ABSTRACT
Intervening proteins, or inteins, are mobile genetic elements that are translated within host polypeptides and removed at the protein level by splicing. In protein splicing, a self-mediated reaction removes the intein, leaving a peptide bond in place. While protein splicing can proceed in the absence of external cofactors, several examples of conditional protein splicing (CPS) have emerged. In CPS, the rate and accuracy of splicing are highly dependent on environmental conditions. Because the activity of the intein-containing host protein is compromised prior to splicing and inteins are highly abundant in the microbial world, CPS represents an emerging form of posttranslational regulation that is potentially widespread in microbes. Reactive chlorine species (RCS) are highly potent oxidants encountered by bacteria in a variety of natural environments, including within cells of the mammalian innate immune system. Here, we demonstrate that two naturally occurring RCS, namely, hypochlorous acid (the active compound in bleach) and N-chlorotaurine, can reversibly block splicing of DnaB inteins from Mycobacterium leprae and Mycobacterium smegmatis in vitro. Further, using a reporter that monitors DnaB intein activity within M. smegmatis, we show that DnaB protein splicing is inhibited by RCS in the native host. DnaB, an essential replicative helicase, is the most common intein-housing protein in bacteria. These results add to the growing list of environmental conditions that are relevant to the survival of the intein-containing host and influence protein splicing, as well as suggesting a novel mycobacterial response to RCS. We propose a model in which DnaB splicing, and therefore replication, is paused when these mycobacteria encounter RCS.
IMPORTANCE Inteins are both widespread and abundant in microbes, including within several bacterial and fungal pathogens. Inteins are domains translated within host proteins and removed at the protein level by splicing. Traditionally considered molecular parasites, some inteins have emerged in recent years as adaptive posttranslational regulatory elements. Several studies have demonstrated CPS, in which the rate and accuracy of protein splicing, and thus host protein functions, are responsive to environmental conditions relevant to the intein-containing organism. In this work, we demonstrate that two naturally occurring RCS, including the active compound in household bleach, reversibly inhibit protein splicing of Mycobacterium leprae and Mycobacterium smegmatis DnaB inteins. In addition to describing a new physiologically relevant condition that can temporarily inhibit protein splicing, this study suggests a novel stress response in Mycobacterium, a bacterial genus of tremendous importance to humans.
KEYWORDS: intein, conditional protein splicing, reactive chlorine species, bleach, chloramines, mycobacteria, DnaB helicase
INTRODUCTION
Inteins are intervening proteins that excise themselves from precursor polypeptides through protein splicing. In this process, the intein is removed and the flanking N and C exteins are joined together with a peptide bond to yield a mature host protein (1–5). In the nearly three decades since the discovery of inteins, thousands have been identified across the genomes of archaea, bacteria, unicellular eukaryotes, phages, and viruses. Inteins are particularly abundant in prokaryotes; they are found in almost one-quarter of bacterial genomes and one-half of archaeal genomes (6).
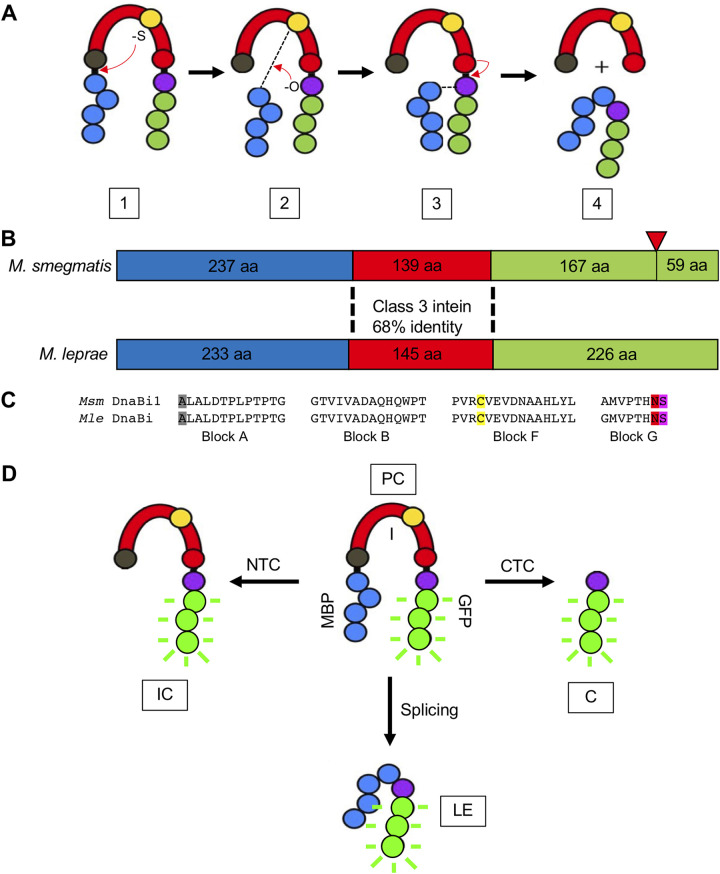
The protein splicing reaction is mediated by conserved residues most often found near the intein-extein junctions. In class 1 protein splicing (7), the first residue of the intein (either cysteine or serine) makes a nucleophilic attack on the preceding peptide bond, leading to an ester/thioester linkage. Next, the first residue of the C extein (either cysteine, serine, or threonine) makes another nucleophilic attack on the ester/thioester formed in step 1, leading to formation of the branched intermediate. Following this, the terminal asparagine of the intein cyclizes to release the intein. Finally, the ester/thioester bond connecting the N and C exteins rearranges to form a peptide bond. Two alternative splicing mechanisms exist (class 2 and class 3), with an overall conservation of intein fold and chemistry but important differences in the catalytic steps. For class 3 inteins, step 1 is carried out by an internal nucleophile (Fig. 1A), with steps 2 to 4 occurring in a manner similar to that for class 1 inteins (7). Off-pathway reactions can also occur, cleaving the N or C extein from the intein prior to ligation.
FIG 1.
M. smegmatis DnaBi1 and M. leprae DnaBi. (A) Mechanism of class 3 protein splicing. Steps 1 to 4 are described in the text. Blue, N extein; red, intein; green, C extein; gray ball, first intein residue; yellow ball, intein initiating nucleophile; red ball, intein terminal asparagine; purple ball, +1 residue; dashed line, thioester linkage. (B) Comparison of M. smegmatis DnaBi1 and M. leprae DnaBi intein insertion positions in DnaB. aa, amino acids. Blue, N extein; red, intein; green, C extein; red inverted triangle, position of second intein within M. smegmatis that is absent in M. leprae. (C) Comparison of splicing blocks between M. smegmatis DnaBi1 and M. leprae DnaBi. Gray, first intein residue; yellow, intein initiating nucleophile; red, intein terminal asparagine; purple, +1 nucleophile. (D) MIG intein activity reporter. Species visible through GFP fluorescence are shown, including unspliced precursor (PC), ligated exteins (LE) from splicing, intein-C extein (IC) from N-terminal cleavage (NTC), and C extein (C) from C-terminal cleavage (CTC).
The ability of inteins to rearrange or cleave peptide bonds in a controlled manner has been exploited in numerous biotechnological applications, including protein purification, segmental isotope labeling, formation of cyclic peptides, incorporation of nonnatural modules into proteins, fabrication of protein arrays, sensor development, imaging, and regulation of protein function in vivo (8, 9). Additionally, new intein-based technologies frequently emerge (10–13). Given the useful chemistry performed by inteins, as well as the ability to leave no trace in the final product, the potential of these elements in protein engineering is tremendous.
Inteins themselves often house an autonomous homing endonuclease (HEN) domain that is not directly involved in protein splicing but rather is involved in horizontal transfer of the intein-coding DNA. Therefore, inteins have been traditionally viewed as selfish genetic elements, the invasiveness of which helps remodel genomes (14, 15). However, several intriguing aspects of intein distribution compel us to rethink the role of these elements in nature. Inteins cluster in particular functional classes of proteins, with over 70% being found in ATP-binding proteins and over 60% being localized in proteins involved in DNA replication, recombination, and repair (6). Remarkably, inteins have even been independently acquired by evolutionarily distinct bacterial and archaeal proteins of equivalent function (6). Further, inteins are typically found within genes essential for host organism survival, with their presence disrupting function. Therefore, successful protein splicing is required for the survival of a significant fraction of all life.
In the past decade, a growing body of evidence has demonstrated that some inteins undergo conditional protein splicing (CPS), whereby splicing rate and formation of off-pathway products are intimately tied to environmental conditions. We and others have challenged the model of pure selfishness and argue that many inteins, particularly inteins that have lost their HEN domain, have transitioned to a form of “altruism.” Several studies have provided compelling examples of CPS in which splicing is highly dependent on environmental conditions. These conditions, which are often crucial for the survival of the host organisms and/or relevant to the function of the intein-containing protein, include pH, divalent cations, redox, reactive oxygen species (ROS)/reactive nitrogen species (RNS), salt, temperature, and even DNA damage (single-stranded DNA [ssDNA]) (16–29). Therefore, some inteins can seemingly modulate protein splicing in response to stress, regulating intein-housing protein activity.
Reactive chlorine species (RCS) modify several biomolecules, including amino acid side chains, nucleotides, and lipids (30, 31). Sulfur-containing amino acid side chains, such as cysteine, are particularly susceptible to RCS oxidation and chlorination, reacting ∼100-fold faster than other biomolecules (30, 31). Many bacteria encounter RCS in nature, such as hypochlorous acid (HOCl), the active compound in bleach, and have evolved specific response pathways (30, 31). For example, cells of the mammalian innate immune system produce RCS to kill foreign bacteria (30, 31). Within neutrophils, myeloperoxidase (MPO) produces millimolar quantities of HOCl from hydrogen peroxide (H2O2) and chloride (31). Chloramines, which are produced upon HOCl reaction with amines, represent another highly reactive, naturally occurring RCS with potent antimicrobial properties (30). For example, N-chlorotaurine (NCT), a derivative of the amino acid taurine, is a relatively long-lived RCS that is naturally produced by granulocytes and monocytes (32). Interestingly, while chloramines are less reactive than HOCl, they are more specific for cysteine oxidation (30).
Here, we demonstrate that two physiologically relevant RCS, namely, NCT and HOCl, can inhibit protein splicing of DnaB inteins from Mycobacterium leprae and Mycobacterium smegmatis. Additionally, we find that this inhibition is fully reversible in the presence of reducing agent. Finally, using an in vivo reporter that directly links intein activity to cell survival in M. smegmatis, we show that DnaB intein splicing can be inhibited within the natural context of the intein. We propose a model whereby RCS can act to reversibly pause protein splicing stress. We argue that the ability of some inteins to detect stress and temporarily pause critical cellular processes, such as DNA replication, may help the intein-containing organism survive and persist under harsh conditions.
RESULTS
The helicase DnaB is the most abundant intein-containing protein in bacteria and is strictly required for DNA replication (6). M. smegmatis and M. leprae each house a class 3 intein within DnaB, referred to as M. smegmatis DnaBi1 and M. leprae DnaBi, respectively. M. smegmatis DnaB also houses a second, full-length intein (Fig. 1B) that splices fully upon expression (25) and does not appear to be subject to CPS. M. smegmatis DnaBi1 and M. leprae DnaBi both localize within the P-loop of the DnaB ATPase domain (Fig. 1B), with the +1 serine of the C extein acting to help coordinate Mg2+-ATP following splicing (25). M. smegmatis DnaBi1 and M. leprae DnaBi are both mini-inteins. Because mini-inteins lack a HEN domain and are no longer capable of endonuclease-mediated mobility, these represent especially intriguing candidates for CPS. M. smegmatis DnaBi1 and M. leprae DnaBi residues are 68% identical overall (Fig. 1B) and splicing blocks are highly conserved, varying only in the first position of block G (Fig. 1C). Further, both M. smegmatis DnaBi1 and M. leprae DnaBi use cysteine as the initiating nucleophile to begin the splicing process. Because these are class 3 inteins, this catalytic cysteine is internal (C-118 for M. smegmatis DnaBi1 and C-124 for M. leprae DnaBi), rather than being the first residue of the intein.
We tested the hypothesis that the activity of these inteins could be blocked by RCS. To measure the potential inhibition of protein splicing, we utilized an established splicing reporter referred to as MIG (MBP-intein-GFP). In the MIG reporter, the intein (surrounded by 10 native extein residues) is flanked by the nonnative exteins maltose-binding protein (MBP) (N extein) and superfolder green fluorescent protein (GFP) (C extein). This reporter can be used to visualize precursor, ligated exteins, and products resulting from off-pathway cleavage (intein-C extein from N terminal cleavage and C extein from C-terminal cleavage) (Fig. 1D). GFP-containing products (i.e., C extein) can be detected using seminative PAGE with fluorescent products measured in the gel (33). Additionally, this reporter has a built-in control for generalized protein misfolding, because the structure of GFP must remain intact in order to maintain the fluorescence signal.
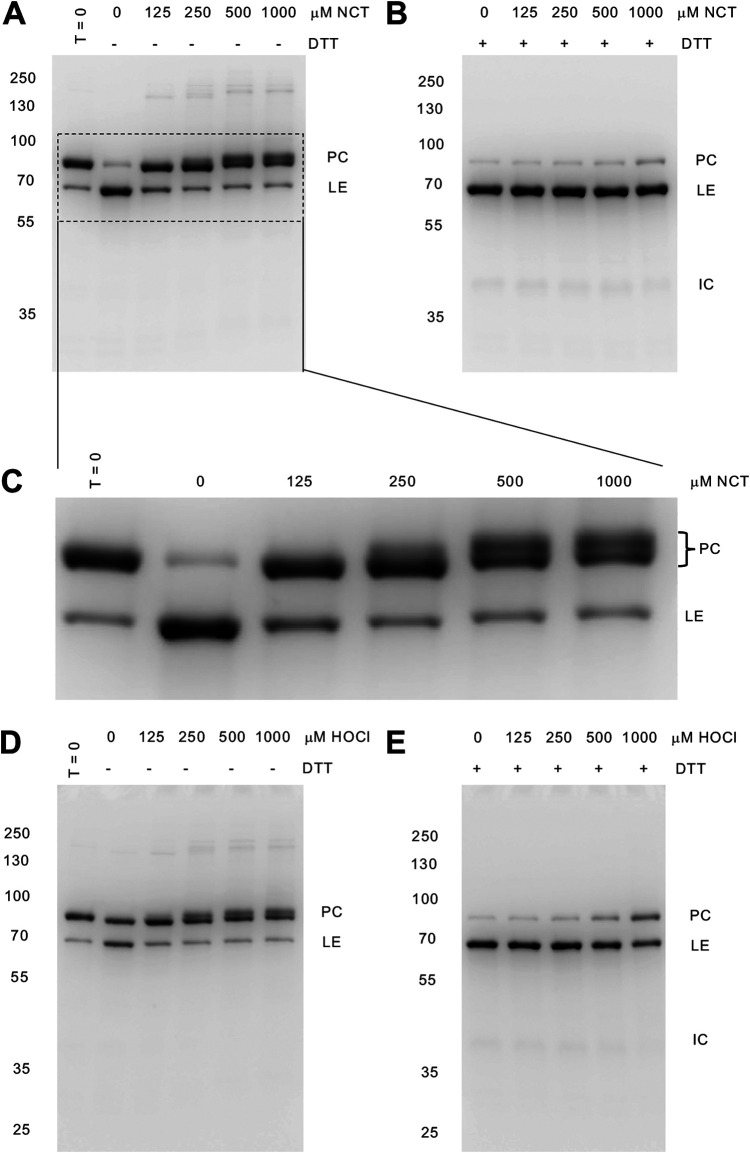
Following expression in the MIG reporter, as observed previously, most M. smegmatis DnaBi1 was in the unspliced precursor (Fig. 2A, C, and D). We next treated M. smegmatis DnaBi1 with two naturally occurring RCS, namely, the chloramine NCT and HOCl. For both of these RCS, we observed substantial inhibition of splicing (Fig. 2A and D). Interestingly, we observed a second precursor band form (Fig. 2C), a pattern previously observed when M. smegmatis DnaBi1 was treated with H2O2 and formed an intramolecular disulfide. We also observed a faint band consistent with an intermolecular disulfide bond between precursors (Fig. 3A). Critical to the model that inteins can serve as “pause buttons” to temporarily block splicing under stress (29), we next asked whether this observed inhibition was reversible. Dithiothreitol (DTT), a reducing agent, is capable of reversing some RCS modifications. To test this, we first incubated lysate with either HOCl or NCT, followed by an excess of the reducing agent DTT, which is expected to reverse oxidative RCS modifications on cysteines (30). Consistent with reversible intein modification, we found that the inhibition of splicing by RCS was fully reversible following treatment with DTT (Fig. 2B and E).
FIG 2.
M. smegmatis DnaBi1 splicing is reversibly inhibited by RCS. (A) NCT treatment of M. smegmatis DnaBi1. The concentrations of NCT are indicated. (B) Excess (50 mM) DTT (reducing agent) reverses the inhibition by NCT and permits splicing. (C) Enlargement of a gel section from panel A. (D) HOCl treatment of M. smegmatis DnaBi1 at the concentrations indicated. (E) Reversal of M. smegmatis DnaBi1 splicing inhibition by HOCl in the presence of excess DTT (50 mM). Each gel is representative of at least three independent experiments. Molecular weights are indicated to the left of the gels, and GFP-containing species are indicated to the right of the gels. PC, unspliced precursor; LE, ligated exteins; IC, intein-C extein.
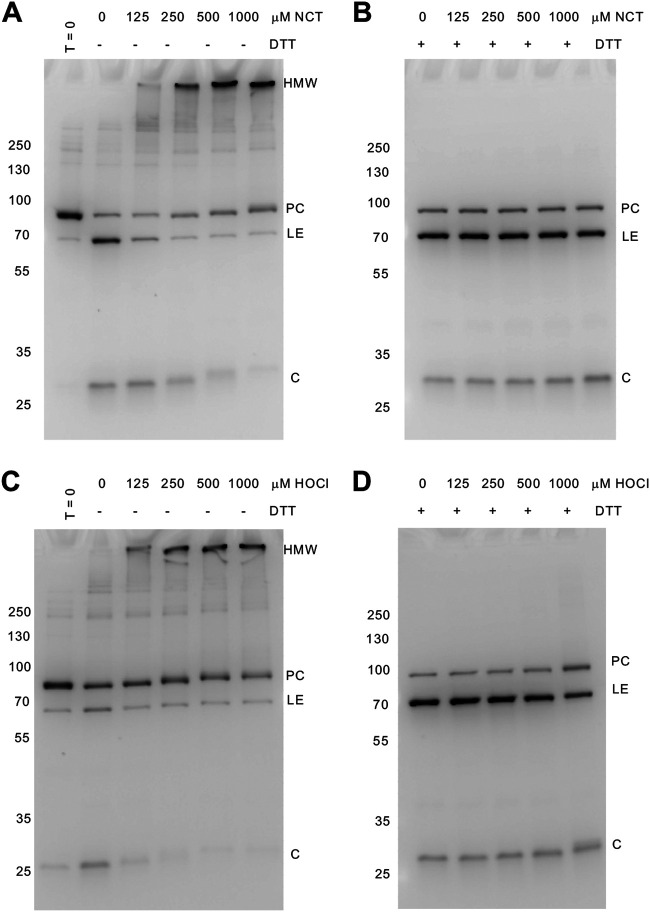
FIG 3.
Reversible inhibition of M. leprae DnaBi activity by RCS. (A) NCT treatment inhibits M. leprae DnaBi splicing. The concentrations of NCT are indicated. (B) DTT in excess (50 mM) reverses the inhibition by NCT. (C) M. leprae DnaBi activity is blocked by HOCl at the indicated concentrations. (D) M. leprae DnaBi activity is restored following excess DTT (50 mM) treatment. Each gel is representative of at least three independent experiments. Molecular weights are indicated to the left of the gels, and GFP-containing species are indicated to the right of the gels. PC, unspliced precursor; LE, ligated exteins; C, C extein.
We next asked whether M. leprae DnaBi activity was also inhibited by RCS. As with M. smegmatis DnaBi1, substantial levels of unspliced precursor remained following expression in the MIG reporter (Fig. 3A and C). Of note, we observed significant C-terminal cleavage, as indicated by the free C extein, for M. leprae DnaBi; this can also be used as a measure of intein activity. Following treatment with NCT and HOCl, M. leprae DnaBi splicing was also fully inhibited (Fig. 3A and C). Unlike M. smegmatis DnaBi1, M. leprae DnaBi appeared to form a high-molecular-weight (HMW) product (Fig. 3A and C). This product is soluble, and GFP in the MIG reporter remains folded. Importantly, as with M. smegmatis DnaBi1, M. leprae DnaBi activity was restored upon incubation with DTT (Fig. 3B and D). This HMW band likely represents several precursors forming intermolecular disulfide bonds. We also observed that RCS changed the migration of the C extein (Fig. 3A and C), likely through modification of the C extein that is reversible with DTT (Fig. 3B and D).
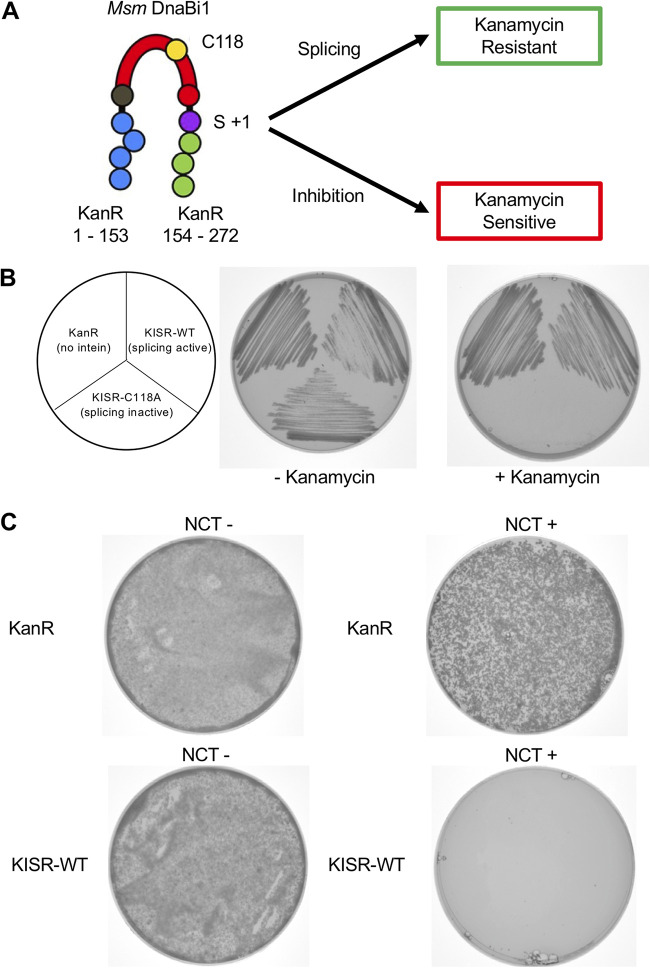
Examination of CPS in vivo has traditionally proved challenging due to the lack of inteins in model organisms. Previously, we developed a splicing reporter that can be used directly within M. smegmatis cells to monitor M. smegmatis DnaBi1 activity. In our kanamycin intein splicing reporter (KISR), M. smegmatis DnaBi1 is inserted between residue 153 and residue 154 of the aminoglycoside phosphotransferase KanR (NCBI reference sequence WP_000018329.1) (Fig. 4A). M. smegmatis DnaBi1 splicing is strictly required for resistance, as mutation of the initiating nucleophile C-118 renders M. smegmatis cells sensitive to kanamycin (27, 34) (Fig. 4B). Oxidative stress (H2O2) and Zn2+ were both shown to reduce M. smegmatis survival in an intein-dependent manner using this reporter, and the results were confirmed in vitro (25, 27).
FIG 4.
RCS inhibit M. smegmatis DnaBi1 splicing-dependent survival in M. smegmatis cells. (A) KISR, in which M. smegmatis DnaBi1 interrupts the KanR protein and splicing is strictly required for resistance to kanamycin. (B) KanR and KISR-WT provide resistance to kanamycin. KISR-C118A, in which the initiating nucleophile C-118 of M. smegmatis DnaBi1 has been mutated, is not resistant to kanamycin. (C) NCT (0.2 mM) dramatically inhibits survival rates of M. smegmatis cells expressing KISR. Equal amounts of KanR-expressing (no intein) or KISR-expressing M. smegmatis cells were plated in the presence of kanamycin and in the presence or absence of NCT. While KanR-expressing and KISR-expressing M. smegmatis cells survive to the same extent in the absence of NCT (left), KISR-expressing cell survival rates are substantially decreased, compared to KanR-expressing cells, in the presence of NCT (right).
To test whether RCS could also inhibit M. smegmatis DnaBi1 activity in vivo, equal numbers of M. smegmatis cells expressing either KanR or KISR with wild-type (WT) M. smegmatis DnaBi1 (KISR-WT) were spread on plates with kanamycin alone or kanamycin with NCT. In the presence of kanamycin alone, M. smegmatis cells expressing KanR or KISR-WT displayed equal survival rates (Fig. 4C). As expected, NCT (0.2 mM) in addition to kanamycin led to reductions in survival rates for both KanR- and KISR-WT-expressing strains (Fig. 4C). In the presence of NCT and kanamycin, however, the survival rate for M. smegmatis with KISR-WT was substantially reduced, compared to the survival rate for M. smegmatis with KanR (Fig. 4C). Based on CFU counts from three independent experiments, this corresponded to an average 91-fold reduction in the survival rate for the KISR-WT-expressing strain. This is similar in magnitude to the effects observed previously with oxidative stress and Zn2+ (25, 27). This observed reduction was variable, with a standard deviation of 46% for the fold reduction, likely due to the volatility of NCT. The overall trend was constant, however, with the KISR-WT-expressing strain always being more sensitive with NCT and kanamycin.
DISCUSSION
DnaB is the most common intein-housing protein in bacteria (6). Previous work demonstrated that mycobacterial DnaB splicing is subject to CPS in the presence of H2O2 and Zn2+, with reversible inhibition of M. smegmatis DnaBi1 occurring in both cases (25, 27). In the case of H2O2 stress, M. smegmatis DnaBi1 forms a reversible, intramolecular disulfide bond that includes the initiating nucleophile C-118 (25). For Zn2+, this metal binds directly to M. smegmatis DnaBi1, with C-118 forming part of the binding pocket (27). Given the finding that RCS are highly reactive with cysteine side chains (30), the tendency of inteins to utilize cysteine for splicing, and the abundance of inteins in the microbial world, our findings in this work demonstrate the potential for RCS sensing by inteins in nature. Importantly, inhibition by RCS of these two DnaB inteins is reversible in the presence of reducing agent (Fig. 2 and 3). Further, our results using our in vivo KISR demonstrate that splicing of M. smegmatis DnaBi1 can be blocked in the mycobacterial cellular environment (Fig. 4).
Discovering new physiologically relevant environmental conditions that influence protein splicing is crucial to understanding the biological roles for inteins in nature. Further, inteins represent attractive drug targets because they are found within essential genes of human pathogens and are absent in metazoans (6). Toward this end, the anticancer drug cisplatin was shown to inhibit the growth of Mycobacterium tuberculosis in an intein-specific manner (35) and was shown by crystallography to bind directly to catalytic residues of the RecA intein (36). Recent evidence suggests that cisplatin also targets an intein within the essential splicesosomal Prp8 protein of the pathogen Cryptococcus neoformans (37, 38). Thus, understanding the conditions that control the splicing of inteins from pathogens such as M. leprae not only yields insights into their responses to stress but also may inform the design of intein-specific inhibitors that selectively kill them.
Bacteria encounter RCS in several natural contexts, and numerous defense mechanisms have been described (30, 31). One such environment is in cells of the mammalian innate immune system. Within the phagolysosome, MPO produces HOCl from H2O2 and chloride (31). MPO, which can represent 5% of the total protein in neutrophils (30), can produce levels of HOCl that reach 25 to 50 mM (39). M. tuberculosis and M. leprae, the agents responsible for tuberculosis and leprosy, respectively, can survive within the phagolysosome (40). The observed concentrations of RCS that inhibit M. smegmatis and M. leprae DnaB splicing are substantially (100 to 200-fold) below the levels of RCS produced within neutrophils, far below the physiological concentrations produced.
RCS are highly reactive with cysteines and can lead to several types of oxidative modification, including disulfide bond formation and production of sulfenic, sulfinic, and sulfonic acids (30). DTT is capable of reversing disulfide bonds and sulfenic acid but not further oxidative states. Because RCS can lead to general protein misfolding, we reason that we are below this threshold, as the MIG reporter requires that GFP remain folded (33). We hypothesize that catalytic cysteines of M. smegmatis DnaBi1 and M. leprae DnaBi are the targets of RCS, leading to either disulfide bonding, as observed previously (25), or sulfenic acid production, both of which are reversible.
DnaB forms a hexamer and couples ATP hydrolysis to the unwinding of the double helix during replication. It is unclear whether M. smegmatis or M. leprae DnaB can hexamerize, bind to DNA, or hydrolyze ATP prior to splicing. The DnaB inteins examined in this study are located in the ATP-coordinating P-loop of the DnaB ATPase domain. For the archaeal recombinase/ATPase RadA from Pyrococcus horikoshii, which also houses an intein within the P-loop, ATPase activity was disrupted without splicing (18). Therefore, ATP hydrolysis, and thus replication, is unlikely without prior splicing. It is unclear, however, whether other activities, such as hexamer formation or DNA binding, are possible as a precursor. Interestingly, the RadA precursor from P. horikoshii retained the ability to bind ssDNA (22).
M. smegmatis DnaBi1 appears to sense multiple oxidative stressors. In the phagolysosome, mycobacteria encounter a variety of oxidants, including H2O2, HOCl, and chloramines such as NCT. Complicating matters, H2O2 is converted to HOCl with chloride by MPO, and chloramines result from HOCl reactions with amines (30). While chloramines and H2O2 are more specific in targeting cysteines, HOCl is more reactive. Regardless of whether ROS or RCS are most import in vivo, both are effective in blocking splicing, and both modifications can be reversed under reducing conditions. Following treatment with H2O2, M. smegmatis DnaBi1 forms an intramolecular disulfide bond between the catalytic C-118 and a noncatalytic cysteine (C-48) (25). Following treatment with NCT and HOCl, a similar patten is observed, suggesting that RCS may also lead to this intramolecular disulfide bond (25).
The M. leprae DnaB intein exhibits a different inhibition pattern upon RCS treatment, resulting in a HMW product (Fig. 3). A key difference between the M. leprae and M. smegmatis inteins is the number of noncatalytic cysteines. While the M. smegmatis intein has a single noncatalytic cysteine (C-48), the M. leprae intein has three (C-48, C-81, and C-110). These cysteines are not found within the conserved splicing blocks (Fig. 1C). The HMW product is consistent in size with a multimer of precursors. Importantly, the HMW product does not appear to represent nonspecific aggregation, as splicing is restored upon DTT treatment, the product remains soluble, and GFP maintains fluorescence. We speculate that this product is most likely a multimer formed by intermolecular disulfide bonds between cysteines on different precursors. It is curious that these extra cysteines are present in the M. leprae DnaB intein, given that they are not conserved with the M. smegmatis DnaB intein or predicted to be directly involved in the splicing mechanism.
Strategies to temporarily pause DNA replication under RCS stress could be beneficial when cells encounter RCS in nature. We propose that these DnaB inteins can sense RCS to pause replisome activity under this stress, to ensure chromosomal integrity. Once this assault has passed and conditions improve, RCS-induced oxidation can be reversed, allowing splicing to occur, which in turn permits immediate replisome activity. Another oxidant, the ROS H2O2, has also been shown to reversibly block M. smegmatis DnaBi1 protein splicing (25). Notably, ROS stress has been shown in human cells to slow the progression of the replication fork in order to protect the genome from damage (41). Indeed, while RCS react more readily with cysteines, they can modify nucleic acids and thus lead to genome damage (30). The ability to detect powerful antimicrobials such as RCS and ROS and quickly pause any new replication could be important for persistence in harsh environments such as within the phagolysosome. Further, bacteria encounter stress from RCS such as HOCl and chloramines in a wide range of environments (30), where this strategy could also be beneficial. Therefore, in our model, DnaB splicing is blocked in the presence of RCS and ROS, allowing an immediate mechanism to halt new replisome formation and thus mitigate genome damage. Then, under more favorable reducing conditions, protein splicing and ultimately replication can resume. In summary, we have demonstrated that the activity of two mycobacterial DnaB inteins can be blocked by RCS, that this inhibition is fully reversible, and that M. smegmatis DnaBi1 splicing is responsive to RCS within M. smegmatis cells. These findings add to the rapidly expanding list of environmental conditions encountered by intein-containing bacteria that modulate protein splicing. Further, this work shows that mycobacterial DnaB inteins are capable of sensing a variety of stress conditions relevant to survival of the organism in nature (25, 27).
MATERIALS AND METHODS
Plasmids and strains.
Plasmids pACYC MIG DnaBi1, pACYC MIG Mle DnaBi, pMBC-283 KanR, and pMBC-283-KanR-DnaBi1WT were reported previously (25, 27). pACYC MIG DnaBi1, which expresses M. smegmatis DnaBi1 in the MIG reporter, and pACYC MIG Mle DnaBi, which expresses M. leprae DnaBi in the MIG reporter, were transformed into Escherichia coli BL21(DE3) cells for protein expression. pMBC-283 KanR and pMBC-283 KanR-DnaBi1WT were transformed into M. smegmatis MC2 155 cells as described (34) for KISR survival assays described below.
Protein expression.
Escherichia coli BL21(DE3) cells expressing either M. smegmatis DnaBi1 or M. leprae DnaBi in the MIG reporter were grown at 37°C in LB with 25 μg/ml chloramphenicol to the mid-logarithmic phase (optical density at 600 nm [OD600] of ∼0.5), and protein expression was induced by the addition 1 mM isopropyl-β-d-1-thiogalactopyranoside (GoldBio). Protein expression proceeded for 1 h at 37°C, cells were pelleted by centrifugation, and pellets were frozen at −20°C.
Protein splicing.
Cells having previously expressed the MIG reporter were resuspended in 1× phosphate-buffered saline (PBS) (137 mM NaCl, 10 mM phosphate, 2.7 mM KCl [pH 7.4]) and lysed by sonication. Insoluble material was removed by centrifugation, and the soluble lysate was used to monitor splicing. Lysates were mixed with either double-distilled water (ddH2O), HOCl, or NCT; following a 60-s incubation, ddH2O or 50 mM DTT was added as indicated in the figure legends. Reaction mixtures were then incubated for ∼20 h at 20°C to 22°C to permit splicing. Finally, products were measured using in-gel GFP fluorescence measurements, as described below.
In-gel GFP fluorescence measurements.
Reaction mixtures were mixed with Laemmli sample buffer (Bio-Rad) to a final concentration of 1×. Samples were not heated, to maintain GFP structure, and products were separated using 8% to 16% Tris-glycine TGX gels (Bio-Rad). Gels were nonreducing unless indicated in the figure legends. Fluorescent products were detected immediately following SDS-PAGE, using an Amersham Imager 680 (GE Healthcare) (33).
KISR survival assays.
M. smegmatis MC2 155 cells housing pMBC-283 KanR or pMBC-283 KanR-DnaBi1WT (25, 27, 33) were grown to stationary phase (∼3 days at 37°C) in Middlebrook 7H9 broth with albumin-dextrose-catalase (ADC) growth supplement, and cells were pelleted and resuspended in Middlebrook 7H9 broth lacking ADC growth supplement. Dilutions of OD600 of 10−2, 10−3, and 10−4 were prepared in Middlebrook 7H9 broth lacking ADC growth supplement, and 100 μl of cells was plated and grown for 5 days at 37°C. Middlebrook 7H10 agar plates lacking oleic-albumin-dextrose-catalase (OADC) growth supplement were prepared fresh on the same day as plating and contained 50 μg hygromycin B (for plasmid maintenance), 150 μg/ml kanamycin sulfate, and, where indicated, 200 μM NCT.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Marlene Belfort and Michael J. Gray for useful discussions, as well as to Markus Nagl for the gift of NCT.
This work was supported by National Institutes of Health/KY INBRE grant P20GM103436, National Institutes of Health grant R15GM143662, and by start-up funds from Murray State University to C.W.L.
Contributor Information
Christopher W. Lennon, Email: clennon1@murraystate.edu.
Kathryn T. Elliott, College of New Jersey
Jan-Ulrik Dahl, Illinois State University.
REFERENCES
- 1.Kane PM, Yamashiro CT, Wolczyk DF, Neff N, Goebl M, Stevens TH. 1990. Protein splicing converts the yeast TFP1 gene product to the 69-kD subunit of the vacuolar H+-adenosine triphosphatase. Science 250:651–657. doi: 10.1126/science.2146742. [DOI] [PubMed] [Google Scholar]
- 2.Hirata R, Ohsumk Y, Nakano A, Kawasaki H, Suzuki K, Anraku Y. 1990. Molecular structure of a gene, VMA1, encoding the catalytic subunit of H+-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem 265:6726–6733. doi: 10.1016/S0021-9258(19)39210-5. [DOI] [PubMed] [Google Scholar]
- 3.Perler FB. 2002. InBase: the intein database. Nucleic Acids Res 30:383–384. doi: 10.1093/nar/30.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novikova O, Topilina N, Belfort M. 2014. Enigmatic distribution, evolution and function of inteins. J Biol Chem 289:14490–14497. doi: 10.1074/jbc.R114.548255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulus H. 2000. Protein splicing and related forms of protein autoprocessing. Annu Rev Biochem 69:447–496. doi: 10.1146/annurev.biochem.69.1.447. [DOI] [PubMed] [Google Scholar]
- 6.Novikova O, Jayachandran P, Kelley DS, Morton Z, Merwin S, Topilina NI, Belfort M. 2016. Intein clustering suggests functional importance in different domains of life. Mol Biol Evol 33:783–799. doi: 10.1093/molbev/msv271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills KV, Johnson MA, Perler FB. 2014. Protein splicing: how inteins escape from precursor proteins. J Biol Chem 289:14498–14505. doi: 10.1074/jbc.R113.540310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood DW, Camarero JA. 2014. Intein applications: from protein purification and labeling to metabolic control methods. J Biol Chem 289:14512–14519. doi: 10.1074/jbc.R114.552653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarmiento C, Camarero JA. 2019. Biotechnological applications of protein splicing. Curr Protein Pept Sci 20:408–424. doi: 10.2174/1389203720666190208110416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Z, Aboualizadeh F, Kroll J, Akula I, Snider J, Lyakisheva A, Tang P, Kotlyar M, Jurisica I, Boxem M, Stagljar I. 2020. Split intein-mediated protein ligation for detecting protein-protein interactions and their inhibition. Nat Commun 11:2440. doi: 10.1038/s41467-020-16299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinto F, Thornton EL, Wang B. 2020. An expanded library of orthogonal split inteins enables modular multi-peptide assemblies. Nat Commun 11:1529. doi: 10.1038/s41467-020-15272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jillette N, Du M, Zhu JJ, Cardoz P, Cheng AW. 2019. Split selectable markers. Nat Commun 10:4968. doi: 10.1038/s41467-019-12891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purde V, Kudryashova E, Heisler DB, Shakya R, Kudryashov DS. 2020. Intein-mediated cytoplasmic reconstitution of a split toxin enables selective cell ablation in mixed populations and tumor xenografts. Proc Natl Acad Sci USA 117:22090–22100. doi: 10.1073/pnas.2006603117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogarten JP, Hilario E. 2006. Inteins, introns, and homing endonucleases: recent revelations about the life cycle of parasitic genetic elements. BMC Evol Biol 6:94. doi: 10.1186/1471-2148-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naor A, Altman-Price N, Soucy SM, Green AG, Mitiagin Y, Turgeman-Grott I, Davidovich N, Gogarten JP, Gophna U. 2016. Impact of a homing intein on recombination frequency and organismal fitness. Proc Natl Acad Sci USA 113:E4654–E4661. doi: 10.1073/pnas.1606416113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills KV, Paulus H. 2001. Reversible inhibition of protein splicing by zinc ion. J Biol Chem 276:10832–10838. doi: 10.1074/jbc.M011149200. [DOI] [PubMed] [Google Scholar]
- 17.Callahan BP, Topilina NI, Stanger MJ, Van Roey P, Belfort M. 2011. Structure of catalytically competent intein caught in a redox trap with functional and evolutionary implications. Nat Struct Mol Biol 18:630–633. doi: 10.1038/nsmb.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topilina NI, Novikova O, Stanger M, Banavali NK, Belfort M. 2015. Post-translational environmental switch of RadA activity by extein-intein interactions in protein splicing. Nucleic Acids Res 43:6631–6648. doi: 10.1093/nar/gkv612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topilina NI, Green CM, Jayachandran P, Kelley DS, Stanger MJ, Piazza CL, Nayak S, Belfort M. 2015. SufB intein of Mycobacterium tuberculosis as a sensor for oxidative and nitrosative stresses. Proc Natl Acad Sci USA 112:10348–10353. doi: 10.1073/pnas.1512777112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciragan A, Aranko AS, Tascon I, Iwaï H. 2016. Salt-inducible protein splicing in cis and trans by inteins from extremely halophilic archaea as a novel protein-engineering tool. J Mol Biol 428:4573–4588. doi: 10.1016/j.jmb.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Reitter JN, Cousin CE, Nicastri MC, Jaramillo MV, Mills KV. 2016. Salt-dependent conditional protein splicing of an intein from Halobacterium salinarum. Biochemistry 55:1279–1282. doi: 10.1021/acs.biochem.6b00128. [DOI] [PubMed] [Google Scholar]
- 22.Lennon CW, Stanger M, Belfort M. 2016. Protein splicing of a recombinase intein induced by ssDNA and DNA damage. Genes Dev 30:2663–2668. doi: 10.1101/gad.289280.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lennon CW, Stanger M, Banavali NK, Belfort M. 2018. Conditional protein splicing switch in hyperthermophiles through an intein-extein partnership. mBio 9:e02304-17. doi: 10.1128/mBio.02304-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lennon CW, Stanger M, Belfort M. 2019. Mechanism of single-stranded DNA activation of recombinase intein splicing. Biochemistry 58:3335–3339. doi: 10.1021/acs.biochem.9b00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley DS, Lennon CW, Li Z, Miller MR, Banavali NK, Li H, Belfort M. 2018. Mycobacterial DnaB helicase intein as oxidative stress sensor. Nat Commun 9:4363. doi: 10.1038/s41467-018-06554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green CM, Li Z, Smith AD, Novikova O, Bacot-Davis VR, Gao F, Hu S, Banavali NK, Thiele DJ, Li H, Belfort M. 2019. Spliceosomal Prp8 intein at the crossroads of protein and RNA splicing. PLoS Biol 17:e3000104. doi: 10.1371/journal.pbio.3000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woods D, Vangaveti S, Egbanum I, Sweeney AM, Li Z, Bacot-Davis V, LeSassier DS, Stanger M, Hardison GE, Li H, Belfort M, Lennon CW. 2020. Conditional DnaB protein splicing is reversibly inhibited by zinc in mycobacteria. mBio 11:e01403-20. doi: 10.1128/mBio.01403-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennon CW, Belfort M. 2017. Inteins. Curr Biol 27:R204–R206. doi: 10.1016/j.cub.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Belfort M. 2017. Mobile self-splicing introns and inteins as environmental sensors. Curr Opin Microbiol 38:51–58. doi: 10.1016/j.mib.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray MJ, Wholey WY, Jakob U. 2013. Bacterial responses to reactive chlorine species. Annu Rev Microbiol 67:141–160. doi: 10.1146/annurev-micro-102912-142520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sultana S, Foti A, Dahl JU. 2020. Bacterial defense systems against the neutrophilic oxidant hypochlorous acid. Infect Immun 88:e00964-19. doi: 10.1128/IAI.00964-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagl M, Arnitz R, Lackner M. 2018. N-Chlorotaurine, a promising future candidate for topical therapy of fungal infections. Mycopathologia 183:161–170. doi: 10.1007/s11046-017-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberger J, II, Lennon CW. 2021. Monitoring protein splicing using in-gel fluorescence immediately following SDS-PAGE. Bio-protocol 11:e4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods D, LeSassier DS, Egbunam I, Lennon CW. 2021. Construction and quantitation of a selectable protein splicing sensor using Gibson assembly and spot titers. Curr Protoc 1:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Zheng Y, Callahan B, Belfort M, Liu Y. 2011. Cisplatin inhibits protein splicing, suggesting inteins as therapeutic targets in mycobacteria. J Biol Chem 286:1277–1282. doi: 10.1074/jbc.M110.171124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan H, Pearson CS, Green CM, Li Z, Zhang J, Belfort G, Shekhtman A, Li H, Belfort M. 2016. Exploring intein inhibition by platinum compounds as an antimicrobial strategy. J Biol Chem 291:22661–22670. doi: 10.1074/jbc.M116.747824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Fu B, Green CM, Liu B, Zhang J, Lang Y, Chaturvedi S, Belfort M, Liao G, Li H. 2019. Cisplatin protects mice from challenge of Cryptococcus neoformans by targeting the Prp8 intein. Emerg Microbes Infect 8:895–908. doi: 10.1080/22221751.2019.1625727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Tharappel AM, Xu J, Lang Y, Green CM, Zhang J, Lin Q, Chaturvedi S, Zhou J, Belfort M, Li H. 2021. Small-molecule inhibitors for the Prp8 intein as antifungal agents. Proc Natl Acad Sci USA 118:e2008815118. doi: 10.1073/pnas.2008815118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Summers FA, Morgan PE, Davies MJ, Hawkins CL. 2008. Identification of plasma proteins that are susceptible to thiol oxidation by hypochlorous acid and N-chloramines. Chem Res Toxicol 21:1832–1840. doi: 10.1021/tx8001719. [DOI] [PubMed] [Google Scholar]
- 40.Holzer TJ, Nelson KE, Crispen RG, Andersen BR. 1986. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect Immun 51:514–520. doi: 10.1128/iai.51.2.514-520.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somyajit K, Gupta R, Sedlackova H, Neelsen KJ, Ochs F, Rask MB, Choudhary C, Lukas J. 2017. Redox-sensitive alteration of replisome architecture safeguards genome integrity. Science 358:797–802. doi: 10.1126/science.aao3172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.