Abstract
This study aimed to investigate the effects of iron, vitamin A (VA) and their interaction on intestinal development and differentiation of cells in suckling piglets. Therefore, 32 Duroc × Landrace × Yorkshire 0-d-old newborn boars with similar body weights were randomly divided into four groups, with eight replicates in each group and one pig in each replicate. All the piglets were breastfed. In addition, the piglets were given normal saline (CON group) or ferrous sulfate (OAFe group) or VA (VA group) or ferrous sulfate and VA (OAFe + VA group) on the 2nd, 7th, 12th, and 17th day, respectively. The piglets were then slaughtered on the 21st day, and intestinal samples were collected. The results showed that: 1) iron (P < 0.001) significantly increased the length, weight, relative weight, and the length to weight ratio of the small intestine. On the other hand, VA had a significant effect on the weight to length ratio (P = 0.015) and relative weight (P < 0.001) of the small intestine; 2) with regard to intestinal morphology, supplementation with iron (P <0.05) had obvious effects on the villus height (VH), crypt depth (CD), villus width (VW), and surface area. Additionally, both VA and interaction of VA and iron increased the VH (P < 0.05) and surface area (P = 0.001). The results also showed that iron (P < 0.01) increased the number of crypt goblet cells, Ki67-positive cells, and endocrine cells. Moreover, both VA and the interaction between VA and iron increased the number of endocrine cells in the villi (P = 0.05); 3) With regard to the mRNA expression levels of stem cell differentiation marker genes, iron (P < 0.05) decreased the expression of trophinin 2 (Trop2), leucine-rich repeat containing G protein-coupled receptor 5 positive (Lgr5+), male-specific lethal 1(Msl1), BMI 1 proto-oncogene, polycomb ring finger (Bmi1), and achaete-scute family bHLH transcription factor 2 (Ascl2). On the other hand, VA increased the expression of Ascl2 (P = 0.001) although the interaction of VA and iron (P < 0.05) had an effect on the expression of secreted phosphoprotein 1 (Spp1) and Bmi1. In addition, VA decreased the gene or mRNA expression of aconitase 1 (Aco1; P < 0.001), transferrin receptor (TFRC; P = 0.001), and solute carrier family 11 member 2 (DMT1; P = 0.003) in the Iron Reactive Element/Iron Regulatory Protein (IRE/IRP) signaling pathway although iron and the interaction of VA and iron had no effect on the genes’ expression. The results therefore showed that VA, iron, and their interaction can promote intestinal development and epithelial cell differentiation in piglets.
Keywords: cell differentiation, intestinal development, iron, jejunum, vitamin A, sucking piglets
Introduction
The intestinal epithelium of piglets often changes significantly in the first month after birth. For example, the gastrointestinal tract grows rapidly, the weight and length of the small intestine increase, the villus height (VH), crypt depth (CD), and mitotic index of the small intestine increase while the activities of maltase and sucrase decrease (Xu et al., 1992; Skrzypek et al., 2005; Zabielski et al., 2008). Notably, the development of villus morphology and function directly affects the absorption of nutrients. In addition, previous study showed that the morphology of the small intestinal mucosa is an important index for measuring the digestive and absorptive capacity of the small intestine (Montagne et al., 2003). Increase of VH can also increase the absorptive capacity of the small intestine. Moreover, deepening of the intestinal crypt indicates a decrease in the differentiation degree of villus epithelial cells in the small intestine, weakening of the digestive and absorptive function of the organ in weaned piglets.
Newborn piglets grow rapidly in the early stages of life, increase the number of iron-related red blood cells and other metabolic activities (Lipinski et al., 2010). They therefore need 7 to 16 mg of iron per day to maintain their body’s activities. Recent studies also showed that iron supplementation can effectively increase the body weight of piglets (Wang et al., 2014), improve the morphology and integrity of the duodenum (Chen et al., 2019), promote intestinal development, and maintain the intestinal mucosa (Pu et al., 2018a). In addition, iron supplementation in lactating sows can increase the pig’s birth weight and reduce mortality, resulting in an increase in piglet weight after weaning (Ducsay et al., 1984; Rincker et al., 2004; Peters and Mahan, 2008a, b).
Vitamin A (VA) is an extremely important and easily deficient fat-soluble vitamin that is necessary for maintaining normal metabolism and function of the body. The role of VA is mainly mediated by transcription factors activated by its active metabolite, and all trans retinoic acid. Moreover, VA is stored as retinol in most diets or mainly in the form of retinol esters (Chambon, 1996; Chawla et al., 2001; Gutierrez-Mazariegos et al., 2011). Previous studies also showed that supplementation with VA can improve the function of the host’s intestinal barrier (Lima et al., 2010), affect intestinal function in weaned piglets by regulating intestinal stem cells (Wang et al., 2020c), and play a key role in protecting intestinal mucosa (Xiao et al., 2019).
Moreover, there is a complex relationship between iron and VA as they reinforce and restrict each other. Previous research showed that adding iron leads to a significant increase in the levels of retinol, the retinol binding protein, and the thyroxine transporter in human serum, indicating that iron could promote the synthesis of the VA transporter and enhance the mobilization as well as utilization of VA (Muñoz et al., 2000). Other studies also suggested that VA can form a complex with iron to maintain the solubility of iron in the intestinal cavity and also prevent phytate and polyphenols from inhibiting iron absorption, hence promoting the absorption of iron. It is therefore possible that there is a synergistic relationship between VA and iron absorption (García-Casal et al., 1998).
Existing evidence suggests that supplementation with iron and VA can promote intestinal development. Therefore, the present study hypothesized that iron, VA, and the interaction between the two nutrients had an effect on intestinal development and cell differentiation in piglets. The study consequently tested the above hypothesis by determining the indices of intestinal organs, intestinal morphology, intestinal enzyme activities, cell proliferation and differentiation as well as the related signaling pathways in piglets.
Materials and Methods
The experimental design and procedures were approved by the Animal Protection and Utilization Committee of Hunan Normal University, Changsha, Hunan Province.
Animals and the experimental design
The study selected a total 32 newborn male piglets (Duroc × Landrace × Yorkshire) with similar body weights, from 4 sows. The sows’ diet was formulated to cover or surpass the nutrient requirements for lactating sows, by the American National Research Council (2012). Thereafter, piglets from the same sow were randomly divided into four groups with eight piglets per treatment group. All piglets were fed with breast milk. In addition, the piglets received oral doses of normal saline (CON group) or 100 mg of ferrous sulfate (OAFe group) or 2,500 IU of VA (VA group) or 100 mg of ferrous sulfate combined with 2,500 IU of VA (OAFe +VA group) on the 2nd, 7th, 12th, and 17th days. Ferrous sulfate solution dissolves ferrous sulfate powder in distilled water and can be taken orally. Additionally, VA (Royal DSM NV, Shanghai, China) was used in the form of retinol acetate which dissolves in distilled water and can also be administered orally. Moreover, the piglets were weighed every week after birth and on the 21st day, the average body weight of piglets in the CON, OAFe, VA, and OAFe +VA groups was 5.11 ± 0.16 kg, 5.33 ± 0.25 kg, 4.99 ± 0.72, and 5.55 ± 0.39 kg, respectively. The piglets were also free to feed and water throughout the experimental period.
Sample collection
On the 21st day, after the piglets had been subjected to an overnight fast, they were euthanized through an overdose (40 mg/kg body weight) of sodium pentobarbital, followed by exsanguination. Thereafter, their abdomens were opened and the small intestine was taken out. The weight of the total length of the small intestine from each piglet was then recorded. Additionally, jejunum samples (at least 2 cm), from which intestinal contents had been removed, were immediately collected and stored in a 4% formalin solution at room temperature. The jejunum segments of the piglets were then cut longitudinally along the intestine, after which the intestinal contents were rinsed with a solution of normal saline. Afterwards, the upper intestinal mucosa was carefully collected with a glass slide, wrapped in tin foil, frozen in liquid nitrogen, and taken back to the laboratory for freezing at −80 °C (Wang et al., 2020a).
Analysis of intestinal morphology
According to the standard paraffin embedding procedure, jejunal samples immersed in a fixed solution of 4% formaldehyde were dehydrated through a series of alcohol gradients. The samples were then treated with xylene to make them transparent, after which they were embedded in paraffin. Thereafter, the paraffin-embedded samples were cut into 4 μm thick slices using a slicer (RM2235; Leica, Germany) then placed on a glass slide (CITOGLAS, Jiangsu, China). Afterwards, the jejunum samples were subjected to hematoxylin and eosin staining then visualized using an optical microscope. Moreover, the Image-Pro Plus 6.0 software (Media Cybernetics, San Diego, CA) was used to measure the VH, CD, and VW in the jejunum. The software was also used to calculate the VH to CD ratio and the fluff surface area. At least 30 complete villi and their associated crypts were selected from each intestinal segment of each piglet and the corresponding average value for each piglet was calculated and used for further analysis (Zong et al., 2018).
Alcian blue-periodic Acid-shiff staining (AB-PAS)
Tissue samples fixed in formalin were dehydrated in a graded series of ethanol solutions and embedded in paraffin wax. 5-μm-thick sections were then obtained using a microtome (RM2235; Leica; Germany), after which they were mounted for AB-PAS staining (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer’s protocol. Thereafter, the stained slides were cover-slipped for examination under a light microscope. In addition, image acquisition was done using the Leica Application Suite version 4.0 Software (Leica DM3000; Wetzlar, Germany). Moreover, the number of goblet cells in both the villi and crypts was counted using the Image-Pro Plus version 6.0 software (Media Cybernetics, San Diego, CA). The mean of 30 values from each sample was then obtained and recorded, for each piglet (Deng et al., 2020).
Immunohistochemistry
Serial slides were processed according to the standard immunohistochemical protocols but with minor modifications. Briefly, the samples were deparaffinized in xylene and rehydrated in a descending series of ethanol. The samples were then heated twice in a microwave and in an aqueous solution of sodium citrate buffer to retrieve the antigen epitopes. The endogenous peroxidase was then blocked in 3% hydrogen peroxidase for 10 min, in the dark. Thereafter, the samples were incubated in diluted bovine serum albumin in a moist chamber, at 37 °C for 30 min. Afterwards, they were incubated in a moist chamber with the following primary antibodies: anti-Ki67 (Abcam, Cambridge, UK, dilution 1:700; incubation 90 min, 37 °C), anti-Chromogranin A (Abcam, Cambridge, UK, dilution 1:700; incubation 90 min, 37 °C) and Cleaved caspase 3 (Cell Signaling Technology, USA, dilution 1:700; incubation 90 min, 37 °C). The samples were then treated with a secondary antibody (ZSBIO, Beijing, China) at 37 °C and the antibody was allowed to bind to the primary antibody for 50 min (ZSBIO). Thereafter, the samples were visualized with Diaminobenzidine (ZSGB-BIO, Beijing, China) to identify the positive cells. They were then counterstained with hematoxylin, dehydrated and mounted. Notably, each step was followed by four 3-min PBS washes, except for blocking. Lastly, a total of 15 microscopic fields under 20× magnification (RM2235; Leica) per sample were used for analysis. The Image-Pro software was then employed to determine the number of labeled cells per field (Wang et al., 2019).
Analysis of intestinal enzyme activities
After the mucosal tissue samples from the jejunum were homogenized in liquid nitrogen, a certain amount of powder was added to the EP tube with 1 mL normal saline for mixing. They were then allowed to stand for 1–2 h, after which centrifugation (3,000 ×g, 4 °C, 10 min) was conducted. Thereafter, the supernatant was taken for analysis of the activities of intestinal maltase, sucrase, lactase, and alkaline phosphatase, using the purchased kits, according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
RNA extraction and cDNA synthesis
Total RNA was isolated from jejunal tissues using the Trizol reagent (TaKaRa). The integrity of RNA was then examined through 1.2% agarose gel electrophoresis where 10 μg/mL of ethidium bromide was used for staining. In addition, RNA quality and quantity were determined using a UV spectrophotometer (Nano-Drop ND-1000; Thermo Fisher Scientific, DE). Thereafter, cDNA was synthesized using an RT reagent Kit and a gDNA Eraser (TaKaRa).
Real-time quantitative PCR (RT-qPCR)
The primer sequences for the selected genes are shown in Table 4. The primers were designed using Primer-BLAST (NCBI) and the primers are listed in Supplementary Table 1. In addition, RT-qPCR was conducted in the Step One Plus TM system (Quant-Studio, Thermo Fisher Scientific). Each reaction consisted of a 5-μL SYBR Green mixture, 0.3 μL each of the positive and negative primers, 3.4 μL of water and 1 μL of 5-fold diluted cDNA, making up a final reaction volume of 10 μL. Moreover, the RT-qPCR amplification procedure was as follows: pre-denaturation at 95 °C for 30 s, 40 cycles of denaturation at 95 °C for 5 s and annealing at 60 °C for 30 s, followed by a melting curve procedure including 95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s. The specific operation steps were performed according to the product manual. Each gene was assessed three times and β-actin was used as the reference. Finally, the relative expression (RE) of the target-gene mRNAs was calculated as follows: RE = 2-ΔΔCt, ΔΔCt = Ct (target gene) -Ct (β-actin) (Wang et al., 2020b)
Table 4.
The mRNA expression of intestinal stem cell markers, Notch signaling pathway, classical RSP and IRE/IRP signaling pathway-related genes1
| Items3 | Treatments2 | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | OAFe | VA | OAFe+VA | VA | OAFe | Interaction | ||
| Expression of intestinal stem cell markers | ||||||||
| Trop2 | 1.70ab | 0.84b | 3.56a | 1.16b | 1.214 | 0.077 | 0.011 | 0.203 |
| Spp1 | 1.37a | 0.34b | 0.73ab | 1.08ab | 0.229 | 0.877 | 0.258 | 0.030 |
| LGR5+ | 0.94b | 0.80b | 1.36a | 0.83b | 0.094 | 0.392 | 0.045 | 0.578 |
| Msl1 | 0.95a | 0.69b | 0.96a | 0.72b | 0.064 | 0.856 | 0.020 | 0.925 |
| Bmi1 | 1.03ab | 0.83bc | 1.30a | 0.75c | 0.059 | 0.271 | 0.000 | 0.045 |
| Ascl2 | 1.14bc | 0.77c | 1.85a | 1.32b | 0.132 | 0.001 | 0.008 | 0.621 |
| Expression of Notch signaling pathway | ||||||||
| Notch1 | 1.07 | 1.01 | 1.10 | 1.02 | 0.061 | 0.831 | 0.543 | 0.223 |
| Notch2 | 1.20 | 0.73 | 1.02 | 0.89 | 0.308 | 0.521 | 0.757 | 0.489 |
| Atoh1 | 1.25 | 0.90 | 0.99 | 0.58 | 0.155 | 0.283 | 0.164 | 0.905 |
| Hes1 | 1.19 | 0.99 | 0.91 | 0.82 | 0.123 | 0.312 | 0.516 | 0.810 |
| Dll4 | 1.07ab | 0.48c | 0.75bc | 1.27a | 0.132 | 0.120 | 0.795 | 0.001 |
| Expression of classical RSP | ||||||||
| RBP2 | 1.08b | 2.12a | 1.94a | 2.46a | 0.190 | 0.018 | 0.003 | 0.291 |
| ALDH1 A3 | 1.02 | 1.07 | 0.99 | 1.08 | 0.062 | 0.944 | 0.600 | 0.871 |
| RAR a | 0.94 | 0.99 | 1.13 | 0.85 | 0.096 | 0.557 | 0.477 | 0.698 |
| RAR b | 0.87 | 1.05 | 0.93 | 1.00 | 0.262 | 0.999 | 0.368 | 0.757 |
| Expression ofIRE/IRP signaling pathway | ||||||||
| Aco1 | 1.04a | 1.18a | 0.70b | 0.63b | 0.075 | 0.000 | 0.710 | 0.275 |
| TFRC | 1.02a | 1.31a | 0.43b | 0.42b | 0.153 | 0.001 | 0.477 | 0.462 |
| DMT1 | 1.04ab | 1.14a | 0.72b | 0.68b | 0.092 | 0.003 | 0.740 | 0.975 |
| IREB2 | 1.02 | 1.04 | 0.75 | 0.99 | 0.071 | 0.138 | 0.246 | 0.316 |
1 n = 8.
2CON, control; OAFe, orally administrated ferrous sulfate; VA, orally administrated vitamin A; OAFe +VA, orally administrated ferrous sulfate and vitamin A.
3Aco1, aconitase 1; Ascl2, achaete-scute family bHLH transcription factor 2; ALDH1 A3, aldehyde dehydrogenase 1 family member A3; Atoh1, atonal bHLH transcription factor 1; Bmi1, BMI 1 proto-oncogene, polycomb ring finger; Dll4, delta-like canonical notch ligand 4; DMT1, Solute carrier family 11 (proton-coupled divalent metal ion transporter), member 2; Hes1, hes family bHLH transcription factor 1; IRE/IRP, iron reactive element/iron regulatory protein; IREB2, iron responsive element binding protein; Lgr5, leucine-rich repeat containing G-protein coupled receptor 5; Msl1, male-specific lethal 1; RARα, retinoic acid receptor α; RARβ, retinoic acid receptor β; RBP2, retinol binding protein 2; RSP, retinol signaling pathway; Spp1, secreted phosphoprotein 1; TFRC, transferrin receptor; Trop2, trophinin 2.
a–cValues in the same row with different superscript letters are significantly different (P < 0.05).
Statistical analysis
The SPSS 20.0 software (IBM Corp., Chicago, IL) was used for statistical analysis. In addition, two-way analysis of variance was used to obtain differences in basic characteristics between the groups. The data was expressed as the standard error of the mean, P < 0.01 means very significant difference, P < 0.05 was considered to be statistically significant, and P > 0.05 means no significant difference.
Results
Intestinal organ indices
The results in Table 1 show that the weight (P < 0.01), length to weight ratio (P < 0.01), and relative weight (P < 0.01) of the small intestine in the three experimental groups were greater than those in the control group. Additionally, iron significantly increased the length (P < 0.001), weight (P < 0.001), relative weight (P < 0.001), and length to weight ratio (P < 0.001) of the small intestine. On the other hand, VA markedly improved the length to weight ratio (P = 0.015) and relative weight (P < 0.001) of the small intestine. However, the organ indices showed that the interaction between VA and iron had no effect.
Table 1.
Effects of VA and Fe on intestinal organ indices1
| Item | Treatments2 | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | OAFe | VA | OAFe+VA | VA | OAFe | Interaction | ||
| Initial weight, kg | 1.71 | 1.76 | 1.75 | 1.72 | 0.022 | 0.993 | 0.873 | 0.413 |
| Final weight, kg | 5.11 | 5.33 | 4.99 | 5.76 | 0.139 | 0.55 | 0.07 | 0.296 |
| Average daily gain | 0.16 | 0.17 | 0.15 | 0.18 | 0.064 | 0.868 | 0.118 | 0.427 |
| Intestine length, m | 7.59b | 9.12a | 7.39b | 9.03a | 0.181 | 0.556 | 0.000 | 0.812 |
| Intestine weight, g | 150.44b | 227.78a | 170.69b | 239.84a | 8.326 | 0.141 | 0.000 | 0.704 |
| Weight: Length, g/m | 19.61c | 24.83ab | 23.04b | 25.79a | 0.579 | 0.015 | 0.000 | 0.158 |
| Relative length3, m/kg | 1.50b | 1.69a | 1.50b | 1.67a | 0.043 | 0.900 | 0.019 | 0.802 |
| Relative weight4, g/kg | 29.43d | 38.75b | 34.30c | 44.22a | 1.395 | 0.000 | 0.000 | 0.804 |
1 n = 8.
2CON, control; OAFe, orally administrated ferrous sulfate; VA, orally administrated vitamin A; OAFe +VA, orally administrated ferrous sulfate and vitamin A.
3The relative length is the length of the small intestine divided by the final body weight.
4The relative weight is the weight of the small intestine divided by the final body weight.
a–dValues in the same row with different superscript letters are significantly different (P < 0.05).
Intestinal morphology and immunohistochemistry
The results of intestinal morphology are shown in Table 2. The findings revealed that iron supplementation significantly increased the villus height (P < 0.05), crypt depth (P < 0.001), villus width (P = 0.001), surface area (P < 0.001), the villus per unit perimeter (P = 0.006), and the crypt per unit perimeter (P < 0.001). Addition of VA also led to significant differences in the villus height (P = 0.007) and surface area (P = 0.001) between the treatment and control groups. Moreover, the interaction of iron and VA significantly increased the height of the villi (P = 0.002) and the surface area (P = 0.001).
Table 2.
Effects of VA and Fe on small intestine morphological indicators1
| Item | Treatments2 | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | OAFe | VA | OAFe+VA | VA | OAFe | Interaction | ||
| VH, μm | 296.41b | 382.96a | 385.03a | 374.74a | 10.22 | 0.009 | 0.013 | 0.002 |
| CD, μm | 179.24b | 222.73a | 172.47b | 222.51a | 5.77 | 0.578 | 0.000 | 0.602 |
| VW, μm | 119.09c | 132.93ab | 123.15bc | 134.01a | 2.05 | 0.458 | 0.001 | 0.665 |
| VH:CD | 1.63 | 1.64 | 1.97 | 1.69 | 0.080 | 0.055 | 0.194 | 0.147 |
| Surface area, μm2 | 108,268b | 159,995a | 152,308a | 160,232a | 4,799.8 | 0.001 | 0.000 | 0.001 |
| Crypt division index3 | 0.123 | 0.124 | 0.140 | 0.142 | 0.005 | 0.081 | 0.883 | 0.977 |
| Unit perimeter villi4 | 0.0045a | 0.0039b | 0.0042ab | 0.0038b | 0.0001 | 0.460 | 0.006 | 0.693 |
| Unit perimeter crypts5 | 0.0111a | 0.0098b | 0.0109a | 0.0093b | 0.0002 | 0.335 | 0.000 | 0.605 |
1 n = 8.
2CON, control; OAFe, orally administrated ferrous sulfate; VA, orally administrated vitamin A; OAFe +VA, orally administrated ferrous sulfate and vitamin A.
3Crypt division index refers to the ratio of crypts in the stage of division in one hundred crypts.
4Unit perimeter villi refer to the perimeter occupied by a certain number of villus.
5Unit perimeter crypts refer to the perimeter occupied by a certain number of crypts.
a–cValues in the same row with different superscript letters are significantly different (P < 0.05).
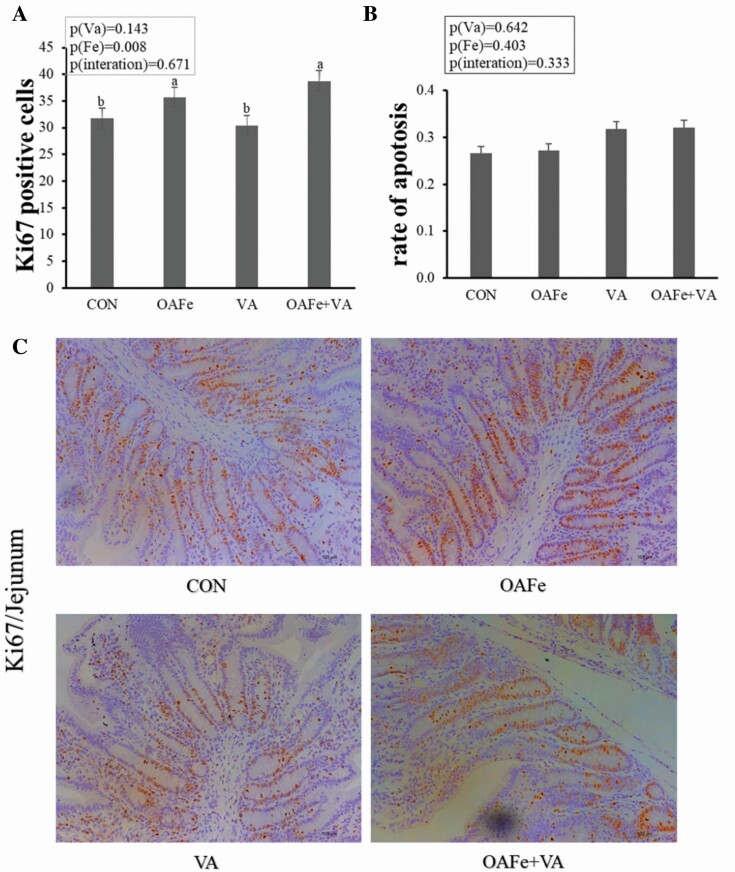
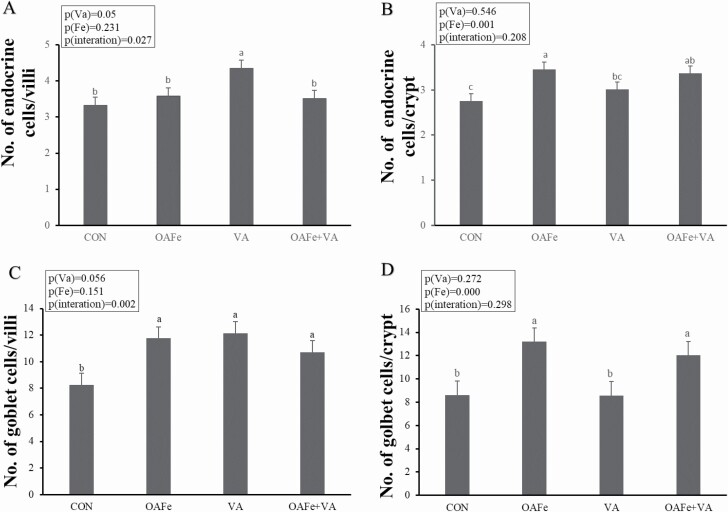
The results in Figures 1 and 2 also show that iron supplementation increased the number of endocrine cells (P = 0.001), goblet cells (P < 0.001), and Ki67-positive cells (P = 0.008) in jejunal crypt. Moreover, VA increased the number of endocrine cells in the villi (P = 0.05) while the interaction between iron and VA significantly affected the number of goblet cells in the villi (P = 0.002).
Figure 1.
Effects of Fe and VA supplementation on proliferation and apoptosis of intestinal cells of piglets. (A) Ki67-positive cells in crypts, (B) apoptotic rate of crypt cells, (C) typical image of Ki67 immunohistochemical staining showed jejunal antibody of piglets (200 ×; n = 8). The data were analyzed by two-way analysis of variance and expressed as mean value. Bars represent SEM (SEM, standard error of mean; n = 8). a,bValues in the same row with different superscript letters are significantly different (P < 0.05).
Figure 2.
Effects of Fe and VA supplementation on the expression of goblet cells and endocrine cells in jejunal villi and crypts of piglets. (A) Villous endocrine cells, (B) crypt endocrine cells, (C) villous goblet cells, (D) crypt goblet cells. a, b, and c: values within a row with different superscripts differ significantly at P < 0.01 or show a tendency toward differing at P < 0.10.
Analysis of intestinal enzyme activities
The results showed that VA enhanced the activities of alkaline phosphatase, maltase, lactase, and sucrose, compared to the control group (Table 3). It also had a significant increase on activities of lactase (P < 0.001) and sucrase (P = 0.015). On the other hand, iron decreased the activities of alkaline phosphatase, maltase, lactase, and sucrose, and had an obvious decrease on the maltase (P = 0.016) compared to the control group. In addition, the interaction of iron and VA has a trend to affect the activity of maltase (P = 0.058).
Table 3.
Effects of VA and Fe on intestinal enzyme activity1
| Item | Treatments2 | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | OAFe | VA | OAFe+VA | VA | OAFe | Interaction | ||
| Alkaline phosphatase | 136.04 | 114.26 | 155.42 | 136.94 | 10.50 | 0.209 | 0.228 | 0.920 |
| Maltase | 23.17b | 20.98b | 36.55a | 19.28b | 5.44 | 0.137 | 0.016 | 0.058 |
| Lactase | 8.12b | 7.15b | 11.95a | 16.60a | 1.02 | 0.000 | 0.343 | 0.373 |
| Sucrase | 129.09b | 116.92b | 158.22a | 165.21a | 9.95 | 0.015 | 0.864 | 0.527 |
1 n = 8.
2CON, control; OAFe, orally administrated ferrous sulfate; VA, orally administrated vitamin A; OAFe +VA, orally administrated ferrous sulfate and vitamin A.
a,bValues in the same row with different superscript letters are significantly different (P < 0.05).
The mRNA expression of genes
The mRNA expression of cell differentiation marker genes in the Notch signaling pathway, retinol signaling pathway (RSP) and iron reactive element/ iron regulatory protein (IRE/IRP) signaling pathway is shown in Table 4. The findings revealed that iron significantly decreased the expression of trophinin 2 (Trop2; P = 0.011), leucine-rich repeat containing G protein-coupled receptor 5 positive (Lgr5+; P = 0.045), male-specific lethal 1 (Msl1; P = 0.020), BMI 1 proto-oncogene, polycomb ring finger (Bmi1; P < 0.001), and achaete-scute family bHLH transcription factor 2 (Ascl2; P = 0.008). In addition, VA markedly increased the mRNA expression of intestinal stem cell marker, Ascl2 (P = 0.001), while the interaction of iron and VA significantly affected the expression of secreted phosphoprotein 1 (Spp1; P = 0.03) and Bmi1 (P < 0.05). Moreover, the interaction between iron and VA only significantly affected the expression of delta like canonical Notch ligand 4 (Dll4; P =0.001) in the Notch signaling pathway. However, iron or VA alone had no effect on the expression of genes in this signaling pathway. Furthermore, both iron (P = 0.003) and VA (P = 0.018) significantly increased the mRNA expression of retinol binding protein 2 (RBP2) in the RSP. Additionally, VA decreased the mRNA expression of genes in the IRE/IRP signaling pathway, including aconitase 1 (Aco1; P < 0.001), transferrin receptor (TFRC; P = 0.001), solute carrier family 11 member 2 (DMT1; P = 0.003). Nonetheless, both iron and the interaction between iron and VA had no effect on the expression of genes in this pathway.
Discussion
The main focus of this study was to explore the effects of iron, VA and the interaction between the two nutrients on intestinal development and cell differentiation in sucking piglets. Iron is one of the most important trace elements in the growth of both livestock and poultry (Zhou et al., 2020). In addition, previous studies showed that iron supplementation can improve the growth performance of piglets and promote intestinal development as well as maturation (Novais et al., 2016; Pu et al., 2018). On the other hand, VA plays a role in maintaining complete intestinal epithelial absorption (Quadro et al., 2000). Presently, the weight and length of the small intestine are used as indicators of the digestive and absorptive capacity of the organ (Li et al., 2018). Moreover, the results from the present study showed that iron supplementation can improve intestinal morphology while VA can significantly increase the weight to length ratio and the relative weight of the small intestine in piglets. According to existing evidence, there is a significant correlation between the length of the small intestine and growth performance in pigs. Additionally, a direct relationship has been observed between the increase in intestinal weight and length and pig weight (Adeola and King, 2006). However, the results showed that the growth performance of piglets was not affected, probably because growth performance is not a sensitive index.
Additionally, it is generally believed that an increase of villus height is the result of an increase in the surface area, which enhances the absorption of available nutrients. In addition, the deeper the crypts, the faster the villi renew. In this study, iron was shown to increase the VH, CD, and surface area of the jejunum in piglets. On the other hand, VA had a positive effect on the VH and surface area. Previous studies showed that iron and VA play a role in promoting the development of intestinal morphology (Wang et al., 2019; Zhuo et al., 2019), consistent with the results reported herein. Moreover, a recent study revealed that the proliferation of intestinal epithelial cells was connected to the VH and CD in weaned piglets (de Lange et al., 2010). Furthermore, Ki67-positive epithelial cells are mainly located in crypts. Therefore, the proliferation of gastrointestinal epithelial cells is often evaluated by counting the number of Ki67-positive cells (Yang et al., 2012; Huygelen et al., 2015). The results from this study showed that iron markedly increased the number of Ki67-positive cells. The increase in VH and CD may therefore be attributed to the proliferation of intestinal cells. In addition, the activity of specific brush border enzymes varies with the VH and CD and can indicate the function and maturity of intestinal epithelial cells. Notably, disaccharidase activity determines the carbohydrate digestive capacity in weaned piglets and is therefore an important marker of intestinal development in animals (Huygelen et al., 2015). Herein, the results showed that iron had an obvious effect on maltase activity. Additionally, VA enhanced the activities of both lactase and sucrase. The interaction of iron and VA also affected the activity of maltase. These results therefore suggested that iron and VA may promote intestinal development.
Moreover, the regulation of cell proliferation in vivo and in vitro is mediated by a combination of growth arrest and cell differentiation (Barker et al., 2007). Therefore, the study assessed the effects of iron and VA on the differentiation of epithelial cells. The results showed that iron increased the number of goblet cells and endocrine cells in crypts. In addition, both VA and the interaction of iron and VA increased the number of endocrine cells and goblet cells in the villi. The results also showed that iron and VA promoted the differentiation of intestinal secretory cells. Furthermore, the study examined the expression of intestinal stem cell marker genes and intestinal stem cell genes through RT-qPCR. It is noteworthy that Lgr5+ specifically labeled columnar cells are recognized as intestinal stem cells and the source of continuous renewal of the intestinal epithelium (Noah and Shroyer, 2013). Additionally, Spp1 and Trop2 are typical fetal stem cell genes while Msl1, Bmi1 and Ascl2 are adult stem cell genes. In this study, the findings revealed that VA increased the expression of Ascl2 in the jejunum, possibly because VA promotes the transformation of fetal stem cells into adult stem cells. Moreover, iron significantly decreased the expression of stem cell genes, probably because it promotes the differentiation of intestinal cells.
Furthermore, the Notch signaling pathway was shown to be crucial in guiding the differentiation of progenitor cells into absorptive or secretive cells. In addition, hes family bHLH transcription factor 1 (Hes1) and atonal bHLH transcription factor 1 (Atoh1) are the main transcription targets of the Notch signaling pathway. The two control the fate of intestinal stem cells by up regulating the expression of Hes1 and inhibiting the expression of Atoh1 (Kazanjian et al., 2010). Moreover, it was reported that Notch1 and Notch2 were mainly expressed in crypt epithelial cells while Dll4 was expressed by a single cell in crypt epithelial cells. In this study, iron or VA alone had no significant effect on the expression of genes in the Notch signaling pathway, possibly because the pathway does not promote the differentiation of intestinal epithelial cells (Kutasy et al., 2016a).
In addition, retinoic acid is an activation form of VA that has a variety of functions in vivo. Notably, retinol can control the proliferation and differentiation of various types of cells. Additionally, the classic RSP can control the fate of cells and the maintenance of various types of stem cells. Therefore, the present study evaluated the expression of four genes which play a central role in the classical retinol retinoic acid pathway. Previous studies showed that increasing the level of retinol can increase the expression of the RSP gene in the lung tissues of newborn mice, which may be related to the activation of RSP by retinol (Kutasy et al., 2016b). In this study, the results showed that both VA and iron increased the expression of RBP2 and promoted the transportation of retinol, possibly promoting the proliferation and differentiation of epithelial cells.
It was also shown that the levels of iron in cells are mainly balanced by the IRE/IRP regulatory system (Muckenthaler et al., 2008). IRP is the main regulatory protein for iron, in vivo. Additionally, TFRC and DMT1 can be directly regulated by IRPs and are at the translation or stable level of mRNA. In this study, iron had no significant effect on the IRE/IRP signaling pathway, because the binding activity of IRE was weakened in iron rich cells. This allowed for the degradation of TFRC mRNA and translation of ferritin mRNA, further inhibiting iron uptake. Moreover, VA affected the expression of Aco1, TFRC, and DMT1 in the IRE/IRP signaling pathway, probably due to the inhibition of IRP activity by VA, which reduced the activity of the transporter.
Conclusion
In summary, this study showed that iron, VA, and the interaction between the two nutrients can promote intestinal development in piglets. In addition, iron can affect cell proliferation and promote intestinal maturation by improving the morphology of the intestine. Moreover, VA stimulates intestinal development by promoting cell differentiation, regulating the expression of stem cell genes and modulating the related signaling pathways. On the other hand, interaction of VA and iron affects intestinal development by improving intestinal morphology and promoting cell differentiation.
Supplementary Material
Acknowledgments
This work was supported by the Hunan Province’s Changsha-Zhuzhou-Xiangtan National Independent Innovation Demonstration Zone projects (Grant No. 2017XK2058), and Hunan Provincial Key Laboratory of Animal Nutritional Physiology and Metabolic Process open fund projects (Grant No. ISA2020113).
Glossary
Abbreviations
- AB-PAS
Alcian Blue-periodic Acid-shiff Staining
- CD
crypt depth
- PCR
polymerase chain reaction
- RT-qPCR
real-time quantitative PCR
- VA
vitamin A
- VH
villus height
- VW
villus width
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Adeola, O., and King D. E.. . 2006. Developmental changes in morphometry of the small intestine and jejunal sucrase activity during the first nine weeks of postnatal growth in pigs. J. Anim. Sci. 84:112–118. doi: 10.2527/2006.841112x [DOI] [PubMed] [Google Scholar]
- Barker, N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., . et al. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449:1003–1007. doi: 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- Chambon, P. 1996. A decade of molecular biology of retinoic acid receptors. Faseb J. 10:940–954. doi: 10.1096/fasebj.10.9.8801176 [DOI] [PubMed] [Google Scholar]
- Chawla, A., Repa J. J., Evans R. M., and Mangelsdorf D. J.. . 2001. Nuclear receptors and lipid physiology: opening the X-files. Science 294:1866–1870. doi: 10.1126/science.294.5548.1866 [DOI] [PubMed] [Google Scholar]
- Chen, X. Y., Zhang X. F., Zhao J., Tang X. Y., Wang F. Q., and Du H. H.. . 2019. Split iron supplementation is beneficial for newborn piglets. Biomed Pharmacother 120:109479. doi: 10.1016/j.biopha.2019.109479 [DOI] [PubMed] [Google Scholar]
- Deng, Q. Q., Shao Y. R., Wang Q. Y., Li J. Z., Li Y. L., Ding X. Q., Huang P. F., Yin J., Yang H. S., and Yin Y. L.. . 2020. Effects and interaction of dietary electrolyte balance and citric acid on the intestinal function of weaned piglets. J Anim Sci 98(5):skaa106. doi: 10.1093/jas/skaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducsay, C. A., Buhi W. C., Bazer F. W., Roberts R. M., and Combs G. E.. . 1984. Role of uteroferrin in placental iron transport: effect of maternal iron treatment on fetal iron and uteroferrin content and neonatal hemoglobin. J. Anim. Sci. 59:1303–1308. doi: 10.2527/jas1984.5951303x [DOI] [PubMed] [Google Scholar]
- García-Casal, M. N., Layrisse M., Solano L., Barón M. A., Arguello F., Llovera D., Ramírez J., Leets I., and Tropper E.. . 1998. Vitamin A and beta-carotene can improve nonheme iron absorption from rice, wheat and corn by humans. J. Nutr. 128:646–650. doi: 10.1093/jn/128.3.646 [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mazariegos, J., Theodosiou M., Campo-Paysaa F., and Schubert M.. . 2011. Vitamin A: a multifunctional tool for development. Semin. Cell Dev. Biol. 22:603–610. doi: 10.1016/j.semcdb.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Huygelen, V., De Vos M., Prims S., Vergauwen H., Fransen E., Casteleyn C., Van Cruchten S., and Van Ginneken C.. . 2015. Birth weight has no influence on the morphology, digestive capacity and motility of the small intestine in suckling pigs. Livest Sci 182:129–136. doi: 10.1016/j.livsci.2015.11.003 [DOI] [Google Scholar]
- Kazanjian, A., Noah T. K., Brown D., Burkart J. T., and Shroyer N. F.. . 2010. Atonal Homolog 1 (ATOH1) is essential for growth and differentiation effects of notch/gamma secretase inhibitors on normal and cancerous intestinal epithelial cells. Gastroenterology 138:S50–S50. doi: 10.1016/S0016-5085(10)60226-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutasy, B., Friedmacher F., Pes L., Coyle D., Doi T., Paradisi F., and Puri P.. . 2016a. Antenatal retinoic acid administration increases trophoblastic retinol-binding protein dependent retinol transport in the nitrofen model of congenital diaphragmatic hernia. Pediatr. Res. 79:614–620. doi: 10.1038/pr.2015.256 [DOI] [PubMed] [Google Scholar]
- Kutasy, B., Friedmacher F., Pes L., Coyle D., Doi T., Paradisi F., and Puri P.. . 2016b. Antenatal retinoic acid administration increases trophoblastic retinol-binding protein dependent retinol transport in the nitrofen model of congenital diaphragmatic hernia. Pediatr. Res. 79:614–620. doi: 10.1038/pr.2015.256 [DOI] [PubMed] [Google Scholar]
- de Lange, C. F. M., Pluske J., Gong J., and Nyachoti C. M.. . 2010. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest Sci 134(1-3):124–134. doi: 10.1016/j.livsci.2010.06.117 [DOI] [Google Scholar]
- Li, S. M., Wang X. G., Qu L., Dou T. C., Ma M., Shen M. M., Guo J., Hu Y. P., and Wang K. H.. . 2018. Genome-wide association studies for small intestine length in an F-2 population of chickens. Ital J Anim Sci 17(2):294–300. doi: 10.1080/1828051x.2017.1368419 [DOI] [Google Scholar]
- Lima, A. A., Soares A. M., Lima N. L., Mota R. M., Maciel B. L., Kvalsund M. P., Barrett L. J., Fitzgerald R. P., Blaner W. S., and Guerrant R. L.. . 2010. Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial. J. Pediatr. Gastroenterol. Nutr. 50:309–315. doi: 10.1097/MPG.0b013e3181a96489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski, P., Starzyński R. R., Canonne-Hergaux F., Tudek B., Oliński R., Kowalczyk P., Dziaman T., Thibaudeau O., Gralak M. A., Smuda E., . et al. 2010. Benefits and risks of iron supplementation in anemic neonatal pigs. Am. J. Pathol. 177:1233–1243. doi: 10.2353/ajpath.2010.091020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne, L., Pluske J. R., and Hampson D. J.. . 2003. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci and Tech 108(1-4):95–117. doi: 10.1016/s0377-8401(03)00163-9 [DOI] [Google Scholar]
- Muckenthaler, M. U., Galy B., and Hentze M. W.. . 2008. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 28:197–213. doi: 10.1146/annurev.nutr.28.061807.155521 [DOI] [PubMed] [Google Scholar]
- Muñoz, E. C., Rosado J. L., López P., Furr H. C., and Allen L. H.. . 2000. Iron and zinc supplementation improves indicators of vitamin A status of Mexican preschoolers. Am. J. Clin. Nutr. 71:789–794. doi: 10.1093/ajcn/71.3.789 [DOI] [PubMed] [Google Scholar]
- Noah, T. K., and Shroyer N. F.. . 2013. Notch in the intestine: regulation of homeostasis and pathogenesis. Annu. Rev. Physiol. 75:263–288. doi: 10.1146/annurev-physiol-030212-183741 [DOI] [PubMed] [Google Scholar]
- Novais, A. K., da Silva C. A., dos Santos R. D. S., Dias C. P., Callegari M. A., and de Oliveira E. R.. . 2016. The effect of supplementing sow and piglet diets with different forms of iron. Rev Bras Zootecn 45(10):615–621. doi: 10.1590/S1806-92902016001000006 [DOI] [Google Scholar]
- Peters, J. C., and Mahan D. C.. . 2008a. Effects of dietary organic and inorganic trace mineral levels on sow reproductive performances and daily mineral intakes over six parities. J. Anim. Sci. 86:2247–2260. doi: 10.2527/jas.2007-0431 [DOI] [PubMed] [Google Scholar]
- Peters, J. C., and Mahan D. C.. . 2008b. Effects of neonatal iron status, iron injections at birth, and weaning in young pigs from sows fed either organic or inorganic trace minerals. J. Anim. Sci. 86:2261–2269. doi: 10.2527/jas.2007-0577 [DOI] [PubMed] [Google Scholar]
- Pu, Y., Li S., Xiong H., Zhang X., Wang Y., and Du H.. . 2018a. Iron Promotes Intestinal Development in Neonatal Piglets. Nutrients 10(6):726. doi: 10.3390/nu10060726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadro, L., Gamble M. V., Vogel S., Lima A. A., Piantedosi R., Moore S. R., Colantuoni V., Gottesman M. E., Guerrant R. L., and Blaner W. S.. . 2000. Retinol and retinol-binding protein: gut integrity and circulating immunoglobulins. J. Infect. Dis. 182(Suppl 1):S97–S102. doi: 10.1086/315920 [DOI] [PubMed] [Google Scholar]
- Rincker, M. J., Hill G. M., Link J. E., and Rowntree J. E.. . 2004. Effects of dietary iron supplementation on growth performance, hematological status, and whole-body mineral concentrations of nursery pigs. J. Anim. Sci. 82:3189–3197. doi: 10.2527/2004.82113189x [DOI] [PubMed] [Google Scholar]
- Skrzypek, T., Valverde Piedra J. L., Skrzypek H., Woliński J., Kazimierczak W., Szymańczyk S., Pawłowska M., and Zabielski R.. . 2005. Light and scanning electron microscopy evaluation of the postnatal small intestinal mucosa development in pigs. J. Physiol. Pharmacol. 56(Suppl 3):71–87. [PubMed] [Google Scholar]
- Wang, J., Li D., Che L., Lin Y., Fang Z., Xu S., and Wu D.. . 2014. Influence of organic iron complex on sow reproductive performance and iron status of nursing pigs. Livest Sci 160:89–96. doi: 10.1016/j.livsci.2013.11.024 [DOI] [Google Scholar]
- Wang, Z., Li J., Wang Y., Wang L., Yin Y., Yin L., Yang H., and Yin Y.. . 2020c. Dietary vitamin A affects growth performance, intestinal development, and functions in weaned piglets by affecting intestinal stem cells. J Anim Sci 98(2):skaa020. doi: 10.1093/jas/skaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q., Wang Y., Hussain T., Dai C., Li J., Huang P., Li Y., Ding X., Huang J., Ji F., . et al. 2020b. Effects of dietary energy level on growth performance, blood parameters and meat quality in fattening male Hu lambs. J. Anim. Physiol. Anim. Nutr. (Berl). 104:418–430. doi: 10.1111/jpn.13278 [DOI] [PubMed] [Google Scholar]
- Wang, L., Yan S., Li J., Li Y., Ding X., Yin J., Xiong X., Yin Y., and Yang H.. . 2019. Rapid Communication: the relationship of enterocyte proliferation with intestinal morphology and nutrient digestibility in weaning piglets. J. Anim. Sci. 97:353–358. doi: 10.1093/jas/sky388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M., Yang C., Wang Q. Y., Li J. Z., Li Y. L., Ding X. Q., Yin J., Yang H. S., and Yin Y. L.. . 2020a. The growth performance, intestinal digestive and absorptive capabilities in piglets with different lengths of small intestines. Animal 14:1196–1203. doi: 10.1017/S175173111900288X [DOI] [PubMed] [Google Scholar]
- Xiao, L., Cui T., Liu S., Chen B., Wang Y., Yang T., Li T., and Chen J.. . 2019. Vitamin A supplementation improves the intestinal mucosal barrier and facilitates the expression of tight junction proteins in rats with diarrhea. Nutrition 57:97–108. doi: 10.1016/j.nut.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Xu, R. J., Mellor D. J., Tungthanathanich P., Birtles M. J., Reynolds G. W., and Simpson H. V.. . 1992. Growth and morphological changes in the small and the large intestine in piglets during the first three days after birth. J. Dev. Physiol. 18:161–172. [PubMed] [Google Scholar]
- Yang, H., Li F., Kong X., Yuan X., Wang W., Huang R., Li T., Geng M., Wu G., and Yin Y.. . 2012. Chemerin regulates proliferation and differentiation of myoblast cells via ERK1/2 and mTOR signaling pathways. Cytokine 60:646–652. doi: 10.1016/j.cyto.2012.07.033 [DOI] [PubMed] [Google Scholar]
- Zabielski, R., Godlewski M. M., and Guilloteau P.. . 2008. Control of development of gastrointestinal system in neonates. J. Physiol. Pharmacol. 59(Suppl 1):35–54. [PubMed] [Google Scholar]
- Zhou, J., Dong Z., Wan D., Wang Q., Haung J., Huang P., Li Y., Ding X., Li J., Yang H., and Yin Y.. . 2020. Effects of iron on intestinal development and epithelial maturation of suckling piglets. J Anim Sci 98(8):1–6. doi: 10.1093/jas/skaa213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo, Z., Yu X., Li S., Fang S., and Feng J.. . 2019. Heme and non-heme iron on growth performances, blood parameters, tissue mineral concentration, and intestinal morphology of weanling pigs. Biol. Trace Elem. Res. 187:411–417. doi: 10.1007/s12011-018-1385-z [DOI] [PubMed] [Google Scholar]
- Zong, E., Huang P., Zhang W., Li J., Li Y., Ding X., Xiong X., Yin Y., and Yang H.. . 2018. The effects of dietary sulfur amino acids on growth performance, intestinal morphology, enzyme activity, and nutrient transporters in weaning piglets. J. Anim. Sci. 96:1130–1139. doi: 10.1093/jas/skx003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.