Abstract
N, N-Dimethylformamide (DMF) can cause liver damage in occupationally exposed workers, but the molecular mechanism of DMF-induced liver damage has not been fully elucidated. Researches have proved that lncRNA plays a major function in chemical-induced liver toxicity and can be used as a biomarker and therapeutic target for liver injury. In order to verify that lncRNA also participates in DMF-induced liver damage, we treated HL-7702 cells with 75 or 150 mM DMF, and obtained lncRNA expression profiles through high-throughput sequencing. Among the differentially expressed lncRNAs, lncRNA SNHG12 was proved to be significantly downregulated in DMF-treated HL-7702 cells and participate in DMF-mediated apoptosis, even under long-term low-dose DMF exposure (5–10 mM, 8 weeks). In addition, according to bioinformatics analysis, miR-218-5p is expected to be a potential target of SNHG12, which was verified by the dual luciferase reporter assay in HEK293FT cells. MiR-218-5p mimic can induce apoptosis in HL-7702 cells. Among the predicted targets of miR-218-5p, protein kinase C epsilon (PRKCE) was reported to be involved in apoptosis, and was indeed downregulated by miR-218-5p mimic in our study. Further experiments showed that changes of the expression of SNHG12 can affect the expression of PRKCE. In the epidemiological study of occupational population, we also found that SNHG12 was downregulated in the serum exosomes of workers exposed to DMF. These results indicated that SNHG12 can mediate DMF-induced apoptosis of HL-7702 cells through miR-218-5p/PRKCE pathway.
Keywords: N, N-Dimethylformamide, SNHG12, miR-218-5p, PRKCE, hepatocyte apoptosis
Graphical Abstract
Graphical abstract. LncRNA SNHG12 regulates DMF-induced hepatic apoptosis through has-miR-218-5p/PRKCE axis. Used Pathway Builder Tool 2.0 software (Protein Lounge Inc.) to draw the graph. DMF, N, N-dimethylformamide
Introduction
N, N-Dimethylformamide (DMF, CAS#68-12-2) is an important organic solvent, which is used in various industries around the world [1]. DMF is often found in industrial waste water and can be easily released into the environment [2]. Both human and animal experiments have shown that DMF can enter the body through inhalation or skin contact, and liver is the main target organ for DMF intoxication [3–5]. Studies [6, 7] have shown that even if exposure level of DMF is lower than expected, it may cause severe liver damage to sensitive population. Animal studies [1, 8] also revealed that liver is the most sensitive target organ for DMF because the biotransformation of DMF is mainly catalyzed in the liver. Consequently, it is inevitable to explore the molecular mechanism of liver injure induced by DMF to find out the biomarkers and therapeutic targets related to DMF exposure. Previous researches reported that the liver toxicity induced by DMF might be mainly due to cellular necrosis and apoptosis [9].
Despite considerable attention to the hepatotoxic effects of DMF, the underlying mechanism of DMF-induced liver injury, especially in the epigenetic mechanism, is still not fully clarified. Epigenetic mechanisms include DNA methylation, histone modifications, and noncoding RNA regulation. Among them, long noncoding RNA (lncRNA) is an RNA transcript greater than 200 nucleotides, which does not directly encode protein but can regulate gene expression at the transcription, post-transcriptional and translational levels [10]. Till now, the most studied aspect of lncRNA function is as the sponge of microRNA (miRNA), which means that lncRNA can competitively bind to miRNA and reduce the binding between miRNA and its target mRNA, increasing the expression of the target genes [11]. LncRNA participates in the regulation of many physiological and pathological processes in the body, and also plays a role in the occurrence of diseases and the toxic effects of chemicals. Existing researches show that lncRNA can be used as the biomarker for certain cancer treatment or prognosis. For example, lncRNA LY6K-AS (CTD-2292P1.4) is a lung adenocarcinoma prognostic biomarker [12]. A study [13] revealed that expression of the serum extracellular vesicles (EVs) lncRNA colon cancer-associated transcript 1 (CCAT1) in gastric cancer (GC) patients was higher than the healthy control group, which indicated that lncRNA CCAT1 may be used as a diagnostic marker for GC. So far, no studies have reported whether lncRNA can be used as a biomarker or therapeutic target for DMF-induced liver injury.
Therefore, we speculated that some lncRNAs may play a regulatory role in DMF-induced liver damage, and may serve as biomarkers of DMF-related toxic effects. We hope to find the lncRNA related to liver injury from the constructed DMF-induced liver injury model and explore its mechanism of action, so as to provide evidence for the treatment of DMF-induced early liver injury. We investigated whether there were lncRNAs that could be used as biomarkers for DMF-induced liver injury in occupational workers exposed to DMF.
Materials and Methods
High-throughput transcriptome sequencing
HL-7702 cells were treated with 0, 75 and 150 mM DMF (sigma, USA) for 48 hours (per group had three samples). Total RNA was extracted by TRIzol reagent (Thermo Fisher Scientific, Inc). Small RNA sample prep kit was used to construct the lncRNA library, and the NEBNext®Ultra™ Directional RNA Library Prep Kit (New England Biolabs) was used to prepare RNA library. Novogene Company (Beijing, China) sequenced these libraries. We obtained expression profiles of lncRNA, mRNA and miRNA from the high-throughput transcriptomic data.
Cell culture
HL-7702 cells and HEK293FT cells were obtained from Procell (Wuhan, China). HL-7702 cells were cultured in RPMI-1640 medium (Gibco, USA), and HEK293FT cells were cultured in Dulbecco’s modified Eagle medium (DMEM) (Gibco, USA). About, 10% fetal bovine serum (Applied Biosystems, USA) was added to both medium. All of the cells were incubated at a temperature of 37°C with 5% CO2.
Quantitative polymerase chain reaction assay
Total RNA was obtained from the HL-7702 cells by TRIzol reagent (Thermo Fisher Scientific, Inc) following the manufacturer’s protocol. We isolated the cytoplasmic and nuclear RNA of HL-7702 cells by the Cytoplasmic & Nuclear RNA Purification Kit (Norgen, Belmont, CA, USA) based on the manufacturer’s manual. About, 1 μg total RNA was used to generate cDNA by ReverTra Ace®qPCR RT Kit (Toyobo, Osaka, Japan). We used cDNA as the template to analyze the gene expression levels by SYBR® Green Realtime PCR Master Mix (Toyobo, Osaka, Japan) on Applied Biosystems QuantStudio7 Flex Real-Time PCR machine (Thermo Fisher Scientific). The relative expression of LncRNA, miRNA and mRNA were normalized to GAPDH or U6. The primer sequences were shown in Table S1-1 (see Supporting Information).
Plasmid construction and transfection
Overexpression of SNHG12 (OE-SNHG12) plasmid was constructed by inserting the complete sequence of SNHG12 into the pcDNA3.1 expression vector. The SNHG12 short-hairpin RNA (shRNA) sequences were designed online (https://rnaidesigner. thermofisher.com/rnaiexpress/). Then we incorporated the oligonucleotides into the pU6 vector. The miR-218-5p mimic and the negative control were obtained from RiboBio (Guangzhou, China). All plasmids and mimic were transfected into cells by ViaFect™ transfection reagent (Promega, Madison, USA) referring to the manufacturer’s protocol. The sequences were shown in Supplementary Data, Table S1-2 and Table S1-3.
Apoptosis analysis
Cellular apoptosis was detected by FITC Annexin V Apoptosis Detection Kit I (BD Biosciences, USA) with flow cytometry according to the manufacturer’s procedure. After collecting HL-7702 cells with corresponding treatments, we suspended 1 × 106 cells and added 5 μl Annexin-V FITC/PI to stain the cells. Flow cytometry (Beckman Coulter) was used to analyze the apoptotic rate of cell samples.
Western blot analysis
Extraction of cellular protein and western blot were performed according to our previous protocol [14]. The primary antibody of rabbit anti-human PRKCE (Affinity, China) was diluted 1:500. Mouse anti-rabbit IgG-HRP antibody (Santa Cruz, USA) and β-actin (proteintech, USA) were diluted 1:1500. The PRKCE protein expression level was normalized relative to β-actin protein level in the same sample, and then compared to the control group.
Dual-luciferase reporter assay
The luciferase reporter assay was carried out to detect the potential interaction between SNHG12 and miR-218-5p. We constructed psi-SNHG12 and Mut-SNHG12 as the reporter plasmids. Then, HEK293FT cells were co-transfected with the reporter gene plasmids and miR-218-5p mimic or the reporter gene plasmids and NC negative control. After 48 h transfection, dual-luciferase reporter assay (Promega, WI) was used to detect the luciferase activities. The PCR primers used to construct the reporter plasmids were listed in Table S1-4 (see Supporting Information).
Population profile
In 2018, we collected peripheral blood and urine samples of 163 workers exposed to DMF in a tannery in Dongguan city, and collected relevant data on DMF exposure levels of workers in different jobs, the information of liver function index and population characteristics of occupational workers. In brief, after collecting 5 mL of venous blood and urine from each participant, the serum was isolated by centrifugation at 1600 × g for 10 min at 4°C. The serum supernatant was stored at −80°C. We used gas chromatography–mass spectrometry (GCMS-QP2010 Plus, Shimadzu) to assay the urine N-methylformamide (NMF, DMF metabolites) as previously described [15]. Each participant was required to obtain written informed consent before their blood and urine samples were collected. The study scheme was approved by the Clinical Research Ethics Committee of Sun Yat-sen University (School of Public Health, Sun Yat-sen University Medical Ethics Committee 2018 No. 036). All experiments were performed according to the World Medical Association Declaration of Helsinki.
Serum Exosomes Isolation
ExoQuick™ Solution (SBI System Biosciences, USA) was used to extract the exosomes from serum samples following the manufacturer’s steps. After the exosomes were re-suspended in 100 μL phosphate buffered saline (PBS), RNA from exosome was extracted by Trizol Regent (Thermo Fisher Scientific, Inc).
Statistical analysis
SPSS 2.0 software was used for statistical processing. According to the normality of the data, the mean ± standard deviation (SD) or the median and quartile [M (P25, P75)] was selected to describe the data. When the data met the homogeneity of variance, one-way ANOVA and Mann–Whitney test were used for pairwise comparison. Categorical variables were expressed by n (%), and χ2 test was used to compare the distribution among groups. Rank sum test was used to compare the non-normal distributed data between groups.
Results
Exposure to DMF induces apoptosis in HL-7702 cells
Our previous [16] research showed that DMF treatment dose-dependently induced apoptosis of HL-7702 cells and increased the expression of cleaved caspase-3 protein, which was also reproduced in this study. We want to know whether the same results can be obtained when cells undergo low-dose long-term DMF exposure in the experiment, so we established a long-term low-dose hepatotoxicity model. Previous study [17] reported that approximately .5 mM DMF and .5 mM NMF in the plasma of cynomolgus monkeys were observed after exposure to DMF vapors of 100 ppm for 13 weeks. In occupationally exposed workers, exposure to about 100 ppm DMF can cause liver damage [18]. Hence, we selected 1, 5 and 10 mM DMF to treat HL-7702 cells for detecting the long-term toxic effect of DMF. As expected, we found that long-term exposure to low-dose DMF can also induce apoptosis in HL-7702 cells. As shown in Figure 1A and B, the 10 mM DMF-exposed group had the highest apoptotic rate after 8-week treatment, which was 7.08% higher than the control group. These results indicated that both long-term low-dose and short-term high-dose DMF exposure can cause apoptosis in HL-7702 cells.
Figure 1.

Long-term low-dose DMF exposure can cause HL-7702 cells apoptosis. (A) The experimental result of flow cytometry in HL-7702 cells exposed to 0, 1, 5 and 10 mM DMF for 2, 4, 6 and 8 weeks. (B) The apoptotic rate (Q2 + Q3) of HL-7702 cells exposed to 0, 1, 5 and 10 mM DMF for 2, 4, 6 and 8 weeks based on the result of flow cytometry. Data were shown as mean ± SD. *P < .05, compared with the control group. DMF, N, N-dimethylformamide. Annexin-FITC: Annexin V-fluorescein isothiocyanate; PI: propidium iodide.
LncRNA expression profile is altered in HL-7702 cells after DMF exposure
To identify whether lncRNA is related to DMF exposure, we extracted RNA of HL-7702 cells respectively from control group, 75 mM DMF group and 150 mM DMF group in triplicate for profile analysis. A total of 2953 annotated lncRNAs were obtained from high-throughput sequencing data. In detail, compared with the control group, there were 153 differentially expressed lncRNAs in 75 mM group and 123 differentially expressed lncRNAs in 150 mM group. There were 78 differentially expressed lncRNAs that exist both in the 75 and 150 mM DMF exposure group (Figure S1A; see Supporting Information). Compared with the control group, the expression of 38 lncRNAs changed more than 2-fold (P adj < .05) in both the 75 mM DMF group and the 150 mM DMF group, and their FPKM values were greater than 1 in all groups. Among them, 11 lncRNAs were upregulated, and 27 lncRNAs were downregulated. Then, we excluded those lncRNAs with only one exon and used co-expression network to analyze the function of the remaining lncRNAs. The results showed that 10 lncRNAs might be involved in the cellular apoptosis process. Next, qRT-PCR was applied to verify the expression trends of these 10 lncRNAs. Seven out of 10 lncRNA were turned out to have consistent expression trends comparing with the high-throughput sequencing data (Figure 2A). Heat map was used to present the cluster analysis of the filtered lncRNAs in high-throughput sequencing data (Figure 2B). The qRT-PCR result was shown in Figure 2C that indicating ENST00000531126.5 (Small nucleolar RNA host gene 12, SNHG12) had a biggest downregulated fold-change after 150 mM DMF treatment. We further detected the change of SNHG12 expression after 0, 1, 5, 10 mM DMF exposure for 2–8 weeks (Figure 2D). The results shown that exposure of 1, 5 or 10 mM DMF to HL-7702 cells for 8 weeks also significantly reduced SNHG12 expression, suggesting that SNHG12 probably plays a role in DMF-induced hepatocyte apoptosis.
Figure 2.
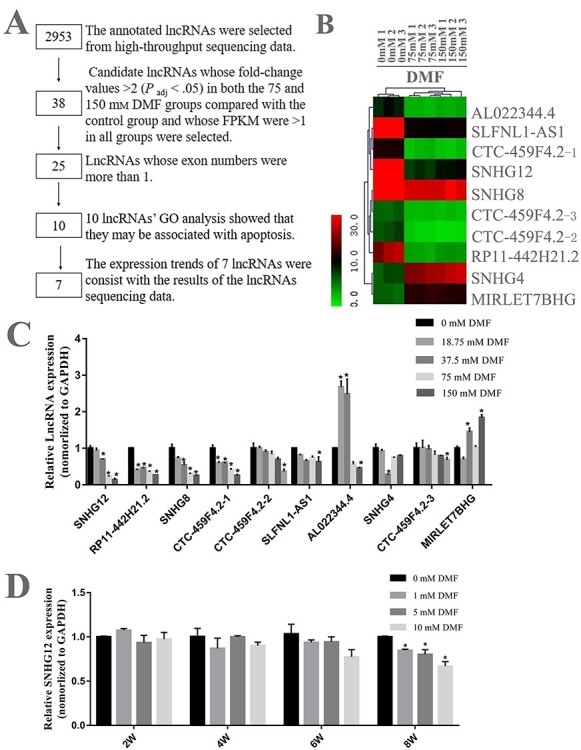
Changes of lncRNA profiles after DMF exposure in HL-7702 cells. (A) The flow chart of screening candidate lncRNA related to DMF-induced hepatotoxicity. (B) The cluster analysis of the heat map shows that the lncRNAs screened have significant differences between different groups. (C) The expression level of selected differential lncRNA was verified in HL-7702 cells by qRT-PCR. (D) Expression levels of SNHG12 in HL-7702 cells exposed to 0, 1, 5 and 10 mM DMF for 2, 4, 6 and 8 weeks were analyzed by qRT-PCR. GAPDH served as an endogenous control. The results of three experiments were shown as mean ± SD. *P < .05, compared to control group. DMF, N, N-dimethylformamide; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
The downregulated expression of SNHG12 is involved in the apoptosis of hepatocytes induced by DMF
In order to verify the function of SNHG12 in DMF-induced hepatocyte apoptosis, we constructed its overexpression vector (Table S1-2; see Supporting Information) and three small double-stranded shRNAs (shRNA-SNHG12–1, shRNA-SNHG12-2, shRNA-SNHG12–3) (Table S1-3; see Supporting Information) for SNHG12. We used qRT-PCR to evaluate the efficiency of SNHG12 knockdown and overexpression (Figure S1B and C; see Supporting Information). ShRNA-SNHG12-3 (also known as sh-SNHG12) showed the strongest inhibitory effect on the expression of SNHG12 and was used in the subsequent experiments. Transfection of sh-SNHG12 into HL-7702 cells for 24 hours and the rate of cell apoptosis was determined, we found the rate of apoptosis in the sh-SNHG12 group was higher than the control group (Figure 3A and B). On the contrary, under the treatment of DMF, the apoptosis rate of the OE-SNHG12 group was reduced by 33.84%, compared to the empty vector plasmid (Figure 3A and C). These results indicated that SNHG12 has a potential anti-apoptotic effect in DMF-induced hepatotoxicity.
Figure 3.

Downregulation of SNHG12 participates in DMF-induced hepatocyte apoptosis. (A) HL-7702 cells were transfected with sh-SNHG12 plasmid or NC control for 24 hours. The OE-SNHG12 plasmid or pcDNA3.1 negative control was transfected into HL-7702 cells for 24 hours, with or without treatment with 150 mM DMF for 48 hours. Cell apoptosis was detected by flow cytometry by Annexin-FITC and PI staining. (B) The apoptosis rate (Q3 + Q2) of HL-7702 cells after knockdown of SNHG12. (C) The apoptosis rate (Q3 + Q2) of HL-7702 cells co-treated with OE-SNHG12 plasmid or pcDNA3.1 negative plasmid and 150 mM DMF. The results were shown as mean ± SD. *P < .05 as compared to the control group. DMF, N, N-dimethylformamide. NC, negative control of sh-SNHG12.
SNHG12 regulates the expression of hsa-miR-218-5p
To explore the mechanism of SNHG12 in DMF-induced hepatocyte apoptosis, we first evaluated the distribution of SNHG12 in HL-7702 cells. We respectively extracted RNA from the cytoplasm, nucleus and the whole cell, and used qRT-PCR to detect the relative expression of SNHG12, U6 and GAPDH in these components. As shown in Figure 4A, the content of SNHG12 in the cytoplasm accounts for about 80% of total SNHG12, which declared SNHG12 mainly exists in the cytoplasm. Hence, we speculated that SNHG12 may act as a competing endogenous RNAs (ceRNA) to regulate DMF-induced hepatotoxicity.
Figure 4.

Hsa-miR-218-5p is the target of SNHG12. (A) Nuclear, cytoplasm and total RNA were separated from HL-7702 cells, relative SNHG12 expression in these fractions was detected using qRT-PCR. (B) Relative expression of hsa-miR-218-5p in HL-7702 cells treated with 0, 18.75, 37.5, 75, 150 mM DMF for 48 hours was detected by qRT-PCR. (C) The predicted binding site of SNHG12 and hsa-miR-218-5p. (D) Dual-luciferase assay was performed to analyze whether miR-218-5p could bind to SHNG12. (E) The expression of hsa-miR-218-5p in HL-7702 cells transfected with OE-SNHG12 was detected by qRT-PCR. (F) The expression of hsa-miR-218-5p in HL-7702 cells transfected with sh-SNHG12 was detected by qRT-PCR. U6 served as an endogenous control. Data were shown as mean ± SD for three experiments. *P < .05, **P < .01 as compared with the control group. DMF, N, N-dimethylformamide; NC, negative control of sh-SNHG12.
We used the starbase 3.0 (http://starbase.sysu.edu.cn/) to predict the potential target miRNAs of SNHG12 and intersect these candidate miRNAs with our miRNA sequencing data. Among them, hsa-miR-514a and hsa-miR-218-5p were selected since their expression levels were increased more than 2-fold after DMF treatment in our miRNA sequencing data. Thus, we further tested the expression of hsa-miR-514a and hsa-miR-218-5p by qRT-PCR and found that only the expression level of hsa-miR-218-5p (Figure 4B) was consistent with the sequencing result. Alignment of the seed sequence of hsa-miR-218-5p with SNHG12 indicated that it might have the potential of binding to SNHG12 (Figure 4C), which was further proved by dual-luciferase reporter gene assay in HEK293FT cells (Figure 4D). In addition, upregulation of hsa-miR-218-5p expression was observed with SNHG12 knockdown in HL-7702 cells (Figure 4E). On the contrary, overexpression of SNHG12 could significantly reduce the expression of hsa-miR-218-5p in HL-7702 cells (Figure 4F). These results indicated that SNHG12 could indeed regulate the expression of hsa-miR-218-5p.
Hsa-miR-218-5p promotes HL-7702 cells apoptosis by regulating PRKCE
To clarify the role of hsa-miR-218-5p on hepatocyte apoptosis, the HL-7702 cells were transfected with hsa-miR-218-5p mimic and the rate of apoptosis was examined. The results demonstrated that the apoptotic rate of HL-7702 cells transfected with hsa-miR-218-5p mimic was significantly higher than that in control group (Figure 5A and B). This revealed that hsa-miR-218-5p might promote the apoptosis of HL-7702 cells. Next, we intersected the predicted genes of miR-218-5p in starbase 3.0 with our high-throughput sequencing data and found that protein kinase C epsilon (PRKCE), endoplasmic reticulum [ER] to nucleus signaling 1 (ERN1) and neural precursor cell expressed developmentally downregulated protein 9 (NEDD9) might be the candidate genes involved in DMF-induced hepatoxicity. Among them, PRKCE has been reported in the literature as a potential target of has-miR-218-5p [19] and was related to apoptosis [20]. Therefore, we used qRT-PCR and western blot analysis to detect the expression of PRKCE in HL-7702 cells after DMF exposure and we found the expression of PRKCE mRNA and protein were gradually decreased with the increase of DMF exposure (Figure S1E and S1F; see Supporting Information). Moreover, the expression of PRKCE mRNA and protein were decreased (Figure 5C and D) when the expression of hsa-miR-218-5p was increased. The results indicated that hsa-miR-218-5p might participate in DMF-induced hepatotoxicity by regulating the expression of PRKCE. Next, the similar experiment was done to confirm the relationship between SNHG12 and PRKCE. The results certified that the overexpression of SNHG12 can increase PRKCE expression with or without the treatment of DMF (Figure 5E and F). On the contrary, the expression of PRKCE was reduced when the SNHG12 was downregulated (Figure 5G and H). Based on the above analysis, we considered that SNHG12 might function as a ceRNA to regulate the expression of PRKCE (the target mRNA of hsa-miR-218-5p) and mediate apoptosis induced by DMF exposure.
Figure 5.

Hsa-miR-218-5p promotes HL-7702 cells apoptosis by regulating PRKCE. (A) HL-7702 cells were transfected with hsa-miR-218-5p mimic or NC control for 24 hours. Cell apoptosis was detected by flow cytometry. (B) The apoptotic rate (Q3 + Q2) of HL-7702 cells based on the result of flow cytometry. Expression levels of PRKCE were detected after transfection of hsa-miR-218-5p mimic (C), OE-SNHG12 (E) and sh-SNHG12 (G) by qRT-PCR. GAPDH served as an endogenous control. Expression levels of PRKCE protein in HL-7702 cells were detected after transfection of hsa-miR-218-5p mimic (D), OE-SNHG12 (F) and sh-SNHG12(H) by western blot analysis. Data were shown as mean ± SD. *P < .05 and **P < .01, compared with the control group. DMF, N, N-dimethylformamide; NC (A, B, C, D), mimic negative control; NC (G, H), negative control of sh-SNHG12
SNHG12 expression in serum exosomes of occupationally exposed population
In order to study whether SNHG12 can be used as a biomarker in occupationally DMF-exposed population, 163 subjects were recruited in this study. According to the DMF level in the working condition of occupationally exposed population, the workers were divided into control group, low-, medium- and high-exposure level groups. There are statistical differences between the four groups in urine N-methylformamide (NMF, DMF metabolites), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (P < .001) (Table 1), suggesting that the degree of liver damage in occupational exposed workers possibly increased with the increase of DMF concentration. The expression of SNHG12 in serum exosomes of 163 workers was detected by qRT-PCR. The results declared that the expression of SNHG12 in serum exosomes of workers was decreased with the increase of DMF in the exposed environment (Figure 6A), which was consistent with the results from in vitro experiment in HL-7702 cells.
Table 1.
Characteristics of occupational exposed population and external exposure
| Control group (n = 40) | Low-exposure group (n = 41) | Medium-exposure Group (n = 40) | High-exposure group (n = 42) | F/X2 | P | |
|---|---|---|---|---|---|---|
| Gender | 4.953 | 0.180 | ||||
| Female | 11 (27.5%) | 6 (14.6%) | 8 (2.0%) | 4 (9.5%) | ||
| Male | 29 (72.5%) | 35 (85.4%) | 32 (8.0%) | 38 (9.5%) | ||
| Age | 1.016 | 0.387 | ||||
| 32.32 ± 1.16 | 33.12 ± 1.06 | 38.23 ± 1.21 | 37.56 ± 1.23 | |||
| Smoke | 0.652 | 0.888 | ||||
| Yes | 16 (4.0%) | 13 (31.7%) | 12 (3.0%) | 15 (35.7%) | ||
| No | 24 (6.0%) | 28 (68.3%) | 28 (7.0%) | 27 (64.3%) | ||
| Alcohol drinking | 1.961 | 0.594 | ||||
| Yes | 21 (52.5%) | 18 (43.9%) | 18 (45.0%) | 24 (57.1%) | ||
| No | 19 (47.5%) | 23 (56.1%) | 22 (55.0%) | 18 (42.9%) | ||
| BMI | 0.415 | 0.742 | ||||
| 21.75 ± .48 | 22.53 ± .64 | 23.04 ± .59 | 22.98 ± .50 | |||
| Working years | 1.811 | 0.147 | ||||
| 3.55 ± .68 | 5.06 ± .87 | 5.30 ± .68 | 3.68 ± .60 | |||
| NMF(μg/L) | 68.560 | <.001 | ||||
| 0 | 406.28 (132.60894.46) | 462.61 (97.021196.93) | 1207.43 (222.943371.21) | |||
| Liver function index | ||||||
| ALT | 19 (13.3 22.5) | 19 (16.0 26.0) | 25 (15.0 33.75) | 41 (28.5 57.0) | 44.717 | <0.001 |
| AST | 22 (18.0 74.6) | 22 (19.0 24.5) | 22 (19.0 25.8) | 30 (24.0 38.25) | 31.328 | <0.001 |
| Total bilirubin | 11.1 (3.7 15.0) | 16.2(12.5 22.0) | 15.4 (12.9 18.3) | 16.15 (13.0 25.3) | 19.845 | <0.001 |
Figure 6.
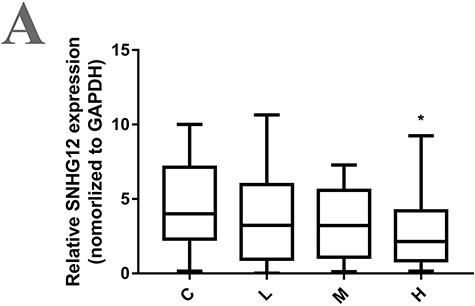
Expression of SNHG12 in serum exosomes of occupationally exposed population. (A) Exosomes were extracted from serum of population exposed to different DMF levels. SNHG12 expression in serum exosomes were detected by qRT-PCR. Results were shown as mean ± SD for all samples in each group. C, control population without DMF exposure; L, population with low concentration of DMF exposure, DMF exposure environment is less than 1 times the threshold; M, population with medium concentration of DMF exposure, DMF exposure environment is 1–2 times the threshold; H, population with high concentration of DMF exposure, DMF exposure environment is more than 2 times the threshold; *P < .05, compared with the control group. Threshold: 30 mg/m3 was recommended as a DMF occupational exposure limit by The American Conference of Governmental Industrial Hygienists.
Discussion
Previous study [18] reported that N, N-dimethylformamide (DMF), an extensively used industrial solvent, can lead to the liver injury of workers. It is necessary to use appropriate cell models to reveal specific molecular mechanisms. However, the information on DMF concentration in human plasma is currently limited. It has been reported that approximately .5 mM DMF and .5 mM NMF in the plasma of cynomolgus monkey was observed after exposure to DMF atmosphere of 100 ppm for 6 hours per day, 5 days per week for 13 weeks. Besides, DMF could be further metabolized into other products, which indicated that the value of DMF in the internal circulatory system /blood should be more than 1 mM. As for human, it has been reported [18] that when workers are exposed to 100 ppm DMF, their livers can be damaged. Since humans and cynomolgus monkeys have similar metabolic patterns, we chose 1, 5 and 10 mM as the low concentrations of DMF to treat HL-7702 cells for 8 weeks based on the previous researches [21, 22] and exposure data in the actual workplace. Since the apoptotic effect induced by long-term low-dose DMF exposure was relatively weaker than that by short-term high-dose exposure, we selected cells treated by 75 and 150 mM DMF for high-throughput sequencing to better screening the lncRNAs related to DMF-induced apoptosis.
In our study, dozens of lncRNAs in HL-7702 cells were altered after DMF exposure. Among those altered hepatic lncRNAs, the change of SNHG12 expression was the most significant. SNHG12 is an lncRNA located on chromosome 1p35.3 [23]. One study [24] reported by Chen et al. demonstrated that SNHG12 can regulate the apoptosis level of cervical cancer through the miR-148a/cyclin-dependent kinase 1 (CDK1) pathway. However, the expression of miRNA-148a-5p and CDK1 were not obviously changed after DMF treatment in HL-7702 cells according to our sequencing data, indicating SNHG12 can regulate apoptosis in different tissues through different pathways and target genes. Intriguingly, one of another SNHG12-associated miRNA hsa-miR-218-5p was upregulated after DMF treatment regulated by SNHG12 and promoted HL-7702 cells apoptosis, demonstrating SNHG12 mediated DMF-induced hepatotoxicity by regulating miRNA-218-5p, not miR-148a.
In this study, SNHG12 was predicted as several miRNAs’ sponge, like miR-514a, miR-330-5p, miR-218-5p, etc. Among them, only the expression of has-miR-218a-5p was affected by DMF exposure based on RNA sequencing data. As we known, the same miRNA can regulate different mRNAs and exert different biological function. MiR-218-5p was reported [25] to play a role in suppressing the progression of retinoblastoma through targeting nucleus accumbens-associated protein 1 (NACC1) and inhibiting the protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway, ultimately leading to the apoptosis and inhibiting cell activity. The present study demonstrated that miR-218-5p can also regulate the expression of PRKCE gene. Further analysis of RNA sequencing data indicated that miR-218-5p regulated the DMF-induced hepatic apoptosis by regulating PRKCE, but not NACC1, because the expression of NACC1 was not altered after DMF treatment. As far as we know, our work is the first study to discover that miR-218-5p can play a regulatory role in the pathway of DMF-induced hepatotoxicity.
Protein kinase C epsilon is an enzyme encoded by human PRKCE gene [26], which plays a pivotal role in various normal physiological processes and the development of many diseases. For instances, previous studies reported that PRKCE can promote the development of non-alcoholic fatty liver (NAFLD) [27] and the deletion of PRKCE in adipose tissue can improve glucose tolerance [28]. Recent evidence suggested that PRKCE can inhibit the apoptotic signal of adult rat ventricular myocytes (ARVM) induced by hyperglycemia [20]. Similarly, we found that PRKCE might be involved in hepatic apoptosis induced by DMF, which provides new insights into the mechanism of DMF-induced liver diseases.
Studies have shown that PRKCE can inhibit apoptosis through mitochondrial pathway. PRKCE attenuates TNF-induced apoptosis by inhibiting Bax activation and mitochondrial translocation [29]. Bax, a member of Bcl-2 family, which is necessary for mitochondrial pore-dependent necrotic cell death by promoting the outer membrane permeability of the mitochondrial permeability transition pore (MPTP) [30]. Meanwhile, early inactivation of ventricular PRKCE may affect mitochondrial translocation of Bad and subsequent mitochondrial destruction and apoptosis in late sepsis [31]. Therefore, our results indicated that SNHG12 may regulate mitochondrial apoptosis signal through miR-218/PRKCE axis.
SNHG12 can be used as a biomarker for the diagnosis and prognosis of a variety of cancers. Lan et al. [32] clarified that the upregulation of SNHG12 was negatively correlated with the survival of patients with hepatocelluarcinoma (HCC), which was helpful for the clinical diagnosis of liver cancer. Our work revealed that the expression of SNHG12 was decreased in serum exosomes of occupationally exposed population when the level of the DMF exposure was increased. This finding indicated that with the increase of DMF exposure, the expression of SNHG12 in the serum exosomes might decrease. However, a much larger population sample should be applied to explore whether SNHG12 can be used as a biomarker in DMF-induced hepatic injury in occupational exposure workers. On the other hand, our results showed that reducing the concentration of DMF in the working environment by enhancing ventilation or using alternative non-toxic materials can reduce the liver damage caused by DMF to workers.
Conclusion
Our result demonstrated that the expression of SNHG12 in the HL-7702 cells was decreased after DMF exposure in a dose-dependent manner. Downregulating the expression of SNHG12 can hinder the inhibitory effect of hsa-miR-218-5p on the expression of PRKCE, and ultimately led to hepatocyte apoptosis (Graphical abstract). These results illustrated the role of SNHG12 and hsa-miR-218-5p in DMF-induced hepatotoxicity, and provide the proof that SNHG12 regulates cellular processes by competing with miR-218-5p, which provided a new perspective for elucidating the hepatotoxicity induced by DMF.
Graphical Abstract.

Supplementary Material
Contributor Information
Ye Liu, School of Public Health, Sun Yat-sen University, Guangzhou 510080, China.
Cuiju Wen, Guangdong Province Hospital for Occupational Disease Prevention and Treatment, Guangzhou 510300, China.
Yangchun Zhang, School of Public Health, Sun Yat-sen University, Guangzhou 510080, China.
Ziqi Liu, School of Public Health, Sun Yat-sen University, Guangzhou 510080, China.
Qianmei He, School of Public Health, Sun Yat-sen University, Guangzhou 510080, China.
Mengxing Cui, School of Public Health, Sun Yat-sen University, Guangzhou 510080, China.
Honghao Peng, School of Public Health, Sun Yat-sen University, Guangzhou 510080, China.
Yuqing Wang, School of Public Health, Sun Yat-sen University, Guangzhou 510080, China.
Xueying Zhang, School of Public Health, Sun Yat-sen University, Guangzhou 510080, China.
Xudong Li, Guangdong Province Hospital for Occupational Disease Prevention and Treatment, Guangzhou 510300, China.
Qing Wang, School of Public Health, Sun Yat-sen University, Guangzhou 510080, China.
Funding
The National Natural Science Foundation of China (grant number: 81673140) and Guangdong Basic and Applied Basic Research Foundation (grant number: 2019A1515011457).
Conflict of interest statement
There is no conflict of interest to declare.
References
- 1. Kim TH, Kim SG. Clinical outcomes of occupational exposure to n,n-dimethylformamide: perspectives from experimental toxicology. Saf Health Work 2011;2(2):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xiao J, Chu S, Tian G et al. An Eco-tank system containing microbes and different aquatic plant species for the bioremediation of N,N-dimethylformamide polluted river waters. J Hazard Mater 2016;320:564–70. [DOI] [PubMed] [Google Scholar]
- 3. Fiorito A, Larese F, Molinari S et al. Liver function alterations in synthetic leather workers exposed to dimethylformamide. Am J Ind Med 1997;32(3):255–60. [DOI] [PubMed] [Google Scholar]
- 4. Hamada M, Abe M, Tokumoto Y et al. Occupational liver injury due to N,N-dimethylformamide in the synthetics industry. Internal Med (Tokyo, Japan) . 2009;48(18):1647–50. [DOI] [PubMed] [Google Scholar]
- 5. Lynch DW, Placke ME, Persing RL et al. Thirteen-week inhalation toxicity of N,N-dimethylformamide in F344/N rats and B6C3F1 mice. Toxicological Sciences : An Off J Soc of Toxicol 2003;72(2):347–58. [DOI] [PubMed] [Google Scholar]
- 6. Nomiyama T, Uehara M, Miyauchi H et al. Causal relationship between a case of severe hepatic dysfunction and low exposure concentrations of N,N-dimethylformamide in the synthetics industry. Ind Health 2001;39(1):33–6. [DOI] [PubMed] [Google Scholar]
- 7. Qi C, Gu Y, Sun Q et al. Low-dose N,N-dimethylformamide exposure and liver injuries in a cohort of chinese leather industry workers. J Occup Environ Med 2017;59(5):434–439. [DOI] [PubMed] [Google Scholar]
- 8. Chieli E, Saviozzi M, Menicagli S et al. Hepatotoxicity and P-4502E1-dependent metabolic oxidation of N,N-dimethylformamide in rats and mice. Arch Toxicol 1995;69(3):165–70. [DOI] [PubMed] [Google Scholar]
- 9. Wang C, Yang J, Lu D et al. Oxidative stress-related DNA damage and homologous recombination repairing induced by N,N-dimethylformamide. JAT 2016;36(7):936–45. [DOI] [PubMed] [Google Scholar]
- 10. Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell 2018;172(3):393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA Interactions. Methods in Molecular Biology (Clifton, NJ) 2016;1402:271–86. [DOI] [PubMed] [Google Scholar]
- 12. Ali MM, Di Marco M, Mahale S et al. LY6K-AS lncRNA is a lung adenocarcinoma prognostic biomarker and regulator of mitotic progression. Oncogene 2021;40(13):2463–2478. [DOI] [PubMed] [Google Scholar]
- 13. Xiao K, Dong Z, Wang D et al. Clinical value of lncRNA CCAT1 in serum extracellular vesicles as a potential biomarker for gastric cancer. Oncol Lett 2021 Jun;21(6):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Z, Zhu W, Liu Z et al. Aberrant expression of miRNA-192-5p contributes to N,N-dimethylformamide-induced hepatic apoptosis. JAT 2020;40(12):1683–1693. [DOI] [PubMed] [Google Scholar]
- 15. He J, Wang P, Zhu JQ et al. Role of urinary biomarkers of N,N-dimethylformamide in the early detection of hepatic injury among occupational exposed workers. Int Arch Occup Environ Health 2010;83(4):399–406. [DOI] [PubMed] [Google Scholar]
- 16. Jiang H, Li R, Zhang Z et al. Retinoid X receptor α (RXRα)-mediated erythroid-2-related factor-2 (NRF2) inactivation contributes to N,N-dimethylformamide (DMF)-induced oxidative stress in HL-7702 and HuH6 cells. JAT 2020;40(4):470–482. [DOI] [PubMed] [Google Scholar]
- 17. Hundley SG, McCooey KT, Lieder PH et al. Dimethylformamide pharmacokinetics following inhalation exposure in monkeys. Drug Chem Toxicol 1993;16(1):53–79. [DOI] [PubMed] [Google Scholar]
- 18. Wang JD, Lai MY, Chen JS et al. Dimethylformamide-induced liver damage among synthetic leather workers. Arch Environ Health 1991;46(3):161–6. [DOI] [PubMed] [Google Scholar]
- 19. Wang H, Zhan M, Xu SW et al. miR-218-5p restores sensitivity to gemcitabine through PRKCE/MDR1 axis in gallbladder cancer. Cell Death Dis . 2017;8(5):e2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malhotra A, Kang BP, Hashmi S et al. PKCepsilon inhibits the hyperglycemia-induced apoptosis signal in adult rat ventricular myocytes. Mol Cell Biochem 2005;268(1–2):169–73. [DOI] [PubMed] [Google Scholar]
- 21. Hundley SG, Lieder PH, Valentine R et al. Dimethylformamide pharmacokinetics following inhalation exposures to rats and mice. Drug Chem Toxicol . 1993;16(1):21–52 sW. [DOI] [PubMed] [Google Scholar]
- 22. Hurtt ME, Placke ME, Killinger JM et al. 13-week inhalation toxicity study of dimethylformamide (DMF) in cynomolgus monkeys. Fundamental and Applied Toxicol : Off j Soc Toxicol 1992;18(4):596–601. [DOI] [PubMed] [Google Scholar]
- 23. Wang P, Chen D, Ma H et al. LncRNA SNHG12 contributes to multidrug resistance through activating the MAPK/Slug pathway by sponging miR-181a in non-small cell lung cancer. Oncotarget 2017;8(48):84086–84101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang C, Shao S, Deng L et al. LncRNA SNHG12 regulates the radiosensitivity of cervical cancer through the miR-148a/CDK1 pathway. Cancer Cell Int 2020;20(1):554. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25. Li L, Yu H, Ren Q. MiR-218-5p suppresses the progression of retinoblastoma through targeting NACC1 and inhibiting the AKT/mTOR signaling pathway. Cancer Manag Res 2020;12:6959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scruggs SB, Wang D, Ping P. PRKCE gene encoding protein kinase C-epsilon-Dual roles at sarcomeres and mitochondria in cardiomyocytes. Gene 2016;590(1):90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samuel VT, Liu ZX, Qu X et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 2004;279(31):32345–53. [DOI] [PubMed] [Google Scholar]
- 28. Brandon AE, Liao BM, Diakanastasis B et al. Protein kinase C epsilon deletion in adipose tissue, but not in liver, improves glucose tolerance. Cell Metab . 2019;29(1):183–191.e7. [DOI] [PubMed] [Google Scholar]
- 29. Lu D, Sivaprasad U, Huang J et al. Protein kinase C-epsilon protects MCF-7 cells from TNF-mediated cell death by inhibiting Bax translocation. Apoptosis : An International Journal on Programmed Cell Death 2007;12(10):1893–900. [DOI] [PubMed] [Google Scholar]
- 30. Karch J, Kwong JQ, Burr AR et al. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. elife 2013;2:e00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsai KL, Liang HJ, Yang ZD et al. Early inactivation of PKCε associates with late mitochondrial translocation of Bad and apoptosis in ventricle of septic rat. J Surg Res 2014;186(1):278–86. [DOI] [PubMed] [Google Scholar]
- 32. Lan T, Ma W, Hong Z et al. Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12) promotes tumorigenesis and metastasis by targeting miR-199a/b-5p in hepatocellular carcinoma. Journal of Experimental & Clinical Ccancer Research : CR 2017;36(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


