Abstract
Background
Many low-grade gliomas (LGG) harbor isocitrate dehydrogenase (IDH) mutations. Although IDH mutation is known to be epileptogenic, the rate of refractory seizures in LGG with IDH mutation vs wild-type had not been previously compared. We therefore compared seizure pharmacoresistance in IDH-mutated and wild-type LGGs.
Methods
Single-institution retrospective study of patients with histologic proven LGG, known IDH mutation status, seizures, and ≥2 neurology clinic encounters. Seizure history was followed until histological high-grade transformation or death. Seizures requiring ≥2 changes in anti-epileptic drugs were considered pharmacoresistant. Incidence rates of pharmacoresistant seizures were estimated using competing risks methodology.
Results
Of 135 patients, 25 patients (19%) had LGGs classified as IDH wild-type. Of those with IDH mutation, 104 (94.5%) were IDH1 R132H; only 6 were IDH2 R172K. 120 patients (89%) had tumor resection, and 14 (10%) had biopsy. Initial post-surgical management included observation (64%), concurrent chemoradiation (23%), chemotherapy alone (9%), and radiotherapy alone (4%). Seizures became pharmacoresistant in 24 IDH-mutated patients (22%) and in 3 IDH wild-type patients (12%). The 4-year cumulative incidence of intractable seizures was 17.6% (95% CI: 10.6%-25.9%) in IDH-mutated and 11% (95% CI: 1.3%-32.6%) in IDH wild-type LGG (Gray’s P-value = .26).
Conclusions
22% of the IDH-mutated patients developed pharmacoresistant seizures, compared to 12% of the IDH wild-type tumors. The likelihood of developing pharmacoresistant seizures in patients with LGG-related epilepsy is independent to IDH mutation status, however, IDH-mutated tumors were approximately twice as likely to experience LGG-related pharmacoresistant seizures.
Keywords: 2-hydroxyglutarate, IDH mutation, low-grade glioma, medically refractory seizure, pharmacoresistant epilepsy
Key Points.
Very few patients with IDH wild-type low-grade glioma experienced pharmacoresistant seizures.
Cumulative incidence of pharmacoresistant seizures is higher in patients with IDH-mutated vs IDH wild-type low-grade glioma.
Importance of the Study.
Seizure control represents an important aspect of treatment in patients with brain tumors. Patients with low-grade glioma who harbor an IDH mutation are known to have higher seizure incidence than those with IDH wild-type and an increase in seizure frequency is often a reason to treat these patients with tumor directed therapy. In our current study, we reviewed our institutional experience with seizure management in low grade glioma patients seen at Memorial Sloan Kettering Cancer Center. We observed 22% of the IDH-mutated patients developed pharmacoresistant seizures, compared to 12% of the IDH wild-type tumors. The likelihood of developing pharmacoresistant seizures in patients with LGG-related epilepsy was independent to IDH mutation status, however, IDH-mutated tumors were approximately twice as likely to experience LGG-related pharmacoresistant seizures. Based on our practice, we advocate that aggressive tumor-directed therapy should be considered in patients with medically refractory seizures regardless of IDH status.
Isocitrate dehydrogenase (IDH) gene mutation is observed in the majority of low-grade gliomas (LGG).1–3 The IDH1 and IDH2 genes encode for cytosolic and mitochondrial IDH, respectively.4 Mutant IDH reduces α-ketoglutarate, a derivative in the Krebs cycle, to 2-hydroxyglutarate (2-HG) (Figure 1).5,6IDH mutation is known to be one of the driver mutations in LGG, and the 2-HG accumulation is oncogenic.1,5,7 Despite its oncogenicity, IDH mutation correlates with better prognosis in gliomas, as IDH wild-type tumors tend to behave similar to higher-grade gliomas such as glioblastoma.1,3,8IDH-mutated gliomas also respond better to tumor-directed treatment than the wild-type gliomas.9,10
Figure 1.
Putative mechanism for epileptogenicity in IDH-mutant tumors.
IDH genes encode for isocitrate dehydrogenase (IDH). Mutant IDH reduces α-ketoglutarate in Krebs cycle to 2-hydroxyglutarate (2-HG), which is structurally similar to glutamate, an excitatory neurotransmitter that is both oncogenic and epileptogenic.
The incidence of seizures in LGG is about 70%-90% but is 59%-74% in IDH mutant compared to 18%-34% in IDH wild-type tumors. The 2-HG is structurally similar to glutamate, an excitatory neurotransmitter, and its accumulation in IDH-mutated gliomas is thought to be responsible for the increased seizure frequency.11,12 Medically refractory epilepsy is currently defined as persistent seizures after treatment with 2 first-line anti-epileptic drugs (AED) and has a negative effect on quality of life and neurocognitive function in patients with LGG.13,14 Although there have been many studies describing the epileptogenicity of IDH mutation in gliomas, the actual prevalence of medically refractory seizures associated with IDH mutation has not been reported.11–13,15 We evaluated seizure pharmacoresistance in IDH-mutated vs wild-type LGG.
Materials and Methods
Study Design and Participants
We undertook a single-institution, retrospective study approved by our Institutional Review Board. From institutional and departmental databases, we identified adult patients (≥18 years) who had histologic proven LGG with known IDH mutation status, seizure diagnosis, and ≥2 encounters in our neurology clinic from October 2005 to March 2020. Chart review was conducted to obtain information on patient demographics, tumor characteristics, and management.
Seizure History
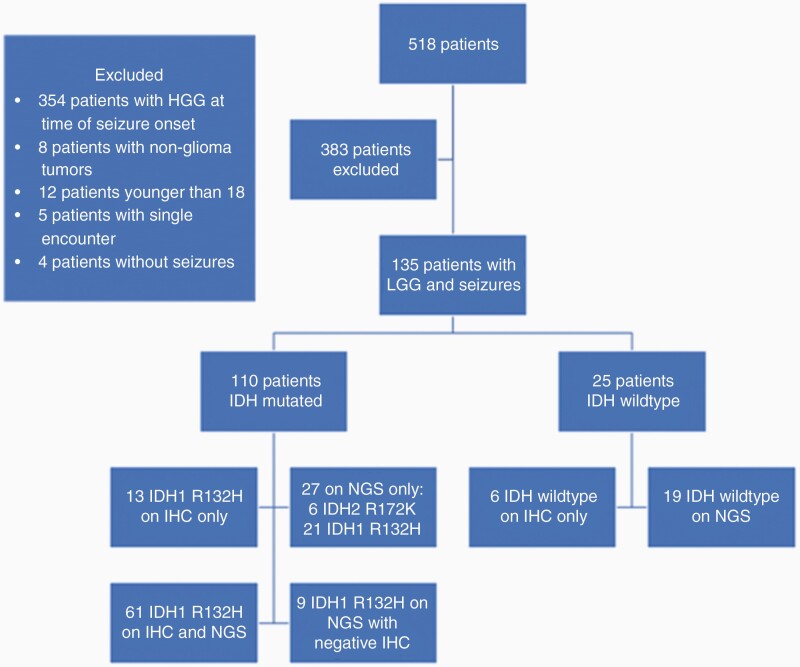
Seizures occurring at any time were included for analysis. Seizure history was followed until histological high-grade tumor transformation, last follow-up, or death. Seizures requiring ≥2 changes in maintenance AED agents were considered pharmacoresistant based on International League Against Epilepsy consensus guideline.16 Scheduled benzodiazepines were considered part of a maintenance regimen. Only AED changes made for reasons of inadequate seizure control were included in this analysis; changes for toxicity were not included. A total of 518 patients were pooled, however, 383 patients were excluded for developing seizures only after transformation to high-grade tumors (Figure 2).
Figure 2.
Consort flow chart for study participants by IDH status.
Abbreviations: IDH, isocitrate dehydrogenase; IHC, immunohistochemistry; LGG, low-grade glioma; NGS, next-generation sequencing.
Statistical Analysis
Simple descriptive statistics such as medians, ranges, and proportions were used to characterize the cohort under study. Variables of interest were compared by IDH mutation status (mutated vs wild-type) using the chi-squared test or Fisher exact test where appropriate for categorical variables and the Wilcoxon 2-sample test for continuous variables.
Incidence rates of pharmacoresistant seizures, defined as ≥2 changes in maintenance AEDs, were calculated using competing risks methodology from the time of histopathological diagnosis of LGG until time of histological high-grade tumor transformation, ≥2 changes in maintenance AED agents, last follow-up, or death. Competing events included histological high-grade tumor transformation and death. Cumulative incidence curves of seizure pharmacoresistance in patients with IDH-mutated and wild-type LGG were compared using Gray’s test.17 Given the small number of patients who experienced an intractable seizure event, multivariable modeling was not feasible
Furthermore, 4-year cumulative incidence rates of seizure pharmacoresistance were determined for both IDH-mutated and wild-type groups. Median overall survival (OS) and 95% confidence intervals (CIs) were determined using the Kaplan-Meier method where follow-up time was calculated from time of histopathological diagnosis of LGG until time of death (event) or last follow-up. OS curves were compared by IDH status using the log-rank test. Analyses were performed in SAS (version 9.4, Cary, NC) and R (version 4.0) software.
Results
IDH Mutation Testing
Initial tumor testing for mutant IDH R132H protein with immunohistochemistry (IHC) was positive in 74 patients (Table 1). Twenty-one tumors were tested with IHC only (13 positive, 6 negative), without further confirmatory testing. The 6 patients with negative IHC for IDH mutation without confirmatory next-generation sequencing were included in the IDH wild-type group based on how they were categorized and treated clinically. Allele-specific PCR confirmed 1 true-positive and 2 true-negative IHC results; however, it found one positive IDH1 R132H tumor which had a previously negative IHC. Gene sequencing confirmed 59 true-positive and 15 true-negative tumors for IDH mutation. Again, gene sequencing found 8 IDH1 R132H mutation which were negative on IHC results.
Table 1.
IDH Mutation Testing (Total n = 135)
| Test Modalities, n (%) | IDH Mutation | ||
|---|---|---|---|
| IDH1 R132H (n = 104, 77%) | IDH2 R172K (n = 6, 4%) | Not Detected (n = 25, 19%) | |
| IHC for mutant IDH1 R132H protein | |||
| IHC only | 13 | NA | 6 |
| Confirmed by allele-specific PCR | 2 | NA | 2 |
| Confirmed by gene sequencing | 59 | NA | 15 |
| Allele-specific PCR only | 5* | 0 | 0 |
| Gene sequencing only | 25* | 6 | 2 |
Abbreviations: IDH, isocitrate dehydrogenase; IHC, immunohistochemistry; NA; not applicable; PCR, polymerase chain reaction.
*IDH1 R132H mutation was detected by PCR (n = 1) and gene sequencing (n = 8) in patients with IHC false-negative tumors.
Five tumors that were tested with only allele-specific PCR revealed IDH1 R132H mutation. Thirty-one tumors showed IDH mutation on gene sequencing only. IDH1 R132H and IDH2 mutations were detected in 25 and 6 patients, respectively. Two patients were wild-type for IDH mutation.
Of our total cohort (n = 135), IDH1 R132H mutation was detected in 104 patients (77%), IDH2 R172K mutation in 6 patients (4%), and 25 patients (19%) did not harbor IDH mutations.
One IDH-mutated LGG also harbored a BRAF mutation. BRAF mutations were not detected in 19 out of 27 IDH wild-type tumors, and there were no data available for the remaining 8 tumors.
Patient Characteristics
In the 110 patients with IDH-mutated LGG, the median age at intervention was 38 years (range, 13-73, P-value <.001 for comparison with IDH wild-type) and 45% were women (n = 50) (Table 2). In 25 patients with IDH wild-type LGG, the median age was 52 years (range, 20-73, P-value <.001 for comparison with IDH-mutated) and 44% were women (n = 11).
Table 2.
Patient Demographics and Tumor Characteristics (Total n = 135)
| IDH-Mutated | IDH Wild-Type | P-valuea | |
|---|---|---|---|
| Patients, n | 110 | 25 | — |
| Median age at intervention (range) | 38 (13-73) | 52 (20-73) | <.001 |
| Women, n (%) | 50 (45%) | 11 (44%) | .8 |
| Intractable seizures, n (%) | 24 (22%) | 3 (12%) | 0.26 |
| LGG histology, n (%) | |||
| Oligodendroglioma | 70 (64%) | 0 (0%) | |
| 1p19q, n (%) | 67 (96%) | N/A | |
| Astrocytoma | 31 (28%) | 21 (84%) | <.001 |
| Glioma | 0 (0%) | 3 (12%) | |
| Mixed/indeterminate | 9 (8%) | 1 (4%) | |
| Initial surgical intervention, n (%) | |||
| Biopsy | 9 (8%) | 5 (20%) | |
| Subtotal resection | 64 (58%) | 19 (76%) | .003 |
| Gross total resection | 36 (33%) | 1 (4%) | |
| Unknown | 1 (1%) | 0 (0%) | |
| Post-surgical initial management, n (%) | |||
| Observation | 78 (71%) | 9 (36%) | |
| Chemotherapy | 12 (11%) | 0 (0%) | — |
| Radiotherapy | 2 (2%) | 3 (12%) | |
| Concurrent chemoradiation | 18 (16%) | 13 (52%) | |
| BRAF mutation, n (%) | 1 (1%) | — | |
| No mutation | 87 (81%) | 19 (70%) | |
| No data | 20 (18%) | 8 (30%) | |
| Dominant tumor site, n (%) | |||
| Gliomatosis | 0 (0%) | 3 (12%) | <.001 |
| Frontal | 65 (59%) | 5 (20%) | |
| Parietal | 15 (14%) | 1 (4%) | |
| Temporal | 27 (24%) | 16 (64%) | |
| Occipital | 3 (3%) | 0 (0%) |
Abbreviations: IDH, isocitrate dehydrogenase; LGG, low-grade glioma; N/A; not applicable.
aWilcoxon 2-sample test for age comparison. Chi-squared or Fisher exact test where appropriate for categorical comparisons with the exception of intractable seizures. Intractable seizures are compared across IDH groups using Gray’s test in the competing risks setting. Post-surgical initial management was compared across IDH groups using Fisher exact test since dates of treatment were not known in all cases.
IDH-mutated LGGs included 70 oligodendrogliomas (64%), 31 astrocytomas (28%), and 9 mixed gliomas (8%). Oligodendrogliomas had 1p/19q co-deletion in 67/70 and all mixed gliomas were 1p/19q. IDH wild-type LGGs comprised 21 astrocytomas (96%), 3 glioma (12%), and 1 mixed gliomas (4%).
IDH-mutated LGGs were located in the frontal (n = 65, 59%), temporal (n = 27, 24%), parietal (n = 15, 14%), and occipital lobes (n = 3, 3%) (Figure 3). IDH wild-type LGGs were located in the temporal (n = 16, 64%), frontal (n = 5, 20%), parietal (n = 1, 4%), and none on occipital lobes. There were 3 IDH wild-type LGGs (12%) with a gliomatosis pattern.
Figure 3.
Tumor location by IDH mutation status.
Out of 110 IDH-mutated LGGs (Mut), tumors were located in frontal (n = 65, 59%), temporal (n = 27, 24%), parietal (n = 15, 14%), and occipital lobes (n = 3, 3%). Tumor location of 25 IDH wild-type (WT) LGGs were temporal (n = 16, 64%), frontal (n = 5, 20%), parietal (n = 1, 4%), and occipital lobes (n = 0). There were 3 (12%) IDH wild-type LGG with gliomatosis pattern. Abbreviations: IDH, isocitrate dehydrogenase; LGGs, low-grade gliomas.
Tumor Management
The initial surgical treatment of IDH-mutated LGGs included biopsy in 9 patients (8%), subtotal resection in 64 (58%), gross total resection in 36 (33%), and unknown in 1. In the IDH wild-type group, 5 patients (20%) had a biopsy, 19 (76%) had a subtotal resection and only 1 (4%) had a gross total resection.
In the IDH-mutated cohort, 78 patients (71%) were observed post-surgically without additional tumor-directed therapy within the subsequent 6 months. In the remaining IDH-mutated patients, post-surgical treatment included temozolomide alone (n = 12, 11%), radiotherapy alone (n = 2, 2%), concurrent chemoradiation (n = 18, 16%), and 1 patient received a vaccine on a clinical trial. For patients receiving radiation, the dose was 4860-6000 cGy.
In the 25 IDH wild-type patients, only 9 (36%) were observed postoperatively. Thirteen received concurrent chemoradiation with temozolomide (52%), and 3 (12%) received radiotherapy alone. None of the IDH wild-type LGGs were treated with chemotherapy alone. The radiation dose was 5040-6000 cGy.
Patient Follow-up and Seizure Pharmacoresistance
Seizures became pharmacoresistant in 24 (22%) IDH-mutated patients, with a median time of 28.3 months (range, 0.1-107.6) from LGG diagnosis to refractory seizures. Three IDH wild-type patients (11%) developed pharmacoresistant epilepsy, with a median time of 47.4 months (range, 4.4-127) from diagnosis. In the 24 IDH-mutated patients with pharmacoresistant seizures, AEDs were changed a median of 3 times (range, 2-7), and 2 times in all IDH wild-type patients with pharmacoresistant seizures. The median follow-up of survivors for the overall cohort was 61.47 months whereas the median time to pharmacoresistant seizures was 30.28 months.
There was no statistically significant difference by IDH type in the incidence of pharmacoresistant epilepsy (P = .263), but a clinically relevant trend of greater incidence in IDH-mutated patients was observed (Supplementary Figure 1). The 4-year cumulative incidence of pharmacoresistant seizures was 17.6% (95% CI: 10.6%-25.9%) in IDH-mutated and 11% (95% CI: 1.3%-32.6%) in IDH wild-type LGGs (Table 3).
Table 3.
Cumulative Incidence Table of Seizure Intractability by IDH Status
| Time | Estimate (%) | Lower 95% CI (%) | Upper 95% CI (%) | Number of Patients at Risk |
|---|---|---|---|---|
| 1 Year | ||||
| Mutant | 6.5 | 2.9 | 12.3 | 96 |
| WT | 4.2 | 0.3 | 18 | 19 |
| 2 Years | ||||
| Mutant | 10.4 | 5.5 | 17.2 | 81 |
| WT | 4.2 | 0.3 | 18 | 12 |
| 3 Years | ||||
| Mutant | 13.8 | 7.9 | 21.4 | 66 |
| WT | 4.2 | 0.3 | 18 | 6 |
| 4 Years | ||||
| Mutant | 17.6 | 10.6 | 25.9 | 49 |
| WT | 11 | 1.3 | 32.6 | 3 |
Abbreviations: CI, confidence interval; IDH, isocitrate dehydrogenase; WT, wild-type.
At the end of study follow-up, 100 IDH-mutated LGG patients (90.9%) and 13 IDH wild-type LGG patients (52%) were still alive, with median follow-up duration of 65.5 months (range, 2.9-210.9) and 24.2 months (range, 3.3-126.9), respectively. Twenty-one IDH-mutated (19%) and 9 IDH wild-type (36%) patients had histologically confirmed high-grade transformation at a median of 36.2 months (range, 3.5-198.0) and 17.6 months (range, 8.8-95.9), respectively, from the time of LGG diagnosis. The median OS in IDH-mutated patients was not reached, which was significantly longer than the 47-month median (95% CI: 27-NR) in IDH wild-type patients (P < .001) (Supplementary Figure 2).
We conducted a post hoc comparison of OS and cumulative incidence of pharmacoresistant seizures between IDH-mutant astrocytomas and IDH wild-type tumors. These results restricted to astrocytomas were similar to the results of the overall cohort. That is, IDH wild-type astrocytoma patients had statistically significantly worse OS (HR = 5.81, 95% CI: 1.99-17.0, P-value = .001) compared with IDH-mutant astrocytoma patients. Additionally, there was no difference in cumulative incidence of intractable seizures between the IDH-mutant and IDH wild-type astrocytoma patients (Gray’s test P-value = .467).
Discussion
Previous studies demonstrated that IDH-mutated LGGs are more likely to develop seizures compared to IDH wild-type LGGs11,12 This is the first study to describe the incidence of pharmacoresistant seizures in relationship to IDH mutation in LGG. While we observed that the incidence of pharmacoresistant epilepsy was not statistically associated with IDH mutation status among these patients, there was a significant clinical trend toward greater seizure pharmacoresistance in the IDH-mutated group, and very few IDH wild-type tumors developed seizure pharmacoresistance at all. We examined the length of follow-up as a surrogate for the number of clinic visits (ie, data points) and median follow-up of survivors for the overall cohort was 61.47 months whereas the median time to pharmacoresistant seizures was 30.28 months. These results bolster our confidence that we captured the majority of events, however, it is conceivable that some events were missed.
There are several confounding factors that may play a role in our study results. First, half the tumors of the IDH-mutated group were oligodendrogliomas. Oligodendrogliomas have long been known to have a higher incidence of seizures than astrocytomas,18 but perhaps this reflects the fact that pure oligodendrogliomas are IDH-mutated, and the mutation may partially contribute to the seizure frequency. According to the 2016 World Health Organization (WHO) classification of tumors of the Central Nervous System, the diagnosis of oligodendroglioma now requires the demonstration of both IDH mutation and 1p19q co-deletion.3 In our cohort, 67/70 IDH-mutated oligodendrogliomas had a 1p19q co-deletion. Those classified as mixed glioma either were 1p19q intact or there was insufficient material for testing.
Second, while most IDH-mutated tumors were oligodendroglioma, the majority of IDH wild-type tumors were astrocytoma. Both histological subtypes preferentially affected the frontal and temporal lobes, which are regions most associated with seizures.13 However, it is interesting that IDH-mutated subtypes predominated in the frontal lobe while the wild-type predominated in the temporal lobe, the most epileptogenic region of the brain.
Third, an important observation was the false-negative rate of IHC testing, suggesting that gene sequencing is essential in all IHC-negative specimens. We were not able to perform confirmatory testing in all our specimens due to tissue acquisition problems across different institutions or paucity of adequate tumor samples. The 2016 WHO classification recommends sequencing of IDH1 codon 132 and IDH2 codon 172 in the absence of positive IDH1 R132H on IHC,3 therefore, more reliable IDH mutation profile should be obtained in future studies.
Last, initial tumor-directed therapy differed between the IDH-mutated and wild-type groups. Gross total resection is associated with improvement in seizure control19–21; however, we observed a higher tendency for pharmacoresistant seizures in IDH-mutated LGG patients despite the higher proportion who achieved gross total resection. Post-surgical tumor management also differed between the 2 groups; the majority of the mutated group had clinical and radiographic surveillance, whereas the wild-type group was aggressively treated with upfront chemotherapy and radiation. This is in accordance with the trend for IDH wild-type LGG to be treated as high-grade gliomas due to their poor prognosis.3,15 More aggressive tumor-directed treatment in IDH wild-type LGG might have resulted in a lower incidence of pharmacoresistant seizures as chemotherapy and radiation have both been shown to reduce seizures in multiple studies.13,22 Median OS was not reached for patient with IDH-mutated LGG, however, patient with wild-type LGG had a median OS of 47 months (95% CI: 27-NR, P < .001). These results, again, validate the notion that IDH mutation is prognostic in patients with LGG.3,15
In this study, the likelihood of developing pharmacoresistant seizures in patients with LGG-related epilepsy is independent to IDH mutation status, however, IDH-mutated tumors were approximately twice as likely to experience LGG-related pharmacoresistant seizures. Based on our practice, we advocate that aggressive tumor-directed therapy should be considered in patients with medically refractory seizures regardless of IDH status. Future studies correlating seizure frequency and quantitative measurements of 2-HG, with tests such as magnetic resonance spectroscopy,23 may provide additional insights into the relationship between IDH mutation and tumor-associated epilepsy.
Supplementary Material
Funding
This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center [Support Grant P30 CA008748].
Conflict of interest statement. The authors declare that they have no conflict of interest.
References
- 1. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11(4):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 4. Pollard PJ, Ratcliffe PJ. Cancer. Puzzling patterns of predisposition. Science. 2009;324(5924):192–194. [DOI] [PubMed] [Google Scholar]
- 5. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17(3):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 10. Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32(8):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liubinas SV, D’Abaco GM, Moffat BM, et al. IDH1 mutation is associated with seizures and protoplasmic subtype in patients with low-grade gliomas. Epilepsia. 2014;55(9):1438–1443. [DOI] [PubMed] [Google Scholar]
- 12. Chen H, Judkins J, Thomas C, et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88(19):1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armstrong TS, Grant R, Gilbert MR, Lee JW, Norden AD. Epilepsy in glioma patients: mechanisms, management, and impact of anticonvulsant therapy. Neuro Oncol. 2016;18(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soffietti R, Baumert BG, Bello L, et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17(9):1124–1133. [DOI] [PubMed] [Google Scholar]
- 15. Buckner J, Giannini C, Eckel-Passow J, et al. Management of diffuse low-grade gliomas in adults - use of molecular diagnostics. Nat Rev Neurol. 2017;13(6):340–351. [DOI] [PubMed] [Google Scholar]
- 16. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. [DOI] [PubMed] [Google Scholar]
- 17. Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 18. Beaumont A, Whittle IR. The pathogenesis of tumour associated epilepsy. Acta Neurochir (Wien). 2000;142(1):1–15. [DOI] [PubMed] [Google Scholar]
- 19. Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108(2):227–235. [DOI] [PubMed] [Google Scholar]
- 20. Englot DJ, Berger MS, Barbaro NM, Chang EF. Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A review. J Neurosurg. 2011;115(2):240–244. [DOI] [PubMed] [Google Scholar]
- 21. Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist. 2014;19(7):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Avila EK, Chamberlain M, Schiff D, et al. Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro Oncol. 2017;19(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de la Fuente MI, Young RJ, Rubel J, Rosenblum M, et al. Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro Oncol. 2016;18(2):283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.