Abstract
Hepatitis B virus (HBV) infection causes hepatocellular carcinoma (HCC). Associations with other cancers are not established. We systematically assessed associations between HBV infection and cancers in the US elderly population. We conducted a case-control study using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database in US adults aged ≥66 years. Cases (N=1,825,316) were people with first cancers diagnosed in SEER registries (1993-2013). Controls (N=200,000) were randomly selected, cancer-free individuals who were frequency-matched to cases on age, sex, race, and calendar year. Associations with HBV infection (ascertained by Medicare claims) were assessed by logistic regression. HBV prevalence was higher in cases than controls (0.6% vs. 0.5%). HBV was positively associated with cancers of the stomach (Adjusted odds ratio [aOR]=1.19; 95% confidence intervals [CI]=1.03-1.37), anus (1.66; 1.17-2.33), liver (10.6; 9.66-11.6), intrahepatic bile ducts (1.67; 1.18-2.37), nasopharynx (2.08; 1.33-3.25), as well as myelodysplastic syndrome (1.26; 1.07-1.49) and diffuse large B cell lymphoma (DLBCL) (1.24; 1.06-1.46). Inverse associations were observed with female breast (aOR=0.86; 95%CI=0.76-0.98) and prostate (0.81; 0.73-0.91) cancers, and chronic lymphocytic leukemia (0.77; 0.62-0.96). Associations were maintained in sensitivity analyses conducted in people without claims for cirrhosis, or hepatitis C or human immunodeficiency virus infections. HBV infection is associated with increased risk of cancers other than HCC, such as bile duct cancers and DLBCL. The biological mechanisms by which HBV may lead to these cancers need to be explored.
Keywords: hepatitis B virus, cancers, SEER-Medicare, elderly, epidemiology
INTRODUCTION
Hepatitis B virus (HBV) infection is a global public health problem with an estimated 2 billion individuals having evidence of ever being infected with the virus and approximately 248 million chronic carriers worldwide.1 HBV is a hepatotropic virus; 12-20% of individuals with chronic HBV infection develop cirrhosis, and 6-15% of those cirrhotic individuals develop hepatocellular carcinoma (HCC).2
Several reports suggest HBV infection may be associated with cancers other than HCC, mostly non-Hodgkin lymphoma (NHL),3, 4 but the subtypes of NHL associated with HBV infection are unclear. HBV infection has also been associated with biliary tract cancers, especially intrahepatic cholangiocarcinoma,5 and pancreatic cancers.6, 7 However, results of those studies have not been consistent.8, 9 Furthermore, most studies were carried out in East Asian countries and included a limited number of cancer cases. HBV infection has also been associated with cancers at other extrahepatic sites (e.g., colorectum, kidney, ovaries).10 Besides the hepatocytes, HBV DNA sequences have been found in extrahepatic tissues such as pancreas, biliary tract, and kidney, as well as lymphatic tissue and peripheral mononuclear cells, thus making associations with cancer biologically plausible.11–16
Population-based studies in the United States (US) that analyzed the association between HBV infection and cancer have mostly focused on hepatobiliary cancers.17–19 HBV infection is currently not considered a major public health problem in the general US population due to widespread implementation of HBV vaccination at birth since 1992.20 However, Medicare-eligible adults over 65 years of age may not have received the vaccine since they were born before 1950. Thus, this population may be at an increased risk of hepatic and extrahepatic cancers.
The Surveillance, Epidemiology, and End Results (SEER)-linked Medicare dataset provides a unique opportunity to explore these associations in a large number of elderly adults with comprehensive cancer ascertainment. We previously systematically assessed the associations between different cancer types and chronic hepatitis C virus (HCV) infection using this database.21 Now, we use the same methodology to thoroughly assess the associations between HBV infection and cancer among a nationally representative cohort of elderly US adults.
MATERIALS AND METHODS
Data source: SEER-Medicare linked database
SEER is a cancer surveillance program that collects demographic and clinical information from 18 US cancer registries covering ~28% of the US population (https://seer.cancer.gov/). Medicare is a federally-funded program that provides health insurance to US adults aged ≥65 years, 97% of whom are eligible for Part-A coverage (hospital inpatient care). Approximately 96% additionally subscribe to Part-B coverage (for physician and outpatient services). Medicare does not receive claims for medical conditions from beneficiaries who elect to enroll in a health maintenance organization (HMO). The SEER-Medicare database is an electronic linkage of SEER and Medicare that successfully links more than 94% of SEER cancer cases ≥65 years of age with their Medicare claims data (1991 onward).22 Claims data for an additional 5% random sample of Medicare beneficiaries residing in SEER geographic areas are available.
Study design and study population
We conducted a case-control study using data from the 2016 SEER-Medicare linkage to determine if HBV was associated with cancer risk.23 Eligible cases were people with a first cancer diagnosis identified in SEER, excluding basal cell and squamous cell skin carcinomas, which are not captured by cancer registries. To ensure adequate ascertainment of HBV status, we required that cases have at least 13 months of Medicare Part-A, Part-B, non-HMO coverage before cancer diagnosis. Therefore, only cases aged ≥66 years were included. Since Medicare did not cover claims for HCV infection before 1992 and HCV infection is an important risk factor for some cancers, we included cases diagnosed 1993 onwards. The 2016 SEER-Medicare linkage includes cancer cases diagnosed through 2013. People whose cancers were diagnosed only at autopsy or by death certificate were excluded. We defined cancer sites using the SEER site recode variable, and histologic subtypes for some cancers using the morphology codes.
We randomly selected 200,000 controls from the 5% random sample of Medicare beneficiaries who were alive and cancer-free as of July 1 of the calendar year of their selection. Like cases, controls were required to have at least 13 months of Part-A, Part-B, non-HMO Medicare coverage prior to their selection. Controls were frequency-matched to cases on age (categories of 66–69, 70–74, 75–79, 80–84, 85–99 years), calendar year of selection, sex, and race (whites, blacks, others). Controls could be sampled repeatedly across multiple calendar years (39,272 controls were sampled more than once) and later become cases.
Ascertainment of HBV and other medical conditions
The International Classification of Diseases, version 9 (ICD-9) codes were used to identify subjects with HBV infection (see Supplementary Table 1 for ICD-9 codes). A diagnosis of HBV infection required at least one inpatient, physician or outpatient claim more than 12 months before cancer diagnosis/control selection. This exclusion period was used to minimize the possibility of differential assessment of HBV in cases as part of medical work-up near the time of their cancer diagnosis. In a sensitivity analysis, we used a stricter definition for HBV infection that required at least one inpatient claim or two physician or outpatient claims at least 30 days apart.
Since human immunodeficiency virus (HIV) and HCV infections are associated with HBV and also increase risk of certain cancers, we identified cases and controls with at least one Medicare claim for HIV or HCV infection any time before death or last follow-up (Supplementary Table 1) to evaluate the impact of these conditions. Cirrhosis, diabetes mellitus, and cholelithiasis were identified using ICD-9 codes that required at least one inpatient claim or two physician/outpatient claims at least 30 days apart, more than 12 months before cancer diagnosis/control selection.
Smoking and alcohol abuse are important cancer risk factors; however direct information on these behaviors is not available in the SEER-Medicare database. Therefore, we used ICD-9 codes for diagnoses related to smoking and alcohol abuse to capture these behaviors. Subjects were classified as smokers or alcohol abusers if at least one specified ICD-9 code was present more than 12 months before cancer diagnosis/control selection.
Socioeconomic status (SES) is an important predictor of cancer incidence. HBV infection is more common in low-income demographic groups due to high prevalence of injection drug use and lower HBV vaccination rates.24 We evaluated potential confounding by SES using three variables available in SEER-Medicare that capture SES based on a person’s residential zip code: median household income, percentage of individuals 25+ years of age with <12 years of education, and percentage of residents living below the poverty line.
Differential cancer screening among HBV-infected and uninfected individuals may lead to confounding of some associations, particularly for screen-detectable cancers. Therefore, we used Medicare claims to obtain information on screening mammograms, prostate specific antigen (PSA), pap smear, and colonoscopy for the period more than 12 months prior to cancer diagnosis/control selection.25
Statistical analyses
We compared the characteristics of cases and controls using chi-squared tests. To select cancer sites for evaluation, we computed the expected number of HBV-infected individuals by multiplying the number of cases for each cancer site by the prevalence of HBV infection in controls in our study (0.48%). We evaluated cancer sites where the expected count of individuals with HBV infection was ≥11 to comply with the SEER-Medicare data use agreement, which requires suppression of cell sizes <11. Major subtypes of NHL were selected for evaluation based on previous reports of their association with HBV or HCV infection. Cancer sites that were not evaluated separately were grouped together as a miscellaneous category. In all, forty-five cancer sites were evaluated in the primary analysis.
We compared HBV prevalence in cases and controls by fitting separate unconditional logistic regression models for each cancer type. Odds ratios were adjusted (aOR) for age, sex, race, year of cancer diagnosis/control selection, average annual number of physician claims more than 12 months before cancer diagnosis/control selection (a measure of healthcare utilization), and smoking status. We adjusted the variance of aORs obtained from these models for repeated selection of some controls across calendar years and inclusion of some controls who later became cases.23 We utilized a two-sided alpha of 0.05 to describe confidence intervals (CI), but to account for multiple testing, we selected cancers for further evaluation using a false discovery rate of 10% according to the Benjamini and Hochberg method.26
Further evaluation included additional adjustment for alcohol abuse, diabetes, duration of Medicare coverage, or SES variables, or by restricting the study population to those without cirrhosis. Finally, we conducted sensitivity analyses, 1) excluding people with claims for HCV or HIV infection, 2) using the more stringent definition of HBV infection, and 3) a combination of both these restrictions.
RESULTS
We included 1,825,316 cancer cases and 200,000 controls; differences between cases and controls along with prevalence of HBV infection among controls are provided in Table 1. Cases and controls were perfectly frequency-matched on age categories, sex, race/ethnicity, and calendar year of cancer diagnosis/control selection. Cases had a slightly longer duration of Medicare coverage and higher average annual number of physician claims. Although differences between cases and controls were small, cases were more likely to have HCV infection, cirrhosis, diabetes mellitus, or cholelithiasis, be smokers or alcohol abusers, and reside in a zip code with characteristics of high SES (Table 1). Cases and controls did not differ with respect to HIV infection.
Table 1:
Characteristics of the study population
| Characteristic | Cancer cases (N=1,825,316) | Controls (N=200,000) | Chi-square p value | HBV prevalence among controls |
|---|---|---|---|---|
|
|
||||
| Number (%) | Number (%) | (%) | ||
| Age, years | _ | |||
| 66-69 | 303,563 (16.6) | 33,262 (16.6) | 0.4 | |
| 70-74 | 469,781 (25.7) | 51,479 (25.7) | 0.6 | |
| 75-79 | 437,146 (24.0) | 47,896 (24.0) | 0.5 | |
| 80-84 | 334,133 (18.3) | 36,607 (18.3) | 0.5 | |
| 85+ | 280,693 (15.4) | 30,756 (15.4) | 0.4 | |
| Sex | _ | |||
| Males | 946,257 (51.8) | 103,681 (51.8) | 0.5 | |
| Females | 879,059 (48.2) | 96,319 (48.2) | 0.4 | |
| Race/ethnicity | _ | |||
| White | 1,551,493 (85.0) | 169,993 (85.0) | 0.3 | |
| Black | 149,888 (8.2) | 16,424 (8.2) | 0.8 | |
| Asian | 51,959 (2.9) | 6,101 (3.1) | 3.4 | |
| Others/Unknown | 71,976 (3.9) | 7,482 (3.7) | 1.3 | |
| Year of cancer diagnosis/control selection | _ | |||
| 1993 - 2000 | 405,500 (22.2) | 44,430 (22.2) | 0.3 | |
| 2001 - 2004 | 418,333 (22.9) | 45,838 (22.9) | 0.4 | |
| 2005 - 2008 | 458,022 (25.1) | 50,188 (25.1) | 0.6 | |
| 2009 - 2013 | 543,461 (29.8) | 59,544 (29.8) | 0.7 | |
| Total part A, part B, non-HMO Medicare coverage (months) | < 0.0001 | |||
| Median (IQR) | 55 (29 - 71) | 54 (30 - 66) | ||
| < 29 | 442,305 (24.2) | 47,073 (23.5) | 0.3 | |
| 29 to 54 | 460,300 (25.2) | 61,511 (30.8) | 0.4 | |
| 55 to 70 | 455,734 (25.0) | 45,127 (22.6) | 0.6 | |
| ≥ 71 | 466,977 (25.6) | 46,289 (23.1) | 0.6 | |
| Average number of physician claims per year | < 0.0001 | |||
| Median (IQR) | 5.72 (2.67 - 10.29) | 5.54 (2.59 - 10.01) | ||
| < 2.67 | 457,293 (25.1) | 51,364 (25.7) | 0.2 | |
| 2.67 to 5.69 | 452,896 (24.8) | 51,041 (25.5) | 0.3 | |
| 5.70 to 10.24 | 456,354 (25.0) | 49,645 (24.8) | 0.5 | |
| ≥ 10.25 | 458,773 (25.1) | 47,950 (24.0) | 1.0 | |
| Hepatitis B * | 10,073 (0.6) | 966 (0.5) | < 0.0001 | _ |
| Hepatitis C † | 29,649 (1.6) | 2,565 (1.3) | < 0.0001 | 10.2 |
| HIV † | 7,899 (0.4) | 879 (0.4) | 0.6625 | 3.5 |
| Cirrhosis ** | 13,058 (0.7) | 797 (0.4) | < 0.0001 | 8.5 |
| Smoking * | 710,539 (38.9) | 67,457 (33.7) | < 0.0001 | 0.7 |
| Alcohol abuse * | 191,289 (10.5) | 14,611 (7.3) | < 0.0001 | 1.0 |
| Diabetes Mellitus ** | 470,524 (25.8) | 49,722 (24.9) | < 0.0001 | 0.9 |
| Cholelithiasis ** | 59,653 (3.3) | 5,942 (3.0) | < 0.0001 | 1.6 |
| Zip code median income (US $) | < 0.0001 | |||
| Median (IQR) | 56,419 (43,377-75,054) | 55,888 (43,152-73,810) | ||
| < 43,331 | 447,466 (24.5) | 49,862 (24.9) | 0.5 | |
| 43,331 - 56,307 | 447,344 (24.5) | 50,364 (25.2) | 0.5 | |
| 56,308 - 75,045 | 448,567 (24.6) | 48,320 (24.2) | 0.5 | |
| ≥ 75,046 | 451,062 (24.7) | 47,702 (23.9) | 0.5 | |
| Missing | 30,877 (1.7) | 3,752 (1.9) | < 0.4 | |
| % Non-high school graduates (age ≥ 25 years) in a zip code | < 0.0001 | |||
| Median (IQR) | 11.15 (6.92-19.00) | 11.18 (6.90-19.15) | ||
| < 6.92 | 448,278 (24.6) | 49,221 (24.6) | 0.3 | |
| 6.92 - 11.14 | 447,941 (24.5) | 48,424 (24.2) | 0.4 | |
| 11.15 - 19.00 | 450,813 (24.7) | 49,037 (24.5) | 0.4 | |
| ≥ 19.01 | 448,354 (24.6) | 49,692 (24.9) | 0.8 | |
| Missing | 29,930 (1.6) | 3,626 (1.8) | < 0.4 | |
| % Living below the poverty line in a zip code | < 0.0001 | |||
| Median (IQR) | 11.53 (6.90-18.28) | 11.62 (6.93-18.41) | ||
| < 6.90 | 448,359 (24.6) | 48,107 (24.1) | 0.4 | |
| 6.90 - 11.53 | 450,093 (24.7) | 48,853 (24.4) | 0.5 | |
| 11.54 - 18.28 | 448,617 (24.6) | 49,584 (24.8) | 0.5 | |
| ≥ 18.29 | 448,317 (24.6) | 49,830 (24.9) | 0.6 | |
| Missing | 29,930 (1.6) | 3,626 (1.8) | < 0.4 | |
At least 1 MEDPAR, NCH, or OUTPT claim, excluding 12 months before the cancer diagnosis/control selection date
At least 1 MEDPAR or 2 NCH/OUTPT claims at least 30 days apart, excluding 12 months before the cancer diagnosis/control selection date
At least 1 MEDPAR, NCH, or OUTPT claim any time after the start of follow-up
The overall prevalence of HBV infection among controls was low (N=966, 0.5%) (Table 1). HBV prevalence increased with calendar year, duration of Medicare coverage, and average annual physician claims. Prevalence was marginally elevated (0.5-1%) among men, blacks, smokers, alcohol abusers, and diabetics. A high HBV prevalence (>1%) was observed among Asians (3.4%), and among those with HCV (10.2%) or HIV infection (3.5%), cirrhosis (8.5%), or cholelithiasis (1.6%). Prevalence was higher among people residing in zip code with higher percentage of non-high school graduates.
Overall, HBV prevalence was higher in cases than controls (0.6% [N=10,073] vs. 0.5%; aOR=1.11; 95%CI=1.03-1.19; p=0.007). Results for the association between HBV infection and each evaluated cancer type are presented in Figure 1. After Benjamini and Hochberg correction, we observed significant positive associations between HBV infection and cancers of the stomach (aOR=1.19; 95%CI=1.03-1.37), anus (1.66; 1.17-2.33), liver (10.6; 9.66-11.6), intrahepatic bile ducts (1.67; 1.18-2.37), and other biliary tract (biliary tract, not otherwise specified; overlapping lesions of the biliary tract; and ampulla of Vater) (1.50; 1.06-2.13), as well as myelodysplastic syndrome (MDS) (1.26; 1.07-1.49), diffuse large B cell lymphoma (DLBCL) (1.24; 1.06-1.46), and miscellaneous cancers (1.25; 1.13-1.39). We observed significant inverse associations between HBV infection and cancers of the female breast (aOR=0.86; 95%CI=0.76-0.98) and prostate (0.81; 0.73-0.91), and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) (0.77; 0.62-0.96). Association with extrahepatic bile duct cancers was borderline significant (aOR=1.34; 95%CI=0.98-1.82). Adjusting for alcohol abuse, diabetes mellitus, duration of Medicare coverage, or SES, or restricting the study population to those who did not have cirrhosis did not substantively affect associations (Supplementary Table 2).
Figure 1:
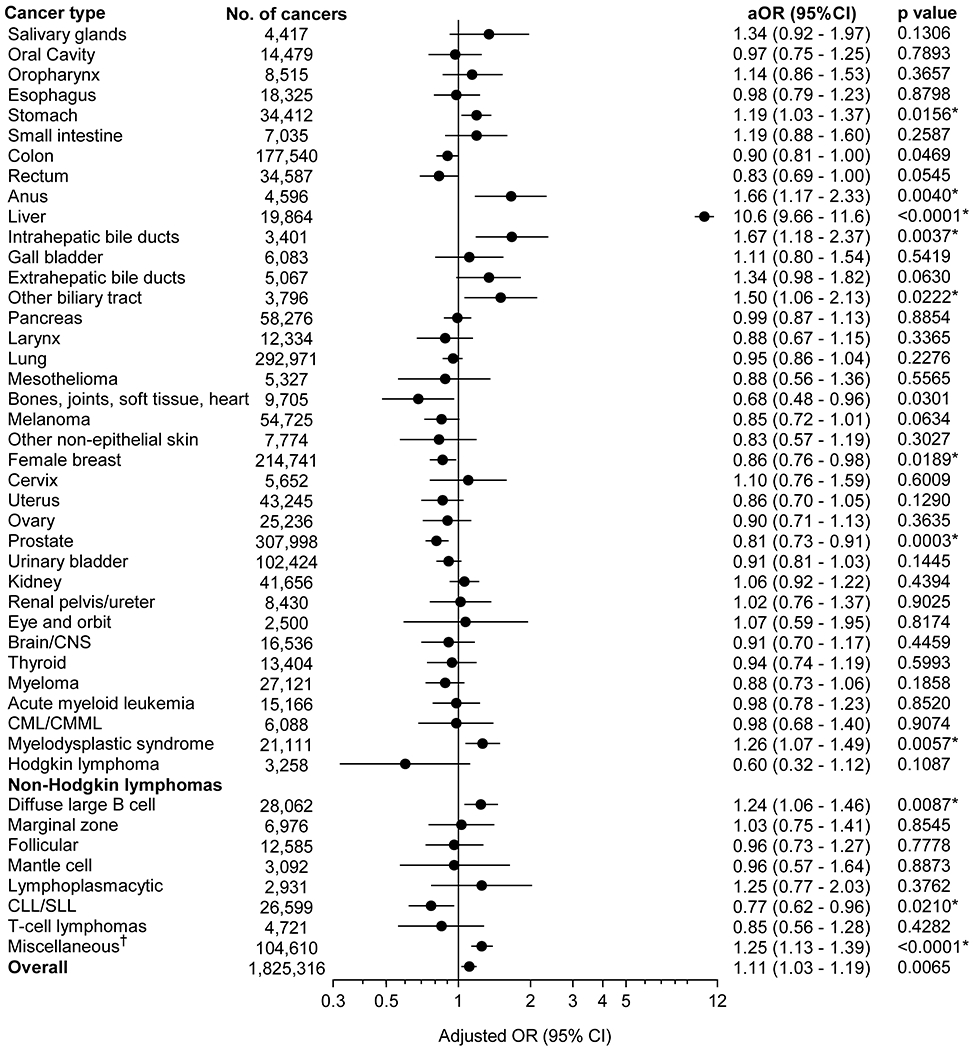
Associations between HBV infection and risk of cancer
The associations with HBV infection are presented for each cancer as an adjusted odds ratio and corresponding 95% confidence interval (horizontal axis, logarithmic scale). Odds ratios are adjusted for age categories (65-69, 70-74, 75-79, 80-84, 85+ years), sex, race/ethnicity, calendar year of cancer diagnosis/control selection (1993-2000, 2001-2004, 2005-2008, 2009-2013), number of physician claims per year (< 2.67, 2.67-5.69, 5.70-10.24, ≥10.25), and smoking status (never/ever).
Abbreviations: aOR, adjusted odds ratio; CI, confidence intervals; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; CNS, central nervous system
* Asterisks indicate cancers that were identified to be significantly associated with HBV infection after correction for multiple comparisons by Benjamini-Hochberg method.
We further explored associations between HBV and individual cancers that formed the “miscellaneous” and “other biliary tract cancers” categories (Table 2). Significant associations were observed for head and neck sites other than oral cavity, oropharynx, or salivary glands (aOR=1.52; 95%CI=1.09-2.10), particularly nasopharynx (2.08; 1.33-3.25) and other gastrointestinal sites (1.40; 1.03-1.90). The most common type of cancer in the “other biliary tract cancers” category was ampulla of Vater cancer. The association of ampulla of Vater cancer with HBV infection was not significant, although there was a suggestive association (22 [0.8%] of 2,731 cases with HBV infection; aOR=1.35; 95%CI=0.88-2.08).
Table 2:
Exploratory analyses to determine cancer types associated with HBV infection from within the miscellaneous category
| Cancer type | No. of cancers | No. of HBV-infected* people (%) | aOR† (95% CI) | p value |
|---|---|---|---|---|
| Miscellaneous sites | 104,610 | 702 (0.67) | 1.25 (1.13 - 1.39) | < 0.0001 |
| Other head and neck sites | 4,141 | 40 (0.97) | 1.52 (1.09 - 2.10) | 0.0128 |
| Nasopharynx | 1,165 | 21 (1.80) | 2.08 (1.33 - 3.25) | 0.0013 |
| Hypopharynx | 2,251 | 15 (0.67) | 1.14 (0.68 - 1.93) | 0.6192 |
| Other gastrointestinal sites | 6,229 | 45 (0.72) | 1.40 (1.03 - 1.90) | 0.0302 |
| Peritoneum/retroperitoneum | 3,945 | 27 (0.68) | 1.47 (0.99 - 2.17) | 0.0533 |
| Other respiratory system sites | 2,845 | 16 (0.56) | 1.10 (0.67 - 1.82) | 0.7011 |
| Other endocrine organs | 1,342 | < 11 (0.75) | 1.17 (0.63 - 2.21) | 0.6161 |
| Other female reproductive organs | 8,594 | 38 (0.44) | 1.03 (0.74 - 1.44) | 0.8587 |
| Other male reproductive organs | 1,820 | 14 (0.77) | 1.31 (0.76 - 2.23) | 0.3291 |
At least 1 MEDPAR, NCH, or OUTPT claim, excluding 12 months before the cancer diagnosis/control selection date
Odds ratios assess the association of HBV with the specified cancer and were adjusted for age categories (66-69, 70-74, 75-79, 80-84, 85+ years), sex, race/ethnicity, calendar year of cancer diagnosis/control selection (1993-2000, 2001-2004, 2005-2008, 2009-2013), average number of physician claims per year (< 2.67, 2.67-5.69, 5.70-10.24, ≥10.25), and smoking status (never/ever)
After excluding people with claims for HCV or HIV infection, aOR estimates were similar to those obtained in the primary analyses for cancers of the stomach, anus, liver, intrahepatic bile ducts, female breast, prostate, and nasopharynx, and DLBCL and CLL/SLL (Table 3). Estimates for the associations with other biliary tract, MDS, and other gastrointestinal sites changed more than 10% and were no longer statistically significant. When we used a stricter definition for HBV infection, associations were maintained for cancers of the anus, liver, intrahepatic bile ducts, breast, and prostate, MDS, and DLBCL, and were borderline significant for CLL/SLL, and nasopharyngeal cancer (Table 3).
Table 3:
Sensitivity analyses to evaluate the associations between HBV infection and selected cancers
| Cancer | No. with HBV infection** (in the primary analyses) | Excluding HCV and HIV infected individuals | Strict HBV* definition | Excluding HCV and HIV-infected individuals plus strict HBV* definition | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. with HBV infection** | aOR† (95% CI) | No. with HBV infection* | aOR† (95% CI) | No. with HBV infection* | aOR† (95% CI) | ||
| Stomach | 288 | 211 | 1.20 (1.01 - 1.41) | 129 | 1.11 (0.90 - 1.38) | 88 | 1.16 (0.90 - 1.50) |
| Anus | 35 | 24 | 1.63 (1.08 - 1.46) | 18 | 1.85 (1.15 - 3.00) | < 11 | 1.64 (0.86 - 3.12) |
| Liver | 1,658 | 421 | 5.37 (4.69 - 6.14) | 1,025 | 12.4 (10.9 - 14.0) | 277 | 8.07 (6.73 - 9.66) |
| Intrahepatic bile duct | 35 | 19 | 1.28 (0.80 - 2.05) | 24 | 2.48 (1.63 - 3.77) | < 11 | 1.62 (0.85 - 3.06) |
| Other biliary tract | 34 | 20 | 1.25 (0.80 - 1.97) | 15 | 1.41 (0.84 - 2.39) | < 11 | 0.89 (0.39 - 2.02) |
| Female breast | 781 | 573 | 0.88 (0.76 - 1.02) | 296 | 0.73 (0.60 - 0.88) | 185 | 0.71 (0.56 - 0.91) |
| Prostate | 1,254 | 879 | 0.81 (0.71 - 0.93) | 505 | 0.70 (0.60 - 0.83) | 319 | 0.68 (0.55 - 0.83) |
| MDS | 189 | 117 | 1.11 (0.91 - 1.37) | 94 | 1.31 (1.03 - 1.66) | 46 | 1.01 (0.73 - 1.41) |
| DLBCL | 184 | 121 | 1.14 (0.93 - 1.39) | 98 | 1.46 (1.16 - 1.83) | 53 | 1.23 (0.91 - 1.67) |
| CLL/SLL | 88 | 61 | 0.76 (0.58 - 0.99) | 37 | 0.71 (0.51 - 1.00) | 22 | 0.66 (0.43 - 1.02) |
| Nasopharynx | 21 | 16 | 2.20 (1.32 - 3.67) | < 11 | 1.84 (0.94 - 3.61) | < 11 | 1.93 (0.85 - 4.41) |
| Other gastrointestinal sites | 45 | 30 | 1.31 (0.90 - 1.91) | 16 | 1.08 (0.65 - 1.78) | < 11 | 0.96 (0.49 - 1.88) |
Abbreviations: aOR, adjusted odds ratio; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; DLBCL, diffuse large B cell lymphoma; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus
At least 1 MEDPAR, or 2 NCH or OUTPT claims at least 30 days apart, excluding 12 months before the cancer diagnosis/control selection date
At least 1 MEDPAR, NCH, or OUTPT claim, excluding 12 months before the cancer diagnosis/control selection date
Odds ratios assess the association of HBV with the specified cancer and were adjusted for age categories (66-69, 70-74, 75-79, 80-84, ≥85 years), sex, race/ethnicity, calendar year of cancer diagnosis/control selection (1993-2000, 2001-2004, 2005-2008, 2009-2013), average number of physician claims per year (< 2.67, 2.67-5.69, 5.70-10.24, ≥10.25), and smoking status (never/ever)
There were no statistically significant differences between average annual number of mammograms or PSA tests between HBV-infected or HBV-uninfected people among the controls (Supplementary Tables 3 and 4). Breast and prostate cancer cases had significantly higher number of mammograms or PSA tests, respectively. After adjusting for cancer screening rates, there was no change in the previously observed association between HBV infection and breast cancer (aOR=0.86; 95%CI=0.76-0.97) or prostate cancer (0.82; 0.73-0.92).
DISCUSSION
Chronic HBV infection is a known cause of HCC. Although HBV vaccination coverage among US children is high, the vaccination rate among US adults is still very low at approximately 25%.27 Prevalence of HBV infection remains high in certain subgroups, such as Asian immigrants and injection drug users.28 As the population ages, HBV-infected people will be at a higher risk of getting chronic liver diseases, including HCC. Thus, it is important to understand the impact of HBV infection in the segment of the US population that is largely unvaccinated and living with HBV for many years. In this first comprehensive and systematic assessment of cancer risk among a large study of nationally-representative, elderly US adults, we could assess the associations of HBV with many cancer types that could not be evaluated in previous studies. We found that chronic HBV infection increases the risk of not only HCC, but also cancers outside of the liver.
The association between chronic HBV infection and intrahepatic cholangiocarcinoma has been previously described; three recent meta-analyses of observational studies reported pooled risk estimates ranging from 2.66 to 5.10.5, 29, 30 Although the causal role of HBV in cholangiocarcinoma development is not clear, detection of HBV DNA and expression of HBx protein in tumor specimens suggests HBV may be directly involved in carcinogenesis.13, 14 Cardinale et al. suggested that the interactions between PTPN3 mutations and HBV proteins may lead to intrahepatic cholangiocarcinoma.31 Another likely explanation is that chronic HBV infection may lead to development of cirrhosis, which is a risk factor for intrahepatic cholangiocarcinoma.18 Notably, we also found a borderline significant association between HBV infection and cancers of the Ampulla of Vater and extrahepatic bile ducts. One previous study had analyzed the association between HBV infection and Ampulla of Vater cancers (total 49 cases, 3 cases with HBV infection) and the results were not significant.32 Though chronic inflammation may play a role, surveillance bias is possible due to differential rates of abdominal imaging for HBV-infected compared to uninfected people.
Some,3, 4 but not all,33, 34 epidemiological studies have reported an association between HBV infection and hematological malignancies, mostly NHLs. Meta-analyses of these observational studies found a significant association with a pooled odds ratio of greater than 2 for NHL.35 HBV can infect lymphocytes and as for the well-known association between HCV infection and NHL, investigators have hypothesized that chronic B-cell antigenic stimulation may play a role in lymphomagenesis in people chronically infected with HBV.36 In our study, we had sufficient power to evaluate specific subtypes of NHL. We found that HBV infection is associated with an increased risk of DLBCL, and a decreased risk of CLL/SLL. CLL/SLL incidence is significantly lower among Asians while HBV infection prevalence is high among this racial group.37 However, the inverse association between HBV and CLL/SLL was present after adjustment for race. MDS is a heterogeneous group of malignancies characterized by ineffective myeloid blood cell production with an increased risk of acute myeloid leukemia.38 HCV infection has been previously found to be associated with both DLBCL and MDS.21 Though we were likely to miss claims for HCV infection, confounding due to HCV infection is improbable because it is rare in the general population, and its associations with DLBCL and MDS were modest.
The associations of HBV infection with stomach and nasopharyngeal cancers are intriguing. Case-control studies from China have reported associations between HBV infection and stomach and nasopharyngeal cancers.39,40 China is endemic for HBV infection and has a high incidence of these cancers. However, the associations that we observed persisted among non-Asians (data not shown). A high prevalence of Helicobacter pylori infection and gastric ulcers has been observed in people with cirrhosis.41 Although chronic inflammation and cirrhosis may play a role in increasing the risk of stomach cancer in HBV-infected people, the association that we observed persisted among non-cirrhotics. An interaction with Epstein Barr virus (EBV) has been proposed as a possible explanation for the association between HBV infection and nasopharyngeal cancer,40 but we could not explore this hypothesis since we did not have information on EBV status of the tumors.
The association that we observed for HBV with anal cancer may be because of confounding by shared risk factors. HBV incidence is high among men who have sex with men (MSM),42 and MSM also have a high prevalence of anal human papillomavirus infection, the cause of anal cancer. MSM also have high prevalence of HIV infection which increases the risk of anal cancer.43 The association between HBV and anal cancer in our study persisted in a sensitivity analysis in which we excluded people with claims for HIV infection, but some claims may have been missed.
The inverse associations between HBV infection and cancers of the breast and prostate were similar to those observed for HCV infection.21 One possible explanation for this association was that HBV-infected individuals may be less likely to get screened for cancer than HBV-uninfected individuals. However, we did not observe any statistically significant differences in the breast or prostate cancer screening rates between HBV-infected and uninfected people, and the associations were maintained despite adjustments for cancer screening. Breast cancer risk is increased in women with high SES,44 while the risk of HBV infection may be lower in these women;24 however, the association persisted after adjusting for SES.
Notably, we did not observe an association between HBV infection and pancreatic cancer. The evidence of such an association has been conflicting.6–9 Although a meta-analysis of these observational studies reported a significant association and a pooled OR of 1.24, it was limited by inadequate adjustment for smoking, alcohol use, and diabetes, significant heterogeneity, and the hospital-based nature of most of the included studies.45
Our study had some limitations. First, as our study was conducted among older people (≥65 years of age), the results may not be generalizable to the entire US population. Second, we assessed HBV infection by Medicare claims, so underascertainment was likely. Furthermore, we did not have information on HBV serology status and we were unable to differentiate between past or active HBV infection. However, the bias due to these issues is likely to be non-differential between cases and controls and would drive the associations towards the null. We also confirmed most of our results by using a stricter definition of HBV infection. Third, we were unable to capture detailed information on smoking, alcohol intake, and other important cancer risk factors such as diet, obesity, and physical activity. Fourth, HCV infection could be underascertained because we determined infection status through ICD-9 codes. Furthermore, many infected people may be unaware of their infection status. Based on the estimates derived from the National Health and Nutrition Examination Survey (NHANES), the prevalence of HCV infection among elderly US individuals is small.46 Any resulting bias is likely to be non-differential. Thus, if undiagnosed HCV infection confounds the association between HBV infection and cancers, the observed estimates would be conservative and towards the null. Finally, since we made multiple comparisons and due to large sample size, it is possible that some of the associations that we observed were due to chance. We tried to minimize this possibility by adjusting the p values, and emphasized only the more significant results.
In conclusion, we observed significant associations between HBV infection and many cancers besides HCC, such as those of the intrahepatic bile ducts, stomach, nasopharynx, and DLBCL. Healthcare providers involved in medical management of liver disease in HBV-infected adults should be aware that infected people maybe at increased risk of selected cancers other than HCC. Further studies are required with better assessment of HBV infection, cancers, and important confounders to strengthen the evidence linking HBV infection to these extrahepatic cancers and elucidate the potential biological mechanisms for carcinogenesis.
Supplementary Material
What’s new?
Hepatitis B virus (HBV) infection is a known cause of hepatocellular carcinoma (HCC), but associations with other cancers have not been established. In the first comprehensive and systematic assessment of the associations between HBV infection and cancers in the elderly US adults using the SEER-Medicare linked database, we report that HBV infection is also associated with cancers other than HCC, such as biliary tract cancers and diffuse large B cell lymphoma.
ACKNOWLEDGEMENTS
We thank Ms. Winnie Ricker, Information Management Services Inc. for assistance with management of the Surveillance, Epidemiology, and End Results (SEER)-Medicare database.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database
FUNDING
This research was supported by the Intramural Research Program of the National Cancer Institute.
Abbreviations:
- aOR
adjusted odds ratio
- CI
confidence intervals
- CLL/SLL
chronic lymphocytic leukemia/small lymphocytic lymphoma
- DLBCL
diffuse large B cell lymphoma
- EBV
Epstein Barr virus
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HMO
health maintenance organization
- ICD
International Classification of Diseases
- MDS
myelodysplastic syndrome
- MSM
men who have sex with men
- NHL
non-Hodgkin lymphoma
- PSA
prostate specific antigen
- SEER
Surveillance, Epidemiology, and End Results
- SES
socioeconomic status
- US
United States
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386: 1546–55. [DOI] [PubMed] [Google Scholar]
- 2.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008;48: 335–52. [DOI] [PubMed] [Google Scholar]
- 3.Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol 2010;11: 827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulcickas Yood M, Quesenberry CP Jr., Guo D, Caldwell, Wells K, Shan J, Sanders L, Skovron ML, Iloeje U, Manos MM. Incidence of non-Hodgkin’s lymphoma among individuals with chronic hepatitis B virus infection. Hepatology 2007;46: 107–12. [DOI] [PubMed] [Google Scholar]
- 5.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 2012;57: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, Davila M, Abbruzzese JL. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol 2008;26: 4557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iloeje UH, Yang HI, Jen CL, Su J, Wang LY, You SL, Lu SN, Chen CJ. Risk of pancreatic cancer in chronic hepatitis B virus infection: data from the REVEAL-HBV cohort study. Liver Int 2010;30: 423–9. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Magnusson M, Torner A, Ye W, Duberg AS. Risk of pancreatic cancer among individuals with hepatitis C or hepatitis B virus infection: a nationwide study in Sweden. Br J Cancer 2013;109: 2917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krull Abe S, Inoue M, Sawada N, Iwasaki M, Shimazu T, Yamaji T, Sasazuki S, Saito E, Tanaka Y, Mizokami M, Tsugane S. Hepatitis B and C Virus Infection and Risk of Pancreatic Cancer: A Population-Based Cohort Study (JPHC Study Cohort II). Cancer Epidemiol Biomarkers Prev 2016;25: 555–7. [DOI] [PubMed] [Google Scholar]
- 10.Kamiza AB, Su FH, Wang WC, Sung FC, Chang SN, Yeh CC. Chronic hepatitis infection is associated with extrahepatic cancer development: a nationwide population-based study in Taiwan. BMC Cancer 2016;16: 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Bai X, Zhang Q, Wen L, Su W, Fu Q, Sun X, Lou Y, Yang J, Zhang J, Chen Q, Wang J, et al. The hepatitis B virus X protein promotes pancreatic cancer through modulation of the PI3K/AKT signaling pathway. Cancer Lett 2016;380: 98–105. [DOI] [PubMed] [Google Scholar]
- 12.Jin Y, Gao H, Chen H, Wang J, Chen M, Li G, Wang L, Gu J, Tu H. Identification and impact of hepatitis B virus DNA and antigens in pancreatic cancer tissues and adjacent non-cancerous tissues. Cancer Lett 2013;335: 447–54. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Wang T, Ye S, Zhao R, Bai X, Wu Y, Abe K, Jin X. Detection of hepatitis B virus DNA in paraffin-embedded intrahepatic and extrahepatic cholangiocarcinoma tissue in the northern Chinese population. Hum Pathol 2012;43: 56–61. [DOI] [PubMed] [Google Scholar]
- 14.Zhou YM, Cao L, Li B, Zhang XZ, Yin ZF. Expression of HBx protein in hepatitis B virus-infected intrahepatic cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2012;11: 532–5. [DOI] [PubMed] [Google Scholar]
- 15.Mason A, Yoffe B, Noonan C, Mearns M, Campbell C, Kelley A, Perrillo RP. Hepatitis B virus DNA in peripheral-blood mononuclear cells in chronic hepatitis B after HBsAg clearance. Hepatology 1992;16: 36–41. [DOI] [PubMed] [Google Scholar]
- 16.Neurath AR, Strick N, Sproul P, Ralph HE, Valinsky J. Detection of receptors for hepatitis B virus on cells of extrahepatic origin. Virology 1990;176: 448–57. [DOI] [PubMed] [Google Scholar]
- 17.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology 2005;128: 620–6. [DOI] [PubMed] [Google Scholar]
- 18.Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, Hassan MM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol 2007;102: 1016–21. [DOI] [PubMed] [Google Scholar]
- 19.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol 2007;5: 1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006;28: 112–25. [DOI] [PubMed] [Google Scholar]
- 21.Mahale P, Torres HA, Kramer JR, Hwang LY, Li R, Brown EL, Engels EA. Hepatitis C virus infection and the risk of cancer among elderly US adults: A registry-based case-control study. Cancer 2017;123: 1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40: Iv-3-18. [DOI] [PubMed] [Google Scholar]
- 23.Engels EA, Pfeiffer RM, Ricker W, Wheeler W, Parsons R, Warren JL. Use of surveillance, epidemiology, and end results-medicare data to conduct case-control studies of cancer among the US elderly. Am J Epidemiol 2011;174: 860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spradling PR, Rupp L, Moorman AC, Lu M, Teshale EH, Gordon SC, Nakasato C, Boscarino JA, Henkle EM, Nerenz DR, Denniston MM, Holmberg SD. Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis 2012;55: 1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Procedure codes for SEER-Medicare Analyses. https://healthcaredelivery.cancer.gov/seermedicare/considerations/procedure_codes.html. Accessed on 1/31/2018
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57: 289–300. [Google Scholar]
- 27.Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, Rodriguez-Lainz A, Fiebelkorn AP. Surveillance of Vaccination Coverage among Adult Populations - United States, 2015. MMWR Surveill Summ 2017;66: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis - United States, 2015. https://www.cdc.gov/hepatitis/statistics/2015surveillance/index.htm. Accessed on 1/26/2018
- 29.Li M, Li J, Li P, Li H, Su T, Zhu R, Gong J. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta-analysis and systematic review. J Gastroenterol Hepatol 2012;27: 1561–8. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Zhao Y, Li B, Huang J, Wu L, Xu D, Yang J, He J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer 2012;12: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardinale V, Alvaro D. PTPN3 mutations and HBV may exert synergistic effects in the origin of the intrahepatic cholangiocarcinoma. Gastroenterology 2014;147: 719–20. [DOI] [PubMed] [Google Scholar]
- 32.Hsing AW, Zhang M, Rashid A, McGlynn KA, Wang BS, Niwa S, Ortiz-Conde BA, Goedert JJ, Fraumeni JF Jr., O’Brien TR, Gao YT. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer 2008;122: 1849–53. [DOI] [PubMed] [Google Scholar]
- 33.Anderson LA, Pfeiffer R, Warren JL, Landgren O, Gadalla S, Berndt SI, Ricker W, Parsons R, Wheeler W, Engels EA. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev 2008;17: 3069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker N, Schnitzler P, Boffetta P, Brennan P, Foretova L, Maynadie M, Nieters A, Staines A, Benavente Y, Cocco P, de Sanjose S. Hepatitis B virus infection and risk of lymphoma: results of a serological analysis within the European case-control study Epilymph. J Cancer Res Clin Oncol 2012;138: 1993–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi HZ, Chen JJ, Cen H, Yan W, Tan XH. Association between infection of hepatitis B virus and onset risk of B-cell non-Hodgkin’s lymphoma: a systematic review and a meta-analysis. Med Oncol 2014;31: 84. [DOI] [PubMed] [Google Scholar]
- 36.Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood 2011;117: 1792–8. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002. Cancer Causes Control 2008;19: 379–90. [DOI] [PubMed] [Google Scholar]
- 38.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer 2007;109: 1536–42. [DOI] [PubMed] [Google Scholar]
- 39.Wei XL, Qiu MZ, Jin Y, Huang YX, Wang RY, Chen WW, Wang DS, Wang F, Luo HY, Zhang DS, Wang FH, Li YH, et al. Hepatitis B virus infection is associated with gastric cancer in China: an endemic area of both diseases. Br J Cancer 2015;112: 1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye YF, Xiang YQ, Fang F, Gao R, Zhang LF, Xie SH, Liu Z, Du JL, Chen SH, Hong MH, Qian CN, Ye W, et al. Hepatitis B virus infection and risk of nasopharyngeal carcinoma in southern China. Cancer Epidemiol Biomarkers Prev 2015;24: 1766–73. [DOI] [PubMed] [Google Scholar]
- 41.Zullo A, Romiti A, Tomao S, Hassan C, Rinaldi V, Giustini M, Morini S, Taggi F. Gastric cancer prevalence in patients with liver cirrhosis. Eur J Cancer Prev 2003;12: 179–82. [DOI] [PubMed] [Google Scholar]
- 42.Falade-Nwulia O, Seaberg EC, Snider AE, Rinaldo CR, Phair J, Witt MD, Thio CL. Incident Hepatitis B Virus Infection in HIV-Infected and HIV-Uninfected Men Who Have Sex With Men From Pre-HAART to HAART Periods: A Cohort Study. Ann Intern Med 2015;163: 673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colon-Lopez V, Shiels MS, Machin M, Ortiz AP, Strickler H, Castle PE, Pfeiffer RM, Engels EA. Anal Cancer Risk Among People With HIV Infection in the United States. J Clin Oncol 2018;36: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robert SA, Strombom I, Trentham-Dietz A, Hampton JM, McElroy JA, Newcomb PA, Remington PL. Socioeconomic risk factors for breast cancer: distinguishing individual- and community-level effects. Epidemiology 2004;15: 442–50. [DOI] [PubMed] [Google Scholar]
- 45.Xu JH, Fu JJ, Wang XL, Zhu JY, Ye XH, Chen SD. Hepatitis B or C viral infection and risk of pancreatic cancer: a meta-analysis of observational studies. World J Gastroenterol 2013;19: 4234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014;160: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


