Abstract
Radiation is considered as a promising insect pest control strategy for minimizing postharvest yield losses. Among various techniques, irradiation is a method of choice as it induces lethal biochemical or molecular changes that cause a downstream cascade of abrupt physiological abnormalities at the cellular level. In this study, we evaluated the effect of 60Co-γ radiation on various developmental stages of Zeugodacus cucurbitae Coquillett and subsequent carry-over effects on the progeny. For this purpose, we treated eggs with 30- and 50-Gy radiation doses of 60Co-γ. We found that radiation significantly affected cellular antioxidants, insect morphology, and gene expression profiles. Our results indicate that in response to various doses of irradiation reactive oxygen species, catalase, peroxidase, and superoxide dismutase activities were increased along with a significant increase in the malondialdehyde (MDA) content. We observed higher mortality rates during the pupal stage of the insects that hatched from irradiated eggs (50 Gy). Furthermore, the life span of the adults was reduced in response to 50 Gy radiation. The negative effects carried over to the next generation were marked by significantly lower fecundity in the F1 generation of the irradiation groups as compared to control. The radiation induced morphological abnormalities at the pupal, as well as the adult, stages. Furthermore, variations in the gene expression following irradiation are discussed. Taken together, our results signify the utility of 60Co-γ radiation for fruit fly postharvest management.
Keywords: Melon Fly, Tephritidae, antioxidant enzymes, reactive oxygen species, apoptosis, gene expression
Over 250 different fruits and vegetables are known to be infested by fruit flies. They are transported as eggs, larvae, or pupae hidden inside fruits, vegetables, and other food packages (Louzeiro et al. 2021). The distribution of Zeugodacus cucurbitae Coquillett, especially on its main hosts, cucumber (Cucumis sativus), watermelon (Citrullus lanatus), and pumpkin (Cucurbita pepo), is primarily across subequatorial Africa, Mauritius, India, Ceylon, Southeast Asia, Japan, and Guam (Ronald et al. 2007, Hu et al. 2008). In China, it is mainly distributed across Hainan, Jiangsu, Yunnan, Fujian, Taiwan, Henan, Shanxi, Gansu provinces, and several other localities (Huang et al. 2020). Among several factors that contribute to the success of Fruit as one of the most economically important pests throughout the world is primarily due to their ability to spread rapidly under favorable environmental conditions. The infestation of fruit flies has led to the disruption of the import and export of food and other agricultural products (Huang et al. 2020). The Consultative Group on International Agricultural Research (CGIAR) proposed a dose of gamma radiation of 150 Gy for fruit flies and 300 Gy for other insects (Follett and Chemistry 2004). However, subsequent work has shown that the effective doses of radiation may vary among species. For example, 232 Gy is recommended for oriental fruit moths (Grapholita molesta) (Hallman 2004), whereas a dose of 100 Gy has been shown to provide a high level of quarantine security against Ceratitis capitata, by preventing adult emergence (Torres-Rivera and Hallman 2007).
Radiation exposure induces sub-lethal biochemical or molecular effects that cause a downstream cascade of abnormal physiological changes. Insects being eukaryotic organisms have developed a sophisticated and complex enzymatic and no-enzymatic antioxidant defence system to prevent damage by reactive oxygen species (ROS) (Felton et al. 1995, Yang et al. 2010). Several antioxidant enzymes prevent damage to cellular organelles, protein and DNA by oxidative detoxification (Felton et al. 1995, Salehi et al. 2020). The most important antioxidant enzymes in insects include the catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), glutathione peroxidase (GPX), and glutathione S-transferase (GST) (Felton et al. 1995, Wang et al. 2001, Dubovskiy et al. 2008). The SOD catalyzes the dismutation of superoxide anions (O2−) into hydrogen peroxide (H2O2) and oxygen (O2), and both CAT and POD convert H2O2 into water and oxygen.
Significant changes in the expression of several genes have been observed in adult insects following treatment with sterilizing doses of radiation at the pupal stage (Chang et al. 2015). Following X-ray irradiation of B. dorsalis pupae, significant variations in the accumulation of some proteins were observed. These include as many as 26 different proteins in males and 31 different proteins in females. These encode the glyceraldehyde-3-phosphate dehydrogenase, fructose-bisphosphate aldolase, larval cuticle protein 2, sarcoplasmic calcium-binding protein alpha-B and A chains, general odorant-binding protein 99b, polyubiquitin, and protein disulfide-isomerase and other key proteins that are impacted by radiation. These proteins act in central energy generating and in pheromone-signal processing pathways. Variations in the accumulation of these proteins contributed to an overall reduction in mating ability and survival (Chang et al. 2015). More recently, in 2018, Li et al. investigated the effects of 200 Gy 60Co-γ radiation on the regulation of antioxidant enzymes of Plutella xylostella. They reported a significant increase in the expression of SOD and catalase (CAT) enzymes with a significant decrease in peroxidase and glutathione S-transferase (GST) enzymes. In addition, they also reported a 2-fold increase in the accumulation of heat shock protein HSP70, along with a significant increase in the accumulation of testis lactate dehydrogenase (LDH) and acid phosphatase (ACP) proteins in male adults, thereby affecting reproduction and survival (Li et al. 2018). These findings indicate significant effects of radiation on various protein levels in the insect body. Radiation-induced morphological abnormalities in insects are common. Tungjitwitayakul et al. (2019) reported that UV-C radiation during the pupal stage affects morphological changes of wings in Tribolium casteneum along with a decrease in adult emergence and insect body mass, and changes in elytra and hind wings such as wrinkled or split elytra and improper folding of hind wings. In another recent paper, Tanaka et al. (2020) describe the morphological abnormalities observed in lepidopteran insects following the Fukushima Dai-ichi Nuclear Power Plant (FDNPP) accident in Japan. They report shrinkage of appendages and aberration of wings as a result of radiation exposure. In addition, it is critical to understand the currently unknown effects of radiation on insect morphology (such as variations in size of elytra, hindwings, and wing shape), physiology, and behavior.
The aims of this study were 1) to assess the physiological effects of radiation and variations in the antioxidant enzyme activity in different life stages of Z. cucurbitae, 2) to study the effects of radiation on the midgut cells of Z. cucurbitae, and 3) to determine the changes in expression of oxidative stress-related genes.
Materials and Methods
Insect Rearing
Zeugodacus cucurbitae was reared under laboratory conditions (temperature: 24 ± 1°С; photoperiod (L:D): 14:10 h; humidity: 50 ± 5%) in the insectary of the School of Plant Protection, Hainan University. Cucumber slices were placed in Petri dishes inside adult fruit fly cages to collect eggs. Adult flies were fed with a mixture of sugar and yeast in a ratio of 3:1. Pupae in a Petri dish were placed in 45 × 45 × 50 cm cages for adult emergence (Ahmad et al. 2021). After hatching, the first instar from the control and irradiated treatments was given an artificial diet consisting of wheat bran (80 g), inactive dry yeast (16 g), sugar (32 g), agar powder (16 g), water (1000 ml), and 3.7 % formalin (HCHO) (10 ml) (Somogyi et al. 2007).
60Co-γ Radiation
For experimental purposes, eggs of approximately 25 h old were irradiated with 60Co-γ at the following doses, under free oxygen at a rate of 1.0 Gy/min: 0 Gy (control), 30 Gy, and 50 Gy (Cai et al. 2018). The experiments were performed in six biological replicates and each replicate comprised 100 eggs in a single glass Petri dish size 100 mm diameter and 15 mm height. The eggs were first covered with a wet filter paper to avoid desiccation and damage to the eggs, as well as facilitate better hatching. Effects of the radiation on the first, second, and third instar, pupae, and adults were assessed through visual observations. The assessment was done every second day, until the adults reached sexual maturity and continued until the emergence. Three of the six replicates were used for collecting data on parameters (egg hatching, development time duration, F1 generation fecundity test, and mortality).
Determination of Antioxidant Enzyme Activities
The antioxidant enzyme activity was determined using specific enzyme extraction kits following the manufacturer’s recommended protocols (Solarbio Life Sciences, Beijing), with slight modifications. Three replicates was performed where in a single replicate 10 larvae of the first, second, and third instars, as well as five 2-d-old pupae were randomly selected from the control and irradiated treatments and washed with distilled water. After washing, enzymes from the pupae and larvae were extracted using the kit for each activity according to the manufacturer’s instructions. First, the samples were homogenized in an extra solution of the CAT enzyme kit. The homogenized samples were then centrifuged at 8000 rpm for 10 min at 4°C, and the supernatant was used for determining antioxidant enzyme activity. Enzyme concentrations were calculated in accordance with the method described in Bradford (1976), utilizing serum albumin as the standard.
CAT activity was calculated using a spectrophotometer at a wavelength of 240 nm following the procedure of a previously reported method (Sezer and Ozalp 2015). H2O2/mg of protein in every second was considered as one unit of CAT activity. CAT activity was described as U/mg protein.
For POD activity, the spectrophotometer was fixed at a wavelength of 470 nm following the protocol of a previously published method (Wang et al. 2013). POD activity was expressed as U/mg protein.
SOD activity was evaluated at a wavelength of 560 nm for 30 s after the addition of xanthine oxidase as start reagent and 3 min after reaction as duplicate samples following the method described in Tian et al. (2013). One unit of SOD activity was expressed as ‘the amount of enzyme required to inhibit half of the xanthine and xanthine oxidase reaction for 1 mg protein extract’. SOD activity was expressed as U/mg protein.
Determination of ROS
Ten larvae of the first, second, and third instars, along with five 2-d-old pupae were randomly selected and washed with distilled water. The samples were homogenized in an extra solution of the CAT enzyme kit according to the company’s instruction. The homogenates were centrifuged at 3000 rpm/min for 5 min at 25°C, and the resultant supernatant was used for the colorimetric quantitative determination of diffused ROS. The common probe 2′, 7′-dichlorofluorescein diacetate was used to detect ROS after incubation of the resultant supernatant (190 μl) diluted with working solution (10 μl) in the presence of 1 mM 2′, 7′-dichlorofluorescein diacetate fluorescent dye in the dark at 37°C for 30 min. ROS was measured with a Tecan Infinite M200 microplate reader (Männedorf, Switzerland) using excitation at 485 nm and emission at 535 nm (Zhang et al. 2019).
Determination of MDA Contents
MDA content was measured according to the instructions of the kits’ manufacturers (Solabrio life sciences, Beijing). A 1-ml portion of a 0.1% tricholoroacetic acid (TCA) solution was added to a 0.1-g sample of flies, and the mixture was centrifuged at 12,500 rpm for 20 min at 5°C. A 0.5-ml portion of the supernatant was then withdrawn and a 1-ml portion of 0.5% thiobarbituric acid (TBA) in 20% TCA was added, before the solution was incubated at 98°C for 30 min. The absorbance at 532 and 600 nm was then measured using a spectrophotometer.
Irradiation of Z. cucurbitae Eggs
About 1-h-old eggs were first incubated for 25 h. Then these eggs were irradiated at 30 and 50 Gy. A sample of 100 eggs kept on a wet filter paper inside a Petri dish was used to determine the hatching percentage for each treatment. At 23 h, the number of un-hatched eggs was recorded under a dissecting microscope. Hatching percentage post-treatment was recorded for at least three replicates of each treatment. Next, we evaluated the quality of the Z. cucurbitae raised from the irradiated eggs, from their first-instar stage until their adult stage. During each stage, the mortality rate was recorded until adult emergence. The flies emergence percentage was calculated at each stage, as the number of emerged (normal plus deformed flies) divided by 100* total number. Insect development time at each stage was evaluated throughout the experiment. To ascertain the fecundity test of the F1 generation, sexually mature 10 male and 20 female flies were kept in adults rearing cages performing three replicates for each treatment and control group and were allowed to mate. Number of eggs laid and hatched were counted for five consecutive days.
Midgut Observation via Transmission Electron Microscopy (TEM)
The midgut was extracted from 20 larvae (third instar), both from the treated and control groups, and visualized under the light microscope. For the TEM examinations, midguts were embedded in 4% agar and fixed in 2.5% glutaraldehyde at 4°C for 4 h. The fixed specimens were treated with 1% OsO4 for 2 h after washing with 0.1 M sodium-phosphate buffer (PB). After 2 h, the samples were again washed with 0.1 M PB and serially dehydrated with ethanol. Finally, the samples were embedded in Epon and cut into approximately 2-µm thick semi-thin sections. Samples were stained in uranyl acetate and lead citrate and the midguts were examined with a transmission electron microscope (JEM-1010, JEOL, Tokyo, Japan) at 100 kV accelerating voltage (Zhang et al. 2019).
Quantitative Real-Time PCR
For real-time PCR analysis, pupal RNA samples were analyzed. Total RNA was extracted from five pupae performing three replicates with a TRIzol RNA Reagent kit (Thermo Scientific, MA, USA). The 3.0-µl samples of RNA were reverse transcribed into cDNA using the Prime Script TM RT reagent kit with gDNA Eraser (Perfect Real Time). Primers Premier 3.0 was used to design the primers (Supp Table S1 [online only]). The SYBRr Premix Ex TaqTM II, 1.0 µl of cDNA, and 0.5 µl of primers were mixed and amplified in a Bio-Rad iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The relative expressions of the genes of treated and untreated samples were calculated by normalization via t-score of the endogenous reference gene EFα1. The expression values were normalized based on the 2−∆∆Ct method (Chen and Wagner 2012).
Statistical Analysis
All data were statistically analyzed using two-way analysis of variance (ANOVA) with Tukey’s test to measure the significant differences between each different group. All the data were quantified using two-way ANOVA with a 0.05 level of significance (95% confidence interval) using GraphPad Prism 8.01 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Effects of Radiation on Catalase Activity
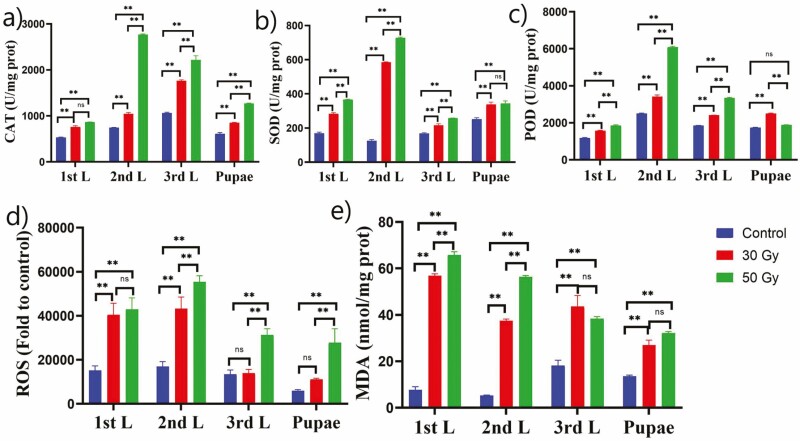
Treatment of Z. cucurbitae eggs with doses (both 30 and 50 Gy) of 60Co-γ radiation led to significant alteration of CAT activity in larval and pupal stages (Fig. 1a). Radiation doses significantly induce the CAT activity compared to the control group. The highest CAT activity was observed at the second-instar post-irradiation with 50-Gy doses, whereas at 30-Gy doses, the highest CAT activity was recorded at the third instar compared to the control group (Fig. 1a).
Fig. 1.
Effects of 60Co-γ irradiation on (a) CAT, (b) SOD, (c) POD, (d) ROS activity, and (e) MDA content of Z. cucurbitae larval stages and pupae at different radiation doses. The values are presented as the mean ± SD. Treatments were compared using two-way ANOVA (Tukey’s test, P < 0.05) with a 0.05 level of significance (95% confidence interval). The symbol (**) represents P < 0.05, (ns) non-significance.
Effects of Radiation on Superoxide Dismutase
Radiation induced SOD radicals in Z. cucurbitae at different instars and pupae, and this SOD activity was probably induced as a response to increased ROS generation. Thus, SOD activity in larval stage and pupae was altered in eggs of Z. cucurbitae irradiated with 30 and 50 Gy. Additionally, SOD activity was substantially increased in the second instar that received a radiation dose, regardless of amount, as shown in Fig. 1b. Based on the following results, irradiation greatly induced the activity of SOD in larvae and pupae. The highest SOD activity was observed at the second instar for both the radiation doses 30 and 50 Gy compared to the control group (Fig. 1b).
Effects of Radiation on Cellular Peroxides
POD activity in larval and pupal stages changed after eggs of Z. cucurbitae were irradiated with 30 Gy and 50 Gy (Fig. 1c). Results showed that irradiation greatly induced the activity of POD in larvae and pupae. The highest significant POD activity was observed at the second instar with both radiation doses 30 and 50 Gy followed by the third instar and pupae compared to the control group (Fig. 1c).
Effects of Radiation on Cellular ROS Levels
The results of this study further indicated that ROS activity was significantly induced by radiation doses of 30 and 50 Gy in Z. cucurbitae larvae and pupae (Fig. 1d). ROS activity in larvae and pupae was significantly increased in Z. cucurbitae that were irradiated with 30 and 50 Gy (Fig. 1d). For both the radiation doses, the highest ROS activity were observed at the second instar followed by the first instar and third instar compared to the control group (Fig. 1d).
Effects of Radiation on MDA Content
MDA activity in larvae and pupae changed after eggs of Z. cucurbitae were irradiated with 30 and 50 Gy (Fig. 1e). Significant induction of MDA content was recorded at both radiation doses 30 and 50 Gy compared to the control group. The highest MDA content was induced at the second instar followed by the first instar and third instar.
Effects of Radiation on Various Life Parameters of Z. cucurbitae
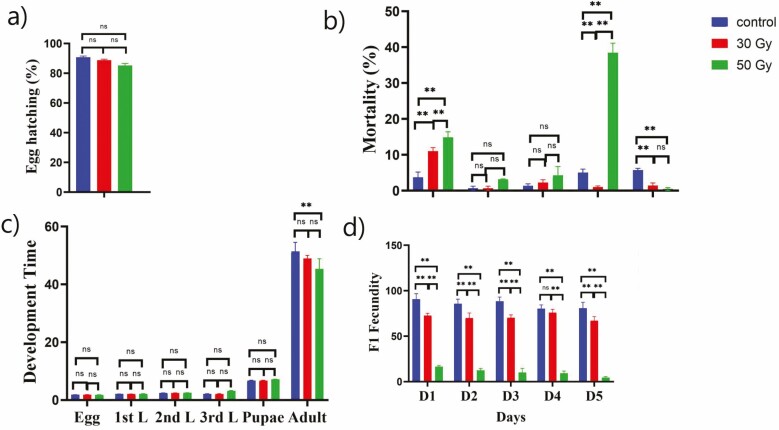
Results indicated that 60Co-γ radiation had no significant effect on the hatching of eggs (Fig. 2a). The egg hatching percentages ranged from 84 to 96% in the control group and from 80 to 89 % in the other treatment groups.
Fig. 2.
Effects of 60Co-γ irradiation on (a) hatching rate (%) of eggs; (b) mortality rate (%) at the larval, pupal and adult stages; (c) development time duration of the larval, pupal and adult stages; and (d) F1 fecundity. The values are presented as the mean ± SD. Treatments were compared using two-way ANOVA (Tukey’s test, P < 0.05) with a 0.05 level of significance (95% confidence interval). The symbol (**) represents P < 0.05, (ns) non-significance.
The mortality of flies at different stages during their life cycle is shown in Fig. 2b. Egg irradiation showed a tendency to increase the mortality at the highest radiation level (50 Gy) at pupal stage approximately (40 %). During the early life stages of Z. cucurbitae, the mortality rates for both females and males hatched from eggs treated with 50 and 30 Gy were higher than that in the control. A significantly increased rate of mortality was recorded during the pupal stage, in which most of the pupae died and turned black or malformed. The mortality rate at the pupal stage was more than 40% higher for flies treated with 50 Gy, as compared to that of the control group. Also significant mortality was recorded for both the radiation doses, as shown in Fig. 2b. Notably, radiation had no significant effect on the development time except for 50 Gy dose at adult stage compared to the non-irradited flies of Z. cucurbitae (Fig. 2c). Fertility test was carried out after the irradiated insects were reared until adulthood and then were allowed for mating. Fertility test for five consecutive days with three biological replicates was recorded. The F1 fecundity has been significantly decreased for all days when parental generation was exposed to the 50 Gy radiation dose (Fig. 2d).
Effects of Irradiation on Morphological Features of Z. cucurbitae
Radiation resulted in significantly higher mortality at the pupal-adult stage. Due to both radiation doses, most of the adults failed to shed their old cuticle and died before the normal emergence, and the old cuticle was found attached to their abdomen and did not completely transition into the adult stage. Both male and female pupae were found to be dead and malformed for both the radiation doses compared to the non-irradiated group (Fig. 3).
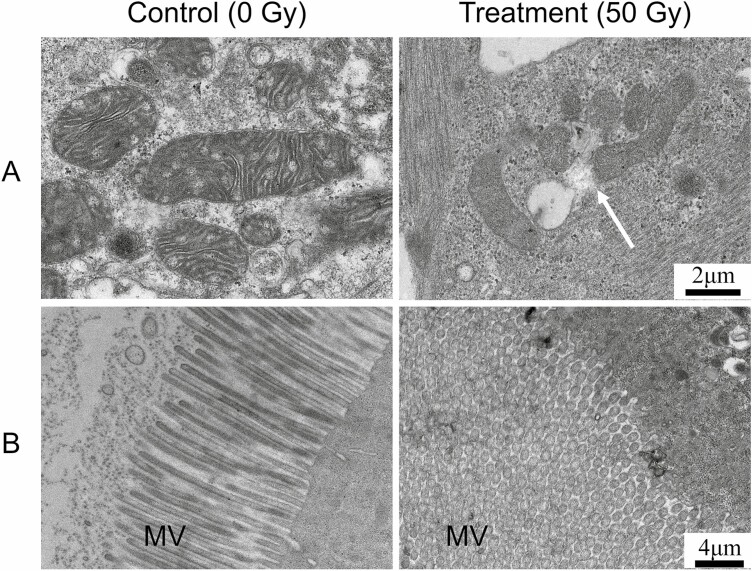
Fig. 3.
Malformation caused by 60Co-γ radiation in pupal-adult stage of Zeugodacus cucurbitae after eggs were exposed to 30 and 50 Gy of 60Co-γ gamma radiation. The arrow shows that flies were failed to shed their old cuticle and were attached with their abdomen.
Effects of Radiation on Z. cucurbitae Midgut Cells
The ultrastructural observations of midgut cells of Z. cucurbitae larvae showed that the mitochondrial structure and microvilli were damaged (Fig. 4A and B). The ROS activity has been significantly increased in larval stages following exposure to the 30 and 50 Gy irradiations, which might affect the mitochondrial structure and microvilli in Z. cucurbitae.
Fig. 4.
Ultrastructural observations of midgut cells of Z. cucurbitae larvae. (A) Observation of the structure of the mitochondria. Magnification: 8000×; scale: 2 μm. (B) Observation of the structure of the microvilli (MV). Magnification: 5000×; scale: 4 μm.
60Co-γ Radiation Induces in Expression of Apoptosis-Related Genes
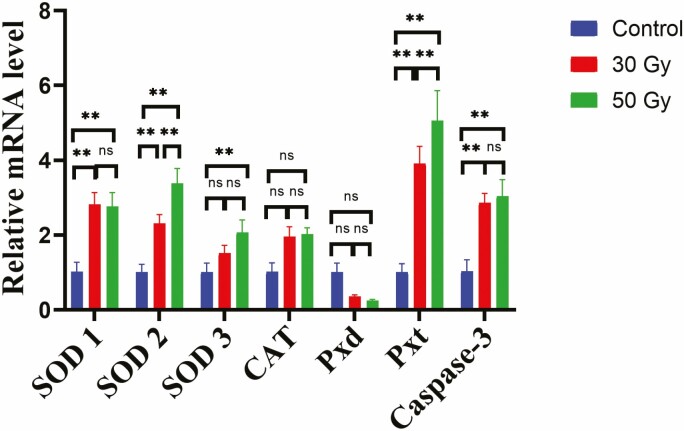
To determine the effect of 60Co-γ radiation at the transcriptional level, the expression of seven apoptosis related genes was measured by RT-qPCR. Among these seven genes, six were significantly upregulated in flies treated with 50 and 30 Gy compared to those in the control (non-irradiated flies), with the exception of SOD-3, which did not differ significantly between the control flies and the 30 Gy (Fig. 5). Pxd expression was significantly downregulated at the 30 and 50 Gy dose compared to that in the control group but the changes are statistically not significant (Fig. 5).
Fig. 5.
Expression levels of seven oxidative stress related genes: Cu/ZnSOD (SOD1), Cu/ZnSOD (SOD2), MnSOD (SOD3), catalase (CAT), peroxidase (Pxd), peroxidase-4 (Pxt), and caspase-3 in the pupal stage of Z. cucurbitae after 60Co-γ radiation. The values are presented as the mean ± SD. Gene expression was compared using two-way ANOVA (Tukey’s test, P < 0.05) with a 0.05 level of significance (95% confidence interval). The symbol (**) represents P < 0.05, (ns) non-significance.
Discussion
Phytosanitary irradiation is the use of ionizing radiation on agricultural commodities, such as fruits and vegetables to inactivate insect pests. The use of this strategy is increasing globally and is now considered a promising pest control measure. In this context, this study was devised to look for the physiological effects of radiation and variations in the antioxidant enzyme activity in different life stages of Z. cucurbitae, and to study the effects of radiation on the midgut cells of Z. cucurbitae and to determine the changes in expression of oxidative stress-related genes.
Our results indicate that radiation could induce the expression of oxidative stress–related genes SOD, CAT, and POD, as well as activate and coordinate with the antioxidants system to counteract the oxidative stress produced by the maximum production of ROS inside the cells. The expression of SOD3 and caspase-3 was significantly upregulated in the irradiated group as well, which may be because of mitochondria and microvilli impairment and damage, and their subsequent involvement in apoptosis. The oxidative stress index was systematically examined to further confirm the hypothesis, wherein, SOD, CAT, and POD activities were significantly increased in different stages of Z. cucurbitae after irradiation. In addition, generally with a low amount of ROS stress, the defense enzyme system increases the activities of enzymes; but, after irradiation, a large amount of ROS was generated and exceeded the range of tolerance of the organisms, resulting in a thorough inhibition of enzyme activities. Our findings indicated that 60Co radiation caused cell death and we concluded that the midgut cells had been subjected to high oxidative stress. In addition, a significant increase of MDA content was recorded in all stages of Z. cucurbitae treated with both 30 and 50 Gy, as compared to that of the control group, an indication of oxidative damage.
The CAT enzyme(s) protect the cells from oxidative stress and increases the life span of insects (Stadtman 1986). In the current study, CAT activity increased dramatically (Fig. 1a) and additionally, there was a significant increase in POD activity across all analyzed developmental stages of Z. cucurbitae after eggs were irradiated with 30 and 50 Gy. Previous studies reported that irradiation increased SOD, CAT, and POD activities to reduce the oxidative stress in Helicoverpa armigera adults (Meng et al. 2009). Moreover, enhanced production of ROS in response to 60Co-γ irradiation may result in the induction of antioxidant enzymes.
The increased activities of SOD, CAT, POD, and MDA under 60Co-γ irradiation might be a defense against oxidative damage owing to ROS accumulation. In previous studies, it was observed that after irradiation, MDA levels were significantly increased in all organs of rats (John et al. 2001). This is consistent with our results, because we found significantly increased MDA content in all larval stages, as well as pupae, of radiation-treated Z. cucurbitae. A radiation treatment of 50 Gy decreased the viability of egg hatching when the irradiated eggs were reared until adulthood and were allowed for mating; the fertility test shows that irradiation reduced significantly eggs laying and hatching for both the radiation doses compared to the non-irradiated group. Further, due to the radiation, highest mortality was observed 40% at pupal adult stage, where the flies failed to shed their old cuticle and were found attached to their abdomen (Fig. 2b). Radiation directly affects insect behavior, biochemistry, and developmental physiology as it significantly increases oxidative stress (Gunn 1998, Mackerness et al. 1999, Mazza et al. 2002). Results of this study confirmed that radiation causes cell damage, which leads to induced DNA damage in organisms via the production of DNA lesions, such as cyclobutane pyrimidine dimmers (CPD) and 6,4-photoproducts or 6,4-pyrimidine-pyrimidones (Sinha et al. 2002, Cadet and Wagner 2013, Douki et al. 2017). The accretion of these DNA lesions inhibits transcription and replication of DNA due to a malformed double-helix structure, which ultimately causes death (Murata and Osakabe 2017).
The morphological and biochemical malformation was observed in this study when eggs were irradiated with 30 and 50 Gy doses, which may be directly linked to a higher production of ROS that caused mitochondrial damage and led to cell death. Indeed, under various types of stress, such as 60Co-γ radiation, ROS levels may augment dramatically and result in oxidative stress (Meng et al. 2009, Nikolić et al. 2015). Thus, the radiation doses reported in this study can be considered to be useful in the context of phytosanitary treatments of fruit commodities to be exported to some country/zone free of fruit fly pests. In our study, it was observed for the first time that the protective enzymes in the fruit fly, Z. cucurbitae, are affected when exposed to radiation, and thus, antioxidants may play a crucial role in maintaining the integrity of the body. Therefore, we assert that irradiation treatments have severe effects on Z. cucurbitae which can be used as a mass storage and transportation of agricultural products.
Conclusion
In conclusion, the results of this study provide important insight into the mass rearing and phytosanitary management against Z. cucurbitae and highlight that a radiation dose of 30 and 50 Gy is the most effective against the melon fly. Additionally, ROS activity significantly increased in all studied stages of Z. cucurbitae, and subsequently, antioxidant enzyme activities and their genes’ expression also significantly increased, thereby confirming our hypothesis that the induction of antioxidant enzyme activities caused the overproduction of ROS. Thus, the radiation doses reported in this study can be considered to be useful in the context of phytosanitary treatments of fruit commodities to be exported to some country/zone free of fruit fly pests. However, the interaction between antioxidant activities and mRNA expression of antioxidant enzymes, along with the factors leading to the differential gene expression or regulation of antioxidant enzyme genes, is still need to be elaborated and further research on this area is needed.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China(31860513 and 31760526) and Natural Science Foundation of Hainan (2019RC121).
Authors’ Contributions
This work was carried out in collaboration between all authors. Author S.A. and Y.L. designed the study, and wrote the experimental procedures and the first draft of the manuscript. Author S.A. performed the studies and supervised by author Y.L. Authors S.A., F.U., and M.J. performed the statistical analysis. Authors A.H., A.A., and S.A. revised the manuscript. All authors read and approved the final manuscript.
References Cited
- Ahmad, S., Jamil M., Fahim M., Zhang S., Ullah F., Lyu B., and Luo Y.. . 2021. RNAi-Mediated Knockdown of Imaginal Disc Growth Factors (IDGFs) genes causes developmental malformation and mortality in melon fly, Zeugodacus cucurbitae. Front. Genet. 12: 691382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, M.. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Cadet, J., and Wagner J.. . 2013. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 5: a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, P., Hong J., Wang C., Yang Y., Yi C., Chen J., and Ji Q.. . 2018. Effects of Co-60 radiation on the activities of three main antioxidant enzymes in Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). J. Asia-Pacific Entomol. 21: 345–351. [Google Scholar]
- Chang, C. L., Villalun M., Geib S. M., Goodman C. L., Ringbauer J., and Stanley D.. . 2015. Pupal X-ray irradiation influences protein expression in adults of the oriental fruit fly, Bactrocera dorsalis. J. Insect Physiol. 76: 7–16. [DOI] [PubMed] [Google Scholar]
- Chen, B., and Wagner A.. . 2012. Hsp90 is important for fecundity, longevity, and buffering of cryptic deleterious variation in wild fly populations. BMC Evol. Biol. 12: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douki, T., von Koschembahr A., and Cadet J.. . 2017. Insight in DNA repair of UV-induced pyrimidine dimers by chromatographic methods. Photochem. Photobiol. 93: 207–215. [DOI] [PubMed] [Google Scholar]
- Dubovskiy, I., Martemyanov V., Vorontsova Y., Rantala M., Gryzanova E., and lupov V.. . 2008. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comp. Biochem. Physiol. C Toxicol. 148: 1–5. [DOI] [PubMed] [Google Scholar]
- Felton, G. W., and Summers C. B.. . 1995. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 29: 187–197. [DOI] [PubMed] [Google Scholar]
- Follett, P. 2004. Irradiation to control insects in fruits and vegetables for export from Hawaii. Radiat. Phys. Chem. 71: 163–166. [Google Scholar]
- Gunn, A. 1998. The determination of larval phase coloration in the African armyworm, Spodoptera exempta and its consequences for thermoregulation and protection from UV light. Entomol. Exp. Appl. 86: 125–133. [Google Scholar]
- Hallman, G. J. 2004. Ionizing irradiation quarantine treatment against oriental fruit moth (Lepidoptera: Tortricidae) in ambient and hypoxic atmospheres. J. Econ. Entomol. 97: 824–827. [DOI] [PubMed] [Google Scholar]
- Hu, J., Zhang J. L., Nardi F., and Zhang R. J.. . 2008. Population genetic structure of the melon fly, Bactrocera cucurbitae (Diptera: Tephritidae), from China and Southeast Asia. Genetica 134: 319. [DOI] [PubMed] [Google Scholar]
- Huang, Y., Gu X., Peng X., Tao M., Peng L., Chen G., and Zhang X.. . 2020. Effect of short-term low temperature on the growth, development, and reproduction of Bactrocera tau (Diptera: Tephritidae) and Bactrocera cucurbitae. J. Econ. Entomol. 113: 2141–2149. [DOI] [PubMed] [Google Scholar]
- John, S., Kale M., Rathore N., and Bhatnagar D.. . 2001. Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J. Nutr. Biochem. 12: 500–504. [DOI] [PubMed] [Google Scholar]
- Li, X., Luo L., Karthi S., Zhang K., Luo J., Hu Q., and Weng Q.. . 2018. Effects of 200 Gy 60Co-γ radiation on the regulation of antioxidant enzymes, Hsp70 genes, and serum molecules of Plutella xylostella (Linnaeus). Molecules 23(5): 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louzeiro, L. R. F., Souza-Filho M. F. D., Raga A., and Gisloti L. J.. . 2021. Incidence of frugivorous flies (Tephritidae and Lonchaeidae), fruit losses and the dispersal of flies through the transportation of fresh fruit. J. Asia-Pacific Entomol. 24: 50–60. [Google Scholar]
- Mackerness, S. A. H., Surplus S., Blake P., John C., Buchanan-Wollaston V., Jordan B., Thomas B.. . 1999. Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: role of signalling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant Cell Environ. 22: 1413–1423. [Google Scholar]
- Mazza, C. A., Izaguirre M. M., Zavala J., Scopel A. L., and Ballaré C. L.. . 2002. Insect perception of ambient ultraviolet-B radiation. Ecol. Lett. 5: 722–726. [Google Scholar]
- Meng, J.-Y., Zhang C.-Y., Zhu F., Wang X.-P., and Lei C.-L.. . 2009. Ultraviolet light-induced oxidative stress: effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 55: 588–592. [DOI] [PubMed] [Google Scholar]
- Murata, Y., and Osakabe M.. . 2017. Developmental phase-specific mortality after ultraviolet-B radiation exposure in the two-spotted spider mite. Environ. Entomol. 46: 1448–1455. [DOI] [PubMed] [Google Scholar]
- Nikolić, T. V., Purać J., Orčić S., Kojić D., Vujanović D., Stanimirović Z., Gržetić I., Ilijević K., Šikoparija B., and Blagojević D. P.. . 2015. Environmental effects on superoxide dismutase and catalase activity and expression in honey bee. Arch. Insect Biochem. Physiol. 90: 181–194. [DOI] [PubMed] [Google Scholar]
- Ronald, F., Jayma L., and Martin K.. . 2007. Bactrocera cucurbitae (Coquillett). Crop. Knowledge. Master. 4: 1–5. [Google Scholar]
- Salehi, B., Azzini E., Zucca P., Maria Varoni E., Anil Kumar N. V., Dini L., Panzarini E., Rajkovic J., Valere Tsouh Fokou P., Peluso I., . et al. 2020. Plant-derived bioactives and oxidative stress-related disorders: a key trend towards healthy aging and longevity promotion. Appl. Sci. 10: 947. [Google Scholar]
- Sezer, B., and Ozalp P.. . 2015. Effects of pyriproxyfen on hemocyte count and morphology of Galleria mellonella. Fresenius Environ. Bull. 24: 621–625. [Google Scholar]
- Sinha, R. P., and Häder D. P.. . 2002. UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 1: 225–236. [DOI] [PubMed] [Google Scholar]
- Somogyi, A., Rosta K., Pusztai P., Tulassay Z., and Nagy G.. . 2007. Antioxidant measurements. Physiol. Meas. 28: R41. [DOI] [PubMed] [Google Scholar]
- Stadtman, E. 1986. Oxidation of proteins by mixed-function oxidation systems: implication in protein turnover, ageing and neutrophil function. Trends Biochem. Sci. 11: 11–12. [Google Scholar]
- Tanaka, S., Kinouchi T., Fujii T., Imanaka T., Takahashi T., Fukutani S., Maki D., Nohtomi A., and Takahashi S.. . 2020. Observation of morphological abnormalities in silkworm pupae after feeding 137CsCl-supplemented diet to evaluate the effects of low dose-rate exposure. Sci. Rep-UK. 10: 16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, Y., Ma W., Wei Q., Luo S., Han X., Chen X., Qiu B., and Ma L.. . 2013. Effects of α-terpineol fumigation on the in vivo antioxidant activity in Zophobas morio (Coleoptera: Tenebrionidae). Acta Entomol. Sin. 56: 47–53. [Google Scholar]
- Torres-Rivera, Z., and Hallman G.. . 2007. Low-dose irradiation phytosanitary treatment against Mediterranean fruit fly (Diptera: Tephritidae). Fla. Entomol. 90: 343–346.
- Tungjitwitayakul, J., Yasanga T., and Tatun N.. . 2019. UV-C radiation during the pupal stage affects morphological changes of wings in Tribolium castaneum (Col; Tenebrionidae). Int. J. Radiat. Biol. 95: 1309–1318. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Oberley L. W., and Murhammer D. W.. . 2001. Antioxidant defense systems of two lipidopteran insect cell lines. Free Radic. Biol. Med. 30: 1254–1262. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Guo J., Chen J., and Ji Q.. . 2013. The changes of the activity of four enzymes in larvae of Bactrocera dorsalis (Hendel) parasitized by Diachasmimorpha longicaudata. Chin. J. Trop. Crops. 34: 335–338. [Google Scholar]
- Yang, L.-H., Huang H., and Wang J.-J.. . 2010. Antioxidant responses of citrus red mite, Panonychus citri (McGregor)(Acari: Tetranychidae), exposed to thermal stress. J. Insect Physiol. 56: 1871–1876. [DOI] [PubMed] [Google Scholar]
- Zhang, J., Ahmad S., Wang L.-Y., Han Q., Zhang J.-C., and Luo Y.-P.. . 2019. Cell death induced by α-terthienyl via reactive oxygen species-mediated mitochondrial dysfunction and oxidative stress in the midgut of Aedes aegypti larvae. Free Radic. Biol. Med. 137: 87–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.