning and memory of rats. As shown in Fig. 4A and B and Supplementary Fig. S1, the latency and swimming distance of male and female rats in 5000 μg/l KIO3 group were significantly longer than those in other groups (P < 0.05, all). In addition, Fig. 4C and D and Supplementary Fig. S2 show that the time of target quadrant of all excess iodine groups in male rats and 5000 μg/l KIO3 groups in female rats was shorter than that in the control group (P < 0.05, all), whereas the distance of target quadrant of male rats and female rats in the 2500 and 5000 μg/l KIO3 groups was lower than that in the control group (P < 0.05, all).
Figure 6.

Effects of excess iodine on the PARP, P53, Bcl 2, CytC, and Cleaved Caspase-3 protein expression levels of male rats (N = 7). (A) Representative image of western blot for the PARP, P53, Bcl 2, Cyt C, and Cleaved Caspase-3 in male rats hippocampus. (B–F) Quantitative analyses of the PAPR, P53, Bcl 2, Cyt C, and Cleaved Caspase-3 expression levels normalized to the internal control GAPDH. *P < 0.05 as compared with the control group, &P < 0.05 as compared with the 500 μg/l KIO3 group, and #P < 0.05 as compared with the 2500 μg/l KIO3 group.
Figure 4.

Effects of excess iodine on the learning and memory ability (N = 6). Each bar denotes as the mean ± SD. (A) The escape latency in the PNT. (B) The swimming distance in the PNT. (C) The time spent in the target quadrant in the SPT. (D) The distance spent in the target quadrant in the SPT. *P < 0.05 as compared with the control group, &P < 0.05 as compared with the 500 μg/l KIO3 group, and #P < 0.05 as compared with the 2500 μg/l KIO3 group.
Effects of excess iodine on the morphological changes of hippocampus
As can be seen in Fig. 5, the contour of hippocampal cells in the control group was clear and normal. However, the morphological changes of hippocampus were observed in all three excess iodine groups. Compared with the control group, the nerve fibers were obviously thickened, especially in the 5000 μg/l KIO3 group, indicating that plenty of hippocampal cells were swollen and nuclear staining was shallow with the disappearance of polarity.
Figure 7.

Effects of excess iodine on the PARP, P53, Bcl 2, Cyt C, and Cleaved Caspase-3 protein expression levels of female rats (N = 7). (A) Representative image of western blot for the PARP, P53, Bcl 2, Cyt C, and Cleaved Caspase-3 in female rats hippocampus. (B–F) Quantitative analyses of the PARP, P53, Bcl 2, Cyt C, and Cleaved Caspase-3 expression levels normalized to the internal control GAPDH. *P < 0.05 as compared with the control group, &P < 0.05 as compared with the 500 μg/l KIO3 group, and #P < 0.05 as compared with the 2500 μg/l KIO3 group.
Effects of excess iodine on apoptosis and protein expression levels of related factors of hippocampal cells
TUNEL test was used to detect the apoptosis of hippocampal cells. Supplementary Figure S3 showed that the number of apoptotic cells was increased in excess iodine exposure groups. Western blot was used to detect protein levels of apoptosis related factors (PARP, P53, and Cleaved Caspase-3) and mitochondrial apoptotic pathway related factors (Bcl 2 and Cyt C).
Figure 6B showed that the PAPR protein expression levels of male rats in the 2500 and 5000 μg/l KIO3 groups were higher than those in the control and 500 μg/l KIO3 groups (P < 0.05, all), and that in the 5000 μg/l KIO3 group was higher than that in the 2500 μg/l KIO3 group (P < 0.05, all). Figure 6C showed that the expression levels of P53 protein of male rats in all excess iodine groups were higher than that in the control group (P < 0.05, all). Figure 6D showed that the expression levels of Bcl2 protein in male rats of the 2500 and 5000 μg/l KIO3 groups were decreased compared with the control group (P < 0.05, all). Figure 6E and F showed that the expression levels of Cyt C and Cleaved Caspase-3 protein in male rats of 5000 μg/l KIO3 group were higher than those in control group (P < 0.05, all), and the expression level of Cleaved Caspase-3 protein in 2500 μg/l KIO3 group was also higher than that in control group (P < 0.05).
In female rats, Fig. 7B, C, and F showed that the expression levels of PAPR, P53 and Cleaved Caspase-3 protein in the 2500 and 5000 μg/l KIO3 groups were significantly higher than those in the control and 500 μg/l KIO3 groups (P < 0.05, all), while the PAPR protein level in the 5000 μg/l KIO3 group was higher than that in the 2500 μg/l KIO3 group (P < 0.05, all). Figure 7D showed that the expression levels of Bcl2 protein in the 2500 and 5000 μg/l KIO3 groups were lower than that in the control group (P < 0.05, all). Compared with the control group, the expression levels of Cyt C protein in the 2500 and 5000 μg/l KIO3 groups were obviously elevated (P < 0.05, all; Fig. 7E).
Effects of excess iodine on NE, E, and 5-HT in hippocampus
Figure 8A showed that there were no difference between the NE concentration in hippocampus of all excess iodine groups and the control group (P > 0.05, all). Figure 8B showed that the concentration of E in hippocampus of male offspring of all excess iodine groups were lower compared with the control group (P < 0.05, all), but there were no statistical difference between different groups in female rats (P > 0.05, all). Figure 8C showed that compared with the control group, the levels of 5-HT in hippocampus of male and female offspring of excess iodine group were decreased (P < 0.05, all), but the levels of 5-HT in hippocampus of male and female offspring of 5000 μg/l KIO3 group showed an upward trend, and that of male rats was higher than those of 500 and 2500 μg/l KIO3 group (P < 0.05, all).
Figure 8.

Effects of excess iodine on levels of NE, E, and 5-HT in the hippocampus (N = 6). (A) The concentration of NE; (B) the concentration of E; (C) the concentration of 5-HT.*P < 0.05 as compared with the control group, &P < 0.05 as compared with the 500 μg/l KIO3 group, and #P < 0.05 as compared with the 2500 μg/l KIO3 group.
Discussion
Eleven countries have reported that the population had excess iodine nutrition, which was mainly caused by water-induced excess iodine [13], especially in China [14]. In 2017, China Health Commission conducted a nationwide survey of water iodine concentration and reported that the median of water iodine exceeded 100 μg/l in 1050 townships, and the highest median of water iodine in townships was 1113.7 μg/l [15] and ~31 million people live in areas with water iodine concentration > 150 μg/l [4].
The concentrations of KIO3 in our study were 500, 2500, and 5000 μg/l (i.e. 296.7, 1483.6, and 2967.3 μg/l iodine ions). According to the body surface area based corrections for drug development, the dose conversion formula of rats and people is: rat dose (mg/kg) = Human equivalent dose (mg/kg) × 6.17 [16]. In this study, the average daily drinking-water volumes of rat and human are estimated to be 30 and 1200 ml, and the body weight is 0.5 and 60 kg, respectively. Therefore, according to the formula, the concentrations of rats exposed to iodine are about the same as that of people exposed to 144.3, 721.4, and 1442.8 μg/l of water iodine, which is similar to the environmental concentration. Moreover, this study began with exposure to maternal rats, which completely simulates the process of excess iodine exposure in the population. The exposure period was up to 6 months, which meet the International Conference on harmony pharmacological rodent long-term exposure time standard [17]. As far as exploring the neurotoxic effect of long-term exposure to excess iodine is concerned, it is more convincing than the results we exposed the rats to excess iodine for 2 or 4 months earlier [11, 12].
The influence of excess iodine on intelligence is still controversial. In the early stage, Qian et al. [18] conducted a meta-analysis of the relationship between high iodine levels and intelligence, no effect of excess iodine on intelligence was found. However, Liu et al. [19] found that exposure to drinking-water with high iodine content could damage children’s intelligence through epidemiological surveys. Zhao [20] concluded that children living in areas with water iodine levels above 300 μg/l or with urinary iodine concentration above 800 μg/l could have low, but obvious, intellectual impairment. Our research team has confirmed that excess iodine can promote the apoptosis of neural cells in vitro [8], reduce learning and memory ability in 2-month-old male [11] and 4-month-old female rats [12]. Except for our group, Zhang et al. [21] exposed the maternal rats to excess iodine, and explored the neural function of the offspring within 45 days after parturition, finding that excess iodine exposure can slightly cause the decrease of learning and spatial memory in pups. In present study, through chronic exposure, it was confirmed again that exposure to excess iodine through drinking-water can lead to the decrease of learning and spatial memory in male and female rats, suggesting that excess iodine can damage intelligence level.
Apoptosis is one of the main mechanisms of target organ damage caused by excess iodine. It has been found that excess iodine could cause apoptosis of rat thyroid cells [22] and spermatozoal cells [23]. Apoptosis, also known as programmed death, is the body’s initiative to remove the damaged cells in order to maintain the stability of the internal environment, however excessive apoptosis can damage the organs [24].Apoptosis can be divided into three pathways: (i) mitochondrial pathway, (ii) endoplasmic reticulum stress pathway, and (iii) death receptor pathway. In mammals, the mitochondrial apoptosis pathway is considered to be at the center of apoptosis. When stimulated by external substances, Bcl2 family proteins in mitochondria will change, resulting in the increase of mitochondrial membrane permeability (MOMP), and the release of Cyt C and other proteins to the cytoplasm, leading to a series of Caspase protein responses, and finally activating apoptosis protein (Cleaved Caspase-3 and PARP) to promote apoptosis. It has been studied that the aggregation of P53 can also cause the increase of MOMP and the activation of mitochondrial apoptosis pathway [25]. In this study, we found that high iodine can increase the protein of Cleaved Caspase-3 and PARP in male and female rats, which confirmed the results of TUNEL, indicating that excess iodine can induce the apoptosis of hippocampal cells. In addition, the mitochondrial apoptosis specific index Bcl2 was decreased and CytC was increased, and the auxiliary index P53 was increased in rat hippocampus cells of excess iodine groups, indicating that the mitochondrial apoptotic pathway was activated.
Monoamine neurotransmitters (including 5-HT, NE, E, etc.) plays a vital part among the process of intellectual development. Growing evidence suggests that 5-HT and E play an important role in learning and memory [26, 27], but the effect of NE remains controversial [28]. Monoamine neurotransmitters are generally positively correlated with intelligence. Ahmadiasl et al. [27] confirmed that exercise can increase the learning ability of rats by increasing the concentration of E in the hippocampus. However, the fluoride-induced brain damage of rats may be related to the decrease of NE and 5-HT [9]. Our study found that the decrease of learning and memory ability of male rats caused by excess iodine may be related to the decrease of E and 5-HT in hippocampus, but the decreased ability in female rats may only be related to the decrease of 5-HT. The trend of the decline of monoamine neurotransmitters caused by excess iodine is also consistent with the declining trend of monoamine neurotransmitters in the brain of Nile Catfish induced by another neurotoxin-aluminum [29].
As a consequence, we provide convincing evidence that long-term exposure to iodide excess can damage the learning and memory ability of male and female rats, and change the structure of the hippocampal cells. In addition, mitochondrial apoptosis may be involved in the decline of learning and memory ability induced by excess iodine of male and female rats. Moreover, the decrease of learning and memory ability may be related to the disorder of monoamine neurotransmitters caused by excess iodine.
Supplementary Material
Contributor Information
Yushan Cui, Institute of Environment and Health, Tianjin Centers for Disease Control and Prevention, 6 Huayue Road, Hedong District, Tianjin 300011, P.R. China.
Bin Zhang, Scientific Fitness and Health Promotion Research Center, China Institute of Sport Science, 11 Tiyuguan Road, Dongcheng District, Beijing 100061, P.R. China.
Zushan Zhang, School of Public Health, Tianjin Medical University, 22 Qixiangtai Road, Heping District, Tianjin 300070, P.R. China.
Junyan Nie, School of Public Health, Tianjin Medical University, 22 Qixiangtai Road, Heping District, Tianjin 300070, P.R. China.
Hongliang Liu, School of Public Health, Tianjin Medical University, 22 Qixiangtai Road, Heping District, Tianjin 300070, P.R. China.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Funding
This work was supported by the National Nature Science Foundation of China (grant nos. 81573107 and 81372934).
References
- 1. Zimmermann MB. Iodine deficiency. Endocr Rev 2009;30: 376–408. [DOI] [PubMed] [Google Scholar]
- 2. Burgi H. Iodine excess. Best Pract Res Clin Endocrinol Metab 2010;24:107–15. [DOI] [PubMed] [Google Scholar]
- 3. Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol 2014;10:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen H, Liu S, Sun D et al. Geographical distribution of drinking-water with high iodine level and association between high iodine level in drinking-water and goitre: a Chinese national investigation. Br J Nutr 2011;106:243–7. [DOI] [PubMed] [Google Scholar]
- 5. Krassas G, Karras SN, Pontikides N. Thyroid diseases during pregnancy: a number of important issues. Hormones 2015;14:59–69. [DOI] [PubMed] [Google Scholar]
- 6. Shrivastava A, Tiwari M, Sinha RA et al. Molecular iodine induces caspase-independent apoptosis in human breast carcinoma cells involving the mitochondria-mediated pathway. J Biol Chem 2006;281:19762–71. [DOI] [PubMed] [Google Scholar]
- 7. Fanning NF, Manning BJ, Buckley J et al. Iodinated contrast media induce neutrophil apoptosis through a mitochondrial and caspase mediated pathway. Br J Radiol 2002;75:861–73. [DOI] [PubMed] [Google Scholar]
- 8. Zhang B, Cui Y, Wang L et al. Autophagy regulates high concentrations of iodide-induced apoptosis in SH-SY5Y cells. Toxicol Lett 2018;284:129–35. [DOI] [PubMed] [Google Scholar]
- 9. Kaur T, Bijarnia RK, Nehru B et al. Effect of concurrent chronic exposure of fluoride and aluminum on rat brain. Drug Chem Toxicol 2009;32:215–21. [DOI] [PubMed] [Google Scholar]
- 10. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984;11:47–60. [DOI] [PubMed] [Google Scholar]
- 11. Zhao L, Zhang B, Cui Y et al. 3-Methyladenine alleviates excessive iodine-induced cognitive impairment via suppression of autophagy in rat hippocampus. Environ Toxicol 2019;34:912–20. [DOI] [PubMed] [Google Scholar]
- 12. Cui Y, Zhang Z, Zhang B et al. Excessive apoptosis and disordered autophagy flux contribute to the neurotoxicity induced by high iodine in Sprague-Dawley rat. Toxicol Lett 2018;297:24–33. [DOI] [PubMed] [Google Scholar]
- 13. Farebrother J, Zimmermann MB, Andersson M. Excess iodine intake: sources, assessment, and effects on thyroid function. Ann N Y Acad Sci 2019;1446:44–65. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Cui Y, Chen C et al. Stopping the supply of iodized salt alone is not enough to make iodine nutrition suitable for children in higher water iodine areas: A cross-sectional study in northern China. Ecotoxicol Environ Saf 2020;188:109930. [DOI] [PubMed] [Google Scholar]
- 15. Bureau of Disease Prevention and Control, National Health Commission of the People’s Republic of China . Investigation Report on Iodine Content of Drinking Water in China, Beijing, China: Bureau of Disease Prevention and Control, National Health Commission of the People's Republic of China 2019. http://www.nhc.gov.cn/jkj/s5874/201905/bb1da1f5e47040e8820b9378e6db4bd3.shtml (12 August 2021 date last accessed).
- 16. Shin JW, Seol IC, Son CG. Interpretation of animal dose and human equivalent dose for drug development. J Korean Orient Med 2010;31:1–7. [Google Scholar]
- 17. DeGeorge JJ, Meyers LL, Takahashi M et al. The duration of non-rodent toxicity studies for pharmaceuticals. International Conference on Harmonication (ICH). Toxicol Sci 1999;49:143–55. [DOI] [PubMed] [Google Scholar]
- 18. Qian M, Yan Y, Chen Z et al. Meta-analysis on the relationship between children's intelligence and factors as iodine deficiency, supplement iodine and excessive iodine. Zhonghua Liu Xing Bing Xue Za Zhi 2002;23:246–9. [PubMed] [Google Scholar]
- 19. Liu HL, Lam LT, Zeng Q et al. Effects of drinking water with high iodine concentration on the intelligence of children in Tianjin, China. J Public Health (Oxf) 2009;31:32–8. [DOI] [PubMed] [Google Scholar]
- 20. Zhao J. Iodine Deficiency and Iodine Excess in Jiangsu Province, China. The Netherlands: Wageningen University, 2001. [Google Scholar]
- 21. Zhang L, Teng W, Liu Y et al. Effect of maternal excessive iodine intake on neurodevelopment and cognitive function in rat offspring. BMC Neurosci 2012;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen W, Man N, Shan Z et al. Effects of long-term exposure to iodine excess on the apoptosis of thyrocytes in Wistar rats. Exp Clin Endocrinol Diabetes 2011;119:1–8. [DOI] [PubMed] [Google Scholar]
- 23. Chandra AK, Chakraborty A. Influence of iodine in excess on seminiferous tubular structure and epididymal sperm character in male rats. Environ Toxicol 2017;32:1823–35. [DOI] [PubMed] [Google Scholar]
- 24. D'Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 2019;43:582–92. [DOI] [PubMed] [Google Scholar]
- 25. Estaquier J, Vallette F, Vayssiere JL et al. The mitochondrial pathways of apoptosis. Adv Exp Med Biol 2012;942:157–83. [DOI] [PubMed] [Google Scholar]
- 26. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci 2012;23:543–53. [DOI] [PubMed] [Google Scholar]
- 27. Ahmadiasl N, Alaei H, Hanninen O. Effect of exercise on learning, memory and levels of epinephrine in rats' hippocampus. J Sports Sci Med 2003;2:106–9. [PMC free article] [PubMed] [Google Scholar]
- 28. Murchison CF, Zhang XY, Zhang WP et al. A distinct role for norepinephrine in memory retrieval. Cell 2004;117:131–43. [DOI] [PubMed] [Google Scholar]
- 29. Khalil SR, Hussein MM. Neurotransmitters and neuronal apoptotic cell death of chronically aluminum intoxicated Nile catfish (Clarias gariepinus) in response to ascorbic acid supplementation. Neurotoxicology 2015;51:184–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


