Abstract
OBJECTIVES
The most used mortality risk prediction models in cardiac surgery are the European System for Cardiac Operative Risk Evaluation (ES) and Society of Thoracic Surgeons (STS) score. There is no agreement on which score should be considered more accurate nor which score should be utilized in each population subgroup. We sought to provide a thorough quantitative assessment of these 2 models.
METHODS
We performed a systematic literature review and captured information on discrimination, as quantified by the area under the receiver operator curve (AUC), and calibration, as quantified by the ratio of observed-to-expected mortality (O:E). We performed random effects meta-analysis of the performance of the individual models as well as pairwise comparisons and subgroup analysis by procedure type, time and continent.
RESULTS
The ES2 {AUC 0.783 [95% confidence interval (CI) 0.765–0.800]; O:E 1.102 (95% CI 0.943–1.289)} and STS [AUC 0.757 (95% CI 0.727–0.785); O:E 1.111 (95% CI 0.853–1.447)] showed good overall discrimination and calibration. There was no significant difference in the discrimination of the 2 models (difference in AUC −0.016; 95% CI −0.034 to −0.002; P = 0.09). However, the calibration of ES2 showed significant geographical variations (P < 0.001) and a trend towards miscalibration with time (P=0.057). This was not seen with STS.
CONCLUSIONS
ES2 and STS are reliable predictors of short-term mortality following adult cardiac surgery in the populations from which they were derived. STS may have broader applications when comparing outcomes across continents as compared to ES2.
REGISTRATION
Prospero (https://www.crd.york.ac.uk/PROSPERO/) CRD42020220983.
Keywords: Mortality, Cardiac surgery, Prediction, European System for Cardiac Operative Risk Evaluation, Society of Thoracic Surgeons
Cardiac surgery carries an inherent risk of perioperative mortality and morbidity.
INTRODUCTION
Cardiac surgery carries an inherent risk of perioperative mortality and morbidity. This varies considerably depending on the patients’ characteristics, baseline pathology and planned surgical intervention. Prediction models have been created [1–6] to quantify this risk. These models are utilized when counselling patients, discussing patients within the multi-disciplinary team, for benchmarking performance and more recently in guidelines for the management of aortic stenosis and deciding between surgical or transcatheter treatments [7, 8]. Present models predominantly quantify the risk of death in the short term. The most cited models are the European System for Cardiac Operative Risk Evaluation (ES) [1, 2, 9] and the Society of Thoracic Surgeons (STS) score [10, 11].
There is no guidance at present on which is the optimum score to utilize in a given clinical or research setting and concerns have arisen regarding the degree of applicability of a specific model to a localized population given the heterogenous populations from which they were originally derived. This leaves clinicians with the difficult decision of choosing which model to utilize when reporting and comparing outcomes. The relative performance of these models is thus the focus of this systematic review. We aim to build on previous work by using dedicated statistical methods to evaluate the comparative discrimination and calibration of the ES2 and STS not only in the wider cardiac surgery spectrum but also as they are applied to specific subgroups of the population. We believe that this is the most thorough comparison of these models.
METHODS
The data and scripts that support the findings of this study are available from the corresponding author upon reasonable request.
Systematic review
We report on the original papers and subsequent external validations available and draw comparisons between the models’ discriminatory power, as defined by the area under the receiver operator curve (AUC) or C-statistic, and their calibration, as defined by the ratio of the observed-to-expected mortality (O:E) within 30 days of the operation or the same hospital admission. Longer-term follow-up data were not included in the analysis to allow parity among studies and with the originally published papers on STS and ES2. A systematic literature review and meta-analysis of the above findings followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [12] and Meta-analysis Of Observational Studies in Epidemiology principles [13].
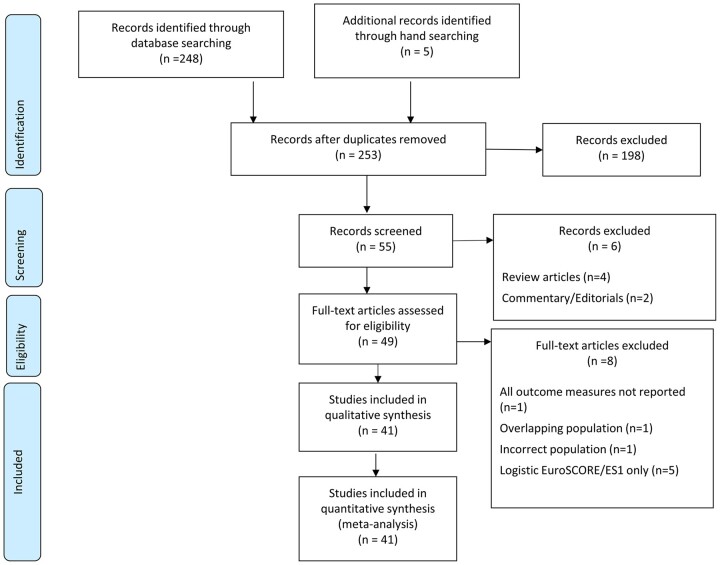
Our librarian conducted a literature search, restricting articles to those translatable into English and referencing adults only, using the described search string (Supplementary Material, Table S1). We also hand-searched the reference lists of papers identified but did not contact the authors. Excluded papers and rationale for exclusion have been noted (Fig. 1 and Supplementary Material, Table S2). If studies performed subgroup analysis such that the AUC or predicted mortality was not available for the whole dataset, then the subgroups were treated as independent populations. Institutes reporting on multiple occasions but utilizing different populations of patients were also treated as independent populations. The search is updated to 29 October 2020. Papers were screened and data extracted independently by 3 reviewers (SS/AD/LD). Outliers and studies with a high risk of bias were included the primary analysis following discussion between 2 authors (SS/UB). SS/UB had full access to all the data in the study and take responsibility for its integrity and the data analysis. The data extraction items were based on the CHARMS checklist [14] and the risk of bias was assessed using the PROBAST tool [15, 16] (Prospero ID: CRD42020220983).
Figure 1:
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart.
Databases searched: MEDLINE (1946 to present), CINAHL (1981 to present), Embase (1974 to present) and EmCare (1946 to present).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram: Fig. 1.
Risk of bias assessment: Supplementary Material, Table S3.
Low risk of bias: 17 papers.
Uncertain risk of bias: 2 papers.
High risk of bias: 24 papers.
Statistical analysis
Data were extracted as frequency and percentage for categorical variables and mean and standard deviation for continuous variables. The outcomes were AUC and O:E. Two separate analyses were conducted. First, we reviewed each score in turn and provided pooled estimates of AUC and O:E for comparison in accordance with previously published guidance [16–18]. It was assumed that variation in these parameters across studies was prone to between-study heterogeneity, due to the varied case-mix of populations studied, and thus, a random effects model was utilized [17]. The standard error of the AUC was calculated using Newcombe Method 4 [19]:
ĉ is the estimated AUC, n is the number of observed events and m is the number of non-events, m* = n* = [1/2 (m + n)] − 1).
Analysis was conducted using R (version 4.0.3). Meta-analysis models were formed using R-package ‘metamisc’ [17] and ‘metafor’ [20] and results displayed as forest plots. We reported 95% prediction interval (PI), which takes into account the between-study heterogeneity [17].
Second, for studies reporting ES2 and STS, we established pooled estimates of discrimination (AUC) and calibration (O:E) for each model and compared the confidence intervals (CIs). The lack of overlap in CIs indicated a marked difference in performance. The differences in AUCs and standard error of the difference in AUCs [6, 21] were calculated per paper and utilized in a meta-analysis with the ‘metafor’ [20] package.
We also conducted stratified analysis by operation, continent and time. All ES2 papers were published after 2011; however, we separated the papers into studies solely reporting on patients operated on in or after 2010 (‘post-2010’) and those that contained data on patients operated on prior to 2010 (‘pre-2010’), on whom the authors had retrospectively calculated the ES2. We repeated the main comparisons stratifying by risk of bias (Supplementary Material, Figs. S1–S4). The presence of small-study effects was verified by visual inspection of the funnel plots (Supplementary Material, Figs. S5 and S6). Statistical heterogeneity was tested using Cochrane Q-test, and extent of statistical consistency was measured with I2, which describes the percentage of the variability in effect estimates due to heterogeneity rather than sampling error (chance).
RESULTS
Study characteristics
A total of 41 studies published between 2004 and 2020 were included the final analysis. The study characteristics are summarized in Table 1. They contained a heterogenous mix of patients, procedures and locations, commonly found in these studies [6, 22, 23]. Twenty studies reported on all operations performed [2, 24–42], 11 reported on aortic valve replacements with or without coronary artery bypass grafts (CABG) [43–53], 8 CABG only [54–61], 2 on mitral valve repair/replacement [62, 63], 2 on unspecified valvular operations [64, 65] and 1 on thoracic aortic [66] operations. A total of 23 were based in Europe [2, 24, 25, 28, 31, 35–39, 42, 46, 48–50, 53–57, 59, 62, 67], 5 in North America (NA) [32, 41, 44, 58, 63], 4 in South America (SA) [26, 30, 34, 47], 8 in Asia [27, 29, 33, 51, 60, 64–66] and 3 in New Zealand (NZ) [40, 52, 61].
Table 1:
Overview of study characteristics
| Author, year Country |
Study period | Sample size | Missing data | Age (years), mean ± SD | Male (%) | Urgency (%) | Case mix (%) | Observed mortality, % (n) | Expected mortality | O:E | AUC |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
1997–2008 | 537 | NR | 70 ± 10 | 100 | Emergency 0.1% | AVR (56% also CABG) | 5.9 (32) | STS 3.6% | STS 1.64 | STS 0.73 |
|
2006–2010 | 2437 | RF presumed absent |
|
79.5 | Urgent 17.8% |
|
1.6 (39) | ES2 2.5% | ES2 0.64 | ES2 0.80 |
|
2006–2010 | 2147 | RF presumed absent |
|
65.8 | Urgent 21.8% |
|
4.3 (92) | ES2 5.0% | ES2 0.86 | ES2 0.75 |
|
May–July 2010 | 22 381 | <1% | 64.7 ± 12.5 | 69.1 |
|
|
3.9 (873) | ES2 3.95% | ES2 0.99 | |
|
2010–2011 | 23 740 | Imputation | 67.1 ± 11.8 | 72.3 |
|
|
3.1 (736) | ES2 3.4% | ES2 0.92 | ES2 0.81 |
|
2006–2010 | 5576 | RF presumed absent |
|
73.9 | Urgent 28.3% |
|
2.2 (101) | ES2 2.0 | ES2 1.1 | ES2 0.79 |
|
2010–2011 | 1090 | NR | 64.5 ± 13.5 | 68.3 |
|
|
3.75 (41) | ES2 3.1% |
ES2 1.2 |
ES2 0.81 |
|
2006–2011 | 933 | Nil |
|
57.5 |
|
|
9.7 (90) | ES2 9.3% | ES2 1.04 | ES2 0.67 |
|
2006–2011 | 1027 | Excluded prior to analysis | 67 ± 9.4 | 77.8 |
|
Isolated CABG | 3.7 (38) | ES2 4.5% | ES2 0.82 | ES2 0.852 |
|
2012–2014 | 2296 | Nil |
|
71.2 | Emergency 11.4% |
|
2.4 (55) | ES2 1.6% | ES2 1.5 | ES2 0.871 |
|
2006–2012 | 7161 | NR | 63 ± 14 | 68 | Urgent 5.7% |
|
5.67 (406) | ES2 5.17% | ES2 1.1 | ES2 0.80 |
|
2014–2017 | 1666 | NR | 65 ± 11 | 76 |
|
CABG 56% | 1.56 (26) | ES2 2.97% | ES2 0.53 | ES2 0.831 |
|
2001–2004 | 692 of 3125 | NR | 65.8 | 0 | NR | Isolated CABG | 2.9 (20) | STS 2.6% | STS 1.1 | STS 0.82 |
|
2001–2004 | 2433 of 3125 | NR | 62.6 | 100 | NR | Isolated CABG | 1.5 (37) | STS 2.1% | STS 0.71 | STS 0.85 |
|
2006–2012 | 1758 | <1%; multiple imputation | 69.8 ± 13.2 | 55 |
|
Isolated AVR | 1.4 (25) |
|
|
|
|
2006–2012 | 12 201 of 13 871 | <1%; multiple imputation | 67.3 ± 11.8 | 68 | NR |
|
1.7 (210) | ES2 2.5% | ES2 0.68 | ES2 0.80 |
|
2006–2012 | 1670 of 13 871 | <1%; multiple imputation | 68.1 ± 11.4 | 74 | NR |
|
8.1 (125) | ES2 6.2% | ES2 1.3 | ES2 0.82 |
|
2005–2010 | 3798 of 4780 | Excluded patients with missing data | 67 ± 10.15 | 62.3 | Emergency 4.63% | CABG 32.4% | 5.7 (215) | ES2 4.46% | ES2 1.27 | ES2 0.85 |
|
2012–2013 | 503 | NR | 66.4 ± 10.3 | 74.8 | Urgent or emergency 15.9% |
|
4.17 (21) | ES2 3.18% | ES2 1.31 | ES2 0.856 |
|
2008–2012 | 250 | NR | 68.6 ± 13.3 | 63.2 | Urgent 7.6% |
|
3.6 (9) | ES2 1.64% | ES2 2.20 | ES2 0.76 |
|
2001–2011 | 1154 | NR | 63.3 | 58.8 | NR |
|
1 (11) |
|
|
|
|
1993–2013 | 461 | NR | 63.5 ± 0.7 | 65 | Emergency 35.4% | Thoracic aortic surgery | 7.2 (33) | ES2 7.4% | ES2 0.97 | ES2 0.770 |
|
2011–2012 | 6293 | 1.6%; replaced with mean values | 67.3 ± 11.2 | 65.9 |
|
Isolated CABG | 4.9 (305) | ES2 4.4% | ES2 1.10 | ES2 0.83 |
|
1999–2005 | 222 | NR | 66.16 | 72.7 | NR | AVR + CABG | 6.3 (14) | ES2 3.99% | ES2 1.58 | ES2 0.77 |
|
2012–2013 | 4034 | Nil | 66.6 ± 12.3 | 63.8 |
|
CABG 25.4% | 6.5 (262) | ES2 5.7% | ES2 1.14 | ES2 0.79 |
|
2011–2012 | 911 | Excluded prior to analysis (61) | 49.37 ± 13.4 | 66.5 |
|
|
5.7 (52) | ES2 2.9% | ES2 1.97 | ES2 0.76 |
|
2001–2010 | 14 432 | RF presumed absent | 65.3 ± 11 | 72.4 |
|
|
3.1 (447) |
|
|
|
|
2011–2012 | 498 | Excluded prior to analysis (39) | 60.48 ± 7.51 | 80.1 | Emergency 1.6% |
|
1.6 (8) |
|
|
|
|
2004–2012 | 428 | Nil | 74.5 ± 3.9 | 65 | Emergency 3.7% | Isolated CABG | 7.9 (34) |
|
|
|
|
2009–2011 | 314 | Nil | 73.4 ± 9.7 (29% ≥80 years) | 59 | Emergency 3% | Severe AS | 5.7 (18) |
|
|
|
|
2002–2008 | 304 | RF presumed absent | 82.1 | 74.3 | Emergency 3.9% | Isolated CABG | 2 (6) |
|
|
|
|
2002–2008 | 608 | RF presumed absent | 63.8 | 84.9 | Emergency 2.6% | Isolated CABG | 1 (6) |
|
|
|
|
2013–2017 | 5222 | Imputation | 60.6 ± 12 | 63.6 |
|
|
7.64 (399) |
|
|
|
|
1996–2001 | 4497 | NR | 66.4 ± 9.3 | 77 |
|
Isolated CABG | 1.89 (85) | STS 1.89% | STS 1.0 | STS 0.71 |
|
2003–2012 | 50 588 | RF presumed absent | 64.7 ± 11.2 | 71.1 | NR |
|
2.1 (1071) |
|
|
|
|
2006–2010 | 2004 | RF presumed absent | 58.3 ± 9.6 | 82.7 |
|
Isolated CABG | 3.8 (76) | ES2 3.72% | ES2 1.02 | ES2 0.836 |
|
2006–2013 |
|
RF presumed absent | 47.36 ± 15.5 | 53.5 | NR | Valve replacement surgery ± CABG | 5.7 (28) |
|
|
|
|
2008–2015 | 1279 | NR | 64 ± 12 | 73 |
|
|
1.95 (25) |
|
|
|
|
2011–2013 | 562 | NR | NR | NR | NR |
|
4.6 (26) |
|
|
|
|
2003–2010 | 106 | RF presumed absent | 83.1 ± 2.2 | 36.8 |
|
Isolated AVR | 5.7 (6) |
|
|
|
|
2006–2011 | 3479 | Imputation | 50 ± 12.4 | 46.2 | NR | Valve surgery only | 3.2 (112) |
|
|
|
|
2010–2012 | 818 | NR | 64.5 ± 10.0 | 79.8 | NR | Isolated CABG | 1.6 (13) |
|
|
|
|
2005–2012 | 620 | NR | 64.8 ± 15.5 | 65.5 |
|
AVR ± CABG | 2.9 (18) |
|
|
|
|
1999–2012 | 1066 | Nil | 68.3 ± 11.5 | 53.8 | NR | AVR ± CABG | 4.2 (45) |
|
|
|
|
2002–2013 | 406 | NR | 71.6 ± 9.9 | 53 | Urgent/emergency 2% | AVR ± CABG | 3.4 (14) |
|
|
|
Bold representation is to highlight the different patient populations AUC: area under the receiver operator curve; AVR: aortic valve replacement; CABG: coronary artery bypass graft; ES: European System for Cardiac Operative Risk Evaluation; MVR: mitral valve repair/replacement; NR: not reported; NZ: New Zealand; O:E: observed-to-expected mortality; PS: prospective; RF: risk factor; RS: retrospective; SD: standard deviation; STS: Society of Thoracic Surgeons.
The necessary data could be derived from 39 studies [2, 24–30, 32–34, 36–40, 42, 46–58, 60–68] (42 independent populations; 190 378 patients, 6254 deaths) on ES2 and 21 studies [28–30, 32–34, 41, 44, 46, 48–52, 57–59, 63–65] (23 independent populations; 92 291 patients; 2477 deaths) on STS score, 18 papers [28–30, 32–34, 46, 48–52, 57, 58, 61, 63–65] (19 independent populations; 84 132 patients; 3455 deaths) comparing ES2 and STS.
Individual model performance
European System for Cardiac Operative Risk Evaluation 2 in individual studies
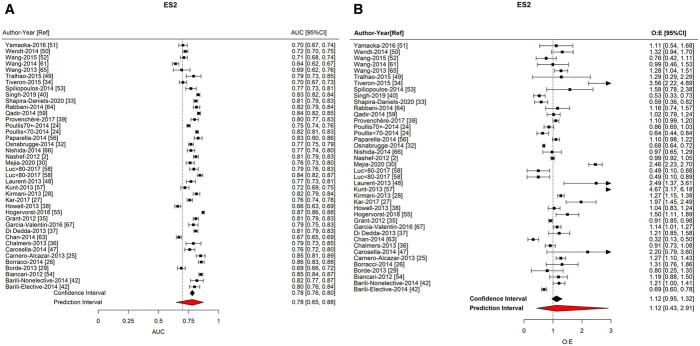
The ES2 showed good discrimination (AUC = 0.782; 95% CI: 0.763–0.800; 95% PI: 0.646–0.875) and calibration (O:E = 1.118; 95% CI: 0.950–1.317; 95% PI: 0.430–2.912) (Fig. 2/Table 2). There was no significant difference in AUC between studies at high and low risks of bias (Supplementary Material, Figs. S1 and S2), between continents nor between studies reporting on patients operated on before and after 2010 (Supplementary Material, Fig. S7).
Figure 2:
Forest plots of meta-analysis of European System for Cardiac Operative Risk Evaluation 2. (A) Area under the receiver operator curve. (B) Observed-to-expected ratio.
Table 2:
Tabulated results of meta-analyses
| Prediction model | Parameter measured | Number of studies | Summary | 95% CI | 95% PI | I 2 |
|---|---|---|---|---|---|---|
| Individual model performance | ||||||
| ES2 | Discrimination (AUC) | 40 | 0.782 | 0.763 to 0.800 | 0.646 to 0.875 | 95.4 |
| Calibration (O:E) | 40 | 1.118 | 0.950 to 1.317 | 0.430 to 2.912 | 97.0 | |
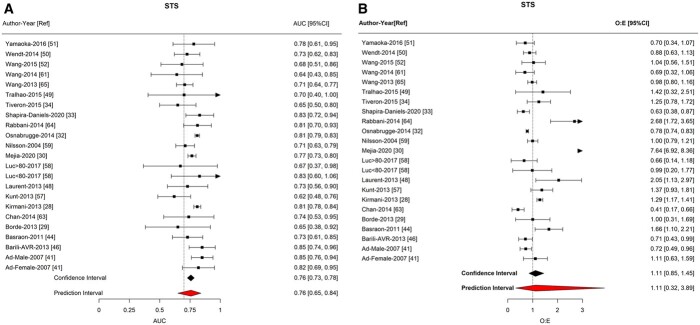
| STS | Discrimination (AUC) | 23 | 0.757 | 0.727 to 0.785 | 0.651 to 0.839 | 56.4 |
| Calibration (O:E) | 23 | 1.111 | 0.853 to 1.447 | 0.0.318 to 3.889 | 96.8 | |
| Parameter measured | Prediction model | Number of studies | Summary | 95% CI | 95% PI | I 2 |
|---|---|---|---|---|---|---|
| Comparison of prediction models | ||||||
| Discrimination (AUC) | ES2 | 19 | 0.756 | 0.728 to 0.783 | 0.623 to 0.854 | 94.6 |
| STS | 19 | 0.752 | 0.720 to 0.781 | 0.638 to 0.839 | 60.8 | |
| Difference | 19 | −0.016 | −0.034 to 0.002 | −0.035 to 0.004 | ||
| Calibration (O:E) | ES2 | 19 | 1.124 | 0.804 to 1.71 | 0.271 to 4.664 | 97.6 |
| STS | 19 | 1.116 | 0.812 to 1.535 | 0.279 to 4.470 | 97.5 | |
AUC: area under the receiver operator curve; CI: confidence interval; ES2: European System for Cardiac Operative Risk Evaluation 2; O:E: observed-to-expected mortality ratio; PI: prediction interval; STS: Society of Thoracic Surgeons.
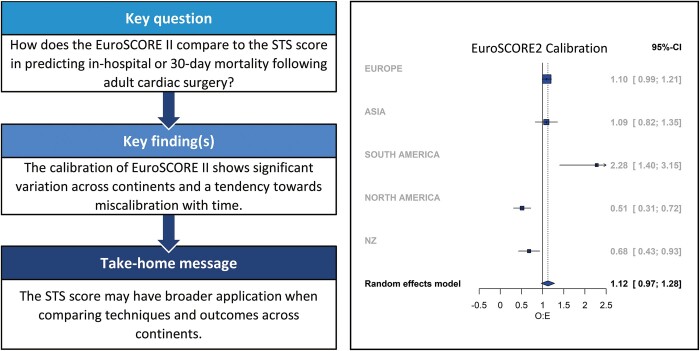
We found that ES2 calibration varied significantly between continents (P < 0.0001). ES2 overestimated risk in NA (O:E = 0.515; 95% CI: 0.312–0.718) and NZ (O:E = 0.680; 95% CI: 0.429–0.931) and under-estimated risk in SA (O:E = 2.279; 95% CI: 1.403–3.155). ES2 had a trend towards risk underestimation in ‘post-2010’ studies (O:E = 1.368; 95% CI: 1.004–1.732) compared to ‘pre-2010’ studies (O:E = 0.991; 95% CI: 0.854–1.128)(P = 0.057) (Table 3/Supplementary Material, Fig. S8). There was statistical evidence of an association between AUC and O:E and the type of operation (P < 0.0001), largely driven by in 1 mitral study (Table 3).
Table 3:
Subgroup analysis of European System for Cardiac Operative Risk Evaluation 2
|
| ||||
|---|---|---|---|---|
| Number of studies | Summary | CI | I 2 | |
| Discrimination (AUC) | ||||
| Summary estimate | 40 | 0.782 | 0.763–0.800 | 95.4 |
| Subgroup analysis | ||||
| By operation (all studies: P < 0.0001; excluding MVR: P = 0.07) | ||||
| AVR ± CABG | 7 | 0.742 | 0.718–0.766 | 64.5 |
| CABG | 7 | 0.789 | 0.730–0.848 | 97.4 |
| MVR | 1 | 0.670 | 0.648–0.692 | – |
| Valve | 2 | 0.759 | 0.639–0.879 | 90.5 |
| Mixed | 22 | 0.790 | 0.768–0.813 | 95.8 |
| Aortic | 1 | 0.759 | 0.739–0.879 | – |
| By continent (P = 0.557) | ||||
| Europe | 21 | 0.793 | 0.771–0.815 | 95.6 |
| North America | 4 | 0.770 | 0.697–0.842 | 97.6 |
| South America | 4 | 0.771 | 0.708–0.835 | 95.3 |
| Asia | 8 | 0.763 | 0.4723–0.803 | 94.6 |
| NZ | 3 | 0.729 | 0.620–0.837 | 98.9 |
| Studies containing patients operated on prior to 2010 (P = 0.397) | ||||
| Pre-2010 | 28 | 0.772 | 0.751–0.793 | 95.3 |
| Post-2010 | 12 | 0.790 | 0.754–0.827 | 97 |
| Calibration (O:E) | ||||
| Summary estimate | 40 | 1.118 | 0.950–1.317 | 97.0 |
| Subgroup analysis | ||||
| By operation (all studies: P < 0.0001; excluding MVR: P = 0.55) | ||||
| AVR ± CABG | 7 | 1.335 | 0.950–1.721 | 58.2 |
| CABG | 7 | 1.267 | 0.449–2.086 | 84.7 |
| MVR | 1 | 0.318 | 0.131–0.515 | – |
| Valve | 2 | 1.249 | 1.046–1.452 | 0 |
| Mixed | 22 | 1.126 | 0.918–1.334 | 95.6 |
| Aortic | 1 | 0.967 | 0.649–1.285 | – |
| By continent (P < 0.0001) | ||||
| Europe | 21 | 1.099 | 0.987–1.211 | 87.2 |
| North America | 5 | 0.515 | 0.312–0.718 | 80.6 |
| South America | 4 | 2.279 | 1.403–3.155 | 83.1 |
| Asia | 8 | 1.087 | 0.824–1.350 | 78.3 |
| NZ | 3 | 0.680 | 0.429–0.931 | 40.8 |
| Studies containing patients operated on prior to 2010 (P = 0.057) | ||||
| Pre-2010 | 28 | 0.991 | 0.854–1.128 | 91 |
| Post-2010 | 12 | 1.368 | 1.004–1.732 | 95.1 |
AUC: area under the receiver operator curve; AVR: aortic valve replacement; CABG: coronary artery bypass graft; CI: confidence interval; MVR: mitral valve repair/replacement; NZ: New Zealand; O:E: observed-to-expected mortality ratio.
Society of Thoracic Surgeons in individual studies
STS demonstrated good discrimination (AUC = 0.757; 95% CI: 0.727–0.785; 95% PI: 0.651–0.839) and calibration (O:E = 1.111; 95% CI: 0.853–1.447; 95% PI: 0.318–3.889; Fig. 3/Table 2). There was a statistically significant correlation between AUC and the continent of the study (P = 0.03; Table 4/Supplementary Material, Fig. S9), with the lower extent of CIs falling noticeably below 0.7 for SA (0.731; 95% CI: 0.627–0.834) and NZ (0.667; 95% CI: 0.532–0.801). There was strong statistical evidence of an association between calibration and operation (P = 0.0018), largely driven by in 1 mitral study (Table 4). There were no significant differences in STS score between continents nor over time.
Figure 3:
Forest plots of meta-analysis of Society of Thoracic Surgeons score. (A) Area under the receiver operator curve. (B) Observed-to-expected ratio.
Table 4:
Subgroup analysis of Society of Thoracic Surgeons
|
| ||||
|---|---|---|---|---|
| Number of studies | Summary | CI | I 2 | |
| Discrimination (AUC) | ||||
| Summary estimate | 23 | 0.757 | 0.727 to 0.785 | 56.4 |
| Subgroup analysis | ||||
| By operation (all studies: P = 0.22; excluding MVR: P = 0.13) | ||||
| AVR ± CABG | 6 | 0.728 | 0.667 to 0.789 | 0 |
| CABG | 7 | 0.745 | 0.772 to 0.821 | 51 |
| MVR | 1 | 0.740 | 0.533 to 0.947 | – |
| Valve | 2 | 0.749 | 0.647 to 0.851 | 58.9 |
| Mixed | 7 | 0.797 | 0.772 to 0.821 | 48.6 |
| Aortic | 0 | – | – | – |
| By continent (P = 0.03) | ||||
| Europe | 6 | 0.751 | 0.684 to 0.818 | 66.6 |
| North America | 7 | 0.809 | 0.792 to 0.827 | 0 |
| South America | 2 | 0.731 | 0.627 to 0.836 | 55 |
| Asia | 6 | 0.758 | 0.699 to 0.817 | 6 |
| NZ | 2 | 0.667 | 0.532 to 0.801 | 0 |
| Studies containing patients operated on prior to 2010 (P = 0.21) | ||||
| Pre-2010 | 19 | 0.773 | 0.742 to 0.805 | 40.6 |
| Post-2010 | 4 | 0.714 | 0.628 to 0.801 | 25.4 |
| Calibration (O:E) | ||||
| Summary estimate | 23 | 1.111 | 0.853 to 1.447 | 96.8 |
| Subgroup analysis | ||||
| By operation (all studies: P = 0.0018; excluding MVR: P = 0.36) | ||||
| AVR ± CABG | 6 | 1.171 | 0.788 to 1.555 | 65.1 |
| CABG | 7 | 0.913 | 0.726 to 1.100 | 41.5 |
| MVR | 1 | 0.414 | 0.171 to 0.658 | – |
| Valve | 2 | 1.763 | 0.102 to 3.425 | 91.3 |
| Mixed | 7 | 1.888 | 0.024 to 3.752 | 98.5 |
| Aortic | 0 | – | – | – |
| By continent (P = 0.42) | ||||
| Europe | 6 | 1.056 | 0.832 to 1.279 | 77.9 |
| North America | 7 | 0.847 | 0.573 to 1.122 | 71 |
| South America | 2 | 4.440 | −1.823 to 10.702 | 99.5 |
| Asia | 6 | 1.230 | 0.640 to 1.820 | 80.8 |
| NZ | 2 | 0.832 | 0.499 to 1.166 | 21.3 |
| Studies containing patients operated on prior to 2010 (P = 0.37) | ||||
| Pre-2010 | 19 | 0.987 | 0.815 to 1.159 | 85.1 |
| Post-2010 | 4 | 2.639 | −0.622 to 5.901 | 99 |
AUC: area under the receiver operator curve; AVR: aortic valve replacement; CABG: coronary artery bypass graft; CI: confidence interval; MVR: mitral valve repair/replacement; NZ: New Zealand; O:E: observed-to-expected mortality ratio.
European System for Cardiac Operative Risk Evaluation 2 versus Society of Thoracic Surgeons in comparative studies
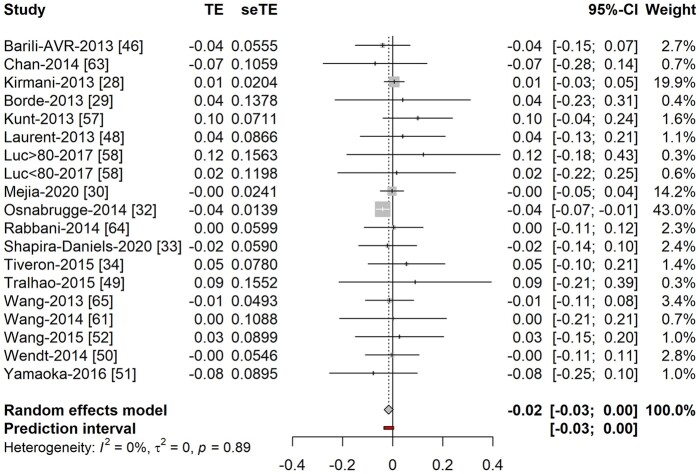
There was no difference in discrimination between ES2 [AUC: 0.756 (95% CI: 0.728–0.783)] and STS [AUC: 0.752 (95% CI: 0.720–0.781)], with no statistically significant difference in the AUC [−0.016 (95% CI: −0.033 to 0.002); P = 0.9; Table 2/Fig. 4]. The pooled estimates of the O:E for the ES2 (1.124; 95% CI: 0.804–1.710) and STS (1.116; 95% CI: 0.812–1.535) were also similar with overlap between their CIs.
Figure 4:
Difference in discrimination of European System for Cardiac Operative Risk Evaluation 2 and Society of Thoracic Surgeons score. TE: difference in C-stastistic; seTE: standard error of difference in C-statistic.
DISCUSSION
We compared the performance of the 2 most used mortality prediction models in adult cardiac surgery-ES2 and STS scores, using measures of discrimination (AUC) and calibration (O:E). Discrimination is a model’s ability to successfully differentiate between those likely and unlikely to experience an event in each population. Calibration describes the certainty with which it can predict the occurrence of an event in an individual. Both should be optimized to have a truly efficient model. Our results build on findings from 3 previous meta-analyses [6, 22, 23] by providing a dedicated statistical technique to quantitatively assess calibration in addition to discrimination and performing extended subgroup analysis.
The most notable finding of our study was that whilst the ES2 and STS performed well across the whole population, there was significant variation in the performance of ES2 between continents. It was shown to work well in the continent from which it was derived (i.e. Europe) but over-predicted risk in NA and NZ and under-predicted risk in SA. The availability of the coefficients for ES2 in the public domain may explain why this is more widely reported and there are substantially more papers from Europe. There was a tendency of ES2 to under-predict risk in papers with patients operated on solely after 2010.
However, the STS score showed good and stable performance in all continents and across both time periods studied. The STS score regression coefficients are not in the public domain and it utilizes far more variables to provide procedure-specific outcome calculations of morbidity and mortality. Consequently, the STS score performance was reported far less frequently. A key difference in the models is that STS is recalibrated annually to ensure the O:E ratio remains around 1 [10, 11].
Analysis of papers providing direct comparisons of calibration of the 2 models suggested a non-significant difference between them. The same predominance of European papers was not seen here and this may account for the discrepancy in our findings. It would have been interesting to evaluate the calibration of these models using the calibration slope or calibration in large, however this is often not reported. The Hosmer–Lemeshow statistic is one of the most widely reported statistics regarding model calibration but does not lend itself to statistical comparison between studies.
Over time the risk profile of patients has increased but operative mortality has decreased and ES has been shown to suffer from poor calibration, especially in those at highest risk [69–73]. The lack of availability of individual patient-level data limited our ability to analyse differential model performance in high and low-risk populations. Further review of these population subgroups would be of clinical importance.
Clinicians need to balance the superior performance of the STS with the relative parsimony and ease of use of ES2. Our findings suggest that ES2 and STS can be used in the populations from which they are derived but that STS may offer advantages when performing comparative research across continents.
Limitations
Bias may have been introduced into the study as we only reviewed articles in English. Abstracts and unpublished works could not be included and may have resulted in publication bias. Small study effects and significant heterogeneity could not be negated despite performing meta-regression, subgroup and sensitivity analyses. We were only able to compare studies in whom the AUC and O:E ratios could be derived, and a large study [74] was excluded due to this. Reclassification metrics have been shown to be a good estimate of model discrimination [75]; however, they were not reported in these studies and the lack of individual patient-level data made their derivation impossible.
The ES2 and STS calibration demonstrated statistically significant differences by type of operation which was driven by a singular study on mitral operations. Most studies evaluated either a mixed population, aortic valve replacements ± CABG or isolated CABG. There were few studies with dedicated performance measures on mitral valve, aortic or off-pump CABG and so the utility of these scoring systems in these subgroups could not be evaluated accurately. With the increasing number of ‘prophylactic’ aortic aneurysm operations being conducted and the emergence of transcatheter mitral interventions the validation of existing risk prediction models in these populations will become increasingly relevant.
Some interventional cardiologists have reported the use of these scoring systems in the prediction of risk in their patients and this is partially reflected in the latest guidelines [7]. We did not review the accuracy of these models in patients undergoing interventional procedures and so cannot comment on their applicability in this setting.
CONCLUSIONS
The results of this meta-analysis validate the use of either ES2 or STS in the prediction of mortality following adult cardiac surgery, especially in the continent from which they were derived. Both scores show good discrimination throughout the populations studied. The STS may be better calibrated when evaluating outcomes across European and North American centres. Future research should focus on analysis of large databases of individual patient-level data to corroborate these findings.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGEMENT
We would like to thank Ms. Joanna Hooper (librarian) for conducting the literature search.
Funding
This work was supported by the Bristol Biomedical Research Centre (NIHR Bristol BRC).
Conflict of interest: none declared.
Author contributions
Shubhra Sinha: Conceptualization; Data curation; Formal analysis; Methodology; Writing—original draft; Writing—review & editing. Arnaldo Dimagli: Data curation; Supervision; Writing—review & editing. Lauren Dixon: Data curation. Mario Gaudino: Supervision; Writing—review & editing. Massimo Caputo: Supervision; Writing—review & editing. Hunaid A. Vohra: Supervision; Writing—review & editing. Gianni Angelini: Funding acquisition; Supervision; Writing—review & editing. Umberto Benedetto: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Writing—original draft.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Guillaume Coutance, Antonio Garcia-Valentin and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- AUC
Area under the receiver operator curve
- CI
Confidence interval
- CABG
Coronary artery bypass grafts
- ES
European System for Cardiac Operative Risk Evaluation
- STS
Society of Thoracic Surgeons
- NZ
New Zealand
- NA
North America
- O:E
Observed-to-expected mortality
- PI
Prediction interval
- SA
South America
REFERENCES
- 1. Nashef SAM, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R.. European System for Cardiac Operative Risk Evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16:9–13. [DOI] [PubMed] [Google Scholar]
- 2. Nashef SAM, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR. et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734–45. [DOI] [PubMed] [Google Scholar]
- 3. Ranucci M, Castelvecchio S, Menicanti L, Frigiola A, Pelissero G.. Risk of assessing mortality risk in elective cardiac operations: age, creatinine, ejection fraction and the law of parsimony. Circulation 2009;119:3053–61. [DOI] [PubMed] [Google Scholar]
- 4. Shahian DM, O'Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB. et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1-coronary artery bypass grafting surgery. Ann Thorac Surg 2009;88:S2–S22. [DOI] [PubMed] [Google Scholar]
- 5. Geissler HJ, Hölzl P, Marohl S, Kuhn-Régnier F, Mehlhorn U, Südkamp M. et al. Risk stratification in heart surgery: comparison of six score systems. Eur J Cardiothorac Surg 2000;17:400–6. [DOI] [PubMed] [Google Scholar]
- 6. Sullivan PG, Wallach JD, Ioannidis JPA.. Meta-analysis comparing established risk prediction models (EuroSCORE II, STS score, and ACEF score) for perioperative mortality during cardiac surgery. Am J Cardiol 2016;118:1574–82. [DOI] [PubMed] [Google Scholar]
- 7. Baumgartner H, Falk V, Bax JJ, De BM, Hamm C, Holm PJ. et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2. [DOI] [PubMed] [Google Scholar]
- 8. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U. et al. ESC/EACTS Guidelines on myocardial revascularization.The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS). G Ital Cardiol 2018;40:87–165. [DOI] [PubMed] [Google Scholar]
- 9. Shanmugam G, West M, Berg G.. Additive and logistic EuroSCORE performance in high risk patients. Interact CardioVasc Thorac Surg 2005;4:299–303. [DOI] [PubMed] [Google Scholar]
- 10. O’Brien SM, Feng L, He X, Xian Y, Jacobs JP, Badhwar V. et al. The Society of Thoracic Surgeons 2018 adult cardiac surgery risk models: part 2—statistical methods and results. Ann Thorac Surg 2018;105:1419–28. [DOI] [PubMed] [Google Scholar]
- 11. Shahian DM, Jacobs JP, Badhwar V, Kurlansky PA, Furnary AP, Cleveland JC. et al. The Society of Thoracic Surgeons 2018 adult cardiac surgery risk models: part 1—background, design considerations, and model development. Ann Thorac Surg 2018;105:1411–8. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G. et al. ; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D. et al. Meta-analysis of observational studies. J Am Med Inform Assoc 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 14. Moons KGM, de Groot JAH, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG. et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med 2014;11:e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS. et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 2019;170:W1–W33. [DOI] [PubMed] [Google Scholar]
- 16. Debray TPA, Damen JAAG, Snell KIE, Ensor J, Hooft L, Reitsma JB. et al. A guide to systematic review and meta-analysis of prediction model performance. BMJ 2017;356: i6460 . [DOI] [PubMed] [Google Scholar]
- 17. Debray TPA, Damen JAAG, Riley RD, Snell K, Reitsma JB, Hooft L. et al. A framework for meta-analysis of prediction model studies with binary and time-to-event outcomes. Stat Methods Med Res 2019;28:2768–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steyerberg EW, Nieboer D, Debray TPA, Houwelingen HC.. Assessment of heterogeneity in an individual participant data meta-analysis of prediction models: an overview and illustration. Stat Med 2019;38:4290–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newcombe RG. Confidence intervals for an effect size measure based on the Mann-Whitney statistic. Part 2: asymptotic methods and evaluation. Stat Med 2006;25:559–73. [DOI] [PubMed] [Google Scholar]
- 20. Viechtbauer W, Viechtbauer W.. Conducting meta-analyses in R with the metafor package. J Stat Soft 2010;36:1–48. [Google Scholar]
- 21. Hanley JA, McNeil BJA.. Method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839–43. [DOI] [PubMed] [Google Scholar]
- 22. Guida P, Mastro F, Scrascia G, Whitlock R, Paparella D.. Performance of the European System for Cardiac Operative Risk Evaluation II: a meta-analysis of 22 studies involving 145,592 cardiac surgery procedures. J Thorac Cardiovasc Surg 2014;148:3049–3057. e1. [DOI] [PubMed] [Google Scholar]
- 23. Biancari F, Juvonen T, Onorati F, Faggian G, Heikkinen J, Airaksinen J. et al. Meta-analysis on the performance of the EuroSCORE II and the society of thoracic surgeons scores in patients undergoing aortic valve replacement. J Cardiothorac Vasc Anesth 2014;28:1533–9. [DOI] [PubMed] [Google Scholar]
- 24. Poullis M, Pullan M, Chalmers J, Mediratta N.. The validity of the original EuroSCORE and EuroSCORE II in patients over the age of seventy. Interact CardioVasc Thorac Surg 2015;20:172–7. [DOI] [PubMed] [Google Scholar]
- 25. Carnero-Alcázar M, Guisasola JAS, Lacruz FJR, Castellanos LCM, Carnicer JC, Medinilla EV. et al. Validation of EuroSCORE II on a single-centre 3800 patient cohort. Interact CardioVasc Thorac Surg 2013;16:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borracci RA, Rubio M, Celano L, Ingino CA, Allende NG, Guerrero RAA.. Prospective validation of EuroSCORE II in patients undergoing cardiac surgery in Argentinean centres. Interact CardioVasc Thorac Surg 2014;18:539–43. [DOI] [PubMed] [Google Scholar]
- 27. Kar P, Geeta K, Gopinath R, Durga P.. Mortality prediction in Indian cardiac surgery patients: validation of European System for Cardiac Operative Risk Evaluation II. Indian J Anaesth 2017;61:157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirmani BH, Mazhar K, Fabri BM, Pullan DM.. Comparison of the EuroSCORE II and Society of Thoracic Surgeons 2008 risk tools. Eur J Cardiothorac Surg 2013;44:999–1005. [DOI] [PubMed] [Google Scholar]
- 29. Borde D, Gandhe U, Hargave N, Pandey K, Khullar V.. The application of European System for Cardiac Operative Risk Evaluation II (EuroSCORE II) and Society of Thoracic Surgeons (STS) risk-score for risk stratification in Indian patients undergoing cardiac surgery. Ann Card Anaesth 2013;16:163–6. [DOI] [PubMed] [Google Scholar]
- 30. Vilca Mejia OA, Borgomoni GB, Zubelli JP, Palma Dallan LR, Alberto Pomerantzeff PM, Praça Oliveira MA. et al. Validation and quality measurements for STS, EuroSCORE II and a regional risk model in Brazilian patients. PLoS One 2020;15:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nilsson J, Algotsson L, Höglund P, Lührs C, Brandt J.. Comparison of 19 pre-operative risk stratification models in open-heart surgery. Eur Heart J 2006;27:867–74. [DOI] [PubMed] [Google Scholar]
- 32. Osnabrugge RL, Speir AM, Head SJ, Fonner CE, Fonner E, Kappetein AP. et al. Performance of EuroSCORE II in a large US database: implications for transcatheter aortic valve implantation. Eur J Cardiothorac Surg 2014;46:400–8. [DOI] [PubMed] [Google Scholar]
- 33. Shapira-Daniels A, Blumenfeld O, Korach A, Rudis E, Izhar U, Shapira OM.. The American Society of Thoracic Surgery score versus EuroSCORE I and EuroSCORE II in Israeli patients undergoing cardiac surgery. Isr Med Assoc J 2019;21:671–5. [PubMed] [Google Scholar]
- 34. Tiveron MG, Bomfim HA, Simplício MS, Bergonso MH, De Matos MPB, Ferreira SM. et al. Desempenho do InsCor e de três escores internacionais em cirurgia cardíaca na Santa Casa de Marília. Braz J Cardiovasc Surg 2015;30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grant SW, Hickey GL, Dimarakis I, Trivedi U, Bryan A, Treasure T. et al. How does EuroSCORE II perform in UK cardiac surgery; an analysis of 23 740 patients from the Society for Cardiothoracic Surgery in Great Britain and Ireland National Database. Heart 2012;98:1568–72. [DOI] [PubMed] [Google Scholar]
- 36. Chalmers J, Pullan M, Fabri B, McShane J, Shaw M, Mediratta N. et al. Validation of EuroSCORE II in a modern cohort of patients undergoing cardiac surgery. Eur J Cardiothorac Surg 2013;43:688–94. [DOI] [PubMed] [Google Scholar]
- 37. Di Dedda U, Pelissero G, Agnelli B, De Vincentiis C, Castelvecchio S, Ranucci M.. Accuracy, calibration and clinical performance of the new EuroSCORE II risk stratification system. Eur J Cardiothorac Surg 2013;43:27–32. [DOI] [PubMed] [Google Scholar]
- 38. Howell NJ, Head SJ, Freemantle N, van der Meulen TA, Senanayake E, Menon A. et al. The new EuroSCORE II does not improve prediction of mortality in high-risk patients undergoing cardiac surgery: a collaborative analysis of two European centres. Eur J Cardiothorac Surg 2013;44:1006–11. [DOI] [PubMed] [Google Scholar]
- 39. Provenchère S, Chevalier A, Ghodbane W, Bouleti C, Montravers P, Longrois D. et al. Is the EuroSCORE II reliable to estimate operative mortality among octogenarians? PLoS One 2017;12:e0187056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singh N, Gimpel D, Parkinson G, Conaglen P, Meikle F, Lin Z. et al. Assessment of the EuroSCORE II in a New Zealand Tertiary Centre. Hear Lung Circ 2019;28:1670–6. [DOI] [PubMed] [Google Scholar]
- 41. Ad N, Barnett SD, Speir AM.. The performance of the EuroSCORE and the Society of Thoracic Surgeons mortality risk score: the gender factor. Interact CardioVasc Thorac Surg 2006;6:192–5. [DOI] [PubMed] [Google Scholar]
- 42. Barili F, Pacini D, Rosato F, Roberto M, Battisti A, Grossi C. et al. In-hospital mortality risk assessment in elective and non-elective cardiac surgery: a comparison between EuroSCORE II and age, creatinine, ejection fraction score. Eur J Cardiothorac Surg 2014;46:44–8. [DOI] [PubMed] [Google Scholar]
- 43. Gummert JF, Funkat A, Osswald B, Beckmann A, Schiller W, Krian A. et al. EuroSCORE overestimates the risk of cardiac surgery: results from the national registry of the German Society of Thoracic and Cardiovascular Surgery. Clin Res Cardiol 2009;98:363–9. [DOI] [PubMed] [Google Scholar]
- 44. Basraon J, Chandrashekhar YS, John R, Agnihotri A, Kelly R, Ward H. et al. Comparison of risk scores to estimate perioperative mortality in aortic valve replacement surgery. Ann Thorac Surg 2011;92:535–40. [DOI] [PubMed] [Google Scholar]
- 45. van Gameren M, Kappetein AP, Steyerberg EW, Venema AC, Berenschot EAJ, Hannan EL. et al. Do we need separate risk stratification models for hospital mortality after heart valve surgery? Ann Thorac Surg 2008;85:921–30. [DOI] [PubMed] [Google Scholar]
- 46. Barili F, Pacini D, Capo A, Ardemagni E, Pellicciari G, Zanobini M. et al. Reliability of new scores in predicting perioperative mortality after isolated aortic valve surgery: a comparison with the society of thoracic surgeons score and logistic EuroSCORE. Ann Thorac Surg 2013;95:1539–44. [DOI] [PubMed] [Google Scholar]
- 47. Carosella V, Mastantuono C, Golovonevsky V, Cohen V, Grancelli H, Rodriguez W. et al. Prospective and multicentric validation of the ArgenSCORE in aortic valve replacement surgery. Comparison with the EuroSCORE I and the EuroSCORE II. Rev Argent Cardiol 2014;82: 5–11. [Google Scholar]
- 48. Laurent M, Fournet M, Feit B, Oger E, Donal E, Thébault C. et al. Simple bedside clinical evaluation versus established scores in the estimation of operative risk in valve replacement for severe aortic stenosis. Arch Cardiovasc Dis 2013;106:651–60. [DOI] [PubMed] [Google Scholar]
- 49. Tralhão A, Campante Teles R, Sousa Almeida M, Madeira S, Borges Santos M, Andrade MJ. et al. Aortic valve replacement for severe aortic stenosis in octogenarians: patient outcomes and comparison of operative risk scores. Rev Port Cardiol 2015;34:439–46. [DOI] [PubMed] [Google Scholar]
- 50. Wendt D, Thielmann M, Kahlert P, Kastner S, Price V, Al-Rashid F. et al. Comparison between different risk scoring algorithms on isolated conventional or transcatheter aortic valve replacement. Ann Thorac Surg 2014;97:796–802. [DOI] [PubMed] [Google Scholar]
- 51. Yamaoka H, Kuwaki K, Inaba H, Yamamoto T, Kato TS, Dohi S. et al. Comparison of modern risk scores in predicting operative mortality for patients undergoing aortic valve replacement for aortic stenosis. J Cardiol 2016;68:135–40. [DOI] [PubMed] [Google Scholar]
- 52. Wang TKM, Choi DHM, Stewart R, Gamble G, Haydock D, Ruygrok P.. Comparison of four contemporary risk models at predicting mortality after aortic valve replacement. J Thorac Cardiovasc Surg 2015;149:443–8. [DOI] [PubMed] [Google Scholar]
- 53. Spiliopoulos K, Bagiatis V, Deutsch O, Kemkes BM, Antonopoulos N, Karangelis D. et al. Performance of EuroSCORE II compared to EuroSCORE I in predicting operative and mid-term mortality of patients from a single center after combined coronary artery bypass grafting and aortic valve replacement. Gen Thorac Cardiovasc Surg 2014;62:103–11. [DOI] [PubMed] [Google Scholar]
- 54. Biancari F, Vasques F, Mikkola R, Martin M, Lahtinen J, Heikkinen J.. Validation of EuroSCORE II in patients undergoing coronary artery bypass surgery. Ann Thorac Surg 2012;93:1930–5. [DOI] [PubMed] [Google Scholar]
- 55. Hogervorst EK, Rosseel PMJ, van de Watering LMG, Brand A, Bentala M, van der Meer BJM. et al. Prospective validation of the EuroSCORE II risk model in a single Dutch cardiac surgery centre. Neth Heart J 2018;26:540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paparella D, Guida P, Di Eusanio G, Caparrotti S, Gregorini R, Cassese M. et al. Risk stratification for in-hospital mortality after cardiac surgery: external validation of EuroSCORE II in a prospective regional registry. Eur J Cardiothorac Surg 2014;46:840–8. [DOI] [PubMed] [Google Scholar]
- 57. Kunt AG, Kurtcephe M, Hidiroglu M, Cetin L, Kucuker A, Bakuy V. et al. Comparison of original EuroSCORE, EuroSCORE II and STS risk models in a Turkish cardiac surgical cohort. Interact CardioVasc Thorac Surg 2013;16:625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luc JGY, Graham MM, Norris CM, Al Shouli S, Nijjar YS, Meyer SR.. Predicting operative mortality in octogenarians for isolated coronary artery bypass grafting surgery: a retrospective study. BMC Cardiovasc Disord 2017;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nilsson J, Algotsson L, Höglund P, Lührs C, Brandt J.. Early mortality in coronary bypass surgery: the EuroSCORE versus the Society of Thoracic Surgeons risk algorithm. Ann Thorac Surg 2004;77:1235–9. [DOI] [PubMed] [Google Scholar]
- 60. Qadir I, Alamzaib SM, Ahmad M, Perveen S, Sharif H.. EuroSCORE vs. EuroSCORE II vs. Society of Thoracic Surgeons risk algorithm. Asian Cardiovasc Thorac Ann 2014;22:165–71. [DOI] [PubMed] [Google Scholar]
- 61. Wang TKM, Li AY, Ramanathan T, Stewart RAH, Gamble G, White HD.. Comparison of four risk scores for contemporary isolated coronary artery bypass grafting. Hear Lung Circ 2014;23:469–74. [DOI] [PubMed] [Google Scholar]
- 62. Barili F, Pacini D, Grossi C, Di Bartolomeo R, Alamanni F, Parolari A.. Reliability of new scores in predicting perioperative mortality after mitral valve surgery. J Thorac Cardiovasc Surg 2014;147:1008–12. [DOI] [PubMed] [Google Scholar]
- 63. Chan V, Ahrari A, Ruel M, Elmistekawy E, Hynes M, Mesana TG.. Perioperative deaths after mitral valve operations may be overestimated by contemporary risk models. Ann Thorac Surg 2014;98:605–10. [DOI] [PubMed] [Google Scholar]
- 64. Rabbani MS, Qadir I, Ahmed Y, Gul M, Sharif H.. Heart valve surgery: EuroSCORE vs. EuroSCORE II vs. Society of Thoracic Surgeons score. Heart Int 2014;9:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang C, Li X, Lu F. L, Xu J, binTang H, Han L. et al. Comparison of six risk scores for in-hospital mortality in Chinese patients undergoing heart valve surgery. Hear Lung Circ 2013;22:612–7. [DOI] [PubMed] [Google Scholar]
- 66. Nishida T, Sonoda H, Oishi Y, Tanoue Y, Nakashima A, Shiokawa Y. et al. The novel EuroSCORE II algorithm predicts the hospital mortality of thoracic aortic surgery in 461 consecutive Japanese patients better than both the original additive and logistic EuroSCORE algorithms. Interact CardioVasc Thorac Surg 2014;18:446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Garcia-Valentin A, Mestres CA, Bernabeu E, Bahamonde JA, Martín I, Rueda C. et al. Validation and quality measurements for EuroSCORE and EuroSCORE II in the Spanish cardiac surgical population: a prospective, multicentre study. Eur J Cardiothorac Surg 2016;49:399–405. [DOI] [PubMed] [Google Scholar]
- 68. Grant SW, Grayson AD, Jackson M, Au J, Fabri BM, Grotte G. et al. Does the choice of risk-adjustment model influence the outcome of surgeon-specific mortality analysis? A retrospective analysis of 14 637 patients under 31 surgeons. Heart 2008;94:1044–9. [DOI] [PubMed] [Google Scholar]
- 69. Michel P, Roques F, Nashef SAM; EuroSCORE Project Group. Logistic or additive EuroSCORE for high-risk patients? Eur J Cardiothorac Surg 2003;23:684–7. [DOI] [PubMed] [Google Scholar]
- 70. Karabulut H, Toraman F, Alhan C, Camur G, Evrenkaya S, Dağdelen S. et al. EuroSCORE overestimates the cardiac operative risk. Cardiovasc Surg 2003;11:295–8. [DOI] [PubMed] [Google Scholar]
- 71. Barmettler H, Immer FF, Berdat PA, Eckstein FS, Kipfer B, Carrel TP.. Risk-stratification in thoracic aortic surgery: should the EuroSCORE be modified? Eur J Cardiothorac Surg 2004;25:691–4. [DOI] [PubMed] [Google Scholar]
- 72. van Straten AHM, Tan E, Hamad MAS, Martens EJ, van Zundert AAJ.. Evaluation of the EuroSCORE risk scoring model for patients undergoing coronary artery bypass graft surgery: a word of caution. Neth Heart J 2010;18:355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sergeant P, De Worm E, Meyns B.. Single centre, single domain validation of the EuroSCORE on a consecutive sample of primary and repeat CABG. Eur J Cardiothorac Surg 2001;20:1176–82. [DOI] [PubMed] [Google Scholar]
- 74. Hu Z, Chen S, Du J, Gu D, Wang Y, Hu S. et al. An in-hospital mortality risk model for patients undergoing coronary artery bypass grafting in China. Ann Thorac Surg 2020;109:1234–42. [DOI] [PubMed] [Google Scholar]
- 75. Enserro DM, Demler OV, Pencina MJ, D'Agostino RB.. Measures for evaluation of prognostic improvement under multivariate normality for nested and nonnested models. Stat Med 2019;38:3817–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.