Abstract
This study investigated the hemato- and genotoxic effects of formaldehyde (FA) and the possible mitigating role of hesperidin (HP) and N-acetylcysteine (NAC), each alone and in combination. Sixty-four adult male albino rats were divided into eight equal groups; the study was conducted for 8 weeks; Group I (negative control: received no medication), Group II (positive control: received distilled water), Group III (received HP 50 mg/kg/day), Group IV (received NAC 50 mg/kg/day), Group V (received FA 10 mg/kg/day), Group VI (FA + HP), Group VII (FA + NAC), and Group VIII (FA + HP + NAC). Groups VI, VII, VIII received the same previously mentioned doses and for the same duration. All treatments were given by intraperitoneal administration. At the end of the study, complete blood count, oxidative stress, histopathological changes, immunohistochemical staining of inducible nitric oxide synthase, and proliferating cell nuclear antigen and genotoxicity by comet assay in the bone marrow of treated rats were assessed. FA administration caused significant hematotoxicity represented by elevated white blood cell numbers and serum malondialdehyde levels and reduced red blood cell numbers, platelets, and serum superoxide dismutase values. Histologically, it induced an increase in fat cell numbers in bone marrow tissue with a widening of marrow spaces and decreased cellularity of hematopoietic cells, megakaryocytes, and granulocytes. FA exposure significantly decreased immunoreactivity for proliferating cell nuclear antigen, whereas the immunoreactivity for inducible nitric oxide synthase was increased. Genotoxicity, as measured by comet assay, revealed a significant increase in comet% and tail length in FA-treated group when compared with other groups. The cotreatment with HP and NAC revealed their ability to protect against hematological changes, oxidative damage, histopathological, and immunohistochemical changes, and genotoxicity induced by FA.
Keywords: formaldehyde, hematotoxicity, immunohistochemistry, genotoxicity, hesperidin, N-acetylcysteine
1. Introduction
Formaldehyde (FA) is a colorless gas that is highly soluble in water, has a pungent odor, and is an irritant in pure form. The solid form of FA is called paraformaldehyde, whereas the liquid form is known as formalin, which can easily produce toxic FA gas [1]. It is present in the body as a normal metabolite in blood, intercellular tissue, and within cells. Its exogenous sources include pharmaceuticals, cosmetics, paper, furniture, cigarette smoke, plywood, and foodstuff [2].
Though, inhalation is still the principal exposure route for FA [3], oral exposure is a common concern as it is illegally added as a preservative called (E240) in different foodstuffs and some drugs. It is also used to reduce bacterial load (especially Salmonella) in poultry feed. The recommended daily exposure to FA from the food of animal and plant origin should not exceed 100 mg/kg food per day [4].
FA is a known human leukemogenic and is hematotoxic in humans and mice. It is believed to induce bone marrow toxicity by affecting myeloid progenitor growth and survival through oxidative damage, apoptosis, and changes in hematopoietic growth factor and receptor levels [5].
Hesperidin (HP) is a bioflavonoid with active pharmacological properties that is present at high levels in citrus fruits such as oranges, lemons, and grapefruits [6]. It has hydrogen radical and hydrogen peroxide removal activities. Hence, it is reported to have lipid-lowering, anti-inflammatory, antioxidant, antihypertensive, anticarcinogenic, neuroprotective, and antiedema properties [7].
N-acetylcysteine (NAC) is an N-acetyl derivative of the amino acid L-cysteine. It is a safe and inexpensive medication. This drug is not found in natural sources, although cysteine is present in some meals like chicken and turkey meats, garlic, yogurt, and eggs [10]. It is considered as an aminothiol-containing antioxidant that has been used broadly as a mucolytic agent, in the treatment of acetaminophen toxicity, as a cytoprotective agent during cancer chemotherapy, in liver diseases, and the prevention of contrast-induced nephropathy [11]. So the present work aimed to evaluate the ameliorative effects of HP and NAC against hematoxic and genotoxic impact of FA exposure.
2. Material and Methods
2.1. Chemicals
-
▪
FA: purchased from Sigma-Egypt (Nasr city, Cairo), in the form of a clear liquid of 37% FA. Its CAS No. is 50-00-0, with molecular weight: 3.026 g/mol.
-
▪
HP: purchased from Sigma-Egypt, like tan or pale yellow powder (25 mg) of HP 95%. Its CAS No. is 520-26-3.
-
▪
NAC: effervescent instant sachets (600 mg each), obtained from Sidico Pharma Industries, Egypt.
-
▪
Distilled water: was obtained from El-Nasr Co., Egypt, and used as a solvent for FA, HP, and NAC.
2.2. Animals and experimental design
The study was carried out on 64 adult male albino rats, 8 rats in each group, each weighing 200–220 g with an average age of 70–80 days. They were obtained from the animal house, Faculty of Medicine, Zagazig University.
The rats received balanced food rich in all stuff necessary to maintain their health before and during drug administration. It consisted of bread, barley, and milk. Water was offered in separate clean containers. All rats were acclimatized for a 2-week period before the experiment.
All animals received humane care in compliance with the Animal Care Guidelines of the National Institutes of Health (NIH), and the local committee approved the design of the experiment.
(Approval number: ZU-IACUC/3/F/102/2018).
2.3. Grouping of animals
The rats were classified into eight groups with eight rats in each group:
Group I (negative control group): rats received only a regular diet and tap water for 8 weeks to measure the basic parameters.
Group II (positive control: vehicle): rats received distilled water 1 ml/day once daily by oral gavage for 8 weeks.
Group III (HP group): rats received HP dissolved in distilled water in a dose of 50 mg/kg/day [12, 68, 69], by intraperitoneal injection for 8 weeks [13].
Group IV (NAC group): rats received NAC dissolved in distilled water in a dose of 50 mg/kg/day [14, 66, 67], by intraperitoneal injection for 8 weeks [15, 16]. The used dose constitutes the rat’s dose corresponding to the human average daily dose (600 mg/day), according to Paget and Barne’s table for conversion of doses [70].
Group V (FA-treated group): rats received FA in a dose of 10 mg/kg/day [17, 76, 77] dissolved in distilled water [13], by intraperitoneal injection [18, 19] for 8 weeks. This dose represents 1/10 of the rat-LD50 according to reference [20].
Group VI (FA + HP group): rats received HP and FA.
Group VII (FA + NAC group): rats received NAC and FA.
Group VIII (FA + HP + NAC group): rats will be given HP, NAC, and FA.
Groups VI, VII, and VIII received the same previously mentioned doses and for the same duration. HP and NAC in these groups were injected independently 2 hours before FA intraperitoneal injection.
At the end of the study (24 hours from the last dose of each substance), rats from each group were subjected to blood samples collection for the estimation of biochemical parameters: complete blood count (CBC) [total white blood corpuscles (WBCs), red blood corpuscles (RBCs), and platelets], malondialdehyde (MDA), and superoxide dismutase (SOD). The rats were euthanized using the cervical dislocation method. Then, the bone marrow was obtained from femurs and processed for histopathological examination, immunohistochemical staining. Comet assay was performed for detection of deoxyribonucleic acid (DNA) damage and genotoxicity.
2.4. Methods
2.4.1. Biochemical study
2.4.1.1. Blood sample collection
Venous blood samples were collected using microcapillary glass tubes from the retro-orbital plexus under sodium thiopental anesthesia 50 mg/kg intraperitoneal [78]. The animals were then sacrificed by cervical dislocation.
2.4.1.2. Assessment of CBC (total WBCs, RBCs, and platelets)
Total WBCs, RBCs, and platelets were measured by the method of impedance technology, which is based on the fact that blood cells are poor conductors of electricity. Cells are diluted with an electrolyte, directed to a moving stream, and pass through a small opening within a transducer (detection device). As each cell passes through the small opening of the aperture, an electrical field exists in the transducer, and when the cells pass through the aperture orifice (small opening), the increase in electrical impedance (resistance) is measured [21].
2.4.1.3. Antioxidant enzymes and lipid peroxidation
For assessment of markers of oxidative stress, blood samples were put into a clean centrifuge tube and incubated at 37°C until blood clotted and then centrifuged for 10 minutes to separate the serum, and a pipette was used to separate the top layer.
2.4.1.4. Assessment of serum MDA level (nmol/ml)
Serum MDA was assayed using MDA biodiagnostic kit according to the method described by Oliveira and Cecchini [22], where MDA can react with thiobarbituric acid in acidic media at a temperature of 95°C for 30 minutes and give pink-colored trimethylene complex. MDA content in the serum was determined from the absorbance on a spectrophotometer at 530 and 572 nm.
2.4.1.5. Assessment of serum SOD (U/ml)
The estimation of the SOD enzyme was carried out by Beauchamp and Fridovich [23], where the substrate used for the assay consists of nitro blue tetrazolium chloride, which reacts with superoxide anions produced on the illumination of riboflavin in the presence of methionine as an electron donor, to produce formazan that is a blue-colored complex. The decrease in the formation of formazan is directly proportional to the amount of SOD in the sample; a 50% decrease in the formation of formazan is taken as one unit of SOD.
2.4.2. Histopathological study
2.4.2.1. The method used for femoral bone dissection and bone marrow isolation
After scarification of rats, it was fixed in a supine position and sprayed with 70% ethanol. A small incision was made to the right of the midline in the lower abdomen, just above the hip; then the incision was extended down the leg and past the ankle joint. The skin was pulled back and the muscles were cut down to the proximal end of the femur. After reaching the femoral head, the femur was dislocated. Then, any additional muscles or connective tissue attached to the femur was removed [24].
2.4.3. Light microscopy
Histopathology slice preparation from the femurs was obtained from two animals in each group. Samples were incubated in the fixing solution [saturated 2,4,6-trinitrophenol: formalin: glacial acetic acid (15:5:1)] at room temperature for 24 hours then stained with hematoxylin and eosin (H&E). The tissues were embedded in paraffin, following by sectioning into slices 10 mm thickness and examined under a light microscope [25].
2.4.4. Immunohistochemistry
Bone marrow sections were processed for immunohistochemical staining and incubated for 1 hour with polyclonal rabbit anti-inducible nitric oxide synthase [anti-iNOS (dilution 1:100)] and anti-proliferating cell nuclear antigen (anti-PCNA) for 2 hours at room temperature in phosphate-buffered saline, pH 6. The peroxidase activity was detected using a 3,3′ diaminobenzidine (DAB) kit (Dako). DAB was used as a chromogen, which is converted into a brown precipitate. Slides were rinsed in distilled water, immersed in hematoxylin for half a minute, then rinsed in tap water until blue. Negative controls were done by applying the same steps but omitting the step of adding the primary antibodies. A positive reaction appears as brown deposits in the cytoplasm for iNOS and as a brown nuclear reaction for PCNA [71, 72].
2.4.5. Morphometric study
Morphometric analysis of sections stained with iNOS and PCNA was done by Leica Qwin 500 Image Analyzer Computer System at Pathology Department, Faculty of Dentistry, Cairo University. The optical density of iNOS and PCNA were assessed and measured in casually chosen five fields/section in different sections from each group.
2.4.6. Single-cell gel electrophoresis (comet) assay
A small number of cells are embedded in a thin agarose layer, lysed, exposed to electrophoresis, and stained with a fluorescent DNA intercalating dye (ethidium bromide). DNA fragments resulting from DNA damage migrate faster than undamaged DNA. A comet-like structure with a head (undamaged DNA) and a tail (DNA fragments) are formed. Alkaline lysis is preferred as it detects single-strand breaks, double-strand breaks, and alkali labile sites [26].
2.5. Statistical analysis
Analysis of data was done using the SPSS program (Statistical Package for Social Science) version 25.0. For normally distributed data, comparison between the four studied groups was analyzed using one-way analysis of variance (ANOVA or F-test) and least significant difference (LSD) test. All data were expressed as mean + standard deviation (SD). P-value <0.05 was considered statistically significant [27].
3. Results
During the study, three rats died in the groups FA, FA + HP, and FA + HP + NAC, one rat from each group. However, there was a nonstatistical significant association between treatment and mortality rates (P > 0.99) determined by the Fisher’s exact test.
Regarding negative control, positive control, HP, and NAC groups: there was no statistically significant difference between these groups in CBC values (total WBCs, RBCs, and platelets), oxidative stress parameters (MDA and SOD), the optical density of iNOS and PCNA, comet%, and tail length. So, we considered the negative control group as the control one for comparison with other groups (Tables 1).
Table 1.
statistical comparison among negative control, positive control, HP and NAC groups as regard CBC, serum (MDA and SOD), comet%, tail length, optical density of iNOS, and optical density of PCNA after 8 weeks of treatment
| Variable | Negative control | Positive control | HP group | NAC group | F | P |
|---|---|---|---|---|---|---|
| WBC (×103/dl) | 7.08 ± .49 | 7.10 ± .49 | 6.95 ± .04 | 7.36 ± .42 | 1.467 | 0.245* |
| RBC (×106/μl) | 4.47 ± .27 | 4.41 ± .26 | 4.61 ± .42 | 4.22 ± .019 | 2.598 | 0.072* |
| Platelets (×103/dl) | 825.5 ± 11.38 | 855 ± 63.47 | 882.5 ± 63.19 | 789.5 ± 25.76 | 2.442 | 0.085* |
| Serum MDA (nmol/l) | 39.5 ± 7.88 | 38.38 ± 8.02 | 39.01 ± 8.05 | 4.09 ± 7.59 | 0.068 | 0.976* |
| Serum SOD (U/ml) | 34.93 ± 1.29 | 33.75 ± 1.41 | 34.16 ± 1.32 | 34.96 ± 1.18 | 1.663 | 0.198* |
| Comet% | 9.58 ± .33 | 9.68 ± .503 | 9.54 ± .49 | 9.43 ± .50 | 0.378 | 0.770* |
| Tail length (μm) | 5.59 ± 1.065 | 5.84 ± .94 | 5.61 ± .91 | 6.075 ± 1.14 | 0.388 | 0.762* |
| Optical density of iNOS | 12.32 ± 2.29 | 12.22 ± 2.29 | 12.11 ± 2.31 | 12.05 ± 2.32 | 0.0216 | 0.995* |
| Optical density of PCNA | 65.95 ± 1.94 | 66.05 ± 1.94 | 65.55 ± 1.93 | 64.99 ± 1.93 | 0.494 | 0.688* |
Eight rats per group.
Data are stated as mean ± SD. P-value < 0.05 is significant.
* P > 0.05, nonsignificant.
3.1. Biochemical results
3.1.1. CBC values (total WBCs, RBCs, and platelets)
Regarding total WBCs levels among the studied groups, there was a highly significant increase in mean WBCs values (p < .001) in FA-treated group when compared with negative control, FA + HP, FA + NAC, and FA + HP + NAC groups (Table 2).
Table 2.
effects of FA, HP, NAC, and their combination on laboratory findings after 8 weeks of treatment
| Variable | Negative control | FA | FA + HP | FA + NAC | FA + HP + NAC |
|---|---|---|---|---|---|
| WBCs (×103/dl) | 7.08 ± .49 | 18.53 ± .96a | 14.28 ± .54a,b | 11.93 ± .60abc | 8.91 ± .73abce |
| RBCs (×106/μl) | 4.47 ± .27 | 2.95 ± .26a | 3.50 ± .21a,b | 4.16 ± .36a,b,c | 4.88 ± .20a,b,c,e |
| Platelets (×103/dl) | 825.5 ± 110 | 647.9 ± 3.9a | 799.9 ± 82#,b | 795.6 ± 42#,b,d | 816 ± 31#,b,d,f |
| Serum MDA (nmol/l) | 39.5 ± 7.88 | 121.88±16a | 82.5 ± 2.8a,b | 67.63±5a,b,c | 52.36±1a,b,c,e |
| Serum SOD (U /ml) | 34.93 ± 1.29 | 8.49 ± .48a | 17.38 ± 2.5a,b | 23.75±3a,b,c | 36.01 ± .9#,b,c,e |
Eight rats per group.
Data are stated as mean ± SD. P-value <0.05 is significant. LSD following ANOVA expressed as letters.
a P < 0.001 significant versus control
b P < 0.001significant versus FA group
c P < 0.001
d P > 0.05 significant versus FA + HP group
e P < 0.001 significant versus FA + NAC group
# P > 0.05 nonsignificant.
There was highly significant decrease (P < .001) in FA + HP, FA + NAC, and FA + HP + NAC groups when compared with the FA group, whereas a highly significant increase (P < 0.001) in the FA + HP + NAC group when compared with the negative control group.
Regarding RBCs levels, there was a high significant decrease in mean RBCs values (P < 0.001) in FA-treated group when compared with negative control, FA + HP, FA + NAC, and FA + HP + NAC groups.
There was a high significant increase (P < 0.001) in the FA + HP, FA + NAC, and FA + HP + NAC groups when compared with the FA group. But there was no significant difference (P > 0.05) in the FA + NAC group when compared with the negative control group.
Regarding platelet levels, there was a highly significant decrease in mean platelet values (P < 0.001) in FA-treated group when compared with negative control group, FA + HP, FA + NAC, and FA + HP + NAC groups.
There was highly significant increase (P < 0.001) in FA + HP, FA + NAC, and FA + HP + NAC groups when compared with the FA group. But there were no significant differences (P > 0.05) in these groups when compared with negative control group and each other.
3.1.2. Oxidative stress parameters: MDA level (nmol/l) and SOD activity (U/ml) in the serum
Regarding MDA levels among the treated groups, there was a highly statistically significant increase in mean values of serum MDA levels in each of the treated groups when compared with the negative control group (P < 0.001; Table 2).
There was a highly significant increase (P < 0.001) in FA-treated group when compared with FA + HP, FA + NAC, and FA + HP + NAC.
Regarding SOD levels, there was a high statistically significant decrease (P < 0.001) in mean values of serum SOD levels in FA, FA + HP, and FA + NAC, but there was a nonsignificant difference (P > 0.05) in FA + HP + NAC when compared with the negative control group.
There was highly significant decrease (P < 0.001) in FA-treated group when compared with FA + HP, FA + NAC, and FA + HP + NAC groups.
3.2. Histopathological results
Histological examination of the control subgroups showed the same histological results, so the negative control group was considered the control group. H&E-stained sections of the control bone marrow slides showed normal histological architecture with a marked cellular crowdedness of various mature and immature hematopoietic cells with normal appearance including the characteristic megakaryocytes, granulocytes, and few fat cells (Fig. 1a).
Figure 1.

(a) H&E-stained sections of the control group (GI, II) showing normal bone marrow histology with variety of hematopoietic cells (H) of normal appearance, megakaryocyte cells (MK), granulocytes (G), and fat cells (F). (b) Examination of H&E-stained sections in rat bone marrow of HP group (GIII) also reveals normal histological structure with normal bone trabeculae (T) and variety of hematopoietic cells (H) with normal appearance of megakaryocyte cells (MK), granulocytes (G) and fat cells (F). (c) H&E-stained sections of NAC group (GIV) showing also normal bone trabeculae (T) and variety of hematopoietic cells (H) with normal appearance of megakaryocyte cells (MK) and fat cells (F). (d) Sections in FA-treated group (GV) reveal increased fat cells (F) in bone marrow with widening of marrow spaces and decreased cellularity of hematopoietic cells (H), megakaryocyte cells (MK), and granulocytes (G).
H&E-stained sections of the HP- and NAC-treated bone marrow slides also showed normal histological appearance with numerous mature and immature hematopoietic cells with normal appearance including the characteristic megakaryocytes, granulocytes with few fat cells (Fig. 1b and c).
H&E-stained sections of the FA-treated bone marrow slides showed increased fat cells with a widening of marrow spaces and decreased cellularity (Figs 1d and2e).
Figure 2.

(e) showing H&E-stained sections in FA-treated group (GV) with increased fat cells (F) in bone marrow with widening of marrow spaces and decreased cellularity of hematopoietic cells (H), megakaryocyte cells (MK), and granulocytes (G). (f) Higher magnification of sections in FA + HP group (GVI) showing improvement in bone marrow changes with decreased fat cells (F) and increased cellular portion of hematopoietic cells (H), megakaryocyte cells (MK), and granulocytes (G). (g) Sections in FA + NAC group (GVII) showing also improvement in bone marrow changes with decreased fat cells (F) and increased cellular portion of hematopoietic cells (H), megakaryocyte cells (MK), and granulocytes (G). (h) Examination of sections of FA + HP + NAC (GVIII) showing more improvement in bone marrow changes with decreased fat cells (F) and increased cellular portion of hematopoietic cells (H), megakaryocyte cells (MK), and granulocytes (G). H&E, ×100 (scale bar 50 μm). Only (f) H&E, ×200 (scale bar 20 μm).
H&E-stained sections of the FA + HP, FA + NAC, and FA + HP + NAC-treated bone marrow slides showed improvement in histological changes with decreased fat cells and increased cellular portion and appearance of numerous mature and immature hematopoietic cells with normal characteristics. The best improvement was in the combined administration of both HP and NAC (Fig. 2f–h).
3.3. Immunohistochemical results
Immunohistochemical examination of the control subgroups, HP and NAC groups showed the same immunohistochemical results. Immunohistochemical reaction for iNOS-stained sections in the bone marrow of the control groups showed weak positive reaction in the cytoplasm of bone marrow cells (Fig 3a). In FA-treated group, a strong positive reaction for iNOS in the cytoplasm of bone marrow cells was seen (Fig. 3b). In FA + HP-treated group, a decrease of iNOS reaction was seen (Fig. 3c). FA + NAC-treated group revealed a decrease of iNOS reaction (Fig. 3d). FA + HP + NAC group showed a marked decrease of iNOS reaction in the cytoplasm of bone marrow cells (Fig. 3e). Immunohistochemically stained sections for PCNA in bone marrow tissue of the control groups showed a strong positive reaction in the nuclei of numerous bone marrow cells (Fig. 4a). In FA-treated group, few cells with positive nuclear immunoreactivity of PCNA in the nuclei of bone marrow cells were seen (Fig. 4b). FA + HP-treated group showed numerous bone marrow cells with positive nuclear immunoreactivity for PCNA (Fig. 4c). FA + NAC-treated group revealed numerous bone marrow cells with positive nuclear immunoreactivity for PCNA (Fig. 4d). FA + HP + NAC group showed a strong positive reaction in the nuclei of most bone marrow cells (Fig. 4e).
Figure 3.

(a) immunohistochemical reaction for iNOS-stained sections in the bone marrow of the control groups showing weak positive reaction in the cytoplasm of bone marrow cells (arrow). (b) In FA-treated group, strong positive reaction for iNOS in the cytoplasm of bone marrow cells is seen (arrow). (c) FA + HP-treated group showing decrease of iNOS reaction (arrow). (d) FA + NAC-treated group reveals decrease of iNOS reaction (arrow). (e) FA + HP + NAC group showing marked decrease of iNOS reaction in the cytoplasm of bone marrow cells (arrow). iNOS, ×400 (scale bar 20 mm).
Figure 4.

(a) immunohistochemical reaction for PCNA-stained sections in the bone marrow of the control groups showing strong positive reaction in the nuclei of numerous bone marrow cells (arrow). (b) In FA-treated group, few cells with positive nuclear immunoreactivity for PCNA in the nuclei of bone marrow cells is seen (arrow). (c) FA + HP-treated group showing numerous bone marrow cells with positive nuclear immunoreactivity for PCNA (arrow). (d) FA + NAC-treated group reveals numerous bone marrow cells with positive nuclear immunoreactivity for PCNA (arrow). (e) FA + HP + NAC group showing strong positive reaction in the nuclei of most bone marrow cells (arrow). PCNA, ×400 (scale bar 20 mm).
3.4. Morphometric results
We observed a significant increase in the optical density of iNOS in the FA-treated group compared with the control, FA + HP, FA + NAC, and FA + HP + NAC-treated groups (P < .05; Table 3).
Table 3.
effects of FA, HP, NAC, and their combination on optical density of iNOS and PCNA immunoreaction after 8 weeks of treatment
| Variable | Negative control | FA | FA + HP | FA + NAC | FA + HP + NAC |
|---|---|---|---|---|---|
| Optical density of iNOS | 12.32 ± 2.29 | 52.35 ± 8.22a | 20.13 ± 2.26a,b | 2.45 ± 2.26a,b | 18.86 ± 2.26a,b |
| Optical density of PCNA | 65.95 ± 1.94 | 14.15 ± 10.53a | 33.05 ± .15a,b | 32.56 ± .15a,b | 35.84 ± .15a,b |
Eight rats per group
Data are stated as mean ± SD. P-value <0.05 is significant. LSD following ANOVA expressed as letters
aSignificant versus control
bSignificant versus FA.
Nevertheless, a significant decrease in the optical density of PCNA in the FA-treated group compared with the control, FA + HP, FA + NAC, and FA + HP + NAC-treated groups (P < 0.05; Table 3).
3.5. Comet assay
There was highly statistically significant increase in mean values of comet% and tail length levels in each of the treated groups when compared with the negative control group (P < 0.001), but a nonsignificant difference is present between FA + HP + NAC and control groups (P > 0.05; Table 4; Fig. 5). There was a high significant increase in mean values of comet% and tail length (P < 0.001) in the FA-treated group when compared with FA + HP, FA + NAC, and FA + HP + NAC groups. Meanwhile, a nonsignificant difference was recorded between FA + HP and FA + NAC groups.
Table 4.
effects of FA, HP, NAC, and their combination on comet parameters after 8 weeks of treatment
| Variable | Negative control | FA | FA + HP | FA + NAC | FA + HP + NAC |
|---|---|---|---|---|---|
| Comet% | 9.58 ± .33 | 13.43 ± 1.2a | 11.26 ± .97a,b | 11.09 ± .78a,b,c | 9.86 ± .83#,b,d,e |
| Tail length (μm) | 5.59 ± 1.065 | 13.1 ± .44a | 1.09 ± 1.9a,b | 10.59 ± .85a,b,c | 5.84 ± 1.38#,b,d,e |
Eight rats per group.
Data are stated as mean ± SD. P-value <0.05 is significant. LSD following ANOVA expressed as letters: aP < 0.001 significant versus control
b P < 0.001significant versus FA group
c P < 0.001
d P > 0.05 significant versus FA + HP group
e P < 0.001 significant versus FA + NAC group
# P > 0.05 nonsignificant.
Figure 5.
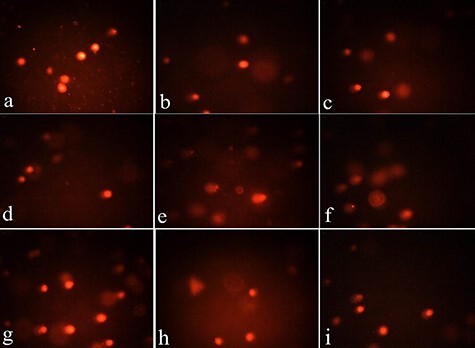
(a–d) microscopic pictures of comet assay in control cells from Groups I–IV showing no comet tails indicating no DNA damage. (e, f) Microscopic pictures from Group V treated with FA showed long DNA tails indicating considerable genotoxicity and DNA damage “the raise in DNA strand breaks was detected as large DNA amounts migrating outside the nucleus, toward the tail of the comet”. (g–i) microscopic pictures from Groups VI–VIII treated with FA along with HP, NAC alone and in combination showing less DNA tails indicating improvement in genotoxicity and DNA damage induced by FA (the best results in combination of both HP and NAC).
4. Discussion
The FA-treated group showed a highly significant increase in mean values of WBCs and a highly significant reduction in RBCs and platelet numbers. This can be explained by the fact that blood cells originate from hematopoietic stem cells and differentiate in the bone marrow. They have a higher sensitivity to chemicals or other environmental factors. So the blood system is more susceptible to damage than the body’s other organs, and one of the clinical significances of damage is an alternation in RBC, WBC, and platelet counts [28].
These results were in line with the results of Mohammed and Hassan [29] who reported that exposure to FA 0.1 ml resulted in a significant increase in WBCs and platelet numbers. These results were attributed to inflammation that accompanied the onset of arthritis induced by FA injection. But there was a significant reduction in RBCs numbers, hemoglobin, and hematocrit with bone marrow suppression or inactive erythropoiesis.
Aishwarya et al. [30] found that there was a marked increase in total WBCs count with reduced RBCs numbers and hemoglobin values in rats with FA-induced arthritis. The significant increase in total leukocyte numbers can be explained by the inflammatory processes that occur in the body after FA exposure that leads to the production of some inflammatory cytokines, which in turn initiate increased proliferation of WBCs [31].
Significant reduction of RBCs values with reduced hemoglobin and hematocrit can be explained by the effects of free radicals and active oxygen state that leads to pancytopenia. Also, defect in the function of the thyroid gland because of toxins secretion by FA, hormones of the thyroid gland has indirect effects on the production of RBCs from bone marrow [32].
A significant decrease in platelet numbers could be attributed to the great effect of cytokines, where it works on the maturation of generated cells of platelets and the effect of FA that cause bone marrow hematopoietic microenvironment damage and bone marrow toxicity [33].
However, in contrast to the results of this study, Zhang et al. [28]; Wei et al. [5], and Bassig et al. [34] reported a significant reduction in WBCs, lymphocytes, and RBCs count with a marked increase in platelets concerning toxicity or alteration in hematopoietic stem cell numbers induced by FA administration. Additionally, Yan Yu et al. [33] described an obvious reduction in WBC and platelet numbers in 40 and 80 mg/m3 inhaled FA concentrations, but there was no change in RBC numbers.
Coadministration of HP and NAC, alone and in combination caused a significant reduction in WBCs levels and a significant increase in RBCs and platelet numbers. Merzoug and Toumi [13] reported that coadministration of HP and FA could alleviate the significant decrease in RBCs and platelet numbers in FA-treated rats, but fail to restore WBCs count.
Afolabi et al. [35] discovered that HP cotreatment caused significant improvement in the anemic and thrombocytopenic effects promoted by exposure to aluminum phosphide in Wister rats. This could be attributed to the effectiveness of HP in the amelioration of oxidative stress in blood and the active osmoregulatory and biomembrane stabilizing properties that help to recover the hematological profile [36, 37].
El-Maddawy and El-Sayed [38] reported the efficient role of NAC in reducing paracetamol hemato- and nephrotoxicity when administered 2 weeks before paracetamol exposure. This could be explained by its free radical-scavenging effect due to its thiol content and its ability to act as an effective glutathione precursor. Increased glutathione levels preserve cellular structures and cause a reduction in oxidative damage and lipid peroxidation [39].
The results of the present study revealed that FA administration caused a significant elevation in serum MDA and a significant reduction in serum SOD. Ciftci et al. [40] reported a significant increase in serum MDA levels after FA exposure and Yan Yu et al. [33] stated that FA exposure caused a decrease in SOD activity and an increase in MDA content with different concentrations of inhaled FA.
Gerin et al. [17] concluded that the reduction in SOD activities and elevated MDA levels specify that FA impairs the pro-oxidant/antioxidant balance in favor of the pro-oxidant system. These results could be explained by the ability of FA to damage biological membranes and causing lipid peroxidation, so MDA level as a peroxidation product was increased; in addition, SOD is being consumed to defend against free radical damage, so SOD activity was decreased. When the body’s antioxidant capacity is depleted, the generation and elimination of free radicals are out of balance [33].
Regarding HP, the results of the present study were following the results of Afolabi et al. [35] and Turk et al. [41] who reported that treatment with HP prevented the aluminum phosphide and sodium arsenite-induced inhibition of SOD activities and the increase in MDA concentrations in the ovary, kidney, and liver. HP has been reported to provide strong cellular antioxidant protection against chemical-induced oxidative damage. The strong anti-inflammatory activity of HP was mainly recognized by its antioxidant defense mechanism and suppression of pro-inflammatory cytokine production [8].
Regarding NAC, Garg et al. [42] demonstrated that NAC successfully diminished oxidative stress and neurodegeneration in aged rats and caused a significant reduction in MDA levels and elevation in SOD activity in these rats. Huang et al. [43] detected the beneficial effects of NAC in improving the activities of antioxidant enzymes as SOD and inhibited MDA formation. NAC detoxifies free radicals by nonenzymatic reactions either by conjugation or reduction. Cellular inflammation and apoptosis can be inhibited by intracellular thiol reductants and free radical scavengers [44].
Light microscopic examination of sections of bone marrow tissues in the FA-treated group, exposed increased adipocytes in bone marrow with a widening of marrow spaces and decreased cellularity. Zhang et al. [28] reported histological changes induced by FA inhalation in the bone marrow in the form of increased numbers of megakaryocytes and myelofibrosis.
Yan Yu et al. [33] demonstrated that a decrease in peroxiredoxin 2 expression, which acts as a key scavenger of reactive oxygen species, was related to the bone marrow toxicity induced by FA. It increases oxidative stress with subsequent toxic effects and inflammatory responses. The above histopathological findings could be explained by the induction of oxidative damage in bone marrow, inflammation, and apoptosis [28].
The protective effects of HP were confirmed by Ahmadi et al. [45] and Hassouna et al. [46]. The hematoprotective effects of HP can be exposed by strong antioxidant and anti-inflammatory properties. It also inhibits lipid peroxidation and NO production, which is considered to be an important pro-inflammatory mediator [47]. HP is associated with inhibition of TNF-α and interleukin-6 production, which plays a vital role in the management of inflammatory disorders and immune modulation [48].
Regarding NAC, Al-Tonbary et al. [49] demonstrated its beneficial role as an antioxidant complementary therapy in children with acute lymphoblastic leukemia that can decrease bone marrow hypoplasia related to chemotherapy. Antioxidant effects of NAC that promote bone marrow protection were related to its ability to act as a precursor of cysteine, which is required in glutathione synthesis, and also due to its ability to restore thiol pools, which in turn regulate the redox state [50].
FA is a water-soluble toxin that diffuses into membranes and directly cross-reacts with DNA–protein chains, so it is believed that FA or its metabolites act like a stimulator that induces iNOS protein synthesis directly [73]. PCNA is a cell cycle regulatory protein marker. It is closely related to DNA synthesis and implicated in the initiation of cell proliferation. FA destroys the proliferation, differentiation of the hematopoietic stem cells, and hematopoietic microenvironment in bone marrow, so it inhibits hematopoietic function and leads to hematopoietic toxicity [74, 75].
Comet assay is a method of single-cell gel electrophoresis assay to detect DNA damage in any tissue of all mammalians. The response of Comet assay can be used as a pointer of carcinogen exposure [51]. FA administration resulted in DNA damage in the form of a significant increase in the tail length and percentage of the tail moment.
These results are following the results of El Nagdy et al. [52] who detected the genotoxic and immunotoxic effects of FA that were dose dependent. Similarly, She et al. [53] demonstrated that the effects of FA on growth inhibition of human blood cells and myeloid progenitor cells revealed that the magnitude of DNA strand breakage increased gradually with increasing FA concentrations.
The molecular basis for possible genotoxicity and carcinogenicity of FA was studied and detected that the possible mechanism is the induction of hydroxyl methyl mono-adducts on adenine, guanine, and cytosine together with methylene crosslinks in DNA [54]. FA generates a variety of DNA lesions including interstrand crosslinks, DNA protein crosslinks, and base adducts. Failure in their repair sensitizes cells to FA and leads to replication defects. Several reports suggested that nucleotide excision repair and homologous recombination have major roles in cellular tolerance to FA [55].
Meanwhile, Neuss et al. [56] exposed that inhalation of FA in a 28 days study with concentrations up to 15 ppm does not lead to genotoxic effects in broncho-alveolar lavage cells of rats. Schmid and Speit [57] concluded that the cytogenetic effects of FA are very unlikely to occur in blood cultures of FA-exposed subjects. These results were explained by the low sensitivity of this approach due to the low mitotic activity of target cells and the poor chromosome morphology [56].
Regarding HP genoprotective effects, the results of the present study coincide with Ahmadi et al. [45], Kalpana et al. [58], Abd El Raouf et al. [59], and Passos et al. [60] who concluded that HP had a potent chemoprotective effect against genotoxicity. It decreased the comet parameters (tail length, tail moment, and %DNA in the tail) after exposure to different toxicants. It led to inhibition of micronuclei and showed powerful anticlastogenic effects.
These results could be attributed to HP antioxidant property, metal chelating effects, and inhibition of the superoxide-derived Fenton reaction, which was an important source of the most reactive hydroxyl radicals. Another explanation for the protective effect of HP on DNA was the direct interaction with DNA and enhancement of DNA repair [59].
Considering NAC, Hamid et al. [61], Foaud et al. [62]; and Yedjou et al. [63] reported the ability of NAC to reduce DNA damage and tail moment percentage, which was increased by different toxic chemicals. These results could be explained by the effects of NAC in preventing apoptosis and oxygen-related genotoxicity by increasing intracellular levels of glutathione and decreasing mitochondrial membrane depolarization [64]. Also, NAC is reported to effectively increasing glutathione, improving T-cell response, and modulating inflammation [65].
The combined use of NAC and HP in the present work improved all the studied parameters better than the single use of each. That is probably due to the additive antioxidant, anti-inflammatory, antiapoptotic, and membrane-stabilizing effects of both.
5. Conclusion
The results of this study revealed that FA exposure induced evident hemato- and genotoxic changes in albino rats, as determined by biochemical, histopathological, immunohistochemical, and comet findings. HP and NAC administration, alone and in combination, could protect from these toxic effects.
6. Recommendations
We recommend that occupationally exposed workers should have time-limited exposure, well-ventilated rooms, and HP and NAC can be used as a nutritional supplement for those workers to reduce FA toxic effects. Also, a restriction to the utilization of FA as a food preservative should be made; further studies with different dosages of HP and NAC are needed to evaluate the potential of these antioxidants.
Contributor Information
Nourhan Mohammed, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Zagazig University, Zagazig, Egypt.
Sahar A Ahmed, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Zagazig University, Zagazig, Egypt.
Nagah I Hegazy, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Zagazig University, Zagazig, Egypt.
Kamal Kashishy, Department of Pathology, Faculty of Medicine, Zagazig University, Zagazig, Egypt.
Conflict of interest statement
None declared.
References
- 1. Safrida S, Syafrianti D, Haryani I. Effect of aloe vera extract in reducing formaldehyde in salted squid (loligoindica) and sensory evaluation. E3S Web Conf 2020;151:1–5. [Google Scholar]
- 2. Kamps J, Hopkinson RJ, Schofield CJ et al. How formaldehyde reacts with amino acids. Commun Chem 2019;2:126. [Google Scholar]
- 3. Wahed P, Razzaq MA, Dharmapuri S et al. Corrales determination of formaldehyde in food and feed by an in-house validated HPLC method. Food Chem 2016;202:476–83. [DOI] [PubMed] [Google Scholar]
- 4. EFSA . Endogenous formaldehyde turnover in humans compared with exogenous contribution from food sources. EFSA J 2014;12:3550. [Google Scholar]
- 5. Wei C, Wen H, Yuan L et al. Formaldehyde induces toxicity in mouse bone marrow and hematopoietic stem/progenitor cells and enhances benzene-induced adverse effects. Arch Toxicol 2016;91:921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kandemir FM, Ozkaraca M, Küçükler S et al. Preventive effects of hesperidin on diabetic nephropathy induced by streptozotocin via modulating TGF-β1 and oxidative DNA damage. Toxin Rev 2017;37:287–93. [Google Scholar]
- 7. Hamdy SM, Shabaan AM, Abdel Latif AKM et al. Protective effect of hesperidin and tiger nut against acrylamide toxicity in female rats. Exp Toxicol Pathol 2017;69:580–8. [DOI] [PubMed] [Google Scholar]
- 8. Haggag Y, el-Ashmawy NE, Okasha KM. Is Hesperidin essential for prophylaxis and treatment of COVID-19 infection? Med Hypothesis 2020;144:109957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jose R, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med 2020;8:e46–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larsson SC, Håkansson N, Wolk A. Dietary cysteine and other amino acids and stroke incidence in women. Stroke 2015;46:922–6. [DOI] [PubMed] [Google Scholar]
- 11. Dodd S, Dean O, Copolov DL et al. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin Biol Ther 2008;8:1955–62. [DOI] [PubMed] [Google Scholar]
- 12. Donia T, Eldaly S, Ali E. Ameliorating oxidative stress and inflammation by Hesperidin and vitamin E in doxorubicin induced cardiomyopathy. Turk J Biochem 2019;44:207–17. [Google Scholar]
- 13. Merzoug S, Toumi M. Effects of hesperidin on formaldehyde-induced toxicity in pregnant rats. EXCLI J 2017;16:400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shahat A, Hassa W, El-Sayed W. N-Acetylcysteine and safranal prevented the brain damage induced by hyperthyroidism in adult male rats. Nutr Neurosci 2020. 10.1080/1028415X.2020.1743917. [DOI] [PubMed] [Google Scholar]
- 15. Mohammadi S. Protective effect of N-acetyl cysteine against Formaldehyde-induced neuronal damage in cerebellum of mice. Pharm Sci 2014;20:61–5. [Google Scholar]
- 16. Mada SB, Musa MA, Garba A et al. Hypolipidemic effect of N-acetylcysteine against dexamethasone-induced hyperlipidemia in rats. Calabar J Health Sci 2019;3:59–67. [Google Scholar]
- 17. Gerin F, Erman H, Erboga M et al. The effects of ferulic acid against oxidative stress and inflammation in Formaldehyde-induced hepatotoxicity. Inflammation 2016;39:1377–86. [DOI] [PubMed] [Google Scholar]
- 18. Jiménez-Villarreal J, Betancourt-Martínez ND, Carranza-Rosales P et al. Formaldehyde induces DNA strand breaks on spermatozoa and lymphocytes of Wistar rats. Cytol Genet 2017;51:65–73. [PubMed] [Google Scholar]
- 19. Aburawi SM, Aburawi SM. Selenium curative and protective action on the histopathological effect of formaldehyde in reproductive system using female albino mice. J Pharmacol 2018;3:39–47. [Google Scholar]
- 20. Material safety data sheet for formaldehyde solution- spectrum chemical. 2013. https://www.Spectrumchemical.com/MSDS/F1080.pdf.
- 21. Graham MD. The Coulter principle: imaginary origins. Cytometry 2013. Part A;83:1057–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oliveira FJA, Cecchini R. Oxidative stress of liver in hamsters infected with Leishmania (L.) chagasi. J Parasitol 2008;6:1067–72. [DOI] [PubMed] [Google Scholar]
- 23. Beauchamp C, Fridovich I. Superoxide dismutase improved assay applicable to acrylamide gels. Anal Biochem 1971;44:276–87. [DOI] [PubMed] [Google Scholar]
- 24. Amend SR, Valkenburg KC, Pienta KJ. Murine hind limb long bone dissection and bone marrow isolation. J Vis Exp 2016;14:53936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bancroft JD, Gamble M. Connective tissue stains. In: Bancroft J (ed). Theory and Practice of Histological Techniques. London: Churchill-Livingston Elsevier, 2008, 135–60. [Google Scholar]
- 26. Jalili P, Huet S, Lanceleur R et al. Genotoxicity of aluminum and aluminum oxide nanomaterials in rats following oral exposure. Nanomaterials 2020;10:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. SPSS I . IBM SPSS statistics for windows version 25. Armonk: IBM SPSS Corp, 2017. [Google Scholar]
- 28. Zhang Y, Liu X, McHale C et al. Bone marrow injury induced via oxidative stress in mice by inhalation exposure to formaldehyde. PLoS One 2013;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohammed Z, Hassan A. Effect of bee venom on some blood and biochemical parameters in formaldehyde induced arthritis male rats in comparison with prednisolone drug. J Phys Conf Ser 2019;2019:1234. [Google Scholar]
- 30. Aishwarya M, Subramaniyan V, Chandiran S et al. Antiarthritic activity of Murraya exotica linn against formaldehyde induced arthritis in Wistar rats. Res J Pharm Biol Chem Sci 2018;9:205. [Google Scholar]
- 31. Hasan A, Jassim H. Effect of treating lactating rats with lead acetate and its interaction with vitamin E or C on neurobehavior, development and some biochemical parameters in their pups. Iraqi J Vet Sci 2011;1:45–52. [Google Scholar]
- 32. Goldstein B. Hematological and toxicological evaluation of formaldehyde as a potential cause of human leukemia. Hum Exp Toxicol 2010;30:725–35. [DOI] [PubMed] [Google Scholar]
- 33. Yan Yu G, Song X-F, Liu Y et al. Inhaled formaldehyde induces bone marrow toxicity via oxidative stress in exposed mice. Asian Pac J Cancer Prev 2014;15:5253–7. [DOI] [PubMed] [Google Scholar]
- 34. Bassig B, Zhang L, Vermeulen R et al. Comparison of hematological alterations and markers of B-cell activation in workers exposed to benzene, formaldehyde and trichloroethylene. Carcinogenesis 2016;37:692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Afolabi O, Oyewo EB, Adeleke GE et al. Mitigation of aluminium phosphide-induced hematotoxicity and ovarian oxidative damage in wistar rats by hesperidin. Am J Biochem 2019;9:7–16. [Google Scholar]
- 36. Bashandy M, Zawahry El, Bashandy SA et al. The protective role of β-Carotene and Hesperidin on some hematological and myocardial measurements against imidacloprid toxicity in albino rats. J Pharm Pharmacol 2017;5:798–806. [Google Scholar]
- 37. Hajialyani M, Hosein Farzaei M, Echeverría J et al. Hesperidin as a neuroprotective agent: a review of animal and clinical evidence. Molecules 2019;24:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. El-Maddawy Z, El-Sayed Y. Comparative analysis of the protective effects of curcumin and N-acetyl cysteine against paracetamol-induced hepatic, renal and testicular toxicity in Wistar rats. Environ Sci Pollut Res 2018;25:3468–79. [DOI] [PubMed] [Google Scholar]
- 39. Sit M, Tosun M, Aktas G et al. Effects of N-acetyl cysteine on lipid levels and on leukocyte and platelet count in rats after splenectomy. Nigerian J Clin Pract 2014;17:343–5. [DOI] [PubMed] [Google Scholar]
- 40. Ciftci G, Aksoy A, Cenesiz S et al. Therapeutic role of curcumin in oxidative DNA damage caused by formaldehyde. Microsc Res Tech 2015;78:391–5. [DOI] [PubMed] [Google Scholar]
- 41. Turk E, Kandemir FM, Yildirim S et al. Protective effect of hesperidin on sodium arsenite-induced nephrotoxicity and hepatotoxicity in rats. Biol Trace Elem Res 2018;189:95–108. [DOI] [PubMed] [Google Scholar]
- 42. Garg G, Singh S, Singh AK et al. N-acetyl L-cysteine attenuates oxidative damage and neurodegeneration in rat brain during aging. Can J Physiol Pharmacol 2018;96:1189–96. [DOI] [PubMed] [Google Scholar]
- 43. Huang S, You J, Wang K et al. N-acetylcysteine attenuates cisplatin-induced acute kidney injury by inhibiting the C5a receptor. Bio Med Res Int 2019;2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Singh V, Keisham A, Bhalla A et al. Efficacy of granulocyte colony-stimulating factor and n-acetylcysteine therapies in patients with severe alcoholic hepatitis. Clin Gastroenterol Hepatol 2018;16:1650–6. [DOI] [PubMed] [Google Scholar]
- 45. Ahmadi A, Hosseinimehr SJ, Naghshvar F et al. Chemoprotective effects of hesperidin against genotoxicity induced by cyclophosphamide in mice bone marrow cells. Arch Pharmacol Res 2008;31:794–7. [DOI] [PubMed] [Google Scholar]
- 46. Hassouna I, Ibrahim H, Abdel Gaffar F et al. Simultaneous administration of hesperidin or garlic oil modulates diazinon-induced hemato- and immunotoxicity in rats. Immunopharmacol Immunotoxicol 2015;37:442–9. [DOI] [PubMed] [Google Scholar]
- 47. Fukuto J, Wink D. Nitric oxide (NO): formation and biological roles in mammalian systems. Metal Ions Biol Syst 2018;2018:547–95. [PubMed] [Google Scholar]
- 48. Dokumacioglu E, Iskender H, Sen TM et al. The effects of hesperidin and quercetin on serum tumor necrosis factor-alpha and interleukin-6 levels in streptozotocin-induced diabetes model. Pharmacogn Mag 2018;14:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Al-Tonbary Y, Al-Haggar M, El-Ashry R et al. Vitamin E and N-acetylcysteine as antioxidant adjuvant therapy in children with acute lymphoblastic leukemia. Adv Hematol 2009;2009:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aldini G, Altomare A, Baron G et al. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res 2018;52:751–62. [DOI] [PubMed] [Google Scholar]
- 51. Bowman L, Castranova V, Ding M. Single cell gel electrophoresis assay (comet assay) for evaluating nanoparticles-induced DNA damage in cells. Methods Mol Biol 2012;906:415–22. [DOI] [PubMed] [Google Scholar]
- 52. El Nagdy S, El Gezawy E, Nasif K. Toxic effects of formaldehyde on cellular immunity and possible relationship to its risk of carcinogenesis: an experimental study. Zagazig J Forensic Med Toxicol 2020;18:140–56. [Google Scholar]
- 53. She Y, Li Y, Liu Y et al. Formaldehyde induces toxic effects and regulates the expression of damage response genes in BM-MSCs. Acta Biochim Biophys Sin 2013;45:1011. [DOI] [PubMed] [Google Scholar]
- 54. Kawanishi M, Matsuda T, Yagi T. Genotoxicity of formaldehyde: molecular basis of DNA damage and mutation. Front Environ Sci 2014;2:36. [Google Scholar]
- 55. Anandarajan V, Noguchi C, Oleksak J et al. Genetic investigation of formaldehyde-induced DNA damage response in Schizosaccharomyces pombe. Curr Genet 2020;66:593–605. [DOI] [PubMed] [Google Scholar]
- 56. Neuss S, Zeller J, Ma-Hock L et al. Inhalation of formaldehyde does not induce genotoxic effects in broncho-alveolar lavage (BAL) cells of rats. Mutat Res/Genetic Toxicol Environ Mutagenesis 2010;695:61–8. [DOI] [PubMed] [Google Scholar]
- 57. Schmid O, Speit G. Genotoxic effects induced by formaldehyde in human blood and implications for the interpretation of biomonitoring studies. Mutagenesis 2007;22:69–74. [DOI] [PubMed] [Google Scholar]
- 58. Kalpana K, Devipriya N, Srinivasan M et al. Evaluating the radioprotective effect of hesperidin in the liver of Swiss albino mice. Eur J Pharmacol 2011;658:206–12. [DOI] [PubMed] [Google Scholar]
- 59. Abd El Raouf A, Girgis SM, Abdou HS et al. Assessment of the protective role of hesperidin against genotoxic and biochemical effects of cytoxan in mice. Biochemistery 2015;9:121–9. [Google Scholar]
- 60. Passos T, Santana EA, da Mota et al. Hesperidin reduces cisplatin-induced DNA damage in bone marrow cells of mice. J Pharm Pharmacol 2017;5:282–8. [Google Scholar]
- 61. Hamid Z, Tan HY, Chow PW et al. The role of N-acetylcysteine supplementation on the oxidative stress levels, genotoxicity and lineage commitment potential of ex vivo murine haematopoietic Stem/Progenitor cells. Sultan Qaboos Univ Med J 2018;18:e130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Foaud M, Lapshina EA, Sudnikovich EY et al. The protective effect of N-acetyl cysteine against carbon tetrachloride toxicity in rats. J Basic Appl Zool 2018;79:1–15. [Google Scholar]
- 63. Yedjou C, Waters D, Tchounwou PB. N-acetyl cysteine protection against lead-induced oxidative stress and genotoxicity in human liver carcinoma (hepg2) cells. Met Ions Biol Med 2008;10:419–24. [PMC free article] [PubMed] [Google Scholar]
- 64. Mokhtari V, Afsharian P, Shahhoseini M et al. A review on various uses of N-acetyl cysteine. Cell J (Yakhteh) 2017;19:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Poe F, Corn J. N-Acetylcysteine: a potential therapeutic agent for SARS-CoV-2. Med Hypotheses 2020;143:109862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sathish P, Paramasivan V, Palani V et al. N-acetylcysteine attenuates dimethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol 2011;654:181–6. [DOI] [PubMed] [Google Scholar]
- 67. Abdel-Salam Q, El-Shamarka M, Omara E. Brain oxidative stress and neurodegeneration in the ketamine model of schizophrenia during antipsychotic treatment: effects of N-acetylcysteine treatment. React Oxygen Species 2018;6:253–66. [Google Scholar]
- 68. Mahmoud A. Hesperidin protects against cyclophosphamide-induced hepatotoxicity by upregulation of PPARγ and abrogation of oxidative stress and inflammation. Can J Physiol Pharmacol 2014;92:717–24. [DOI] [PubMed] [Google Scholar]
- 69. Aly M, Galaly S, Moustafa N et al. Hesperidin protects against diethylnitrosamine/carbon tetrachlorideinduced renal repercussions via up-regulation of Nrf2/HO-1 signaling and attenuation of oxidative stress. J Appl Pharm Sci 2017;7:007–14. [Google Scholar]
- 70. Paget G, Barnes J. Interspecies dosage conversion scheme in evaluation of results and quantitative application in different species. Eval Drug Act: Pharmacometrics 1964;1:160–2. [Google Scholar]
- 71. FAC R, VCC G, RdM N et al. Cell sources of inflammatory mediators present in bone marrow areas inside the meniscus. PLoS One 2019;14:e0226986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Abdel Mohsen A, Salama N, Rashed L et al. Comparative histological study on the effect of adipose derived stem cells versus their conditioned medium on indomethacin induced enteritis in adult female albino rats. Egypt J of Histol 2018;41:503–19. [Google Scholar]
- 73. Speit G. The implausibility of systemic genotoxic effects measured by the comet assay in rats exposed to formaldehyde. J Proteome Res 2006;5:2523–4. [DOI] [PubMed] [Google Scholar]
- 74. Park JM, Yang SW, Yu KR et al. Modification of PCNA by ISG15 plays a crucial role in termination of error-prone translesion DNA synthesis. Mol Cell 2014;54:626–38. [DOI] [PubMed] [Google Scholar]
- 75. Ge J, Honglian Y, Xianxian L et al. Combined exposure to formaldehyde and PM2.5: hematopoietic toxicity and molecular mechanism in mice. Environ Int 2020;144:106050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ezati D, Vardiyan R, Talebi A et al. L-Carnitine reduces the negative effects of formalin on sperm parameters, chromatin condensation and apoptosis in mice: an experimental study. IJRM 2020;18:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Afrigan L, Jafari Anarkooli I, Sohrabi D et al. The effect of hydroethanolic extract of Matricaria chamomilla on the reproductive system of male rats exposed to formaldehyde. Andrologia 2019;51:e13362. [DOI] [PubMed] [Google Scholar]
- 78. Chatterjee T. Handbook of Laboratory Mice and Rats. Kolkata, India: Department of Pharmaceutical Technology, Jadavpur University, 1993, 157. [Google Scholar]


