Abstract
Background
Cardiovascular sequelae of coronavirus disease 2019 (COVID-19) infection have been explored by clinicians and researchers all over the world.
Objective
The purpose of this study was to evaluate the incidence of atrioventricular block (AV) in patients hospitalized for COVID-19 and its association between in-hospital morbidity and mortality.
Methods
In-hospital electrocardiograms (ECGs) of 438 patients were compared with their prior or baseline ECGs to ascertain the development of new onset AV block. Patients who developed new AV blocks were then followed at 30 and 90 days post-discharge to check for resolution of AV block. Demographic characteristics, clinical characteristics, and complications during their hospital stay were evaluated. Major complications including respiratory failure requiring oxygen supplementation and mechanical ventilation, sepsis, deep vein thrombosis, elevated troponins, hospital and intensive care unit (ICU) length of stay, as well as death were compared between those who developed new onset AV blocks and those who did not.
Results
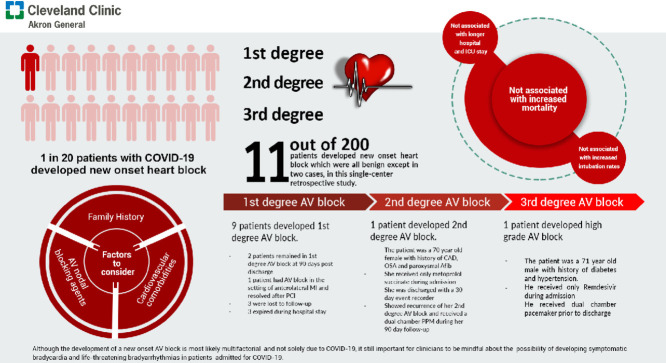
Based on our single center study, the incidence of new onset AV blocks among patients admitted for COVID-19 during the study period was 5.5 cases per 100 patients. New onset AV blocks were not associated with longer hospital and ICU length of stay, increased intubation rates, or increased mortality.
Conclusion
Although the development of a new onset AV block is most likely multifactorial and not solely due to COVID-19, it is still important for clinicians to be mindful about the possibility of developing symptomatic bradycardia and life-threatening arrhythmias in patients admitted for COVID-19. This can be achieved by appropriate rhythm monitoring in-patient but the need for a cardiac event monitor upon discharge is unlikely to be necessary. Careful history taking, including family and drug use history is also of great importance as emerging drug therapies for COVID-19 have potential arrhythmogenic effects.
Keywords: COVID-19, Pandemic, Cardiology, Atrioventricular block, Arrhythmias
Graphical abstract
Introduction
Since the first case of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2 virus) was reported at the end of 2019, coronavirus disease 2019 (COVID-19) has become a serious threat to global public health. Although the disease mainly affects the respiratory system, its effects on the cardiovascular system are increasingly being recognized [1,2]. While myocardial damage, as shown by increased high sensitivity troponin and fulminant myocarditis, has been reported, cardiovascular complications such as malignant arrhythmias are still largely unknown. In one study of 138 hospitalized patients with COVID-19, arrhythmia (not further specified) was reported in 17% of total patients and in 16 of 36 patients admitted to the intensive care unit (ICU) [1]. Therefore, an arrhythmogenic effect of COVID-19 could be expected, potentially contributing to disease outcome. With growing data in the literature reporting the incidence of arrhythmias in COVID-19 patients, the incidence of atrioventricular (AV) blocks has not been well reported.
Bradycardias due to either sinus node disease or AV blocks are an important cause of age-related cardiovascular morbidity [3]. Presentation is typically with symptoms of dizziness, syncope, or effort intolerance and either an abnormal resting electrocardiogram (ECG) or rhythm monitoring can help to establish the diagnosis. While first degree AV blocks are benign and do not show an interruption of the AV conduction, second and third-degree AV blocks are considered life-threatening as they translate to interruption of AV conduction, and even complete dissociation of the atrial and ventricular conduction, requiring emergent permanent pacemaker placement (PPM). While PPM implantation for all degrees of heart block may have symptomatic benefit, implantation in the context of Mobitz II, other higher degree AV blocks and third-degree heart block, has proven survival benefit [3].
Acknowledging the lack of data concerning these AV blocks in patients admitted for COVID-19, the objective of this study was to evaluate the incidence of AV blocks and its effect on disposition and in-hospital outcomes among patients with COVID-19.
Methods
Selection criteria
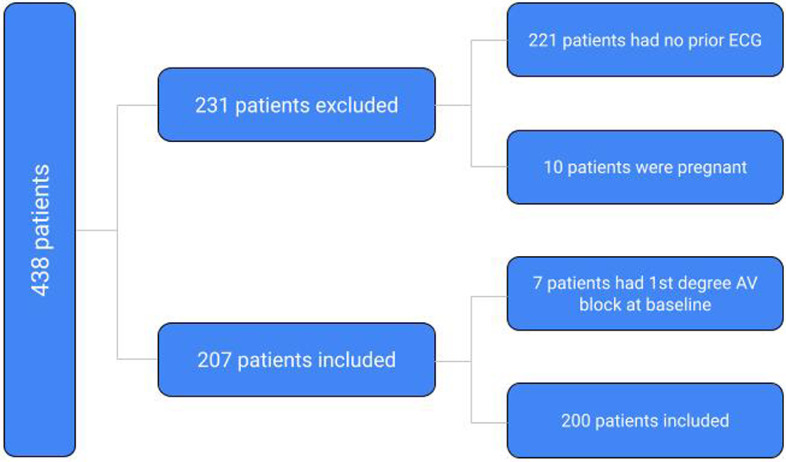
This retrospective study was approved by the local institutional review board with a waiver of informed consent because of the minimal risk to participants. Patients admitted with a diagnosis of COVID-19 as determined by a positive reverse transcription polymerase chain reaction test between March 1st, 2020 to August 31st, 2020 were included in the study. Subjects were excluded if they did not have prior ECGs for comparison, had a prior documented AV block, if they were discharged from the emergency department after testing positive for SARS-COV-2, were pregnant, or were less than 18 years of age. Charts of the 438 identified subjects were reviewed. The patients’ ECGs prior to hospital admission and during their hospital stay were compared. Out of 438 patients admitted for COVID-19, only 207 patients met the inclusion criteria and were included in the final analysis. Based on their prior ECGs, 7 of these 200 patients had first degree AV block; 17 out of 200 patients (8.5%) had atrial fibrillation on presentation but without AV block and were not included in the final analysis; 3 out of 200 (1.5%) had atrial flutter but without AV block and were also excluded (Fig. 1 ).
Fig. 1.
Method of selecting the final number of patients included for analysis. AV, atrioventricular; ECG, electrocardiogram.
Data collection and outcomes
Data, including patient demographics and clinical endpoints, were collected from electronic medical records. Abstracted data included race, gender, age, prior ECG, admission ECG, family history of sudden cardiac death, cardiomyopathies, past medical histories, blood type, and electrolyte abnormalities. Outcomes evaluated included dispositions (death, discharge, hospice) and in-hospital complications including respiratory failure, anemia requiring transfusion, acute kidney injury, non ST-elevation myocardial infarction (NSTEMI), deep vein thrombosis, pulmonary embolism, pneumonia, sepsis, and cerebrovascular accident. Lengths of ICU and hospital stay were also collected.
Statistical analysis
Descriptive statistics, as well as inferential statistics were performed, as appropriate. Categorical variables were described using frequencies and percentages, and p-values obtained using chi square tests or Fisher's exact tests, as applicable. Continuous variables were described using medians and interquartile range, or mean and SD according to their distribution and p-values obtained from t-tests or Wilcoxon rank sum tests. Dispositions and in-hospital complications were compared between those who developed new onset heart blocks and those who did not. ICU length of stay and hospital length of stay were also compared between the two groups. Due to the small number of patients who developed new onset heart blocks during the study period, multiple logistic regression analysis was not performed. Analyses were performed using SAS® Software (version 9.4; Cary, NC, USA). A significance level of 0.05 was assumed.
Results
Baseline patient characteristics
Among the study population, 110 ten subjects (55%) were female, and the median age was 68 years old with a standard deviation of ±16.15 years. One hundred twenty-three (62%) were Caucasian, 67 (34%) were African American, and 6 (3%) were either Asian or Hispanic.
Patients who were admitted with COVID-19 were predominantly blood group A. Sixty-four percent of the patients had a normal sinus rhythm prior to admission, while 10% of the patients had atrial fibrillation prior to admission. At admission, normal sinus rhythm was observed in 46.5% of these patients, atrial fibrillation was observed in 8.5% of patients. Rhythms, such as atrial flutter (2%), atrial tachycardia and junctional rhythm were much less common (<1%). The most common comorbidity among admitted patients was hypertension n=139 (69.5%), followed by diabetes n=75 (37.5%), coronary artery disease n=46 (23%), obesity n=41 (21%), and heart failure n=37 (18.5%). Of the patients included in the study, 17 (8.5%) had a known history of hypothyroidism all of which were controlled on presentation with no therapeutic intervention (see Table 1 ).
Table 1.
Demographics and characteristics of participants (n=200)
| Variable | Number or Percentage (%) |
|---|---|
| Race | |
| Caucasian | n= 123 (61.5%) |
| African-American | n= 67 (33.5%) |
| Hispanic | n = 2 (1%) |
| Asian | n= 4 (2%) |
| Other | n= 4 (2%) |
| Gender | |
| Male | n= 90 (45%) |
| Female | n=110 (55%) |
| Age | Mean 67.80 |
| Prior ECG | |
| Normal | n = 128 (64%) |
| Atrial Fibrillation | n =19 (9.5%) |
| Atrial Flutter | n = 2 (1%) |
| Others | n = 51 (25.5%) |
| Admission ECG | |
| Normal | n = 93 (46.5%) |
| Atrial Fibrillation | n = 17 (8.59%) |
| Atrial Flutter | n = 3 (1.52%) |
| Junctional | n =1 (0.51%) |
| Atrial Tachycardia | n = 1 (0.51%) |
| Other | n = 85 (42.93%) |
| New Onset AV Block | |
| Yes | n =11 (5.53%) |
| No | n = 189 (94.5%) |
| PR Interval Increased (from prior ECG) | |
| Yes | n =13 (6.53%) |
| No | n = 187 (93.5%) |
| Disposition | |
| Discharged | n = 161 (80.5%) |
| Hospice | n = 3 (1.51%) |
| Deceased | n = 33 (16.58%) |
| AMA | n = 3 (1.51%) |
| Family History | |
| Sudden Cardiac Death | n = 2 (1%) |
| Cardiomyopathies | n = 2 (1%) |
| 1None | n = 196 (98%) |
| Past medical History | |
| Coronary Artery Disease | n = 46 (23%) |
| Atrial fibrillation/Atrial flutter | n = 36 (18%) |
| Hypertension | n = 139 (69.5%) |
| Chronic Obstructive Pulmonary Disease | n = 35 (17.5%) |
| Asthma | n = 28 (14%) |
| Diabetes | n = 75 (37.5%) |
| Chronic Kidney Disease | n= 31 (15.5%) |
| End Stage Renal Disease on Hemodialysis | n= 9 (4.5%) |
| PCI or CABG | n= 10 (5%) |
| Seizure Disorders | n= 8 (4%) |
| Sarcoidosis | n= 1 (0.5%) |
| Amyloidosis | n= 2 (1%) |
| Peripheral Venous Disease | n= 10 (5%) |
| Thyroid Disorders | n= 17 (8.5%) |
| Anxiety | n= 11 (5.5%) |
| Depression | n=19 (9.5%) |
| Obstructive Sleep Apnea | n=25 (12.5%) |
| Heart Failure | n=37 (18.5%) |
| Valvular Heart Disease | n=8 (4%) |
| Liver Disease | n=5 (2.5%) |
| Polysubstance use | n=5 (2.5%) |
| Obesity | n=41 (20.5%) |
| History of ICD placement | n=6 (3%) |
| Chronic Anemia | n=13 (6.5%) |
| Others | n=107 (53.5%) |
| None | n=7 (3.5%) |
| Transferred to ICU? | |
| Yes | n= 58 (29.15%) |
| No | n= 141 (70.85%) |
| Blood Type | |
| A | n=8 (9.41%) |
| A NEGATIVE | n=4 (4.71%) |
| A POSITIVE | n=21 (24.71%) |
| AB | n=2 (2.35%) |
| AB POSITIVE | n=3 (3.53%) |
| B | n=5 (5.88%) |
| B POSITIVE | n=5 (5.88%) |
| O | n=17 (20%) |
| O NEGATIVE | n=3 (3.53%) |
| O POSITIVE | n=17 (20%) |
1ECG = Electrocardiogram, AV = Atrioventricular, AMA = Left Against Medical Advice
Incidence of Atrioventricular Blocks
Of the 200 patients included, 11 (5.5%) patients were confirmed to have ECG changes consistent with a new onset AV block. The incidence of AV blocks among COVID-19 patients was reported to be 5.5 cases per 100 patients. Nine patients were reported to have first-degree AV block. One patient was reported to be with a high-degree AV block requiring pacemaker placement. The patient was a 71-year-old Caucasian male who initially presented with fatigue and generalized weakness at an outside facility. He did not require oxygen supplementation. He subsequently developed a high-degree AV block with ventricular response in the 20s. He was started on dexamethasone and remdesivir while at the outside facility, which was discontinued immediately after the occurrence of the heart block. Another patient had a transient second-degree Mobitz Type 2 AV block. The patient was a 70-year-old African American female with vascular dementia who was asymptomatic and was previously on metoprolol succinate which was continued during admission. Prior to discharge, she spontaneously converted to sinus rhythm and did not require a permanent pacemaker placement. She received dexamethasone therapy only. She was discharged with a 30-day event recorder and was found to have recurrence of her second-degree Mobitz Type 2 AV block. A dual chamber pacemaker was then placed.
Two patients remained in first-degree AV block at 90-day follow-up and no intervention was made. One patient showed resolution of the first-degree AV block after percutaneous coronary intervention (PCI). Out of the remaining 6 patients, 3 expired during hospital admission and 3 were lost to follow-up (see Tables 2 and 3 )
Table 2.
Characteristics of admitted patients with COVID-19 who developed atrioventricular blocks on admission.
| Patient | Age | Past medical history | PR interval and heart rate prior to admission | Rhythm on admission | PR interval and heart rate during admission | Medications during hospitalization | Intervention on discharge |
|---|---|---|---|---|---|---|---|
| 1 | 70 | CAD s/p remote CABG, Obesity, HTN, OSA, Paroxysmal Atrial Fibrillation | PR = 0.192 HR 53 | Sinus bradycardia w/ 1st degree AV Block (Transient 2nd degree AV Block which resolved prior to discharge) | PR = 0.220 HR 59 | Lorazepam, Ondansetron, Nitroglycerin, Metoprolol tartrate, ASA, Atorvastatin, Apixaban | 30 day event recorder |
| 2 | 65 | HTN, HLD, OSA | PR = 0.186 HR 86 | Sinus rhythm with 1st degree AV Block, anterolateral MI | PR = 0.214 HR 70 | ASA, Ticagrelor, Atorvastatin, Carvedilol, Lisinopril, Acyclovir | PCI with drug eluting stent in the proximal LAD |
| 3 | 61 | CAD, ESRD, ischemic cardiomyopathy, s/p ICD placement 1 year prior to admission for 10 prevention,cocaine abuse | PR = 0.180 HR 90 | Sinus rhythm with 1st degree AV Block, prolonged QT | PR = 0.214 HR 74 | ASA, Atorvastatin, Carvedilol, Cefepime, Clopidogrel, Isosorbide mononitrate, Hydralazine, Spironolactone | None |
| 4 | 93 | OSA, T2DM, HTN, CKD,TIA | PR = 0.180 HR 68 | Sinus rhythm with 1st degree AV Block | PR = 0.222 HR 70 | Furosemide,albuterol, dexamethasone, Remdesivir, piperacillin-tazobactam, vancomycin, quetiapine | Expired |
| 5 | 62 | HTN, T2DM, CKD Stage III | PR = 0.186 HR 83 | Sinus rhythm with 1st degree AV Block | PR = 0.220 HR 90 | Azithromycin, hydroxychloroquine, furosemide, zosyn, vancomycin, albuterol, atorvastatin, gabapentin, metoprolol | 2 week event monitor |
| 6 | 44 | Latent TB, completed treatment | PR = 0.178 HR 99 | Sinus rhythm with 1st degree AV Block, RBBB, LAD | PR = 0.354 HR 72 | Azithromycin, ceftriaxone, furosemide, Remdesivir, tocilizumab, albuterol, dexamethasone, propofol, fentanyl | None |
| 7 | 75 | CAD s/p PCI, HFrEF, T2DM, Dementia, OSA1 | PR = 0.155 HR 75 | Sinus rhythm with 1st degree AV Block, prolonged QT -> turned to Afib with RVR | PR = 0.212 HR 98 | Furosemide, metoprolol, amiodarone, levofloxacin, synthroid, lorazepam, piperacillin-tazobactam, vancomycin, trazodone, olanzapine | None |
| 8 | 55 | T2DM, OSA, Morbid obesity, HFpEF, hypothyroidism | PR = 0.188 HR 81 | Sinus bradycardia w/ 1st degree AV Block | PR = 0.222 HR 59 | Dexamethasone, Remdesivir, furosemide, levothyroxine, losartan | None |
| 9 | 71 | T2DM, HTN, HLD | PR = 0.14 HR 88 | Sinus bradycardia with high degree AV Block | PR = 0.158 HR 45 | Amlodipine, lisinopril, simvastatin, dexamethasone, Remdesivir | PPM placement; received remdesivir but stopped during admission due to heart block |
| 10 | 72 | CAD s/p PCI, HFpEF, Afib, thyroid cancer | PR = 0.180 HR 77 | Sinus bradycardia with 1st degree AV Block -> turned into Afib with RVR | PR = 0.220 HR 63; Then became A fib HR = 90 | Vancomycin, cefepime, norepinephrine, levetiracetam | None. Patient Expired |
| 11 | 55 | T2DM, HTN, HFpEF | PR = 0.173 HR 92 | Sinus rhythm with 1st degree AV block | PR = 0.210 HR 60 | Vancomycin, piperacillin-tazobactam, dexamethasone, Remdesivir | None |
1CAD = Coronary Artery Disease, CABG = Coronary Artery Bypass Graft, HTN = Hypertension, HLD = Hyperlipidemia, OSA = Obstructive Sleep Apnea, AV = Atrioventricular, HR = Heart Rate, ESRD = End Stage Renal Disease, PCI = Percutaneous Coronary Intervention, LAD = Left Anterior Descending Artery, MI = Myocardial Infarction, ICD = Implantable Cardiac Defibrillator, T2DM = Type 2 Diabetes Mellitus, CKD = Chronic Kidney Disease, TIA = Transient Ischemic Attack, TB = Tuberculosis, RBBB = Right Bundle Branch Block, HFrEF = Heart Failure with reduced Ejection Fraction, HFpEF = Heart Failure with preserved Ejection Fraction, Afib = Atrial Fibrillation, PPM = Permanent Pacemaker, RVR = Rapid Ventricular Rate
Table 3.
PR interval, heart rate and rhythm at 30 days and 90 days post discharge of patients with atrioventricular blocks on admission.
| Patient | Age | Rhythm 30 days post discharge | PR interval and HR at 30 days post discharge | Rhythm 90 days post discharge | Intervention |
|---|---|---|---|---|---|
| 1 | 70 | Sinus bradycardia w/ Second degree AV block Type 2 | PR = 0.272 HR = 46 | Atrial Fibrillation with Second degree AV Block | Dual chamber permanent pacemaker placed with His Bundle pacing |
| 2 | 65 | Normal sinus rhythm with T wave inversions in the anterolateral leads | PR = 0.192 HR 78 | Normal sinus rhythm with T wave inversions in the anterolateral leads (no significant change from baseline at 1 month) | Continue Dual Antiplatelet therapy |
| 3 | 61 | Normal sinus rhythm, with prolonged QT | PR = 0.180 HR 86 | Sinus rhythm with 1st degree AV Block, prolonged QT | Continued guideline directed medical therapy for chronic systolic heart failure |
| 4 | 93 | N/A. Patient expired1 | N/A. Patient expired | N/A. Patient expired | N/A. Patient expired |
| 5 | 62 | Normal sinus rhythm with no AV block | PR = 0.190 HR 105 | Sinus rhythm with 1st degree AV block | None |
| 6 | 44 | Sinus rhythm with 1st degree AV Block | PR = 0.210 HR 58 | Lost to follow up | Lost to follow up |
| 7 | 75 | N/A. Patient expired | N/A. Patient expired | N/A. Patient expired | N/A. Patient expired |
| 8 | 55 | Lost to follow up | Lost to follow up | Lost to follow up | Lost to follow up |
| 9 | 71 | Atrial sensed ventricular paced rhythm | Atrial sensed ventricular paced rhythm | Atrial sensed ventricular paced rhythm | Atrial sensed ventricular paced rhythm |
| 10 | 72 | N/A. Patient expired | N/A. Patient expired | N/A. Patient expired | N/A. Patient expired |
| 11 | 55 | Lost to follow up | Lost to follow up | Lost to follow up | Lost to follow up |
1HR = heart rate, AV = Atrioventricular, N/A = Not Applicable
Association between morbidity and mortality
Among patients admitted with COVID-19, ninety-one developed respiratory failure requiring oxygen supplementation in the form of regular and hi-flow nasal cannula, noninvasive positive pressure ventilation (see Table 4 ). In this same subset of patients, 29 required mechanical ventilation and subsequent admission to the ICU. New onset heart block was seen in six (6.5%) of these patients who developed respiratory failure. Troponin elevation was observed in seven of the patients. Out of these seven patients, only one had a new onset heart block in the setting of anterior myocardial infarction. The same patient underwent PCI which revealed a 90% occlusion in the proximal left anterior descending artery. The six other cases of NSTEMI were from demand supply mismatch (Type 2) (see Table 5 ). Acute kidney injury was observed in 33 patients and 2 (6%) of the 33 had a new onset heart block. No incidence of new onset AV block was found in the other complications including sepsis, anemia requiring transfusion, deep vein thrombosis, pulmonary embolism, and stroke (see Table 5).
Table 4.
Clinical characteristics of participants (n=200)
| Variable | Number or Percentage (%) |
|---|---|
| Complications | |
| Respiratory Failure | n=91 (45.5%) |
| Anemia Requiring Transfusion | n= 7 (3.5%) |
| Acute Kidney Injury | n= 33 (16.5%) |
| NSTEMI Types I and II | n= 7 (3.5%) |
| Deep Vein Thrombosis | n= 5 (2.5%) |
| Pulmonary Embolism | n =2 (1%) |
| Pneumonia | n= 49 (24.5%) |
| Sepsis | n= 26 (13%) |
| CVA | n =2 (1%) |
| Death | n= 16 (8%) |
| Others | n= 84 (42%) |
| Intervention | |
| Pacemaker Placement | n= 1 (0.5%) |
| Coronary Angiography | n= 1 (0.5%) |
| Mechanical Ventilation | n= 29 (14.5%) |
| BiPAP | n= 4 (2%) |
| HFNC | n= 11 (5.5%) |
| Oxygen supplementation | n= 84 (42%) |
| ICD Placement | n= 1 (0.5%) |
| Hemodialysis | n= 6 (3%) |
| No intervention | n= 87 (43.5%) |
| Electrolyte Abnormalities | |
| Hyperkalemia (>5.1 mEq/L) | n= 13 (6.5%) |
| Hypokalemia (< 3.5 meq/L) | n= 77 (38.5%) |
| Hypomagnesaemia (<1.6 mg/dL) | n= 15 (7.5%) |
| Hypophosphatemia (<2.5 mg/dL) | n= 9 (4.5%) |
| Hypocalcaemia (< 8.5 mg/dL) | n= 34 (17%) |
| Hypercalcemia (> 10.1 mg/dL) | n= 2 (1%) |
| Hyponatremia (<136 mEq/L) | n= 56 (28%) |
| Hypernatremia (> 145 mEq/L) | n= 24 (12%) |
| No abnormality | n= 57 (28.5%) |
1PCI = Percutaneous Coronary Intervention, CABG = Coronary Artery Bypass Graft, ICD = Implantable Cardiac Defibrillator, ICU = Intensive Care Unit
Table 5.
Comparisons of outcomes between those who developed new onset AV blocks and those who did not.
| Variable | Overall (n=200) | AV Block (n=11) | No AV Block (n=189) |
|---|---|---|---|
| Complications | |||
| Respiratory Failure | 91(45.00) | 6(54.55) | 85(45.21) |
| Anemia Requiring Transfusion | 7(3.50) | 0(0.00) | 7(3.72) |
| AKI | 33(16.50) | 2(18.18) | 31(16.49) |
| NSTEMI Types 1 and 11 | 7(3.50) | 1(9.09) | 6(3.19) |
| DVT | 5(2.50) | 0(0.00) | 5(2.66) |
| PE | 2(1.00) | 0(0.00) | 2(1.06) |
| Pneumonia | 49(24.40) | 2(18.18) | 47(25.00) |
| Sepsis | 26(13.00) | 0(0.00) | 26(13.83) |
| CVA | 2(1.00) | 0(0.00) | 2(1.06) |
| Others | 84(42.00) | 4(36.36) | 80(42.55) |
| Disposition | |||
| Discharged | 160(80.40) | 8(72.73) | 152(80.85) |
| Hospice | 3(1.51) | 0(0.00) | 3(1.60) |
| Deceased | 33(16.58) | 2(18.18) | 31(16.49) |
| AMA | 3(1.51) | 1(9.09) | 2(1.06) |
| ICU Length of stay | 8.05(7.56) | 4.00(3.51) | 8.61(7.81) |
| Hospital length of Stay | 8.98(8.35) | 7.64(8.67) | 9.06(8.36) |
NSTEMI = Non-ST Elevation Myocardial Infarction, CVA = Cerebrovascular Accident, BIPAP = Bi-level Positive Airway Pressure, ICD = Implantable Cardiac Defibrillator, HFNC = Hi-flo Nasal Cannula
There was no difference in in-hospital mortality among patients who developed AV blocks compared with patients who did not [2 (18.18) vs. 31 (16.49), p-value = 1.00]. There was no difference in ICU length of stay [4.00 (3.51) vs. 8.61 (7.81), p-value = 0.1219] or hospital length of stay [7.64 (8.67) vs. 9.06 (8.36), p-value = 0.2568] between the two groups (see Table 6 ).
Table 6.
Comparison of major outcomes between those who developed new onset AV blocks and those who did not.
| Variable | AV Block (n=11) | No AV Block (n=189) | P value |
|---|---|---|---|
| ICU Length of stay | 4.00(3.51) | 8.61(7.81) | 0.1219 |
| Hospital length of Stay | 7.64(8.67) | 9.06(8.36) | 0.2568 |
| Death | |||
| Yes | 2(18.18) | 31(16.49) | 1.000 |
| No | 9(81.82) | 157(83.51) | |
| Respiratory Failure | |||
| Yes | 6(54.55) | 85(45.21) | 0.5459 |
| No | 5(45.45) | 103(54.79) | |
| Pneumonia | |||
| Yes | 2(18.18) | 47(25.00) | 0.2662 |
| No | 9(81.82) | 141(75.00) | |
| Mechanical Ventilation | |||
| Yes | 0(0.00) | 29(15.43) | 0.3724 |
| No | 11(100.00) | 159(84.57) |
1AKI = Acute kidney Injury, NSTEMI = Non-ST Elevation Myocardial Infarction, DVT = Deep Vein Thrombosis, PE = Pulmonary Embolism, CVA = Cerebrovascular Accident, AMA = Left Against Medical Advice, ICU = Intensive Care Unit
2ICU = Intensive Care Unit
Discussion
Cardiovascular morbidity in patients with COVID-19 has been reported to include the following: myocardial injury, takotsubo cardiomyopathy, atrial arrhythmias, acute myocarditis, and even pulseless electrical activity arrest [4,5,6,7,8,10,11]. Lau et al. demonstrated that sinus tachycardia was the most common cardiovascular SARS‐CoV finding, with an overall incidence of 72%; and significant sinus bradycardia was seen in 18 (14.9%) patients. Unlike tachycardia which manifests to be persistent, bradycardia was somewhat transient [12].
There have been a few reports regarding the incidence of cardiac arrhythmias in COVID-19, but whether or not the virus specifically affects the conduction system of the heart, is still largely unknown. Additionally, the effects of new onset AV blocks in terms of patient outcomes remain unclear.
Our study showed that arrhythmias, particularly new onset AV blocks can occur in COVID-19 patients. Several mechanisms have been proposed regarding the pathophysiology of arrhythmias in this subset of patients [13], [14], [15]. Given that angiotensin-converting enzyme 2, the entry receptor for the causative coronavirus SARS-CoV-2, is expressed in multiple extrapulmonary tissues, including the cardiomyocytes, direct viral tissue damage causing electrical system conduction is a plausible mechanism of injury [13]. It is unclear whether inflammation, and resulting edema play a role in the development of transient AV blocks, and whether healing with fibrosis ultimately leads to permanent conduction system damage. Effects of the virus on ion channels have not been studied to our knowledge.
One postulated theory is that the infection in the pericardium causes massive edema and ischemia. Myocardial fibrosis from the recent viral infection can predispose patients to arrhythmias [14], particularly ventricular tachycardias. Elevation in cardiac and inflammatory markers may reflect a direct effect of the virus on the cardiac conduction system. Lastly, proinflammatory cytokines can also predispose these patients to arrhythmogenicity [14]. Other theories postulate worsening/further deterioration of preexisting cardiovascular debility in the setting of a viral infection, due to increasing metabolic demand in the setting of reduced cardiac reserve. This imbalance, along with an accentuated inflammatory response and myocardial damage, could raise the risk of acute coronary syndromes, heart failure, and arrhythmias [5]. Pre-existing conduction system disease or infiltrative disease are unlikely to be the underlying cause for the patients who were found to have first-degree AV blocks, as most of the patients did not have known infiltrative disease or conduction system disease, and had normal ECG findings prior to admission.
Patients who were admitted with COVID-19 infection were predominantly blood group A. This is consistent with an associated reported for SARS-COV-1, in which O blood groups were less common among SARS patients [17]. Our findings are also consistent with the results reported by Zhao et al. [18] where non-O types appear to be at a greater risk of infection and the meta-analysis performed by Pourali et al. [19] which showed that individuals with blood group A are at higher risk for COVID-19 infection while those with blood group O are at a lower risk.
This study also showed that there was no significant difference in morbidity and mortality in patients who developed new onset AV blocks compared to those who did not. Specifically, new onset AV blocks were not associated with longer hospital and ICU length of stay, increased intubation rates, or increased mortality. Our results are in line with prior reports that have illustrated that the various cardiac arrhythmias associated with COVID-19 infection do not necessarily correlate with the extent of the lung injury of these patients [9,10]. However, the results of this study were contrary to those of a small cross-sectional cohort study by Shi et al., where they reported higher mortality and intubation rates in patients with COVID-19 who developed concomitant conduction abnormalities [16]. This study was reported among 416 hospitalized patients in a single center in Wuhan, China.
Although the results of this study showed that most of the new onset AV blocks identified were clinically benign and did not result in an increase in overall mortality, it is notable to mention that one patient developed a high-grade AV block that required pacemaker placement. This patient had no pre-existing electrophysiologic issues to our knowledge. This finding is similar to previously reported rare cases about younger patients, with no underlying medical, cardiac, or drug history, who developed high-grade AV blocks while being hospitalized for COVID-19 [3,4]. Although the development of a new onset AV block is most likely multifactorial and not solely due to COVID-19, it is still important for clinicians to be mindful about the possibility of developing symptomatic bradycardia and life-threatening arrhythmias in patients admitted for COVID-19. This can be achieved by appropriate rhythm monitoring in-patient but the need for a cardiac event monitor upon discharge is unlikely to be necessary. Careful history taking, including family and drug use history is also of great importance as emerging drug therapies for COVID-19 have potential arrhythmogenic effects [17].
The results of this study also suggest that viral injury arrhythmias, specifically, AV blocks, sinus bradycardia, and atrial fibrillation, are likely the consequence of systemic illness and not solely the direct effects of COVID-19 infection. Many of the patients who developed AV blocks had underlying comorbidities and cerebrovascular diseases, such as hypertension, coronary artery disease, heart failure, prior stenting or bypass surgery, obstructive sleep apnea, and obesity.
While this study assessed the occurrence of new onset AV blocks and their association with morbidity and mortality, there were limitations. The sample size in this study is relatively small and a larger sample size from multiple centers may confer a much better estimation of the true relationship between COVID-19 and arrhythmias. There is also no control group (those without COVID-19) used to compare the incidence of new onset AV blocks. Another limitation would be that baseline medications as well as preexisting bundle branch block conferring risks to development of high-grade AV block were not factored into the study. Next, for patients who developed AV blocks, an underlying conduction system disease could not be entirely excluded as some patients were lost to follow-up after discharge and did not undergo an electrophysiology study. Moreover, this study design did not take into account the use of hydroxychloroquine and azithromycin in these patients. The investigators also did not look into QT prolongation in patients who were admitted for COVID-19 and in patients who developed heart blocks. Another limitation of the study is that the authors focused on AV blocks and did not take into account sick sinus syndrome (SSS) as the cause of bradycardias in these patients. Further studies are needed which will take into account SSS and AV blocks as a cause of bradyarrythmias, in patients with COVID-19.
Conclusion
COVID-19 is known to be associated with not only respiratory, but also cardiac injury. The results presented in this study show that there is an association between COVID-19 and new onset AV blocks, but direct causality cannot be ascertained as the development of new onset AV blocks is likely multifactorial. Moreover, the development of new onset AV blocks in patients with COVID-19 is mostly clinically benign and likely does not affect immediate and long-term patient outcomes. As such, the decision to conduct appropriate rhythm monitoring in-patient should only be considered on a case-by-case basis - depending on severity of illness and preexisting cardiac conditions. Future studies are needed to better understand the mechanisms underlying the effects of COVID-19 on the heart, as well as how to best treat arrhythmias associated with COVID-19 in the setting of potentially arrhythmogenic emerging drug therapies for COVID-19. Further studies should focus on a broader spectrum of demographic factors to determine their association with AV blocks in this condition; and also clinical outcomes in COVID-19 patients randomized to specific therapies such as steroids, tocilizumab, and remdesivir.
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Source of funding
None.
References
- 1.Wu CI, Postema PG, Arbelo E, Behr ER, Bezzina CR, Napolitano C, et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm. 2020;17(9):1456–1462. doi: 10.1016/j.hrthm.2020.03.024. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He J, Wu B, Chen Y, Tang J, Liu Q, Zhou S, et al. Characteristic electrocardiographic manifestations in patients with COVID-19. Can J Cardiol. 2020;36(6):966.e1–966.e4. doi: 10.1016/j.cjca.2020.03.028. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuyun MF, Squire IB, Ng GA, Samani NJ. Evidence for reduced susceptibility to cardiac bradycardias in South Asians compared with Caucasians. Heart. 2018;104:1350–1355. doi: 10.1136/heartjnl-2017-312374. [DOI] [PubMed] [Google Scholar]
- 4.Azarkish M, Laleh Far V, Eslami M, Mollazadeh R. Transient complete heart block in a patient with critical COVID-19. Eur Heart J. 2020;41:2131. doi: 10.1093/eurheartj/ehaa307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kir D, Mohan C, Sancassani R. HEART BRAKE-An unusual cardiac manifestation of coronavirus disease 2019 (COVID-19) JACC Case Rep. 2020;2:1252–1255. doi: 10.1016/j.jaccas.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochi AN, Tagliari AP, Forleo GB, et al. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Jul 1;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061–1069. doi: 10.1001/jama.2020.1585. Erratum in: JAMA. 2021 Mar 16;325(11):1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rattanawong P, Shen W, El Masry H, Sorajja D, Srivathsan K, Valverde A, et al. Guidance on short-term management of atrial fibrillation in coronavirus Disease 2019. J Am Heart Assoc. 2020 Jul 21;9(14) doi: 10.1161/JAHA.120.017529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavri BB, Kloo J, Farzad D, Riley JM. Behavior of the PR interval with increasing heart rate in patients with COVID-19. Heart Rhythm. 2020;17:1434–1438. doi: 10.1016/j.hrthm.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochav SM, Coromilas E, Nalbandian A, Ranard LS, Gupta A, Chung MK, et al. Cardiac Arrhythmias in COVID-19 infection. Circ Arrhythm Electrophysiol. 2020;13(6) doi: 10.1161/CIRCEP.120.008719. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabri A, Kalra A, Kumar A, Alameh A, Adroja S, Bashir H, et al. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020 Jul 1;3(7) doi: 10.1001/jamanetworkopen.2020.14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau ST, Yu WC, Mok NS, Tsui PT, Tong WL, Cheng SW. Tachycardia amongst subjects recovering from severe acute respiratory syndrome (SARS) Int J Cardiol. 2005;100:167–169. doi: 10.1016/j.ijcard.2004.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, Cooper LT, Jr, Chahal CAA. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peretto G, Sala S, Rizzo S, De Luca G, Campochiaro C, Sartorelli S, et al. Arrhythmias in myocarditis: state of the art. Heart Rhythm. 2019;16(5):793–801. doi: 10.1016/j.hrthm.2018.11.024. May. [DOI] [PubMed] [Google Scholar]
- 17.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 Jul 1;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakkireddy DR, Chung MK, Gopinathannair R, Patton KK, Gluckman TJ, Turagam M, et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020;17(9):e233–e241. doi: 10.1016/j.hrthm.2020.03.028. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Y, Cheng G, Chui CH, Lau FY, Chan PK, Ng MH, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005 Mar 23;293(12):1450–1451. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]