Abstract
Inflammatory demyelination characterizes the initial stages of multiple sclerosis, while progressive axonal and neuronal loss are coexisting and significantly contribute to the long-term physical and cognitive impairment. There is an unmet need for a conceptual shift from a dualistic view of multiple sclerosis pathology, involving either inflammatory demyelination or neurodegeneration, to integrative dynamic models of brain reorganization, where, glia-neuron interactions, synaptic alterations and grey matter pathology are longitudinally envisaged at the whole-brain level. Functional and structural MRI can delineate network hallmarks for relapses, remissions or disease progression, which can be linked to the pathophysiology behind inflammatory attacks, repair and neurodegeneration. Here, we aim to unify recent findings of grey matter circuits dynamics in multiple sclerosis within the framework of molecular and pathophysiological hallmarks combined with disease-related network reorganization, while highlighting advances from animal models (in vivo and ex vivo) and human clinical data (imaging and histological). We propose that MRI-based brain networks characterization is essential for better delineating ongoing pathology and elaboration of particular mechanisms that may serve for accurate modelling and prediction of disease courses throughout disease stages.
Keywords: multiple sclerosis, brain networks, demyelination, neurodegeneration, neuroinflammation
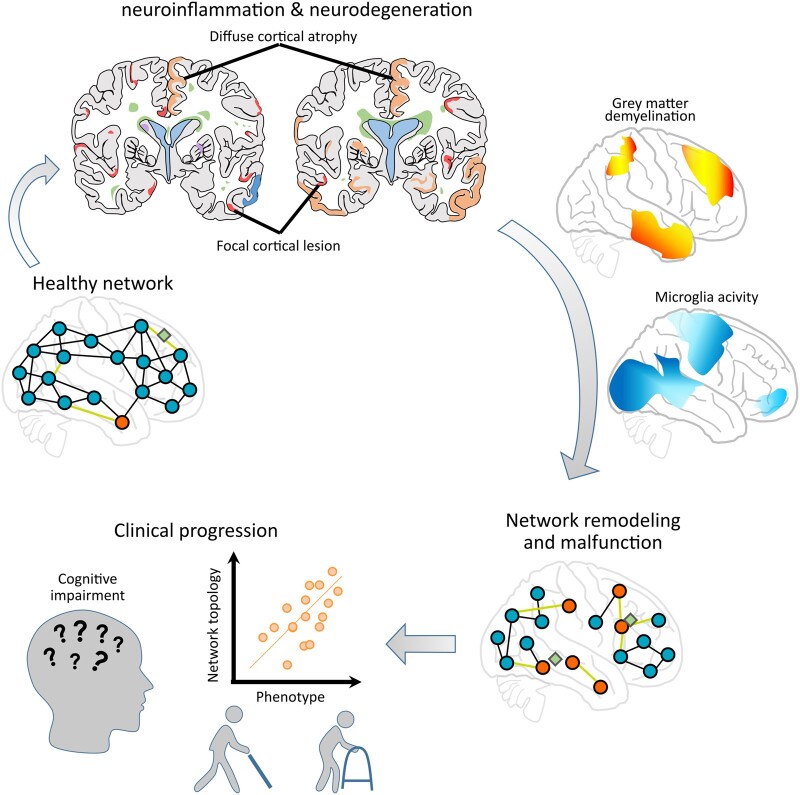
Groppa, Gonzalez-Escamilla et al. propose to end the dualistic view of multiple sclerosis as either neuroinflammatory or neurodegenerative, by conjointly addressing demyelination, microglia and network reorganization that can be compensatory or maladaptive. All presented in the light of disease progression and MRI quantifiable pathological processes, including GM lesions and atrophy.
Graphical Abstract
Graphical Abstract.
Introduction
For many years, multiple sclerosis has been considered as a chronic inflammatory demyelinating disease of the white matter (WM). However, during the last decade, the grey matter (GM) has been postulated to have a major role in multiple sclerosis pathophysiology.1–3 These findings have created a dualistic view of multiple sclerosis as primary either neuroinflammatory or neurodegenerative. Nonetheless, in multiple sclerosis, both inflammatory demyelination and neurodegeneration are present from the early phase of the disease.4
Here, we propose a unifying framework to link local inflammatory demyelination and neurodegeneration with global structural and functional brain network dynamics that translate into the clinical phenotype of multiple sclerosis. We further discuss how network reorganization reflects mechanisms of maladaptation and potential compensation, and highlight how persistent neurodegeneration in the cortex drives the disease course from prominent early inflammation with clinical relapses to later progressive stages and compartmentalized inflammation. We argue that MRI techniques can sensitively (though indirectly) depict neuroinflammatory and neurodegenerative pathology in multiple sclerosis.5–7
In all, we address how the brain responds to the immune-mediated network disruption in an attempt to reduce functional multiple sclerosis-mediated damage. Through this review, we describe two pathological processes which can be indirectly quantified by MRI: (i) acute focal inflammatory activity and (ii) diffuse GM damage, and show how they can be used to define and detect vulnerable brain circuits as the stepping stone for future studies to design interventions in the time window that precedes functional impairment.
Defining an integrative framework in multiple sclerosis
In response to inflammation and neurodegeneration, repair processes emerge and functional–structural networks reorganize (Fig. 1). ‘Reorganization’, thus, refers to the capacity of the CNS to adjust to injury or disease, which at the cellular scale involves several mechanisms, such as intrinsic neuronal modification, axonal regeneration, sprouting, dendritic arborization and synaptic plasticity.8 At the circuitry scale, reorganization leads to the formation or recruitment of functionally homologous neural pathways.9–12 Furthermore, neuron-axonal damage may secondarily alter cortical excitability in the interconnected areas or within networks.13–15 This damage disinhibits intracortical connections and increases excitability (thus synaptic adaptation), which at the network scale may temporarily compensate for the impact of localized injury. However, these processes could further predispose the brain to network vulnerability and precipitate neurodegeneration.13,16 The latter mechanism is referred as maladaptation.
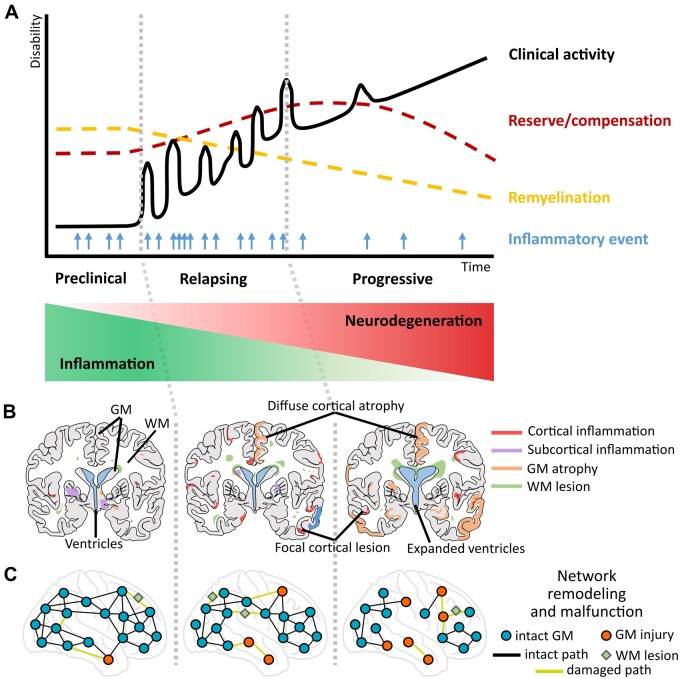
Figure 1.
An integrative hypothetical framework of the progression of multiple sclerosis pathology with evolving neurodegeneration through grey matter (GM) pathology (involving cortical and subcortical GM) that can be characterized via MRI-based network measures. (A) The y-axis of the graph shows the progression of neurological/clinical disability over time (x-axis); the black continuous line denotes clinical activity with relapsing events that cause inflammatory damage; the light blue arrows denote frequency of new inflammatory episodes from preclinical to progressive stages as recorded by MRI; the yellow dashed line denotes the slowly progressing decline in remyelination capacities of brain tissue, while more resources of reserve and compensation become necessary (red dashed line). (B) Coronary lesion maps of GM damage: cortical (red) and subcortical (purple) GM inflammation, increasing GM atrophy (orange) and white matter (WM) lesions (green) relating to distinct stages of multiple sclerosis progression. (C) Representation of network remodelling and malfunction corresponding to different stages of multiple sclerosis disease progression. Blue circles and black lines represent GM regions and their connecting paths without signs of pathology; red circles indicate GM regions affected in multiple sclerosis; green diamonds illustrate WM lesions; light green lines represent damaged connections between brain regions. Two main GM contributors are considered for this model: (i) acute or sustained focal inflammatory activity (red and purple) and (ii) continuous diffuse GM damage (atrophy; orange), both of which can indirectly but closely mirror ongoing tissue processes quantified by MRI. The network approach allows the detection of reorganization and malfunction in the cortex while highlighting its role in the transition from inflammatory demyelination to persistent neurodegeneration and progressive disease stages. This model implies that besides the primary neuroinflammatory origin of immune attacks on brain circuitry in multiple sclerosis, there is a high possibility for focal demyelination, diffuse GM tissue damage or incomplete restoration and emerging neurodegeneration to translate into altered cortical activity. The abnormal cortical activity, thus, drives the involvement of cortico-cortical and cortico-subcortical circuits, accelerates the network dysfunction and spreads secondary malfunction to the interconnected sites.
Functional impairment in multiple sclerosis is highly heterogeneous and ranges from acute focal deficits (secondary to inflammatory lesions) to incomplete clinical recovery after relapses. Symptoms of functional impairment in multiple sclerosis, such as fatigue, neurocognitive deficits (concerning e.g. attention, memory and executive functions) and neuropsychiatric symptoms (e.g. depression and anxiety) may result from damage spreading across the brain WM and GM. Often, these symptoms develop in the absence of acute relapses or early in the disease course before the first attack, and may not completely recover even after an attack has subsided.
An incomplete clinical recovery following acute relapses can only partly explain the increase in clinical disability. A growing body of evidence suggests that the disability accumulation may result from insidious neurodegeneration within the GM.6,17,18 Early neuroinflammatory activity in GM (and WM) is accompanied by glial activation19 and blood–brain barrier (BBB) dysfunction,20 which play an important role in neurodegeneration from early stages onward. Although the effects of neurodegeneration in GM are becoming widely recognized, the underlying mechanisms are intensely debated. Cortical neurodegeneration co-exists with focal inflammation from the onset of multiple sclerosis, but neural function deteriorates and brain circuits are disrupted as disease progresses.21 Nonetheless, the mechanisms that drive the transition to chronic progressive disease phases are unknown.
MRI plays a critical role in tracking the dynamics of brain networks and in predicting disability milestones. In addition to the primary neuroinflammatory pathology, focal demyelination, diffuse GM tissue damage or incomplete restoration and emerging neurodegeneration can all alter cortical activity in multiple sclerosis. This abnormal cortical activity drives aberrant cortico-cortical and cortico-subcortical communication, accelerates network dysfunction and secondarily disrupts interconnected sites functionally (see Fig. 1 for a detailed description of our proposed hypothetical model). MRI-detectable atrophy that affects remote areas of the deep GM structures (thalamus, basal ganglia) and parieto-temporal regions indicates how the brain is affected at the network level.22
Modern network science has provided robust and biologically relevant insights into the behavioural aspects of brain circuitry organization and dynamics. Structural network properties can be assessed regionally and globally using morphometric MRI measures, including cortical thickness and volume, or based on microstructural tissue properties derived from diffusion imaging. In parallel, functional networks depict the relationship between physiological signals in time, reflecting synchronized activity among locations that are commonly assessed using techniques such as functional magnetic resonance imaging (fMRI). Therefore, network measures can capture relevant disease-related anatomical and functional components and can closely mirror disease trajectories and the spread of pathology across the brain.9,23,24 Network measures have been robustly applied to closely depict progression of clinical symptoms in multiple sclerosis10 and therapeutic outcomes.25 Despite these findings, attempts to inclusively model neuroinflammation and neurodegeneration to explain brain circuit functioning at a global scale have remained scarce.
Essential pathological hallmarks of GM inflammation and degeneration in multiple sclerosis
In multiple sclerosis, both GM and WM lesions show areas of demyelination and, thus, their most characterizing difference appear to lie on the only minor number of infiltrating immune cells in GM lesions,3,26 this is despite the GM has been shown to have larger amounts of demyelination than in WM.27
Recent evidence suggests that cortical GM abnormalities in multiple sclerosis may even precede WM inflammatory activity. For example, studies on experimental autoimmune encephalomyelitis, an animal model of CNS inflammatory demyelination used in multiple sclerosis research, have shown that pathogenic T cells interact with meningeal antigen-presenting cells leading to cytokine production and T cell infiltration of pial vessel walls and surrounding parenchyma.28,29 Since the meningeal membrane of the brain is essential to maintain GM integrity, inflammatory processes or evolving meningeal damage may mirror or potentiate GM functional abnormalities. Concordantly BBB abnormalities and infiltration of adaptive immune cells have been shown as indicators of cortical GM pathology.20,30 In this line, a recent study on human tissue samples showed that meningeal inflammation induces the microglia to acquire two distinct phenotypes that differentially associate with neurodegeneration in the progressive multiple sclerosis cortex in a time-dependent manner, and that microglia eventually lose its initial protective properties contributing to neuronal damage.31 An alternative possibility is that efficient restoration of the myelin is delivered when proinflammatory microglia is depleted followed by repopulation of pro-regenerative microglia.32 Regardless of the exact mechanism, the topography of cortical demyelination seem to follow meningeal inflammation.33,34 Noteworthy preclinical models are only an approximation of the key pathological features of multiple sclerosis: inflammation, demyelination, axonal loss and gliosis, and in experimental autoimmune encephalomyelitis, CNS lesions are more pronounced in the brainstem and spinal cord. Thus, caution is advised when translating experimental findings to human disease.
Concerning neurodegeneration, axonal loss is observed within the GM tissue related to demyelinating inflammatory lesions3; however, its extension goes beyond lesions and led to the observed patterns of atrophy.35,36 Thus, a primary role needs to be considered for neurodegeneration, even at the most initial phases of disease.
GM lesions are considered clinically relevant37 and are classified according to their location: leukocortical, intracortical, subpial and pancortical lesions.38 All types of GM lesions are related to neuroinflammation and have infiltrates of T and B cells, and activated macrophages and microglia,38 presenting a decreasing T1 MRI signal over time, which may represent irreversible acute neural tissue damage (e.g. ongoing injury). Cortical lesions in early multiple sclerosis frequently extend along the subpial surface of the cortex and cerebellum, and are topologically related to diffuse and local inflammatory aggregates in adjacent meninges and microglia at the border between demyelinated and myelinated GM tissue39,40 (Fig. 2). Subpial lesions, the most common demyelinated cortical lesions, seem unique to multiple sclerosis since they are not present in other inflammatory diseases (reviewed in Kuhlmann et al.37). These lesions can be large and are often found on neighbouring cortical banks within a sulcus (‘kissing lesions’), which strongly suggests leptomeningeal inflammation as the origin of these lesions.34,41 GM lesions, therefore, might represent the link between chronic inflammation and neurodegeneration in multiple sclerosis. Subpial lesions accumulate over time, involving ∼15–30% of the cortex in early disease phases [e.g. relapsing–remitting multiple sclerosis (RRMS)] and up to 60% of the cortex in progressive phases.42 MRI evaluations have depicted the distribution of GM lesions to be predominantly in frontal and temporal cortical regions with a predilection for motor areas.43
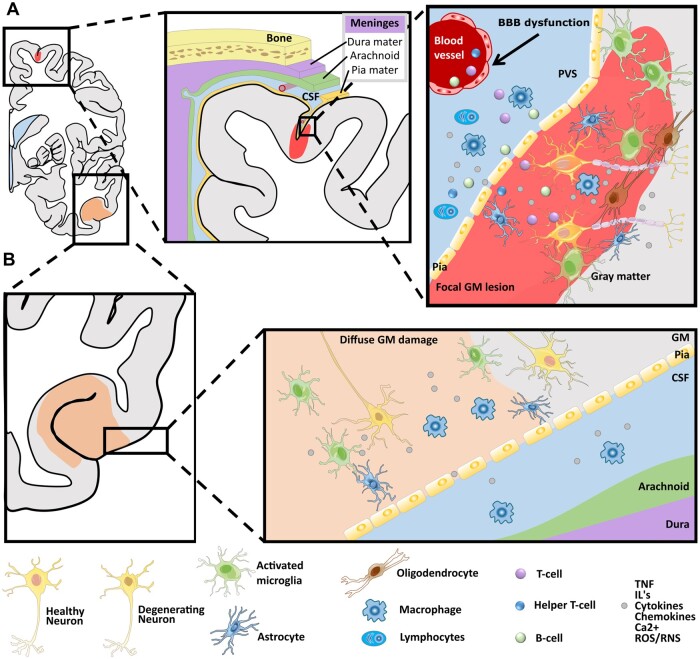
Figure 2.
Simplified schematic depiction of cortical involvement in multiple sclerosis. (A) Focal grey matter lesions (light red) frequently extend along the subpial surface of the cortex. They are topologically related to inflammatory aggregates in adjacent meninges and microglia/macrophages lead to demyelination of the cortex, which is related to a cascade that involves microglial activation and subsequent adaptive immunity via (i) perivascular infiltration of T-cells and B-cells into the GM and/or (ii) infiltration of cytokines, interferon (IFN) and chemokines released by macrophages and other inflammatory cells, such as lymphocytes. (B) Diffuse GM damage (light orange) is mainly related to degeneration of cell bodies and it may be related directly or indirectly to the diffuse microglial activation, which in turn affects trans-synaptic communication leading alterations in network topology. The presence of phagocytic cells may, in first instance, be a reparative process leading to stabilization of the inflammatory response by removal of cellular debris, but it may, however, fail and then lead to incomplete tissue repair. GM = grey matter; WM = white matter; CSF = cerebrospinal fluid; BBB = blood–brain barrier; PVS = perivascular space.
Involvement of glia in the cortical pathophysiology of multiple sclerosis
The role of glial cells in the pathophysiology of cortical degeneration in multiple sclerosis is an essential factor that has been neglected over the years.44 Although all three glial cell types (microglia, astrocytes and oligodendrocytes) are considered to play a potential role in multiple sclerosis pathology, current advances in human genomic mapping have highlighted microglia to be involved in the altered susceptibility to develop multiple sclerosis, where enrichment for multiple sclerosis genes is seen in human microglia but not in astrocytes or neurons.45 However, a particular link with neurodegeneration remains to be elucidated. While early work has addressed the role of glia in acute lesions, research has recently switched emphasis to the interactions between microglial and neuronal cell architecture within the GM.46,47 Yet, microglia dynamics should be further addressed for the long-term characterization of neurodegeneration.
When comparing GM appearing normal on conventional MRI (NAGM; which may be histopathologically abnormal) and WM between patients with multiple sclerosis and non-neurological control donors, microglia depicts a clear region-specific profile of gene expression and transcription; WM microglia show increased lipid metabolism and GM microglia show glycolysis and iron homeostasis.48 These tissue-specific transcriptome signatures still share some similarities between mice and human microglia states, as well as neurodegeneration and de-/remyelination associated microglia,49 further suggesting that microglia are a principal component in brain pathology and therefore have the potential to be a therapeutic target.
Microglia–neuron interactions are mediated by a numerous range of cell surface receptors and signalling molecules50,51; among which ATP and potassium play a pivotal role. Under physiological conditions, microglia–neuron interactions shape synaptic connectivity52,53 and mediate synaptic plasticity,54 thereby promoting harmonious neural circuitry activity and regulating neural network excitability.55 Therefore, it follows that we must consider microglia influence into the neural tissue dynamics, impacting on neural networks potentially both beneficially and detrimentally, which may ultimately affect the MRI-quantifiable brain circuitry. In animal models, some evidence already exist in which fMRI connectivity shows a direct effect of microglia-induced inflammation that is mediated by cytokines, leading to increased connectivity between the cerebellar cortex and prefrontal regions.56 This microglia-induced aberrant excitability pattern was further associated with impaired motor behaviour. These changes may partly alter plasticity and contribute to observed hyperexcitability in RRMS57,58 and in animal models.13
In the context of neurodegeneration, microglial cells may amplify neuronal damage, which can deteriorate neuronal function and accelerate neuronal death through release of proinflammatory cytokines, proteases, and glutamate and recruit reactive T cells.59 During this process, microglia actively clear cellular debris from neurons, their processes and synaptic structures,60 and alteration in this mechanism is related to impaired remyelination.61
There is also growing interest in other sorts of myeloid cells in multiple sclerosis pathology, such as border-associated macrophages, which may be potent at producing pathology.62
GM pathology related to network remodelling
Cortical lesions and NAGM are to various extents, and on an ongoing basis, related to neuronal and glial cell death, decreased synaptic density and axonal damage.31,63–65 Besides demyelination and MRI-detectable cortical lesions, reduced synaptic density may also occur in parallel. In support to our hypothesis, histopathological analyses in multiple sclerosis brain tissue have described a reduced absolute axonal density across cortical layers in the demyelinated but not in the cortical NAGM.66 Cortical layer IV contains neurons receiving thalamocortical afferents or intra-hemispheric cortico-cortical connections, while cortical layer V contains large pyramidal neurons projecting their axons to subcortical structures and the spinal cord. Remarkably, a comparison of stained sections from multiple sclerosis patients and control subjects revealed a significantly reduced axonal density in layers IV-V within the NAGM of multiple sclerosis patients. In MRI studies, multiple sclerosis patients have been shown to present reduced neurite density when compared to healthy controls, with SPMS having more widespread abnormalities than RRMS in both GM lesions and NAGM67 that parallels reduction in myelin water fraction (MWF).68 The pattern of selective damage provides evidence that disconnection occurs during disease and seems to be closely related to either primary alterations through direct damage or secondary functional alterations due to the reorganization of interconnected brain areas.
The expanded view on synaptic pathology within the cortex challenges our current view of multiple sclerosis pathology as a unique neuroinflammatory or neurodegenerative process, and poses important questions for disease progression and our search for new therapeutic strategies. First, there seems to be direct primary synaptic damage that is driven by immunopathogenic mechanisms with the immediate participation of activated microglia. For example, in multiple sclerosis cortex, a generalized loss of dendritic spines has been found independently from axonal loss.66 Furthermore, synapses are found to be detached from their post-synaptic neuron and enclosed by microglia and astrocyte processes in the hippocampus and cerebellum of multiple sclerosis patients.69,70 A second possibility is that the synaptic reorganization could be a consequence of reduced afferent inputs through injured axons crossing regions with focal inflammation.64,70
Although the exact mechanisms underlying GM damage in multiple sclerosis remain elusive, a recent study has evidenced that focal active GM lesions present an important synaptic loss of about 58.9%, while inactive lesions are found to have less synaptic loss (12.6%) as compared to adjacent NAGM.71 Additionally, the authors reported that synaptic loss equally affected excitatory (glutamatergic) and inhibitory (GABAergic) synapses. Accordingly, acute synaptic pathology has been found to relate to acute damage to dendrites in animal models.72 Recently, by taking advantage of animal models of diffuse demyelination, we have detected severe functional alterations in GM as a consequence of myelin loss in the interconnected subcortico-cortical pathways within the auditory thalamocortical system.73 This is supportive to the suggestions that the non-selective synaptic loss occurs during inflammatory demyelination, while the reduced loss synapses in chronically demyelinated GM rather suggest synaptic reorganization.71 Such widespread synaptic remodelling may be reflected as modification in the MRI-derived network topology.
We have shown that remyelination can have a different compartment-dependent functional regeneration potential.73 This is concordant with previous reports of more robust remyelination GM part than in its WM counterpart where also differences in cellular density and activation exist.74–76 However, the efficiency of remyelination is variable, with most efficient lesion repair in early multiple sclerosis stages and limited upon age and disease progression.38,77 Thus, network malfunction may be related to altered or ultimate failure in cortical remyelination and synaptic dysfunction, which we proposed is linked to altered glial function. This hypothesis is based on recent histological findings evidencing that in progressive multiple sclerosis and mouse models, selective loss of parvalbumin fast-spiking interneurons and their connections occurs secondary to cortical demyelination, and quickly translate into a functional neurophysiological impairment of the inhibitory circuitry.65 Noteworthy, it is remarkably difficult to discriminate between purely neuronal and glia-mediated mechanisms of neurodegeneration due to their very intimate cross-talk.
MRI detected GM alterations and disease progression
We have described the GM to present two distinct patterns that characterize multiple sclerosis damage: (i) focal lesions, including processes of focal inflammation and demyelination; and (ii) diffuse pathology (such as atrophy and spreading inflammation). Both patterns are robustly detectable by MRI78 but to different extents.6
Despite recent advances, GM lesions cannot be easily detected; significant variation occurs between MRI readers, even with advanced MRI techniques.79 MRI can identify 18–20% of cortical lesions confirmed by pathological studies.80,81 The number of lesions detected varies depending on the MRI sequence and field strength of the scanner.82,83 Moreover, even sequences sensitive for focal GM involvement (e.g. double inversion recovery or phase-sensitive inversion recovery) miss a large amount of cortical lesions detected in histopathological assessments, leading to false negative results.84,85 Thus, measures of diffuse GM pathology are more reliable and reproducible across research reports.86,87 By measuring the cortical integrity or the thickness of the cortical mantle, processes of neurodegeneration in multiple sclerosis are sensitively captured with MRI.6 Decrease of GM density is a surrogate measure of disease-related atrophy that is visible very early in the disease course88–90 and spreads as the disease continues.91 Brain atrophy occurs at a rate of 0.5–1.35% per year in multiple sclerosis92,93 and plays an essential role in driving neurological disability and cognitive dysfunction.94–96 GM atrophy in multiple sclerosis has been demonstrated to be non-uniform across the cortical mantle, with cross-sectional studies depicting some areas to be more susceptible to loss of structural integrity than others,36,97 predominantly affecting temporal, insular, cingulate and parietal cortices at the earliest disease phases (Fig. 3, upper row), and extending to central, post-central and cerebellar cortices at later disease stages. At the subcortical level, involvement of deep GM nuclei, mainly the thalamus, basal ganglia and hippocampus has also been evidenced.22,97–99 Such patterns of cortical and subcortical vulnerability correlate with demyelinating lesion load.100 MRI-detectable GM pathology can be already sensitively measured in the early stages of multiple sclerosis—even before WM lesions are detected24 or WM network reorganization occurs.101 Assuming that GM atrophy is a selective process, the exact mapping of GM vulnerability and atrophy across stages of multiple sclerosis remains to be investigated in longitudinal studies that include patients who are at the earliest stages of the disease. Moreover, although GM atrophy is currently a major hallmark of long-term disability and provides a quantifiable variable for clinical evaluation and a primary or secondary variable for clinical studies,91 the mechanisms linking brain tissue damage and accumulating clinical disability still remain poorly understood. Importantly, histopathological studies lack of temporal data, and therefore, it is not yet possible to build accurate models that resemble predict clinical progression.
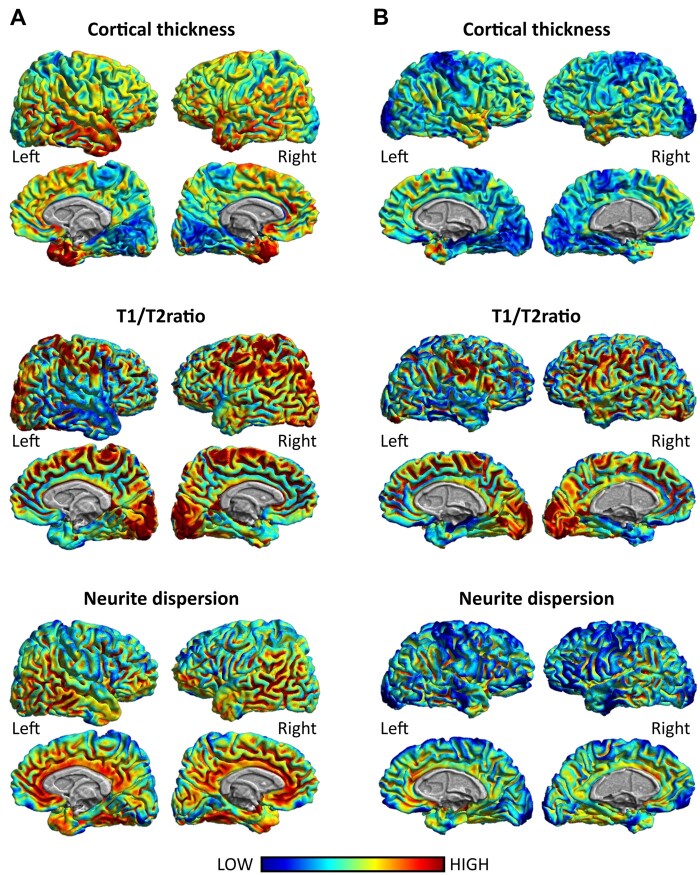
Figure 3.
Example of regional distribution of non-invasive and complementary MRI markers of cortical involvement: cortical thickness, T1/T2 ratio and neurite dispersion. Notice the similarity in the cortical distribution of cortical thickness, T1/T2 ratio and neurite dispersion around the frontal, parietal and temporal cortices across markers. (A) The cortical distribution of these markers in a 36-year-old healthy individual. (B) The cortical distribution of these markers of an age-matched multiple sclerosis patient at disease onset (EDSS: 2; disease duration: 2 years).
Inflammation-induced brain network reorganization
During the initial stages of multiple sclerosis, when damage and the subsequent inflammatory response are transient, different tissue repair mechanisms (e.g. lesion resolution and remyelination) involving neurons and glial cells are ongoing. However, in the presence of persistent inflammation, the remyelination process (particularly in the GM, as discussed above) is severely impaired and cannot prevent neuronal cell death.61,80,102 Despite the exact dynamics between inflammatory responses, MRI assessment and lesion resolution still need further clarification and consolidation, what is clear here is that inflammation plays a crucial role in neural tissue reorganization processes that present inverted patterns to neurodegeneration throughout the disease course (Fig. 1A). Despite inflammation is not the only factor triggering neurodegeneration, evidence from animal models show that sustained inflammatory responses, comprising microglia and astrocytes, have a prevalent role in disease progression.103,104
Two possible primary causes of inflammation-related GM pathology may be considered: (i) the presence of inflammatory cellular infiltrates in the meningeal covering and/or in the adjacent perivascular spaces releasing cytotoxic inflammatory mediators such as tumour necrosis factor or interferon into the GM tissue40,72—these processes play a crucial role to shape GM pathology by regulating microglial activity; or (ii) triggered adaptive and/or innate immune responses within the GM that lead to abnormal firing activity of neurons, microglia activation and astrocyte or oligodendrocyte reaction culminating in neurodegeneration.35 These alterations might involve or be related to disturbed homeostasis of excitatory and/or inhibitory neurotransmitters, ion signalling and cytokine release (Fig. 2).
Structural network alterations and neurodegeneration
Structural networks can be modelled to infer the organization of WM pathways as derived from diffusion MRI or inter-regional statistical associations of GM morphometric measures (e.g. cortical thickness or volume). Here, we will focus on the latter which is commonly defined by computing the spatial correlation between pairs of regions across subjects.
The principle for modelling morphometric networks, so-called covariance networks, is that the associations (i.e. similarities) between regions reflect shared underlying physical and functional properties. In this manner, even remote regions engaged in common functions show greater macrostructural similarity than non-functionally related neighbouring regions.105 It thereby becomes possible to accurately capture the complexity of tissue characteristics over regions while disentangling key organizational principles between brain tissue compartments, and allows to examine the link between structural damage and its functional consequences23 by addressing wider spatial maps of structural properties and associating these with clinical variables or task performance measures.7,100,106,107
Such analyses have identified time-dependent structural abnormalities in multiple sclerosis patients108,109 and have emphasized heterogeneity in subpopulations of multiple sclerosis patients.24 Pathological processes occur in early phases of the disease and induce direct disruption of connectivity, information flow and interregional communication, which later translate into sensory,110 motor111 or cognitive106,112 dysfunction.
Functional MRI activity and network reorganization supporting compensation
The next type of brain networks, called functional networks, is derived from fMRI, commonly acquired during resting-state. This type of network evaluates the time-coupling, i.e. statistical correlation of time signals, in blood oxygenation between GM regions. In the human brain, regions with the strongest functional connections have a relatively small concentration of synapses and glial cells,113 implying that functional connectivity largely relies on myelinated cortical axons. Hence, abnormal BOLD signal and derived resting-state connectivity can be considered proxies of immune- and degeneration-induced brain reorganization and glial dysfunction in multiple sclerosis patients. Supporting evidence exists in which structural network reorganization, based on diffusion tensor imaging, precedes functional reorganization.114
Although recent work defining concepts of network measures as derived from graph theory, the leading approach to quantify networks, are available.10,109 First, the network efficiency reflects the networks’ integrative capacity for information processing across brain regions. Thus, the increase in efficiency implies a reorganized functional integration of the network topology. Next, the network degree and centrality reflects the connectedness of individual brain regions, where core regions, called the ‘hubs’, are usually more connected then the rest and damage to these regions would have a greater impact to the network structure.
Recent studies addressing the dynamics of network topology in RRMS patients showed increased local and nodal efficiencies in early disease stages and no statistically significant differences compared to controls 2 years later.115 Moreover, after 2 years, the multiple sclerosis patients included in the study presented decreased local and global connectivity that strongly correlated with lesion load,115 suggesting that the functional connectivity increase at baseline was counteracted by a widespread accumulation of macroscopic WM lesions during the disease course. Notably, the patients were not in the initial stages of multiple sclerosis but had been diagnosed with the disease 10 years before study participation. In this context, the reported longitudinal breakdown of functional compensation at more advanced disease stages has been described as network collapse.10,116,117 A more recent study assessed the potential brain functional reorganization at rest in patients with CIS and reported that network metrics, such as degree and centrality, indicate network reorganization over the course of 1 year.118 In this study, the bilateral hippocampus, post-ventral cingulate cortex and left parieto-occipital sulcus exhibited significantly higher connectivity in CIS patients compared to controls, while the right middle occipital gyrus and the left posterior segment of the lateral fissure had lower connectivity. The preserved global network efficiency and absence of cognitive decline found for CIS patients compared to age-matched healthy controls suggest that increased connectivity patterns could be an early sign of network compensatory mechanisms.118 Indeed, increased functional connectivity has been associated with better cognitive performance in multiple sclerosis patients,119 while reduced connectivity has been related to the severity of cognitive impairment and structural damage of the underlying neural circuits.120 Thus, the presence of network reorganization in very early disease stages, accompanied by normal cognitive performance, is suggestive of a recruitment mechanism of reserve or plasticity to compensate for structural damage.106,121–123 However, these network re-arrangements can actually be maladaptive due to the loss of proper functional coupling or an expression of network vulnerability.124,125
Brain networks and maladaptation
Several possible mechanisms may explain how neurodegeneration and network dynamics interrelate.126–128 First, the disease directly damages regions within a network, promoting either focal or widespread disruption by propagating the pathological effects via neural connections to other areas (‘prion-like’ spread). Second, inflammation can target the neural connections themselves, for instance, via demyelination and axonal injury, leading to abnormal connectivity that later turns into widespread network organization impairment. Indeed, both mechanisms contribute to network malfunction, in which case accumulation and exchange of focal neurodegeneration and synaptic injury could lead to ‘network collapse’. In line with the first hypothesis, models employing the graph theory framework have suggested that neurodegeneration proceeds through epicentres (hubs) and that the connectivity strength of these epicentres predicts the regional localization of the atrophy.126,129 Notably, the brain network hubs are not only important because their topological centrality supports integrative network processing and adaptive responses, but also because their high connectivity makes them particularly vulnerable to disease progression.130 Therefore, damage to hub regions is more likely to be symptomatic than damage to other network nodes. A non-human primate study showed that even if brain damage does not primarily target hub regions, hubs have an increased likelihood of being connected to the site of primary insult, making them more likely to suffer from diaschisis (sudden change of function in part of the brain).131 This study proposes a relatively high neuron to non-neuronal cell ratio in hubs, which perhaps indicates less support for each neuron from glial or other cell types, as one contributing factor for hub susceptibility.131 However, studies on the relationships between cell densities and hub properties are inconclusive both in humans and in non-human primates.131–133 Additionally, damage to different brain regions may induce diverse patterns of plasticity.131,134 The mechanisms underlying the increased vulnerability of hubs to injury in comparison to other regions requires further study.
Taken together, we hypothesize that network failure or malfunction may contribute to and even drive disease progression. Early network failure, marked by reduced functional connectivity or increased structural heterogeneity, is followed by increased connectivity at distant network sites. The increased connectivity, whether compensatory or maladaptive, could be one of the mechanisms potentiating the negative effects of neurodegeneration, related to the increased neural activity in these regions,13,16,135 which eventually leads to increased GM vulnerability. Further studies are warranted to investigate the mechanisms of network reorganization and their relationship with neurodegeneration, disease progression and physical and cognitive disability in multiple sclerosis.
Alterations of specific brain networks and clinical symptoms
The most common brain networks that show altered functional properties in multiple sclerosis when compared to healthy individuals are the following: the visual network, the sensorimotor network and the default mode network.136 Altered functional connectivity in these networks or their components is often associated with clinical disability,111,137,138 but is also inversely related to cognitive performance at different disease stages,11,139 as well as other neurological symptoms (e.g. fatigue, visual problems, depression and sleep disturbance), see Ref.136 for a recent review. Additionally, the preserved cognitive performance of patients in the early disease stages may be linked with preserved functional–structural network coupling.114 Since increased functional connectivity is associated with both decreases and increases in functional performance, the outcome (adaptive or maladaptive) depends on both the neuroanatomical location of networks and their functions. Thus, more studies are needed, probably incorporating further MRI modalities, to fully understand the importance of network-specific reorganization in multiple sclerosis.
Increased network vulnerability and clinical progression
The complex GM tissue alterations observed in multiple sclerosis that contribute to disability progression and cognitive impairment140 are also visible at the level of the disrupted topological architecture of structural and functional brain networks in different multiple sclerosis stages.10 Abnormal network connectivity strongly involves frontal, cingulate middle temporal, sensorimotor and thalamic areas, reviewed in Ref.10 Such network disruptions have revealed delimited temporal patterns of increased and decreased network connectivity that can be related to physical and cognitive impairment. Specifically, cognitive decline has been associated with increased modularity, clustering and network centrality.10
In line with the idea that network properties can provide essential information about the degree of disease progression, alterations in network topology can be attributed to inflammation-induced microstructural processes. These can lead either to decreased (impaired) connectivity due to structural alterations in specific regions or to increased connectivity due to more similar patterns of ongoing pathology. The increased regional structural homogeneity of the human cerebral cortex is related to its cellular composition, with a higher number of glial cells per neuron resulting in an average glia/neuron ratio of 1.48 across cortical layers.141 According to this relationship, local variations in neuronal density across sites within the GM of the human cortical mantle are also correlated with local variations in the glia/neuron ratio.142 This association implies that the cellular composition across the cortex is relatively homogeneous, and that inflammatory processes exert a homogenizing effect over these regions. Nevertheless, in neurodegeneration, the spatial spreading of molecular neuropathology, causative of functional impairment may be strongly modulated by the regional vulnerability. This hypothesis arises from the heterogeneous distribution of neural cell densities,143 laminar differentiation144,145 and long-range anatomical and functional connectivity146,147 across cortical locations.
Advanced imaging techniques imaging
The possibility of inducing remyelination, either through pharmacological treatments148 or behavioural interventions through experience-dependent myelin plasticity,149 is an emerging focus in clinical trials. Therefore, robust in vivo markers to assess myelination could enable diagnosis and treatment monitoring for a wide range of conditions.
Macromolecules in the myelin have diamagnetic properties, which influence the local magnetic field strength, making it possible to indirectly assess myelin by means of different sequences, including the longitudinal relaxation rate R1, the transverse relaxation rate R2, the effective transverse relaxation rate R2*, quantitative susceptibility mapping, MWF, as well as magnetization transfer (MT; as quantified by the magnetization transfer ratio). Despite heterogeneous reports, each of these imaging modalities present a fairly good association with the underlying histology (see Refs.150–152 for recent review and meta-analyses) and are sensitive to cortical demyelination, remyelination, neuronal density and axonal loss and can each be applied to independently predict future disability progression in the long-term.153 However, no consensus still exist in terms of their validity and specificity to myelin.
Among all MRI sequences, the recently introduced T1w/T2w ratio154 presents high test–retest reliability for differentiating between myelinated or demyelinated cortex, as defined by anti-proteolipid protein antibody immunostainings on post-mortem tissue samples from multiple sclerosis patients,155 and to differentiate between patients at different stages of multiple sclerosis and healthy controls.156 In the latter study, the most striking finding was the decreased myelin content in the posterior cingulate cortex in multiple sclerosis patients, which correlated with dendrite density from post-mortem histopathology. Conversely, Nakamura et al.155 evidenced strong correlations between proxies of myelin content (T1w/T2w ratio and MT) in regions such as the temporal, frontal, cingulate and parietal cortices where myelin status was identified via post-mortem tissue staining. In general, it is highly plausible that cortical demyelination follows a similar spatial spreading pattern as neurodegeneration, where the frontal-cingulate and temporal cortices are slightly more affected than the parietal and occipital regions1,75 (Fig. 3, middle row). Within the temporal lobe, the hippocampus seems to be strongly involved,6,157,158 whereas among the subcortical deep GM structures, involvement of the thalamus, putamen, caudate and globus pallidus has been reported.91,99,159
The lipidic structure of the myelin hinders local water diffusion, as measured by diffusion-MRI (dMRI). One way to characterize the microstructural information from dMRI is to use biophysical models, such as neurite orientation dispersion and density imaging (NODDI)160 that is arguably becoming the most popular model currently. NODDI has three scalar parameters: neurite density index (NDI), orientation dispersion index and free water fraction. NODDI parameters have been observed to correlate with clinical disability.161 Moreover, NDI (Fig. 3, lower row) was observed to be reduced in RRMS patients and even more in progressive multiple sclerosis. Thus, the use of NODDI in multiple sclerosis may offer the opportunity to reveal axonal damage in the NAGM and predict clinical outcomes. However, the large scanning times required for a dMRI suitable for NODDI hamper its current application in clinical settings. Moreover, NODDI parameters are needed to be validated with different histological stainings to evaluate its underlying biology.
Recently, also amyloid PET tracers have been successfully used for assessing myelin integrity in humans.162,163 Combining the spatial resolution of MRI with the quantitative power of PET may further improve reliable quantification of myelin invivo. However, more studies are still needed in this field.
Concluding remarks, open questions and perspectives
In this review, we presented brain network developments to interrelate the pathological processes of inflammatory demyelination and neurodegeneration with clinical manifestations of multiple sclerosis. We proposed a framework that embraces structural and functional network disruption to explain the spread of multiple sclerosis pathology across the brain, providing key insights into the pathways of neurodegeneration and neuroinflammation in multiple sclerosis. This framework underlines why some brain areas are more vulnerable to multiple sclerosis damage and how networks of regions (circuits) contribute to disease progression. This overarching brain network theoretical framework may help us to classify patients into mechanistic subgroups by providing information on the type of molecular pathology and on the nature of the affected network. Overall, brain network dynamics assist us in deciphering the interplay between neurodegeneration and neuroinflammation opening new windows to evaluate clinical manifestations of multiple sclerosis, disease progression and, in the future, development of possible therapeutic interventions.
Moving forward, we must gain better insights on the mechanisms underlying the potential protective and deleterious effects of network reorganization in response to innate immunity and neurodegeneration. Reaching this goal may be facilitated by mechanistic in vitro studies, further analysis of mouse models and multimodal longitudinal imaging data in humans throughout their disease course. Moreover, integrated structural and functional connectivity measures together with clinical scales for functional performance and molecular markers of microglial activity during the progression of multiple sclerosis will increase the potential for better delineating adaptive and maladaptive mechanisms on an individual level and enable future development of novel therapeutic routes for multiple sclerosis patients.
Acknowledgements
We thank Rosalind Gilchrist and Cheryl Ernest for proofreading the manuscript.
Funding
This study was supported by a grant from the German Research Foundation (DFG; CRC-TR-128 to S.G. and S.G.M.).
Competing interests
The authors report no competing interests.
Glossary
- BBB =
blood–brain barrier
- BOLD =
blood oxygen level-dependent
- CIS =
clinically isolated syndrome
- CNS =
central nervous system
- DMN =
default mode network
- fMRI =
functional magnetic resonance imaging
- GM =
grey matter
- NAGM =
normal-appearing grey matter
- MT =
magnetization transfer
- RRMS =
relapsing–remitting multiple sclerosis
- SMN =
sensorimotor network
- WM =
white matter
References
- 1. Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ.. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropath Exp Neur. 2003;62(7):723–732. [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y, Wu IW, Tosun D, Foster E, Schuff N;. Parkinson’s Progression Markers Initiative. Progression of regional microstructural degeneration in Parkinson's disease: A multicenter diffusion tensor imaging study. PLoS One. 2016;11(10):e0165540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamagata K, Hatano T, Okuzumi A, et al. Neurite orientation dispersion and density imaging in the substantia nigra in idiopathic Parkinson disease. Eur Radiol. 2016;26(8):2567–2577. [DOI] [PubMed] [Google Scholar]
- 4. Mahad DH, Trapp BD, Lassmann H.. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–193. [DOI] [PubMed] [Google Scholar]
- 5. Ciccarelli O, Barkhof F, Bodini B, et al. Pathogenesis of multiple sclerosis: Insights from molecular and metabolic imaging. Lancet Neurol. 2014;13(8):807–822. [DOI] [PubMed] [Google Scholar]
- 6. Filippi M, Brück W, Chard D, et al. ; Attendees of the Correlation between Pathological and MRI findings in MS workshop. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2019;18(2):198–210. [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez-Escamilla G, Ciolac D, De Santis S, et al. Gray matter network reorganization in multiple sclerosis from 7-Tesla and 3-Tesla MRI data. Ann Clin Transl Neurol. 2020;7(4):543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lassmann H. Multiple sclerosis pathology: Inflammation versus neurodegeneration. In: Arnon R, Miller A, (eds.) Translational neuroimmunology in multiple sclerosis. Elsevier, Cambridge, Massachusetts; 2016:3–13. [Google Scholar]
- 9. Fleischer V, Groger A, Koirala N, et al. Increased structural white and grey matter network connectivity compensates for functional decline in early multiple sclerosis. Mult Scler. 2017;23(3):432–441. [DOI] [PubMed] [Google Scholar]
- 10. Fleischer V, Radetz A, Ciolac D, et al. Graph theoretical framework of brain networks in multiple sclerosis: A review of concepts. Neuroscience. 2019;403:35–53. [DOI] [PubMed] [Google Scholar]
- 11. Eijlers AJ, Meijer KA, Wassenaar TM, et al. Increased default-mode network centrality in cognitively impaired multiple sclerosis patients. Neurology. 2017;88(10):952–960. [DOI] [PubMed] [Google Scholar]
- 12. Meijer KA, Eijlers AJC, Geurts JJG, Schoonheim MM.. Staging of cortical and deep grey matter functional connectivity changes in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2018;89(2):205–210. [DOI] [PubMed] [Google Scholar]
- 13. Ellwardt E, Pramanik G, Luchtman D, et al. Maladaptive cortical hyperactivity upon recovery from experimental autoimmune encephalomyelitis. Nat Neurosci. 2018;21(10):1392–1403. [DOI] [PubMed] [Google Scholar]
- 14. Cerina M, Narayanan V, Gobel K, et al. The quality of cortical network function recovery depends on localization and degree of axonal demyelination. Brain Behav Immun. 2017;59:103–117. [DOI] [PubMed] [Google Scholar]
- 15. Narayanan V, Cerina M, Gobel K, et al. Impairment of frequency-specific responses associated with altered electrical activity patterns in auditory thalamus following focal and general demyelination. Exp Neurol. 2018;309:54–66. [DOI] [PubMed] [Google Scholar]
- 16. Trapp BD, Stys PK.. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8(3):280–291. [DOI] [PubMed] [Google Scholar]
- 17. Di Filippo M, Portaccio E, Mancini A, Calabresi P.. Multiple sclerosis and cognition: Synaptic failure and network dysfunction. Nat Rev Neurosci. 2018;19:599–609. [DOI] [PubMed] [Google Scholar]
- 18. Cree BAC, Hollenbach JA, Bove R, et al. ; University of California, San Francisco MS-EPIC Team. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mañé-Martínez MA, Olsson B, Bau L, et al. Glial and neuronal markers in cerebrospinal fluid in different types of multiple sclerosis. J Neuroimmunol. 2016;299:112–117. [DOI] [PubMed] [Google Scholar]
- 20. Kroth J, Ciolac D, Fleischer V, et al. Increased cerebrospinal fluid albumin and immunoglobulin A fractions forecast cortical atrophy and longitudinal functional deterioration in relapsing-remitting multiple sclerosis. Mult Scler. 2019;25(3):338–343. [DOI] [PubMed] [Google Scholar]
- 21. Haider L, Zrzavy T, Hametner S, et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain. 2016;139(Pt 3):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deppe M, Kramer J, Tenberge JG, et al. Early silent microstructural degeneration and atrophy of the thalamocortical network in multiple sclerosis. Hum Brain Mapp. 2016;37(5):1866–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He Y, Chen ZJ, Evans AC.. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17(10):2407–2419. [DOI] [PubMed] [Google Scholar]
- 24. Muthuraman M, Fleischer V, Kolber P, Luessi F, Zipp F, Groppa S.. Structural brain network characteristics can differentiate CIS from early RRMS. Front Neurosci. 2016;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ciolac D, Luessi F, Gonzalez-Escamilla G, et al. Selective brain network and cellular responses upon dimethyl fumarate immunomodulation in multiple sclerosis. Front Immunol. 2019;10:1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sitz A, Hoevels M, Hellerbach A, et al. Determining the orientation angle of directional leads for deep brain stimulation using computed tomography and digital x-ray imaging: A phantom study. Med Phys. 2017;44(9):4463–4473. [DOI] [PubMed] [Google Scholar]
- 27. Gilmore CP, Donaldson I, Bo L, Owens T, Lowe J, Evangelou N.. Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: A comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J Neurol Neurosurg Psychiatry. 2009;80(2):182–187. [DOI] [PubMed] [Google Scholar]
- 28. Rossi B, Constantin G.. Live imaging of immune responses in experimental models of multiple sclerosis. Front Immunol. 2016;7:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schirmer L, Velmeshev D, Holmqvist S, et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature. 2019;573(7772):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schafflick D, Xu CA, Hartlehnert M, et al. Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nat Commun. 2020;11(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hellerbach A, Dembek TA, Hoevels M, et al. DiODe: Directional orientation detection of segmented deep brain stimulation leads: A sequential algorithm based on CT imaging. Stereotact Funct Neurosurg. 2018;96(5):335–341. [DOI] [PubMed] [Google Scholar]
- 32. Lloyd AF, Davies CL, Holloway RK, et al. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat Neurosci. 2019;22(7):1046–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bevan RJ, Evans R, Griffiths L, et al. Meningeal inflammation and cortical demyelination in acute multiple sclerosis. Ann Neurol. 2018;84(6):829–842. [DOI] [PubMed] [Google Scholar]
- 34. Kolber P, Droby A, Roebroeck A, et al. A “kissing lesion”: In-vivo 7T evidence of meningeal inflammation in early multiple sclerosis. Mult Scler J. 2017;23(8):1167–1169. [DOI] [PubMed] [Google Scholar]
- 35. Calabrese M, Magliozzi R, Ciccarelli O, Geurts JJ, Reynolds R, Martin R.. Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci. 2015;16(3):147–158. [DOI] [PubMed] [Google Scholar]
- 36. Steenwijk MD, Geurts JJ, Daams M, et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain. 2016;139(Pt 1):115–126. [DOI] [PubMed] [Google Scholar]
- 37. Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H.. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathologica. 2017;133(1):13–24. [DOI] [PubMed] [Google Scholar]
- 38. Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I.. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: A retrospective autopsy cohort analysis. Acta Neuropathologica. 2018;135(4):511–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134(Pt 9):2755–2771. [DOI] [PubMed] [Google Scholar]
- 40. Lucchinetti CF, Popescu BF, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365(23):2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Absinta M, Vuolo L, Rao A, et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology. 2015;85(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Honce JM. Gray matter pathology in MS: Neuroimaging and clinical correlations. Mult Scler Int. 2013;2013:627870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reinacher P, Krüger M, Coenen V, et al. Determining the orientation of directional deep brain stimulation electrodes using 3D rotational fluoroscopy. Am J Neuroradiol. 2017;38(6):1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lassmann H, van Horssen J.. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim Biophys Acta. 2016;1862(3):506–510. [DOI] [PubMed] [Google Scholar]
- 45. International Multiple Sclerosis Genetics Consortium I. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365(6460):eaav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mallucci G, Peruzzotti-Jametti L, Bernstock JD, Pluchino S.. The role of immune cells, glia and neurons in white and gray matter pathology in multiple sclerosis. Prog Neurobiol. 2015;127:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dendrou CA, Fugger L, Friese MA.. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–558. [DOI] [PubMed] [Google Scholar]
- 48. van der Poel M, Ulas T, Mizee MR, et al. Transcriptional profiling of human microglia reveals grey-white matter heterogeneity and multiple sclerosis-associated changes. Nat Commun. 2019;10:1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Masuda T, Sankowski R, Staszewski O, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;568(7751):E4. [DOI] [PubMed] [Google Scholar]
- 50. Wohleb ES. Neuron-microglia interactions in mental health disorders: “For Better, and For Worse”. Front Immunol. 2016;7:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T.. Bidirectional microglia-neuron communication in health and disease. Front Cell Neurosci. 2018;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. [DOI] [PubMed] [Google Scholar]
- 53. Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ferrini F, De Koninck Y.. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013;2013:429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yamamoto M, Kim M, Imai H, Ohtsuki G. Microglia-triggered hyperexcitability in the cerebellum depresses animal behaviors. bioRxiv. 2018:353730.
- 57. Mori F, Nistico R, Nicoletti CG, et al. RANTES correlates with inflammatory activity and synaptic excitability in multiple sclerosis. Mult Scler. 2016;22(11):1405–1412. [DOI] [PubMed] [Google Scholar]
- 58. Zipser CM, Premoli I, Belardinelli P, et al. Cortical excitability and interhemispheric connectivity in early relapsing-remitting multiple sclerosis studied with TMS-EEG. Front Neurosci. 2018;12:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brown GC, Vilalta A.. How microglia kill neurons. Brain Res. 2015;1628(Pt B):288–297. [DOI] [PubMed] [Google Scholar]
- 60. D'Albis T, Haegelen C, Essert C, Fernández-Vidal S, Lalys F, Jannin P.. PyDBS: An automated image processing workflow for deep brain stimulation surgery. Int J Comput Assist Radiol Surg. 2015;10(2):117–128. [DOI] [PubMed] [Google Scholar]
- 61. Johansson JD, Alonso F, Wardell K.. Patient-specific simulations of deep brain stimulation electric field with aid of in-house software ELMA. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:5212–5216. [DOI] [PubMed] [Google Scholar]
- 62. Prineas JW, Lee S.. Multiple sclerosis: Destruction and regeneration of astrocytes in acute lesions. J Neuropathol Exp Neurol. 2019;78(2):140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Friese MA. Widespread synaptic loss in multiple sclerosis. Brain. 2016;139(Pt 1):2–4. [DOI] [PubMed] [Google Scholar]
- 64. Trapp BD, Vignos M, Dudman J, et al. Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: A retrospective study. Lancet Neurol. 2018;17(10):870–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Noecker AM, Choi KS, Riva-Posse P, Gross RE, Mayberg HS, McIntyre CC.. StimVision software: Examples and applications in subcallosal cingulate deep brain stimulation for depression. Neuromodulation. 2018;21(2):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jurgens T, Jafari M, Kreutzfeldt M, et al. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain. 2016;139(Pt 1):39–46. [DOI] [PubMed] [Google Scholar]
- 67. Lauro PM, Vanegas-Arroyave N, Huang L, et al. DBSproc: An open source process for DBS electrode localization and tractographic analysis. Hum Brain Mapp. 2016;37(1):422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang Q, Akram H, Muthuraman M, et al. Normative vs. patient-specific brain connectivity in deep brain stimulation. NeuroImage. 2021;224:117307. [DOI] [PubMed] [Google Scholar]
- 69. Albert M, Barrantes-Freer A, Lohrberg M, et al. Synaptic pathology in the cerebellar dentate nucleus in chronic multiple sclerosis. Brain Pathol. 2017;27(6):737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dutta R, Chang A, Doud MK, et al. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol. 2011;69(3):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vercellino M, Marasciulo S, Grifoni S, et al. Acute and chronic synaptic pathology in multiple sclerosis gray matter. Mult Scler. 2021. doi: 10.1177/13524585211022174 [DOI] [PubMed] [Google Scholar]
- 72. Magliozzi R, Howell OW, Reeves C, et al. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68(4):477–493. [DOI] [PubMed] [Google Scholar]
- 73. Cerina M, Narayanan V, Delank A, et al. Protective potential of dimethyl fumarate in a mouse model of thalamocortical demyelination. Brain Struct Funct. 2018;223(7):3091–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Glaser FM, Ruck T.. Translational animal models for MS and related neuroimmunological disorders. In: Groppa S, Meuth S, (eds.) Translational methods for multiple sclerosis research. Springer, Humana, New York, NY; 2021:13–27. [Google Scholar]
- 75. Albert M, Antel J, Bruck W, Stadelmann C.. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ciolac D, Groppa SA, Gonzalez-Escamilla G.. Translational characterization of the glia role in multiple sclerosis. In: Groppa S, Meuth S, (eds.) Translational methods for multiple sclerosis research. Springer, Humana, New York, NY; 2021:61–76. [Google Scholar]
- 77. Patrikios P, Stadelmann C, Kutzelnigg A, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129(Pt 12):3165–3172. [DOI] [PubMed] [Google Scholar]
- 78. Bischof A, Caverzasi E, Cordano C, Hauser SL, Henry RG.. Advances in imaging multiple sclerosis. Semin Neurol. 2017;37(5):538–545. [DOI] [PubMed] [Google Scholar]
- 79. Gonzalez-Escamilla G, Groppa S.. 7 Tesla MRI will soon be helpful to guide clinical practice in multiple sclerosis centres - No. Mult Scler. 2021;27(3):362–363. [DOI] [PubMed] [Google Scholar]
- 80. Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43. [DOI] [PubMed] [Google Scholar]
- 81. Seewann A, Kooi EJ, Roosendaal SD, et al. Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology. 2012;78(5):302–308. [DOI] [PubMed] [Google Scholar]
- 82. De Santis S, Bastiani M, Droby A, et al. Characterizing microstructural tissue properties in multiple sclerosis with diffusion MRI at 7 T and 3 T: The impact of the experimental design. Neuroscience. 2019;403:17–26. [DOI] [PubMed] [Google Scholar]
- 83. Gracien RM, Reitz SC, Wagner M, et al. Comparison of two quantitative proton density mapping methods in multiple sclerosis. Magma. 2017;30(1):75–83. [DOI] [PubMed] [Google Scholar]
- 84. Droby A, Fleischer V, Carnini M, et al. The impact of isolated lesions on white-matter fiber tracts in multiple sclerosis patients. NeuroImage Clin. 2015;8:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gracien RM, Reitz SC, Hof SM, et al. Assessment of cortical damage in early multiple sclerosis with quantitative T2 relaxometry. NMR Biomed. 2016;29(4):444–450. [DOI] [PubMed] [Google Scholar]
- 86. Guo C, Ferreira D, Fink K, Westman E, Granberg T.. Repeatability and reproducibility of FreeSurfer, FSL-SIENAX and SPM brain volumetric measurements and the effect of lesion filling in multiple sclerosis. Eur Radiol. 2019;29(3):1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. McGuire SA, Wijtenburg SA, Sherman PM, et al. Reproducibility of quantitative structural and physiological MRI measurements. Brain Behav. 2017;7(9):e00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Azevedo CJ, Overton E, Khadka S, et al. Early CNS neurodegeneration in radiologically isolated syndrome. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Geurts JJ, Calabrese M, Fisher E, Rudick RA.. Measurement and clinical effect of grey matter pathology in multiple sclerosis. Lancet Neurol. 2012;11(12):1082–1092. [DOI] [PubMed] [Google Scholar]
- 90. Perez-Miralles F, Sastre-Garriga J, Tintore M, et al. Clinical impact of early brain atrophy in clinically isolated syndromes. Mult Scler. 2013;19(14):1878–1886. [DOI] [PubMed] [Google Scholar]
- 91. Eshaghi A, Marinescu RV, Young AL, et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain. 2018;141:1665–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. De Stefano N, Stromillo ML, Giorgio A, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(1):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Filippi M, Agosta F.. Imaging biomarkers in multiple sclerosis. J Magn Reson Imaging. 2010;31(4):770–788. [DOI] [PubMed] [Google Scholar]
- 94. Calabrese M, Poretto V, Favaretto A, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain. 2012;135(Pt 10):2952–2961. [DOI] [PubMed] [Google Scholar]
- 95. Nocentini U, Bozzali M, Spano B, et al. Exploration of the relationships between regional grey matter atrophy and cognition in multiple sclerosis. Brain Imaging Behav. 2014;8(3):378–386. [DOI] [PubMed] [Google Scholar]
- 96. Pitteri M, Romualdi C, Magliozzi R, Monaco S, Calabrese M.. Cognitive impairment predicts disability progression and cortical thinning in MS: An 8-year study. Mult Scler. 2017;23(6):848–854. [DOI] [PubMed] [Google Scholar]
- 97. Calabrese M, Reynolds R, Magliozzi R, et al. Regional distribution and evolution of gray matter damage in different populations of multiple sclerosis patients. PloS One. 2015;10(8):e0135428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Haider L, Simeonidou C, Steinberger G, et al. Multiple sclerosis deep grey matter: The relation between demyelination, neurodegeneration, inflammation and iron. J Neurol Neurosurg Psychiatry. 2014;85(12):1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Vercellino M, Masera S, Lorenzatti M, et al. Demyelination, inflammation, and neurodegeneration in multiple sclerosis deep gray matter. J Neuropathol Exp Neurol. 2009;68(5):489–502. [DOI] [PubMed] [Google Scholar]
- 100. Muthuraman M, Fleischer V, Kroth J, et al. Covarying patterns of white matter lesions and cortical atrophy predict progression in early MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Radetz A, Koirala N, Kramer J, et al. Gray matter integrity predicts white matter network reorganization in multiple sclerosis. Hum Brain Mapp. 2020;41(4):917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rodriguez EG, Wegner C, Kreutzfeldt M, et al. Oligodendroglia in cortical multiple sclerosis lesions decrease with disease progression, but regenerate after repeated experimental demyelination. Acta Neuropathol. 2014;128(2):231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jiang Z, Jiang JX, Zhang GX.. Macrophages: A double-edged sword in experimental autoimmune encephalomyelitis. Immunol Lett. 2014;160(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang J, Wang J, Wang J, Yang B, Weng Q, He Q.. Targeting microglia and macrophages: A potential treatment strategy for multiple sclerosis. Front Pharmacol. 2019;10:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Alexander-Bloch A, Giedd JN, Bullmore E.. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14(5):322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gamboa OL, Tagliazucchi E, von Wegner F, et al. Working memory performance of early MS patients correlates inversely with modularity increases in resting state functional connectivity networks. Neuroimage. 2014;94:385–395. [DOI] [PubMed] [Google Scholar]
- 107. Tur C, Kanber B, Eshaghi A, et al. Clinical relevance of cortical network dynamics in early primary progressive MS. Mult Scler. 2020;26:442–456. [DOI] [PubMed] [Google Scholar]
- 108. Fleischer V, Koirala N, Droby A, et al. Longitudinal cortical network reorganization in early relapsing-remitting multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419838673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chard DT, Alahmadi AAS, Audoin B, et al. ; MAGNIMS Study Group. Mind the gap: From neurons to networks to outcomes in multiple sclerosis. Nat Rev Neurol. 2021;17(3):173–184. [DOI] [PubMed] [Google Scholar]
- 110. Basile B, Castelli M, Monteleone F, et al. Functional connectivity changes within specific networks parallel the clinical evolution of multiple sclerosis. Mult Scler J. 2014;20(8):1050–1057. [DOI] [PubMed] [Google Scholar]
- 111. Dogonowski AM, Siebner HR, Sorensen PS, et al. Resting-state connectivity of pre-motor cortex reflects disability in multiple sclerosis. Acta Neurol Scand. 2013;128(5):328–335. [DOI] [PubMed] [Google Scholar]
- 112. Rocca MA, Valsasina P, Meani A, Falini A, Comi G, Filippi M.. Impaired functional integration in multiple sclerosis: A graph theory study. Brain Struct Funct. 2016;221(1):115–131. [DOI] [PubMed] [Google Scholar]
- 113. Wen J, Goyal MS, Astafiev SV, Raichle ME, Yablonskiy DA.. Genetically defined cellular correlates of the baseline brain MRI signal. Proc Natl Acad Sci U S A. 2018;115(41):E9727–E9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Koubiyr I, Besson P, Deloire M, et al. Dynamic modular-level alterations of structural-functional coupling in clinically isolated syndrome. Brain. 2019; 142:3428–3439. [DOI] [PubMed] [Google Scholar]
- 115. Faivre A, Robinet E, Guye M, et al. Depletion of brain functional connectivity enhancement leads to disability progression in multiple sclerosis: A longitudinal resting-state fMRI study. Mult Scler. 2016;22(13):1695–1708. [DOI] [PubMed] [Google Scholar]
- 116. Cerqueira JJ, Compston DAS, Geraldes R, et al. Time matters in multiple sclerosis: Can early treatment and long-term follow-up ensure everyone benefits from the latest advances in multiple sclerosis? J Neurol Neurosurg Psychiatry. 2018;89(8):844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Schoonheim MM, Meijer KA, Geurts JJ.. Network collapse and cognitive impairment in multiple sclerosis. Front Neurol. 2015;6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Koubiyr I, Deloire M, Besson P, et al. Longitudinal study of functional brain network reorganization in clinically isolated syndrome. Mult Scler. 2020;26:188–200. [DOI] [PubMed] [Google Scholar]
- 119. Loitfelder M, Filippi M, Rocca M, et al. Abnormalities of resting state functional connectivity are related to sustained attention deficits in MS. PLoS One. 2012;7(8):e42862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Rocca MA, Valsasina P, Absinta M, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74(16):1252–1259. [DOI] [PubMed] [Google Scholar]
- 121. Castellazzi G, Debernard L, Melzer TR, et al. Functional connectivity alterations reveal complex mechanisms based on clinical and radiological status in mild relapsing remitting multiple sclerosis. Front Neurol. 2018;9:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Droby A, Yuen KS, Muthuraman M, et al. Changes in brain functional connectivity patterns are driven by an individual lesion in MS: A resting-state fMRI study. Brain Imaging Behav. 2016;10(4):1117–1126. [DOI] [PubMed] [Google Scholar]
- 123. Lopez-Gongora M, Escartin A, Martinez-Horta S, et al. Neurophysiological evidence of compensatory brain mechanisms in early-stage multiple sclerosis. PloS One. 2015;10(8):e0136786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hawellek DJ, Hipp JF, Lewis CM, Corbetta M, Engel AK.. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc Natl Acad Sci U S A. 2011;108(47):19066–19071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tona F, Petsas N, Sbardella E, et al. Multiple sclerosis: Altered thalamic resting-state functional connectivity and its effect on cognitive function. Radiology. 2014;271(3):814–821. [DOI] [PubMed] [Google Scholar]
- 126. Drzezga A. The network degeneration hypothesis: Spread of neurodegenerative patterns along neuronal brain networks. J Nucl Med. 2018;59(11):1645–1648. [DOI] [PubMed] [Google Scholar]
- 127. Poudel GR, Harding IH, Egan GF, Georgiou-Karistianis N.. Network spread determines severity of degeneration and disconnection in Huntington's disease. Hum Brain Mapp. 2019;40:4192–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Raj A, Powell F.. Models of network spread and network degeneration in brain disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(9):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW.. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73(6):1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Crossley NA, Mechelli A, Scott J, et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137(Pt 8):2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Froudist-Walsh S, Browning PG, Young JJ, et al. Macro-connectomics and microstructure predict dynamic plasticity patterns in the non-human primate brain. eLife. 2018;7:e34354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Perin R, Telefont M, Markram H.. Computing the size and number of neuronal clusters in local circuits. Front Neuroanat. 2013;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shimono M. Non-uniformity of cell density and networks in the monkey brain. Sci Rep. 2013;3:2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ganguly K, Poo MM.. Activity-dependent neural plasticity from bench to bedside. Neuron. 2013;80(3):729–741. [DOI] [PubMed] [Google Scholar]
- 135. Ksiazek-Winiarek DJ, Szpakowski P, Glabinski A.. Neural plasticity in multiple sclerosis: The functional and molecular background. Neural Plast. 2015;2015:307175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tahedl M, Levine SM, Greenlee MW, Weissert R, Schwarzbach JV.. Functional connectivity in multiple sclerosis: Recent findings and future directions. Front Neurol. 2018;9:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Faivre A, Rico A, Zaaraoui W, et al. Assessing brain connectivity at rest is clinically relevant in early multiple sclerosis. Mult Scler J. 2012;18(9):1251–1258. [DOI] [PubMed] [Google Scholar]
- 138. Liu Y, Wang H, Duan Y, et al. Functional brain network alterations in clinically isolated syndrome and multiple sclerosis: A graph-based connectome study. Radiology. 2017;282(2):534–541. [DOI] [PubMed] [Google Scholar]
- 139. Louapre C, Perlbarg V, Garcia-Lorenzo D, et al. Brain networks disconnection in early multiple sclerosis cognitive deficits: An anatomofunctional study. Hum Brain Mapp. 2014;35(9):4706–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Reich DS, Lucchinetti CF, Calabresi PA.. Multiple sclerosis. N Engl J Med. 2018;378(2):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Herculano-Houzel S. The glia/neuron ratio: How it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62(9):1377–1391. [DOI] [PubMed] [Google Scholar]
- 142. Ribeiro PF, Ventura-Antunes L, Gabi M, et al. The human cerebral cortex is neither one nor many: Neuronal distribution reveals two quantitatively different zones in the gray matter, three in the white matter, and explains local variations in cortical folding. Front Neuroanat. 2013;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH.. Neuron densities vary across and within cortical areas in primates. Proc Natl Acad Sci U S A. 2010;107(36):15927–15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Palomero-Gallagher N, Zilles K.. Cortical layers: Cyto-, myelo-, receptor- and synaptic architecture in human cortical areas. NeuroImage. 2019;197:716–741. [DOI] [PubMed] [Google Scholar]
- 145. Wagstyl K, Lepage C, Bludau S, et al. Mapping cortical laminar structure in the 3D BigBrain. Cereb Cortex. 2018;28(7):2551–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Pandya D, Petrides M, Cipolloni PB.. Cerebral cortex: Architecture, connections, and the dual origin concept. New York, NY: Oxford University Press; 2015. [Google Scholar]
- 147. Huntenburg JM, Bazin PL, Goulas A, Tardif CL, Villringer A, Margulies DS.. A systematic relationship between functional connectivity and intracortical myelin in the human cerebral cortex. Cereb Cortex. 2017;27(2):981–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Stankoff B, Jadasz JJ, Hartung H-P, Küry P, Zalc B, Lubetzki C.. Repair strategies for multiple sclerosis: Challenges, achievements and perspectives. Curr Opin Neurol. 2016;29(3):286–292. [DOI] [PubMed] [Google Scholar]
- 149. Purger D, Gibson EM, Monje M.. Myelin plasticity in the central nervous system. Neuropharmacology. 2016;110(Pt B):563–573. [DOI] [PubMed] [Google Scholar]
- 150. Lazari A, Lipp I.. Can MRI measure myelin? Systematic review, qualitative assessment, and meta-analysis of studies validating microstructural imaging with myelin histology. NeuroImage. 2021;117744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Mancini M, Karakuzu A, Cohen-Adad J, Cercignani M, Nichols TE, Stikov N.. An interactive meta-analysis of MRI biomarkers of myelin. eLife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. van der Weijden CWJ, García DV, Borra RJH, et al. Myelin quantification with MRI: A systematic review of accuracy and reproducibility. NeuroImage. 2021;226:117561. [DOI] [PubMed] [Google Scholar]
- 153. Cortese R, Collorone S, Ciccarelli O, Toosy AT.. Advances in brain imaging in multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419859722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Glasser MF, Van Essen DC.. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci. 2011;31(32):11597–11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Nakamura K, Chen JT, Ontaneda D, Fox RJ, Trapp BD.. T1-/T2-weighted ratio differs in demyelinated cortex in multiple sclerosis. Ann Neurol. 2017;82(4):635–639. [DOI] [PubMed] [Google Scholar]
- 156. Righart R, Biberacher V, Jonkman LE, et al. Cortical pathology in multiple sclerosis detected by the T1/T2-weighted ratio from routine magnetic resonance imaging. Ann Neurol. 2017;82(4):519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Geurts JJ, Bo L, Roosendaal SD, et al. Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2007;66(9):819–827. [DOI] [PubMed] [Google Scholar]
- 158. Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R.. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009;19(2):238–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Vercellino M, Plano F, Votta B, Mutani R, Giordana MT, Cavalla P.. Grey matter pathology in multiple sclerosis. J Neuropathol Exp Neurol. 2005;64(12):1101–1107. [DOI] [PubMed] [Google Scholar]
- 160. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC.. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61(4):1000–1016. [DOI] [PubMed] [Google Scholar]
- 161. Spano B, Giulietti G, Pisani V, et al. Disruption of neurite morphology parallels MS progression. Neurol Neuroimmunol Neuroinflamm. 2018;5(6):e502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wei W, Poirion E, Bodini B, et al. Predicting PET-derived myelin content from multisequence MRI for individual longitudinal analysis in multiple sclerosis. NeuroImage. 2020;223:117308. [DOI] [PubMed] [Google Scholar]
- 163. Zeydan B, Lowe VJ, Schwarz CG, et al. Pittsburgh compound-B PET white matter imaging and cognitive function in late multiple sclerosis. Mult Scler. 2018;24(6):739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]