Abstract
Background
The associations between vitamin D and coronavirus disease 2019 (COVID-19) infection and clinical outcomes are controversial. The efficacy of vitamin D supplementation in COVID-19 is also not clear.
Methods
We identified relevant cohort studies that assessed the relationship between vitamin D, COVID-19 infection and associated death and randomized controlled trials (RCTs) that reported vitamin D supplementation on the outcomes in patients with COVID-19 by searching the PubMed, EMBASE, and medRxiv databases up to June 5th, 2021. Evidence quality levels and recommendations were assessed using the GRADE system.
Results
Eleven cohort studies with 536,105 patients and two RCTs were identified. Vitamin D deficiency (< 20 ng/ml) or insufficiency (< 30 ng/ml) was not associated with an significant increased risk of COVID-19 infection (OR for < 20 ng/ml: 1.61, 95% CI: 0.92–2.80, I2 = 92%) or in-hospital death (OR for < 20 ng/ml: 2.18, 95% CI: 0.91–5.26, I2 = 72%; OR for < 30 ng/ml: 3.07, 95% CI: 0.64–14.78, I2 = 66%). Each 10 ng/ml increase in serum vitamin D was not associated with a significant decreased risk of COVID-19 infection (OR: 0.92, 95% CI: 0.79–1.08, I2 = 98%) or death (OR: 0.65, 95% CI: 0.40–1.06, I2 = 79%). The overall quality of evidence (GRADE) for COVID-19 infection and associated death was very low. Vitamin D supplements did not significantly decrease death (OR: 0.57, I2 = 64%) or ICU admission (OR: 0.14, I2 = 90%) in patients with COVID-19. The level of evidence as qualified using GRADE was low.
Conclusions
Current evidence suggested that vitamin D deficiency or insufficiency was not significantly linked to susceptibility to COVID-19 infection or its associated death. Vitamin D supplements did not significantly improve clinical outcomes in patients with COVID-19. The overall GRADE evidence quality was low, we suggest that vitamin D supplementation was not recommended for patients with COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12937-021-00744-y.
Keywords: Vitamin D, COVID-19, Meta-analysis, Nutrition
Introduction
Recent studies have highlighted that the mean plasma vitamin D level is significantly lower in patients who tested positive for COVID-19 than in patients who tested negative [1]. Rhodes et al. [2] found that patients with COVID-19 residing in all countries that lie below 35 degrees north latitude have relatively low death. These results suggest that low vitamin D levels are associated with increased COVID-19 infection rates and worse outcomes. Several studies have reported that serum vitamin D deficiency is associated with an increased risk of COVID-19 positivity and worse outcomes (e.g., severe COVID-19 and in-hospital death) [3–5]. However, a larger cohort-based study from the UK Biobank showed a nonsignificant association after adjustment for confounders (COVID-19 infection HR: 1.00; p = 0.89; death HR: 0.98; p = 0.69) [6]. Two other studies also found no association between vitamin D and COVID-19 positivity [7, 8]. Therefore, the impact of vitamin D on COVID-19 risk and clinical outcomes remains controversial, and a definite conclusion has not been reached. Thus, we performed a meta-analysis to clarify the association between serum vitamin D level, COVID-19 risk and associated death and assessed the effect of vitamin D supplements on clinical outcomes in patients with COVID-19 by pooling current evidence from clinical trials.
Methods
The present study was performed according to Preferred Reporting Items for Systematic reviews and Meta-Analyses Statement (PRISMA) guidelines (Supplemental Table S1). The protocol of this meta-analysis was not registered.
Literature search and study selection
Two authors (X.L. and J.C.) independently searched several databases (PubMed, EMBASE, and medRxiv) using the following groups of keywords with no language restrictions up to June 5th, 2021,: 2019-novel coronavirus, SARS-CoV-2, COVID-19, 2019-nCoV, vitamin D, death, severe and ICU. The details of the search strategy are described in Supplemental Table S2. We also searched the reference lists of relevant publications to identify further studies. The following inclusion criteria were used: 1) human studies that were published as original articles; 2) reports designed as cohorts with estimated effects (multivariate-adjusted) and 95% confidence interval (CI) results that reported the association between vitamin D, COVID-19 risk, and death; and 3) clinical trials (randomized controlled design) that assessed vitamin D supplementation on clinical outcomes in patients with COVID-19. Case-control, cross-sectional were excluded. When multiple papers reporting on the same study were identified, the most informative or complete article was included.
Data extraction and statistical analysis
Two authors independently extracted all data from the included studies. Discrepancies and disagreements were resolved via collegial discussion. The following information was extracted: first author, country, publication year, gender, mean or median age, study design, sample size, vitamin D level, OR or RR with the 95% CI for each category (results adjusted according to most potential confounders), and adjusted variables.
Effect measures were transformed to their natural logarithms (logOR), and the standard errors (SElog[OR]) were calculated from the corresponding 95% CI. We calculated study-specific slopes (vitamin D per 10 ng/ml increase), and 95% CIs from the natural logs of the reported RRs and CIs across categories of vitamin D levels [9, 10]. Cochran Q and I2 statistics were used to detect statistical heterogeneity between studies. The overall quality of the included studies was assessed with the Newcastle-Ottawa quality assessment scale (NOS), and an NOS score ≥ 6 was considered high quality [11]. The risk of bias for the trials was assessed with the Cochrane risk of bias tool. The GRADE methodology was used to evaluate the quality of the body of retrieved evidence (GRADEpro, https://gdt.gradepro.org/app/#projects). All statistical analyses were performed using Review Manager version 5.3 (The Cochrane Collaboration 2014; Nordic Cochrane Center Copenhagen, Denmark). All statistical tests were double-sided, and P < 0.05 was considered statistically significant.
Results
Study selection
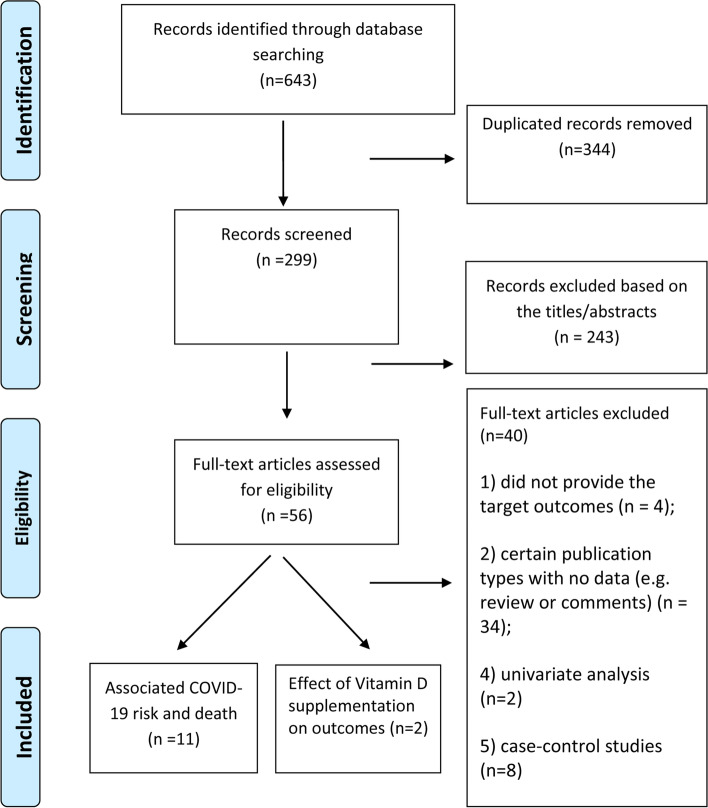
As shown in Fig. 1, we identified 643 studies in the initial database search. After the removal of duplicates (n = 344) and studies with insufficient information (n=283) on vitamin D and COVID-19, a total of 13 [3, 5, 6, 12–21] studies were included.
Fig. 1.
Flow chart of study selection in this meta-analysis
Study characteristics and quality
Each study is listed in Table 1. Of the 13 studies, 11 [3, 5, 6, 12–15, 17–19, 22] cohorts with 536,105 patients assessed the COVID-19 risk and death, and two [16, 20] RCTs investigated the effect of vitamin D supplementation on outcomes in patients with COVID-19. The mean patient age ranged from 49 to 69 years. Ten [3, 5, 12–15, 17–19, 22] studies were based on hospitalized patients, and one [6] study was based on a prospective cohort (UK Biobank). Most studies [3, 14, 18–20, 22] (n = 6) were performed in the US, 5 [5, 6, 13, 15, 16] studies were conducted in Europe, 1 [17] study was performed in Asia, and 1 [12] study was performed in Australia. Four [3, 6, 23, 24] studies reported vitamin D levels and COVID-19 positivity, and eight [5, 6, 12–15, 17, 22] studies reported the association between vitamin D levels and death. The overall quality of the observational studies was acceptable (NOS score ≥ 6) (Supplemental Table S4). Among the two RCTs were [16, 20] had a low risk of bias (Supplemental Table S5).
Table 1.
Basic characteristics of included articles reporting the association between vitamin D and COVID-19 infection and death, effect of vitamin D supplement on clinical outcomes in patients with COVID-19
| Author, publication year, country | Study design, Follow up | Source of patients | Sample size (N) | Mean age (years), male | Definition of Vitamin D exposure | Expose level of vitamin D | OR (95% CI)a | Adjustments for confounders |
| Hastie, 2020, UK [6, 8] | Prospective cohort study | UK Biobank | 341,484 | 49, 48% | Conducted between 2006 and 2010 |
Per 10 ng/ml < 20 ng/ml > 20 ng/ml Per 10 nmol/L < 20 ng/ml > 20 ng/ml |
COVID-19 infection 1.00 (0.89–1.12) 1.06 (0.89–1.26) Ref Death 0.95 (0.79–1.15) 1.02 (0.75–1.38) Ref |
Age, sex, ethnicity, month of assessment, Townsend deprivation quintile, household income, BMI category, smoking status, diabetes, systolic blood pressure, diastolic blood pressure, self-reported health rating, and long-standing illness, disability or infirmity |
| Hastie, 2020, UK [6, 8] | Retrospective cohort study | University of Chicago Medicine | 498 | 49,25% | Within 1 year before their first COVID-19 tests |
< 20 ng/ml > 20 ng/ml |
COVID-19 infection 1.77 (1.12–2.81) Ref |
Age, sex, ethnicity, race, employee status. Hypertension, DM, chronic pulmonary disease, pulmonary circulation disorders, depression, CKD, liver disease, comorbidities with immunosuppression, BMI |
| Radujkovic,2020, Germany [5] | Retrospective cohort study | Medical university Hospital of Heidelberg | 185 | 60,51% | At the time of admission and SARS-CoV-2 testing |
< 20 ng/ml > 20 ng/ml |
Death 11.27 (1.48–85.55) Ref |
Age, gender, and any comorbidities |
| Charoenngam,2021, US [14] | Retrospective cohort study | Boston University Medical Center | 287 | 62,47% | Measured at within 48 h after admission |
< 30 ng/ml > 30 ng/ml |
Death Ref 0.62 (0.28–1.41) |
Age, sex, BMI, insurance, race, smoking, alcohol drinking, type 2 DM, hypertension, dyslipidemia, CAD, cerebrovascular disease, COPD, asthma, CKD, ESRD, malignancy, HIV infection, and heart failure. |
| Carpagnano, 2020, Italy [13] | Retrospective cohort study | Hospital Policlinic of Bari | 42 | NA | At admission |
< 10 ng/ml > 10 ng/ml |
Death 5.68 (1.14–28.97) Ref |
Age, higher levels of creatinine, troponin, and IL-6 |
| Katz D,2020, US [18] | Retrospective cohort study | UF health centers | 887 | NA, NA | October 1, 2015, through June, 30, 2020, for vitamin D deficiency |
< 20 ng/ml > 20 ng/ml |
COVID-19 infection 2.27 (1.79–2.87) Ref |
Age, sex, malabsorption, PA, dental diseases, race, periodontal disease status, DM, obesity |
| Kaufman, 2020, US [19] | Retrospective cohort study | National clinical laboratory | 191,779 | 54, 22% | Most recent vitamin D level | Per 10 ng/ml |
COVID-19 infection 0.85 (0.84–0.86) |
Male, northern and central latitudes, predominately black non-Hispanic zip codes, and predominately Hispanic zip codes |
| Angelidi,2020, US [22] | Retrospective cohort study | 2 tertiary academic medical centers | 144 | 66, 64% | Hospital personnel at Regular intervals |
< 30 ng/ml > 30 ng/ml Per 10 ng/ml |
Death 8.33 (1.6–50) Ref 0.54(0.35–0.82) |
Age, BMI, ARB or ACEI, in-hospital drug treatment, CRP, smoking, heart failure, CAD, diabetes, hypertension, C-reactive protein level, and corticosteroids |
| De Smet,2021, Germany [15] | Retrospective cohort study | AZ Delta General Hospital | 186 | 69,58% | Measured in patients with COVID-19 on admission and within 24 h |
< 20 ng/ml > 20 ng/ml |
Death 3.87(1.30–11.55) Ref |
Age, higher CT severity score, presence of chronic lung |
| AlSafar, 2021, Australia [12] | Retrospective cohort study | Abu Dhabi, or Rashed hospital in Dubai. | 464 | 46.6,80% | At recruitment |
< 20 ng/ml > 20 ng/ml |
Death 1.71 (0.66, 4.43) Ref |
Age, sex, and comorbidities, BMI |
| Karahan, 2021, Turkey [17] | Retrospective cohort study | Health Sciences University | 149 | 64, 54% | NA | Per 10 ng/ml |
Death 0.93(0.88–0.98) |
Age, smoking, hyperlipidemia, DM, CKD, Chronic AF, congestive heart failure, acute kidney injury, CRP, lymphocyte count, white blood cell count, serum albumin |
| Author, publication year, country | Design | Sample size (male%); mean age(years) | Number of participants in intervention and control groups | 25(OH)D assay | Mean baseline 25(OH)D concentrations, nmol/L (SD) | Oral dose of vitamin D in the intervention group | Control group | Outcome |
| Murai,2021, US [20] | Multicenter double-blind RCT | 240(56),56.3 |
intervention = 120; control = 120 |
NA |
21.0 (10.2) 20.6 (8.1) |
Single dose of 200,000 IU | Placebo |
Death Admission to ICU |
| Castillo,2020, Spain [16] | Signal center RCT | 76(59%),53.0 |
intervention = 50; control = 26 |
NA | NA | Single dose of oral calcifediol (0.532 mg) | Without Calcifediol treatment |
Death Admission to ICU |
OR odd ratio, UCLA University of California Los Angeles, UK United Kingdom, US Unite Status, SES residential socioeconomic status, CKD chronic kidney diseases, ICU intensive care unit, AF atrial fibrillation, BMI body mass index, ARB Angiotensin Receptor Blocker, ACEI angiotensin converting enzyme inhibitors, CRP C-reactive protein, DM diabetes mellitus, CAD coronary heart disease, NA not available, RCT randomized controlled trial, ESRD end-stage renal disease, HIV human immunodeficiency virus
aHazard ratio and incidence rate ratio were treated as odd ratio
The effect of low vitamin D level on COVID-19
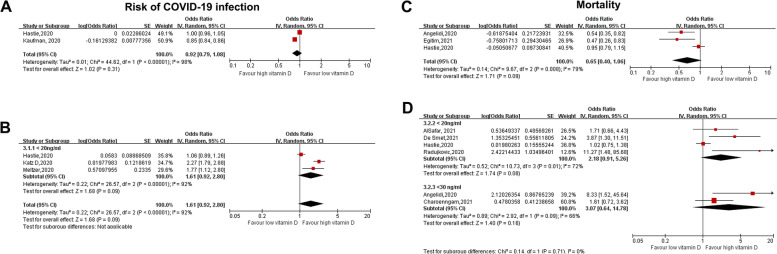
The categorical analysis revealed that vitamin D deficiency (< 20 ng/ml) or insufficiency (< 30 ng/ml) was not associated with an significant increased risk of COVID-19 infection (OR for < 20 ng/ml: 1.61, 95% CI: 0.92–2.80, I2 = 92%) or in-hospital death (OR for < 20 ng/ml: 2.18, 95% CI: 0.91–5.26, I2 = 72%; OR for < 30 ng/ml: 3.07, 95% CI: 0.64–14.78, I2 = 66%) (Fig. 2B&D). When vitamin D level was analyzed as a continuous variable, each 10 ng/ml increase in vitamin D level was not associated with a significant decreased risk of COVID-19 inflection (OR: 0.92, 95% CI: 0.79–1.08, I2 = 98%) or death (OR: 0.65, 95% CI: 0.40–1.06, I2 = 79%) (Fig. 2A&C).
Fig. 2.
Forest plot showing the association between serum vitamin D level and risk of COVID-19 infection and death in patients with COVID-19. A-B: COVID-19 infection, vitamin D was analyzed as a categorical variable (A: upper) or continuous variable (B: lower). C-D: Death, vitamin D was analyzed as a categorical variable (C: upper) or continuous variable (D: lower). (Continuous variable: vitamin D per 10 ng/ml increase). Abbreviations: COVID-19, coronavirus disease 2019; OR, odds ratio; CI, confidence interval; IV, inverse variance; SE, standard error
Effect of vitamin D supplements on ICU admission or death
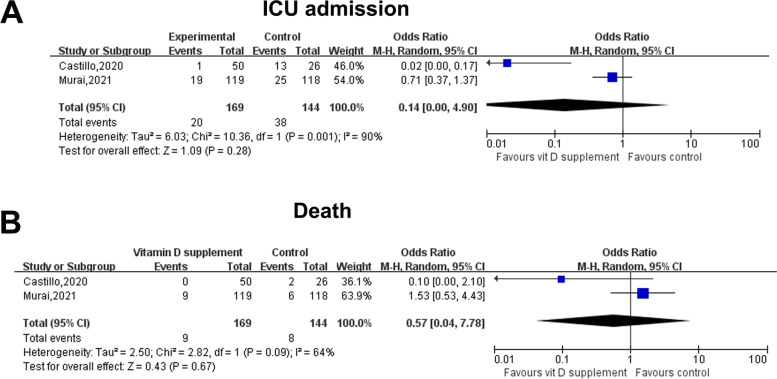
Two [16, 20] RCTs including 233 hospitalized patients with COVID-19 (vitamin D supplement, n = 169; control n = 164) assessed the effect of vitamin D supplements on clinical outcomes in patients with COVID-19. Vitamin D supplements did not significantly decrease death (OR: 0.57, 95% CI: 0.04–7.78, I2 = 64%) or ICU admission (OR: 0.14, 95% CI: 0.00–4.90, I2 = 90%) in hospitalized patients with COVID-19 (Fig. 3).
Fig. 3.
Forest plot showing the effect of vitamin D supplements on ICU admission and death in hospitalized patients with COVID-19. A: ICU admission; B: Death. Abbreviations: COVID-19, coronavirus disease 2019; OR, odds ratio; CI, confidence interval; IV, inverse variance; SE, standard error
Publication bias
Publication bias was not assessed because of the limited studies (n < 10) according to the guidelines [25].
Grade
The overall evidence for the RCTs and observational studies was qualified using GRADE. Very little confidence showed that low vitamin D supplementation contributed to an increased risk of COVID-19 or COVID-19-related death. Furthermore, only low-quality evidence supports the benefits of vitamin D supplementation on death or ICU admission in patients with COVID-19. The GRADE tables are described in detail in Supplemental Tables S6 and S7.
Discussion
The present study showed that: (i) vitamin D deficiency (< 20 ng/ml) or insufficiency (< 30 ng/ml) was not associated with a significantly increased risk of COVID-19 infection or in-hospital death (P = 0.56). (ii) A 10 ng/ml increase in serum vitamin D was not significantly linked to an increased risk of COVID-19 infection or in-hospital death. (iii) Vitamin D supplements did not improve clinical outcomes in patients with COVID-19. Overall, our study suggested no significant association between vitamin D level, COVID-19 infection, and outcomes and no benefit of vitamin D supplementation in hospitalized patients with COVID-19.
Comparisons with previous studies and further research
In contrast, several meta-analysis studies found a positive correlation between low serum vitamin D levels and worse clinical outcomes [26, 27]. However, they included case-control studies or reported unadjusted estimate effects, which might cause greater bias. For example, findings from the UK Biobank found a positive association between low vitamin D and COVID-19 infection, but the association was not significant after adjusting for confounders [6]. The present study included only cohort studies and multivariate-adjusted studies, which should reduce the potential bias. Evidence from a few Mendelian randomization studies also showed that vitamin D status did not causally affect susceptibility to and the severity of COVID-19 infection [28, 29]. Overall, these results strongly suggested no association between serum vitamin D and COVID-19.
Our results also support the recommendation of joint guidance of insufficient evidence of vitamin D for the treatment of COVID-19 [30]. Although a small random controlled trial showed that oral vitamin D supplementation helped achieve SARS-CoV-2 RNA negativity in greater proportion and decreased inflammatory markers [21], we did not find a significant benefit of vitamin D supplements by combining available evidence from RCTs on clinical outcomes in patients with COVID-19, which was consistent with two quasi-experimental studies [31, 32]. The overall quality of evidence for death or ICU admission was low, suggesting no recombination of vitamin D supplementation for hospitalized patients with COVID-19. However, all of the trials included a low or unclear percentage of patients with 25-hydroxyvitamin D deficiency. The benefits of vitamin D supplementation in patients with vitamin D deficiency should be further studied. Further studies with higher quality should also determine whether preventive or early vitamin D3 supplementation would be useful. (NCT04535791; NCT04482673; NCT04407286).
Strengths and limitations
The strength of the present study lies in the study design, which included only cohort studies and RCTs. This study is the first meta-analysis to use the GRADE system to evaluate the quality of the evidence. The present study also has several limitations. Our meta-analysis had high heterogeneity, which might be derived from the study design and variability in baseline characteristics. For example, several studies have shown that the death rate for black patients with COVID-19 was higher than the rate for white patients with COVID-19 [33, 34]. Second, many factors modulate vitamin D status, including genetic polymorphisms, age, health, sun exposure behavior, and season [11]. Although we included only studies that performed multivariable analysis, some potential risk factors were not fully adjusted, which affected our results. Therefore, further research should adjust for additional confounding factors to verify the results.
Conclusion
Based on current evidence, vitamin D deficiency or insufficiency was not significantly linked to susceptibility to COVID-19 infection or its associated death. Vitamin D supplements did not significantly improve clinical outcomes in patients with COVID-19, and the overall GRADE evidence quality was low, which suggested that vitamin D supplementation was not recommended for patients with COVID-19.
Supplementary Information
Additional file 1: Table S1. Table S2. Detailed description of the search strategy. Supplemental Table S4. Quality assessment of the included observational studies. Supplemental Table S5. Quality assessment of the included RCT trials. Table S6. GRADE table observational studies. Table S7. GRADE table for RCTs.
Acknowledgments
We acknowledge all people who fought against COVID-19.
We acknowledge the grant support from Guangzhou Science Technology Bureau (202102010007).
Abbreviations
- OR
Odd ratio
- COVID-19
Corona Virus Disease 2019
- RAAS
Renin-angiotensin-aldosterone system
- CIs
Confidence intervals
- NOS
Newcastle-Ottawa quality assessment scale
Authors’ contributions
Guarantor of the article: XL. Authors’ contributions: XL contributed to the study concept and design. K.B-M and JC contributed to database research, acquisition of data, and statistical analyses. All of the authors reviewed and approved the final manuscript.
Funding
This work was supported in part by the National Natural Science Foundation of China (No. 81760050, 81760048, 82100347) and the Jiangxi Provincial Natural Science Foundation for Youth Scientific Research (No. 20192ACBL21037).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing financial interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jie Chen and Kaibo Mei contributed equally to this work.
Contributor Information
Chunhua Zheng, Email: zch6595@163.com.
Xiao Liu, Email: liux587@mail.sysu.edu.cn.
References
- 1.Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020;13:1373–1380. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes JM, Subramanian S, Laird E, Kenny RA. Editorial: low population death from COVID-19 in countries south of latitude 35 degrees north supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51:1434–1437. doi: 10.1111/apt.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3:e2019722. doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendy A, Apewokin S, Wells AA, Morrow AL. Factors Associated with Hospitalization and Disease Severity in a Racially and Ethnically Diverse Population of COVID-19 Patients. medRxiv. 2020.2020.06.25.20137323. 10.1101/2020.06.25.20137323.
- 5.Radujkovic A, Hippchen T, Tiwari-Heckler S, Dreher S, Boxberger M, Merle U. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients. 2020;12:2757. [DOI] [PMC free article] [PubMed]
- 6.Hastie CE, Pell JP, Sattar N. Vitamin D and COVID-19 infection and death in UK biobank. Eur J Nutr. 2020;60:545–8. [DOI] [PMC free article] [PubMed]
- 7.Raisi-Estabragh Z, McCracken C, Bethell MS, Cooper J, Cooper C, Caulfield MJ, Munroe PB, Harvey NC, Petersen SE. Greater risk of severe COVID-19 in black, Asian and minority ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK biobank. J Public Health (Oxf) 2020;42:451–460. doi: 10.1093/pubmed/fdaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastie CE, Mackay DF, Ho F, Celis-Morales CA, Katikireddi SV, Niedzwiedz CL, Jani BD, Welsh P, Mair FS, Gray SR, et al. Vitamin D concentrations and COVID-19 infection in UK biobank. Diabetes Metab Syndr. 2020;14:561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C, Thabane L, Liu T-Z, Li L, Borhan S, Sun X. Flexible piecewise linear model for investigating doseresponse relationship in meta-analysis: methodology, examples, and comparison. PeerJ Preprints. 2018;6:e27277v1. doi: 10.1111/jebm.12339. [DOI] [PubMed] [Google Scholar]
- 10.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Wang W, Tan Z, Zhu X, Liu M, Wan R, Hong K. The relationship between vitamin D and risk of atrial fibrillation: a dose-response analysis of observational studies. Nutr J. 2019;18:73. doi: 10.1186/s12937-019-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AlSafar H, Grant WB, Hijazi R, Uddin M, Alkaabi N, Tay G, Mahboub B, Al Anouti F. COVID-19 disease severity and death in relation to vitamin D status among SARS-CoV-2-positive UAE residents. Nutrients. 2021;13:1714. doi: 10.3390/nu13051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpagnano GE, Di Lecce V, Quaranta VN, Zito A, Buonamico E, Capozza E, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Investig. 2020;44:765–71. [DOI] [PMC free article] [PubMed]
- 14.Charoenngam N, Shirvani A, Reddy N, Vodopivec DM, Apovian CM, Holick MF. Association of Vitamin D Status with Hospital Morbidity and Death in adult hospitalized patients with COVID-19. Endocr Pract. 2021;27:271–278. doi: 10.1016/j.eprac.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and death. Am J Clin Pathol. 2021;155:381–388. doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcala Diaz JF, Lopez Miranda J, Bouillon R, Quesada Gomez JM. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and death among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karahan S, Katkat F. Impact of serum 25(OH) vitamin D level on death in patients with COVID-19 in Turkey. J Nutr Health Aging. 2021;25:189–196. doi: 10.1007/s12603-020-1479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz J, Yue S, Xue W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition. 2021;84:111106. doi: 10.1016/j.nut.2020.111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, Silva CBR, Franco AS, Macedo MB, Dalmolin HHH, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J. 2020;postgradmedj-2020-139065. [DOI] [PubMed]
- 22.Angelidi AM, Belanger MJ, Lorinsky MK, Karamanis D, Chamorro-Pareja N, Ognibene J, Palaiodimos L, Mantzoros CS. Vitamin D status is associated with in-hospital death and mechanical ventilation: a cohort of COVID-19 hospitalized patients. Mayo Clin Proc. 2021;96:875–886. doi: 10.1016/j.mayocp.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang TS, Ding Y, Freund MK, Johnson R, Schwarz T, Yabu JM, et al. Prior diagnoses and medications as risk factors for COVID-19 in a Los Angeles Health System. medRxiv. 2020.07.03.20145581. 10.1101/2020.07.03.20145581.
- 24.Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green I, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287:3693–702. [DOI] [PMC free article] [PubMed]
- 25.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 [updated March 2017]: The Cochrane Collaboration; 2017. Available from http://handbook.cochrane.org.chanpter: 10.4.3.1 Recommendations on testing for funnel plot asymmetry. 2011.
- 26.Pereira M, Dantas Damascena A, Galvão Azevedo LM, de Almeida OT, da Mota SJ. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020;1–9. Epub ahead of print. [DOI] [PubMed]
- 27.Petrelli F, Luciani A, Perego G, Dognini G, Colombelli PL, Ghidini A. Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies. J Steroid Biochem Mol Biol. 2021:105883. [DOI] [PMC free article] [PubMed]
- 28.Patchen BK, Clark AG, Gaddis N, Hancock DB, Cassano PA. Genetically predicted serum vitamin D and COVID-19: a Mendelian randomisation study. BMJ Nutr Prev Health. 2021;4:213–25. [DOI] [PMC free article] [PubMed]
- 29.Amin HA, Drenos F. No evidence that vitamin D is able to prevent or affect the severity of COVID-19 in individuals with European ancestry: a Mendelian randomisation study of open data. BMJ Nutr Prev Health. 2021;4:42–8. [DOI] [PMC free article] [PubMed]
- 30.Vimaleswaran KS, Forouhi NG, Khunti K. Vitamin D and covid-19: British Medical Journal Publishing Group; 2021.
- 31.Annweiler C, Hanotte B, de l'Eprevier CG, Sabatier JM, Lafaie L, Celarier T. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J Steroid Biochem Mol Biol. 2020;204:105771. doi: 10.1016/j.jsbmb.2020.105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Annweiler G, Corvaisier M, Gautier J, Dubee V, Legrand E, Sacco G, et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12.3377. [DOI] [PMC free article] [PubMed]
- 33.Milam AJ, Furr-Holden D, Edwards-Johnson J, Webb B, Patton JW, Ezekwemba NC, Porter L, Davis T, Chukwurah M, Webb AJ, et al. Are clinicians contributing to excess African American COVID-19 deaths? Unbeknownst to them, they may be. Health Equity. 2020;4:139–141. doi: 10.1089/heq.2020.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khunti K, Singh AK, Pareek M, Hanif W. Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020;369:m1548. doi: 10.1136/bmj.m1548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Table S2. Detailed description of the search strategy. Supplemental Table S4. Quality assessment of the included observational studies. Supplemental Table S5. Quality assessment of the included RCT trials. Table S6. GRADE table observational studies. Table S7. GRADE table for RCTs.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.