Abstract
Coadministration of human secretory IgA (sIgA) together with subtherapeutic vancomycin enhanced survival in the Clostridioides difficile infection (CDI) hamster model. Vancomycin (5 or 10 mg/kg × 5 days) plus healthy donor plasma sIgA/monomeric IgA (TID × 21 days) or hyperimmune sIgA/monomeric IgA (BID × 13 days) enhanced survival. Survival was improved compared to vancomycin alone, P = .018 and .039 by log-rank Mantel-Cox, for healthy and hyperimmune sIgA, respectively. Passive immunization with sIgA (recombinant human secretory component plus IgA dimer/polymer from pooled human plasma) can be administered orally and prevents death in a partially treated CDI hamster model.
Keywords: Clostridioides difficile infection, secretory IgA, immunotherapy
Clostridioides difficile infection (CDI) is a major cause of antibiotic-associated diarrhea, nosocomial disease, and morbidity. CDI does not resolve in most patients with cessation of the causative antibiotic but does resolve in most with cessation of the causative antibiotic plus oral vancomycin. Approximately 25% of patients treated with oral vancomycin experience recurrent disease. The latter individuals require additional antibiotic treatment directed against C. difficile. However, in this context nonantibiotic prophylaxis and treatments for CDI would facilitate antibiotic stewardship.
Secretory immunoglobulin A (sIgA) is the principal intestinal antibody class. It is protective and not toxic. Many patients with relapsed CDI lack circulating antibodies to the C. difficile toxins [1, 2]. Such patients appear most likely to benefit from passive immunization with intravenous IgG [3, 4]. IgG directed against toxins A (TcdA) and B (TcdB) of C. difficile is present in the general population [5]. Toxin-specific circulating IgA titers are similar to those of toxin-specific IgG [6].
Cohn fraction III (CFxIII) precipitate is a discarded byproduct of the recovery of IgG from pooled donor plasma using cold ethanol fractionation [7]. Tons are discarded annually. The antigenic specificity of plasma IgA is similar to that of circulating IgG [8]. Recombinant human secretory component combines with IgA dimers and polymers [9, 10]. Healthy donor plasma IgA binds to C. difficile toxins A and B [10]. We propose that recovered IgA has the potential to be a new orally administered immunoglobulin therapy for the treatment of CDI. Proof of principle requires the demonstration of efficacy of sIgA administered orally in an animal model of CDI.
METHODS
IgA was isolated by a proprietary affinity chromatography method from naturally hyperimmune healthy human plasma from 2 donors enrolled in the plasma donation program of the Ruhr Plasma Center, Bochum, Germany (www.ruhrplasma.de). One donor had high concentrations of anti-TcdA IgA and the other had high concentrations of anti-TcdB IgA. These IgA antibodies were shown to neutralize their respective toxins using a cell-based assay performed by tcgBiomics, GmbH, Bingen, Germany. As we determined using enzyme-linked immunosorbent assays (ELISAs) [10] (data not shown), the IgA derived from the naturally hyperimmune donors possessed from 4 to 8 times the concentrations of anti-TcdA and anti-TcdB IgA as the IgA derived from US pooled plasma CFxIII precipitate (Biotest Pharmaceutical Corporation) (see below).
IgA was also recovered from CFxIII precipitate from the pooled plasma of up to 5000 donors (personal communication, Biotest). Precipitate was resuspended in phosphate-buffered saline (PBS), then viral inactivated by solvent-detergent treatment with 1% tri (N butyl) phosphate, 1% Triton X-100 [11]. IgA was then isolated by jacalin affinity chromatography [12]. Separation of IgA monomer, dimer, and polymer by size exclusion chromatography was performed using a GE HiLoad 16–600 Superdex 200 column on a fast protein liquid chromatography BioCAD Workstation (Applied Biosystems) to ascertain the percentage of polymer, dimer, and monomeric IgA.
Secretory IgA Preparation
Recombinant human secretory component (rhSC; University of Michigan High Throughput Protein Lab) was added to the combined hyperimmune anti-TcdA and anti-TcdB IgA (80% monomer and 20% dimer) in a 1:1 molar ratio, solutions in PBS, and adjusted to a concentration of 1 mg/mL. On average, the ratios of IgA monomer (60%) and dimer plus polymer (40%) recovered from CFxIII precipitate differed from the ratios recovered from the hyperimmune IgA. rhSC was added stoichiometrically to the IgA dimer and polymer IgA, which then forms sIgA from the nonmonomeric IgA [9]. IgA lacking J chain and secretory component is monomeric. We did not separate monomeric IgA from polymeric IgA for these proof-of-principle experiments.
Hamster Model of C. difficile Infection
To more closely approximate human disease in which patients usually survive and would likely continue to receive antibiotic treatment, we modified the hamster model of CDI by treating the animals with subtherapeutic courses of vancomycin. The hamsters were infected on day 0. On day 1, at 24 hours after infection, all animals received a single subcutaneous injection of clindamycin (10 mg/kg) and were then treated with either vancomycin 10 mg/kg (hyperimmune IgA) or 5 mg/kg (pooled donor IgA) from days 2 through 6. The hyperimmune monomeric IgA and semisynthetic sIgA mixture described above was administered 2 times daily by 1-mL gavage at a concentration of 1 mg/mL. IgA administration began the night before the C. difficile challenge (day −1) and continued through day 13. Surviving animals (9 of 9) were euthanized on day 28. The pooled healthy donor monomeric IgA and semisynthetic sIgA mixture solution was adjusted to a concentration of 7 mg/mL and administered 3 times daily by 1-mL gavage. IgA administration began the night before the C. difficile challenge (day −1) and continued through day 19. Surviving animals were euthanized on day 21. We began administration of sIgA before the C. difficle spore challenge to maximize the likelihood of successful experiments.
Naturally hyperimmune healthy human plasma from 2 anonymized donors enrolled in the plasma donation program of the Ruhr Plasma Center, Bochum, Germany (www.ruhrplasma.de) was obtained from the Ruhr Plasma Center. Pooled healthy donor plasma IgA was recovered from CFxIII precipitate, which is a discarded byproduct of the recovery of IgG from pooled donor plasma. CFxIII precipitate was obtained from the Biotest Pharmaceuticals Corporation. All animal use and care protocols were filed with University of North Texas College of Pharmacy Institutional Animal Care and Use Committee. Animal care standards were based on National Institutes of Health standards and were in effect for this project. Any moribund animal encountered during daily observation of animals were humanely euthanized consistent with American Veterinary Medical Association guidelines.
RESULTS
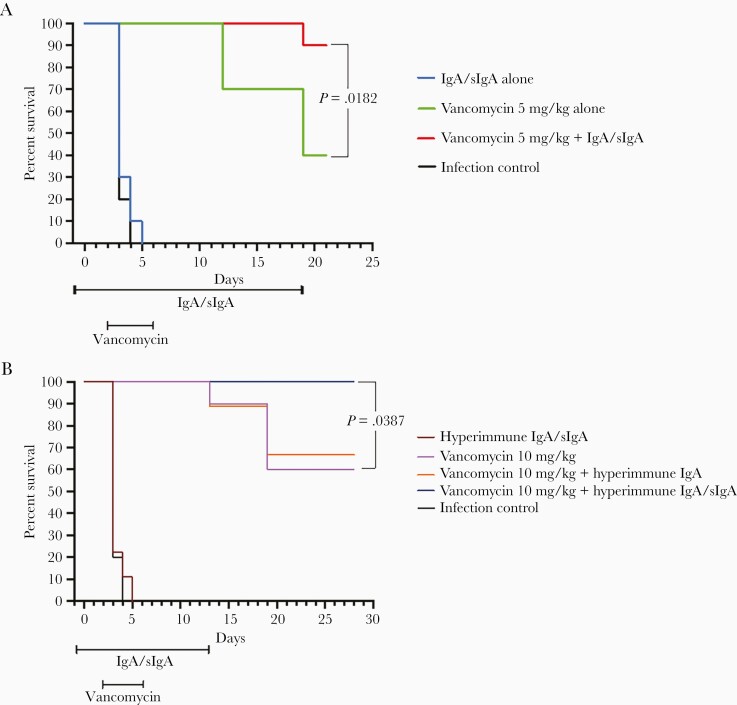
Infection resulted in 100% mortality of untreated controls by day 4. Administration of vancomycin alone, at 5 mg/kg or 10 mg/kg, resulted in 70% or 90% survival by day 13 and 40% or 60% survival at day 21, respectively. The combination of vancomycin (5 mg/kg daily for 5 days) plus pooled healthy donor plasma-derived sIgA/monomeric IgA (3 times daily for 21 days) (Figure 1A) or naturally hyperimmune plasma sIgA/monomeric IgA (2 times daily for 13 days) (Figure 1B) enhanced survival of infected animals as compared to either alone. There was 90% and 100% overall survival using pooled plasma sIgA and hyperimmune IgA, respectively, and a delay in mortality as compared to sIgA/monomeric IgA alone or vancomycin alone. The survival curves for the pooled sIgA/monomeric IgA plus vancomycin group and the hyperimmune sIgA/monomeric IgA plus vancomycin group were significantly improved compared to vancomycin alone (P = .018 and .039 by log-rank [Mantel-Cox], respectively Figure 1). One group of animals was also treated with vancomycin 10 mg/kg from days 2 through 6 together with hyperimmune plasma IgA but lacking semisynthetic sIgA. The orally administered IgA lacking semisynthetic sIgA did not confer a survival advantage as compared to animals that received vancomycin without IgA (Figure 1B).
Figure 1.
Kaplan-Meier survival curves. A, Vancomycin, 5 mg/kg, vs vancomycin plus orally administered pooled healthy donor IgA/sIgA, 7 mg/mL, 3 times daily. IgA survival advantage as compared to animals that received vancomycin without IgA (log-rank test, z = 2.36; P = .0182). B, Vancomycin, 10 mg/kg, vs vancomycin plus orally administered hyperimmune IgA/sIgA, 1 mg/mL, 2 times daily. IgA survival advantage as compared to animals that received vancomycin without IgA/sIgA (log-rank test, z = 2.07; P = .0387). x-axis is time in days; y-axis is % of animals alive. Abbreviation: sIgA, secretory immunoglobulin A. P value bracket shows Vancomycin 10 mg/kg + hyperimmune IgA/sIgA vs Vancomycin 10 mg/kg alone.
DISCUSSION
The hamster model is the gold standard animal model for the study of CDI. However, CDI in hamsters is more severe than the infection in humans. The subtherapeutic doses of vancomycin used in such experiments result in CDI in the hamster that closely models the disease in humans. People with CDI are treated with antibiotics (eg, vancomycin, metronidazole, and fidaxomicin). This treatment is usually successful. However, a supplemental treatment would be valuable in situations where a patient does not recover with antibiotic administration alone. The successful proof-of-principle experiments shown here indicate that the prophylactic oral administration of sIgA derived from pooled healthy donor plasma, along with an antibiotic, could provide an added recovery benefit in such a situation.
The secretory component in sIgA provides protection from digestion and also anchors the secretory antibody to the mucus layer [13], which probably prolongs its intestinal transit time. Polyclonal sIgA antibodies may also facilitate the correction of the dysbiosis that predisposes to CDI. While the deficiency of sIgA is associated with dysbiosis, the correction of dysbiosis by the provision of sIgA remains to be demonstrated empirically. The role of sIgA in mucosal homeostasis has been reviewed [14, 15].
Batch-to-batch variation in potency is a concern because of the variability in antibody titer from person to person. However, this variability can be compensated for by use of a cell-based toxin neutralization assay to measure relative potencies of different batches after scale-up for human administration.
The clinical uses of the oral administration of sIgA in the context of CDI remain to be determined experimentally in clinical phase 2 and 3 trials. A possible use of sIgA might be prophylactic administration to patients at high risk of developing CDI, for example, frail individuals who are being exposed to multiple broad-spectrum antibiotics. We have demonstrated prevention of CDI in all of 9 animals that received vancomycin 10 mg/kg and in 9 of 10 animals that received vancomycin 5 mg/kg because the sIgA administration was begun 24 hours before the challenge with C. difficile spores. Support for such sIgA use in high-risk patients includes the observation that people who possess anti-toxin antibodies are less likely to experience relapse of CDI [3, 4]. People who possess antibodies to TcdA are likely to also have antibodies to the other C. difficile toxins. The healthy human sIgA antibodies used in these experiments included antibodies to the C. difficile toxins [10] (current data not shown).
Our successful proof-of-principle experiments indicate that such clinical use will likely be successful.
Clinical trials will be needed to determine efficacy of concomitant use of sIgA along with vancomycin, metronidazole, or fidaxomicin in patients (1) in whom these antibiotics have not previously been completely effective in eliminating disease, or (2) patients who have experienced relapse of disease. Prevention of relapse of CDI was found in mouse experiments performed by others [16]. Orally administered sIgA, together with continuing appropriate antibiotic treatment, would be expected to still be effective when partial resistance to the anti-C. difficile antibiotic develops during antibiotic administration. Unlike bacterial acquisition of resistance to antibiotics during treatment with antibiotics, oral administration of sIgA will not induce bacterial resistance to the IgA nor to the antibiotic.
CONCLUSION
Our successful proof-of-principle experiments demonstrate that sIgA is likely to be efficacious as a prophylactic treatment for CDI. We have also demonstrated that a monomeric IgA/sIgA mixture derived from pooled plasma CFxIII precipitate significantly enhances survival in a hamster model of CDI. A successful confirmatory experiment was accomplished using naturally hyperimmune plasma-derived monomeric IgA/sIgA mixture at a lower dose.
Notes
Acknowledgment. Dr. David Aronoff (Vanderbilt), Dr. Nicholas Mantis (NYS Dept of Health, Albany) and Dr. Paul Montgomery (former Chair of Immunology, Emeritus, Wayne State University School of Medicine, Detroit) have graciously served as consultants to us on this project.
Financial support. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grant numbers 1R43DK109729-01A1 and 3R43DK109729-01A1S1); Business Accelerator Award from Ann Arbor SPARK, Ann Arbor, MI; and by PreviPharma, GmbH, Mannheim, Germany.
Potential conflicts of interest . M. R. S. is the President of Secretory IgA, Inc; reports other from Secretory IgA, Inc., during the conduct of the study; and is the coinventor of issued US patent 7 597 891 Synthesis of Human Secretory IgA for the treatment of Clostridium difficile associated diseases, issued US patent 8 021 645 Synthesis of human secretory IgA and IgM and the formation of a medicament therefrom, and US patent allowed application 15/205 359 Treatment of celiac disease, C. difficile infection, food intolerance and food allergy with secretory IgA/IgM. S. C. B. is the Chief Scientific Officer of Secretory IgA, Inc; and coinventor of issued US patent 8 021 645 Synthesis of human secretory IgA and IgM and the formation of a medicament therefrom. S. T. K. is the Chief Medical Officer of PreviPharma, GmbH; and reports other from PreviPharma, GmbH outside the submitted work. S. T. K., H. R. G., and M. M. are coinventors of pending US patent WO/2020/173928A1 Epitopes of Clostridium difficile toxins A and B and uses thereof. C. v. E.-S. and K. G. are employees of tcgBIOMICS, GmbH outside the submitted work. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 2000; 342:390–7. [DOI] [PubMed] [Google Scholar]
- 2. Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 2001; 357:189–93. [DOI] [PubMed] [Google Scholar]
- 3. Leung DY, Kelly CP, Boguniewicz M, Pothoulakis C, LaMont JT, Flores A. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr 1991; 118:633–7. [DOI] [PubMed] [Google Scholar]
- 4. Cone LA, Lopez C, Tarleton HL, et al. A durable response to relapsing Clostridium difficile colitis may require combined therapy with high-dose oral vancomycin and intravenous immune globulin. Infect Dis Clin Pract 2006; 14:217–20. [Google Scholar]
- 5. Bacon AE 3rd, Fekety R. Immunoglobulin G directed against toxins A and B of Clostridium difficile in the general population and patients with antibiotic-associated diarrhea. Diagn Microbiol Infect Dis 1994; 18:205–9. [DOI] [PubMed] [Google Scholar]
- 6. von Eichel-Streiber A, Paik W, Knight K, et al. Induction of antitoxin responses in Clostridium-difficile-infected patients compared to healthy blood donors. Anaerobe 2016; 41:91–103. [DOI] [PubMed] [Google Scholar]
- 7. Cohn EJ, Strong LE. Preparation and properties of serum and plasma proteins; a system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc 1946; 68:459–75. [DOI] [PubMed] [Google Scholar]
- 8. Goodman JW. Immunoglobulins I: structure and function. In: Stites DP, Stobo JD, Fudenberg HH, Wells JV, eds. Basic and clinical immunology, 5th ed. Los Altos, CA: Lange Medical Publications, 1984. [Google Scholar]
- 9. Longet S, Miled S, Lötscher M, Miescher SM, Zuercher AW, Corthésy B. Human plasma-derived polymeric IgA and IgM antibodies associate with secretory component to yield biologically active secretory-like antibodies. J Biol Chem 2013; 288:4085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simon MR, Chervin SM, Brown SC, Polyclonal antibody therapies for Clostridium difficile infection. Antibodies 2014; 3:272–88. [Google Scholar]
- 11. Hellstern P, Solheim BG. The use of solvent/detergent treatment in pathogen reduction of plasma. Transfus Med Hemother 2011; 38:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kabir S. Jacalin: a jackfruit (Artocarpus heterophyllus) seed-derived lectin of versatile applications in immunobiological research. J Immunol Methods 1998; 212:193–211. [DOI] [PubMed] [Google Scholar]
- 13. Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthésy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 2002; 17:107–15. [DOI] [PubMed] [Google Scholar]
- 14. Mantis NJ, Rol N, Corthésy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 2011; 4:603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pietrzak B, Tomela K, Olejnik-Schmidt A, Mackiewicz A, Schmidt M. Secretory IgA in intestinal mucosal secretions as an adaptive barrier against microbial cells. Int J Mol Sci 2020; 21: 9254–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelly CP, Chen X, Miescher S, et al. , inventors. Prevention of infection. US patent 9 932 392. 3 April 2018. [Google Scholar]