ABSTRACT
Avian pathogenic Escherichia coli (APEC), a subgroup of extraintestinal pathogenic E. coli (ExPEC), causes colibacillosis in chickens and is reportedly implicated in urinary tract infections and meningitis in humans. A major limitation for the current ExPEC antibiotic therapy is the development of resistance, and antibacterial drugs that can circumvent this problem are critically needed. Here, we evaluated eight novel membrane-affecting anti-APEC small molecule growth inhibitors (GIs), identified in our previous study, against APEC infection in chickens. Among the GIs tested, GI-7 (the most effective), when administered orally (1 mg/kg of body weight), reduced the mortality (41.7%), severity of lesions (62.9%), and APEC load (2.6 log) in chickens. Furthermore, GI-7 administration at an optimized dose (60 mg/liter) in drinking water also reduced the mortality (14.7%), severity of lesions (29.5%), and APEC load (2.2 log) in chickens. The abundances of Lactobacillus and oleate were increased in the cecum and serum, respectively, of GI-7-treated chickens. Pharmacokinetic analysis revealed that GI-7 was readily absorbed with minimal accumulation in the tissues. Earlier, we showed that GI-7 induced membrane blebbing and increased membrane permeability in APEC, suggesting an effect on the APEC membrane. Consistent with this finding, the expression of genes essential for maintaining outer membrane (OM) integrity was downregulated in GI-7-treated APEC. Furthermore, decreased levels of lipopolysaccharide (LPS) transport (Lpt) proteins and LPS were observed in GI-7-treated APEC. However, the mechanism of action of GI-7 currently remains unknown and needs further investigation. Our studies suggest that GI-7 represents a promising novel lead compound that can be developed to treat APEC infection in chickens and related human ExPEC infections.
IMPORTANCE APEC is a subgroup of ExPEC, and genetic similarities of APEC with human ExPECs, including uropathogenic E. coli (UPEC) and neonatal meningitis E. coli (NMEC), have been reported. Our study identified a novel small molecule growth inhibitor, GI-7, effective in reducing APEC infection in chickens with an efficacy similar to that of the currently used antibiotic sulfadimethoxine, notably with an 8-times-lower dose. GI-7 affects the OM integrity and decreases the Lpt protein and LPS levels in APEC, an antibacterial mechanism that can overcome the antibiotic resistance problem. Overall, GI-7 represents a promising lead molecule/scaffold for the development of novel antibacterial therapies that could have profound implications for treating APEC infections in chickens, as well as human infections caused by ExPECs and other related Gram-negative bacteria. Further elucidation of the mechanism of action of GI-7 and identification of its target(s) in APEC will benefit future novel antibacterial development efforts.
KEYWORDS: APEC, ExPEC, small molecule, Lpt system, LPS, Lactobacillus, oleate, lipopolysaccharide, LptD/E
INTRODUCTION
Avian pathogenic Escherichia coli (APEC), a subgroup of extraintestinal pathogenic E. coli (ExPEC), is a foodborne zoonotic pathogen and a source of extraintestinal infections in humans (1–4). In particular, APEC shares a genetic similarity with human ExPECs (uropathogenic E. coli [UPEC] and neonatal meningitis E. coli [NMEC]), possesses UPEC- and NMEC-associated virulence genes, and is able to cause urinary tract infections and meningitis in rodent models (1). Furthermore, the detection of APEC-specific colicin V (ColV) plasmids in human ExPEC isolates suggests a possible zoonotic transmission of APEC from poultry to humans (4). Moreover, poultry hatcheries and poultry products are regarded as reservoirs for antibiotic-resistant E. coli strains (5). Therefore, the consumption of contaminated poultry products could contribute to the transfer of antibiotic-resistant E. coli strains, as well as antibiotic resistance genes (ARGs), to human pathogens, which can make human infections difficult to treat (1, 5). Thus, APEC is a pathogen of significant importance to human health.
APEC is a very common bacterial pathogen of poultry, including chickens, turkeys, and ducks, and of many other avian species (6, 7). It is widely prevalent in all age groups of chickens (9.52 to 36.73%), with specifically high prevalence in adult layer birds (36.73%) (8). It is estimated that at least 30% of the commercial flocks in the United States at any point in time are affected by APEC (9). APEC causes a wide range of localized and systemic infections in poultry, including yolk sac infection, omphalitis, respiratory tract infection, swollen head syndrome, septicemia, polyserositis, coligranuloma, enteritis, cellulitis, and salphingitis, commonly referred to as avian colibacillosis (10). Colibacillosis can result in high morbidity and mortality (up to 20%), decreased meat and egg production, and increased condemnation (up to 43%) of carcasses at slaughter (1, 11, 12), thus leading to extensive economic losses in all facets of the global poultry industry (13).
At present, antibiotics (tetracyclines, sulfonamides, and aminoglycosides) are commonly used to control colibacillosis in poultry (14). However, evidence exists that APEC strains are becoming more resistant to these antibiotics, indicating that the control of colibacillosis is likely to become even more challenging in the future (9). Up to 92% of APEC isolates characterized in the United States, Europe, and Australia were resistant to three or more antibiotics, particularly against tetracyclines, aminoglycosides, and sulfonamides (13). Recently, APEC isolates with the mcr-1 gene, which confers resistance to colistin (one of the drugs of last resort to treat human infectious diseases), have been detected (15). Furthermore, APEC isolates with extended-spectrum-β-lactamase (ESBL) genotypes with resistance to β-lactam antibiotics have been also detected (16, 17). Moreover, the current vaccine (Poulvac E. coli) designed to prevent avian colibacillosis is only effective against homologous APEC serotype O78 challenge and fails to confer protection against heterologous serotypes (O1, O2, O8, O15, O35, O109, and O115) commonly associated with colibacillosis (13). Therefore, there is an urgent need for the development of new anti-APEC therapeutics for the effective control of this key endemic avian disease, including control of emerging antibiotic-resistant APEC infections.
Our previous study identified 11 small molecule growth inhibitors (GIs) with bactericidal activity against APEC (18). Previously, these GIs were designated SM1 to SM11 (18); however, in the current study they are referred as GI-1 to GI-11. Previously, using microscopy and in vitro studies (crystal violet uptake and loss of 260-/280-nm-absorbing materials) we showed that these GIs induced membrane defects like membrane blebbing, disruption of membrane, and increased membrane permeability (18), suggesting an effect on the APEC membrane. Antibacterial compounds that target the outer membrane (OM) of Gram-negative bacteria can counter the resistance problem (19, 20). The OM acts as a shield for bacteria by providing a permeability barrier, thus precluding the entry of antibiotics in bacteria (19, 21). The OM in Gram-negative bacteria is critical for viability and virulence and plays a key role in resistance to antibiotics (19, 21). The OM is composed of lipopolysaccharides (LPS), β-barrel proteins, phospholipids, and lipoproteins (21). These components are transported from the inner membrane (IM) and assembled at the OM by different assembly/transport complexes, including LPS transport (Lpt), β-barrel assembly (Bam), maintenance of lipid asymmetry (Mla), and localization of lipoproteins (Lol) complexes (21). Inhibition of the function of these transport/assembly complexes leads to a defective OM; therefore, the OM is an attractive target for the development of effective antibacterial drugs to circumvent the resistance problem (21). Different OM-targeting antibacterial compounds have been identified with activity against multidrug-resistant (MDR) Gram-negative pathogens, particularly E. coli and Pseudomonas aeruginosa (19, 21). The β-hairpin macrocyclic peptides (JB-95, murepavadin, and L27-11) and thanatin (insect-derived antimicrobial peptide) target the Lpt pathway, specifically LptD, thus inhibiting the transport of LPS to the OM (22–25). Similarly, darobactin (antibiotic derived from Photorhabdus), MRL-494 (synthetic compound), and LlpA (lectin-like bacteriocin) target the Bam complex, particularly BamA, resulting in inhibition of the assembly of OM proteins (26–28). Arenicin-3, a peptide derived from Arenicola marina, inhibits the Mla pathway, specifically MlaC, leading to perturbation of the OM phospholipid equilibrium (29). Notably, resistance is less likely to occur against OM-targeting antibacterial compounds, as they avoid the most common mechanisms for resistance development in bacteria, which are the entry barriers that prevent access by antibiotics and the efflux pumps that eliminate antibiotics from bacterial cells (19, 21). Moreover, OM-acting antibacterial compounds can make resistant bacteria susceptible to antibiotics when used in combination with antibiotics (19, 20, 30). Therefore, antibacterial compounds targeting the OM are promising leads for the development of novel therapeutics to circumvent the resistance problem in ExPECs and other Gram-negative bacteria.
In this study, we evaluated the efficacy and safety of eight membrane-affecting anti-APEC small molecule GIs identified in our earlier study (18). We extensively evaluated the most effective GI, GI-7, for its effect on the cecal microbiome and serum metabolome of chickens (31, 32). We optimized the delivery of GI-7 in drinking water of chickens and assessed the efficacy and safety under conditions mimicking the field settings. Furthermore, we explored the potential mechanism of action (MOA) of GI-7 by using bacterial cytological profiling (BCP) (18), gene expression, and immunoblot studies followed by in silico docking studies (22, 33, 34). Our findings demonstrate that GI-7 affects the OM integrity and decreases the Lpt protein and LPS levels in APEC through a mechanism that remains currently unknown and therefore warrants further investigation. Overall, GI-7 represents a promising antibacterial compound and scaffold for the development of novel antibacterial therapies for effectively treating APEC and other ExPEC infections and circumventing the antibiotic resistance problem.
RESULTS
Three GIs reduced the mortality, lesion severity, and APEC load in chickens.
We tested eight growth inhibitors (GIs) belonging to five different chemical scaffolds (pyrrolidinyl, GI-2, GI-3 and GI-7; imidazole, GI-6; quinoline, GI-8 and GI-9; piperidine, GI-1; and nitrophenyl, GI-10) (Fig. 1A) that were identified in our previous study (18) against APEC infection in chickens. In our earlier study, by screening 4,182 compounds, a total of 40 compounds were identified as having an inhibitory effect on the growth of APEC serotype O78 at a 100 μM concentration. The eight GIs selected in this study were effective against APEC in vitro, in cultured epithelial/macrophage cells, and in wax moth larva. These compounds were minimally toxic or nontoxic to eukaryotic cells and wax moth larva (18). The MICs of these GIs against APEC O78 ranged from 12.5 μM to 200 μM (GI-6 and GI-8, 12.5 μM; GI-7, GI-9, and GI-10, 25 μM; GI-2 and GI-3, 100 μM; and GI-1, 200 μM) (18). To measure the mortality reduction in chickens, the mortality rates observed in GI-treated groups were subtracted from the mortality rate in the positive-control (PC; infected and vehicle-treated) group (58.3%). GI-7 and GI-10 reduced the mortality by 41.7% and GI-6 reduced the mortality by 38.3% compared to the PC group (Fig. 1B). GI-2, GI-9, and GI-1 were less effective and reduced the mortality by 25%, 25%, and 8.3%, respectively. Consistent with the reduction of mortality, GI-7, GI-10, and GI-6 also prolonged the survival of chickens (P ≤ 0.1) (Fig. 1C).
FIG 1.
(A) Structures of growth inhibitors (GIs) tested against APEC infection in chickens in this study. (B) Mortality (%) in GI-treated groups compared to PC (infected and vehicle-treated; positive control) group. (C) Survival curve of chickens in PC- and GI-treated groups. *, P ≤ 0.1; log rank test.
The severity of the APEC lesions in liver, heart, lung, and air sacs of chickens was assessed, and the mean lesion score of each organ was calculated for each group. The cumulative mean lesion score was calculated by adding the mean lesion score of each organ. The PC (infected and vehicle-treated) chickens had a cumulative mean lesion score of 6.2, and only GI-7-treated chickens had a significantly lower (P < 0.05) cumulative mean lesion score of 2.3, while GI-2- and GI-6-treated chickens had cumulative mean lesion scores of 5.3 (P > 0.05) (Table 1). The treatment of APEC-infected chickens with GI-2, GI-6, and GI-7 reduced the lesion severity in liver, heart, air sacs, and lung by 14.5% to 62.9% (on average) in infected chickens (Table 1). The highest reduction in lesion severity was observed in the GI-7-treated group (52.9% to 83.3%), followed by the GI-6-treated (20% to 40%) and GI-2-treated (12% to 33.3%) groups. GI-7 treatment significantly (P < 0.05) reduced the severity of liver lesions by 60%, heart lesions by 60%, lung lesions by 83.3%, and air sac lesions by 52.9% (Table 1). Images showing the APEC lesions in internal organs of GI-7-treated (most effective GI) and PC chickens are displayed in Fig. 2A.
TABLE 1.
APEC lesion scores and reduction of lesion severity in GI-treated chickens
| Trial, treatmenta | Mean lesion score (% reduction in severity) inb: |
Cumulative lesion score | |||
|---|---|---|---|---|---|
| Liver | Heart | Lung | Air sacs | ||
| Efficacy | |||||
| GI-1 | 2.0 (−20) | 2.0 (4) | 0.7 (33.3) | 1.5 (−5.9) | 6.2 |
| GI-2 | 1.7 (0) | 1.8 (12) | 0.7 (33.3) | 1.2 (17.7) | 5.3 |
| GI-3 | 2 (−20) | 2 (4) | 1 (0) | 1.8 (−29.4) | 6.8 |
| GI-6 | 1 (40) | 1.7 (20) | 0.7 (33.3) | 2 (−41.2) | 5.3 |
| GI-7 | 0.7 (60)* | 0.8 (60)* | 0.2 (83.3)* | 0.7 (52.9)* | 2.3 |
| GI-8 | 2.8 (−68) | 3.8 (−82.4) | 2 (−100) | 3 (−111.8) | 11.6 |
| GI-9 | 2.5 (−50) | 3.3 (−60) | 2 (−16.7) | 2.7 (−88.2) | 9.7 |
| GI-10 | 1.3 (20) | 2.2 (−4) | 1 (0) | 1.7 (−17.6) | 6.2 |
| PC | 1.7 | 2.1 | 1 | 1.4 | 6.2 |
| GI-7 dose-response | |||||
| 20 mg/liter | 2 (0) | 2.7 (−9.5) | 1.8 (10) | 2.3 (1.4) | 8.8 |
| 40 mg/liter | 2 (0) | 2.6 (−5.4) | 1.4 (30) | 1.4 (27.1) | 7.7 |
| 60 mg/liter | 1.7 (13.6) | 2.1 (15.2) | 1.4 (36.3)* | 1.7 (33.8)* | 6.6 |
| PC | 2 | 2.5 | 2 | 2.3 | 8.8 |
| Comparative efficacy | |||||
| GI-7 | 2.3 (7.2) | 3.2 (10.2)* | 1.3 (29.5)*** | 3.0 (9.9) | 9.8 |
| SDM | 2.0 (19.2)* | 2.8 (20.8)** | 1.2 (36.9)*** | 2.5 (25.7)** | 8.5 |
| PC | 2.5 | 3.6 | 1.8 | 3.7 | 11.3 |
PC, positive control (infected and vehicle-treated chickens); SDM, sulfadimethoxine.
Statistical significance compared to PC chickens is shown by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 2.

(A) APEC lesions in PC (infected and vehicle-treated; positive control) and GI-7-treated groups. Images of the internal organs of all the chickens from the PC and GI-7 groups are included. In the PC group, chickens 1, 2, and 3 showed severe APEC lesions (yellow arrows), while only chicken 1 in the GI-7 groups showed mild APEC lesions. (B) Mean APEC loads in PC and GI-treated chickens. APEC loads were quantified in internal organs (liver, heart, lung, and kidney) of chickens and are displayed as the mean of all organs in each chicken. Red symbols show APEC loads in chickens that died within 7 days postinfection (dpi), black symbols show APEC loads in live chickens sacrificed and necropsied at 7 dpi, and horizontal bars show the mean value for each group. *, P < 0.05; Mann-Whitney U test.
The APEC burden was quantified in different internal organs (liver, heart, lung, and kidney) of the chickens. Treatment with GI-2, GI-6, GI-7, and GI-10 reduced the APEC loads in internal organs by 1.2 to 2.6 log/g of tissue (average APEC load across all internal organs combined) compared to the APEC load in the PC (infected and vehicle-treated) group (Fig. 2B). The average APEC load was determined by calculating the mean APEC load for all internal organs of each chicken. GI-7 most effectively reduced the APEC loads in internal organs (2.3 to 3.2 log) (P < 0.05), followed by GI-10 (0.8 to 2.4 log), GI-6 (1.2 to 1.9 log), and GI-2 (0.8 to 1.7 log) (Fig. 2B and Table S4 in the supplemental material). GI-7 treatment significantly (P < 0.05) reduced the APEC load in the liver by 2.5 log, in the lung by 2.4 log, in the heart by 2.3 log, and in the kidney by 3.2 log (Table S4). None of the GIs affected the body weight gain (BWG) of chickens (P > 0.05) compared to the BWG in the NC (noninfected and nontreated) group, except GI-2 (Table S5). GI-7-treated chickens had BWG similar to that of NC chickens.
Resistance to GIs was not observed in APEC isolated from treated chickens.
Randomly selected APEC colonies (n = 24/group) isolated from internal organs of chickens treated with GI-6, GI-7, and GI-10 were tested for their resistance against GIs. The same MICs and minimal bactericidal concentrations (MBCs) were observed before and after the treatment for all three GIs that showed impacts against APEC infection in chickens as described above (MICs/MBCs were as follows: GI-6, 12.5/25 μM; GI-7, 25/50 μM; and GI-10, 25/50 μM) (18). This is consistent with our earlier in vitro results where no resistance to GI-7 was detected when a high inoculum of APEC (109 CFU) was incubated with twice the lethal concentration (2× MBC) of GI-7 or passaged 15 times with a sublethal concentration (0.75× MIC) of GI-7 (18).
Lactobacillus abundance was significantly increased in GI-7-treated chickens.
We investigated the effects of GIs on gut microbiota through metagenomic analysis of the cecal microbiota of chickens (31). GI-treated chickens displayed a cecum microbial composition that was similar to that of the NC (noninfected and nontreated) group at the phylum level, except for the GI-10-treated group (Fig. 3A). The Firmicutes abundance was reduced (89% to 71%) and the Proteobacteria abundance was increased (11% to 29%) in GI-10-treated chickens. At the class level, the Bacilli abundance was increased (4% to 14%) in GI-7-treated chickens, while the Clostridia abundance was decreased (77% to 49%) in GI-10-treated chickens. Similar trends of increased Lactobacillales (4% to 14%) in GI-7-treated chickens and decreased Clostridiales (77% to 49%) in GI-10-treated chickens were observed at the order level. At the family level, the Lactobacillaceae population was significantly increased (1% to 11%) in GI-7-treated chickens, while the Lachnospiraceae population was significantly decreased (68% to 36%) in GI-10-treated chickens (Fig. 3B and Table S6). Specifically, Lactobacillus species (Lactobacillus zeae, 0% to 7%, and Lactobacillus helveticus, 0% to 4%) abundances were increased in GI-7-treated chickens and Lachnoclostridium (29.7% to 8.1%) abundance was decreased in GI-10-treated chickens (Table 2). Bacterial genera Blautia, Shuttleworthia, and Butyricicoccus were uniquely detected in GI-7-treated chickens, whereas Bifidobacterium (species longum), CAG-56, Clostridioides, Negativibacillus, and UBA1819 were uniquely detected in GI-10-treated chickens (Table 2). Ruminococcaceae member UCG-005 was uniquely detected in GI-6-treated chickens (Table 2). Interestingly, the abundance of Flavonifractor was increased in all GI-treated groups compared to its abundance in the PC (infected and vehicle-treated) group (Table 2). The microbial richness was significantly (P < 0.05) higher in GI-10-treated chickens than in GI-7-treated, PC, and NC groups (Fig. S4A). The microbial communities in GI-10-treated chickens were dissimilar (clustered differently) to those in NC, PC, GI-6-treated, and GI-7-treated chickens (Fig. 3C).
FIG 3.

(A) Microbial relative abundances at the phylum level in growth inhibitor (GI)-treated, NC (noninfected and nontreated; negative control), and PC (infected and vehicle-treated; positive control) groups. (B) Microbial relative abundances at the family level in GI-treated, NC, and PC groups. *, P < 0.05; ***, P < 0.001; Mann-Whitney U test. (C) Bray-Curtis dissimilarity graph comparing the microbial similarity/dissimilarity between GI-treated, PC, and NC groups.
TABLE 2.
Microbial composition in different treatment groups at genus level
| Microbe | Abundance (%) in cecal microbiota of indicated treatment group |
||||
|---|---|---|---|---|---|
| NC | GI-6 | GI-10 | GI-7 | PC | |
| Escherichia -Shigella | 8.9 | 6.5 | 26.6 | 12.3 | 8.5 |
| [Clostridium] inoculum group | 1.3 | 0.0 | 2.8 | 0.8 | 0.0 |
| Erysipelatoclostridium | 0.0 | 0.0 | 1.1 | 0.0 | 0.0 |
| [Eubacterium] coprostanoligenes group | 1.5 | 0.0 | 0.0 | 1.5 | 0.0 |
| UBA1819 | 0.0 | 0.0 | 1.1 | 0.0 | 0.0 |
| Ruminococcaceae UCG-014 | 0.0 | 1.2 | 0.4 | 0.0 | 0.0 |
| Ruminococcaceae UCG-005 | 0.0 | 2.7 | 0.0 | 0.0 | 0.0 |
| Ruminiclostridium 9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 |
| Oscillibacter | 0.5 | 1.7 | 0.8 | 3.0 | 5.1 |
| Negativibacillus | 0.0 | 0.0 | 1.6 | 0.0 | 0.0 |
| Flavonifractor | 3.0 | 5.0 | 7.7 | 3.8 | 0.0 |
| Caproiciproducens | 0.4 | 0.7 | 0.3 | 0.0 | 1.5 |
| Butyricicoccus | 0.0 | 0.0 | 0.0 | 4.0 | 2.1 |
| Anaerotruncus | 1.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| Clostridioides | 0.0 | 0.0 | 0.7 | 0.0 | 0.0 |
| [Ruminococcus] torques group | 9.0 | 27.3 | 13.9 | 24.8 | 15.2 |
| [Ruminococcus] gauvreauii group | 1.3 | 0.0 | 0.03 | 3.0 | 0.00 |
| Tyzzerella | 2.1 | 0.5 | 0.4 | 0.0 | 0.7 |
| Shuttleworthia | 0.0 | 0.0 | 0.0 | 1.7 | 0.0 |
| Sellimonas | 0.0 | 1.0 | 0.0 | 0.0 | 1.6 |
| Lachnoclostridium | 29.7 | 15.9 | 8.1 | 13.0 | 18.5 |
| CAG-56 | 0.0 | 0.0 | 0.7 | 0.0 | 0.0 |
| Blautia | 0.0 | 0.0 | 0.0 | 1.8 | 0.0 |
| Anaerostipes | 0.4 | 0.0 | 1.4 | 0.0 | 2.0 |
| Clostridium sensu stricto 1 | 0.0 | 2.9 | 0.3 | 0.0 | 0.0 |
| Lactobacillus | 1.4 | 1.5 | 1.9 | 11.02a,b | 1.6 |
| Enterococcus | 2.6 | 0.8 | 0.0 | 2.9 | 0.0 |
| Bacillus | 0.0 | 0.0 | 3.0 | 0.6 | 0.0 |
| Bifidobacterium | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 |
Significantly (P < 0.05) altered in GI-treated group compared to NC (noninfected and nontreated; negative control) group.
Significantly (P < 0.05) altered in GI-treated group compared to PC (infected and vehicle-treated; positive control) group.
No significant alterations in the microbial composition were observed between the PC and NC groups, except for an increased abundance of Peptostreptococcaceae (0% to 3.7%) and decreased abundance of Enterococcaceae (2.6% to 0%) in PC chickens (Fig. 3B). Specifically, Oscillibacter (0.5% to 5.1%), Sellimonas (0% to 1.6%), and Anaerostipes (species butyraticus; 0.4% to 2%) were increased while Anaerotruncus (1.6% to 0%), Flavonifractor (3% to 0%), Enterococcus (species cecorum; 2.6% to 0%), and Tyzzerella (2.1% to 0.7%) were decreased in APEC-infected chickens (Table 2).
Oleate was elevated in GI-7-treated chickens and l-arginine was elevated in APEC-infected chickens.
The metabolomic profiles of the serum of chickens treated with GIs were determined to assess the effects of GIs on the serum metabolites of chickens (32). The PC (infected and vehicle-treated) chickens had elevated levels (1.5- to 5.3-fold) of l-arginine, IMP, acetoacetate, (5)-methylmalonate-semialdehyde, and 2-oxobutanoate compared to the levels in NC (noninfected and nontreated) chickens (Table 3). Only a few metabolites were altered in GI-7-treated chickens. The level of oleate was elevated (2-fold; P < 0.05) in GI-7-treated chickens, while the level of l-saccharopine was decreased (greater than −2.7-fold; P < 0.05) compared to the levels in NC and PC chickens (Table 3). The levels of sucrose, α,α-trehalose, geranyl diphosphate, β-nicotinate d-ribonucleotide, l-saccharopine, 4-(-3-pyridyl)-butanoate, urate, l-lysine, 5-amino-1-(5-phospho-d-ribosyl)imidazole-4-carboxamide, coproporphyrinogen III, inosine, and thyronamine were elevated (1.9- to 5.4-fold; P < 0.05) in GI-10-treated chickens compared to the levels in NC chickens (Table 3). Compared to PC chickens, the levels of 4S/7S-hydroperoxy 17R-docosahexaeonate, aspirin-triggered resolvins D1/D2/D3/D4, thymine, 2′-deoxyinosine, 4-imidazolacetate, and uracil were elevated (2.1- to 3.8-fold; P < 0.05) in GI-10-treated chickens (Table 3). Interestingly, the levels of xanthine and xanthosine were elevated (3.7 to 5.6-fold; P < 0.05) in GI-10-treated chickens compared to the levels in both NC and PC chickens (Table 3). Similarly, nicotine-glucuronide, 4-hydroxy-4(3-pyridyl)-butanoate, 3-methoxy-4-hydroxyphenylglycoaldehyde, homovanillate, and d-sorbitol levels were elevated (1.4 to 2.7-fold; P < 0.05) in GI-6-treated chickens compared to the levels in NC and PC chickens (Table 3). Overall, only the gamma-linolenate biosynthesis pathway was affected by GI-7 treatment, while the urate biosynthesis/inosine 5′-phosphate degradation, adenosine nucleotide degradation, purine ribonucleoside degradation to ribose-1-phosphate, nicotine degradation IV/III, tRNA charging, lysine degradation I (saccharopine), and guanosine nucleotide degradation pathways were affected by GI-10 and GI-6 treatments. Principal-component analysis (PCA) revealed no distinct clustering of metabolome profiles between the treatment groups (Fig. 3C and Fig. S4B).
TABLE 3.
Metabolites that were significantly altered in APEC-infected and growth inhibitor-treated chickens
| Metabolitea | Fold change in indicated treatment groupb |
|||
|---|---|---|---|---|
| GI-6 | GI-10 | GI-7 | PC | |
| Xanthine | 2.8 | 3.7 | NA | −1.5 |
| Xanthosine | 3.6 | 5.6 | NA | 1.0 |
| 3-Mercaptopyruvate | 2.1 | 1.9 | NA | 2.0 |
| Sucrose | 2.4 | 2.6 | NA | 1.7 |
| Linoleate | NA | NA | −1.8 | −2.2 |
| α,α-Trehalose | 2.4 | 2.6 | NA | 1.7 |
| Nicotine-glucuronide | 2.7 | 1.5 | NA | 1.6 |
| 4-Hydroxy-4-(3-pyridyl)-butanoate | 1.9 | 1.3 | NA | 1.4 |
| 3-Methoxy-4-hydroxyphenylglycoaldehyde | 1.9 | 1.3 | NA | 1.4 |
| 19-Hydroxytestosterone | NA | NA | −2.4 | −3.2 |
| Homovanillate | 1.9 | 1.3 | NA | 1.4 |
| Geranyl diphosphate | 3.7 | 5.4 | NA | 3.6 |
| β-Nicotinate d-ribonucleotide | 3.7 | 5.4 | NA | 3.6 |
| l-Saccharopine | 2.2 | 3.0 | NA | 1.7 |
| 4-(3-Pyridyl)-butanoate | 1.8 | 1.9 | NA | 1.4 |
| Oleate | NA | NA | 2.0 | 1.4 |
| Urate | 2.5 | 3.0 | NA | 2.1 |
| l-Lysine | 1.8 | 2.2 | NA | 1.4 |
| 5-Amino-1-(5-phospho-d-ribosyl)imidiazole-4-carboxamide | 2.6 | 3.3 | NA | 2.1 |
| d-Sorbitol | 2.0 | 1.3 | NA | 1.4 |
| 15S-Hydroxypentaenoate | NA | NA | −1.3 | −1.4 |
| l-Arginine | 2.0 | 2.7 | 1.0 | 3.2 |
| 4S-Hydroperoxy,17R-H-docosahexaenoate | −1.1 | 1.2 | NA | −1.7 |
| 7S-Hydroperoxy,17R-H-docosahexaenoate | −1.1 | 1.2 | NA | −1.7 |
| Aspirin-triggered resolvin D1 | −1.1 | 1.2 | NA | −1.7 |
| (15R)-Hydroxypentaenoate | NA | NA | −1.3 | −1.4 |
| Aspirin-triggered resolvin D2 | −1.1 | 1.2 | NA | −1.7 |
| Aspirin-triggered resolvin D3 | −1.1 | 1.2 | NA | −1.7 |
| Aspirin triggered resolvin D4 | −1.1 | 1.2 | NA | −1.7 |
| Coproporphyrinogen III | 2.5 | 2.9 | NA | 2.0 |
| Inosine | 2.1 | 2.4 | NA | 1.5 |
| α-Carboxyethylhydroxychroman | NA | NA | −2.8 | 1.0 |
| Thyronamine | 2.1 | 2.4 | NA | 1.5 |
| Thymine | 1.1 | 1.3 | NA | −2.8 |
| 2′-Deoxyinosine | 1.1 | 1.3 | NA | −2.8 |
| 4-Imidazolacetate | 1.1 | 1.3 | NA | −2.8 |
| Cytidine | 1.8 | 2.6 | NA | −1.1 |
| l-Saccharopine | NA | NA | −2.8 | 1.0 |
| IMP | 1.8 | 1.6 | NA | 2.0 |
| Uracil | 1.2 | 1.6 | NA | −1.6 |
| Acetoacetate | 3.2 | 2.9 | NA | 5.3 |
| (S)-Methylmalonate-semialdehyde | 3.2 | 2.9 | NA | 5.3 |
| 2-Oxo-butanote | 3.2 | 2.9 | NA | 5.3 |
Boldface indicates the metabolites that were significantly (P < 0.05) altered as analyzed using Progenesis QI.
Fold change was calculated in comparison to the abundance of the metabolite in serum of NC (noninfected and nontreated; negative control) chickens. Fold increase (for metabolite with abundance higher than NC group) = abundance of metabolite in GI or PC (infected and vehicle-treated chickens; positive control) group/abundance of metabolite in NC group. Fold decrease (for metabolite with abundance lower than NC group) = abundance of metabolite in NC group/abundance of metabolite in GI or PC group. NA, not available.
GI-7 administered at 60 mg/liter in drinking water reduced mortality, lesion severity, and APEC load in chickens.
Since GI-7 was the most effective GI when administered orally in chickens, we selected GI-7 for further evaluation. To simulate the current field practice in the poultry industry, GI-7 was administered in drinking water for 7 days at doses of 20 mg/liter, 40 mg/liter, and 60 mg/liter. GI-7 treatments reduced the mortality by 10% to 50.9%, based on the dose, compared to that in the PC (infected and vehicle-treated; 60% mortality) group (Fig. 4A). As described above, the mortality rates observed in GI-7-treated groups were subtracted from that of the PC group. The GI-7 60-mg/liter treatment reduced the mortality by 50.9%, whereas the GI-7 40-mg/liter and GI-7 20-mg/liter treatments reduced the mortality by 50% and 10%, respectively. Furthermore, the GI-7 40-mg/liter and GI-7 60-mg/liter treatments significantly (P < 0.05) prolonged the survival of chickens (Fig. 4B).
FIG 4.
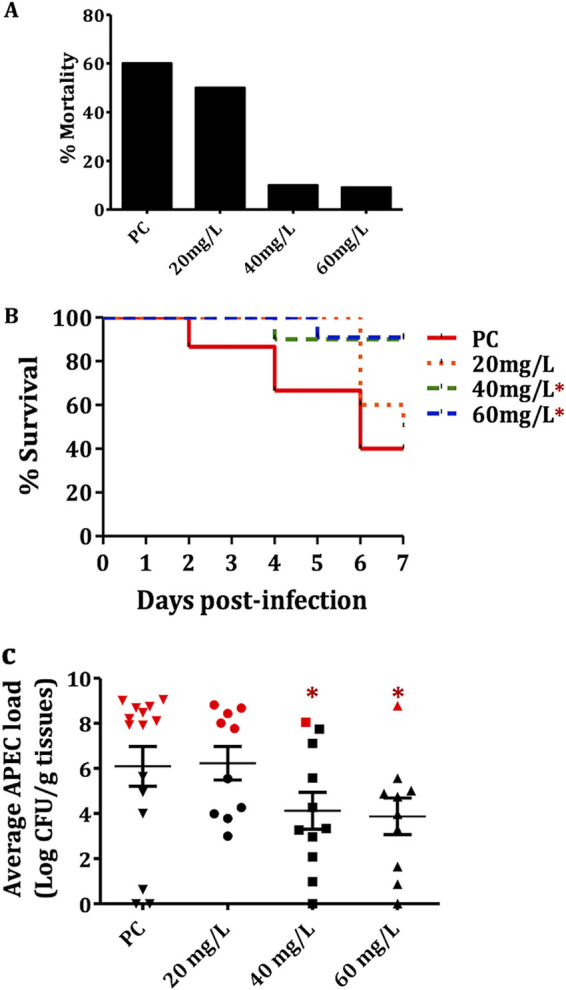
(A) Mortality (%) in GI-7 20-mg/liter, GI-7 40-mg/liter, and GI-7 60-mg/liter treatment groups compared to PC (infected and vehicle-treated; positive control) group. (B) Survival curve of chickens in PC and GI-7 20-mg/liter, GI-7 40-mg/liter, and GI-7 60-mg/liter treatment groups. *, P < 0.05; log-rank test. (C) APEC loads in PC and GI-7 20-mg/liter, GI-7 40-mg/liter, and GI-7 60-mg/liter treatment groups. APEC loads were quantified in internal organs (liver, heart, lung and kidney) of chickens and are displayed as the mean of all organs in each chicken. Red symbols show APEC loads in chickens that died within 7 days postinfection (dpi), and black symbols show APEC loads in live chickens sacrificed and necropsied at 7 dpi. *, P < 0.05; Student’s t test.
Treatments with GI-7 (40 mg/liter and 60 mg/liter) reduced the severity of APEC lesions in the internal organs of chickens by up to 36.3% compared to the severity of APEC lesions in the PC group (infected and vehicle-treated) (Table 1). The PC chickens had a cumulative mean lesion score of 8.8, whereas the GI-7 60 mg/liter-treated chickens had a cumulative mean lesion score of 6.6. GI-7 40 mg/liter- and GI-7 20 mg/liter-treated chickens had cumulative mean lesion scores of 7.7 and 8.8, respectively. The highest reductions in lesion severity were observed in the GI-7 60 mg/liter-treated group (13.6% to 36.3%) (Table 1). GI-7 60-mg/liter treatment reduced the severity of liver lesions by 13.6%, heart lesions by 15.2%, lung lesions by 36.3% (P < 0.05), and air sac lesions by 33.8% (P < 0.05) (Table 1).
Treatments with GI-7 (40 mg/liter and 60 mg/liter) significantly (P < 0.05) reduced the APEC loads in the different internal organs of chickens, by 1.6 to 2.5 log (Fig. 4C and Table S4). GI-7 60-mg/liter treatment significantly (P < 0.05) reduced the APEC load in the liver by 2.4 log, in the lung by 1.6 log, in the heart by 2.4 log, and in the kidney by 2.5 log (Table S4).
A follow-up study conducted using 80 mg/liter of GI-7 did not result in a better anti-APEC effect in chickens compared to the effect of treatment at 60 mg/liter (data not shown); therefore, 60 mg/liter was used for further studies.
GI-7 showed efficacy comparable to that of currently used antibiotic SDM without affecting BWG and feed conversion ratio (FCR) in chickens.
We compared the efficacy of GI-7 with that of sulfadimethoxine (SDM; a currently used antibiotic) in chickens under conditions simulating field conditions. GI-7 was administered at 60 mg/liter (optimized dose), whereas SDM was administered at its therapeutic dose (0.05%; 495.3 mg/liter). GI-7 reduced the mortality of chickens by 14.7% and SDM treatment reduced the mortality by 11.9% compared to that of the PC (infected and vehicle-treated; 41.2% mortality) group (Fig. 5A). As described above, the mortality rates observed in GI-7- and SDM-treated groups were subtracted from the mortality rate of the PC group. Both GI-7 and SDM treatments significantly (P < 0.1) increased the survival of chickens (Fig. 5B).
FIG 5.
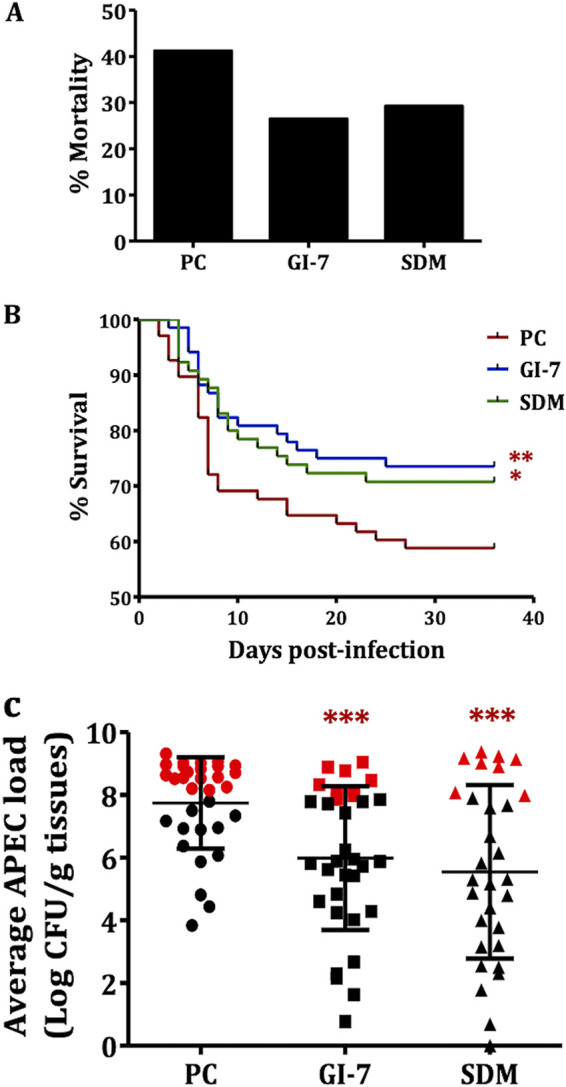
(A) Mortality (%) in GI-7- and sulfadimethoxine (SDM)-treated groups compared to PC (infected and vehicle-treated; positive control) group. (B) Survival curve of chickens in GI-7- and SDM-treated groups compared to PC group. *, P < 0.1; **, P < 0.05; log-rank test. (C) APEC loads in PC, GI-7-treated, and SDM-treated groups. APEC loads were quantified in internal organs (liver, heart, lung, and kidney) of chickens and are displayed as the mean of all organs in each chicken. Red symbols show APEC loads in chickens that died within 7 days postinfection (dpi), and black symbols show APEC loads in live chickens sacrificed and necropsied at 7 dpi. ***, P < 0.001; Student’s t test.
GI-7 and SDM treatments also significantly (P < 0.001) reduced the APEC load at 7 days postinfection (dpi) in the internal organs of chickens, by 1.6 to 2.5 log (GI-7, 1.6 to 2.0 log; SDM, 1.9 to 2.5 log) (Fig. 5C and Table S4). No significant difference in the APEC loads was observed between GI-7 and SDM treatments. Similarly, GI-7 and SDM treatments also decreased the severity of APEC lesions in the internal organs of chickens at 7 dpi (Table 1). The PC chickens had a cumulative mean lesion score of 11.6, whereas the GI-7- and SDM-treated chickens had cumulative mean lesion scores of 9.8 and 8.5, respectively. The reductions in lesion severity ranged from 7.2% to 29.5% in the GI-7-treated group and from 19.2% to 36.9% in SDM-treated group (Table 1). No significant difference in the APEC lesions was observed between GI-7 and SDM treatments.
No significant effect on the body weight gain (BWG) and feed conversion ratio (FCR) was observed with GI-7 and SDM treatments (Fig. S5A), which corroborates our earlier findings that GI-7 is minimally toxic to eukaryotic cells and wax moth larvae (18). Overall BWGs of 2,432.4, 2,408.1, 2,312.8, and 2,501.2 g were obtained in the GI-7, SDM, PC (infected and vehicle-treated), and NC (noninfected and not-treated) groups, respectively. Similarly, overall FCRs of 1.45, 1.49, 1.51, and 1.45 were obtained in the GI-7, SDM, PC, and NC groups, respectively (Fig. S5B).
GI-7 was rapidly absorbed upon oral delivery in chickens, with minimal accumulation in tissues.
Pharmacokinetic (PK) analysis of the plasma of GI-7-treated chickens revealed that GI-7 is absorbed rapidly (0.5 h) compared to the rate of absorption of sulfadimethoxine (SDM) (2 h) (Fig. 6A). Similarly, GI-7 is excreted by 24 h, whereas no excretion of SDM was observed until 24 h. The peak plasma concentration (Cmax, 434.2 ± 388.5 ng/ml [mean ± standard deviation]) of GI-7 was reached at 8 h (time to maximum concentration [Tmax]) postadministration, while the Cmax and Tmax for SDM were 993.6 ± 166.9 ng/ml and 24 h, respectively. Interestingly, no cumulative accumulation of GI-7 in plasma was observed after repeated administration, whereas accumulation was observed with SDM (data not shown). These data indicate that GI-7 has a shorter half-life than SDM.
FIG 6.

(A) Pharmacokinetic profile showing concentrations (ng/ml) of GI-7 and SDM in plasma of chickens at different time points (0, 0.5, 1, 2, 4, 8, 12, and 24 h) postadministration. (B) Concentrations (ppm/g) of GI-7 and SDM residues in tissues (muscle, liver, and kidney) of chickens. DPLT, days post-last treatment; SDM, sulfadimethoxine.
The residue analysis of GI-7 in chicken tissues showed relatively less or no accumulation of GI-7 compared to the accumulation of SDM, except in the liver (Fig. 6B). In the muscle, concentrations of 0.04 ± 0.03, 0.01 ± 0.01, and 0.0 ± 0.0 ppm/g tissue of GI-7 were observed at 2, 5, and 31 days post-last treatment (DPLT), respectively, while concentrations of 0.2 ± 0.1, 0.02 ± 0.02, and 0.0 ± 0.0 ppm/g tissue of SDM were observed at 2, 5, and 31 DPLT, respectively. Similarly, in the kidney, residue levels of 0.7 ± 0.6, 0.3 ± 0.3, and 0.0 ± 0.0 ppm/g tissue were observed in GI-7-treated chickens at 2, 5, and 31 DPLT, respectively, while residue levels of 1.0 ± 0.2, 1 ± 0.1, and 0.0 ± 0.0 ppm/g tissue of SDM were observed at 2, 5, and 31 DPLT, respectively. On the other hand, in the liver, residue levels of 0.5 ± 0.4, 0.2 ± 0.2, and 0.0 ± 0.0 ppm/g tissue of GI-7 were observed at 2, 5, and 31 DPLT, respectively, while residue levels of 0.2 ± 0.04, 0.04 ± 0.02, and 0.0 ± 0.0 ppm/g tissue of SDM were observed at 2, 5, and 31 DPLT, respectively. The residue quantitation of GI-7 in tissues of chickens showed GI-7 residues below the limits permitted by the Food and Drug Administration for antibiotics (35), which ensures the safety of GI-7 from the public health standpoint (36).
Expression of genes essential for OM integrity was downregulated and levels of Lpt proteins were decreased in GI-7-treated APEC.
Based on the similarity in BCPs (bacterial cytological profiles; accumulation of membrane-like material in the form of knobs inside the cells) observed between GI-7 (18), thanatin, and JB-95 (reported Lpt inhibitors) (22, 23), we investigated the effect of GI-7 on the expression of lpt genes by reverse transcription-quantitative PCR (RT-qPCR) and on total membrane proteins by SDS-PAGE (under nonreducing conditions) followed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of the excised ∼21.4-kDa (expected size of LptE protein) gel fragments. GI-7 treatment downregulated the expression of the lptD gene by 12.90-fold, while the lptE, lptA, and lptC genes were downregulated by 5.39-, 5.13-, and 4.99-fold, respectively, compared to their expression in untreated APEC (Fig. 7A). Furthermore, downregulation of lptD was higher than that of other OM genes (bamA, lolB, mlaA, and pbgA). Downregulation of expression by 7.72-, 1.92-, 9.94-, and 5.75-fold was observed for genes bamA, lolB, mlaA, and pbgA, respectively. Furthermore, in SDS-PAGE (nonreducing conditions), a decreased level of an ∼21.4-kDa (expected size of LptE) protein was observed with GI-7 treatment (Fig. 7B). Additionally, slightly faster mobility of an approximately 89-kDa protein (expected size of LptD) was observed in GI-7-treated APEC compared to its mobility in untreated APEC (Fig. 7B), suggesting the possible formation of an LptD intermediate (or nonfunctional LptD). The LptD intermediate with slightly faster mobility was observed in E. coli when LptE was limited (37). LC-MS/MS analysis also showed a depleted level (or low exponentially modified protein abundance index [emPAI]) of LptE in GI-7-treated APEC (emPAI of 8.46) compared to the level (emPAI of 13.84) in untreated APEC. Furthermore, immunoblot studies performed using anti-LptD and anti-LptE antibodies (GenScript) showed that GI-7 treatment decreased the LptE level and showed LptD with slightly faster mobility compared to that in untreated APEC, which might represent a nonfunctional LptD intermediate, as previously observed when LptE is limited (Fig. 7C) (37). A decreased level of another protein, of ∼22 kDa, which is the expected size of LptC (21.7 kDa), was also observed with GI-7 treatment (Fig. 7B). No noticeable changes in the cytoplasmic protein profiles were observed between GI-7-treated and untreated APEC in SDS-PAGE (data not shown).
FIG 7.
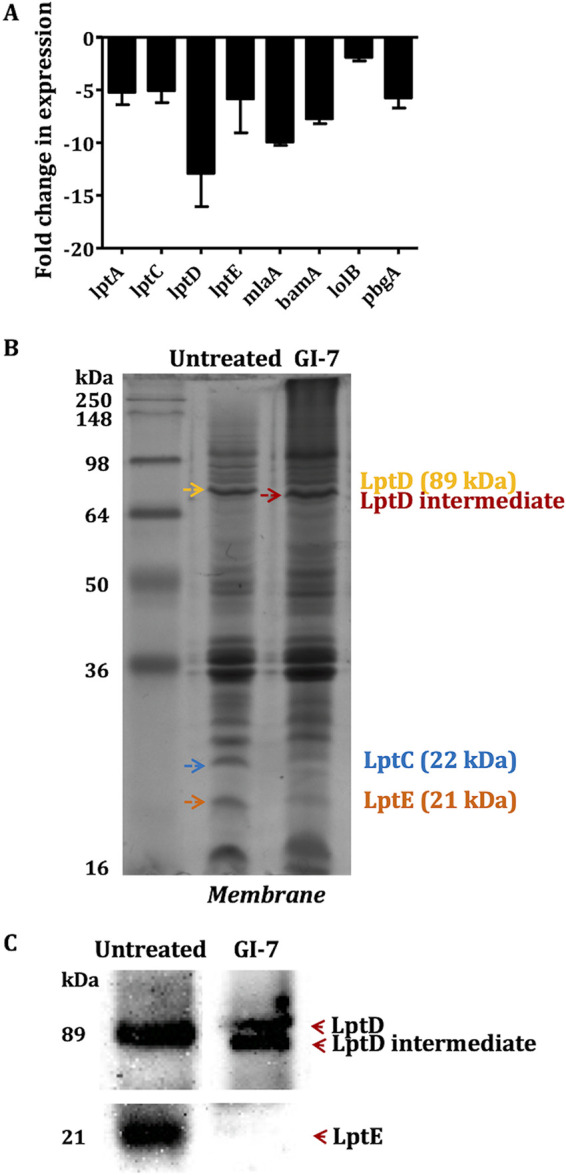
(A) Effects of GI-7 treatment on the expression of lpt and other genes essential for maintaining outer membrane (OM) integrity as determined by RT-qPCR. Fold change was calculated using the ΔΔCT method. (B) Effects of GI-7 treatment on total membrane proteins as determined by SDS-PAGE and staining with Coomassie blue R-250. Yellow arrow shows expected size of LptD, red arrow shows potential LptD intermediate, orange arrow shows expected size of LptE, and blue arrow shows expected size of LptC. (C) LptD and LptE immunoblots performed using anti-LptD and anti-LptE antibodies showing formation of potential LptD intermediate and depleted LptE level in GI-7-treated APEC compared to untreated APEC.
GI-7 decreases OM LPS level and is predicted to interact with Tyr244, Lys234, and Glu733 residues of LptD.
In silico docking studies using Autodock predicted that GI-7 is likely to bind in the LptD pocket between the β1 and β26 strands, wherein LPS enters the lumen channel (Fig. 8A and Fig. S6A) (38). GI-7 is predicted to interact with key amino acid residues (i) Tyr244, which is flanked by Pro246 and Pro231 at the lateral gate of the lumen, and (ii) Lys234, which is next to Thr236 inside the lumen of LptD (Fig. 8A and Fig. S6B and C). Additionally, docking using Autodock Vina predicted that GI-7 is also likely to bind with Glu733, in addition to Tyr244 and Lys234 (Fig. S7A). The predicted hydrogen bonding interactions of GI-7 with the Tyr244 and Lys234 residues of LptD are portrayed in Fig. S7B and C. The docking of GI-2 and GI-3, which have structures similar to that of GI-7 (Fig. 1A) but are less effective against APEC (18), revealed lower predicted binding affinities of GI-2 (−6.75 kcal/mol) and GI-3 (−7.26 kcal/mol) with LptD compared to that of GI-7 (−9.05 kcal/mol) (Table S7). Furthermore, the binding affinities of GI-6 (−4.75 kcal/mol) and GI-10 (−5.61 kcal/mol), which have different structures (Fig. 1A) than GI-7, are even lower than those of GI-2 and GI-3, suggesting that GI-7 and other compounds with structural similarity to GI-7 might specifically bind to LptD.
FIG 8.

(A) Predicted binding mode of GI-7 in the pocket of LptD. GI-7 is predicted to interact with key amino acid residues (i) Tyr244, which is flanked by Pro231 and Pro246 (shown in red) at the lateral gate of LptD, and (ii) Lys234, which is next to Thr236 (shown in yellow) inside the lumen of LptD. (B and C) Effect of GI-7 treatment on the level of outer membrane lipopolysaccharide (LPS; yellow arrow) as visualized by Coomassie staining (B) and quantitated by a total carbohydrate assay kit (C). ***, P < 0.0001; Student’s t test.
To further support that GI-7 affects the Lpt levels and, thereby, likely the LPS transport, we determined the LPS level in the OM of APEC treated with GI-7. A lower level of LPS was observed in OM of APEC treated with GI-7 than in OM of untreated APEC (Fig. 8B). This was further confirmed by quantitating the LPS levels using the total carbohydrate assay kit, which revealed a significantly (P < 0.0001) lower level of LPS in OM of GI-7-treated APEC than in OM of untreated APEC (Fig. 8C).
DISCUSSION
Previously, we identified eight small molecule growth inhibitors (GIs) (Fig. 1A) with promising in vitro (epithelial, macrophage, and red blood cells) and in vivo (wax moth larva model) activities against APEC (18). In this study, we show three GIs containing pyrrolidinyl (GI-7), nitrophenyl (GI-10), and imidazole (GI-6) scaffolds as having protective effects against APEC infection in chickens (Fig. 1 and 2). Among the three GIs, GI-7 was the most effective when administered orally and also showed efficacy when delivered in drinking water at a 60-mg/liter dose (Fig. 4 and 5), a dose 8 times lower than the standard dose of sulfadimethoxine (SDM), an antibiotic currently used to treat APEC infection in chickens. The delivery of GI-7 in drinking water simulates the current antibiotic treatment practice for treating APEC infection in the poultry industry (14). Notably, the dose of GI-7 (60 mg/liter) is relatively lower (3 to 75 times) than the doses of antibiotics commonly used in the poultry industry (sulfadimethoxine, 495 mg/liter; sulfaquinoxaline, 200 mg/liter; chlortetracycline, 4.5 g/liter; and ampicillin, 1.65 g/liter) (14). This has a significant advantage from the perspective of treatment costs, as well as the accumulation of drug residues in tissues of chickens, a parameter essential for the safety of food for human consumption (36). Furthermore, no in vivo resistance to GI-7 was detected in APEC isolated from chickens treated with GI-7. Moreover, resistance was also not detected in vitro in our earlier study when APEC was incubated with a lethal concentration or passaged with a sublethal concentration of GI-7 (18). Our findings are therefore noteworthy in the current situation, where high levels of resistance to available antibiotics have been reported (17) and there are increased restrictions on the use of medically important antibiotics in food-producing animals (39). The lack of APEC resistance to GI-7 might be due to the membrane-targeting mechanism of GI-7 (18), although a more thorough search for and characterization of GI-7-resistant mutants is necessary to facilitate industrial application of GI-7 as an anti-APEC therapeutic in the future. Membrane-targeting antibacterial drugs are less likely to acquire resistance and are also effective against antibiotic-resistant strains (20, 40). The APEC strain used in our chicken studies is resistant to ampicillin and tetracycline, two of the antibiotics most commonly used in food animals (18). Furthermore, our earlier study also showed that GI-7 can potentiate the effects of other antibiotics (colistin, tetracycline, and ciprofloxacin) (41) and, thus, can synergistically enhance the efficacy of current antibiotics. Therefore, combination therapy with GI-7 has a great potential to lower the dose of antibiotics needed for the treatment, which thereby can mitigate the emergence of antibiotic resistance (42) and potential transmission of antibiotic resistance genes (ARGs) to humans (43).
Studies including bacterial cytological profiling (18), expression of lpt genes, LptD/E immunoblot analysis, LPS quantitation in the OM, and in silico docking were performed to explore the potential mechanism of GI-7 (Fig. 7 and 8 and Fig. S6 and S7). GI-7 affected the OM integrity and decreased the Lpt protein and LPS levels in APEC. LPS is critical for bacterial growth, virulence, survival, and maintenance of the OM permeability barrier (44). Furthermore, LPS is an essential component for OM biogenesis/assembly in Gram-negative bacteria (44). The altered LPS level compromises the OM permeability barrier, thus resulting in loss of bacterial viability and growth (37). The OM permeability barrier is also responsible for inducing bacterial resistance against antibiotics by hindering antibiotics from reaching their intracellular targets (19, 21). Moreover, LPS plays a key role in virulence and pathogenesis of bacteria in the host (45, 46). Therefore, the LPS transport pathway is regarded as a novel and attractive target for the development of new antibacterial drugs, as well as to counter antibiotic resistance in Gram-negative pathogens (19, 21). LPS transport from the IM to the OM is mediated by the Lpt complex (LptABCDEFG) (37, 47). LptB2FGC transports LPS from the IM to the periplasmic space, followed by transport across the periplasmic space by LptA and, finally, to the OM by LptD/E (37). The interaction of LptD with LptE (i.e., the LptD/E complex) is crucial for the transport of LPS to the OM in Gram-negative pathogens (48). Our studies show that GI-7 treatment might induce the formation of LptD intermediate, as shown by slightly faster migration than for mature wild-type LptD in SDS-PAGE and an additional LptD band (or new species of LptD) in immunoblot analysis (37). The formation of LptD intermediate was also reported previously when LptE was depleted, similar to what we observed in our study, or in LptD mutants (LptDΔ330-352 and LptDΔ529-538) (37). LptE is essential for the proper assembly and maturation of functional LptD with the proper arrangement of disulfide bonds (37, 46, 49). The LptD intermediate, which lacks native disulfide bond(s), is nonfunctional and incapable of transporting LPS from the periplasmic space to the OM, resulting in defective bacterial OM (37, 49). The nonfunctional LptD intermediate is formed when LptE is limited and results in depleted LPS levels in the OM (37, 49). We showed a lower level of LPS in the OM of APEC treated with GI-7, in support of decreased Lpt protein levels in APEC treated with GI-7. Based on these data, a hypothetical mechanism of action (MOA) of GI-7 is displayed in Fig. S8. However, future studies that incorporate control antibiotics, including antibiotics already known to act through the Lpt system (for example, murepavadin) and antibiotics affecting OM integrity without interacting with the Lpt system (for example, colistin), are necessary to validate the Lpt system as a target of GI-7. Furthermore, the effect of GI-7 on the Lpt system could be indirect or secondary to other mechanism(s) of GI-7. Therefore, further investigation through direct GI-7–LptD/E binding studies using biophysical methods like nuclear magnetic resonance (NMR) and X-ray crystallography (XRC) are needed to validate GI-7’s interaction with the Lpt system. It is also important to recognize that GI-7 has an antibacterial effect on Gram-positive bacteria as well (18), suggesting that it might also function through other targets, characteristic of membrane-acting antibacterials (40). Therefore, a comprehensive analysis is needed to better define the MOA(s) of GI-7. In fact, a preliminary thermal proteome profile (TPP) (50) of GI-7-treated APEC indicated LptD/E as a potential target of GI-7; however, multiple other proteins with increased abundance were also identified, suggesting potential interaction of GI-7 with other targets (data not shown).
Docking studies predicted that GI-7 is likely to interact with LptD (Fig. 8 and Fig. S6 and S7). Recently, many antibacterials targeting the Lpt complex, especially LptD and LptA, have been identified (22–25, 51). Murepavadin and L27-11, the peptidomimetic antibiotics effective against MDR Pseudomonas, bind to the periplasmic segment of LptD and block LPS transport to the OM (24, 25). Similarly, JB-95, a macrocyclic peptide highly effective against MDR E. coli, downregulates LptD, thus affecting OM biogenesis (22). Likewise, thanatin, an insect-derived antimicrobial peptide, binds to LptA and LptD, which leads to inhibition of LPS transport and, subsequently, OM assembly in E. coli (23). GI-7 was also effective against antibiotic-resistant APEC strains (18), which supports LptD as the potential target for its antibacterial action. Resistance mechanisms against LptD inhibitors are not widely reported, although a recent report has suggested that mutations in pmrB can confer cross-resistance against an LptD inhibitor, POL7080, in P. aeruginosa (52). Our study suggests that LptD/E is a valuable druggable target, and confirmation of the direct interaction of GI-7 with LptD/E using biophysical methods like surface plasmon resonance (SPR) and isothermal titration calorimetry (ITC), as well as site-directed mutagenesis studies, will pave the way for the development of novel and effective antibacterial drugs. The downregulation of expression of other OM genes, particularly bamA and mlaA, might be an off-target/secondary effect of lptD inhibition by GI-7. Decreased levels of BamA and multiple other OM proteins were also observed with JB-95 (LptD inhibitor) treatment in E. coli (22). Similarly, downregulation of MlaA and LptD proteins was observed concurrently in LPS-deficient Acinetobacter baumannii (53).
GI-7 did not significantly alter the microbial composition (at the phylum level) of the cecum of chickens (Fig. 3A), which might suggest a minimal impact on the host microbiota (54). Furthermore, the increased Lactobacillus (L. zeae and L. helveticus) population in GI-7-treated chickens (Fig. 3B and Table 2) might be responsible for the better anti-APEC activity of GI-7 compared to that of other GIs. The antibacterial effects of L. zeae and L. helveticus have been reported previously (55–59). Pretreatment with L. zeae strain LB1 protected Caenorhabditis elegans from enterotoxigenic E. coli (ETEC) infection by upregulating the production of antimicrobial peptides and host defense molecules (55) and inhibiting the enterotoxin expression (56). Similarly, feeding fermented feed containing L. zeae reduced the Salmonella enterica serovar Typhimurium strain DT104 burden in experimentally challenged weaned pigs (57). Likewise, L. helveticus strain SP-27 reduced S. Typhimurium infection in human patients (58) and prevented the death of neonatal mice caused by Citrobacter rodentium infection (59). GI-7 also did not significantly alter the serum metabolites (Table 3 and Fig. S3), which might be due to the minimal impact of GI-7 on the cecal microbiota of chickens (60). Oleate is the major serum metabolite elevated in chickens treated with GI-7 (Table 3). Oleate is required for the induction of innate immune defenses and protection against diverse bacterial pathogens (P. aeruginosa, Enterococcus faecalis, and Serratia marcescens) in C. elegans (61). Furthermore, oleate possesses significant antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA), Helicobacter pylori, and Mycobacteria (62). Oleate interferes with fatty acid synthesis through inhibition of the bacterial enoyl-acyl carrier protein reductase (FabI) (62).
In our study, we infected the chickens with 107 CFU of APEC using the subcutaneous (s.c.) APEC infection model, which is a reproducible and commonly used acute infection model causing higher mortality and rapid progression of disease in chickens (63). However, under field conditions, chickens usually get exposed to lower APEC levels (102 to 103 CFU) through oral or aerosol routes and the infection progresses relatively more slowly (10, 63). Furthermore, the presence of concurrent viral (Newcastle, infectious bronchitis, and avian influenza virus) and Mycoplasma infections and stress (high CO2, NH3, and humidity) predispose the chickens to APEC infection (10). Therefore, future investigations involving the natural routes of APEC infection or naturally infected chickens will be an important consideration to advance the development of GI-7 for therapeutic application in poultry farms. Approaches complementary to GI-7 might be advantageous for the complete protection of chickens against APEC. Recent studies have reported the potential use of immunomodulators like Toll-like receptor (TLR) agonists, NOD-like receptor agonists (NLR), innate defense regulator (IDR) peptides, probiotics, and surfactins as adjuvants for antibacterial therapy (64–67). GI-7 can also be combined with antibiotics currently being used in the poultry industry to enhance colibacillosis control, since GI-7 showed synergistic activity with different antibiotics (e.g., colistin, tetracycline, and ciprofloxacin) in our earlier study (41). Additionally, in our separate studies, we have identified beneficial bacteria and peptides (68) and APEC virulence inhibitors (33) displaying anti-APEC activity, which can be combined to provide better protection against APEC infection in chickens.
In summary, this study identified a promising novel small molecule growth inhibitor, GI-7, effective against APEC infection in chickens. Our studies showed that GI-7 affected the OM and decreased the Lpt protein and LPS levels in APEC. Antibacterial drugs targeting the bacterial OM can overcome the resistance problem, and thus, the OM represents a promising target for novel antibacterial development against Gram-negative pathogens. Furthermore, with APEC being genetically similar to human ExPECs, our findings can have profound implications in developing new antibacterial drugs against human ExPECs. Our future studies will focus on improving the efficacy of GI-7 using medicinal chemistry methods. We will also validate the Lpt system (LptD/E) as a target of GI-7 by using biophysical methods to measure the binding affinity of GI-7 to LptD/E proteins and site-directed mutagenesis studies to determine the essentiality of LptD residues predicted to bind with GI-7.
MATERIALS AND METHODS
Ethics in animal experimentation.
All chicken experiments conducted in this study were approved by The Ohio State University Institutional Animal Care and Use Committee (IACUC) (protocol number 2010A00000149). Chickens were euthanized using CO2 following American Veterinary Medical Association (AVMA) guidelines. Approved husbandry practices were followed throughout the experiments.
Efficacy of GIs against APEC infection in chickens.
The efficacy of GIs was assessed using commercial broiler chickens (n = 6/group). A total of eight GIs (GI-1, GI-2, GI-3, GI-6, GI-7, GI-8, GI-9, and GI-10) (Fig. 1A) identified in our previous high-throughput study (18) were tested. The detailed experimental approach is displayed in Fig. S1A. GI compounds (ChemBridge, San Diego, CA) dissolved in dimethyl sulfoxide (DMSO) were administered orally using a syringe, twice a day, from day 4 (1 day before APEC infection) to day 8 (3 days postinfection [dpi]). The dose of each GI is described in Table S1. The doses corresponded to 50× the in vitro minimum bactericidal concentrations (MBCs) of the GIs and were selected based on previous studies (69, 70). Chickens that were infected and vehicle-treated (positive control [PC]) and noninfected and nontreated (negative control [NC]) were included as controls. On day 5, chickens were infected subcutaneously (s.c.) with rifampin-resistant (Rifr) APEC serotype O78 (18) (1 × 107 CFU/chicken). This dose, which resulted in consistent productive infection, was selected based on a preliminary study with different infection routes (s.c., intratracheal, and intra-airsac) and doses (106, 107, and 108 CFU/chicken) to determine the appropriate route and dose for establishment of APEC infection in chickens (Table S2). To prepare the APEC inoculum for infection, Rifr APEC O78 (50 μg/ml rifampin) was grown to logarithmic phase in LB medium, washed twice with phosphate-buffered saline (PBS), and adjusted to an optical density at 600 nm (OD600) of 0.1 (5 × 107 CFU/ml). The clinical signs and mortality were recorded until 7 dpi. Chickens that died during this period were necropsied on the same day, lesions in internal organs (liver, heart, lung, and air sacs) were scored as described previously (63, 71), and the APEC loads were quantified in internal organs (liver, heart, lung, and kidney) by plating the tissue homogenates on MacConkey agar plates containing 50 μg/ml rifampin (18). At 7 dpi (day 12), all live birds were euthanized, lesions were scored, and the APEC loads in internal organs were quantified as described above. The body weight of chickens was measured before GI treatment (day 3) and at the day of necropsy (day 12).
Evaluation of resistance acquisition in vivo.
To test whether APEC developed resistance against GIs (GI-7, GI-10, and GI-6) posttreatment in chickens, APEC colonies (n = 24/GI-treated group) cultured from different organs were randomly selected from the agar plates at the end of the experiments, grown in M63 minimal medium (37°C and 200 rpm), and tested for growth inhibition by GIs at their respective MICs and MBCs as described previously (18).
Effect of GIs on cecal microbiota of chickens.
To investigate the impact of GIs on the cecal microbiota of chickens, a 16S rRNA-based metagenomic study was conducted as described previously (69, 72). Cecal microbiota was analyzed for only those GIs (GI-7, GI-10, and GI-6) that had an impact on APEC infection in the experiment described above. For the metagenomic analysis, the QIIME 2 (Quantitative Insights Into Microbial Ecology 2) bioinformatics platform (73, 74) was used. Details of methods are included in the supplemental material. The statistical difference (P < 0.05) in the taxonomic composition between different groups was determined using the Mann-Whitney U test. The alpha and beta diversity were analyzed using the Kruskal-Wallis test and permutational multivariate analysis of variance (PERMANOVA) (P < 0.05), respectively.
Effect of GIs on serum metabolome of chickens.
To investigate the impact of GIs on the serum metabolites of chickens, the untargeted serum metabolome profile was determined using liquid chromatography-mass spectrometry (LC-MS) at the Campus Chemical Instrumentation Center, Mass Spectrometry and Proteomics Facility, The Ohio State University (CCIC, MS&P Facility, OSU) (https://live-ccic.pantheonsite.io/MSP). Metabolome profiling was performed on a Thermo Orbitrap LTQ XL instrument in positive-mode analysis with high-performance liquid chromatography (HPLC) separation on a Thermo Scientific RLCS ultimate 3000 LC system using a Poroshell 120 SB-C18 (2 by 100 mm, 2.7-μm particle size) column. The significantly (P < 0.05, Kruskal-Wallis test) altered metabolites and the associated metabolic pathways were identified using XCMS Online (https://xcmsonline.scripps.edu/) and expressed as the fold changes in abundance compared to the abundance in NC (noninfected and nontreated) chickens. Principal-component analysis (PCA) was performed to compare the metabolome profiles between the treatment groups. Progenesis QI software (Waters/Nonlinear Dynamics) was used to validate the results. Details of methods are included in the supplemental material.
Dose optimization of GI-7 in drinking water.
Among the GIs tested, GI-7 showed the most potent anti-APEC activity in chickens when administered orally in the pilot experiment described above; therefore, we optimized the dose of GI-7 for drinking water delivery in chickens (a common industry practice). GI-7 was administered at different doses (20 mg/liter, 40 mg/liter, and 60 mg/liter), and chickens were challenged (s.c.) with Rifr APEC O78 (5 × 106 CFU/chicken). Prior to selection of these doses, we conducted a preliminary study using 20-mg/liter, 200-mg/liter, and 500-mg/liter doses (doses similar to those of antibiotics currently used in the poultry industry). Only 20 mg/liter showed a promising result in reducing mortality, lesions, and APEC load (data not shown), and higher doses were toxic to chickens; therefore, we tested further using 20-mg/liter, 40-mg/liter, and 60-mg/liter doses. The experiment was conducted using commercial broiler chickens (n = 11/group) as described above following procedures described in Fig. S1A. GI-7 was administered in drinking water containing 0.05% DMSO from day 4 to day 10 (a total of 7 days). The amount of drinking water was adjusted daily based on the age and requirement of chickens (https://www.thepoultrysite.com/articles/broiler-water-consumption). The clinical signs and mortality, lesion scores, and APEC loads were determined as described above. We also tested GI-7 at an 80-mg/liter dose in a follow-up study.
Comparative efficacies of GI-7 and sulfadimethoxine in large chicken trial simulating field conditions (floor trial).
The optimized dose of GI-7 (60 mg/liter) and therapeutic dose of sulfadimethoxine (SDM; 0.05% or 495.3 mg/liter) were used to compare the efficacies of GI-7 and SDM in commercial broiler chickens (n = 70/group) raised on built-up floor litter. GI-7 and SDM were administered in drinking water for 7 consecutive days from day 5 (1 day prior to infection) to day 11 (5 dpi). The detailed experimental approach is displayed in Fig. S1B. On day 6, chickens were infected (s.c.) with Rifr APEC O78 (5 × 106 CFU/chicken). At day 13 (7 dpi), half of the chickens from each group, and at day 42 (slaughter age), 10 chickens from each group were necropsied and lesions and APEC loads were assessed as described above. The body weight of the chickens was measured weekly, and feed intake was recorded daily to compare the effects of GI-7 and SDM on the body weight gain (BWG) and feed conversion ratio (FCR).
Pharmacokinetic (PK) study.
The absorption and excretion kinetics of GI-7 upon oral delivery in chickens (from experimental set-up as described for the floor trial above) were determined using LC-MS (CCIC, MS&P Facility, OSU) as described previously (75). Five chickens from each group were sacrificed at 0, 0.5, 1, 2, 4, 8, 12, 24, 84, and 180 h posttreatment, and plasma samples were mixed with cold methanol (MeOH) followed by the addition of 10 μl of 1 μg/ml internal drug standard (heavy-labeled phenylalanine dissolved in H2O and 0.1% formic acid [FA]). Proteins were removed by ultracentrifugation, and 5-μl amounts of supernatants were run using the Agilent Poroshell 120 SB-C18 column (2 by 100 mm, 2.7-μm particle size). The quantitation of GI-7 in plasma was performed by calibrating standard solutions of GI-7 containing different concentrations (0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, and 5 μg/ml) in LC-MS analyses run as described above (Fig. S2A and B). Plasma samples from chickens treated with SDM were used for comparison. GI-7 was monitored at 439.2→232.1 m/z at 20 V collision energy (CE) and sulfadimethoxine at 311.1→156 m/z at 30 V CE. Details of the methods are included in the supplemental material.
Quantification of GI-7 residue in tissues.
The GI-7 residue in chicken tissues (muscle, liver, and kidney) was quantified using LC-MS (CCIC, MS&P Facility, OSU) as described previously (76). Chickens (n = 5/time point) from an experimental set-up as described above for the floor trial were sacrificed at 2 days (day 13; 2 DPLT), 5 days (day 16; 5 DPLT), and 31 days (day 42; 31 DPLT) post-last treatment. The quantitation of GI-7 in chicken tissues was performed by calibrating against the standard solutions of GI-7 containing different concentrations (0.1, 0.5, 1, 5, 10, 50, 100, 500, and 1,000 ng/ml) (Fig. S2C and D). Tissue samples from chickens treated with SDM were used for comparison. GI-7 and SDM peaks were monitored at 439.2→232.1 m/z and 311.1→156 m/z, respectively, as described above. Details of the methods are included in the supplemental material.
Gene expression analysis.
To understand the potential mechanism of GI-7’s antibacterial effect, the expression of lpt genes (lptD, lptE, lptA, and lptC) was quantitated using RT-qPCR as described previously (33, 41). These genes were selected based on the similarity of the bacterial cytological profile (BCP; accumulation of membrane-like material in the form of knobs inside the cells) induced by GI-7 (18) to the BCPs of thanatin (23) and JB-95 (22), which were identified as Lpt inhibitors in E. coli (22, 23). We also quantitated the expression of other genes essential for maintaining OM integrity (bamA, mlaA, lolB, and pbgA) (21). An APEC O78 culture grown overnight was adjusted to an OD600 of 0.5 and treated with GI-7 for 4 h to achieve ∼50% growth inhibition (8 replicates, 200 μl each) (Fig. S3) at 37°C with shaking at 200 rpm as previously described (22, 77, 78). An APEC O78 culture treated with 1% DMSO was used as a control. Total RNA was extracted using the RNeasy minikit (Qiagen), and 5 μg of purified RNA was used to synthesize cDNA using the RT2 first strand kit (Qiagen). The RT-qPCR was performed using Maxima SYBR green/ROX qPCR master mix (Thermo Fisher) in a RealPlex2 Mastercycler (Eppendorf) with a 55°C annealing temperature. The primers (Table S3) were designed using the PrimerQuest tool and obtained from Integrated DNA Technologies (IDT). The data were normalized to the housekeeping gene encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and relative fold changes were calculated using the cycle threshold (ΔΔCT) method (79). Two independent experiments were conducted.
Protein expression and LC-MS/MS analysis.
To support the gene expression findings, the levels of total membrane proteins upon GI-7 treatment were investigated as described previously (22). A GI-7-treated culture of APEC O78 was prepared as described above, and the fractions of cytoplasmic and total membrane proteins were prepared using ultracentrifugation as described previously (80). The concentration of fractionated proteins was normalized, and 25 μg of proteins were run on 12% SDS-PAGE under nonreducing (in the absence of β-mercaptoethanol) conditions (37). The gel was stained with Coomassie blue R-250, the bands of ∼21.4 kDa (expected size of LptE) were excised from both untreated and GI-7-treated lanes of the gel, and in-gel digestion was performed as previously described (81). LC-MS/MS of the digested samples was performed on the Thermo Scientific Orbitrap Fusion MS instrument equipped with an EASY-Spray source operated in positive ion mode (CCIC, MS&P Facility, OSU). The Thermo Scientific UltiMate 3000 RSLCnano system containing an Acclaim PepMap 100 (75 μm by 2 cm) trap column and Easy-Spray nano separation column (PepMap RSLC C18, 3 μM, 100 Å, 75 μm by 150 mm; Thermo Scientific) was used for injection and elution of samples. For the proteomics database search, the E. coli Uniprot protein database with trypsin/P (selected as the enzyme) was used with the fragment mass tolerance set to 0.5 Da. Mascot Daemon (Matrix Science version 2.6.0) and Scaffold Proteome software were used for the MS/MS data analysis. The results were filtered to a 1% false discovery rate and a minimum of 2 peptides per protein identification. The exponentially modified protein abundance index (emPAI) (82) was also calculated to compare the LptE levels between untreated and GI-7-treated gel fragments.
LptD/E immunoblot analysis.
For immunoblot analysis, total membrane proteins (25 μg) resolved by SDS-PAGE under nonreducing (in the absence of β-mercaptoethanol) conditions (37) as described above were electrotransferred onto an Immun-Blot polyvinylidene difluoride (PVDF) membrane (Bio-Rad) and probed for LptD or LptE using anti-LptD (1:20,000) and anti-LptE (1:30,000) polyclonal rabbit antibodies (GenScript) and goat anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary antibody (Sigma-Aldrich). Anti-LptD and anti-LptE antibodies were generated at GenScript using keyhole limpet hemocyanin (KLH)-conjugated peptides as the antigens (LptD epitope, QLHQKEAPGQPEPVC, and LptE epitope, ELLDKETTRKDVPSC). The epitopes were selected using the OptimumAntigen design tool (GenScript). The transferred membrane was developed with Clarity Western ECL substrate (Bio-Rad) and visualized in a FluorChem Q imager (ProteinSimple).
LPS analysis.
To further support the results from the above-described studies showing that GI-7 affects the levels of Lpt proteins, LPS was extracted from untreated and GI-7-treated APEC (as described above) using an LPS isolation kit (BioVision, Inc., Milpitas, CA) following the manufacturer’s protocol. Extracted LPS (normalized based on the OD600 before extraction) was separated on a 4-to-20% gradient SDS-PAGE gel and stained with Coomassie blue R-250 to visualize the LPS levels (83). In addition, LPS was also quantitated using a total carbohydrate assay kit (Cell Biolabs, Inc., San Diego, CA) following the manufacturer’s protocol. Two independent experiments with six replicates in each experiment were performed.
Docking studies.
Autodock 4.0 (84) was employed in GI-7 docking studies using the protein structure with PDB code 4RHB, and Discovery Studio Visualizer along with Chimera was used for protein-ligand interaction. Graphical User Interface for AutoDock Tools (ADT) was used for preparation of pdbqt files for protein and ligand and for grid box creation. AutoGrid was employed for the preparation of the grid map, and the grid size was set to 60 by 60 by 60 xyz points with grid spacing at 0.375 Å. Both protein and ligand were considered rigid during docking, and the outcomes of docking with 1.0 Å of positional root-mean-square deviation (RMSD) were clustered together. The docking pose with the most favorable, i.e., lowest, energy or binding affinity was aligned with the protein structure and further analyzed using Biovia Discovery Studio Visualizer. In order to determine the LptD-specific binding of GI-7, docking was also conducted with GI-2 and GI-3, which have structures similar (Fig. 1A) to that of GI-7 (18), and with GI-10 and GI-6, which have different structures (Fig. 1A) than GI-7 (18) but showed impacts against APEC infection in chickens. AutoDock Vina 1.5.6 (34) was used to further support the predicted interactions of GI-7 with LptD. PyMOL (The PyMOL Molecular Graphics System, version 2.3.4; Schrodinger LLC) was used for the visualization.
Statistical analysis.
The statistical significance of the effects of treatments on the survival of chickens was analyzed using the log-rank test (P < 0.05). The statistical significance (P < 0.05) of the effects of treatments on the reduction of APEC lesions and APEC loads was calculated using the Mann-Whitney U test or Student’s t test after testing normality using the Shapiro-Wilk test. The statistical comparison of APEC lesions, APEC loads, BWGs, and FCRs between treatment groups was performed using the Kruskal-Wallis test (P < 0.05). The statistical comparison (P < 0.05) of LPS levels was performed using Student’s t test. GraphPad Prism 5.02 was used for the analysis.
ACKNOWLEDGMENTS
We thank Dhanashree Lokesh, Sochina Ranjit, and Dhwani Parsana for technical assistance. We thank Wilbur Ouma for bioinformatics assistance. We thank Thomas J. Silhavy for kindly providing us anti-LptD and anti-LptE antibodies. We thank the MCIC and MS&P Facility of The Ohio State University for analysis of chicken samples for microbiome and metabolomic studies. We thank Juliette Hanson, Megan Strother, Sara Tallmadge, and Ronna Wood for animal care assistance. We are grateful to Tim Barman for providing the antibiotic used in this study.
The research in the Rajashekara laboratory was supported by the U.S. Department of Agriculture (USDA) National Institute for Food and Agriculture (NIFA) (grants number 2015-68004-23131 and 2020-6701-31401), the Technology Commercialization Office (TCO) of The Ohio State University, and the Ohio State Innovation Foundation.
Footnotes
Supplemental material is available online only.
Contributor Information
Gireesh Rajashekara, Email: rajashekara.2@osu.edu.
Gustavo H. Goldman, Universidade de Sao Paulo
REFERENCES
- 1.Mellata M. 2013. Human and avian extraintestinal pathogenic Escherichia coli: infections; zoonotic risks, and antibiotic resistance trends. Foodborne Pathog Dis 10:916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markland SM, LeStrange KJ, Sharma M, Kniel KE. 2015. Old friends in new places: exploring the role of extraintestinal E. coli in intestinal disease and foodborne illness. Zoonoses Public Health 62:491–496. doi: 10.1111/zph.12194. [DOI] [PubMed] [Google Scholar]
- 3.Bélanger L, Garenaux A, Harel J, Boulianne M, Nadeau E, Dozois CM. 2011. Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol Med Microbiol 62:1–10. doi: 10.1111/j.1574-695X.2011.00797.x. [DOI] [PubMed] [Google Scholar]
- 4.Liu CM, Stegger M, Aziz M, Johnson TJ, Waits K, Nordstrom L, Gauld L, Weaver B, Rolland D, Statham S, Horwinski J, Sariya S, Davis GS, Sokurenko E, Keim P, Johnson JR, Price LB. 2018. Escherichia coli ST131-H22 as a foodborne uropathogen. mBio 9:e00470-18. doi: 10.1128/mBio.00470-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osman KM, Kappell AD, Elhadidy M, ElMougy F, El-Ghany WAA, Orabi A, Mubarak AS, Dawoud TM, Hemeg HA, Moussa IMI, Hessain AM, Yousef HMY. 2018. Poultry hatcheries as potential reservoirs for antimicrobial-resistant Escherichia coli: a risk to public health and food safety. Sci Rep 8:5859. doi: 10.1038/s41598-018-23962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dho-Moulin M, Fairbrother JM. 1999. Avian pathogenic Escherichia coli (APEC). Vet Res 30:299–316. [PubMed] [Google Scholar]
- 7.Kathayat D, Lokesh D, Ranjit S, Rajashekara G. 2021. Avian pathogenic Escherichia coli (APEC): an overview of virulence and pathogenesis factors, zoonotic potential, and control strategies. Pathogens 10:467. doi: 10.3390/pathogens10040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutful Kabir SM. 2010. Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int J Environ Res Public Health 7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson TJ, Wannemuehler Y, Doetkott C, Johnson SJ, Rosenberger SC, Nolan LK. 2008. Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J Clin Microbiol 46:3987–3996. doi: 10.1128/JCM.00816-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dziva F, Stevens MP. 2008. Colibacillosis in poultry: unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol 37:355–366. doi: 10.1080/03079450802216652. [DOI] [PubMed] [Google Scholar]
- 11.Guabiraba R, Schouler C. 2015. Avian colibacillosis: still many black holes. FEMS Microbiol Lett 362:fnv118. doi: 10.1093/femsle/fnv118. [DOI] [PubMed] [Google Scholar]
- 12.Landman WJM, van Eck JHH. 2015. The incidence and economic impact of the Escherichia coli peritonitis syndrome in Dutch poultry farming. Avian Pathol 44:370–378. doi: 10.1080/03079457.2015.1060584. [DOI] [PubMed] [Google Scholar]
- 13.Ghunaim H, Abu-Madi MA, Kariyawasam S. 2014. Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: potentials and limitations. Vet Microbiol 172:13–22. doi: 10.1016/j.vetmic.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Agunos A, Léger D, Carson C. 2012. Review of antimicrobial therapy of selected bacterial diseases in broiler chickens in Canada. Can Vet J 53:1289–1300. [PMC free article] [PubMed] [Google Scholar]
- 15.Lima Barbieri N, Nielsen DW, Wannemuehler Y, Cavender T, Hussein A, Yan S-G, Nolan LK, Logue CM. 2017. mcr-1 identified in avian pathogenic Escherichia coli (APEC). PLoS One 12:e0172997. doi: 10.1371/journal.pone.0172997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen RH, Bisgaard M, Löhren U, Robineau B, Christensen H. 2014. Extended-spectrum β-lactamase-producing Escherichia coli isolated from poultry: a review of current problems, illustrated with some laboratory findings. Avian Pathol 43:199–208. doi: 10.1080/03079457.2014.907866. [DOI] [PubMed] [Google Scholar]
- 17.Nhung NT, Chansiripornchai N, Carrique-Mas JJ. 2017. Antimicrobial resistance in bacterial poultry pathogens: a review. Front Vet Sci 4:126. doi: 10.3389/fvets.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kathayat D, Helmy YA, Deblais L, Rajashekara G. 2018. Novel small molecules affecting cell membrane as potential therapeutics for avian pathogenic Escherichia coli. Sci Rep 8:15329. doi: 10.1038/s41598-018-33587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehman KM, Grabowicz M. 2019. Countering gram-negative antibiotic resistance: recent progress in disrupting the outer membrane with novel therapeutics. Antibiotics 8:163. doi: 10.3390/antibiotics8040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacNair CR, Brown ED. 2020. Outer membrane disruption overcomes intrinsic, acquired, and spontaneous antibiotic resistance. mBio 11:e01615-20. doi: 10.1128/mBio.01615-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi U, Lee CR. 2019. Antimicrobial agents that inhibit the outer membrane assembly machines of gram-negative bacteria. J Microbiol Biotechnol 29:1–10. doi: 10.4014/jmb.1804.03051. [DOI] [PubMed] [Google Scholar]
- 22.Urfer M, Bogdanovic J, Lo Monte F, Moehle K, Zerbe K, Omasits U, Ahrens CH, Pessi G, Eberl L, Robinson JA. 2016. A peptidomimetic antibiotic targets outer membrane proteins and disrupts selectively the outer membrane in Escherichia coli. J Biol Chem 291:1921–1932. doi: 10.1074/jbc.M115.691725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vetterli SU, Zerbe K, Müller M, Urfer M, Mondal M, Wang S-Y, Moehle K, Zerbe O, Vitale A, Pessi G, Eberl L, Wollscheid B, Robinson JA. 2018. Thanatin targets the intermembrane protein complex required for lipopolysaccharide transport in Escherichia coli. Sci Adv 4:eaau2634. doi: 10.1126/sciadv.aau2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andolina G, Bencze LC, Zerbe K, Muller M, Steinmann J, Kocherla H, Mondal M, Sobek J, Moehle K, Malojcic G, Wollscheid B, Robinson JA. 2018. A peptidomimetic antibiotic interacts with the periplasmic domain of LptD from Pseudomonas aeruginosa. ACS Chem Biol 13:666–675. doi: 10.1021/acschembio.7b00822. [DOI] [PubMed] [Google Scholar]
- 25.Werneburg M, Zerbe K, Juhas M, Bigler L, Stalder U, Kaech A, Ziegler U, Obrecht D, Eberl L, Robinson JA. 2012. Inhibition of lipopolysaccharide transport to the outer membrane in Pseudomonas aeruginosa by peptidomimetic antibiotics. Chembiochem 13:1767–1775. doi: 10.1002/cbic.201200276. [DOI] [PubMed] [Google Scholar]
- 26.Hart EM, Mitchell AM, Konovalova A, Grabowicz M, Sheng J, Han X, Rodriguez-Rivera FP, Schwaid AG, Malinverni JC, Balibar CJ, Bodea S, Si Q, Wang H, Homsher MF, Painter RE, Ogawa AK, Sutterlin H, Roemer T, Black TA, Rothman DM, Walker SS, Silhavy TJ. 2019. A small-molecule inhibitor of BamA impervious to efflux and the outer membrane permeability barrier. Proc Natl Acad Sci USA 116:21748–21757. doi: 10.1073/pnas.1912345116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imai Y, Meyer KJ, Iinishi A, Favre-Godal Q, Green R, Manuse S, Caboni M, Mori M, Niles S, Ghiglieri M, Honrao C, Ma X, Guo JJ, Makriyannis A, Linares-Otoya L, Böhringer N, Wuisan ZG, Kaur H, Wu R, Mateus A, Typas A, Savitski MM, Espinoza JL, O’Rourke A, Nelson KE, Hiller S, Noinaj N, Schäberle TF, D’Onofrio A, Lewis K. 2019. A new antibiotic selectively kills Gram-negative pathogens. Nature 576:459–464. doi: 10.1038/s41586-019-1791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghequire MGK, Swings T, Michiels J, Buchanan SK, De Mot R. 2018. Hitting with a BAM: selective killing by lectin-like bacteriocins. mBio 9:e02138-17. doi: 10.1128/mBio.02138-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott AG, Huang JX, Neve S, Zuegg J, Edwards IA, Cain AK, Boinett CJ, Barquist L, Lundberg CV, Steen J, Butler MS, Mobli M, Porter KM, Blaskovich MAT, Lociuro S, Strandh M, Cooper MA. 2020. An amphipathic peptide with antibiotic activity against multidrug-resistant Gram-negative bacteria. Nat Commun 11:3184. doi: 10.1038/s41467-020-16950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q, Cebrián R, Montalbán-López M, Ren H, Wu W, Kuipers OP. 2021. Outer-membrane-acting peptides and lipid II-targeting antibiotics cooperatively kill Gram-negative pathogens. Commun Biol 4:31. doi: 10.1038/s42003-020-01511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maier L, Typas A. 2017. Systematically investigating the impact of medication on the gut microbiome. Curr Opin Microbiol 39:128–135. doi: 10.1016/j.mib.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Tolstikov V. 2016. Metabolomics: bridging the gap between pharmaceutical development and population health. Metabolites 6:20. doi: 10.3390/metabo6030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helmy YA, Deblais L, Kassem II, Kathayat D, Rajashekara G. 2018. Novel small molecule modulators of quorum sensing in avian pathogenic Escherichia coli (APEC). Virulence 9:1640–1657. doi: 10.1080/21505594.2018.1528844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trott O, Olson AJ. 2010. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Code of Federal Regulations. 2020. Title 21 – Food and drugs. Chapter I – Food and Drug Administration. Subchapter E – Animal drugs, feeds, and related products. Part 556 -- Tolerance for residues of new animal drugs in food. 21 CFR 556. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm. [Google Scholar]
- 36.Mund MD, Khan UH, Tahir U, Mustafa B-E, Fayyaz A. 2017. Antimicrobial drug residues in poultry products and implications on public health: a review. Int J Food Properties 20:1433–1446. doi: 10.1080/10942912.2016.1212874. [DOI] [Google Scholar]
- 37.Chng S-S, Xue M, Garner RA, Kadokura H, Boyd D, Beckwith J, Kahne D. 2012. Disulfide rearrangement triggered by translocon assembly controls lipopolysaccharide export. Science 337:1665–1668. doi: 10.1126/science.1227215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sperandeo P, Martorana AM, Polissi A. 2017. The lipopolysaccharide transport (Lpt) machinery: a nonconventional transporter for lipopolysaccharide assembly at the outer membrane of Gram-negative bacteria. J Biol Chem 292:17981–17990. doi: 10.1074/jbc.R117.802512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maron DF, Smith TJS, Nachman KE. 2013. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Global Health 9:48. doi: 10.1186/1744-8603-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mingeot-Leclercq M-P, Décout J-L. 2016. Bacterial lipid membranes as promising targets to fight antimicrobial resistance, molecular foundations and illustration through the renewal of aminoglycoside antibiotics and emergence of amphiphilic aminoglycosides. Med Chem Commun 7:586–611. doi: 10.1039/C5MD00503E. [DOI] [Google Scholar]
- 41.Kathayat D, Antony L, Deblais L, Helmy YA, Scaria J, Rajashekara G. 2020. Small molecule adjuvants potentiate colistin activity and attenuate resistance development in Escherichia coli by affecting pmrAB system. Infect Drug Resist 13:2205–2222. doi: 10.2147/IDR.S260766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang KL, Caffrey NP, Nóbrega DB, Cork SC, Ronksley PE, Barkema HW, Polachek AJ, Ganshorn H, Sharma N, Kellner JD, Ghali WA. 2017. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health 1:e316–e327. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Economou V, Gousia P. 2015. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect Drug Resist 8:49–61. doi: 10.2147/IDR.S55778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang G, Meredith TC, Kahne D. 2013. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr Opin Microbiol 16:779–785. doi: 10.1016/j.mib.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertani B, Ruiz N. 2018. Function and biogenesis of lipopolysaccharides. EcoSal Plus 2018. doi: 10.1128/ecosalplus.ESP-0001-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo Sciuto A, Martorana AM, Fernández-Piñar R, Mancone C, Polissi A, Imperi F. 2018. Pseudomonas aeruginosa LptE is crucial for LptD assembly, cell envelope integrity, antibiotic resistance and virulence. Virulence 9:1718–1733. doi: 10.1080/21505594.2018.1537730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Gu Y, Dong H, Wang W, Dong C. 2015. Trapped lipopolysaccharide and LptD intermediates reveal lipopolysaccharide translocation steps across the Escherichia coli outer membrane. Sci Rep 5:11883. doi: 10.1038/srep11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Botos I, Majdalani N, Mayclin SJ, McCarthy JG, Lundquist K, Wojtowicz D, Barnard TJ, Gumbart JC, Buchanan SK. 2016. Structural and functional characterization of the LPS transporter LptDE from gram-negative pathogens. Structure 24:965–976. doi: 10.1016/j.str.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chimalakonda G, Ruiz N, Chng SS, Garner RA, Kahne D, Silhavy TJ. 2011. Lipoprotein LptE is required for the assembly of LptD by the beta-barrel assembly machine in the outer membrane of Escherichia coli. Proc Natl Acad Sci USA 108:2492–2497. doi: 10.1073/pnas.1019089108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin JK, II, Sheehan JP, Bratton BP, Moore GM, Mateus A, Li SH, Kim H, Rabinowitz JD, Typas A, Savitski MM, Wilson MZ, Gitai Z. 2020. A dual-mechanism antibiotic kills gram-negative bacteria and avoids drug resistance. Cell 181:1518–1532.e14. doi: 10.1016/j.cell.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naclerio GA, Sintim HO. 2020. Multiple ways to kill bacteria via inhibiting novel cell wall or membrane targets. Future Med Chem 12:1253–1279. doi: 10.4155/fmc-2020-0046. [DOI] [PubMed] [Google Scholar]
- 52.Romano KP, Warrier T, Poulsen BE, Nguyen PH, Loftis AR, Saebi A, Pentelute BL, Hung DT. 2019. Mutations in pmrB confer cross-resistance between the LptD inhibitor POL7080 and colistin in Pseudomonas aeruginosa. Antimicrob Agents Chemother 63:e00511-19. doi: 10.1128/AAC.00511-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chamoun S, Welander J, Martis-Thiele MM, Ntzouni M, Claesson C, Vikström E, Turkina MV. 2021. Colistin dependence in extensively drug-resistant Acinetobacter baumannii strain is associated with ISAjo2 and ISAba13 insertions and multiple cellular responses. Int J Mol Sci 22:576. doi: 10.3390/ijms22020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao J, Carter RA, Vuagniaux G, Barbier M, Rosch JW, Rock CO. 2016. A pathogen-selective antibiotic minimizes disturbance to the microbiome. Antimicrob Agents Chemother 60:4264–4273. doi: 10.1128/AAC.00535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou M, Liu X, Yu H, Yin X, Nie S-P, Xie M-Y, Chen W, Gong J. 2018. Cell signaling of Caenorhabditis elegans in response to enterotoxigenic Escherichia coli infection and Lactobacillus zeae protection. Front Immunol 9:1745. doi: 10.3389/fimmu.2018.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou M, Yu H, Yin X, Sabour PM, Chen W, Gong J. 2014. Lactobacillus zeae protects Caenorhabditis elegans from enterotoxigenic Escherichia coli-caused death by inhibiting enterotoxin gene expression of the pathogen. PLoS One 9:e89004. doi: 10.1371/journal.pone.0089004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin F, Farzan A, Wang QC, Yu H, Yin Y, Hou Y, Friendship R, Gong J. 2014. Reduction of Salmonella enterica serovar typhimurium DT104 infection in experimentally challenged weaned pigs fed a lactobacillus-fermented feed. Foodborne Pathog Dis 11:628–634. doi: 10.1089/fpd.2013.1676. [DOI] [PubMed] [Google Scholar]
- 58.Piatek J, Sommermeyer H, Bernatek M, Ciechelska-Rybarczyk A, Oleskow B, Mikkelsen LS, Barken KB. 2019. Persistent infection by Salmonella enterica servovar Typhimurium: are synbiotics a therapeutic option?—A case report. Benef Microbes 10:211–217. doi: 10.3920/BM2018.0080. [DOI] [PubMed] [Google Scholar]
- 59.Gareau MG, Wine E, Reardon C, Sherman PM. 2010. Probiotics prevent death caused by Citrobacter rodentium infection in neonatal mice. J Infect Dis 201:81–91. doi: 10.1086/648614. [DOI] [PubMed] [Google Scholar]
- 60.Zarrinpar A, Chaix A, Xu ZZ, Chang MW, Marotz CA, Saghatelian A, Knight R, Panda S. 2018. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun 9:2872. doi: 10.1038/s41467-018-05336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson SM, Cheesman HK, Peterson ND, Salisbury JE, Soukas AA, Pukkila-Worley R. 2019. The fatty acid oleate is required for innate immune activation and pathogen defense in Caenorhabditis elegans. PLoS Pathog 15:e1007893. doi: 10.1371/journal.ppat.1007893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng CJ, Yoo J-S, Lee T-G, Cho H-Y, Kim Y-H, Kim W-G. 2005. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett 579:5157–5162. doi: 10.1016/j.febslet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 63.Antão E-M, Glodde S, Li G, Sharifi R, Homeier T, Laturnus C, Diehl I, Bethe A, Philipp H-C, Preisinger R, Wieler LH, Ewers C. 2008. The chicken as a natural model for extraintestinal infections caused by avian pathogenic Escherichia coli (APEC). Microb Pathog 45:361–369. doi: 10.1016/j.micpath.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Nicholls EF, Madera L, Hancock RE. 2010. Immunomodulators as adjuvants for vaccines and antimicrobial therapy. Ann N Y Acad Sci 1213:46–61. doi: 10.1111/j.1749-6632.2010.05787.x. [DOI] [PubMed] [Google Scholar]
- 65.Hancock RE, Nijnik A, Philpott DJ. 2012. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol 10:243–254. doi: 10.1038/nrmicro2745. [DOI] [PubMed] [Google Scholar]
- 66.Allen HK, Trachsel J, Looft T, Casey TA. 2014. Finding alternatives to antibiotics. Ann N Y Acad Sci 1323:91–100. doi: 10.1111/nyas.12468. [DOI] [PubMed] [Google Scholar]
- 67.Liu J, Wang X, Shi W, Qian Z, Wang Y. 2019. Sensitization of avian pathogenic Escherichia coli to amoxicillin in vitro and in vivo in the presence of surfactin. PLoS One 14:e0222413. doi: 10.1371/journal.pone.0222413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kathayat D, Closs G, Jr, Helmy YA, Lokesh D, Ranjit S, Rajashekara G. 2021. Peptides affecting outer membrane lipid asymmetry system (MlaA-OmpC/F) reduce avian pathogenic Escherichia coli (APEC) colonization in chickens. Appl Environ Microbiol 87:e00567-21. doi: 10.1128/AEM.00567-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deblais L, Helmy YA, Kathayat D, Huang H-C, Miller SA, Rajashekara G. 2018. Novel imidazole and methoxybenzylamine growth inhibitors affecting Salmonella cell envelope integrity and its persistence in chickens. Sci Rep 8:13381. doi: 10.1038/s41598-018-31249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deblais L, Helmy YA, Kumar A, Antwi J, Kathayat D, Acuna UM, Huang H-C, de Blanco EC, Fuchs JR, Rajashekara G. 2019. Novel narrow spectrum benzyl thiophene sulfonamide derivatives to control Campylobacter. J Antibiot (Tokyo) 72:555–565. doi: 10.1038/s41429-019-0168-x. [DOI] [PubMed] [Google Scholar]
- 71.Peighambari SM, Julian RJ, Gyles CL. 2000. Experimental Escherichia coli respiratory infection in broilers. Avian Dis 44:759–769. doi: 10.2307/1593047. [DOI] [PubMed] [Google Scholar]
- 72.Helmy YA, Kathayat D, Ghanem M, Jung K, Closs G, Jr, Deblais L, Srivastava V, El-Gazzar M, Rajashekara G. 2020. Identification and characterization of novel small molecule inhibitors to control Mycoplasma gallisepticum infection in chickens. Vet Microbiol 247:108799. doi: 10.1016/j.vetmic.2020.108799. [DOI] [PubMed] [Google Scholar]
- 73.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sang K, Hao H, Huang L, Wang X, Yuan Z. 2016. Pharmacokinetic–pharmacodynamic modeling of enrofloxacin against Escherichia coli in broilers. Front Vet Sci 2:80. doi: 10.3389/fvets.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gajda A, Posyniak A, Tomczyk G. 2014. LC-MS/MS analysis of doxycycline residues in chicken tissues after oral administration. Bull Vet Inst Pulawy 58:573–579. doi: 10.2478/bvip-2014-0089. [DOI] [Google Scholar]
- 77.Shaw KJ, Miller N, Liu X, Lerner D, Wan J, Bittner A, Morrow BJ. 2003. Comparison of the changes in global gene expression of Escherichia coli induced by four bactericidal agents. J Mol Microbiol Biotechnol 5:105–122. doi: 10.1159/000069981. [DOI] [PubMed] [Google Scholar]
- 78.Kaldalu N, Mei R, Lewis K. 2004. Killing by ampicillin and ofloxacin induces overlapping changes in Escherichia coli transcription profile. Antimicrob Agents Chemother 48:890–896. doi: 10.1128/AAC.48.3.890-896.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 80.Sandrini SM, Haigh R, Freestone PPE. 2014. Fractionation by ultracentrifugation of gram negative cytoplasmic and membrane proteins. Bio Protoc 4:e1287. doi: 10.21769/BioProtoc.1287. [DOI] [Google Scholar]
- 81.Shevchenko A, Tomas H, Havli J, Olsen JV, Mann M. 2006. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 82.Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, Mann M. 2005. Exponentially modified protein abundance index (EMPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics 4:1265–1272. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- 83.Kuzio J, Kropinski AM. 1983. O-antigen conversion in Pseudomonas aeruginosa PAO1 by bacteriophage D3. J Bacteriol 155:203–212. doi: 10.1128/jb.155.1.203-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. 2009. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download SPECTRUM00006-21_Supp_1_seq12.pdf, PDF file, 1 MB (1.1MB, pdf) .



