Abstract
COVID-19 vaccines have brought us a ray of hope to effectively fight against deadly pandemic of COVID-19 and hope to save lives. Many vaccines have been granted emergency use authorizations by many countries. Post-authorization, a wide spectrum of neurological complications is continuously being reported following COVID-19 vaccination. Neurological adverse events following vaccination are generally mild and transient, like fever and chills, headache, fatigue, myalgia and arthralgia, or local injection site effects like swelling, redness, or pain. The most devastating neurological post-vaccination complication is cerebral venous sinus thrombosis. Cerebral venous sinus is frequently reported in females of childbearing age, generally following adenovector-based vaccination. Another major neurological complication of concern is Bell’s palsy that was reported dominantly following mRNA vaccine administration. Acute transverse myelitis, acute disseminated encephalomyelitis, and acute demyelinating polyneuropathy are other unexpected neurological adverse events that occur as result of phenomenon of molecular mimicry. Reactivation of herpes zoster in many persons, following administration of mRNA vaccines, has been also recorded. Considering the enormity of recent COVID-19-vaccinated population, the number of serious neurological events is miniscule. Large collaborative prospective studies are needed to prove or disprove causal association between vaccine and neurological adverse events occurring vaccination.
Keyword: COVID-19; SARS-COV-2; Vaccination; Cerebral venous sinus thrombosis; Thrombocytopenia
SARS-CoV-2 is a novel coronavirus that can rapidly affect human beings and can result in coronavirus disease (COVID-19). COVID-19 is dominantly characterized by lung damage and hypoxia. The first case of COVID-19, in Wuhan, China, was reported on December 8, 2019. Later, the World Health Organization announced COVID-19 as a worldwide health emergency, on January 30, 2020. On March 11, 2020, COVID-19 was declared a pandemic. As per the latest World Health Organization report, there were 196,553,009 confirmed cases as on August 1, 2021 along with 4,200,412 deaths [1].
Early this year, COVID-19 vaccines has brought a ray of hope to effectively fight against this deadly pandemic and save precious human lives. Currently, four major vaccine types are being used. These vaccine types include viral vector-based vaccines, COVID-19 mRNA-based vaccines, inactivated or attenuated virus vaccine, and protein-based vaccines. In viral vector-based vaccines, adenovirus is used to deliver a part of SARS-COV-2 genome to human cells. Human cells use this genetic material to produce SARS-COV-2 spike protein. Human body recognizes this protein to start a defensive response. The mRNA-based vaccines consist of SARS-COV-2 RNA. Once introduced, genetic material helps in making SARS-COV-2-specific protein. This protein is recognized by human body to start defensive immune reaction. In inactivated or attenuated vaccines, killed or attenuated SARS-COV-2 virus triggers immune response. Protein-based vaccines use the spike protein or its fragments for inciting immune response. These COVID-19 vaccines have received emergency approvals in different countries for human use [2]. As per the latest World Health Organization report, until August 1, 2021, globally, a total of 3,839,816,037 COVID-19 vaccine doses have been globally administered [1].
In fact, all kinds of vaccines are associated with the risk of several serious neurological complications, like acute disseminated encephalomyelitis, transverse myelitis, aseptic meningitis, Guillain-Barré syndrome, macrophagic myofasciitis, and myositis. Influenza vaccine has been found associated with narcolepsy in young persons. Several pathogenic mechanisms, like molecular mimicry, direct neurotoxicity, and aberrant immune reactions, have been ascribed to explain these vaccines associated with neurological complications [3]. Even COVID-19 vaccines are not free from neurological complications. In this article, we have focused on the neurological complications following COVID-19 vaccination that were reported after their emergency use authorizations.
Search strategy
We reviewed available data regarding neurological complications (post-authorization) described following the World Health Organization–approved COVID-19 vaccination. We classified COVID-19 vaccination associated with neurological complications in two broad groups: (1) common but mild and (2) rare but severe. We searched PubMed, Google, and Google Scholar databases using the keywords “COVID‐19” or “SARS‐CoV‐2” and “vaccination” or “vaccine,” to identify all published reports on neurological complications of COVID‐19 vaccines. We in this review will focus on spectrum of published neurological adverse events following COVID-19 vaccination. Last search was done on August 1, 2021.
Mild neurological events
Neurological adverse events following COVID-19 vaccination are generally mild and transient, like fever/chills, headache, fatigue, myalgia and arthralgia, or local injection site effects like swelling, redness, or pain. These mild neurological symptoms are common following administration of all kinds of COVID-19 vaccines.
Anxiety-related events, like feeling of syncope and/or dizziness, are particularly common. For example, Centers for Disease Control and Prevention, in a report published on April 30, 2021, recorded 64 anxiety-related events (syncope in 17) among 8,624 Janssen COVID-19 vaccine recipients. None of the event was labeled as serious [4].
In Mexico (data available in form of preprint) among 704 003 subjects who received first doses of the Pfizer-BioNTech mRNA COVID-19 vaccine, 6536 adverse events following immunization were recorded. Among those, 4258 (65%) had at least one neurologic manifestation, mostly (99.6%) mild and transient. These events included headache (62·2%), transient sensory symptoms (3·5%), and weakness (1%). In this study, there were only 17 serious adverse events, seizures (7), functional syndromes (4), Guillain-Barré syndrome (3), and transverse myelitis (2) [5].
In South Korea, Kim and co-workers collected data of post-vaccination adverse events following first dose of adenovirus vector vaccine ChAdOx1 nCoV-19 (1,403 subjects) and mRNA vaccine BNT162b2 (80 subjects) vaccinations. Data were collected daily for 7 days after vaccination. Authors noted that 91% of adenovirus-vectored vaccine and 53% of mRNA vaccine recipients had mild adverse reactions, like injection-site pain, myalgia, fatigue, headache, and fever [6]. A mobile-based survey among healthcare workers (265 respondents) who received both doses of the BNT162b2 mRNA vaccine was conducted. The most common adverse effects were muscle ache, fatigue, headache, chills, and fever. Adverse reactions were higher after the second dose compared with that after the first dose [7].
Headache
Headache is one of the most frequent mild neurological complaints reported by a large number of COVID-19 vaccine recipients, soon after they receive vaccine.
A review of headache characteristic noted that among 2464 participants, headache begun 14.5 ± 21.6 h after AstraZeneca adenovirus vector vaccine COVID-19 vaccination and persisted for 16.3 ± 30.4 h. Headaches, in majority, were moderate to severe in intensity and generally localized to frontal region. Common accompanying symptoms were fatigue, chills, exhaustion, and fever [8]. In a multicenter observational cohort study, Göbel et al. recorded clinical characteristic of headache occurring after the mRNA BNT162b2 mRNA COVID-19 vaccination. Generally, headache started 18.0 ± 27.0 h after vaccination and persisted for 14.2 ± 21.3 h. In majority, the headaches were bifrontal or temporal, dull aching character and were moderate to severe in intensity. The common accompanying symptoms were fatigue, exhaustion, and muscle pain [8].
Severe neurological adverse events
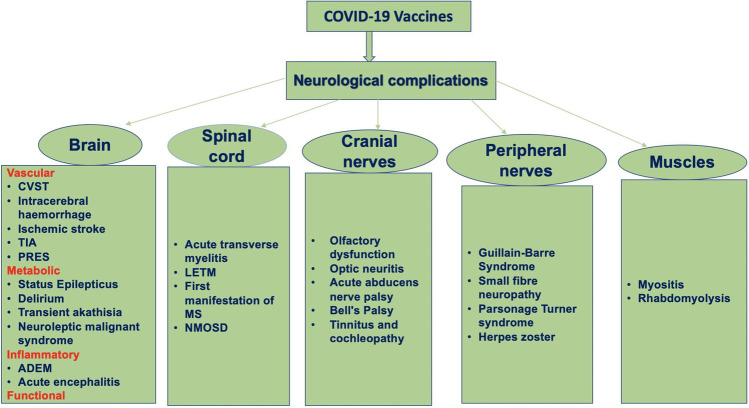
Serious adverse reaction following immunization is defined as a post-vaccination event that are either life-threatening, requires hospitalization, or result in severe disability. The World Health Organization listed Guillain-Barré syndrome, seizures, anaphylaxis, syncope, encephalitis, thrombocytopenia, vasculitis, and Bell’s palsy as serious neurologic adverse events. Instances of serious adverse events following COVID-19 vaccinations are continuously pouring in the current scientific literature and are source of vaccine hesitancy in many persons [9] (Fig. 1).
Fig. 1.
A flow diagram depicts the spectrum of severe neurological complications following COVID-19 vaccinations (ADEM, acute disseminated encephalomyelitis; CVST, cerebral venous sinus thrombosis; LETM, longitudinally extensive transverse myelitis; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorders; PRES, posterior reversible encephalopathy syndrome; TIA, transient ischemic attacks)
Functional neurological disorders
Functional neurological disorders are triggered by physical/emotional stress following an injury, medical illness, a surgery, or vaccination. Functional neurological disorders often remain misdiagnosed despite extensive workup.
After availability of COVID-19 vaccine, many YouTube videos depicted continuous limb and trunk movements and difficulty walking immediately after COVID-19 vaccine administration. These videos were of concern as they were the source of “vaccine hesitancy” [10]. Kim and colleagues reviewed several such social media videos demonstrating motor movements consistent with functional motor symptoms occurring after administration of COVID-19 vaccine. Motor movements were bizarre asynchronous and rapidly variable in frequency and amplitude consistent with functional neurological disorder. The Functional Neurological Disorder Society has lately clarified that movement disorder is consistent with functional in nature. The spread of these videos are important because these functional disorders created concerns for vaccine hesitancy [11].
Several other kinds of functional neurological disorders have also been reported. Butler and colleagues described two young ladies, who presented with functional motor deficits mimicking stroke. Both these patients had variability in weakness and had many non-specific symptoms. A detailed workup and neuroimaging failed to demonstrate any specific abnormality [12]. Ercoli and colleagues described a middle-aged man who, immediately after vaccine administration, reported bilateral facial paralysis along with failure to blink. These manifestations resolved quickly within 40 min. Immediately after administration of second dose of vaccine, he complained of respiratory distress and swollen tongue. Again, all these symptoms resolved quickly following treatment with corticosteroids, however, he developed new symptoms in the form of right hemiparesis. Two weeks later, he developed facial hypoesthesia. A detailed workup of the patient failed to demonstrate any abnormality. A diagnosis of functional neurological disorder was, finally, made [13].
Cerebral vascular events
As a matter of concern, increasing number of reports about adenoviral vector vaccine-induced cerebral vascular adverse events, like cerebral venous thrombosis, arterial stroke, and intracerebral hemorrhage, is getting published in leading medical journals. These reports are alarming as post-vaccination vascular events culminate either in severe disability or death. Vaccine-induced cerebral vascular adverse events are generally associated with severe immune-mediated thrombotic thrombocytopenia. Thrombocytopenia generally clinically manifests within 5 to 30 days after administration of adenovirus vector-based vaccines. In post-vaccination thrombotic thrombocytopenia, a picture similar to that of heparin-induced thrombocytopenia is encountered. When heparin binds platelet factor 4, there is generation of antibodies against platelet factor 4. Antibodies against platelet factor 4 result in platelet destruction and trigger the intravascular blood clotting [14]. The post-mortem examination, in patients with vaccine-induced thrombocytopenia, demonstrated extensive involvement of large venous vessels. Microscopic findings showed vascular thrombotic occlusions occurring in the vessels of multiple body organs along with marked inflammatory infiltration [15]. The vector-based vaccines contain genetic material of SARS-COV-2 that is capable of encoding the spike glycoprotein. Possibly, leaked genetic material binds to platelet factor 4 that subsequently activates formation of autoantibodies. These autoantibodies destroy platelets [16, 17].
Cerebral venous thrombosis
Cerebral venous thrombosis is the one of the most feared devastating COVID-19 vaccine-associated neurological complication. Cerebral venous thrombosis should be suspected in all vaccinated patients, who has persistent headache. Headache is generally unresponsive to the analgesics, and some patients may have focal neurological deficits. Affected patients are generally females of younger ages (Table 1) [18–46].
Table 1.
Clinical, magnetic resonance imaging findings, and outcome details of patients who developed cerebral venous sinus thrombosis after vaccination against SARS-CoV-2
| Reference | Neurological complications | Country | Age/sex | Vaccine type | Duration of onset after vaccination | Clinical features | Neuroimaging | Treatment given |
|---|---|---|---|---|---|---|---|---|
| Castelli et al. [18] | Cerebral venous sinus thrombosis | Italy | 50/M | COVID-19 vaccine AstraZeneca | 10 days | Severe headache, right hemiparesis, unsteady gait, and visual impairment of 4 days Patient needed ICU care and mechanical ventilation | Intra-parenchymal hemorrhage CT angiography = left transverse and sigmoid venous sinuses thrombosis | Fibrinogen concentrate (10 g total) and platelet (4 units total) a bilateral decompressive craniectomy |
| D’Agostino et al. [19] | Cerebral venous thrombosis and disseminated intravascular coagulation | Italy | 54/F | The AstraZeneca vaccine | 12 days | Altered sensorium and hemiparesis Myocardial infarction | Multiple subacute lobar hemorrhages basilar artery thrombosis associated with the superior sagittal sinus thrombosis Bilateral adrenal hemorrhage | Intensive care unit |
| Scully et al. (report of 23 patients) [20] | Thrombocytopenia (23 patients) Cerebral venous thrombosis (13 patients) | London | 12 years (Median) | ChAdOx1 nCoV-19 vaccine (AstraZeneca) | 6 to 24 days | 13 patients with cerebral venous thrombosis | Not available | Not available |
| Franchini et al. [21] | Cerebral venous thrombosis | Italy | 50/M | COVID-19 vaccine AstraZeneca | 7 days | Coma thrombocytopenia | Intra-parenchymal hemorrhage Angiography cerebral venous sinus thrombosis | Intensive care unit |
| Mehta et al. [22] | Cerebral venous sinus thrombosis | UK | 32/M | Vaxzevria vaccine | 9 days | Thunderclap headache Left hemiparesis, left-sided incoordination Thrombocytopenia and rapidly evolving coma | Superior sagittal sinus and cortical vein thrombosis and significant cortical edema with small areas of parenchymal and subarachnoid hemorrhage | Intensive care unit |
| 25/M | Vaxzevria vaccine | 6 days | Headache hemiparesis, left hemisensory loss Seizures, agitation, decerebrate posturing, reduced GCS Thrombocytopenia | Superior sagittal sinus thrombosis with extension into the cortical veins and hemorrhage in lobar and sub-arachnoid locations | Intensive care unit | |||
| Bersinger et al. [23] | Cerebral venous sinus thrombosis | France | 21/F | ChAdOx1 nCoV-19 vaccine | 9 days | Headaches, seizures, hemiplegia, expressive aphasia, and no pupillary abnormalities and altered sensorium The platelet count was 61,000 per cubic millimeter | CT of the head showed massive thrombosis in the deep and superficial cerebral veins, thrombosis of the left jugular vein, and left frontoparietal venous hemorrhagic infarction | A selective arterial embolization was performed immediately after decompressive craniectomy IV immunoglobulin Fondaparinux |
| Ramdeny et al. [24] | Cerebral venous sinus thrombosis | United Kingdom | 54/M | COVID-19 Vaccine AstraZeneca | 21 days | Worsening headache, bruising and unilateral right calf swelling Thrombocytopenia D-dimer = 60,000 ng/ml Anti-platelet factor 4 | Cerebral venous sinus thrombosis | Intravenous immunoglobulin |
| Zakaria et al. [25] | Cerebral venous sinus thrombosis | Malaysia | 49/M | First dose of mRNA SARS-CoV-2 vaccine | 16 days | New onset of mild to moderate headache and giddiness | CT) of the brain showed cordlike hyperattenuation within the left transverse and sigmoid sinus suggestive of cord or dense clot sign CT cerebral venography a long segment-filling defect and empty delta sign within the superior sagittal sinus extending into the torcula Herophili, left transverse sinus, and sigmoid sinus to proximal internal jugular vein | Subcutaneous Clexane improved |
| Ryan et al. [26] | Cerebral venous sinus thrombosis | Ireland | 35/F | AZD1222 (COVID-19 Vaccine AstraZeneca) | 10 days | Headache thrombocytopenia bruising and petechiae Antibody to platelet factor 4 | MR venogram showed cerebral venous sinus thrombosis | Apixaban |
| Graf et al. [27] | Cerebral venous sinus thrombosis | Germany | 29/M | ChAdOx1 nCov-19, AstraZeneca | 9 days | Severe headache and hematemesis thrombocytopenia | Complete thrombosis of the left transverse and sigmoid sinus down to the left proximal jugular vein Temporo-parietal intracranial hemorrhage CT angiography revealed extensive thrombosis of the mesenteric and portal vein | High-dose immunoglobulins Argatroban |
| George et al. [28] | Cerebral venous sinus thrombosis | USA | 40/F | ChAdOx1 nCov-19, AstraZeneca | 7 days | Headache thrombocytopenia Antibody to platelet factor 4 | Venous thrombosis involving the left transverse sigmoid sinus and internal jugular vein | A direct thrombin inhibitor (bivalirudin) Intravenous immune globulin (IVIG) |
| Jamme et al. [29] | Cerebral venous sinus thrombosis | France | 69/F | First dose of Oxford–AstraZeneca vaccine | 11 days | Headache associated with behavioral symptoms | Bilateral frontal hemorrhage cerebral venous thrombosis of the left internal jugular vein, sigmoid sinus, and superior sagittal sinus | None |
| Tiede et al. (report of 5 patients) [30] | Cerebral venous sinus thrombosis | Germany | 41 and 67 years All females | ChAdOx1 COVID-19 vaccine (AZD1222, Vaxzevria) | 5 to 11 days after first vaccination | Cerebral venous sinus thrombosis (CVST), splanchnic vein thrombosis (SVT), arterial cerebral thromboembolism, and thrombotic microangiopathy thrombocytopenia Autoantibodies against platelet factor 4 | Brain hematomas infarcts, presence of thrombi in major vessels | Intravenous immunoglobulin or corticosteroids Argatroban |
| Schulz et al. (report of 45 cases) [31] | Cerebral venous thrombosis | Germany | 46.5 years (mean)/35 females | BNT162b2, ChAdOx1, and mRNA-1273 | Within 30 days of vaccination | Thrombocytopenia in all patients | Cerebral venous thrombosis | Intravenous immunoglobulins, plasmapheresis, corticosteroids, anticoagulants |
| Bourguignon et al. [32] | A report three patients one had cerebral venous sinus thrombosis | Canada | 69/M | ChAdOx1 nCov-19, AstraZeneca | 12 days | Diabetes mellitus, hypertension, obstructive sleep apnea, recently diagnosed prostate cancer Headache and confusion left-sided weakness Thrombocytopenia Autoantibodies against platelet factor 4 | Right middle cerebral-artery stroke with hemorrhagic transformation Right cerebral transverse and sigmoid sinuses, right internal jugular vein, hepatic vein, and distal lower-limb vein; pulmonary embolism | Intravenous immunoglobulin Plasmapheresis |
| Gattringer et al. [33] | Cerebral venous sinus thrombosis | Austria | 39/F | The first vaccination with ChAdOx1 nCov-19 (AstraZeneca) | 8 days | Headache since 2 days thrombocytopenia (84 × 10 [8]/L) | Left sigmoid/transverse sinus thrombosis without brain parenchymal involvement | Intravenous immunoglobulin |
| Ikenberg et al. [34] | Cerebral venous sinus thrombosis | Germany | early 30 s/F | The first dose of ChAdOx1 nCov-19 (AstraZeneca) | Headache Gait ataxia, and amnestic difficulties as well as aphasia Thrombocytopenia of 37 000/µL | CVST of the left transverse and sigmoidal sinus with a left-temporal and left-cerebellar intracerebral hemorrhage | Intravenous immunoglobulin argatroban | |
| Clark et al. [35] | Cerebral venous sinus thrombosis | USA | 40/F | The Ad26.COV2.S (Johnson & Johnson/ Jansen) vaccine | 5 days | Worsening headaches thrombocytopenia | Cerebral venous sinus thrombosis involving the left transverse and sigmoid sinuses, extending into the left internal jugular vein | Bivalirudin infusion Intravenous immunoglobulin |
| Bonato et al. [36] | Cerebral venous sinus thrombosis | Italy | 26/F | ChAdOx1 nCoV-19 vaccine | 14 days | headache non-responsive to drugs right-sided weakness and visual disturbances rapidly deteriorated with decreased consciousness | Multifocal venous thrombosis with bilateral occlusion of parietal cortical veins, straight sinus, vein of Galen, internal cerebral veins, and inferior sagittal sinus. Right parietal and left frontoparietal lobes an extensive venous infarction with hemorrhagic transformation Platelet-factor 4 (PF4)–heparin IgG antibodies – elevated thrombocytopenia | Dexamethasone Intravenous immunoglobulin argatroban |
| Wang et al. [37] | Cerebral venous sinus thrombosis | Taiwan | 41/F | First vaccination with ChAdOx1 nCoV-19 | 7 days | Fever and headache thrombocytopenia positive anti-PF4 antibodies | MR venography revealed cerebral venous sinus thrombosis | Intravenous immunoglobulin |
| Dutta et al. [38] | Cerebral venous sinus thrombosis | India | 51/M | First-dose of COVISHIELD | 6 days | Headache double vision papilledema Platelet count was normal | MR venography revealed thrombosis in superior sagittal sinus and transverse sinus | Low-molecular-weight heparin |
| Aladdin et al. [39] | Cerebral venous sinus thrombosis | Saudi Arabia | 36/F | First dose of the ChAdOx1 nCoV-19 vaccine | 14 days | Vomiting and severe headache left upper limb weakness thrombocytopenia Disseminated intravascular coagulation | Brain computed tomography (CT) scan showed superior sagittal thrombosis with thickened cortical veins and bilateral hypodensities in the parietal lobes | Low-molecular-weight heparin ICU care |
| Lavin et al. (a series of 4 patients) [40] | Cerebral venous sinus thrombosis | Ireland | 29/F 38/M 50/F 35/F | Vaxzevria vaccine (ChAdOx1 nCoV-19, AstraZeneca) | 10 days 16 days 23 days 14 days | Visual disturbance followed by a headache, nausea, vomiting, bruising and petechiae severe thunderclap headache, nausea and vomiting headache, persistent bruising and petechiae all had thrombocytopenia | Dural venous sinus thrombosis in one patient only other had abdominal abnormalities | Intravenous immunoglobulin |
| Tølbøll Sørensen et al. [41] | Cerebral venous sinus thrombosis | UK | 30/F | ChAdOx1 nCoV-19 | Headache and general malaise portal vein thrombosis thrombocytopenia and consumption coagulopathy Anti-platelet antibodies were detected | Normal | Tinzaparin | |
| Fan et al. [42] (a series of 3 patients) | Cerebral venous sinus thrombosis | Singapore | 54/M 62/F 60/F | BNT162b2 mRNA vaccination | 1 day 9 days 8 days | Severe headache and vomiting and acute left hemiparesis Headache and vomiting Right ataxic hemiparesis There was no thrombocytopenia | A large right temporo-parietal lobe intraparenchymal hemorrhage Acute right cerebral bleed involving occipital and temporal lobes associated with subarachnoid hemorrhage Venous infarct in bilateral perirolandic gyri Venogram confirmed cerebral venous sinus thrombosis in all three | Low-molecular-weight heparin decompressive craniectomy |
| Suresh and Petchey [43] | Cerebral venous sinus thrombosis | UK | 27/M | ChAdOx1 nCOV-19 vaccine | 2 days | Worsening headache and new homonymous hemianopia Thrombocytopenia Anti-platelet antibodies were detected | Acute parenchymal bleed with subdural extension CT venogram confirmed significant cerebral venous sinus thrombosis | Dabigatran and intravenous immunoglobulins |
| Dias et al. (a series of 2 patients) [44] | Cerebral venous sinus thrombosis | Portugal | 47/F 67/F | BNT162b2 mRNA SARS-CoV-2 vaccine | 6 days 3 days | Headache, nausea and photophobia a sudden left motor deficit Sudden right lower limb clonic movements, followed by motor deficit, loss of consciousness and headache There was no thrombocytopenia Anti-platelet antibodies were not detected | MRI with venography revealed thrombosis of superior sagittal, right lateral, transverse, sigmoid sinuses, and jugular vein and left sigmoid sinus, together with right frontal subarachnoid hemorrhage and a cortical venous infarct Brain MRI showed thrombosis of high convexity cortical veins, superior sagittal, right transverse, and sigmoid sinus and jugular vein | Acetazolamide and enoxaparin Levetiracetam 500 mg bid and enoxaparin |
| Guan et al. [45] | Cerebral venous sinus thrombosis | Taiwan | 52/M | The first dose of ChAdOx1 nCov-19 (AstraZeneca) | 10 days | Nausea and thunderclap headache thrombocytopenia Platelet factor 4 antibodies detected | Hyperdensity of the sinus, including cord sign and dense vein sign at the left transverse and sigmoid sinuses CT venogram revealed CVST at the left transverse sinus and sigmoid sinuses and thrombosis of the left internal jugular vein | Apixaban Outcome not provided |
| Varona et al. [46] | Cerebral venous sinus thrombosis and primary adrenal insufficiency | Spain | 47/M | Adenoviral (ChAdOx1) vector-based COVID-19 vaccine | 10 days | Headache, somnolence, and mild confusion Blateral segmentary pulmonary embolism Thrombocytopenia Anti-platelet antibodies were detected | Consistent with cerebral venous thrombosis | Intravenous immunoglobulins and subcutaneous fondaparinux hydrocortisone Patient improved |
In Europe, since March 2021, cases of cerebral venous thrombosis started pouring in following COVID-19 vaccination, particularly after administration of viral vector based (AstraZeneca ChAdOx1 nCoV-19 and the Johnson and Johnson Ad26. COV2.S) vaccines [22]. Scully and colleagues recently reported findings of 23 patients, who presented with thrombosis and thrombocytopenia (platelet counts below 10 × 109/L). These patients developed thrombosis and thrombocytopenia 6 to 24 days after they received the first dose of the viral vector-based vaccines. In a significant observation, authors, in majority of patients, demonstrated the presence of autoantibodies against platelet factor 4. Additionally, D-dimer levels were found elevated [20]. Tiede and co-workers reported five German cases of prothrombotic immune thrombocytopenia after vaccination with viral vector-based vaccine (Vaxzevria). In these patients, acute vascular events clinically manifested as cerebral venous sinus thrombosis, splanchnic vein thrombosis, arterial cerebral thromboembolism, and/or thrombotic microangiopathy within 2 weeks post vaccination. All five patients had low platelet counts and markedly raised D-dimer. In all, autoantibodies against platelet factor 4 were also demonstrated [30].
Pottegård et al. in Denmark and Norway evaluated incidence of arterial events, venous thromboembolism, thrombocytopenia, and bleeding among vaccinated population. The vaccinated cohorts comprised of 148,792 Danish people and 132,472 persons from Norway. All has received their first dose of viral vector-based vaccine (ChAdOx1-S). An excess rate of venous thromboembolism (like cerebral venous thrombosis) was observed among vaccine recipients, within 28 days of vaccine administration. Authors estimated an increased rate for venous thromboembolism corresponding to 11 excess events per 100,000 vaccinations with 2.5 excess cerebral venous thrombosis events per 100,000 vaccinations [47].
Krzywicka et al., from the Netherlands, collected data of 213 cases with post-vaccination (187 after adenoviral vector vaccines and 26 after a mRNA vaccine) cerebral venous sinus thrombosis; they noted thrombocytopenia in 107/187 (57%) post-vaccination cerebral venous sinus thrombosis cases. Thrombocytopenia was not recorded in any of patients, who received an mRNA-based vaccine. Cerebral venous sinus thrombosis after adenoviral vector vaccines carried poorer prognosis. Approximately, 38% (44/117) patients in adenoviral vector vaccine group died, while in mRNA vaccine group, 20% (2/10) had died [48].
Recently published National Institute for Health and Care Excellence (NICE) guidelines recommend that the patients with clinical diagnosis of vaccine-induced immune thrombocytopenia and thrombosis should be treated with intravenous administration of human immunoglobulin, at a dose of 1 g/kg. If there is no response or there is further deterioration, second dose of human immunoglobulin should be given. In patients with insufficient response, methylprednisolone 1 g intravenously for 3 days or dexamethasone 20 to 40 mg for 4 days can be used [49].
Heparin needs to be avoided, instead alternative anticoagulants like argatroban, bivalirudin, fondaparinux, rivaroxaban, or apixaban should be used for anticoagulation [49–51]. NICE guidelines further recommend that patients with very low platelet count should be treated either alone with a argatroban or a combination of argatroban and platelet transfusion [49].
Arterial events
Several acute arterial events, like arterial thrombosis, intracerebral hemorrhage, transient global amnesia, and spinal artery ischemia, have also been reported following vaccination [31].
Simpson and colleagues, in Scotland, estimated the incidence of vaccine-associated thrombocytopenia and vascular events following administration of first dose of viral vector-based vaccine (ChAdOx1) or mRNA (BNT162b2 Pfizer-BioNTech or mRNA-1273 Moderna) vaccination. First dose of viral vector-based vaccine was associated with small enhanced risk of idiopathic thrombocytopenic purpura; in addition, up to 27 days after vaccination, there was possibility of an increased risk for thromboembolic and hemorrhagic events. No such adverse associations were noted with mRNA vaccines [52]. The reports of COVID-19 vaccine-related intracerebral hemorrhage and ischemic stroke are summarized in Table 2 [53–61].
Table 2.
Clinical, neuroimaging and outcome details of patients who suffered strokes (other than cerebral venous thrombosis) after vaccination against SARS-CoV-2
| Reference | Neurological complication | Country | Age/sex | Vaccine type | Duration after vaccination | Clinical features | Neuroimaging | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Athyros and Doumas [53] | Intracerebral hemorrhage | Greece | 71/F | Moderna anti-COVID-19 vaccine | 3 days | Right hemiplegia, aphasia, agnosia Acute hypertensive crisis | Left basal ganglia hemorrhage | Clonidine, furosemide | Died |
| Bjørnstad-Tuveng [54] | Intracerebral hemorrhage | Norway | Thirties/F | AstraZeneca’s vaccine ChAdOx1 nCoV-19 | 9 days | Slurred speech, left hemiparesis, and reduced consciousness | Right intracerebral hemorrhage on CT, thrombosis in transverse sinus and pulmonary artery on postmortem | ICU management | Died |
| de Mélo Silva et al. [55] | Intracerebral hemorrhage with intraventricular extension | Brazil | 57/F | ChAdOx1 nCoV-19 vaccine | 5 days | Left hemiparesis, vomiting, and somnolence | A large right deep frontal lobe parenchymal hematoma | ICU management Decompressive craniectomy | Survived with disabilities |
| Bayas et al. [56] | Bilateral superior ophthalmic vein thrombosis, ischemic stroke, and immune thrombocytopenia | Germany | 55/F | SARS-CoV-2— ChAdOx1 nCoV-19 | 10 days | Flu-like illness, diplopia, vision loss, a transient, mild, right-sided hemiparesis, and aphasia, focal seizures | MRI showed superior ophthalmic vein thrombosis An MRI showed an ischemic stroke in the left parietal lobe, middle cerebral artery territory, with restricted diffusion | Intravenous dexamethasone Anticoagulants | Improved |
| Al-Mayhani et al. [57] | Ischemic stroke with thrombocytopenia | London | 35/F 37/F 43/F | ChAdOx1 nCoV-19 vaccine ChAdOx1 nCoV-19 vaccine ChAdOx1 nCoV-19 vaccine | 11 days 12 days 21 days | Left face, arm, leg weakness and drowsiness Headache, left visual field loss, confusion, left arm weakness Dysphasia | Right middle-cerebral artery infarct Bilateral acute border zone infarcts Left middle-cerebral artery infarct | Decompressive hemicraniectomy Intravenous immunoglobulin Intravenous immunoglobulin | Died Improved Stable |
| Blauenfeldt et al. [58] | Ischemic stroke | Denmark | 60/M | mRNA-based vaccine BNT162b2 (Pfizer/BIOTECH) | 7 days | Bilateral adrenal hemorrhages A massive right sided ischemic stroke Thrombocytopenia Platelet factor 4 (PF‐4) reactive antibodies | Angiography showed occlusion of the right internal. Carotid artery | Intensive care unit | Palliative care |
| Malik et al. [59] | transient ischemic attack | USA | 43/F | Johnson and Johnson COVID-19 Ad26.COV2.S vaccination | 10 days | Headache, fever, body aches, chills, mild dyspnea and light-headedness thrombocytopenia numbness and tingling of her face and right arm | Right internal carotid artery (ICA) thrombus | Fondaparinux | Improved |
| Finsterer and Korn [60] | Aphasia | Austria | 52/M | The second dose of an mRNA-based SARS-CoV-2 vaccine | 7 days | Sudden-onset reading difficulty and aphasia motor aphasia with paraphasia | A lobar bleeding in the left temporal lobe | Supportive | Improved |
| Walter et al. [61] | Ischemic stroke Main stem occlusion of middle cerebral artery | Germany | First dose ChAdOx1 nCov-19 vaccine | acute headache, aphasia, and hemiparesis Platelet count and fibrinogen level were normal | Main stem occlusion of middle cerebral artery A wall-adherent, non-occluding thrombus in the ipsilateral carotid bulb was noted | Within 1 h after start of IV thrombolysis | Thrombus dissolved and patient improved |
Intracerebral hemorrhage
Athyros and Doumas reported a 71-year-old female. who developed intracerebral hemorrhage after she received the first dose of the Moderna mRNA vaccine.
On the third post-vaccination day, the patient developed right hemiplegia, aphasia, and agnosia along with accelerated hypertension. Computed tomography revealed a hematoma in the left basal ganglia. On the 9th day, she died [53].
In another report, Bjørnstad-Tuveng et al. described a young woman, who had a fatal cerebral event following vaccination with AstraZeneca’s ChAdOx1 nCoV-19 vaccine. She was found to have severe thrombocytopenia. The patient died the next day of the event. Post-mortem examination revealed antibodies against platelet factor 4 and the presence of small thrombi in the transverse sinus, frontal lobe, and pulmonary artery [54].
Acute ischemic stroke
Bayas and co-workers described a case that presented with superior ophthalmic vein thrombosis, ischemic stroke, and immune thrombocytopenia, after administration of viral vector-based vaccine. Intravenous dexamethasone resulted in marked improvement in platelet count [56]. Al-Mayhani et al. described three cases of vaccine-induced thrombotic thrombocytopenia, all presented with arterial strokes. Authors opined that young patients with arterial stroke after receiving the COVID-19 vaccine should always be evaluated for vaccine-induced thrombotic thrombocytopenia. Other laboratory tests, like platelet count, D-dimers, fibrinogen level, and testing for platelet factor 4 antibodies, should also be performed [57].
Blauenfeldt et al. described a 60-year-old woman, who presented with intractable abdominal pain, 7 days after receiving the adenoviral (ChAdOx1) vector-based COVID-19 vaccine. Abdominal computed tomography revealed bilateral adrenal necrosis. Later, a massive right cerebral infarction, secondary to occlusion of the right internal carotid artery, occurred that led to death of the patient. Blood tests showed thrombocytopenia, elevated in D-dimer and platelet factor 4 antibodies [58].
Many reports of acute brain disorders like encephalopathy, seizures, acute disseminated encephalopathy, neuroleptic malignant syndrome, and post-vaccine encephalitis were described secondary to COVID-19 vaccine. These are summarized in Table 3 [62–75].
Table 3.
Clinical, neuroimaging and outcome details of patients who presented with an acute brain disorder (other than cerebral venous thrombosis and arterial stroke) after vaccination against SARS-CoV-2
| Reference | Neurological complication | Country | Age/sex | Vaccine type | Duration after vaccination | Clinical features | Neuroimaging | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Baldelli et al. [62] | Reversible encephalopathy | Italy | 77/M | The first dose of ChAdOx1 nCoV-19 vaccine (AstraZeneca) | 1 day | Delirium A significant increase of interleukin (IL)-6 in both CSF and serum | Normal | Corticosteroids | |
| Aladdin and Shirah [63] | New-onset refractory status epilepticus | Saudi Arabia | 42/F | ChAdOx1 nCoV-19 vaccine | 10 days | Headache and fever first-ever generalized tonic–clonic seizure lorazepam, levetiracetam, and phenytoin failed to control | Increase in the signal on FLAIR images at bilateral hippocampi and insula | Midazolam and propofol Plasma exchange | Improved |
| Ghosh et al. [64] | Seizures | India | 68/M | Covishield vaccine | 4 days | Focal onset non-motor seizure | Periventricular leukoaraiosis and cortical atrophy | brivaracetam | Improved |
| Liu et al. [65] (two cases) | Associated with non-convulsive status epilepticus | USA | 86/F 73/M | Moderna COVID-19 vaccine | 7 days 21 days | Diastolic dysfunction, chronic kidney disease and diabetes mellitus with acute encephalopathy Acute confusion with visual hallucinations EEG demonstrated non-convulsive focal status epilepticus Acute encephalopathy with non-convulsive status epilepticus | Normal | Antiepileptic therapy and ICU care | Both improved |
| Naharci and Tasc [66] | Delirium | Turkey | 88/F | first dose of CoronaVac–-an inactivated COVID-19 vaccine | Acute confusion, hallucinations, agitation, and sleep disturbance | None | Haloperidol and trazodone | Improved | |
| Salinas et al. [67] | Transient akathisia | USA | 36/F | Pfizer-BioNTech vaccine | Within 24 h of second dose | Restless body syndrome had fever after 5 h of motor restlessness resolved after 24 h | None | None | Improved |
| Zavala-Jonguitud et al. [68] | Delirium | Mexico | 89/M | The first dose of BNT162b2 RNA vaccine | 24 h | Acute confusion, fluctuating attention, anxiety and inversion of the sleep–wake cycle History of type 2 diabetes mellitus, hypertension, stage III‐b chronic kidney disease, prostatic hyperplasia | Not done | Quetiapine | Improved |
| Alfishawy et al. [69] | Neuroleptic malignant syndrome | Kuwait | 74/F | BNT162b2 mRNA COVID-19 vaccine | 16 days | Old case of dementia and bipolar disorder and was receiving memantine, donepezil, and quetiapine presented with fever, delirium, rigidity, and elevated CPK | Normal | Symptomatic | Improved |
| Ozen Kengngil et al. [70] | Acute disseminated encephalomyelitis like MRI lesions | Turkey | 46/F | Inactivated SARS-CoV-2 vaccine of Sinovac | 1 Month | Seizures, normal examination | T2, FLAIR hyperintensity in thalamus, and corona radiata | Methyl prednisolone | No recurrence of seizures |
| Cao and Ren [71] | Acute disseminated encephalomyelitis | China | 24/F | SARS-CoV-2 Vaccine (Vero Cell), Inactivated | 2 weeks | Somnolence and memory decline, MMSE-11 inflammatory changes in CSF | T2/FLAIR white matter hyperintensity in both temporal lobes | IV immunoglobulin | Improved |
| Raknuzzaman et al. [72] | Acute disseminated encephalomyelitis | Bangladesh | 55/M | BNT162b2 mRNA COVID-19 vaccine | 3 weeks | Delirium followed by loss of consciousness | T2/FLAIR white matter hyperintensities in periventricular region | Methyl prednisolone | Improved |
| Torrealba-Acosta et al. [73] | Acute encephalitis, myoclonus and Sweet syndrome | USA | 77/M | mRNA-1273 vaccine | 1 day | Confusion, fever and generalized rash; later headache, dizziness and double vision leading to severe encephalopathy Intermittent orofacial movements and upper extremity myoclonus CSF showed increased cells and protein. Skin biopsy showed vasculitis changes | Normal | Methylprednisolone | Improved |
| Vogrig et al. [74] | Acute disseminated encephalomyelitis | Italy | 56/F | Pfizer-BioMTech COVID-19 vaccine (Comirnaty) | 2 weeks | Horizontal gaze-evoked nystagmus, Mild weakness on left upper limb, left hemi-ataxic gait | T2/FLAIR white matter hyperintensity in left cerebellar peduncle prednisone improved FLAIR sequences were observed, the largest in the left centrum semiovale | Prednisone | Improved |
| Zuhorn et al. [75] | Postvaccinal encephalitis Similar to autoimmune encephalitis | Germany | 21/F | ChAdOx1 nCov-19 vaccine the first dose | 5 days | Headache and progressive neurological symptoms including attention and concentration difficulties and a seizure CSF lymphocytic pleocytosis EEG slow delta rhythm | Normal | Prednisone | Improved |
| 63/F | ChAdOx1 nCov-19 vaccine | 6 days | Gait disorder, a vigilance disorder and a twitching all over her body Opsoclonus-myoclonus syndrome CSF lymphocytic pleocytosis EEG slow delta rhythm | Normal | Methylprednisolone | Improved | |||
| 63/M | ChAdOx1 nCov-19 vaccine | 8 days | Isolated aphasia and fever CSF lymphocytic pleocytosis EEG normal | Normal | None | Mild improvement despite no treatment | |||
Encephalopathy
Some patients developed encephalopathy following administration of COVID-19 vaccines. Acute encephalopathy is defined as rapidly evolving disorder of the brain. Acute encephalopathy clinically manifests either with delirium, decreased consciousness, or coma.
Delirium
Delirium is characterized with fluctuating disturbance in attention and awareness. Zavala-Jonguitud and Pérez-García described an 89-year-old man, who developed delirium after mRNA vaccination. Within 24 h, patient developed confusion, fluctuating attention, anxiety, and inversion of the sleep–wake cycle. Patient had many comorbidities (diabetes mellitus, hypertension, and chronic kidney disease). Patient improved after he was treated with quetiapine [68].
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome is a life-threatening complication of many antipsychotic drugs characterized by fever, altered mental status, muscle rigidity, and autonomic dysfunction. In an isolated report, neuroleptic malignant syndrome, in a 74-year-old female with dementia and bipolar disorder 16 days after COVID-19 vaccination, has been described [69].
Acute disseminated encephalomyelitis
Acute disseminated encephalomyelitis (ADEM) is an acute inflammatory demyelinating disorder of the central nervous system. In the majority, ADEM is a post-infectious entity; in many cases, it even develops after vaccination [76]. In two cases, acute disseminated encephalomyelitis following COVID-19 vaccination has been reported. In first such case a 46-year-old woman received Sinovac inactivated SARS-CoV-2 vaccine before onset of clinical manifestations. Patient was presented with seizures, and magnetic resonance imaging revealed multiple, discrete T2/FLAIR periventricular. hyperintense lesions. Patient improved following methylprednisolone treatment [70] Another patient was a 24-year-old female who presented with encephalopathy along with limb weakness of 1-day duration. Two weeks prior, patient was vaccinated with inactivated SARS-CoV-2 vaccine. Magnetic resonance imaging revealed multiple, discrete T2/FLAIR hyperintense lesions in the brain. Patient improved following treatment with antiepileptics and intravenous immunoglobulins [71].
Post-vaccinal encephalitis
Zuhorn et al. reported a case series 3 patients, who presented with post-vaccinal encephalitis, akin to autoimmune encephalitis, 7 to 11 days after administration of adenovirus-based ChAdOx1 nCov-19 vaccine. All patients fulfilled the diagnostic criteria for possible autoimmune encephalitis. One interesting case had presented with opsoclonus-myoclonus syndrome. Two patients presented with cognitive decline, seizures, and gait disorder. Neuroimaging did not reveal any abnormality. CSF pleocytosis was noted in all three patients. All patients responded well to corticosteroids [75].
Transverse myelitis
Acute transverse myelitis is an inflammatory spinal cord disorder that clinically manifests with the paraparesis/quadriparesis, transverse sensory level, and bowel or bladder dysfunction. Acute transverse myelitis usually is a postinfectious disorder. Magnetic resonance imaging demonstrates T2/FLAIR hyperintensity extending several spinal cord segments. Autoimmunity via mechanism of molecular mimicry is usually responsible for spinal cord dysfunction. Adenoviral vector-based COVID-19 vaccines are more frequently associated with causation of transverse myelitis. In isolated cases, even inactivated virus vaccine and mRNA-based vaccines had precipitated acute demyelination spinal cord syndromes, like multiple sclerosis and neuromyelitis optica. Reports of myelitis associated with vaccination for SARS-CoV-2 are summarized in Table 4 [77–83].
Table 4.
Clinical, neuroimaging, and outcome details of patients who presented with spinal cord involvement after vaccination against SARS-CoV-2
| Reference | Neurological complication | Country | Age/sex | Vaccine type | Duration after vaccination | Clinical features | Neuroimaging | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Malhotra et al. [77] | Transverse myelitis | India | 36/M | Viral-vectored, recombinant ChAdOX1 nCoV-19 Covishield vaccine (AstraZeneca vaccine by Serum Institute of India) | On the 8th post-vaccination day | Abnormal sensations in lower limbs with truncal level | T2-hyperintense lesion in the dorsal aspect of spinal cord at C6 and C7 vertebral levels | Methylprednisolone | Improved |
| Fitzsimmons and Nance [78] | Transverse myelitis | USA | 63/M | Second dose of the Moderna vaccine | Within 1 day | Lower back pain, paresthesia in both feet, and pain in lower extremities difficulty in walking and urinary retention | Increased T2 cord signal seen in the distal spinal cord and conus | Intravenous immunoglobulin and methylprednisolone | Improved |
| Tahir et al. [79] | Transverse myelitis | USA | 44/F | Ad26.COV2.S (Johnson & Johnson) vaccine | 10 days | Cervical cord transverse myelopathy CSF increased cells | Increased T2 cord signal seen in the spinal cord extending from the C2-3 segment into the upper thoracic region | Plasma exchange and methylprednisolone | Improved |
| Pagenkopf and Südmeyer [80] | Longitudinally extensive transverse myelitis | Germany | 45/M | First dose COVID-19-vaccine (AZD1222, AstraZeneca) | 11 days | Thoracic back pain and urinary retention | T2 hyperintense signal of the spinal cord with wide axial and longitudinal extent reaching from C3 to Th2 | Prednisolone | Improved |
| Helmchen et al. [81] | Optic neuritis with longitudinal extensive transverse myelitis in stable multiple sclerosis | Germany | 40/F | Astra Zeneca, COVID19 Vaccine®; Vaxzevria | 2 weeks | Blindness paraplegia, with absent tendon reflexes in the legs, incontinence, and a sensory deficit for all qualities below Th5. CSF showed severe pleocytosis and elevated protein | Increased longitudinal centrally located signal intensities throughout the thoracic spinal cord | Corticosteroids and plasmapheresis | Improved |
| Havla et al. [82] | First manifestation of multiple sclerosis | Germany | 28/F | Pfizer-BioNTech COVID-19 vaccine | 6 days first dose | Myelitis oligoclonal bands | MRI revealed multiple (> 20), partially confluent lesions with spatial dissemination but no gadolinium enhancement. Contrast-enhancing lesion at the T6 level, suggestive of myelitis | Methylprednisolone and plasma exchange | Improved |
| Chen et al. [83] | Neuromyelitis optica spectrum disorder | China | Middle-aged female | The first dose of inactivated virus vaccine | 3 days | Dizziness and unsteady walking AQP4-positive | MRI scanning of the brain revealed area postrema and bilateral hypothalamus lesions | Methylprednisolone | Improved |
Malhotra and colleagues reported a 36-year-old patient, who had short-segment myelitis 21 days after first dose of adenoviral vector-based (Oxford/AstraZeneca, COVISHIELD™) vaccine. Patient recovered completely after treatment with methylprednisolone [77]. Fitzsimmons and Nance reported another patient of acute transverse myelitis following Moderna vaccine (an mRNA vaccine). The 63-year-old patient developed symptoms of acute myelopathy within 24 h of vaccination. MRI revealed increased T2 cord signal seen in the distal spinal cord and conus. Patient improved considerably following treatment with methylprednisolone and intravenous immunoglobulin [78].
Earlier, in phase III trial of Oxford/AstraZeneca vaccine, 2 patients had developed transverse myelitis. One of the case of transverse myelitis was reported 14 days after booster vaccination. The expert committee considered that this case was the most likely an idiopathic, short segment transverse myelitis. The second case was reported 68 days post-vaccination. Experts believed that in this case, transverse myelitis was not likely to be associated with vaccination. This patient was earlier diagnosed as a case of multiple sclerosis [84, 85].
The pathogenesis of acute transverse myelitis following COVID-19 vaccination remains unknown. Possibly, SARS-CoV-2 antigens present in the COVID-19 vaccine or its adenovirus adjuvant induce immunological reaction in the spinal cord. The occurrence of 3 reported acute transverse myelitis adverse effects among 11,636 participants in the vaccine trials was considered high and a cause of concern [86].
Bell’s palsy
Several cases of Bell’s palsy have occurred following COVID-19 vaccination. (Table 5) [87–95]. The instances of Bell’s palsy are most often associated with mRNA vaccines [96]. Vaccine-associated Bell’s palsy generally responds very well to the oral corticosteroids. The exact pathogenesis remains speculative.
Table 5.
Summary of reported patients, who suffered from Bell’s palsy after vaccination against SARS-CoV-2
| Reference | Neurological complication | Country | Age/sex | Vaccine type | Duration after vaccination | Clinical features | Neuroimaging | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Shemer et al. (a report of 9 cases) [87] | Bell’s palsy | Israel | 35–86 (M = 5 and F = 4) | BNT162b2 SARS-CoV-2 vaccine | 4–30 days after first dose 3 received 2nd dose | Acute facial weakness One had herpes zoster ophthalmicus and herpes zoster oticus | None | Corticosteroids | Not given |
| Repajic et al. [88] | Bell's palsy | USA | 57/F | Pfizer-BioNTech COVID-19 A messenger RNA (mRNA) vaccine | 36 h after second dose | 3 previous episodes of Bell’s palsy ageusia Facial weakness | None | Prednisone | Improved |
| Colella et al. [89] | Bell’s palsy | Italy | 37/M | mRNA vaccine BNT162b2 | 5 days after first dose | Acute facial weakness | Not done | Corticosteroids | Improved |
| Martin-Villares et al. [90] | Bell’s palsy | Spain | 34/F | Moderna COVID-19 vaccine | 2 days | Grade III facial palsy She developed a right Bell’s palsy in 2012 during pregnancy (5th month) | None | Corticosteroids | Improved |
| Nishizawa et al. [91] | Bell’s palsy | Japan | 62/F | Ad26.COV2.S vaccination | 20 days | House-Brackmann score 4 Bell’s Palsy | Normal | None | None |
| Gómez de Terreros et al. [92] | Bell’s palsy | Spain | 50/M | Pfizer-BNT162b2 mRNA vaccine | 9 days | Muscle weakness on the left side of his face | Normal | Corticosteroids | Improved |
| Burrows et al. [93] | Sequential contralateral facial nerve palsies | UK | First and second doses of the Pfizer-BioNTech COVID-19 vaccine | Right palsy, 5 h Left palsy after 2 days | Two discrete contralateral episodes of Bell’s palsy | Normal | Prednisolone | Improved both the time | |
| Obermann et al. [94] | Bell’s palsy | Germany | 21/F | First dose of SARS-CoV-2 mRNA vaccine Comirnaty (BNT162b2, BioNTech/Pfizer) | 2 day | Facial muscle paralysis SARS-CoV-2 antibodies were present in blood and CSF | Normal | Prednisolone | Improved |
| Iftikhar et al. [95] | Bell’s palsy | Qatar | 36/M | Second dose of the mRNA-1273 vaccine | 1 day | Facial palsy | Normal | Prednisolone | Improved |
In a case–control study, Shemer et al. compared clinical parameters of patients with Bell’s palsy following mRNA vaccination with that of patients with Bell’s palsy without vaccination. Out of 37 patients, 21 had received vaccination. Bell’s palsy developed within 2 weeks following first dose of COVID-19 vaccination. There was no difference in any of the clinical parameter between vaccinated or unvaccinated groups [97].
Earlier, in the Pfizer-BioNTech clinical trial, which included 44,000 participants, 4 people had Bell’s palsy. No case of Bell’s palsy was reported in the placebo arm. In the Moderna trial, which included 30,400 participants, 3 vaccine recipients reported Bell’s palsy. One person was in the placebo arm [98]. An article, published in the Lancet, analyzed the combined phase 3 data of Pfizer and Moderna trials and noted that the rate of Bell’s palsy was higher than expected [98].
Other cranial nerve involvement
In isolated instances, mRNA vaccines were found associated with olfactory dysfunction and sixth cranial nerve palsy (Table 6) [99–104].
Table 6.
Summary of reported patients, who suffered from cranial nerve involvement (other than Bell’s palsy) after vaccination against SARS-CoV-2
| Reference | Neurological complication | Country | Age/sex | Vaccine type | Duration after vaccination | Clinical features | Neuroimaging | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Konstantinidis et al. [99] Report of 2 patients | Olfactory dysfunction | Greece | Both female | Pfizer-BioNTech BNT162b2 | 3 and 5 days after second dose | Hyposmia after their second dose | None | Olfactory training | Improved |
| Keir et al. [100] | Phantosmia | USA | 57/F | Pfizer-BioNTech COVID-19 vaccination Second dose | None | Feeling weak, fatigued, with random episodes of ‘‘smelling smoke’’ associated with hyposmia | Postcontrast CT demonstrates faint enhancement left olfactory tract MRI enhancement of the left greater than right olfactory bulb and bilateral olfactory tracts | None | None |
| Reyes-Capo et al. [101] | Acute abducens nerve palsy | USA | 59/F | Pfizer-BioNTech COVID-19 vaccine | 2 days | Fever for 1 day followed by diplopia | Normal MRI of brain and orbits | Not available | Sensory-motor examination remained unchanged in recent follow-up |
| Parrino et al. [102] | Tinnitus | Italy | 37/F 63/ 30/M | BNT162b2 mRNA-vaccine | 7-h first dose 20 h 7 days | Sudden unilateral tinnitus | Normal MRI | Corticosteroids, in two | Improved all |
| Tseng et al. [103 ] PMID: 34,297,133 | Reversible tinnitus and cochleopathy | Taiwan | 32/M | First dosage of the AstraZeneca COVID-19 vaccine | 5 h | High-pitch tinnitus and disturbed the normal hearing high fever with chills and myalgia | Not done | Corticosteroids | Improved |
| Narasimhalu et al. [104] | Trigeminal and cervical radiculitis | Singapore | 52/F | Pfizer-BioNTech vaccination (tozinameran) | 3 h first dose | Numbness, swelling and pain over the left face and neck | MRI of trigeminal nerve revealed thickening and perineural sheath enhancement of the V3 segment of the left trigeminal nerve The MRI of the cervical spine revealed spondylotic changes | Pregabalin | Improved |
Olfactory dysfunction
Olfactory dysfunction is the most frequent neurological complication of COVID-19. Konstantinidis and colleagues reported two cases of smell impairment after second dose of the BioNTechBNT162b2 vaccine (Pfizer) administration [51].
Keir and colleagues reported phantosmia following administration of Pfizer COVID-19 vaccine. Patient complained of constantly “smelling smoke” and headaches. MRI of brain of the patient showed enhancement of the olfactory bulbs and bilateral olfactory tracts [100].
Abducens nerve palsy
Reyes-Capo et al. reported a 59-year-old lady, who presented with an abducen nerve palsy 2 days post-vaccination (Pfizer-BioNTech mRNA vaccine). Neuroimaging in this patient was normal..
Otologic manifestations
A variety of otologic manifestations has been noted following COVID-19 vaccination. Parrino and colleagues described three patients with sudden unilateral tinnitus following BNT162b2 mRNA vaccine administration. Tinnitus rapidly resolved in 2 cases. Wichova and colleagues in a retrospective review recorded 30 patients, who either had significantly exacerbated otologic symptoms or had a new symptom after getting mRNA vaccine. Post-vaccination otologic manifestations included hearing loss with tinnitus, dizziness, or with vertigo. In some patients, with Menière's disease or autoimmune inner ear disease, vaccine led to exacerbation of the pre-existing otologic symptoms [102,105].
Acute vision loss
Santovito and Pinna reported an unusual patient, who developed acute visual impairment following the 2nd dose of the Pfizer-BioNTech COVID-19 vaccine. Prior to visual symptoms, patient experienced unilateral headache. He also reported mild confusion, asthenia, and profound nausea. His symptoms got relieved after taking analgesics. Possibly, patient had an acute attack of migraine with aura that got precipitated by the vaccine [106].
Guillain-Barré syndrome
Guillain-Barré syndrome is a post-infectious disorder of peripheral nerve manifesting with lower motor neuron type of sensory-motor quadriparesis. Acute motor weakness is frequently preceded by an antecedent microbial infection. There are numerous reports indicating that COVID-19 infection can trigger Guillain-Barré syndrome. The US Food and Drug Administration has recently expressed its concern regarding a possible association between the Johnson and Johnson COVID-19 vaccine with Guillain-Barré syndrome [107].
After emergency use approvals, all kinds of COVID-19 vaccines were found associated with Guillain-Barré syndrome. Adenovector-based vaccines were more frequently associated with Guillain-Barré syndrome. Earlier, in phase 3 trial of Johnson and Johnson adenovirus vector-based COVID-19 vaccine, 2 patients developed Guillain-Barré syndrome. One patient belonged to vaccine group and other to placebo group. Both patients had Guillain-Barré syndrome within 2 weeks of receiving injections. The Guillain-Barré syndrome in the vaccine arm was preceded by chills, nausea, diarrhea, and myalgia [108, 109].
Post-vaccination Guillain-Barré syndrome generally affects older adults within 2 weeks of vaccine administration. Clinical presentation is similar to acute demyelinating neuropathy; nerve conduction studies show demyelinating pattern, and CSF examination shows cyto-albuminic dissociation. Many patients present only with facial diplegia. Response to immunotherapy is generally good. (Table 7) [110–126].
Table 7.
Summary of reported patients, who developed an acute peripheral nerve disorder after vaccination against SARS-CoV-2
| Reference | Neurological complication | Country | Age/sex | Vaccine type | Duration after vaccination | Clinical features | Neuroimaging | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Waheed et al. [110] | Guillain-Barré syndrome | USA | 82/F | Pfizer-BioNTech COVID-19 A messenger RNA (mRNA) vaccine | 2 weeks | Areflexic paraparesis with distal sensory loss CSF showed albuminocytologic dissociation | enhancement of cauda equina nerve roots | IV immunoglobulin | Improved |
| Márquez Loza et al. [111] | Guillain-Barré syndrome | USA | 60/F | Johnson & Johnson, d26.COV2.S, a recombinant adenovirus serotype 26 (Ad26) vector vaccine | 2 weeks | Ophthalmoplegia, facial diplegia and Areflexic quadriparesis CSF showed albuminocytologic dissociation | Enhancement of cauda equina nerve roots | IV immunoglobulin | Improved |
| Patel et al. [112] | Guillain-Barré syndrome | UK | 37/M | COVID-19 ChAdOx1 vaccine adenovirus-vectored vaccine Oxford AstraZeneca | 2 weeks | Symmetrical, progressive ascending muscle weakness areflexic bilaterally in the lower limbs | Cauda equina nerve root enhancement | Intravenous immunoglobulin | Improved |
| Razok et al. [113] | Guillain-Barré syndrome | Qatar | 73/M | Pfizer-BioNTech COVID-19 vaccine | 20 days Second dose | Acute bilateral lower limb weakness | None | IVIG | Improved |
| Ogbebor et al. [114] | Guillain-Barré syndrome | US | 86/3F | Pfizer-BioNTech COVID-19 vaccine | 1 day | Weakness in her bilateral lower extremities and by day 6, she could no longer walk CSF = a protein 162 mg/dL and glucose (49 mg/dL) | None | Intravenous immunoglobulin | Improved |
| Finsterer [115] | Exacerbating Guillain-Barré syndrome | Austria | 32/M | A vector-based COVID-19 vaccine | 8 days | Paresthesia and dysphagia bilateral frontal and nuchal headache | None | Intravenous immunoglobulin | Improved |
| Marammatom et al. [116] Report of 7 cases | Guillain-Barré syndrome | India | ChAdOx1-S/nCoV-19 adenovector-based vaccine | Within 2 weeks of the first dose | All patients progressed to areflexic quadriplegia 2 cases required mechanical ventilation All 7 cases had bilateral facial paresis Four patients (57%) also developed other cranial neuropathies (4th and 5th) | In two patients, MRI brain and spine were normal | Intravenous immunoglobulin | One recovered Rest six still bed bound | |
| Allen et al. [117] Report of 4 cases | Guillain-Barré syndrome variant | UK | 20–57 all males | Oxford-AstraZeneca SARS-CoV2 vaccine | Within 3 weeks | Facial weakness in 1 facial diplegia in 3 areflexic quadriparesis in 1 Cyto-albuminic dissociation in all | MRI of the brain and whole spine with contrast showed enhancement of the facial nerve within the right internal auditory canal | Intravenous immunoglobulin, oral steroids, or no treatment | All improved |
| Kohli et al. [118] | Guillain-Barré syndrome | India | 71/M | Covishield, AstraZeneca, University of Oxford | 6 days | Areflexic quadriparesis with bulbar palsy NCV- demyelinating pattern | None | Intravenous immunoglobulin and mechanical ventilation | Improved |
| Azam et al. [119] | Guillain-Barré syndrome | UK | 67/M | The first dose of the AstraZeneca COVID-19 | 15 days |
Areflexic quadriparesis with facial diplegia NCV- demyelinating pattern |
Normal | Intravenous immunoglobulin | Improved |
| Hasan et al. [120] | Guillain-Barré syndrome | UK | 62/F | First dose of the Oxford/AstraZeneca COVID-19 vaccine | Weakness of bilateral lower limbs preceded by paresthesia and numbness a flaccid-type paraplegia NCV- demyelinating pattern CSF-albumin-cytological dissociation | Normal | Intravenous immunoglobulin | The patient remains in the ICU | |
| Theuriet et al. [121] | Guillain-Barré syndrome | France | 72/M | First dose of ChAdOx1 nCoV-19 vaccine (VaxZevria/Oxford-AstraZeneca) | 3 weeks | Areflexic quadriparesis with facial diplegia NCV- demyelinating pattern | None | Intravenous immunoglobulin | The patient remains in the ICU |
| Bonifacio et al. [122] (A series of 5 cases) | Guillain-Barré syndrome | UK | 43/M 51 M 53/M 66/m 71/f | Vaxzevria AstraZeneca, University of Oxford COVID-19 vaccine | 11 days 7 days 7 days 8 days 12 days | Bilateral facial weakness with paresthesia variant of Guillain-Barré syndrome NCV- demyelinating pattern in 4 patients | Bilateral contrast enhancement along whole facial nerve in 3 patients | Intravenous immunoglobulin Was given in 2 patients | All improved |
| Nasuelli et al. [123] | Guillain-Barré syndrome | Italy | 59/M | ChAdOx1 nCoV-19 vaccine | 10 days | Areflexic quadriparesis with facial diplegia NCV- demyelinating pattern in 4 patients CSF-albumin-cytological dissociation | Normal | Intravenous immunoglobulin | Improved |
| Jain et al. [124] | Guillain-Barré syndrome | USA | 65/F | Ad26.COV2.S (Johnson & Johnson) vaccine | 19 days | Facial diplegia | Normal | Intravenous immunoglobulin And plasmapheresis | Improved |
| McKean and Chircop [125] | Guillain-Barré syndrome | Malta | 48/M | Vaxzevria AstraZeneca, University of Oxford COVID-19 vaccine First dose | 10 days | Facial diplegia and severe back pain ascending paresthesia and bilateral progressive areflexic lower limb weakness. CSF-albumin-cytological dissociation NCV multifocal sensorimotor demyelinating polyneuropathy | Normal | Intravenous immunoglobulin and oral prednisolone | Improved |
| Bonifacio et al. [126] (a report of 5 cases) | Guillain-Barré syndrome | UK | |||||||
| Waheed et al. [127] | Small fiber neuropathy | USA | 57/F | Pfizer-BioNTech COVID-19 A messenger RNA (mRNA) vaccine (Second dose) | Subacute onset | Intense burning dysesthesias in the feet gradually spreading to the calves and minimally into the hands (Nerve biopsy proved small fiber neuropathy) | None | Gabapentin | Symptomatic improvement |
Proposed pathogenesis of Guillain-Barré syndrome is an autoantibody-mediated immunological damage of peripheral nerves via mechanism of molecular mimicry between structural components of peripheral nerves and the microorganism. Lately, several cases of Guillain-Barré syndrome following COVID-19 vaccination have also been reported.
Small fiber neuropathy
Waheed et al. described a 57-year-old female, who presented with painful neuropathy following administration of the mRNA COVID-19 vaccine. Patient subacutely presented with intense peripheral burning sensations. Electrodiagnostic studies were normal. Skin biopsy proved small fiber neuropathy. Patient responded to gabapentin.(Table 7) [127].
Parsonage-Turner syndrome
Parsonage-Turner syndrome or neuralgic amyotrophy is clinically manifested with acute unilateral shoulder pain followed by brachial plexopathy. Parsonage-Turner syndrome is usually triggered by any infection, surgery, or rarely vaccination. In many reports, Parsonage-Turner syndrome has been described following COVID-19 vaccination.(Table 8) [128–130].
Table 8.
Summary of reported patients, who developed neuralgic amyotrophy after vaccination against SARS-CoV-2
| Reference | Neurological complication | Country | Age/sex | Vaccine type | Duration after vaccination | Clinical features | Neuroimaging | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Mahajan et al. [128] | Parsonage-Turner syndrome | USA | 50/M | COVID-19 BNT162b2 vaccination | 7 days | Sudden onset of severe left periscapular pain after first dose One week after the second dose, the patient developed left hand grip and left wrist extension weakness. Electromyography showed decreased motor unit recruitment | Normal | Corticosteroids | Improved |
| Diaz-Segarra et al. [129] | Painless idiopathic neuralgic amyotrophy | USA | 35/F | Pfizer-BioNTech COVID-19 vaccine | 9 days | New-onset painless left arm weakness, numbness, and paresthesias | Cervical spine computed tomography showed mild degenerative changes without foraminal narrowing | High-dose prednisone | Improved |
| Antonio Crespo Burillo et al. [130] | Parsonage-Turner syndrome | Spain | 38/M | Vaxzevria (AstraZeneca) | 4 days | Shoulder and arm pain Electrophysiology suggested brachial plexopathy | MRI of the shoulder revealed a mild left subacromial tendinopathy | Methylprednisolone | Improved |
Herpes zoster
Herpes zoster occurs following reactivation of varicella zoster virus. Patients with herpes zoster present with the classic maculopapular rash, which is unilateral, confined to a single dermatome. The rash disappears in 7 to 10 days. Postherpetic neuralgia is the frequent complication of herpes zoster, which is noted in 1 in 5 patients. McMahon and co-workers recorded 414 cutaneous reactions to mRNA COVID-19 vaccines, and 5 (1.9%) were diagnosed with herpes zoster [131]. Other types of COVID-19 vaccines are infrequently associated with post-vaccination reactivation of herpes zoster. It has been suggested that vaccine-induced immunomodulation, resulting in dysregulation of T cell function, is responsible for reactivation of herpes zoster virus [132, 133]. Reports of herpes zoster reactivation after vaccine against SARS-CoV-2 are summarized in Table 9 [134–142].
Table 9.
Summary of reported patients, who developed Herpes zoster after vaccination against SARS-CoV-2
| Reference | Neurological complication | Country | Age/sex | Vaccine type | Duration after vaccination | Clinical features | Neuroimaging | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Tessas and Kluger [134] | Herpes zoster | Finland | 44/M | BNT162b2 mRNA COVID-19 vaccine | 7 days | Herpetiform vesicular and erythematous rash on the left upper back | None | Oral valacyclovir | Improved |
| Rodríguez-Jiménez et al. [135] A report of 5 cases | Herpes zoster | Spain | 39–58 F = 3 | BNT162b2 mRNA COVID-19vaccine (Pfizer) | 1–16 (4 less than 7 days) | Painful herpetiform dermatomal rash | None | None | None |
| Eid et al. [136] | Herpes zoster | Lebanon | 79/M | mRNA COVID vaccine | 6 days | Painful herpetiform dermatomal rash | None | Antiviral treatment | Improved |
| Bostan and Yalici-Armagan [137] | Herpes zoster | Turkey | 78/M | Inactivated COVID-19 vaccine | Erythematous, painful, and pruritic lesions on chest | ||||
| Furer et al. [138] (a report of 6 cases) | Herpes zoster | Israel | 36–61 All females | BNT162b2 mRNA vaccination | 3 -14 days | All had autoimmune inflammatory rheumatic diseases Herpes zoster ophthalmicus in one Truncal herpes zoster in others | Not done | NA | NA |
| Aksu and Öztürk et al. [139] | Herpes zoster | Turkey | 68/M | The inactivated COVID-19 vaccine | 5 days | multiple pinheaded vesicular lesions upon an erythematous base occupying an area on his right mammary region and back corresponding to T3–T5 dermatomes | Not done | Valacyclovir paracetamol | Improved |
| Chiu et al. [140] (a report of 3 cases) | Herpes zoster | Taiwan | 71/M 46/M 42/M | Pfizer-BNT162b2 mRNA and Moderna mRNA-1273 | 2 days 7 days 2 days | Erythematous papules and vesicle in dermatomal pattern | Not done | Oral acyclovir | All improved |
| Alpalhão and Filipe et al. [141] (a report of 4 cases) | Herpes zoster | Portugal | NA | Pfizer’s Comirnaty™ vaccine AstraZeneca Vaxzevria™ vaccine | 3–6 days | Erythematous papules and vesicle in dermatomal pattern | Not done | Valacyclovir | All improved |
| Channa et al. [142] | Herpes zoster | USA | 81/M | mRNA-1273 (Moderna) Covid-19 vaccine | 3 days | A dermatomal rash | Not done | Not available | Not available |
Myositis and rhabdomyolysis
There are reports, which have indicated that COVID-19 vaccines have potential to damage the skeletal muscles as well (Table 10) [143–147]. Tan and colleagues described a patient with a known carnitine palmitoyltransferase-II deficiency disorder, who developed fever, vomiting, shortness of breath, frank haematuria, myalgia and muscle weakness within four hours of receiving AstraZeneca COVID-19 vaccine [143]. Theodorou and colleagues described a 56-year-old woman who, 8 days after a second dose of vaccine administration, developed severe left upper arm pain along restricted shoulder movements. Her serum creatine kinase was elevated suggesting skeletal muscle damage. MRI revealed severely edematous deltoid muscles. Contrast-enhanced imaging demonstrated enhancement of deltoid muscles suggestive of myositis [146].
Table 10.
Summary of reported patients, who developed an acute muscular disorder following vaccination against SARS-CoV-2
| Reference | Neurological complication | Country | Age/sex | Vaccine type | Duration after vaccination | Clinical features | Neuroimaging | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Tan et al. [143] | Rhabdomyolysis in a patient with Carnitine palmitoyltransferase II deficiency | UK | 27/M | COVID-19 vaccine AstraZeneca | 5 h | Fever, vomiting, shortness of breath, frank hematuria, and myalgia CK concentration of 105,000 U/L and deranged liver function tests (ALT 300 U/L and AST 1496 U/L) | None | Continuous intravenous dextrose 10% and a high carbohydrate diet | Improved |
| Mack et al. [144] | Rhabdomyolysis | USA | 80/M | Second dose of Moderna COVID-19 vaccine | 2 days | Generalized body aches, nausea, and vomiting elevated CK | None | IV fluids | Improved |
| Nassar et al. [145] | Rhabdomyolysis | USA | 21/M | First Pfizer/BioNTech COVID-19 vaccine | 1 day | Severe back pain with radiation to his left lateral thigh Creatinine phosphokinase (CPK) level more than 22,000 U/L | Normal | IV fluids | Improved |
| Theodorou et al. [146] | Myositis | Greece | 56/F | Modified mRNA COVID-19 vaccine | 8 days after second dose | There was tenderness over the deltoid muscle, guarding, and decreased abduction of the shoulder and arm along with elevated CPK | On MRI, the deltoid muscle was edematous. On contrast enhancement, muscle exhibited enhancement indicating inflammation | Symptomatic | Improved |
| Godoy et al. [147] | Myositis ossificans | Brazil | 51/M | 3 months | Right upper arm pain, soreness and palpable mass | Intramuscular nodule n the proximal fibers of the brachii muscle with perilesional muscle edema One week later, CT showed a hypoattenuating intramuscular nodule with internal calcifications | NSAIDs | Improved |
Conclusion
Post-authorization, a wide spectrum of serious neurological complications has been reported following COVID-19 vaccination. The most devastating neurological complication is cerebral venous sinus thrombosis that has been reported in females of childbearing age following adenovector-based vaccines. Another major neurological complication of concern is Bell’s palsy that was reported dominantly following mRNA vaccine administration. Transverse myelitis, acute disseminated encephalomyelitis, and Guillain-Barré syndrome are other severe unexpected post-vaccination complications that can occur as result of molecular mimicry and subsequent neuronal damage. Most of other serious neurological complications are reported in either in form of isolated case reports or small cases series. A causal association of these adverse events is controversial; large collaborative prospective studies are needed to prove causality.
Declarations
Ethical approval
None
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ravindra Kumar Garg, Email: garg50@yahoo.com.
Vimal Kumar Paliwal, Email: dr_vimalkpaliwal@rediffmail.com.
References
- 1.World Health Organization. 12 January 2021. WHO Coronavirus (COVID-19) Dashboard. &It; The World Health Organization https://covid19.who.int/. Accessed 1 Aug 2021.
- 2.Jeff Craven. 10 June 2021. COVID-19 vaccine tracker. &It; https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker. Accessed 16 June 2021.
- 3.Piyasirisilp S, Hemachudha T. Neurological adverse events associated with vaccination. Curr Opin Neurol. 2002;15(3):333–338. doi: 10.1097/00019052-200206000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Hause AM, Gee J, Johnson T, et al. Anxiety-related adverse event clusters after Janssen COVID-19 vaccination - five U.S. mass vaccination sites, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):685–688. doi: 10.15585/mmwr.mm7018e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Grimshaw M, Ceballos-Liceaga SE, Hernández-Vanegas LE, et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: a nationwide descriptive study. Clin Immunol. 2021;229:108786. doi: 10.1016/j.clim.2021.108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SH, Wi YM, Yun SY, et al. Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci. 2021;36(14):e107. doi: 10.3346/jkms.2021.36.e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YW, Lim SY, Lee JH, et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in Korea. J Korean Med Sci. 2021;36(21):e153. doi: 10.3346/jkms.2021.36.e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Göbel CH, Heinze A, Karstedt S, et al. 2021 Headache attributed to vaccination against COVID-19 (coronavirus SARS-CoV-2) with the ChAdOx1 nCoV-19 (AZD1222) vaccine: a multicenter observational cohort study [published online ahead of print, 2021 Jul 27]. Pain Ther. 1–22. [DOI] [PMC free article] [PubMed]
- 9.Module 3. Adverse events following immunization. https://www.who.int/vaccine_safety/initiative/tech_support/Part-3.pdf?ua=1. Accessed 19 June 2021.
- 10.Ng JH, Chaudhuri KR, Tan EK. Functional neurological disorders and COVID-19 vaccination. Ann Neurol. 2021;90(2):328. doi: 10.1002/ana.26160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DD, Kung CS, Perez DL. Helping the public understand adverse events associated with COVID-19 vaccinations: lessons learned from functional neurological disorder. JAMA Neurol. 2021;78(7):789–790. doi: 10.1001/jamaneurol.2021.1042. [DOI] [PubMed] [Google Scholar]
- 12.Butler M, Coebergh J, Safavi F, et al. 2021 Functional neurological disorder after SARS-CoV-2 vaccines: two case reports and discussion of potential public health implications [published online ahead of print, 2021 Jul 15]. J Neuropsychiatry Clin Neurosci. appineuropsych21050116. 10.1176/appi.neuropsych.21050116 [DOI] [PMC free article] [PubMed]
- 13.Ercoli T, Lutzoni L, Orofino G, Muroni A, Defazio G. 2021 Functional neurological disorder after COVID-19 vaccination [published online ahead of print, 2021 Jul 29]. Neurol Sci.;1–2. 10.1007/s10072-021-05504-8 [DOI] [PMC free article] [PubMed]
- 14.Iba T, Levy JH, Warkentin TE. 2021 Recognizing vaccine-induced immune thrombotic thrombocytopenia [published online ahead of print, 2021 Jul 13]. Crit Care Med. 10.1097/CCM.0000000000005211 [DOI] [PMC free article] [PubMed]
- 15.Pomara C, Sessa F, Ciaccio M, et al. 2021 Post-mortem findings in vaccine-induced thrombotic thombocytopenia [published online ahead of print, 2021 May 20]. Haematologica. 10.3324/haematol.2021.279075. [DOI] [PMC free article] [PubMed]
- 16.Ledford H. COVID vaccines and blood clots: five key questions. Nature. 2021;592(7855):495–496. doi: 10.1038/d41586-021-00998-w. [DOI] [PubMed] [Google Scholar]
- 17.McGonagle D, De Marco G, Bridgewood C. Mechanisms of Immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 Infection. J Autoimmun. 2021;121:102662. doi: 10.1016/j.jaut.2021.102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castelli GP, Pognani C, Sozzi C, Franchini M, Vivona L. Cerebral venous sinus thrombosis associated with thrombocytopenia post-vaccination for COVID-19. Crit Care. 2021;25(1):137. doi: 10.1186/s13054-021-03572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Agostino V, Caranci F, Negro A, et al. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J Pers Med. 2021;11(4):285. doi: 10.3390/jpm11040285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa210538512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franchini M, Testa S, Pezzo M, et al. Cerebral venous thrombosis and thrombocytopenia post-COVID-19 vaccination. Thromb Res. 2021;202:182–183. doi: 10.1016/j.thromres.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta PR, Apap Mangion S, Benger M, et al. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination - a report of two UK cases. Brain Behav Immun. 2021;95:514–517. doi: 10.1016/j.bbi.2021.04.006.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bersinger S, Lagarde K, Marlu R, Pernod G, Payen JF. Using nonheparin anticoagulant to treat a near-fatal case with multiple venous thrombotic lesions during ChAdOx1 nCoV-19 vaccination-related vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2021;49(9):e870–e873. doi: 10.1097/CCM.0000000000005105. [DOI] [PubMed] [Google Scholar]
- 24.Ramdeny S, Lang A, Al-Izzi S, Hung A, Anwar I, Kumar P. Management of a patient with a rare congenital limb malformation syndrome after SARS-CoV-2 vaccine-induced thrombosis and thrombocytopenia (VITT) [published online ahead of print, 2021 Jun 7]. Br J Haematol. 2021. 10.1111/bjh.17619. [DOI] [PMC free article] [PubMed]
- 25.Zakaria Z, Sapiai NA, Ghani ARI. Cerebral venous sinus thrombosis 2 weeks after the first dose of mRNA SARS-CoV-2 vaccine. Acta Neurochir (Wien) 2021;163(8):2359–2362. doi: 10.1007/s00701-021-04860-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan E, Benjamin D, McDonald I, et al. AZD1222 vaccine-related coagulopathy and thrombocytopenia without thrombosis in a young female. Br J Haematol. 2021;194(3):553–556. doi: 10.1111/bjh.17530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graf T, Thiele T, Klingebiel R, Greinacher A, Schäbitz WR, Greeve I. 2021 Immediate high-dose intravenous immunoglobulins followed by direct thrombin-inhibitor treatment is crucial for survival in Sars-Covid-19-adenoviral vector vaccine-induced immune thrombotic thrombocytopenia VITT with cerebral sinus venous and portal vein thrombosis [published online ahead of print, May 22]. J Neurol. 2021;1–3. 10.1007/s00415-021-10599-2 [DOI] [PMC free article] [PubMed]
- 28.George G, Friedman KD, Curtis BR, Lind SE. 2021 Successful treatment of thrombotic thrombocytopenia with cerebral sinus venous thrombosis following Ad26.COV2.S vaccination [published online ahead of print, 2021 May 14]. Am J Hematol. 10.1002/ajh.26237. [DOI] [PubMed]
- 29.Jamme M, Mosnino E, Hayon J, Franchineau G. Fatal cerebral venous sinus thrombosis after COVID-19 vaccination. Intensive Care Med. 2021;47(7):790–791. doi: 10.1007/s00134-021-06425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiede A, Sachs UJ, Czwalinna A, et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccine [published online ahead of print, 2021 Apr 28]. Blood. 2021;blood.2021011958. 10.1182/blood.2021011958. 13
- 31.Schulz JB, Berlit P, Diener HC, Gerloff C, Greinacher A, Klein C. 2021 COVID-19 vaccine-associated cerebral venous thrombosis in Germany. medRxiv.04.30.21256383; doi: 10.1101/2021.04.30.21256383. 14 [DOI] [PMC free article] [PubMed]
- 32.Bourguignon A, Arnold DM, Warkentin TE, et al. 2021 Adjunct immune globulin for vaccine-induced thrombotic thrombocytopenia [published online ahead of print, 2021 Jun 9]. N Engl J Med. 10.1056/NEJMoa2107051. [DOI] [PMC free article] [PubMed]
- 33.Gattringer T, Gressenberger P, Gary T, Wölfler A, Kneihsl M, Raggam RB. 2021 Successful management of vaccine-induced immune thrombotic thrombocytopenia-related cerebral sinus venous thrombosis after ChAdOx1 nCov-19 vaccination [published online ahead of print, 2021 Jul 8]. Stroke Vasc Neurol.svn-2021–001142. 10.1136/svn-2021-001142 [DOI] [PMC free article] [PubMed]
- 34.Ikenberg B, Demleitner AF, Thiele T, et al. Cerebral venous sinus thrombosis after ChAdOx1 nCov-19 vaccination with a misleading first cerebral MRI scan. Stroke Vasc Neurol. 2021;8:svn-2021–001095. doi: 10.1136/svn-2021-001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark RT, Johnson L, Billotti J, et al. Early outcomes of bivalirudin therapy for thrombotic thrombocytopenia and cerebral venous sinus thrombosis after Ad26.COV2.S vaccination. Ann Emerg Med. 2021;S0196 0644(21):00342 5. doi: 10.1016/j.annemergmed.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonato S, Artoni A, Lecchi A, et al. 2021 Massive cerebral venous thrombosis due to vaccine-induced immune thrombotic thrombocytopenia [published online ahead of print, 2021 Jul 15]. Haematologica. 10.3324/haematol.2021.279246 [DOI] [PMC free article] [PubMed]
- 37.Wang RL, Chiang WF, Shyu HY, et al. COVID-19 vaccine-associated acute cerebral venous thrombosis and pulmonary artery embolism. QJM. 2021;10:hcab185. doi: 10.1093/qjmed/hcab185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutta A, Ghosh R, Bhattacharya D, et al. Anti-PF4 antibody negative cerebral venous sinus thrombosis without thrombocytopenia following immunization with COVID-19 vaccine in an elderly non-comorbid Indian male, managed with conventional heparin-warfarin based anticoagulation. Diabetes Metab Syndr. 2021;15(4):102184. doi: 10.1016/j.dsx.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aladdin Y, Algahtani H, Shirah B. Vaccine-induced immune thrombotic thrombocytopenia with disseminated intravascular coagulation and death following the ChAdOx1 nCoV-19 Vaccine. J Stroke Cerebrovasc Dis. 2021;30(9):105938. doi: 10.1016/j.jstrokecerebrovasdis.2021.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavin M, Elder PT, O'Keeffe D, et al. Vaccine-induced immune thrombotic thrombocytopenia (VITT) - a novel clinico-pathological entity with heterogeneous clinical presentations [published online ahead of print, 2021 Jun 22]. Br J Haematol. 2021. 10.1111/bjh.17613. [DOI] [PMC free article] [PubMed]
- 41.Tølbøll Sørensen AL, Rolland M, Hartmann J, et al. A case of thrombocytopenia and multiple thromboses after vaccination with ChAdOx1 nCoV-19 against SARS-CoV-2. Blood Adv. 2021;5(12):2569–2574. doi: 10.1182/bloodadvances.2021004904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan BE, Shen JY, Lim XR, et al. Cerebral venous thrombosis post BNT162b2 mRNA SARS-CoV-2 vaccination: a black swan event. Am J Hematol. 2021;96(9):E357–E361. doi: 10.1002/ajh.26272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suresh P, Petchey W. ChAdOx1 nCOV-19 vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis (CVST) BMJ Case Rep. 2021;14(6):e243931. doi: 10.1136/bcr-2021-243931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dias L, Soares-Dos-Reis R, Meira J, et al. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J Stroke Cerebrovasc Dis. 2021;30(8):105906. doi: 10.1016/j.jstrokecerebrovasdis.2021.105906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan CY, Tsai SH, Fan JS, Lin YK, Kao CC. 2021 A rare case of a middle-age Asian male with cerebral venous thrombosis after COVID-19 AstraZeneca vaccination [published online ahead of print, Jul 8]. Am J Emerg Med. 2021;S0735–6757(21)00571–4. 10.1016/j.ajem.2021.07.011 [DOI] [PMC free article] [PubMed]
- 46.Varona JF, García-Isidro M, Moeinvaziri M, Ramos-López M, Fernández-Domínguez M. 2021 Primary adrenal insufficiency associated with Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia (VITT) [published online ahead of print, Jul 10]. Eur J Intern Med. 2021;S0953–6205(21)00236–3. 10.1016/j.ejim.2021.06.025 [DOI] [PMC free article] [PubMed]
- 47.Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krzywicka K, Heldner MR, Sánchez van Kammen M, et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European Medicines Agency. Eur J Neurol. 2021;28(11):3656–3662. doi: 10.1111/ene.15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Institute for Health and Care Excellence. 29 July 2021. COVID-19 rapid guideline: vaccine-induced immune thrombocytopenia and thrombosis (VTT). &It; https://www.nice.org.uk/guidance/ng200/resources/covid19-rapid-guideline-vaccineinduced-immune-thrombocytopenia-and-thrombosis-vitt-pdf-51036811744. Accessed 2 Aug 2021. [PubMed]
- 50.Krzywicka K, Heldner MR, Sánchez van Kammen M, et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European Medicines Agency. Eur J Neurol. 2021;28(11):3656 3662. doi: 10.1111/ene.15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bersinger S, Lagarde K, Marlu R, Pernod G, Payen JF. Using nonheparin anticoagulant to treat a near-fatal case with multiple venous thrombotic lesions during ChAdOx1 nCoV-19 vaccination-related vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2021;49(9):e870–e873. doi: 10.1097/CCM.0000000000005105. [DOI] [PubMed] [Google Scholar]
- 52.Simpson CR, Shi T, Vasileiou E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Athyros VG, Doumas M. 2021 A possible case of hypertensive crisis with intracranial haemorrhage after an mRNA Anti-COVID-19 vaccine [published online ahead of print, 2021 May 21]. Angiology.;33197211018323. 10.1177/00033197211018323 [DOI] [PubMed]
- 54.Bjørnstad-Tuveng TH, Rudjord A, Anker P. 2021 Fatal cerebral haemorrhage after COVID-19 vaccine. Fatal hjerneblødning etter covid-19-vaksine. Tidsskr Nor Laegeforen. 2021;141. 10.4045/tidsskr.21.0312. [DOI] [PubMed]
- 55.de Mélo Silva ML Jr, Lopes DP. 2021 Large hemorrhagic stroke after ChAdOx1 nCoV-19 vaccination: a case report [published online ahead of print, 2021 Jul 17]. Acta Neurol Scand. 10.1111/ane.13505. [DOI] [PMC free article] [PubMed]
- 56.Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet. 2021;397(10285):e11. doi: 10.1016/S0140-6736(21)00872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Mayhani T, Saber S, Stubbs MJ, et al. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J Neurol Neurosurg Psychiatry. 2021;92(11):1247–1248. doi: 10.1136/jnnp-2021-326984. [DOI] [PubMed] [Google Scholar]
- 58.Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost. 2021;19(7):1771–1775. doi: 10.1111/jth.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malik B, Kalantary A, Rikabi K, Kunadi A. Pulmonary embolism, transient ischaemic attack and thrombocytopenia after the Johnson & Johnson COVID-19 vaccine. BMJ Case Rep. 2021;14(7):e243975. doi: 10.1136/bcr-2021-243975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finsterer J, Korn M. 2021 Aphasia seven days after second dose of an mRNA-based SARS-CoV-2 vaccine [published online ahead of print, 2021 Jun 24]. Brain Hemorrhages. 10.1016/j.hest.2021.06.001. [DOI] [PMC free article] [PubMed]
- 61.Walter U, Fuchs M, Grossmann A, et al. 2021 Adenovirus-vectored COVID-19 vaccine-induced immune thrombosis of carotid artery: a case report [published online ahead of print, 2021 Jul 26]. Neurology. 10.1212/WNL.0000000000012576. [DOI] [PubMed]
- 62.Baldelli L, Amore G, Montini A, et al. Hyperacute reversible encephalopathy related to cytokine storm following COVID-19 vaccine. J Neuroimmunol. 2021;358:577661. doi: 10.1016/j.jneuroim.2021.577661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aladdin Y, Shirah B. New-onset refractory status epilepticus following the ChAdOx1 nCoV-19 vaccine. J Neuroimmunol. 2021;357:577629. doi: 10.1016/j.jneuroim.2021.577629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh R, Dubey S, Roy D, Mandal A, Naga D, Benito-León J. Focal onset non-motor seizure following COVID-19 vaccination: a mere coincidence? Diabetes Metab Syndr. 2021;15(3):1023–1024. doi: 10.1016/j.dsx.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu BD, Ugloini C, Jha P. Two cases of post-Moderna COVID-19 vaccine encephalopathy associated with nonconvulsive status epilepticus. Cureus. 2021;13(7):e16172. doi: 10.7759/cureus.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naharci MI, Tasci I. 2021 Delirium in a patient with Alzheimer’s dementia following COVID-19 vaccination [published online ahead of print, 2021 Jul 10]. Psychogeriatrics. 10.1111/psyg.12747. [DOI] [PMC free article] [PubMed]
- 67.Salinas MR, Dieppa M. Transient akathisia after the SARS-Cov-2 vaccine. Clin Park Relat Disord. 2021;4:100098. doi: 10.1016/j.prdoa.2021.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zavala-Jonguitud LF, Pérez-García CC. Delirium triggered by COVID-19 vaccine in an elderly patient. Geriatr Gerontol Int. 2021;21(6):540. doi: 10.1111/ggi.14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alfishawy M, Bitar Z, Elgazzar A, Elzoueiry M. 2021 Neuroleptic malignant syndrome following COVID-19 vaccination [published online ahead of print, Feb 20]. Am J Emerg Med. 2021;S0735–6757(21)00117–0. 10.1016/j.ajem.2021.02.011 [DOI] [PMC free article] [PubMed]
- 70.Ozgen Kenangil G, Ari BC, Guler C, Demir MK. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol Belg. 2021;121(4):1089–1091. doi: 10.1007/s13760-021-01699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao L, Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg. 2021;1:1–3. doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raknuzzaman M, Jannaty T, Hossain MB, Saha B, Dey SK, Shahidullah M. 2021 Post Covid 19 vaccination acute disseminated encephalomyelitis: a case report in Bangladesh. Int J Med Sci Clin Res Studies 3. 10.47191/ijmscrs/v1-i3-01
- 73.Torrealba-Acosta G, Martin JC, Huttenbach Y, et al. Acute encephalitis, myoclonus and Sweet syndrome after mRNA-1273 vaccine. BMJ Case Rep. 2021;14(7):e243173. doi: 10.1136/bcr-2021-243173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vogrig A, Janes F, Gigli GL. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021;208:106839. doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zuhorn F, Graf T, Klingebiel R, Schäbitz WR, Rogalewski A. Postvaccinal encephalitis after ChAdOx1 nCov-19. Ann Neurol. 2021;90(3):506–511. doi: 10.1002/ana.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pellegrino P, Carnovale C, Perrone V, et al. Acute disseminated encephalomyelitis onset: evaluation based on vaccine adverse events reporting systems. PLoS One. 2013;8(10):e77766. doi: 10.1371/journal.pone.0077766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malhotra SH, Gupta P, Prabhu V, Garg RK, Dandu H, Agarwal V. COVID-19 vaccination-associated myelitis [published online ahead of print, 2021 Mar 31]. QJM. 2021;hcab069. 10.1093/qjmed/hcab069 [DOI] [PMC free article] [PubMed]
- 78.Fitzsimmons, William and Nance, Christopher S., Sudden onset of myelitis after COVID-19 vaccination: an under-recognized severe rare adverse event (May 5, 2021). Available at SSRN: https://ssrn.com/abstract=3841558 or 10.2139/ssrn.3841558
- 79.Tahir N, Koorapati G, Prasad S, et al. SARS-CoV-2 vaccination-induced transverse myelitis. Cureus. 2021;13(7):e16624. doi: 10.7759/cureus.16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pagenkopf C, Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J Neuroimmunol. 2021;358:577606. doi: 10.1016/j.jneuroim.2021.577606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Helmchen C, Buttler GM, Markewitz R, Hummel K, Wiendl H, Boppel T. 2021 Acute bilateral optic/chiasm neuritis with longitudinal extensive transverse myelitis in longstanding stable multiple sclerosis following vector-based vaccination against the SARS-CoV-2 [published online ahead of print, Jun 15]. J Neurol. 2021;1–6. 10.1007/s00415-021-10647-x [DOI] [PMC free article] [PubMed]
- 82.Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. 2021 First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine [published online ahead of print, 2021 Jun 11]. J Neurol. 1–4. 10.1007/s00415-021-10648-w [DOI] [PMC free article] [PubMed]
- 83.Chen S, Fan XR, He S, Zhang JW, Li SJ. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol Sci. 2021;42(9):3537–3539. doi: 10.1007/s10072-021-05427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chagla Z. In adults, the Oxford/AstraZeneca vaccine had 70% efficacy against COVID-19 >14 d after the 2nd dose. Ann Intern Med. 2021;174(3):JC29. doi: 10.7326/ACPJ202103160-029. [DOI] [PubMed] [Google Scholar]
- 85.Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute transverse myelitis (ATM):clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front Immunol. 2021;26(12):653786. doi: 10.3389/fimmu.2021.653786.PMID:33981305;PMCID:PMC8107358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shemer A, Pras E, Hecht I. Peripheral facial nerve palsy following BNT162b2 (COVID-19) vaccination. Isr Med Assoc J. 2021;23(3):143–144. [PubMed] [Google Scholar]
- 88.Repajic M, Lai XL, Xu P, Liu A. Bell's Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav Immun Health. 2021;13:100217. doi: 10.1016/j.bbih.2021.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colella G, Orlandi M, Cirillo N. 2021 Bell’s palsy following COVID-19 vaccination [published online ahead of print, 2021 Feb 21]. J Neurol. 1–3. doi:10.1007/s00415-021-10462-4 [DOI] [PMC free article] [PubMed]
- 90.Martin-Villares C, Vazquez-Feito A, Gonzalez-Gimeno MJ, de la Nogal-Fernandez B. 2021 Bell’s palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report [published online ahead of print, 2021 May 25]. J Neurol. 1–2. doi:10.1007/s00415-021-10617-3 [DOI] [PMC free article] [PubMed]
- 91.Nishizawa Y, Hoshina Y, Baker V. 2021 Bell’s palsy following the Ad26.COV2.S COVID-19 vaccination [published online ahead of print, 2021 May 20]. QJM.;hcab143. doi:10.1093/qjmed/hcab143 [DOI] [PMC free article] [PubMed]
- 92.Gómez de Terreros Caro G, Díaz SG, Alé MP, Gimeno LM. 2021 PARÁLISIS DE BELL TRAS VACUNACIÓN COVID19: A PROPÓSITO DE UN CASO [BELL´S PALSY FOLLOWING COVID-19 VACCINATION: A CASE REPORT] [published online ahead of print, 2021 Apr 12]. Neurologia (Engl Ed). 10.1016/j.nrl.2021.04.004. [DOI] [PMC free article] [PubMed]
- 93.Burrows A, Bartholomew T, Rudd J, Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021;14(7):e243829. doi: 10.1136/bcr-2021-243829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Obermann M, Krasniqi M, Ewers N, Fayad J, Haeberle U. Bell’s palsy following COVID-19 vaccination with high CSF antibody response. Neurol Sci. 2021;42(11):4397–4399. doi: 10.1007/s10072-021-05496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iftikhar H, Noor SU, Masood M, et al. Bell’s palsy after 24 hours of mRNA-1273 SARS-CoV-2 vaccine. Cureus. 2021;13(6):e15935. doi: 10.7759/cureus.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ozonoff A, Nanishi E, Levy O. 2021 Bell's palsy and SARS-CoV-2 vaccines-an unfolding story – authors’ reply [published online ahead of print, 2021 Jun 7]. Lancet Infect Dis S1473–3099(21)00323–6. doi:10.1016/S1473-3099(21)00323-6 [DOI] [PMC free article] [PubMed]
- 97.Shemer A, Pras E, Einan-Lifshitz A, Dubinsky-Pertzov B, Hecht I. Association of COVID-19 vaccination and facial nerve palsy: a case-control study. JAMA Otolaryngol Head Neck Surg. 2021;147(8):739–743. doi: 10.1001/jamaoto.2021.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21(4):450–452. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. 2021 Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination [published online ahead of print, 2021 May 28]. Int Forum Allergy Rhinol. 10.1002/alr.22809. [DOI] [PMC free article] [PubMed]
- 100.Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133–137. doi: 10.1097/RMR.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 101.Reyes-Capo DP, Stevens SM, Cavuoto KM. 2021 Acute abducens nerve palsy following COVID-19 vaccination [published online ahead of print, 2021 May 24]. J AAPOS S1091–8531(21)00109–9. doi:10.1016/j.jaapos.2021.05.003 [DOI] [PMC free article] [PubMed]
- 102.Parrino D, Frosolini A, Gallo C, De Siati RD, Spinato G, de Filippis C. Tinnitus following COVID-19 vaccination: report of three cases. Int J Audiol. 2021;13:1–4. doi: 10.1080/14992027.2021.1931969. [DOI] [PubMed] [Google Scholar]
- 103.Tseng PT, Chen TY, Sun YS, Chen YW, Chen JJ. 2021 The reversible tinnitus and cochleopathy followed first-dose AstraZeneca COVID-19 vaccination [published online ahead of print, 2021 Jul 23]. QJM hcab210. doi:10.1093/qjmed/hcab210 [DOI] [PMC free article] [PubMed]
- 104.Narasimhalu K, Lee WC, Salkade PR, De Silva DA. Trigeminal and cervical radiculitis after tozinameran vaccination against COVID-19. BMJ Case Rep. 2021;14(6):e242344. doi: 10.1136/bcr-2021-242344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wichova H, Miller ME, Derebery MJ. Otologic manifestations after COVID-19 vaccination: the house ear clinic experience. Otol Neurotol. 2021;42(9):e1213–e1218. doi: 10.1097/MAO.0000000000003275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Santovito LS, Pinna G. Acute reduction of visual acuity and visual field after Pfizer-BioNTech COVID-19 vaccine 2nd dose: a case report. Inflamm Res. 2021;70(9):931–933. doi: 10.1007/s00011-021-01476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dyer O. Covid-19: Regulators warn that rare Guillain-Barré cases may link to J&J and AstraZeneca vaccines. BMJ. 2021;374:n1786. doi: 10.1136/bmj.n1786. [DOI] [PubMed] [Google Scholar]
- 108.Márquez Loza AM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Guillain- Barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality [published online ahead of print, 2021 Apr 6]. Neurology. 2021. 10.1212/WNL.0000000000011881. [DOI] [PubMed]
- 109.FDA Briefing Document Janssen Ad26.COV2.S Vaccine for the prevention of COVID-1.26 February 2021. Vaccines and Related Biological Products Advisory Committee Meeting. &It; https://www.fda.gov/media/146217/download. Accessed 24 June 2021
- 110.Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13(2):e13426. doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Márquez Loza AM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. 2021 Guillain-Barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality [published online ahead of print, 2021 Apr 6]. Neurology. 10.1212/WNL.0000000000011881. [DOI] [PubMed]
- 112.Patel SU, Khurram R, Lakhani A, Quirk B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021;14(4):e242956. doi: 10.1136/bcr-2021-242956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Razok A, Shams A, Almeer A et al. 2021 Post-COVID-19 vaccine Guillain-Barré syndrome; first reported case from Qatar. Authorea. May 07,. DOI: 10.22541/au.162041666.65803989/v1 [DOI] [PMC free article] [PubMed]
- 114.Ogbebor O, Seth H, Min Z, Bhanot N. Guillain-Barré syndrome following the first dose of SARS-CoV-2 vaccine: a temporal occurrence, not a causal association. IDCases. 2021;24:e01143. doi: 10.1016/j.idcr.2021.e01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Finsterer J. Exacerbating Guillain-Barré syndrome eight days after vector-based COVID-19 vaccination. Case Rep Infect Dis. 2021;2021:3619131. doi: 10.1155/2021/3619131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maramattom BV, Krishnan P, Paul R, et al. Guillain-Barré syndrome following ChAdOx1-S/nCoV-19 vaccine. Ann Neurol. 2021;90(2):312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 117.Allen CM, Ramsamy S, Tarr AW, Tighe PJ, Irving WL, Tanasescu R, Evans JR. Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol. 2021 doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 118.Kohli S, Varshney M, Mangla S, Jaiswal B, Chhabra PH. Guillain-Barré syndrome after COVID-19 vaccine: should we assume a causal Link? International Journal of Medical and Pharmaceutical Case Reports. 2021;14(1):20–24. doi: 10.9734/ijmpcr/2021/v14i130124. [DOI] [Google Scholar]
- 119.Azam S, Khalil A, Taha A. Guillain-Barré syndrome in a 67-year-old male post COVID-19 vaccination (Astra Zeneca) American Journal of Medical Case Reports. 2021;9(8):424–427. doi: 10.12691/ajmcr-9-8-10. [DOI] [Google Scholar]
- 120.Hasan T, Khan M, Khan F, Hamza G. Case of Guillain-Barré syndrome following COVID-19 vaccine. BMJ Case Rep. 2021;14(6):e243629. doi: 10.1136/bcr-2021-243629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Theuriet J, Richard C, Becker J, Pegat A, Bernard E, Vukusic S. 2021 Guillain-Barré syndrome following first injection of ChAdOx1 nCoV-19 vaccine: First report. Rev Neurol (Paris) S0035–3787(21)00585–3 doi:10.1016/j.neurol.2021.04.005 [DOI] [PubMed]
- 122.Bonifacio GB, Patel D, Cook S, et al. 2021 Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine [published online ahead of print, 2021 Jul 14]. J Neurol Neurosurg Psychiatry jnnp-2021–327027. doi:10.1136/jnnp-2021-327027 [DOI] [PubMed]
- 123.Nasuelli NA, De Marchi F, Cecchin M, et al. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine. Neurol Sci. 2021;42(11):4747–4749. doi: 10.1007/s10072-021-05467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jain E, Pandav K, Regmi P, et al. Facial diplegia: a rare, atypical variant of Guillain-Barré syndrome and Ad26.COV2.S Vaccine. Cureus. 2021;13(7):e16612. doi: 10.7759/cureus.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McKean N, Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. 2021;14(7):e244125. doi: 10.1136/bcr-2021-244125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bonifacio GB, Patel D, Cook S, et al. 2021 Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine [published online ahead of print, 2021 Jul 14]. J Neurol Neurosurg Psychiatry jnnp-2021–327027. doi:10.1136/jnnp-2021-327027 [DOI] [PubMed]
- 127.Waheed W, Carey ME, Tandan SR, Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021;64(1):E1–E2. doi: 10.1002/mus.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mahajan S, Zhang F, Mahajan A, Zimnowodzki S. Parsonage Turner syndrome after COVID-19 vaccination. Muscle Nerve. 2021;64(1):E3–E4. doi: 10.1002/mus.27255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Diaz-Segarra N, Edmond A, Gilbert C, Mckay O, Kloepping C, Yonclas P. 2021 Painless idiopathic neuralgic amyotrophy after COVID-19 vaccination: a case report [published online ahead of print, 2021 Apr 22]. PM R.;10.1002/pmrj.12619 [DOI] [PMC free article] [PubMed]
- 130.Antonio Crespo Burillo J, Martínez CL, Arguedas CG, Pueyo FJM. Neuralgia amiotrófica secundaria a vacuna contra COVID-19 Vaxzevria (AstraZeneca) [Amyotrophic neuralgia secondary to Vaxzevria (AstraZeneca) COVID-19 vaccine] Neurologia. 2021;36(7):571–572. doi: 10.1016/j.nrl.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arora P, Sardana K, Mathachan SR, Malhotra P. 2021 Herpes zoster after inactivated COVID-19 vaccine: a cutaneous adverse effect of the vaccine [published online ahead of print, 2021 Jun 2]. J Cosmet Dermatol. 10.1111/jocd.14268. [DOI] [PMC free article] [PubMed]
- 133.Lladó I, Fernández-Bernáldez A, Rodríguez-Jiménez P 2021. "Varicella zoster virus reactivation and mRNA vaccines as a trigger". Reply to: Herpes-Zoster reactivation after mRNA-1273 (Moderna) SARS-CoV-2 Vaccination [published online ahead of print, 2021 Jul 22]. JAAD Case Rep. 10.1016/j.jdcr.2021.07.011. [DOI] [PMC free article] [PubMed]
- 134.Tessas I, Kluger N. Ipsilateral herpes zoster after the first dose of BNT162b2 mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(10):e620–e622. doi: 10.1111/jdv.17422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: Report of 5 cases. JAAD Case Rep. 2021;12:58–59. doi: 10.1016/j.jdcr.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eid E, Abdullah L, Kurban M, Abbas O. Herpes zoster emergence following mRNA COVID-19 vaccine. J Med Virol. 2021 doi: 10.1002/jmv.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20(6):1566–1567. doi: 10.1111/jocd.14035. [DOI] [PubMed] [Google Scholar]
- 138.Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. 2021 Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series [published online ahead of print, 2021 Apr 12]. Rheumatology (Oxford) keab345. doi:10.1093/rheumatology/keab345 [DOI] [PMC free article] [PubMed]
- 139.Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? Clin Exp Vaccine Res. 2021;10(2):198–201. doi: 10.7774/cevr.2021.10.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chiu HH, Wei KC, Chen A, Wang WH. 2021 Herpes zoster following COVID-19 vaccine: report of 3 cases [published online ahead of print, 2021 Jul 22]. QJM.;hcab208. 10.1093/qjmed/hcab208 [DOI] [PMC free article] [PubMed]
- 141.Alpalhão M, Filipe P. Herpes Zoster following SARS-CoV-2 vaccination - a series of 4 cases. J Eur Acad Dermatol Venereol. 2021;35(11):e750–e752. doi: 10.1111/jdv.17555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Channa L, Torre K, Rothe M. Letter to the editor: Herpes-Zoster reactivation after mRNA-1273 (Moderna) SARS-CoV-2 Vaccination. JAAD Case Rep. 2021;15:60–61. doi: 10.1016/j.jdcr.2021.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tan A, Stepien KM, Narayana STK. Carnitine palmitoyltransferase II deficiency and post-COVID vaccination rhabdomyolysis. QJM. 2021;19:hcab077. doi: 10.1093/qjmed/hcab077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mack M, Nichols L, Guerrero DM. Rhabdomyolysis secondary to COVID-19 vaccination. Cureus. 2021;13(5):e15004. doi: 10.7759/cureus.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nassar M, Chung H, Dhayaparan Y, et al. COVID-19 vaccine induced rhabdomyolysis: Case report with literature review. Diabetes Metab Syndr. 2021;15(4):102170. doi: 10.1016/j.dsx.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Theodorou DJ, Theodorou SJ, Axiotis A, Gianniki M, Tsifetaki N. COVID-19 vaccine-related myositis. QJM. 2021;1146(424):425. doi: 10.1093/qjmed/hcab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Godoy IRB, Rodrigues TC, Skaf A. Myositis ossificans following COVID-19 vaccination. QJM. 2021;9:hcab161. doi: 10.1093/qjmed/hcab161. [DOI] [PMC free article] [PubMed] [Google Scholar]