Primary central nervous system lymphoma (PCNSL) is a rare tumor that can be difficult to diagnose as it can mimic other CNS diseases. Tissue biopsy is the gold standard for diagnosis, but this is not always feasible if the lesions are in deep structures of the brain. Tissue samples may also be non-diagnostic if steroids have been administered prior to the procedure. Genomic testing of circulating tumor DNA (ctDNA) in the cerebrospinal fluid is a technique that may provide an alternative method for diagnosis. Prior case reports and studies have shown a correlation between PCNSL and MYD88 mutation, but patients were treated only in the setting of a diagnostic biopsy. Here we present a case of a patient with inconclusive biopsy results whose CSF ctDNA revealed a MYD88 mutation suggestive of PCNSL and guided her treatment with high-dose methotrexate.
Case Report
A 65-year-old woman with metastatic malignant peripheral nerve sheath tumor (MPNST) on active treatment with a novel clinical trial drug and no known germline mutations presented with 6 weeks of nausea, worsening gait, and falls. MRI of the brain revealed nonenhancing white matter abnormalities (Figure 1). Cerebrospinal fluid (CSF) cell count and protein were normal, cytology demonstrated atypical lymphoid cells, and flow cytometry noted an abnormal mature B-cell population that could not be further characterized. CSF viral studies, CSF oligoclonal bands, and both CSF and serum paraneoplastic panels were negative. PET scan of the body showed no evidence of new disease, and ophthalmologic examination was normal. Her clinical trial drug was held, but she worsened symptomatically over the next month with new intermittent diplopia, difficulty holding up her head, and inability to walk. Repeat MRI brain revealed new enhancing lesions, and repeat CSF studies again showed atypical cells with an abnormal B-cell population but normal cell count and protein. The differential diagnosis included an inflammatory or autoimmune process, drug-related process from her MPNST treatment, or a new neoplastic process. Given the deep location of the enhancing lesions, brain biopsy was deferred. She was treated with high-dose methylprednisolone for a presumed autoimmune/inflammatory versus drug-related process.
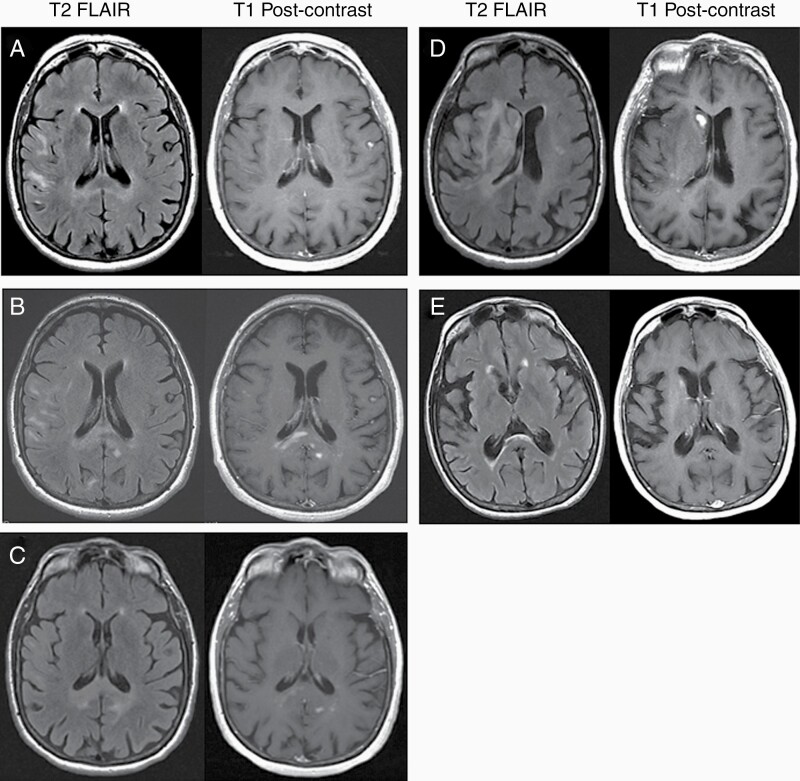
Figure 1.
Changes in MRI brain over time. T2 FLAIR precontrast and T1 postcontrast images between initial presentation of symptoms and post-treatment with high-dose methotrexate and cytarabine consolidation. (A) Initial presentation, no contrast-enhancing lesions present. (B) One month after initial presentation, new contrast-enhancing lesions in the corpus callosum. (C) Post high-dose steroid administration, mild reduction in size of enhancing lesions. (D) Just prior to treatment with high-dose methotrexate. (E) Post-treatment with 8 high-dose methotrexate infusions and consolidation with cytarabine.
Over the next several months, her brain MRI showed worsening fluctuations in enhancing and nonenhancing lesions (Figure 1). Brain biopsy of a new enhancing portion revealed gliosis with mild–moderate parenchymal and perivascular mixed inflammatory infiltrate. Genomic testing of the biopsy sample using MSK-IMPACT was nondiagnostic. As there was no evidence of malignancy, she was started on intravenous immunoglobulin (IVIG) and discharged on a prednisone taper. Her symptoms continued to worsen with progressive MRI changes, and she was treated with plasma exchange followed by IVIG and rituximab.
Given her continued lack of improvement, MSK-IMPACT was performed on the CSF and resulted in the discovery of MYD88, CD58, HIST1H1C, KMT2D, and LCK mutations suggestive of a lymphoid malignancy like primary central nervous system lymphoma (PCNSL). Based on these results, she was treated with 8 doses of rituximab and methotrexate with complete radiographic resolution of disease (Figure 1). She was consolidated with high-dose cytarabine and is now functionally independent.
Discussion
This case is an example of the importance of CSF ctDNA analysis in the diagnosis of PCNSL in patients who have suspicious lesions in areas that are difficult to biopsy or have nondiagnostic biopsies. PCNSL is a rare type of extranodal non-Hodgkin lymphoma located in the central nervous system that can be difficult to diagnose. Initial clinical presentations and radiographic findings can mimic other disorders such as primary brain tumors, demyelinating diseases, autoimmune or paraneoplastic syndromes, or central nervous system infections.1 More than 90% of PCNSL lesions enhance on MRI brain, and the gold standard for diagnosis is via biopsy of a lesion.2 In this case, the patient’s treatment with a clinical trial drug with unknown side effects along with her fluctuating symptoms of diplopia and weakness made an autoimmune, inflammatory, or demyelinating process higher on the differential over a second uncommon malignancy with a rare atypical presentation like PCNSL. In addition, her nonenhancing FLAIR hyperintense lesions were in the deep structures of the brain, making biopsy less appealing in the setting of lower clinical suspicion.
Clinicians have attempted to rely on CSF for diagnosis of PCNSL as it is less invasive than biopsy, but neither CSF cell count nor protein levels appear to reliably predict the presence of PCNSL.2 In addition, the diagnostic yield in CSF cytology or flow cytometry is often low and do not produce corresponding results. In a prospective study of 123 B-cell lymphoma patients, only 22% of patients had CSF flow cytometry that identified neoplastic B cells, and 8% of patients had conventional CSF cytology with malignant or suspicious cells present.3 Other studies have shown that the presence of monoclonal B cells in the CSF may not be diagnostic of clinically significant CNS involvement by lymphoma, as they can also be seen in inflammatory and demyelinating conditions, making it difficult to interpret CSF flow cytometry results.4 Atypical cells on cytology can also be observed in patients with infections and autoimmune/inflammatory disorders.5,6 Although this patient had CSF cytology with atypical cells and CSF flow cytometry showing an abnormal B-cell population, it was difficult to interpret these results given that an autoimmune/inflammatory process was high on the differential. In this setting, these results alone could not justify a diagnosis of PCNSL and exposure to high-dose methotrexate with its associated side effects including nephrotoxicity, hepatotoxicity, and myelosuppression.7
The diagnosis was made by performing next-generation molecular testing on ctDNA in the CSF with MSK-IMPACT, a FDA-authorized DNA sequencing panel targeting over 400 oncogenes and tumor suppressor genes identifying mutations, copy number alterations, and gene fusions.8 The presence of MYD88, CD58, HIST1H1C, KMT2D, and LCK in the patient’s CSF made the molecular diagnosis of PCNSL and guided treatment. Although these mutations have all been linked to hematologic malignancies (Table 1), MYD88 is of particular interest as it occurs in at least 35% of patients with PCNSL. Fontanilles et al. reported that MYD88 had a diagnostic sensitivity of 24% for PCNSL but a specificity of 100%.9 More recently, Ferreri et al. report a sensitivity of 94% and specificity of 98% for the MYD88 mutation status and IL-10 levels in the CSF.10 Given the specificity of MYD88 reported in the literature at the time the patient was diagnosed, she was treated with high-dose methotrexate and rituximab even though her brain biopsy was inconclusive.
Table 1.
MSK-IMPACT Report From CSF ctDNA
| Gene | Alternate Name | Type | Alteration | Location | Mean Allele Frequency | Function | Citation |
|---|---|---|---|---|---|---|---|
| MYD88 | None | Missense mutation | L265P (c.794T>C) | Exon 5 | 28.3% | Adaptor protein | Fontanilles et al.9 Ferreri et al.10 |
| KMT2D | MLL2, CAGL114, TNRC21m ALR | Frameshift deletion | P1769Lfs*16 (c.5306del) | Exon 22 | 29.2% | Tumor suppressor and methyltransferase | Ortega-Molina et al.11 |
| CD58 | LFA3 | Frameshift insertion | H44Ffs*20 (c.126_129dupTTTC) | Exon 2 | 14.1% | Cell surface adhesion molecule expressed in immune cells | Cao et al.12 |
| HIST1H1C | H1-2, H1s-1, H1F2, H1c, H1.2 | Missense mutation | Q95E (c.283C>G) | Exon 1 | 14.9% | Histone H1 variant binding to linker DNA between nucleosomes | Okosun et al.13 |
| LCK | None | Nonsense mutation | Q87* (c.259C>T) | Exon 4 | 20.0% | Tyrosine kinase regulating T-cell receptor signaling | Tan et al.14 |
Somatic alterations detected in ctDNA from the patient’s CSF. Overall sequence coverage was 59×.
Although next-generation molecular sequencing allowed for the diagnosis to be made, 1 major drawback is the amount of time it takes to generate the results. Molecular diagnostics can take weeks to months to result whereas frozen pathology from a brain biopsy can confirm the diagnosis before the surgery is finished leading to faster treatment and clinical improvement for the patient. Given how quickly PCNSL patients can deteriorate, rapid diagnosis is paramount to their care. At the time of this diagnosis, MSK-IMPACT took 3 weeks to result, though this has improved over the years. Newer technology such as droplet digital PCR can identify mutations like MYD88 and can be resulted faster, but they can only be utilized if the clinician is highly suspicious of PCNSL.15 In this report, the patient’s diagnosis was unknown in the setting of her MPNST, and the benefit of using a test like MSK-IMPACT is that it targets a wide variety of oncogenes and tumor suppressor genes to help narrow down a diagnosis. This case is an example of how ctDNA can be used to guide treatment of PCNSL in the setting of an inconclusive traditional workup.
Funding
National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Conflict of interest statement. None declared.
Authorship Statement. All authors cared for the patient clinically, discussed her case, and contributed to the final manuscript.
References
- 1. Chiavazza C, Pellerino A, Ferrio F, Cistaro A, Soffietti R, Rudà R. Primary CNS lymphomas: challenges in diagnosis and monitoring. Biomed Res Int. 2018;2018:3606970. doi: 10.1155/2018/3606970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scott BJ, Douglas VC, Tihan T, Rubenstein JL, Josephson SA. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013;70(3):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quijano S, Lopez A, Manuel Sancho J, et al. Identification of leptomeningeal disease in aggressive B-cell non-Hodgkin’s lymphoma: improved sensitivity of flow cytometry. J Clin Oncol. 2009;27(9):1462–1469. [DOI] [PubMed] [Google Scholar]
- 4. Nowakowski GS, Call TG, Morice WG, Kurtin PJ, Cook RJ, Zent CS. Clinical significance of monoclonal B cells in cerebrospinal fluid. Cytometry B Clin Cytom. 2005;63(1):23–27. [DOI] [PubMed] [Google Scholar]
- 5. Manucha V, Zhao F, Rodgers W. Atypical lymphoid cells in cerebrospinal fluid in acute Epstein Barr virus infection: a case report demonstrating a pitfall in cerebrospinal fluid cytology. Acta Cytol. 2008;52(3):334–336. [DOI] [PubMed] [Google Scholar]
- 6. de la Monte S, Gupta PK, Hutchins GM. Polymorphous exudates and atypical mononuclear cells in the cerebrospinal fluid of patients with Sjogren’s syndrome. Acta Cytol. 1985;29(4):634–637. [PubMed] [Google Scholar]
- 7. Holmboe L, Andersen AM, Mørkrid L, Slørdal L, Hall KS. High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br J Clin Pharmacol. 2012;73(1):106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fontanilles M, Marguet F, Bohers É, et al. Non-invasive detection of somatic mutations using next-generation sequencing in primary central nervous system lymphoma. Oncotarget. 2017;8(29):48157–48168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferreri AJM, Calimeri T, Lopedote P, et al. MYD88 L265P mutation and interleukin-10 detection in cerebrospinal fluid are highly specific discriminating markers in patients with primary central nervous system lymphoma: results from a prospective study. Br J Haematol. 2021;193(3):497–505. [DOI] [PubMed] [Google Scholar]
- 11. Ortega-Molina A, Boss IW, Canela A, et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat Med. 2015;21(10):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao Y, Zhu T, Zhang P, et al. Mutations or copy number losses of CD58 and TP53 genes in diffuse large B cell lymphoma are independent unfavorable prognostic factors. Oncotarget. 2016;7(50):83294–83307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okosun J, Bödör C, Wang J, et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet. 2014;46(2):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan Y, Timakhov RA, Rao M, et al. A novel recurrent chromosomal inversion implicates the homeobox gene Dlx5 in T-cell lymphomas from Lck-Akt2 transgenic mice. Cancer Res. 2008;68(5):1296–1302. [DOI] [PubMed] [Google Scholar]
- 15. Hiemcke-Jiwa LS, Minnema MC, Radersma-van Loon JH, et al. The use of droplet digital PCR in liquid biopsies: a highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid. Hematol Oncol. 2018;36(2):429–435. [DOI] [PubMed] [Google Scholar]