Summary
Background
Background The second wave of the COVID-19 pandemic was more aggressive in Brazil compared to other countries around the globe. Considering the Brazilian peculiarities, we analyze the in-hospital mortality concerning socio-epidemiological characteristics of patients and the health system of all states during the first and second waves of COVID-19.
Methods
We performed a cross-sectional study of hospitalized patients with positive RT-PCR for SARS-CoV-2 in Brazil. Data was obtained from the Influenza Epidemiological Surveillance Information System (SIVEP-Gripe) and comprised the period from February 25, 2020, to April 30, 2021, separated in two waves on November 5, 2020. We performed a descriptive study of patients analyzing socio-demographic characteristics, symptoms, comorbidities, and risk factors stratified by age. In addition, we analyzed in-hospital and intensive care unit (ICU) mortality in both waves and how it varies in each Brazilian state.
Findings
Between February 25, 2020 and April 30, 2021, 678 235 patients were admitted with a positive RT-PCR for SARS-CoV-2, with 325 903 and 352 332 patients for the first and second wave, respectively. The mean age of patients was 5965 (IQR 480 - 720). In total, 379 817 (5600%) patients had a risk factor or comorbidity. In-hospital mortality increased from 3481% in the first to 3930% in the second wave. In the second wave, there were more ICU admissions, use of non-invasive and invasive ventilation, and increased mortality for younger age groups. The southern and southeastern regions of Brazil had the highest hospitalization rates per 100 000 inhabitants. However, the in-hospital mortality rate was higher in the northern and northeastern states of the country. Racial differences were observed in clinical outcomes, with White being the most prevalent hospitalized population, but with Blacks/Browns (Pardos) having higher mortality rates. Younger age groups had more considerable differences in mortality as compared to groups with and without comorbidities in both waves.
Interpretation
We observed a more considerable burden on the Brazilian hospital system throughout the second wave. Furthermore, the north and northeast of Brazil, which present lower Human Development Indexes, concentrated the worst in-hospital mortality rates. The highest mortality rates are also shown among vulnerable social groups. Finally, we believe that the results can help to understand the behavior of the COVID-19 pandemic in Brazil, helping to define public policies, allocate resources, and improve strategies for vaccination of priority groups.
Funding
Coordinating Agency for Advanced Training of Graduate Personnel (CAPES) (C.F. 001), and National Council for Scientific and Technological Development (CNPq) (No. 309537/2020-7).
Keywords: COVID-19, SARS-CoV-2, In-hospital mortality, Pandemic response, Healthcare
Research in context.
Evidence before this study
We conducted a PubMed search for studies describing the behavior of the first and second waves of COVID-19 in hospitals worldwide. The search terms used were: “COVID-19” OR “SARS-CoV-2” OR “COVID” AND “hospital” OR “critical care” OR “ICU” AND “wave” AND “mortality”. For the most part, studies from other countries show that the second wave of the COVID-19 pandemic was less aggressive. However, the socio-epidemiological characteristics and in-hospital mortality during the two waves of the pandemic in Brazil are still shallow, and the previous studies focus on the most general characteristics, especially for the second wave.
Added value of this study
In this analysis, we evaluated 678 235 hospitalizations during the first and second waves in Brazil. We observed a more considerable burden on the hospital system in the second wave and the prevalence of the P.1 (Gamma) variant with an increase in hospitalization and mortality rates. In addition, in-hospital mortality showed a considerable increase from the first to the second wave, especially in patients between 20-60 years. The data also showed social and access differences to the health system, with higher mortality rates in more vulnerable social groups. Finally, the highest mortality rates were observed in the Brazilian states of the north and northeast regions.
Implication of all the available evidence
It was expected that in Brazil, as reported in other countries, the second wave would be less severe due to investment and the experience of the first wave. Nevertheless, our analysis demonstrated that Brazil faced a second wave more severe than the first one. In general, even with some states in the north and northeast having a higher number of hospital beds per 100 000 inhabitants than other states in Brazil, this increase could not reduce lethality compared to the numbers in the southern region. These differences between in-hospital mortality evidence that the opening of new hospital beds was not enough to mitigate the effects of the pandemic in Brazil. As alternatives, it is necessary to adopt effective non-pharmacological measures, better primary care protocols, qualification of health professionals, and a national policy to fight the pandemic. Another point that deserves attention is implementing initiatives to reach the most vulnerable population in society. Also, monitoring through genomic analysis of confirmed SARS-CoV-2 cases needs to be expanded in Brazil for better pandemic management.
Alt-text: Unlabelled box
Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic started in Wuhan Province, China, spreading globally and impacting the countries in different ways. The world has more than 190 375 116 cases, 4 088 293 deaths, and around 2 billion (1340% of the population) people fully vaccinated (until July 22, 2021). Brazil had its first case of COVID-19 on February 25, 2020,1, and since then faced 19 376 574 cases, 542 214 deaths, and 35 168 730 (1726% of the population) people fully vaccinated (until July 22, 2021).2
The high transmissibility of SARS-CoV-2 caused a rapid increase in the number of infected individuals. These high numbers added to the broad spectrum of symptoms, and the rapid evolution to severe cases has made the COVID-19 pandemic unique in comparison to others already faced.3 The contagion and evolution characteristics of the COVID-19 pandemic generated an overload on healthcare systems worldwide. The responses have been different and dependent on the capacity of each system to restructure and absorb a growing demand for supplies and intensive care unit (ICU) beds.4
In this paper, we investigated the sociodemographic characteristics of patients between the two waves of COVID-19. The main characteristics evaluated were symptoms, risk factors, use of hospital resources, notifications, admissions, and mortality in each Brazilian state. To that end, we employ a dataset from the national surveillance system (SIVEP-Gripe) that reports on hospitalized patients in Brazil with severe acute respiratory infections (SARI) due to COVID-19. We also intend to analyze the prevalence of variants reported throughout the pandemic in Brazil. Considering the context, our study aims to assist in decision-making in public health policies and can be used as a starting point in other health scenarios worldwide.
Methods
Study design and data sources
We performed a descriptive study of publicly available epidemiological data relating to hospitalizations of SARI with COVID-19 in Brazil. The analysis was based on SIVEP-Gripe data from February 25, 2020, until April 30, 2021. SIVEP-Gripe is an official anonymized system from the Brazilian Ministry of Health for the notification of hospitalized cases and deaths from SARI in Brazil.5 We included all patients registered in SIVEP-Gripe who had been admitted to the hospital, had a positive RT-PCR test result for SARS-CoV-2, and had an outcome of discharge or death related to COVID-19.
We divided the SIVEP-Gripe data into three sets: whole pandemic, first and second waves. The first wave consists of patients admitted by COVID-19 from February 25, 2020, to November 5, 2020. For the second wave, we considered patients admitted by COVID-19 from November 6, 2020, to April 30, 2021. We use as the definition of an epidemic wave an increasing number of cases with a defined peak followed by a period with a defined valley.6 Therefore, we described it as an epidemic wave. In this period, the division of waves was based on the point with the lowest 7-day moving average for cases confirmed by COVID-19 in Brazil.
As complementary information to our study, we surveyed the genomic analyzes collected from patients with SARS-CoV-2 variants. Genomic analyzes were obtained from the Global Initiative on Sharing Avian Influenza Database (GISAID), totaling 12 586 cases.7 The cases brought correspond to the genomic analyzes collected during the same period as the SIVEP-Gripe data. In Brazil, the Oswaldo Cruz Foundation (Fiocruz) manages the SARS-CoV-2 Genomic Surveillance, centralizing the results of all analyzes that are sent to GISAID.8
Due to the continental proportions and different responses of the Brazilian states, we evaluated the population characteristics and active hospital beds in each state. Therefore, we used population estimates from the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística, IBGE) for the year 2020.9 Finally, we analyzed the number of hospital beds and ICU beds for the two periods comprising the end of the first (October 2020) and second waves (April 2021) of COVID-19 in each Brazilian state from the TABNET database.10 A detailed description of the information for each dataset is provided in Appendix 1 (pp 1–2).
Statistical analysis
Data was presented using descriptive statistics, including frequency, distribution, and characteristics related to hospitalized cases reported to the SIVEP-Gripe. For continuous variables, we calculated the mean, median, standard deviation, and interquartile range. In turn, for categorical variables, we analyzed frequency and percentage. We stratified patients into seven age groups based on the patient’s admission date and birth, as follows: 0–19, 20–39, 40–49, 50–59, 60–69, 70–79. These age groups are in line with similar studies in the literature 11, 12.
We looked at hospitalized SARI patients characteristics in the two waves of the COVID-19 pandemic. In this sense, we considered the incidence and frequency of the following variables: sociodemographic characteristics, symptoms, comorbidities, and risk factors at the time of hospitalization. Comparisons between the first and second waves of COVID-19 were reported in percentages, percentage differences, and with a confidence interval of 995%. In addition, we analyzed incidence and mortality rates for hospitalizations, ICU admission, and the use of noninvasive and invasive ventilation. Finally, we analyzed hospitalization rates, beds, and deaths per population in each state in Brazil.
To assess the behavior of hospitalizations and deaths related to the percentage of prevalence of the main variants of SARS-CoV-2 during the pandemic in Brazil, we grouped genomic analyses and hospitalizations by epidemiological week. We considered the genomic analysis from the 8th epidemiological week based on the first official notification of SARS-CoV-2 in Brazil (Feb 25, 2020). As our study considers periods related to the years 2020 and 2021, the epidemiological weeks of 2021 were considered a sequence of the epidemiological weeks of 2020. In this way, we analyzed the frequency of the main variants per epidemiological week of collection and the cumulative incidence of hospitalizations and deaths based on the patient’s admission date.
Following ethically agreed principles on open data, this analysis did not require ethical approval in Brazil. Furthermore, we disregarded records with missing data in the analysis. In this situation, we provide the number of patients considered in the respective table or figure. Analyzes were conducted using the Python programming language and the Pandas and NumPy libraries.
Role of the funding source
The funders had no role in any decision about the manuscript.
Results
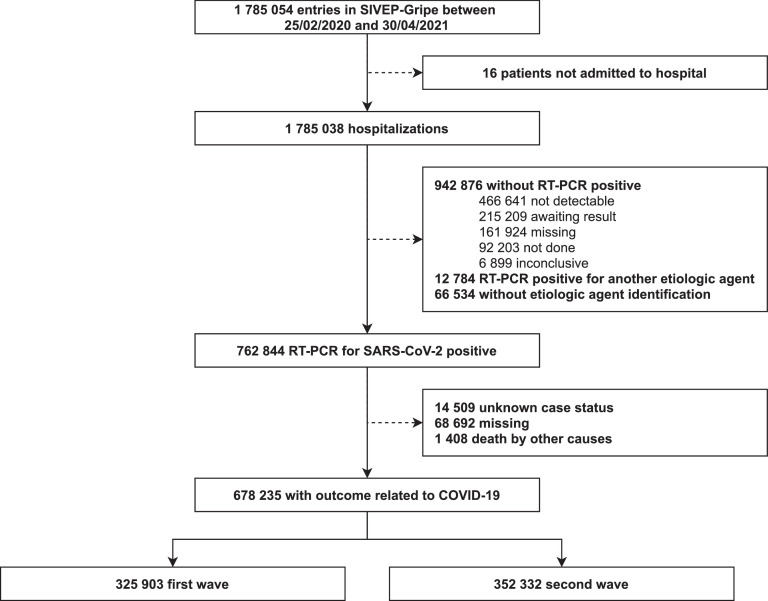
Between February 25, 2020 and April 30, 2021, 1 785 054 cases were reported in SIVEP-Gripe (Fig. 1). Among them, 1 785 038 (9999%) cases were hospitalized, and 762 844 (4273%) had a positive RT-PCR for SARS-CoV-2. Of the 762 844 cases with positive RT-PCR test, 678 235 (8890%) cases had an outcome related to COVID-19. Therefore, our final sample was 678 235 cases, divided into two waves: first wave with 325 903 (4805%) cases and second wave with 352 332 (5195%) cases.
Figure 1.
Study profile. Note: The continuous line represents the number of cases that met each of the cutoff criteria. Meanwhile, the dashed line informs the number of patients that were removed from the original dataset. The analysis period considered is from February 25, 2020, to April 30, 2021. We considered all hospitalizations in this period and divided them into two waves. The first wave corresponds to the period from February 25, 2020, to November 5, 2020. The second wave corresponds to the period from November 6, 2020, to April 30, 2021. SARS-CoV-2 = Severe acute respiratory syndrome coronavirus 2. RT-PCR = Reverse transcription polymerase chain reaction.
Table 1 presents the characterization of our data. The mean population age for the whole pandemic was 59 years (IQR 480 - 720). Cases were distributed among seven age groups, but in general, there was a slight predominance of cases in the age group 60 to 69 years (148 302 (2187%)). In both waves, the number of males hospitalized by COVID-19 was higher than that of females (Table 2). Male patients represented 5613% and 5499% of the hospitalizations in the first and second waves, respectively. In-hospital mortality rates were slightly higher for males than for females during the whole pandemic (3760% vs. 3657% of deaths, respectively).13 The difference is higher during the first wave (3372% of female death vs. 3566% of male death).
Table 1.
Patient characteristics stratified by wave.
| Whole pandemic (n = 678 235) | First wave (n = 325 903) | Second wave (n = 352 332) | |
|---|---|---|---|
| Age, in years | |||
| Min - Max | 0 - 116 | 0 - 116 | 0 - 114 |
| Mean (SD) | 5965 (1763) | 5969 (1828) | 5961 (1701) |
| Median (IQR) | 61 (480 - 720) | 61 (470 - 730) | 61 (480 - 720) |
| Age groups, in years | |||
| 0 to 19 | 10 152 (150%) | 5 983 (184%) | 4 169 (118%) |
| 20 to 39 | 82 214 (1212%) | 40 842 (1253%) | 41 372 (1174%) |
| 40 to 49 | 96 950 (1429%) | 45 996 (1411%) | 50 954 (1446%) |
| 50 to 59 | 128 931 (1901%) | 59 966 (1840%) | 68 965 (1957%) |
| 60 to 69 | 148 303 (2187%) | 67 562 (2073%) | 80 741 (2292%) |
| 70 to 79 | 121 879 (1797%) | 58 145 (1784%) | 63 734 (1809%) |
| 80 + | 89 806 (1324%) | 47 409 (1455%) | 42 397 (1203%) |
| Sex (n = 678 159) | 678 159 (9999%) | 325 857 (9999%) | 352 302 (9999%) |
| Female | 301 521 (4446%) | 142 942 (4387%) | 158 579 (4501%) |
| Male | 376 638 (5554%) | 182 915 (5613%) | 193 723 (5499%) |
| Self-reported race/ethnicity (n = 534 956) | 534 956 (7887%) | 245 170 (7523%) | 289 786 (8225%) |
| White | 298 887 (5587%) | 128 382 (5236%) | 170 505 (5884%) |
| Black/Brown | 228 134 (4265%) | 112 545 (4590%) | 115 589 (3989%) |
| Asian | 6 934 (130%) | 3 575 (146%) | 3 359 (116%) |
| Indigenous | 1 001 (019%) | 668 (027%) | 333 (011%) |
| Scholarity (n = 239 709) | 239 709 (3534%) | 115 260 (3537%) | 124 449 (3532%) |
| Illiterate | 13 744 (573%) | 7 260 (630%) | 6 484 (521%) |
| Elementary School | 62 953 (2626%) | 30 355 (2634%) | 32 598 (2619%) |
| Middle School | 45 712 (1907%) | 21 736 (1886%) | 23 976 (1927%) |
| High School | 77 903 (3250%) | 37 101 (3219%) | 40 802 (3279%) |
| College / University | 39 397 (1644%) | 18 808 (1632%) | 20 589 (1654%) |
| Risk factors (n = 379 817) | 379 817 (5600%) | 184 309 (5655%) | 195 508 (5549%) |
| Chronic Cardiovascular Disease | 241 695 (6363%) | 116 563 (6324%) | 125 132 (6400%) |
| Diabetes mellitus | 171 845 (4524%) | 85 713 (4651%) | 86 132 (4406%) |
| Obesity | 54 066 (1423%) | 19 879 (1079%) | 34 187 (1749%) |
| Chronic Kidney Disease | 26 516 (698%) | 14 858 (806%) | 11 658 (596%) |
| Chronic Neurological Disease | 26 059 (686%) | 14 334 (778%) | 11 725 (600%) |
| Other Chronic Pneumatopathy | 25 121 (661%) | 13 491 (732%) | 11 630 (595%) |
| Asthma | 18 467 (486%) | 9 261 (502%) | 9 206 (471%) |
| Immunodeficiency or Immunodepression | 17 525 (461%) | 9 821 (533%) | 7 704 (394%) |
| Chronic Liver Disease | 5 884 (155%) | 3 158 (171%) | 2 726 (139%) |
| Chronic Hematological Disease | 5 132 (135%) | 2 934 (159%) | 2 198 (112%) |
| Down syndrome | 1 823 (048%) | 863 (047%) | 960 (049%) |
| Puerperal | 1 492 (039%) | 812 (044%) | 680 (035%) |
| Without risk factors | 298 418 (4400%) | 141 594 (4345%) | 156 824 (4451%) |
| Symptoms (n = 658 529) | 658 529 (9709%) | 315 995 (9696%) | 342 534 (9722%) |
| Dyspnea | 487 205 (7398%) | 230 661 (7300%) | 256 544 (7490%) |
| Cough | 472 161 (7170%) | 234 597 (7424%) | 237 564 (6935%) |
| Oxygen saturation 95% | 429 751 (6526%) | 191 834 (6071%) | 237 917 (6946%) |
| Fever | 404 358 (6140%) | 209 651 (6635%) | 194 707 (5684%) |
| Respiratory discomfort | 387 116 (5878%) | 184 994 (5854%) | 202 122 (5901%) |
| Fatigue | 122 379 (1858%) | 28 759 (910%) | 93 620 (2733%) |
| Sore throat | 112 758 (1712%) | 55 780 (1765%) | 56 978 (1663%) |
| Diarrhea | 93 356 (1418%) | 45 063 (1426%) | 48 293 (1410%) |
| Vomit | 53 293 (809%) | 26 002 (823%) | 27 291 (797%) |
| Loss of taste | 50 978 (774%) | 15 040 (476%) | 35 938 (1049%) |
| Loss of smell | 50 606 (768%) | 15 016 (475%) | 35 590 (1039%) |
| Abdominal pain | 26 770 (407%) | 7 239 (229%) | 19 531 (570%) |
| Without symptoms | 19 706 (291%) | 9 908 (304%) | 9 798 (278%) |
Notes: SD = Standard deviation. IQR = Interquartile range. Race/ethnicity declared by the patient: Branca (White); Amarela (Asian); Preta (Black)/Parda (Brown); and, Indígena (Indigenous). We only consider puerperal or parturient women up to 50 years of age.
Table 2.
Mortality stratified by sociodemographic characteristics and wave.
| Whole pandemic |
First Wave |
Second Wave |
Second wave vs first wave |
|||||
|---|---|---|---|---|---|---|---|---|
| Cases | Deaths | Cases | Deaths | Cases | Deaths | Difference | 995% CI | |
| Sex | ||||||||
| Female | 301 521 (4446%) | 110 256 (3657%) | 142 942 (4387%) | 48 205 (3372%) | 158 579 (4501%) | 62 051 (3913%) | 541% | 06 - 575 |
| Male | 376 638 (5554%) | 141 627 (3760%) | 182 915 (5613%) | 65 227 (3566%) | 193 723 (5499%) | 76 400 (3944%) | 378% | 47 - 409 |
| Total | 678 159 | 251 883 (3714%) | 325 857 (4805%) | 113 432 (3481%) | 352 302 (5195%) | 138 451 (3930%) | 378% | 26 - 472 |
| Self-reported race/ethnicity | ||||||||
| White | 298 887 (5587%) | 110 230 (3688%) | 128 382 (5236%) | 43 540 (3391%) | 170 505 (5884%) | 66 690 (3911%) | 520% | 85 - 555 |
| Black/Brown | 228 134 (4265%) | 93 867 (4115%) | 112 545 (4590%) | 43 982 (3908%) | 115 589 (3989%) | 49 885 (4316%) | 408% | 67 - 448 |
| Asian | 6 934 (130%) | 2 585 (3728%) | 3 575 (146%) | 1 278 (3575%) | 3 359 (116%) | 1 307 (3891%) | 316% | 88 - 544 |
| Indigenous | 1 001 (019%) | 374 (3736%) | 668 (027%) | 253 (3787%) | 333 (011%) | 121 (3634%) | -153% | 77 - 486 |
| Total | 534 956 | 207 056 (3871%) | 245 170 (4583%) | 89 053 (3632%) | 289 786 (5417%) | 118 003 (4072%) | 440% | 14 - 466 |
| Scholarity | ||||||||
| Illiterate | 13 744 (573%) | 7 847 (5709%) | 7 260 (630%) | 4 118 (5672%) | 6 484 (521%) | 3 729 (5751%) | 079% | 87 - 245 |
| Elementary School | 62 953 (2626%) | 31 171 (4951%) | 30 355 (2634%) | 14 219 (4684%) | 32 598 (2619%) | 16 952 (5200%) | 516% | 38 - 594 |
| Middle School | 45 712 (1907%) | 19 166 (4193%) | 21 736 (1886%) | 8 482 (3902%) | 23 976 (1927%) | 10 684 (4456%) | 554% | 63 - 644 |
| High School | 77 903 (3250%) | 24 219 (3109%) | 37 101 (3219%) | 10 201 (2750%) | 40 802 (3279%) | 14 018 (3436%) | 686% | 21 - 751 |
| College / University | 39 397 (1644%) | 10 326 (2621%) | 18 808 (1632%) | 4 127 (2194%) | 20 589 (1654%) | 6 199 (3011%) | 817% | 30 - 903 |
| Total | 239 709 | 92 729 (3868%) | 115 260 (4808%) | 41 147 (3570%) | 124 449 (5192%) | 51 582 (4145%) | 575% | 36 - 614 |
| Geographic zone | ||||||||
| Urban | 584 140 (9666%) | 215 310 (3686%) | 281 272 (9681%) | 97 349 (3461%) | 302 868 (9653%) | 117 961 (3895%) | 434% | 09 - 458 |
| Rural | 18 316 (303%) | 8 024 (4381%) | 8 367 (288%) | 3 525 (4213%) | 9 949 (317%) | 4 499 (4522%) | 309% | 65 - 453 |
| Periphery | 1 842 (030%) | 767 (4164%) | 888 (031%) | 364 (4099%) | 954 (030%) | 403 (4224%) | 125% | 25 - 574 |
| Total | 604 298 | 224 101 (3708%) | 290 527 (4808%) | 101 238 (3485%) | 313 771 (5192%) | 122 863 (3916%) | 431% | 07 - 455 |
Note: We only consider data that have an informed outcome. Proportions are calculated based on complete records for sex, self-reported race/ethnicity, education, and geographic area of residence. Data on race/ethnicity were collected as self-reported race or skin colour, classified as Branco (White), Preto (Black), Pardo (Brown), Amarelo (Asian), or Indígena (Indigenous) The percentages of cases are calculated based on the total for each stratification (whole pandemic, first wave, or second wave). Percentages of deaths are calculated based on the total number of cases for each sociodemographic characteristics stratified by whole pandemic, first wave, or second wave.
There was a predominance of hospitalized White individuals, representing 5587% of the total data, followed by the Black/Brown (Pardos) population (4265%) (Table 2). Asian and Indigenous people represented 149% of the cases in our data. The mortality rate was higher among Black/Brown (Pardos) people and decreased inversely to the educational level. Furthermore, the mortality rate is 30% higher among illiterate people compared with those with a college degree. The number of hospitalized patients from rural and peripheral areas represents around 3% of the total data. However, these populations have mortality rates about 6% higher than the urban population.
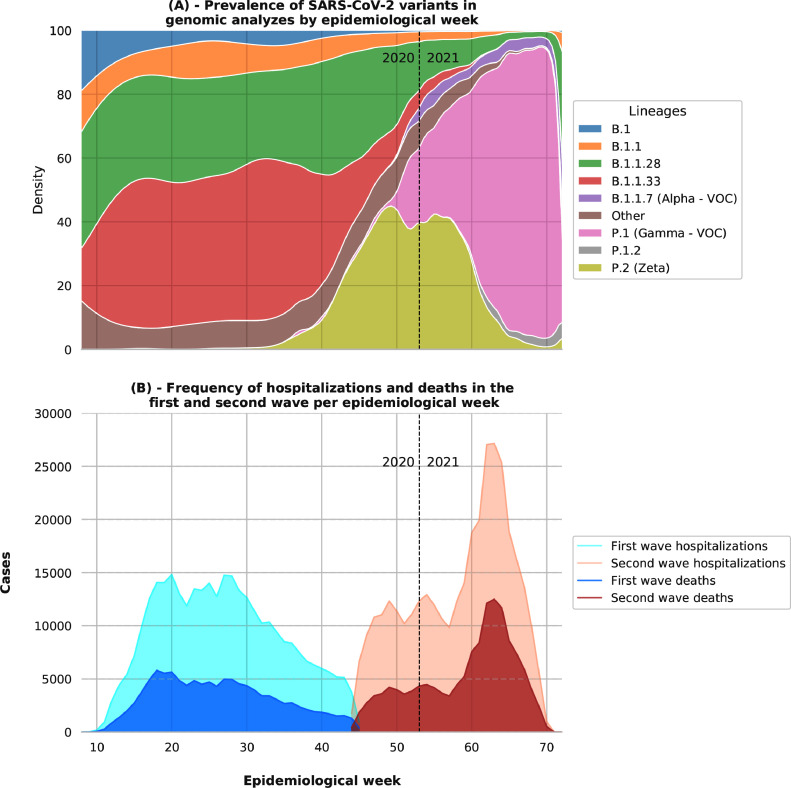
During the first wave, according to genomic analyzes (Fig. 2), the variants with the highest prevalence in Brazil were B.1.1.28 (2030%) and B.1.1.33 (1035%). At the end of the first wave, genomic analyzes indicate a prevalence of approximately 20% of the P.2 (Zeta) variant in SARS-CoV-2 cases in Brazil. During the second wave, the P.2 and P.1 (Gamma) variants were the most prevalent. The P.2 variant had the highest prevalence during 50th to 60th epidemiological weeks (20%). From the 60th epidemiological week onwards, the P.1 variant ranged from 60% to approximately 90% of the variants circulating in Brazil.
Figure 2.
Prevalence of variants and frequency of hospitalizations and deaths from SARS-CoV-2 in Brazil. Note: the genomic analyzes considered were carried out in Brazil and made available in the GISAID database. The analysis period considered is from February 25, 2020, to April 30, 2021. We considered all hospitalizations in this period and divided them into two waves. The first wave corresponds to the period from February 25, 2020 to November 5, 2020. The second wave corresponds to the period from November 6, 2020 to April 30, 2021. With a programmatic objective, the World Health Organization (WHO) classified the variants as Variants of Concern (VOC) or Variants of Interest (VOI), depending on their transmissibility, virulence, clinical presentation, their ability to impact the epidemiological picture of the pandemic, and its power to reduce the effectiveness of public health measures, diagnostic tests, therapeutic measures or vaccines.14
The first case of SARS-CoV-2 in Brazil was identified in the 8th epidemiological week (February 25, 2020).1 From the first case to the peak of the pandemic to the first wave (Fig. 2) was just 12 weeks. During 20th to 30th epidemiological weeks, Brazil had 15 000 hospitalizations and 5 000 deaths from COVID-19 per week. In the 30th to 44th epidemiological weeks, corresponding to the first wave, there is a substantial decline in cases and deaths. The end of this period also characterizes the division between the first and second waves of our study. In the second wave, between 60th to 70th weeks, Brazil reached the maximum peak for the pandemic, with more than 25 000 hospitalizations and 10 000 deaths per epidemiological week.
Of the 678 235 hospitalizations, there were 251 910 deaths (3714%) (Table 3). Among these deaths, 113 444 (4503%) occurred in the first wave and 138 466 (5497%). ICU admissions correspond for 245 304 (3617%) of the SARI cases reported in SIVEP-Gripe during the whole pandemic. In the first wave, 115 018 (4689%) cases were admitted to the ICU, with 64 589 (5616%) deaths. In the second wave, 130 286 (5311%) cases were admitted to the ICU, with 83 781 (6431%) deaths.
Table 3.
Use of hospital resources stratified by age group and wave.
| Age group | Whole pandemic |
First wave |
Second wave |
Second wave vs first wave |
||||
|---|---|---|---|---|---|---|---|---|
| Cases | Deaths | Cases | Deaths | Cases | Deaths | Difference | 995% CI | |
| Hospitalizations stratified by age group and wave | ||||||||
| 0 to 19 | 10 152 (150%) | 969 (954%) | 5 983 (184%) | 548 (916%) | 4 169 (118%) | 421 (1010%) | 094% | 22 - 212 |
| 20 to 39 | 82 214 (1212%) | 11 666 (1419%) | 40 842 (1253%) | 4 530 (1109%) | 41 372 (1174%) | 7 136 (1725%) | 616% | 68 - 663 |
| 40 to 49 | 96 950 (1429%) | 19 375 (1998%) | 45 996 (1411%) | 7 574 (1647%) | 50 954 (1446%) | 11 801 (2316%) | 669% | 19 - 719 |
| 50 to 59 | 128 931 (1901%) | 36 310 (2816%) | 59 966 (1840%) | 14 759 (2461%) | 68 965 (1957%) | 21 551 (3125%) | 664% | 15 - 712 |
| 60 to 69 | 148 303 (2187%) | 61 602 (4154%) | 67 562 (2073%) | 26 166 (3873%) | 80 741 (2292%) | 35 436 (4389%) | 516% | 66 - 566 |
| 70 to 79 | 121 879 (1797%) | 64 746 (5312%) | 58 145 (1784%) | 29 743 (5115%) | 63 734 (1809%) | 35 003 (5492%) | 377% | 21 - 433 |
| 80 + | 89 806 (1324%) | 57 242 (6374%) | 47 409 (1455%) | 30 124 (6354%) | 42 397 (1203%) | 27 118 (6396%) | 042% | 21 - 105 |
| Total | 678 235 | 251 910 (3714%) | 325 903 (4805%) | 113 444 (3481%) | 352 332 (5195%) | 138 466 (3930%) | 449% | 26 - 472 |
| ICU admissions stratified by age group and wave | ||||||||
| 0 to 19 | 2 825 (115%) | 626 (2216%) | 1 616 (140%) | 349 (2160%) | 1 209 (093%) | 277 (2291%) | 131% | 77 - 444 |
| 20 to 39 | 22 458 (916%) | 7 640 (3402%) | 10 310 (896%) | 2 807 (2723%) | 12 148 (932%) | 4 833 (3978%) | 1255% | 33 - 1378 |
| 40 to 49 | 29 454 (1201%) | 12 293 (4174%) | 13 096 (1139%) | 4 522 (3453%) | 16 358 (1256%) | 7 771 (4751%) | 1298% | 86 - 1409 |
| 50 to 59 | 44 359 (1808%) | 22 938 (5171%) | 19 865 (1727%) | 8 916 (4488%) | 24 494 (1880%) | 14 022 (5725%) | 1237% | 43 - 1329 |
| 60 to 69 | 58 258 (2375%) | 37 891 (6504%) | 25 934 (2255%) | 15 498 (5976%) | 32 324 (2481%) | 22 393 (6928%) | 952% | 74 - 1030 |
| 70 to 79 | 51 553 (2102%) | 38 165 (7403%) | 24 424 (2123%) | 17 186 (7037%) | 27 129 (2082%) | 20 979 (7733%) | 696% | 21 - 772 |
| 80 + | 36 397 (1484%) | 28 817 (7917%) | 19 773 (1719%) | 15 311 (7743%) | 16 624 (1276%) | 13 506 (8124%) | 381% | 98 - 464 |
| Total | 245 304 | 148 370 (6048%) | 115 018 (4689%) | 64 589 (5616%) | 130 286 (5311%) | 83 781 (6431%) | 815% | 76 - 854 |
| Use of non-invasive respiratory stratified by age group and wave | ||||||||
| 0 to 19 | 3 332 (101%) | 186 (558%) | 1 780 (123%) | 98 (551%) | 1 552 (083%) | 88 (567%) | 016% | 40 - 176 |
| 20 to 39 | 38 124 (1150%) | 3 394 (890%) | 15 923 (1105%) | 1 157 (727%) | 22 201 (1185%) | 2 237 (1008%) | 281% | 24 - 337 |
| 40 to 49 | 48 541 (1464%) | 6 037 (1244%) | 20 404 (1415%) | 2 126 (1042%) | 28 137 (1502%) | 3 911 (1390%) | 348% | 90 - 406 |
| 50 to 59 | 65 108 (1964%) | 11 696 (1796%) | 27 533 (1910%) | 4 444 (1614%) | 37 575 (2006%) | 7 252 (1930%) | 316% | 57 - 375 |
| 60 to 69 | 72 200 (2178%) | 20 875 (2891%) | 30 180 (2094%) | 8 170 (2707%) | 42 020 (2243%) | 12 705 (3024%) | 317% | 50 - 383 |
| 70 to 79 | 58 118 (1753%) | 23 710 (4080%) | 25 707 (1783%) | 10 049 (3909%) | 32 411 (1730%) | 13 661 (4215%) | 306% | 26 - 386 |
| 80 + | 46 083 (1390%) | 25 874 (5615%) | 22 626 (1570%) | 12 589 (5564%) | 23 457 (1252%) | 13 285 (5664%) | 100% | 09 - 190 |
| Total | 331 506 | 91 772 (2768%) | 144 153 (4348%) | 38 633 (2680%) | 187 353 (5652%) | 53 139 (2836%) | 156% | 26 - 187 |
| Use of invasive respiratory stratified by age group and wave | ||||||||
| 0 to 19 | 1 133 (082%) | 553 (4881%) | 660 (108%) | 306 (4636%) | 473 (062%) | 247 (5222%) | 586% | 04 - 1170 |
| 20 to 39 | 10 227 (743%) | 6 182 (6045%) | 4 244 (692%) | 2 288 (5391%) | 5 983 (784%) | 3 894 (6508%) | 1117% | 24 - 1309 |
| 40 to 49 | 14 394 (1046%) | 9 715 (6749%) | 5 845 (954%) | 3 598 (6156%) | 8 549 (1120%) | 6 117 (7155%) | 999% | 42 - 1157 |
| 50 to 59 | 24 226 (1760%) | 18 104 (7473%) | 10 002 (1632%) | 6 957 (6956%) | 14 224 (1863%) | 11 147 (7837%) | 881% | 69 - 994 |
| 60 to 69 | 35 197 (2557%) | 29 266 (8315%) | 14 988 (2445%) | 11 917 (7951%) | 20 209 (2647%) | 17 349 (8585%) | 634% | 53 - 714 |
| 70 to 79 | 31 998 (2325%) | 28 460 (8894%) | 14 643 (2389%) | 12 680 (8659%) | 17 355 (2273%) | 15 780 (9092%) | 433% | 63 - 503 |
| 80 + | 20 463 (1487%) | 18 999 (9285%) | 10 915 (1781%) | 10 036 (9195%) | 9 548 (1251%) | 8 963 (9387%) | 192% | 22 - 263 |
| Total | 137 638 | 111 279 (8085%) | 61 297 (4453%) | 47 782 (7795%) | 76 341 (5547%) | 63 497 (8318%) | 523% | 80 - 565 |
Note: We only consider data that have an informed outcome. Proportions are calculated based on complete records of ICU admission, use of non-invasive ventilation, and invasive mechanical ventilation. ICU = Intensive Care Unit
The overall mortality for cases that used non-invasive ventilation was 2768% (Table 3). Of the hospitalized patients, 137 638 (2029%) required invasive mechanical ventilation, with a mortality rate of 7795% and 8318% for the first and second waves, respectively. 158 389 (6582%) of ICU admissions occurred on the same day of hospitalization (Table 4). The overall mortality rate in the ICU during the pandemic was 6038%. After one day of hospitalization, 24 875 cases were admitted to the ICU. For patients who had a late admission to the ICU, more than eight days after hospitalization, the mortality rate was 7917%.
Table 4.
Mortality rate per days from hospitalization to admission to the ICU.
| Days until admission to the ICU | Whole pandemic |
First Wave |
Second wave |
Second wave vs first wave |
||||
|---|---|---|---|---|---|---|---|---|
| ICU admission | Deaths | ICU admission | Deaths | ICU admission | Deaths | Difference | 995% CI | |
| 0 | 158 389 (6582%) | 90 015 (5683%) | 77 759 (6856%) | 41 279 (5309%) | 80 630 (6339%) | 48 736 (6044%) | 735% | 687 - 784 |
| 1 | 24 875 (1034%) | 15 086 (6065%) | 11 860 (1046%) | 6 771 (5709%) | 13 015 (1023%) | 8 315 (6389%) | 680% | 558 - 801 |
| 2 | 14 836 (617%) | 9 711 (6546%) | 6 176 (545%) | 3 782 (6124%) | 8 660 (681%) | 5 929 (6846%) | 722% | 567 - 879 |
| 3 | 10 818 (450%) | 7 218 (6672%) | 4 417 (389%) | 2 737 (6197%) | 6 401 (503%) | 4 481 (7000%) | 803% | 622 - 986 |
| 4 | 7 788 (324%) | 5 271 (6768%) | 3 215 (283%) | 2 013 (6261%) | 4 573 (360%) | 3 258 (7124%) | 863% | 651 - 1076 |
| 5 | 5 620 (234%) | 3 933 (6998%) | 2 274 (200%) | 1 461 (6425%) | 3 346 (263%) | 2 472 (7388%) | 963% | 717 - 1210 |
| 6 | 4 037 (168%) | 2 901 (7186%) | 1 644 (145%) | 1 079 (6563%) | 2 393 (188%) | 1 822 (7614%) | 1051% | 765 - 1337 |
| 7 | 2 910 (121%) | 2 165 (7440%) | 1 125 (099%) | 784 (6969%) | 1 785 (140%) | 1 381 (7737%) | 768% | 439 - 1101 |
| 8 + | 11 355 (472%) | 8 989 (7916%) | 4 955 (437%) | 3 646 (7358%) | 6 400 (503%) | 5 343 (8348%) | 990% | 838 - 1143 |
| Total | 240 628 | 145 289 (6038%) | 113 425 (4714%) | 63 552 (5603%) | 127 203 (5286%) | 81 737 (6426%) | 823% | 784 - 862 |
Note: We only consider data that have an informed outcome. Only patients admitted to the ICU were considered. Days until admission to the ICU were calculated based on the date of hospitalization and admission to the ICU.
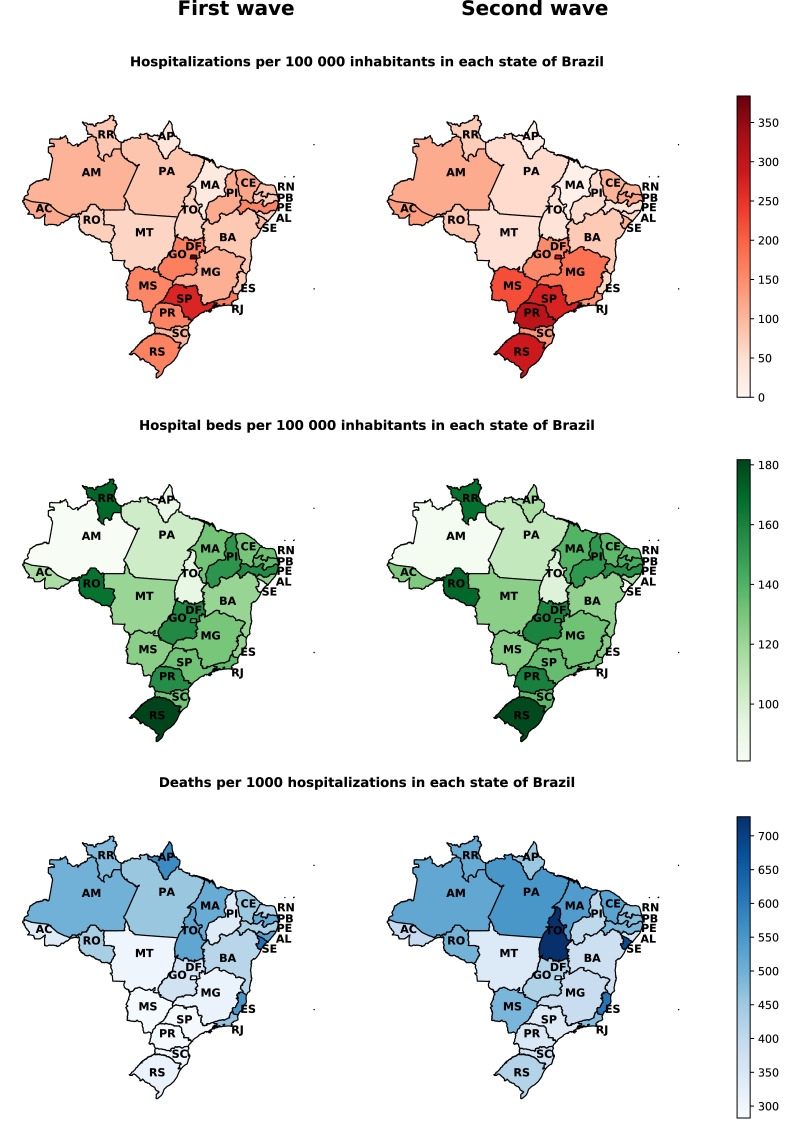
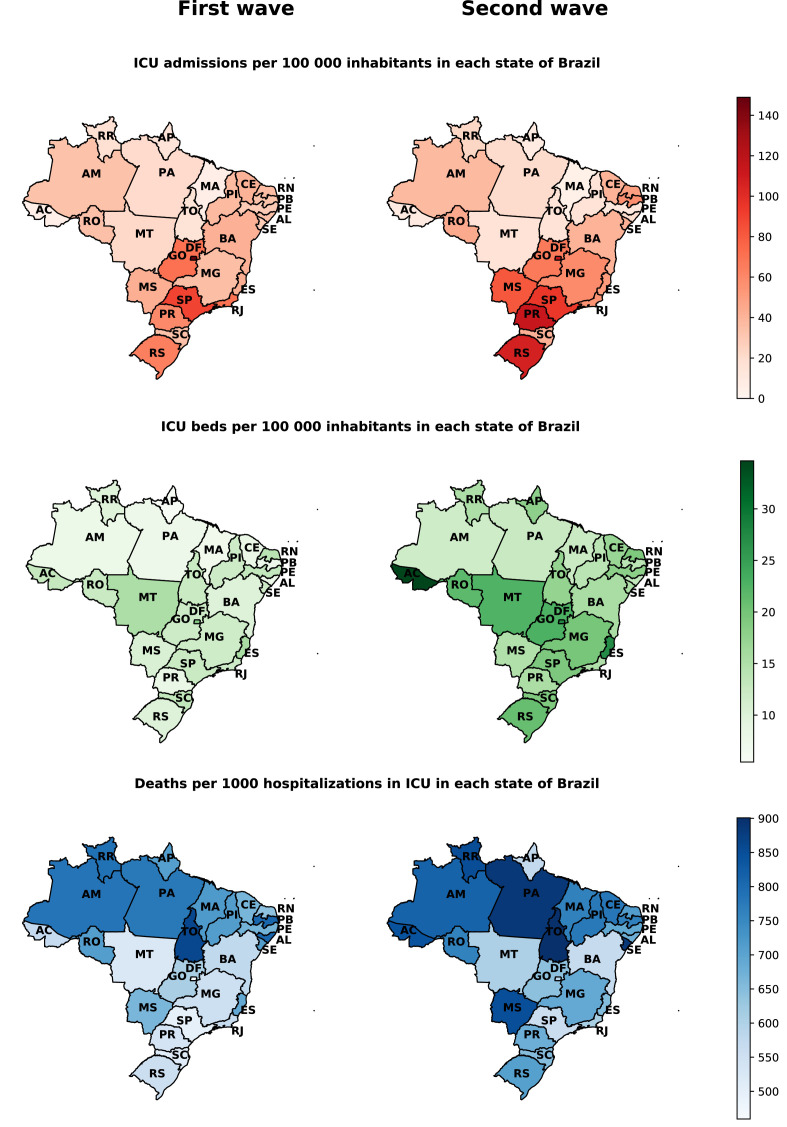
The highest admissions rates in hospital beds and ICUs per 100 000 inhabitants in Brazil during the first and second wave are mainly concentrated in the states of Distrito Federal (DF), São Paulo (SP), Paraná (PR), and Rio Grande do Sul (RS) (Figs. 3 and 4). The overall mortality rate in the first and second wave for every 1 000 hospitalizations is higher mainly in the states of the North and Northeast region of the country, with emphasis on the increase in the mortality rate in almost all states in the second wave (Fig. 3).
Figure 3.
Hospital admission rates, hospital beds per 100 000 inhabitants, and in-hospital mortality per 1 000 admissions due to COVID-19 in the first and second wave, stratified by the Brazilian state. Note: the population considered for each state is an official estimate from Brazilian government agencies. The number of hospital beds considered was based on data for the first wave of October 2020 and the second wave of April 2021. The number of hospital beds per state was obtained from TABNET. Hospitalizations and deaths were assigned to each state according to hospital location.
Figure 4.
ICU admission rates, ICU beds per 100 000 inhabitants, and in-ICU mortality per 1 000 admissions due to COVID-19 in the first and second wave, stratified by the Brazilian state. Note: the population considered for each state is an official estimate from Brazilian government agencies. The number of ICU beds considered was based on data for the first wave of October 2020 and the second wave of April 2021. The number of ICU beds per state was obtained from TABNET. ICU admissions and deaths were assigned to each state according to hospital location.
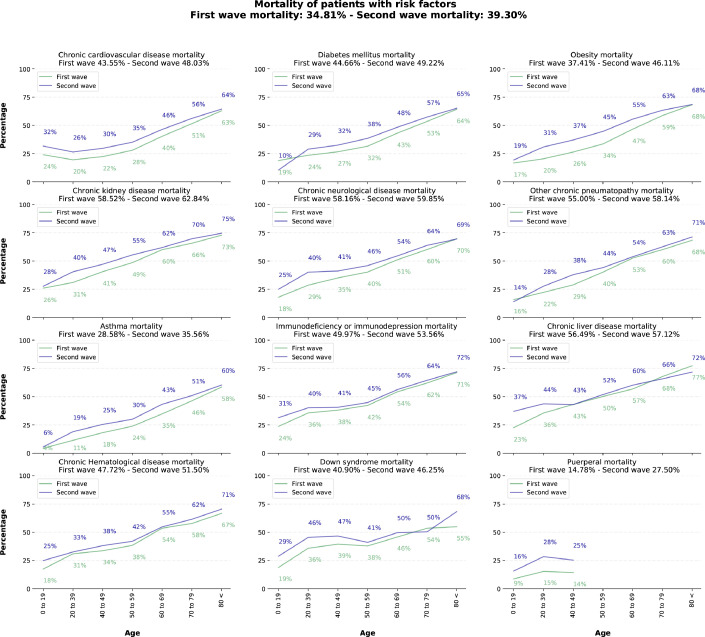
Analyzing the mortality rates between the groups with and without risk factors, we can observe a more considerable mortality rate among younger age groups, especially in the 20–39 age group (Appendix 1 (p 30)). In addition, there was an increase in the prevalence of mortality for all comorbidities evaluated in the second wave (Fig. 5). Cardiovascular disease was the most prevalent among the risk factors analyzed in this study. Furthermore, diabetes-related mortality increased from 4466% to 4922% from the first to the second wave. The most considerable differences in mortality for risk factors between the first and second wave were for puerperal women, with an increase of 1272%, and obese patients, with 870%.
Figure 5.
In-hospital mortality rate of patients with comorbidities in the first and second wave of the COVID-19 pandemic in Brazil. Note: We only considered patients with comorbidities informed at the time of admission. Death rates are calculated based on patients with reported outcomes. We only consider puerperal or parturient women up to 50 years of age.
According to the Table 1, COVID-19 presented symptoms were predominantly respiratory and constitutional, such as fever and fatigue. Oxygen saturation 95% is more frequent for elderly patients. In general, there was a relative predominance in percentage terms of constitutional, respiratory, neurological, and gastrointestinal symptoms among patients with 60 to 79 years. The increase in gastrointestinal, neurological symptoms and oxygen desaturation in the second wave is evident.
Discussion
We analyzed 678 235 patients hospitalized with SARI due to COVID-19 between February 25, 2020 and April 30, 2021. Data was taken from a nationwide public dataset. The second wave, in our study, corresponds to a shorter period compared to the first wave. However, there were more patients admitted to the hospital during the second wave. In the first wave, the highest number of hospitalizations occurred during the 27th epidemiological week, reaching 14 825 hospitalizations. Meanwhile, in early 2021, during the second wave, Brazil faced a substantial and rapid increase in hospital admissions, reaching a peak of 27 154 admissions during the 63rd epidemiological week, which corresponds to an increase of 8316% in the number of hospitalizations as compared to the first wave.
A meta-analysis of the 3 111 714 globally reported COVID-19 cases indicates that males have a higher chance of contracting the disease and having death as outcome.15 Numbers collected between January 1, 2020 until June 1, 2020, demonstrated that male patients have almost three times the odds of requiring ICU admission and higher odds of death compared to females.15 Our data also demonstrates a greater male demand for ICU beds in both waves. However, when we compare the mortality rates of patients admitted to ICUs, Brazilian women are slightly higher. In the second wave, when we analyze the use of invasive mechanical ventilation, we observed mortality rates reaching 8317% (Appendix 1 (pp 4–5)). These rates are extremely high in comparison to studies from other countries, which indicate a mortality between 4790% (patients up to 40 years old) and 7710% (71–80 years).16 For patients over 80 years old and who used invasive mechanical ventilation, the mortality rate (8440%) is similar to those described in another study.16
Differently from other races/ethnicities, during the second wave, the mortality among Indigenous people showed lower values (Table 2). This fact may have occurred because the vaccination of this group began in January 2021. A United Kingdom (UK) study noted a robust association between Non-White race and COVID-19 mortality.17 Despite the Black/Brown (Pardos) race being the predominant race in the Brazilian population,18 the data indicates that it represents 4265% of hospital admissions, while the White population represented 5587%. These numbers can be related to that, historically, the Brazilian Black/Brown (Pardos) population has disadvantages regarding housing conditions, income distribution, education, and also less access to health systems.19
Races/ethnicities and education are strong predictors of mortality, being jointly associated with the region of residence as social determinants for differences in access to health services in Brazil.12 In our study, 325% of all cases of hospitalized COVID-19 patients attended high school. However, the highest mortality rate is seen in the illiterate population (a mortality rate among illiterates of 5709% in the whole pandemic) followed by the population who attended elementary school (4951% of deaths), middle school (4193% of deaths), high school (3109% of deaths), and college (2621%). The pattern of mortality considering education remained the same in both waves, with mortality rates increasing inversely proportional to the level of education. Furthermore, the Brazilian death registration system shows an increase in the disparity of deaths in COVID-19 concerning the education level.20
The people from rural areas, despite representing a small portion of the studied data, have higher mortality rates, during the whole pandemic, than the urban population. This is probably because the regions furthest away from urban centers often do not have adequate health infrastructure and resources. Therefore, the delay and complications during the patient transfer to hospitals with better infrastructure may be related to higher mortality in the rural population. People who live on the peripheries of cities and those from rural areas, mostly with lower educational levels, with more considerable household crowding, and lower-income, also presented higher in-hospital mortality in other Brazilian studies.12, 20
Among the main variants detected in Brazil are P.1 and P.2. P.1, considered Variants of Concern (VOC), had its first case in the 40th epidemiological week in October 2020 (Fig. 2). P.2 was identified for the first time in April 2020 in the 16th epidemiological week (Fig. 2). The increase in the prevalence of P.1 and P.2 among sequenced variants coincides with the beginning of the second wave in Brazil. The association between the variants and the increase in cases and hospitalizations was evidenced in the city of Manaus, Amazonas (AM), where the P.1 variant was first reported.21 The P.2 variant, carrying the E484K mutation, being a variant derived from the B.1.1.28 lineage, was the most prevalent variant among the sequenced variants of patients who developed symptoms in the state of Rio de Janeiro.22 These strains have been spreading rapidly, and both P.2 and P.1 were recently found in documented cases of SARS-CoV-2 re-infection.23 Variants P.1 and P.2 accounted for approximately 75% of the variants sequenced in Brazil around the 55th epidemiological week. From the 60th epidemiological week onwards, the P.1 variant was predominant for the cases sequenced in Brazil.
Thus far, there is not much information about the transmissibility of the P.1 variant. However, it shares several acquired mutations from B.1.1.7 (Alpha), first identified in the UK and considered to be VOC, and which appears to be associated with increased transmissibility of SARS-CoV-2 in several countries.24 B.1.1.7 was also associated with a higher risk of hospitalization compared to other variants.25 In addition, B.1.1.7 showed an increase in severity for adults over 30.26
The prevalence of P.1 corresponds to the increase in hospitalizations for SARI in Brazil (Table 3). Comparing both waves, it was observed similar percentages of hospitalizations for all age groups. However, there were higher percentages of deaths among hospitalized patients at the second wave for several age groups, especially among 20 to 69 years old. The same happened when looking at ICU admissions—for the major age groups, there was a slight increase in percentages of patients admitted in ICU at the second wave, but a higher difference when looking at deaths. The increase in hospitalization and death rates in younger groups during the second period may be partially related to the vaccination campaign in Brazil, which at first prioritized the elderly age groups. Another hypothesis is related to the behavior of P.1 in younger age groups. According to a study conducted in eight European countries, considering P.1 cases was observed between 3.0 and 13.1 times higher odds of hospitalization in the age groups 20–39, 40–59, and 60–79, as well as a 2.9–13.9 times higher odds of ICU admission (40–59, 60–79, and 80 + age groups).27
These evidences suggest that the second wave was more severe and presented higher lethality rates in Brazil than the first one. In the second wave, a higher number of patients demanding ICUs admission, and invasive mechanical ventilation. These results are impressive, especially regarding the worsening outcomes seen for young age groups at the second wave. Previous studies have already described that the second wave around the world had affected a higher percentage of young people than the first one.28, 29 However, in contrast to what was seen in Brazil, most countries reported better outcomes for patients at the second wave. One study from Japan, for example, showed that severe respiratory conditions, the need for invasive mechanical ventilation, and mortality rates were higher during the first wave of the disease.28 In Spain, Germany, Italy, and Belgium, similar results were obtained.29, 30, 31 The discrepancies between Brazil and other countries are probably related to the lower preparation of the healthcare system to deal with the second wave and the inefficiency of the preventive social actions, such as social distancing and vaccination.11, 21 Another hypothesis for a more severe second wave in Brazil compared to other countries is the emergence and prevalence of P.1, which has higher rates of transmission and hospital admissions.27, 32
Discrepancies on COVID-19 hospitalizations and mortality between the two waves were also observed among Brazilian states, possibly due to social, political, and economic differences. When looking at state differences for hospitalizations, we can observe that the southern states, such as Rio Grande do Sul (RS), Santa Catarina (SC), and Paraná (PR) presented higher hospitalization rates per 100 000 inhabitants in the second wave (Fig. 3). Moreover, almost all states presented higher rates per 100 000 inhabitants and deaths per 1 000 in ICU admissions at the second wave. It is important to note, however, that the mortality rate for 1 000 ICU admissions is higher in northern states, which present lower Human Development Indexes and weakened health assistance quality.33 The Brazilian states considerably increased the number of hospital and ICU beds per 100 000 inhabitants from the first to the second wave. However, we can observe in the Figs. 3 and 4, especially in Northern Brazil, that despite the increase in hospital beds, the mortality rates in the second wave compared to the first wave were considerably superiors. In summary, the results described support the hypothesis that the increase in hospital and ICU beds alone is not enough to prevent deaths from COVID-19. As evidenced by other articles, joint efforts are needed to increase the capacity of beds with an adequate hospital structure with qualified professionals, medical supplies, supplemental oxygen, and the adoption of non-pharmacological measures to contain the advance of outbreaks of COVID-19.34, 35, 36, 37, 38
For Brazil, the regional differences observed during the COVID-19 pandemic could also be due to social and health inequities, local strategies regarding social distancing and preventive actions, quality and readiness of health services, availability of supplies and medical resources, different SARS-COV-2 variant prevalence, treatment strategies, as well as the availability of qualified human resources. These hypotheses are corroborated by a report of the UK Health Foundation.39 indicating that hospital capacity, especially beds, was the main determinant of differences in COVID-19 deaths across the world. The study further demonstrates that countries with higher bed capacity were more likely to lockdown earlier to reduce transmission and, hence, the burden on the health system.39
Another issue concerning COVID-19 prognosis is related to the waiting time for hospital and ICU admission. Table 4 shows that the percentage of deaths increases in all ages when the number of days until hospital and ICU admission increases. Saito et al. (2021) observed a lower mortality rate in Japan in their second wave even when stratifying data for age and severity of symptoms at admission, which they suggested could be due to the shorter time between disease onset and hospital admission at the second wave.28 In this sense, the efficiency of bed management is also an important factor when dealing with the pandemic.40 A retrospective observational cohort with patients hospitalized in Lombardy, Italy, observed an increase in mortality with the increase in the interval between the first symptoms and admission to the ICU, from six days onwards.41
Patients with comorbidities are usually associated with higher mortality among cases of COVID-19.42 Our data showed a high prevalence of comorbidities among patients who died and increased mortality between the first and second waves. The most prevalent comorbidity in our data was cardiovascular disease, followed by diabetes, which is similar to the findings of other studies.42, 43 We observed that individuals with kidney disease had considerably higher mortality, with rates reaching 6284% in the second wave. Survival analysis performed in Italian cohort studies showed an association between older age and decreased glomerular filtration rate with a higher risk of death.44
The difference between COVID-19 mortality in the population with or without risk factors during the whole pandemic is more considerable among the younger age groups (Appendix (p 30)). The difference between mortality percentages is inversely proportional to age and practically does not exist in the age group above 80. Specifically for diabetes, a decrease in the risk of mortality in adults has already been identified in Mexico as the patients’ age increases. The study also indicates that there is no association between mortality due to COVID-19 and diabetes in patients over 80 years old.45 Another point that can influence mortality for patients with comorbidities is the reduction in the continuous care of chronic diseases.46
Telemedicine was one of the ways found during the pandemic for the continuity of care for chronic patients.46 However, the population with lower income and scholarly, which also is the one most affected by comorbidities in Brazil.47also has less access to telemedicine. Thus, the association of comorbidities with less access to primary care may have contributed to higher mortality rates observed in lower-income or less-educated groups. Furthermore, a study in the southern region of Brazil during the pandemic showed an association of multimorbidity with a greater chance of impaired management of chronic diseases. The study also related a reduction in access to medication and the search for face-to-face care among low-income people.48
In January 2021, P.1 was the main COVID-19 variant identified in the Amazon region. It is important to highlight that there was an increase in mortality in the same period compared to April and May 2020 in this region, especially among women and between people with 20 to 59 years old.32 In this way, we can hypothesize that the differences found in mortality and the presented symptoms (gastrointestinal, neurological, and respiratory symptoms—including desaturation) from the first to the second wave were related to the variant P.1. In fact, variant P.1 can be 1.7 and 2.4 times more transmissible than Amazon non-P1 lineages. Also, the infections are 1.2 to 1.9 times more likely to result in mortality in the period after the emergence of P.1 than before, even considering that inferred cross-immunity and inadequate medical care access can considerably impact the estimates of this relative risk.32
The present study has some limitations, as described next. The data analyzed in this article are observational and comprise an arbitrary subset of all SARI cases due to COVID-19 hospitalized in Brazil during February 25, 2020, to April 30, 2021. Furthermore, our study was limited to describing the intragroup behavior of the pandemic using an analysis based on crude rates without age standardization. We believe that crude rates can help determine the specific burden and needs of services for certain populations. Although Brazil centralizes information on SARI cases and outcomes due to COVID-19, the lack of information on patient care limited our ability to provide details of patients’ evolution during treatment. There may be some losses in the notification of patients admitted by SARI, as the notification is performed manually by health professionals due to the lack of interoperability of hospital systems with the national notification system. Symptoms and comorbidities refer only to information collected and sent to SIVEP-Gripe by the hospitals. Furthermore, it is not possible to analyze the severity of the disease at the time of admission. Serological/clinical-epidemiological tests can be used to confirm the diagnosis of COVID-19 in Brazil. Therefore, mortality rates may be overestimated, as we only analyzed patients with positive RT-PCR for SARS-CoV-2. Finally, due to the deficiency in the genetic sequencing of confirmed cases of SARS-CoV-2, we cannot only attribute the increase in hospital burden to the new variants.
In conclusion, this study analyzes a Brazilian national dataset of COVID-19 hospitalized cases with SARI. We described the clinical and socio-demographic characteristics of patients hospitalized in Brazil stratified into the first and second waves. Despite efforts to increase the hospital structure during the pandemic, the second wave of COVID-19 in Brazil exhibited a higher in-hospital mortality rate in almost all ages, races/ethnicities, and social groups. We hope that our study can help to understand better a load of COVID-19 in the hospital system in Brazil and contribute to the definition of public policies. Finally, efforts are needed to improve the surveillance system for SARS-CoV-2 variants in Brazil, along with campaigns to encourage population vaccination.
Contributors
FAZ, BD, JNS, NTB, APA, MLRI, CAC, GOR, and APWCM participated in the design and concept of the study. FAZ, HCB, IGS, and LB acquired data. FAZ and BD did statistical analyses. FAZ, BD, JNS, NTB, APA, MLRI, and APWCM wrote the first version of the manuscript. CAC, and GOR supervised the study. CAC, GOR, RSA, RRR, SJR contributed to reviewing and editing the manuscript. All authors had full access to all data in the study, participated in data interpretation, revised the manuscript, and approved the final version of the manuscript for publication.
Data sharing
All de-identified data, including individual participant data, are publicly available. The data sources are described in the manuscript and in appendix (p 1).
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of Competing Interests
The authors declare no conflicts of interest.
Acknowledgments
The authors would like to thank the Coordinating Agency for Advanced Training of Graduate Personnel (CAPES) (C.F. 001), and National Council for Scientific and Technological Development (CNPq) (No. 309537/2020-7).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2021.100107
Supplementary materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
References
- 1.Burki T. COVID-19 in Latin America. The Lancet Infectious Diseases. 2020;20(5):547–548. doi: 10.1016/S1473-3099(20)30303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of COVID-19-studies needed. New England Journal of Medicine. 2020;382(13):1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 4.Taylor L. COVID-19: is Manaus the final nail in the coffin for natural herd immunity? BMJ. 2021;372 doi: 10.1136/bmj.n394. [DOI] [PubMed] [Google Scholar]
- 5.DATASUS, Ministry of Health. SRAG 2021 - Severe acute respiratory syndrome database - including data from COVID-19. 2021. https://opendatasus.saude.gov.br/dataset/bd-srag-2021(accessed on April 30, 2021).
- 6.Salyer S.J., Maeda J., Sembuche S., Kebede Y., Tshangela A., Moussif M., et al. The first and second waves of the COVID-19 pandemic in Africa: a cross-sectional study. The Lancet. 2021;397(10281):1265–1275. doi: 10.1016/S0140-6736(21)00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Global Challenges. 2017;1(1):33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Health. Vigilância genômica do vírus SARS-CoV-2 no âmbito da SVS/MS. 2021. http://bvsms.saude.gov.br/bvs/publicacoes/vigilancia_genomica_SARS-CoV-2_ambito_SVS.pdf(accessed on June 17, 2021).
- 9.Brazilian Institute of Geography and Statistics. Estimativas da população. 2021a. https://www.ibge.gov.br/estatisticas/sociais/populacao/9103-estimativas-de-populacao.html?=&t=downloads(accessed on April 30, 2021).
- 10.Datasus, Ministry of Health. Portal da saúde - SUS. 2021. http://www2.datasus.gov.br/DATASUS/index.php?area=0204id=11663(accessed on April 30, 2021).
- 11.Ranzani O.T., Bastos L.S., Gelli J.G.M., Marchesi J.F., Baião F., Hamacher S., et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. The Lancet Respiratory Medicine. 2021;9(4):407–418. doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peres I.T., Bastos L.d.S.L., Gelli J.G.M., Marchesi J.F., Dantas L.F., Antunes B., et al. Sociodemographic factors associated with COVID-19 in-hospital mortality in Brazil. Public Health. 2021;192:15–20. doi: 10.1016/j.puhe.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raimondi F., Novelli L., Ghirardi A., Russo F.M., Pellegrini D., Biza R., et al. Covid-19 and gender: lower rate but same mortality of severe disease in women-an observational study. BMC Pulmonary Medicine. 2021;21(1):1–11. doi: 10.1186/s12890-021-01455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO announces simple, easy-to-say labels for SARS-CoV-2 variants of interest and concern. 2021. http://www.emro.who.int/media/news/who-announces-simple-easy-to-say-labels-for-sars-cov-2-variants-of-interest-and-concern.html(accessed on June 24, 2021).
- 15.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nature Communications. 2020;11(1):1–10. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim Z.J., Subramaniam A., Reddy M.P., Blecher G., Kadam U., Afroz A., et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. a meta-analysis. American Journal of Respiratory and Critical Care medicine. 2021;203(1):54–66. doi: 10.1164/rccm.202006-2405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bray I., Gibson A., White J. Coronavirus disease 2019 mortality: a multivariate ecological analysis in relation to ethnicity, population density, obesity, deprivation and pollution. Public Health. 2020;185:261–263. doi: 10.1016/j.puhe.2020.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brazilian Institute of Geography and Statistics. Conheça o Brasil - População. 2021b. https://educa.ibge.gov.br/jovens/conheca-o-brasil/populacao/18319-cor-ou-raca.html(accessed on June 29, 2021).
- 19.Brazilian Institute of Geography and Statistics. Desigualdades sociais por cor ou raça no Brasil. 2021c. https://biblioteca.ibge.gov.br/visualizacao/livros/liv101681_informativo.pdf(accessed on June 29, 2021).
- 20.Ribeiro K.B., Ribeiro A.F., Veras M.A.d.S.M., de Castro M.C. Social inequalities and COVID-19 mortality in the city of São Paulo, Brazil. International Journal of Epidemiology. 2021 doi: 10.1093/ije/dyab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabino E.C., Buss L.F., Carvalho M.P., Prete C.A., Crispim M.A., Fraiji N.A., et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. The Lancet. 2021;397(10273):452–455. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voloch C.M., da Silva Francisco Jr R., de Almeida L.G., Cardoso C.C., Brustolini O.J., Gerber A.L., et al. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. Journal of Virology. 2021;95(10):e00119–21. doi: 10.1128/JVI.00119-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Resende P.C., Gräf T., Paixão A.C.D., Appolinario L., Lopes R.S., Mendonça A.C.d.F., et al. A potential SARS-CoV-2 variant of interest (VOI) harboring mutation E484K in the spike protein was identified within lineage B.1.1.33 circulating in Brazil. Viruses. 2021;13(5):724. doi: 10.3390/v13050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538) doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bager P., Wohlfahrt J., Fonager J., Rasmussen M., Albertsen M., Michaelsen T.Y., et al. Risk of hospitalisation associated with infection with SARS-CoV-2 lineage B.1.1.7 in Denmark: an observational cohort study. The Lancet Infectious Diseases. 2021 doi: 10.1016/S1473-3099(21)00290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyberg T., Twohig K.A., Harris R.J., Seaman S.R., Flannagan J., Allen H., et al. Risk of hospital admission for patients with SARS-CoV-2 variant B.1.1.7: cohort analysis. BMJ. 2021;373 doi: 10.1136/bmj.n1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funk T., Pharris A., Spiteri G., Bundle N., Melidou A., Carr M., et al. Characteristics of SARS-CoV-2 variants of concern B.1.1.7, B.1.351 or P.1: data from seven EU/EEA countries, weeks 38/2020 to 10/2021. Eurosurveillance. 2021;26(16):2100348. doi: 10.2807/1560-7917.ES.2021.26.16.2100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito S., Asai Y., Matsunaga N., Hayakawa K., Terada M., Ohtsu H., et al. First and second COVID-19 waves in Japan: comparison of disease severity and characteristics. The Journal of Infection. 2020 doi: 10.1016/j.jinf.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollinedo-Gajate I., Villar-Álvarez F., de los Ángeles Zambrano-Chacón M., Núñez-García L., de la Dueña-Muñoz L., López-Chang C., et al. First and second waves of coronavirus disease 2019 in Madrid, Spain: clinical characteristics and hematological risk factors associated with critical/fatal illness. Critical Care Explorations. 2021;3(2) doi: 10.1097/CCE.0000000000000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coccia M. The impact of first and second wave of the covid-19 pandemic in society: comparative analysis to support control measures to cope with negative effects of future infectious diseases. Environmental Research. 2021;197:111099. doi: 10.1016/j.envres.2021.111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soriano V., Ganado-Pinilla P., Sanchez-Santos M., Gómez-Gallego F., Barreiro P., de Mendoza C., et al. Main differences between the first and second waves of COVID-19 in Madrid, Spain. International Journal of Infectious Diseases. 2021;105:374–376. doi: 10.1016/j.ijid.2021.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faria N.R., Mellan T.A., Whittaker C., Claro I.M., Candido D.d.S., Mishra S., et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372(6544):815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Souza C.D.F., Machado M.F., do Carmo R.F. Human development, social vulnerability and COVID-19 in Brazil: a study of the social determinants of health. Infectious Diseases of Poverty. 2020;9(1):1–10. doi: 10.1186/s40249-020-00743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karagiannidis C., Windisch W., McAuley D.F., Welte T., Busse R. Major differences in ICU admissions during the first and second COVID-19 wave in Germany. The Lancet Respiratory Medicine. 2021;9(5):e47–e48. doi: 10.1016/S2213-2600(21)00101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahbazi F., Khazaei S. Socio-economic inequality in global incidence and mortality rates from coronavirus disease 2019: an ecological study. New Microbes and New Infections. 2020;38:100762. doi: 10.1016/j.nmni.2020.100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang L.-L., Tseng C.-H., Ho H.J., Wu C.-Y. Covid-19 mortality is negatively associated with test number and government effectiveness. Scientific Reports. 2020;10(1):1–7. doi: 10.1038/s41598-020-68862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sen-Crowe B., Sutherland M., McKenney M., Elkbuli A. A closer look into global hospital beds capacity and resource shortages during the COVID-19 pandemic. journal of Surgical Research. 2021;260:56–63. doi: 10.1016/j.jss.2020.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Z. Analysis of hospital resource availability and COVID-19 mortality across the United States. Journal of Hospital Medicine. 2021;16(4):211–214. doi: 10.12788/jhm.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocks S., Idriss O. Did hospital capacity affect mortality during the pandemics first wave. London: The Health Foundation. 2020 [Google Scholar]; https://www.health.org.uk/news-and-comment/charts-and-infographics/did-hospital-capacity-affect-mortality-during-the-pandemic(accessed on June 24, 2021)
- 40.Pecoraro F., Luzi D., Clemente F. The efficiency in the ordinary hospital bed management: a comparative analysis in four European countries before the COVID-19 outbreak. PLoS ONE. 2021;16(3):e0248867. doi: 10.1371/journal.pone.0248867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Internal Medicine. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izcovich A., Ragusa M.A., Tortosa F., Lavena Marzio M.A., Agnoletti C., Bengolea A., et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS ONE. 2020;15(11):e0241955. doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iftimie S., López-Azcona A.F., Vicente-Miralles M., Descarrega-Reina R., Hernández-Aguilera A., Riu F., et al. Risk factors associated with mortality in hospitalized patients with SARS-CoV-2 infection. a prospective, longitudinal, unicenter study in Reus, Spain. PLoS ONE. 2020;15(9):e0234452. doi: 10.1371/journal.pone.0234452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Castelnuovo A., Bonaccio M., Costanzo S., Gialluisi A., Antinori A., Berselli N., et al. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre italian CORIST study. Nutrition, Metabolism and Cardiovascular Diseases. 2020;30(11):1899–1913. doi: 10.1016/j.numecd.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woolcott O.O., Castilla-Bancayán J.P. The effect of age on the association between diabetes and mortality in adult patients with COVID-19 in Mexico. Scientific Reports. 2021;11(1):1–10. doi: 10.1038/s41598-021-88014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kendzerska T., Zhu D.T., Gershon A.S., Edwards J.D., Peixoto C., Robillard R., et al. The effects of the health system response to the COVID-19 pandemic on chronic disease management: a narrative review. Risk Management and Healthcare Policy. 2021;14:575. doi: 10.2147/RMHP.S293471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monteiro C., Macário E., Sardinha L., Gouvea E., Silva L., Oliveira M., et al. Ministry of Health; 2020. Vigitel Brasil 2019: Vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico : estimativas sobre frequência e distribuição sociodemográfica de fatores de risco e proteção para doenças crônicas nas capitais dos 26 estados brasileiros e no Distrito Federal em 2019. [Google Scholar]
- 48.Leite J.S., Feter N., Caputo E.L., Doring I.R., Cassuriaga J., Reichert F.F., Silva M.C.d., Rombaldi A.J. Managing noncommunicable diseases during the COVID-19 pandemic in brazil: findings from the PAMPA cohort. Ciência & Saúde Coletiva. 2021;26:987–1000. doi: 10.1590/1413-81232021263.39232020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/