Abstract
Objective
The Consolidated Standards of Reporting Trials (CONSORT) recommends reporting adverse events (AEs) and dropouts (DOs) with their definitions. The purpose of this study was to identify how AEs and DOs were reported in randomized controlled trials of therapeutic exercise for knee osteoarthritis (OA).
Methods
Data sources were the Cochrane Library, Embase, PubMed, and CINAHL. Databases were searched to identify randomized controlled trials of therapeutic exercise for knee OA published from January 1, 1980, through July 23, 2020. Researchers independently extracted participant and intervention characteristics and determined whether a clear statement of and reasons for AEs and DOs existed. The primary outcome was exercise-related harm. Physiotherapy Evidence Database (PEDro) scoring described study quality and risk of bias. Descriptive and inferential statistics characterized results. Meta-analysis was not performed due to data heterogeneity.
Results
One hundred and thirteen studies (152 arms) from 25 countries were included, with 5909 participants exercising. PEDro scores ranged from 4 to 9. Exercise intensity was not specified in 57.9% of exercise arms. Fifty studies (44.2%) included an AE statement and 24 (21.2%) reported AEs, yielding 297 patients. One hundred and three studies (91.2%) had a DO statement. Sixteen studies (15.5%) provided reasons for DOs that could be classified as AEs among 39 patients, yielding a 13.1% increase in AEs. Thus, 336 patients (6.0%) experienced exercise-related harm among studies with a clear statement of AEs and DOs. A significant difference existed in misclassification of DOs pre- and post-CONSORT-2010 (12.2% vs 3.1%; 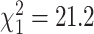 ).
).
Conclusions
In some studies, the reason for DOs could be considered AEs, leading to potential underreporting of harm. Improvements in reporting of harm were found pre- and post-CONSORT-2010. Greater clarity regarding AE and DO definitions and therapeutic exercise intensity are needed to determine safe dosing and mode of therapeutic exercise for knee OA.
Impact
More adherence to the CONSORT statement is needed regarding reporting of and defining of AEs, DOs, and therapeutic exercise intensity; however, despite this, therapeutic exercise seems to be associated with minimal risk of harm.
Keywords: Adult, Exercise Therapy, Knee Osteoarthritis, Rehabilitation, Risk Assessment
Introduction
Therapeutic exercise is a major component of the conservative management of knee osteoarthritis (OA).1–3 Evidence suggests that the mechanical loading associated with exercise can reduce inflammation and cartilage degradation,4 improve physical function and muscle strength, and reduce joint pain.5–7 Therapeutic exercise is the “systematic performance or execution of planned physical movements or activities intended to remediate or prevent impairments of body functions and structures, enhance activities and participation, reduce risk, optimize overall health, and enhance fitness and well-being.”8 Prescription of therapeutic exercise includes information regarding the intensity, frequency, duration, and mode of exercise along with the type of supervision used to ensure proper dosing and technique. Without a complete description of therapeutic exercise interventions in clinical trials, clinicians and patients cannot reliably implement therapeutic exercises shown to be effective, and researchers cannot replicate findings or extend the research in this area.9
Although therapeutic exercise is perceived as a less risky intervention for managing knee OA symptoms compared with invasive interventions such as surgery and nonsteroidal anti-inflammatory drugs, it does require active participation by the patient and substantial time commitment. The potential exists for exercise to lead to increased knee pain or exercise-related injury (eg, falls).10–12 Thus, it is important for patients to receive detailed instruction in the prescription of therapeutic exercise from physical therapists or other appropriate health professionals. In this process, patients should be informed about exercise-related symptoms (eg, temporary muscle discomfort) that are to be expected and not harmful, and the potential for and the incidence of risk of exercise-related adverse events (AEs). Disclosure of harm from therapeutic exercise is also a necessary component of informed consent in clinical trials of therapeutic exercise.
The Consolidated Standards of Reporting Trials (CONSORT) statement for reporting clinical trials13 is a universally accepted method for enhancing the consistency of reporting components of and problems with clinical trials.14 Originally published in 1996,15 the CONSORT statement has been updated 3 times,13,15,16 with the most recent version being published in 2010.13 In 2004, CONSORT published a statement specifically addressing the reporting of harm.17 This statement specifies that AEs and dropouts (DOs) should be reported separately along with their corresponding reasons and that AEs should be classified by severity.
AEs are defined as any negative outcome resulting either directly or indirectly from the assigned treatment.18 More recently the PRISMA Harms checklist provides guidance on how systematic reviews should address the reporting on harms.19 The PRISMA Harms group created a new term, “adverse effects,” which is defined as “an unfavorable outcome that occurs during or after the use of a drug or other intervention but is not necessarily caused by it.” Adverse effects become AEs when the causality of the unfavorable outcome can potentially be attributed to the intervention. Finally, study-related harm is considered to be the totality of adverse consequences occurring during or after an intervention.19
Patient withdrawal (or dropout) from studies due to harm-related reasons can occur, but DOs can also occur and not be associated with harm attributed to the intervention. Understanding why patients decide not to continue in a study is important to determine whether the intervention has any harmful impact, because their reasons for dropping out could indicate their inability to tolerate the intervention.17 Thus, clear definitions of both DOs and AEs are needed. However, there is little guidance regarding how to define and categorize AEs and DOs in therapeutic exercise trials,20 and this lack of consistency may lead to overlap in reasons for AEs and DOs. As such, it is difficult to ascertain the risk of harm in therapeutic exercise as well as the most effective dosage, intensity, and mode of therapeutic exercise for patients with knee OA.
Exercise-related harm has been investigated in a number of systematic reviews11,21–25 but each review operationalized harm differently and assessed exercise-related harm in different populations. For example, Sherrington et al12 examined whether exercise performed by older adults reduced their risk of falls. Neimeijer et al11 examined whether exercise therapy among adult patients, regardless of health condition, led to a 1.19 increased risk of nonserious AEs. None of these studies specifically addressed AEs in adults with arthritis. In a review of harm reporting in clinical trials within rheumatic diseases, Ethgen et al18 compared pharmacological trials with nonpharmacological trials and found pharmacological trials were 5.2 times more likely to discuss harms than nonpharmacological trials. Additionally, Quicke et al22 performed a systematic review of physical activity interventions in adults aged 45 years and older with knee pain to examine safety-related outcomes and found no serious safety issues in studies that used primarily low-intensity physical activity interventions.
This study aimed to identify how AEs, and DOs are defined in randomized controlled trials of therapeutic exercise among adults with knee OA and whether statements of AEs and DOs were included in manuscripts, to better describe the frequency and severity of therapeutic exercise-related harm, as recommended in the CONSORT Harms statement.17 Additionally, this study aimed to characterize the attributes of the study populations and therapeutic exercise interventions and to ascertain the incidence rate of therapeutic exercise–related harm in these studies. We hypothesized that the classification of AEs and DOs would be inconsistent across studies and that studies published prior to the CONSORT 2010 consensus statement13 would be less likely to mention AEs and consistently define AEs and DOs than studies published after this statement. Based on our findings, we provide recommendations for documenting exercise intervention details and the use of operational definitions of DOs and AEs, along with methods for reporting these in clinical trials of therapeutic exercise. We also discuss how our recommendations align with or add to the current CONSORT13 and CONSORT Harms17 reporting guidelines.
Methods
Data Sources and Searches
Four researchers (M.D.I, J.v.H., S.C., J.B.) used the American Physical Therapy Association’s (APTA’s) definition of therapeutic exercise8 to conduct a systematic review of randomized controlled trials of therapeutic exercise for managing knee OA symptoms. We searched the Cochrane Library, CINAHL, PubMed, and Embase databases for peer-reviewed randomized controlled trials conducted in adults diagnosed with knee OA and published in English between January 1, 1980 and July 23, 2020 using the following search terms: (knee osteoarthritis OR knee arthritis) AND (exercise OR exercise therapy OR strengthening OR aerobics OR anaerobic OR dynamic exercise OR isotonic OR isometric OR isokinetic OR physical therapy). These particular search terms were used in an effort to include every relevant type of therapeutic exercise. Studies that compared nonpharmacological interventions with therapeutic exercise were eligible provided the therapeutic exercise intervention arm included only therapeutic exercise. Because we used the APTA definition of therapeutic exercise,8 exercise was broadly defined and could include tai chi, yoga, strengthening, flexibility, aerobic (on land or in water), balance, or agility training, etc. We excluded studies that: (1) enrolled patients with nonspecified knee pain, (2) enrolled patients with other forms of arthritis (hip OA, rheumatoid arthritis, etc.) and did not separately report patient outcomes by diagnosis (either primary outcomes or for DOs and AEs), and (3) were of unsupervised exercise or studies where the level of supervision was unspecified. We used the PRISMA harms guidelines19 for the development and reporting of this systematic review (see Suppl. Appendix 1). This systematic review was registered with and approved by PROSPERO, the international prospective register of systematic reviews.26
Study Selection
Four researchers (M.D.I., J.v.H., S.C., J.B.) individually screened 4649 titles and 386 abstracts for eligibility and eliminated studies that did not meet the inclusion criteria. In instances where article eligibility was unclear, the researchers discussed the rationale for inclusion and after deliberating, came to a consensus about whether or not the study met inclusion/exclusion criteria. Figure 1 provides the flowchart portraying the process of study elimination. Two hundred and thirty-four full-text articles were screened for eligibility and the article reference lists were examined for eligible studies. Prior to the final data analysis, the search was rerun to determine whether any relevant studies had been missed.
Figure 1.
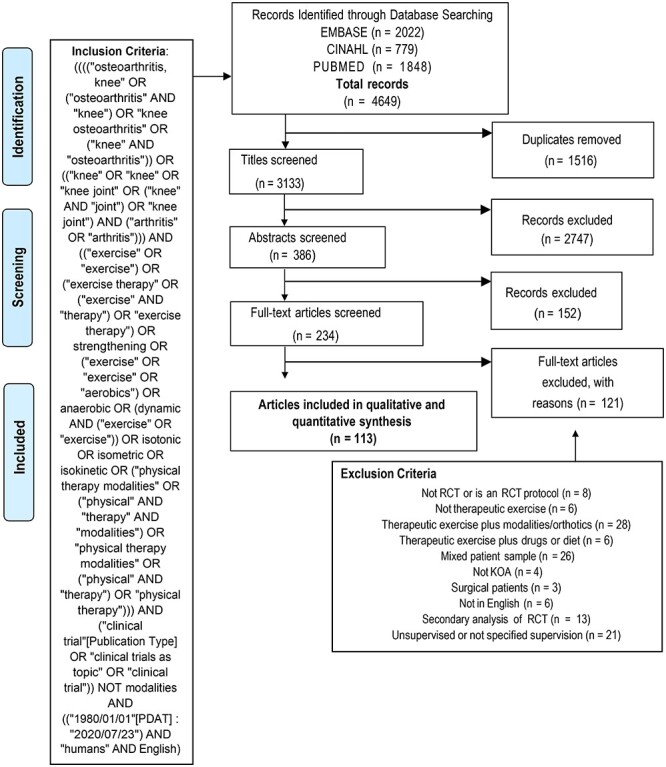
PRISMA flow diagram of randomized studies of therapeutic exercise for knee osteoarthritis.
Data Extraction and Quality Assessment
The research team (J.v.H., M.D.I., S.C., J.B., K.J.) extracted data from the final 113 studies (Suppl. Appendices 2 and 3) according to predefined criteria, using a standardized form. This form included the following data elements: year of publication, country where study was conducted, total number of subjects in exercise arm(s), program length, exercise intensity and how intensity was assessed (eg, BORG scale, 70% of 1 repetition max), frequency and mode of exercise and duration of exercise sessions, the amount of exercise supervision, whether there was a clear statement of AEs, the number and type of AEs (related to therapeutic exercise and not related to therapeutic exercise), whether there was a clear statement of DOs, the number of DOs, and reasons for DOs. The results section of each article was thoroughly annotated to ensure that a definition of AEs associated with therapeutic exercise was clearly reported in the narrative, tables, or figures and not overlooked. In instances where a DO could be considered an AE or an adverse effect, we recorded that outcome as harm.
We used the first assessment after the exercise intervention as the primary endpoint for the collection of AEs and DOs. Many studies did not record AEs and DOs at each follow-up assessment, rather, they reported these outcomes at the first assessment period post exercise intervention and then summarized the outcomes across all evaluation timepoints.
The PEDro scoring method27 was used to independently rate the internal validity of the studies and identify risk of bias. Specific data elements examined included: eligibility, random allocation, concealed allocation, baseline comparability, subject blinded, therapist blinded to allocation, assessor blinded to allocation, measures of key outcomes, intention to treat, results comparison, and point estimate of variability. One point was given to each item, except for study eligibility criteria, which is not included in the PEDro score. These points were then summed to create a single score for each article27 ranging from 0 to 10.When disagreements occurred between the raters regarding the PEDro scoring, the researchers discussed and came to a consensus regarding the scoring attributed to the articles (Suppl. Appendix 4).
Data Synthesis and Analysis
We conducted both a qualitative and quantitative synthesis of the studies. A meta-analysis was not conducted due to study heterogeneity and the focus on counts of outcomes vs intervention effect sizes.28 To evaluate therapeutic exercise data, data were grouped into categories of exercise modes (strengthening, aerobic, etc.), frequency per week, and session durations (unspecified, 1–2 times per week, etc.). In cases where a range of values was provided for any of these variables, the average was calculated, or the number was rounded up to the next category. Next, the percentage of studies using various modes of exercise, along with exercise intensity level and how intensity was assessed, duration, and frequency was calculated. We stratified studies based on the timeframe of the publication of the CONSORT-2010 statement using a cut point of 2011 (eg, by pre-CONSORT-2010 statement and post-CONSORT-2010 statement publication).13
We calculated the percentage of AEs and DOs occurring among the group of individuals exercising within each study or each study arm (when more than 1 exercise program was being tested). In studies where it was difficult to ascertain the reasons for DOs due to data being reported across intervention groups and during the follow-up periods when no therapeutic exercise was provided, we coded those data as unspecified reasons for DOs. We documented all reasons for DOs and specifically identified when the reason for dropping out of a study was an exercise-related harm (ie, DOs that met the definition of an AE were reclassified as AEs). For example, if exercise-related knee pain was given as a reason for someone refusing to continue in the study, then the DO was reclassified as an AE. Additionally, AEs and newly reclassified DOs were categorized by severity using the following operational definition: severe (fracture, permanent damage, disability, or death) and nonserious (muscle strain, soreness, fall not related to exercise program).29
χ2 tests were used to determine whether differences existed regarding a clear statement of AEs (Y/N) and DOs (Y/N) and regarding the misclassification of dropouts, pre- and post-publication of the CONSORT-2010 statement.13 Finally, we examined the incidence rate of nonserious and severe AEs plus exercise-related DOs, along with their 95% CIs. To accomplish this, we calculated exposure time (n = 224,480 person hours of exercise) as the duration of exercise sessions (minutes) × frequency of sessions per week × length of exercise program (number of weeks) among studies that included these data. Forty-seven (30.9%) study arms with unspecified total weeks of exercise training or/and unspecified frequency per week or/and unspecified duration of individual exercise sessions were excluded from the incidence rate calculation. The statistical significance level for all tests was defined as a P value of .05. Data analysis was conducted using SPSS for Mac 25.0 (SPSS Inc., Chicago, IL, USA).
Role of the Funding Source
The funders played no role in the design, conduct, or reporting of this study.
Results
Study Characteristics and Study Quality
Of the included 113 studies conducted in 25 countries, 54 (47.8%) were published from countries in North America and Western Europe. These studies included 152 exercise arms, yielding 5909 subjects exercising. The median number of patients per therapeutic exercise arm was 28 (range 8–209) (Suppl. Appendix 2). The PEDro scores of these studies ranged from 4 to 9. There were 2 studies30,31 that had a PEDro score of 4. With respect to risk of bias, subject blinding was performed in 15 (13.3%) of studies, and assessors were blinded in 79 studies (69.9%) (Suppl. Appendix 4).
Attributes of Therapeutic Exercise
Thirty-eight studies included more than 1 exercise arm within the study (Suppl. Appendix 3). Most exercise interventions were prescribed for a period of 6 and up to 12 weeks (32.9%), and the most common frequency examined was 3 times per week (52.6%). In 61.2% of exercise arms, the duration of each exercise session was 30 minutes up to and including 60 minutes. Of the 152 supervised exercise arms, 112 were supervised throughout the program. Strengthening exercise, whether progressive strengthening or not, was the most common mode of exercise (30.3%), followed by various combinations of exercise modes. The assessment of exercise intensity was not specified in 57.9% of the study arms. Because there were too few studies that specified exercise intensity and had reported AEs, we were not able to determine whether exercise intensity was a causative factor for AEs (Tab. 1).
Table 1.
Attributes of Therapeutic Exercise Interventions (N = 152) in Randomized Controlled Trials of Exercise for Adults (N = 5909) With Knee Osteoarthritisa
| Category | No. of Study Arms (%) | No. of Exercisers (%) |
|---|---|---|
| Total weeks of exercise training | ||
| Unspecified | 1 (0.7) | 30 (0.5) |
| <6 wk | 20 (13.2) | 682 (11.5) |
| ≥6 wk and <12 wk | 50 (32.9) | 1406 (23.8) |
| 12 wk | 48 (31.6) | 2110 (35.7) |
| >12 wk to <52 wk | 27 (17.8) | 1145 (19.4) |
| ≥52 wk | 6 (3.9) | 536 (9.1) |
| Frequency per week | ||
| Unspecified | 6 (3.9) | 147 (2.5) |
| 1-2× | 52 (34.2) | 1899 (32.1) |
| 3× | 80 (52.6) | 3255 (55.1) |
| 4-5× | 11 (7.2) | 511 (8.7) |
| >5× | 3 (2.0) | 97 (1.6) |
| Duration of individual exercise sessions | ||
| Unspecified | 43 (28.3) | 1393 (23.6) |
| <30 min | 14 (9.2) | 695 (11.8) |
| ≥30 min to ≤45 min | 41 (27.0) | 1430 (24.2) |
| >45 to ≤60 min | 52 (34.2) | 2098 (35.5) |
| >60 min | 2 (1.3) | 293 (4.9) |
| Supervised sessions | ||
| Yes, partial | 40 (26.3) | 1796 (30.4) |
| Yes | 112 (73.7) | 4113 (69.6) |
| Modes of exercise | ||
| Unspecified | 2 (1.3) | 116 (1.9) |
| Strengthening (progressive or not) | 46 (30.3) | 1534 (26.0) |
| Aerobic (including walking) | 7 (4.6) | 148 (2.5) |
| Balance and neuromuscular training | 9 (5.9) | 260 (4.4) |
| Tai chi, yoga, and other nontraditional exercise | 18 (11.8) | 551 (9.3) |
| Any combined aerobic, flexibility, ROM, strengthening | 26 (17.1) | 1342 (22.7) |
| Aquatic or aquatic and land exercises | 15 (9.9) | 539 (9.1) |
| Combined balance, ROM, flexibility, strengthening | 27 (17.8) | 1249 (21.1) |
| Physical therapy plus all modes of exercise | 2 (1.3) | 170 (2.9) |
| Assessment of exercise intensity | ||
| Unspecified | 88 (57.9) | 3816 (64.6) |
| Perceived exertion scale | 21 (13.8) | 597 (10.1) |
| % heart rate reserve or maximal heart rate | 16 (10.5) | 651 (11.0) |
| % strength performance test | 24 (15.8) | 680 (11.5) |
| Not well described | 3 (2.0) | 165 (2.8) |
Some studies had more than 1 exercise program being tested. ROM = range of motion.
Reporting of AEs
Fifty studies (44.2%) included a statement of AEs, and 24 studies (21.2%) reported exercise-related AEs, for a total of 297 AEs (Tab. 2). Although the proportion of studies that included a statement of AEs increased pre- and post-CONSORT-201013 (37.2% vs 48.6%), this difference was not statistically significant (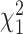 = 1.4; P = .24). We found no established definition of the severity of AEs nor classification of AEs by severity in many of these randomized controlled trials of therapeutic exercise. The following symptoms were recorded as AEs within the 113 studies: dizziness, back pain, hip pain, knee pain, falls/fractures, and “unspecified” injury during strength training. Among the 50 studies with a statement of AEs, 14 (28.0%) studies classified AEs by severity. To determine the total number of nonserious and severe AEs across all studies, we used a modified operational definition of severity of AEs by Vincent et al,29 and found 286 (96.3%) nonserious AEs and 11 (3.7%) serious AEs reported in these studies (Suppl. Appendix 3).
= 1.4; P = .24). We found no established definition of the severity of AEs nor classification of AEs by severity in many of these randomized controlled trials of therapeutic exercise. The following symptoms were recorded as AEs within the 113 studies: dizziness, back pain, hip pain, knee pain, falls/fractures, and “unspecified” injury during strength training. Among the 50 studies with a statement of AEs, 14 (28.0%) studies classified AEs by severity. To determine the total number of nonserious and severe AEs across all studies, we used a modified operational definition of severity of AEs by Vincent et al,29 and found 286 (96.3%) nonserious AEs and 11 (3.7%) serious AEs reported in these studies (Suppl. Appendix 3).
Table 2.
Summary of Adverse Event and Dropout Reporting in Clinical Trials of Therapeutic Exercise (N = 113)a
| Category | Number (%) |
|---|---|
| Total number of subjects in exercise arm | 5909 |
| Studies with a clear statement of AEs | 50 (44) |
| Studies that reported exercise-related AEs occurring among those subjects who were allocated to exercise | 24 (21.2) |
| Studies that reported exercisers experiencing an AE NOT related to exercise (among those with statement about AEs) | 5 (12.5) |
| Total number of exercisers in studies that had a clear statement of AEs | 2856 |
| Number of exercisers who had an exercise-related AE in exercise study arms that had a clear statement about AEs | 297 (10.4) |
| Number of exercisers who had an AE NOT related to exercise in studies that had a clear statement about AEs | 58 (2.0) |
| Studies with a clear statement of DOs | 103 (91.2) |
| Studies that reported DOs among studies that had a clear statement of DOs | 76 (73.9) |
| Studies in which reasons for DOs could be considered AEs (eg, back pain, knee pain, neck pain, leg pain, wrist pain) among all studies that had a statement of DOs | 16 (15.5) |
| Total number of exercisers in studies that had a clear statement of DOs | 5562 |
| Number of exercisers who dropped out where DO reason could be considered an AE among all exercisers in studies that reported DOs | 39 (0.7) |
| Number of exercisers who dropped out where DO reason was not considered harm | 605 (10.9) |
| Total number of exercisers in studies that had a statement of AEs or a statement about DOs | 5579 |
| Number of exercisers who dropped out where reasons for DO could be interpreted as an exercise-related AE plus total number of exercise-related AEs reported | 336 (6.0) |
AE = adverse event; DO = dropout.
Reporting of DOs
One hundred and three studies (91.2%) included a clear statement regarding DOs. There was no significant difference in the inclusion of a statement regarding DOs, pre- and post-CONSORT-201013 (88.4% vs 92.9%; 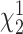 = 0.66; P = .42). Eighty studies (77.7%) reported DOs occurring during the exercise intervention (Tab. 2). Sixty-one studies (54.0%) reported using an intention-to-treat approach to analysis (Suppl. Appendix 4). The reasons for subject DOs during the intervention period included: heart problems, neck and back pain, leg and knee pain, patient did not like the exercise intervention, time commitment required, and chlorine sensitivity. The most common reason for dropping out was a musculoskeletal pain. Sixteen studies (15.5%) gave reasons for DOs that were similar to types of AEs reported in other studies of therapeutic exercise (Tab. 3).
= 0.66; P = .42). Eighty studies (77.7%) reported DOs occurring during the exercise intervention (Tab. 2). Sixty-one studies (54.0%) reported using an intention-to-treat approach to analysis (Suppl. Appendix 4). The reasons for subject DOs during the intervention period included: heart problems, neck and back pain, leg and knee pain, patient did not like the exercise intervention, time commitment required, and chlorine sensitivity. The most common reason for dropping out was a musculoskeletal pain. Sixteen studies (15.5%) gave reasons for DOs that were similar to types of AEs reported in other studies of therapeutic exercise (Tab. 3).
Table 3.
Comparison Between Types of Adverse Events and Reasons for Dropping Out of Studies Among Patients Who Exerciseda
| Types of Adverse Events Reported | Reasons for Dropping Out of Study That Are Similar to Reported Adverse Events | Reasons for Dropout That Are Not Similar to Adverse Events |
|---|---|---|
| Back pain Hip pain Leg pain Falls/fractures Knee pain Muscle strain/soreness Swollen knees Varices Dizziness Osteoarthritis flare/pain Injury during strength training |
Back pain Knee pain Neck pain Leg pain Wrist pain |
Did not like type of exercise Bad general health Required joint injection Chlorine sensitivity Unable to attend sessions NSAID taken after allocation Heart problems Discontinued intervention—no reason |
a NSAID = nonsteroidal anti-inflammatory drug.
On a subject-level, 39 of 644 (6.1%) individuals who dropped out did so for reasons that could be considered exercise-related AEs, yielding 336 patients (6.0%) experiencing exercise-related AEs. None of the 39 reclassified DOs could be considered severe AEs. There was a significant difference in misclassification of DOs pre- and post-CONSORT-201013 (12.2% vs 3.1%; χ21 = 21.2; P < .00001), wherein studies published prior to the statement had more misclassification of DOs than those published after the statement.
Association Between Therapeutic Exercise and Severity of AEs
Due to study heterogeneity with respect to exercise program attributes or lack of specificity of exercise program data, it was not possible to conduct a meta-analysis. However, among the 105 exercise arms with complete information regarding exposure time there were 248 episodes of exercise-related harm. The incidence rate per 100,000 exercise hours for nonserious AEs/DOs (n = 237) was 105.6 (95% CI = 101.1–110.3). For severe AEs/DOs (n = 11), the incidence rate was 4.9 (95% CI = 4.0–6.0) per 100,000 exercise hours. Regardless of severity, the incidence rate for all AEs/DOs was 110.5 (95% CI = 105.9–115.3) per 100,000 exercise hours.
Discussion
We found that 44.2% of the 113 randomized controlled trials across the 40-year period included a statement regarding AEs. This result is similar to data in another systematic review by Quicke et al22 that examined 49 studies of physical activity interventions among adults aged 45 years and older with knee pain and reported 45% of studies included information about AEs. Ethgen et al18 examined harm reporting in pharmacological and nonpharmacological studies for the management of rheumatic disease including exercise for OA and found 47.3% of nonpharmacological studies included a statement of AEs. These data are concerning and suggest adherence to CONSORT reporting guidelines is poor in studies of exercise for OA. There is a possibility that researchers examining therapeutic exercise for OA management believe that it is safe and focus less on the reporting of intervention-related harm compared with researchers engaged in drug trial studies18,32,33 or spinal manipulation,34 where transient discomfort can be experienced immediately following the procedure, though serious AEs associated with its use have not been identified. Additionally, unlike pharmacological interventions, which have strict regulatory guidelines for testing, therapeutic exercise is not held to that same level of scrutiny.18,32,33 This low prevalence of AE statements suggests that harm reporting in clinical trials of therapeutic exercise may receive less attention than issues related to internal consistency and study efficacy. Although some transient musculoskeletal sequelae can be expected at the beginning of an exercise program, it is important to inform patients that transient discomfort may be expected to reduce their fears and decrease the impact of fear avoidance behaviors. But, if musculoskeletal pain is significant enough to lead patients to discontinue exercise and drop out of the study, then we propose that reasons for dropping out should also be considered an AE.
The CONSORT harm statement requires researchers to classify the severity of AEs.17 We found 14 of the 50 studies that included a statement about AEs classified AEs by severity. This low rate of adherence to the CONSORT harm statement is similar to rates reported in other studies of nonpharmacological interventions in arthritis.18,22 For example, Ethgen et al found 16.2% of nonpharmacological studies reported severity of AEs, compared to 59.7% of pharmacological studies.18 In this review, we classified all AEs and found 11 (3.7%) serious AEs. Thus, specific attention to reporting severity of AEs in clinical trials of therapeutic exercise in knee OA is warranted.
Given that exercise requires active participation of patients over an extended period of time and adherence rates are known to be low, researchers often use intention-to-treat analysis to account for DOs. Thus, we found a much higher rate (91.2%) of DO statements, and there was no statistically significant difference in these statements pre-and post-CONSORT-2010.13 However, among the 16 studies that reported DOs, the intervention-related reasons for dropping out were reported as AEs in other studies. These data support the hypothesis that there is no consistency regarding DO classification across studies of therapeutic exercise, leading to a potential for greater exercise-related harm than currently reported. To enhance the credibility of studies of therapeutic exercise in knee OA, it is important for authors to report operational definitions of AEs and DOs within their methods. Additionally, many studies did not report the exact timing of DOs across the study timeframe. We agree the CONSORT Harms Statement17 is the gold standard for reporting of harms in randomized controlled trials. Based on the results of this systematic review, we provide additional recommendations for documenting exercise interventions and reporting the number of DOs and AEs at each time point. We emphasize that it is important to clarify whether an AE led to a patient dropping out of the study. Finally, because knee pain is a symptom of knee OA, it is important to be able to determine whether knee pain experienced during a study intervention reaches a threshold that qualifies it for being defined as a “harm” (Fig. 2).
Figure 2.

Additional recommendations for reporting of harm in clinical trials of therapeutic exercise for knee osteoarthritis to complement the CONSORT harms statement. KOA = knee osteoarthritis.
We also aimed to determine whether AEs and DOs were classified differently in studies published prior to vs after the CONSORT-2010 statement.13 We were able support our hypothesis that reporting of AEs was affected by the COSNORT statement, because there was a statistically significant difference in inclusion of statements regarding AEs before and after its publication. Thus, CONSORT has improved the manner in which RCTs are reported, and these positive impacts can only help to improve our ability to evaluate the evidence for therapeutic exercise in the management of knee OA.
There is agreement across health professionals that therapeutic exercise, although providing benefits presents greater than minimal risk (eg, may cause fatigue or musculoskeletal pain beyond what would normally be experienced by the subjects in daily activities).35
Therapeutic exercise should be prescribed to patients in the same manner as medications and along with clear instructions to ensure therapeutic exercises are performed in the correct manner (ie, dose, frequency, intensity, mode, and supervision).21 Within these studies, therapeutic exercise interventions were not fully described. Researchers consistently reported the frequency of exercise per week and total length of the exercise program. Not surprisingly, the most common frequency used was 3 times per week, because this is often considered the minimal frequency for obtaining benefits from exercise.20,21 However, the duration of individual sessions was difficult to discern in many cases, especially when exercises were prescribed as a number of repetitions for a series of exercises. This limited our ability to synthesize the data from these studies with other studies that provided the duration of the exercise session. There were also many different modes of exercise and combinations of modes of exercise, making it difficult to discern what types of exercise may be less harmful in specific subgroups of patients. Interestingly, we found assessment of exercise intensity was not reported in 57.9% of the exercise programs used in these clinical trials, despite the fact it is common knowledge that intensity of exercise has been associated with risk of harm.36 It is important to include all dimensions of therapeutic exercise and identify which subgroups of patients benefit the most from various therapeutic exercise prescriptions.37 It was beyond the scope of this article to try to determine which type of therapeutic exercise is less harmful for these patients. We did attempt to ascertain the incidence rate of severe and nonserious AEs among these studies of therapeutic exercise programs and found the rate was low.
Limitations and Strengths
There exists a risk of misclassification bias of study-reported AEs because each research team used their own definition of AEs. Additionally, our reclassification of DOs as AEs may be at risk of misclassification bias because the details regarding DOs were vague. However, we used a standardized approach to categorize the reasons for DOs and a published definition of AEs.29 Although we did not assess interrater reliability for coding of study elements, the research team a priori developed and used a standardized protocol for data extraction and conducted data extraction separately before coming to a consensus regarding coding. Because we included only studies published in English, we may not be including all relevant studies. To examine AEs and DOs by exercise dose, we calculated incidence ratios, combining modes of exercise and calculating the summative exposure. However, data were limited regarding exercise attributes, and many studies used mixed modes of exercise, which prevented subgroup analysis of AEs and DOs by mode of exercise.
With respect to study strengths, only randomized controlled trials of therapeutic exercise without concurrent inclusion of modalities or medications were included to ensure that harm, when it occurred, was attributed only to therapeutic exercise. We also used a universally accepted method, PEDro,27 for scoring study quality. The search was repeated a second time prior to data extraction to confirm the accuracy of the included studies. Data were extracted regarding all aspects of the therapeutic exercise interventions to determine the association between exercise attributes and potential harm. However, the level of missing data regarding interventions prohibited an evaluation of exercise dose and potential harm. Finally, due to the heterogeneity of the studies, a meta-analysis could not be performed.
Conclusions
Although evidence suggests that therapeutic exercise is a safe treatment option for managing knee OA symptoms,1–3 there is room for improvement in the reporting of AEs and DOs in trials of therapeutic exercise. AEs and DOs may be underreported and not sufficiently represent the risk associated with therapeutic exercise for knee OA. Greater clarity regarding AE definitions and adherence to the CONSORT Harm statement17 is needed to best determine safe dosing of therapeutic exercise for knee OA. Exercise intensity is frequently not specified in randomized controlled trials of therapeutic exercise and the duration of the individual exercise sessions is often missing. Greater details regarding the interventions must be included in published articles to ensure proper implementation of exercises for the treatment of knee OA. We recommend establishing an operational definition of AEs and DOs for therapeutic exercise trials and that researchers use the Template for Intervention Description and Replication (TIDier),9 CONSORT statements,13,17 and Consensus on Exercise Reporting Template (CERT)38 to describe their interventions details and record AEs and DOs. Results of this article can be used to inform the design and reporting of trials of therapeutic exercise.
Supplementary Material
Contributor Information
Johan von Heideken, Department of Women’s and Children’s Health, Karolinska Intitutet, Stockholm, Sweden.
Sana Chowdhry, College of Health Professions, Department of Health Sciences, Northeastern University, Boston, Massachusetts, USA.
Joanna Borg, College of Health Professions, Department of Health Sciences, Northeastern University, Boston, Massachusetts, USA.
Khara James, Department of Rehabilitation and Movement Sciences, Northeastern University, Boston, Massachusetts, USA.
Maura D Iversen, Department of Women’s and Children’s Health, Karolinska Intitutet, Stockholm, Sweden; Department of Rehabilitation and Movement Sciences, Northeastern University, Boston, Massachusetts, USA; College of Health Professions, Departments of Public Health and Physical Therapy and Movement Sciences, Sacred Heart University, Fairfield, Connecticut, USA; Section of Clinical Sciences, Division of Rheumatology, Inflammation and Immunity, Brigham and Women’s Hospital, Department of Medicine, Harvard Medical School, Boston, Massachusetts, USA.
Author Contributions
Concept/idea/research design: M.D. Iversen
Writing: J. von Heideken, S. Chowdhry, K. James, M.D. Iversen
Data collection: J. von Heideken, S. Chowdhry, J. Borg, M.D. Iversen
Data analysis: J. von Heideken, S. Chowdhry, J. Borg, K. James, M.D. Iversen
Project management: M.D. Iversen
Funding
M.D. Iversen was supported in part by grants from the National Institutes of Health (R03 AR057133-0) and National Institute of Arthritis and Musculoskeletal and Skin Diseases-Multidisciplinary Clinical Research Center (NIAMS-MCRC) (P60 AR047782).
Systematic Review Registration
The protocol for this systematic review was registered on PROSPERO 2019 (CRD42019136191) and is available in full on the National Institute for Health Research Health Technology Assessment (NIHR HTA) program website (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019136191).
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. Rausch Osthoff AK, Niedermann K, Braun J, et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77:1251–1260. [DOI] [PubMed] [Google Scholar]
- 2. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27:1578–1589. [DOI] [PubMed] [Google Scholar]
- 3. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465–474. [DOI] [PubMed] [Google Scholar]
- 4. Fu S, Thompson CL, Ali A, et al. Mechanical loading inhibits cartilage inflammatory signalling via an HDAC6 and IFT-dependent mechanism regulating primary cilia elongation. Osteoarthr Cartil. 2019;27:1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pisters MF, Veenhof C, van Meeteren NL, et al. Long-term effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a systematic review. Arthritis Rheum. 2007;57:1245–1253. [DOI] [PubMed] [Google Scholar]
- 6. Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622–636. [DOI] [PubMed] [Google Scholar]
- 8. American Physical Therapy Association . APTA guide to physical therapist practice 3.0. Updated 2014. Accessed September 15, 2021. http://guidetoptpractice.apta.org.
- 9. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 10. Bosomworth NJ. Exercise and knee osteoarthritis: benefit or hazard? Can Fam Physician. 2009;55:871–878. [PMC free article] [PubMed] [Google Scholar]
- 11. Niemeijer A, Lund H, Stafne SN, et al. Adverse events of exercise therapy in randomised controlled trials: a systematic review and meta-analysis. Br J Sports Med. 2020;54:1073–1080. [DOI] [PubMed] [Google Scholar]
- 12. Sherrington C, Fairhall N, Wallbank G, et al. Exercise for preventing falls in older people living in the community: an abridged Cochrane systematic review. Br J Sports Med. 2020;54:885–891. [DOI] [PubMed] [Google Scholar]
- 13. Schulz KF, Altman DG, Moher D, for the CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falci SG, Marques LS. CONSORT: when and how to use it. Dental Press J Orthod. 2015;20:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637–639. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–1194. [PubMed] [Google Scholar]
- 17. Ioannidis JP, Evans SJ, Gøtzsche PC, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141:781–788. [DOI] [PubMed] [Google Scholar]
- 18. Ethgen M, Boutron I, Baron G, Giraudeau B, Sibilia J, Ravaud P. Reporting of harm in randomized, controlled trials of nonpharmacologic treatment for rheumatic disease. Ann Intern Med. 2005;143:20–25. [DOI] [PubMed] [Google Scholar]
- 19. Zorzela L, Loke YK, Ioannidis JP, et al. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ. 2016;i157:352. [DOI] [PubMed] [Google Scholar]
- 20. Liu CJ, Latham N. Adverse events reported in progressive resistance strength training trials in older adults: 2 sides of a coin. Arch Phys Med Rehabil. 2010;91:1471–1473. [DOI] [PubMed] [Google Scholar]
- 21. Iversen MD. Managing hip and knee osteoarthritis with exercise: what is the best prescription? Ther Adv Musculoskelet Dis. 2010;2:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quicke JG, Foster NE, Thomas MJ, Holden MA. Is long-term physical activity safe for older adults with knee pain?: a systematic review. Osteoarthr Cartil. 2015;23:1445–1456. [DOI] [PubMed] [Google Scholar]
- 23. Wayne PM, Berkowitz DL, Litrownik DE, Buring JE, Yeh GY. What do we really know about the safety of tai chi?: a systematic review of adverse event reports in randomized trials. Arch Phys Med Rehabil. 2014;95:2470–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. García-Hermoso A, Ramirez-Vélez R, Sáez de Asteasu ML, et al. Safety and effectiveness of long-term exercise interventions in older adults: a systematic review and meta-analysis of randomized controlled trials. Sports Med. 2020;50:1095–1106. [DOI] [PubMed] [Google Scholar]
- 25. Bricca A, Harris LK, Jager M, Smith SM, Juhl CB, Skou ST. Benefits and harms of exercise therapy in people with multimorbidity: a systematic review and meta-analysis of randomised controlled trials. Ageing Res Rev. 2020;63:101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iversen MD, von Heideken J, Borg J, Chowdhry S. Reporting of adverse events in randomized controlled trials of therapeutic exercise for knee osteoarthritis: a systematic review. PROSPERO 2019 CRD42019136191. November 2019. Accessed September 15, 2021. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019136191.
- 27. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 28. Mosteller F, Colditz GA. Understanding research synthesis (meta-analysis). Annu Rev Public Health. 1996;17:1–23. [DOI] [PubMed] [Google Scholar]
- 29. Vincent KR, Vasilopoulos T, Montero C, Vincent HK. Eccentric and concentric resistance exercise comparison for knee osteoarthritis. Med Sci Sports Exerc. 2019;51:1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hurley MV, Scott DL. Improvements in quadriceps sensorimotor function and disability of patients with knee osteoarthritis following a clinically practicable exercise regime. Br J Rheumatol. 1998;37:1181–1187. [DOI] [PubMed] [Google Scholar]
- 31. Isaramalai SA, Hounsri K, Kongkamol C, et al. Integrating participatory ergonomic management in non-weight-bearing exercise and progressive resistance exercise on self-care and functional ability in aged farmers with knee osteoarthritis: a clustered randomized controlled trial. Clin Interv Aging. 2018;13:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raisch DW, Troutman WG, Sather MR, Fudala PJ. Variability in the assessment of adverse events in a multicenter clinical trial. Clin Ther. 2001;23:2011–2020. [DOI] [PubMed] [Google Scholar]
- 33. National Institutes of Health . Guidance on reporting adverse events to instructional review boards for NIH-supported multicenter clinical trials. NIH. June 1999. Accessed September 15, 2021. https://grants.nih.gov/grants/guide/notice-files/not99-107.html.
- 34. Ernst E. Prospective investigations into the safety of spinal manipulation. J Pain Symptom Manag. 2001;21:238–242. [DOI] [PubMed] [Google Scholar]
- 35. U.S. Department of Health and Human Services . Electronic Code of Federal Regulations, §46.102 Definitions for purposes of this policy. 1998. Accessed September 15, 2021. https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=83cd09e1c0f5c6937cd9d7513160fc3f&pitd=20180719&n=pt45.1.46&r=PART&ty=HTML#se45.1.46_1102.
- 36. Kesaniemi YK, Danforth E Jr, Jensen MD, Kopelman PG, Lefèbvre P, Reeder BA. Dose-response issues concerning physical activity and health: an evidence-based symposium. Med Sci Sports Exerc. 2001;33:S351–S358. [DOI] [PubMed] [Google Scholar]
- 37. Page P, Hoogenboom B, Voight M. Improving the reporting of therapeutic exercise interventions in rehabilitation research. Int J Sports Phys Ther. 2017;12:297–304. [PMC free article] [PubMed] [Google Scholar]
- 38. Slade SC, Dionne CE, Underwood M, Buchbinder R. Consensus on exercise reporting template (CERT): explanation and elaboration statement. Br J Sports Med. 2016;50:1428–1437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


