Abstract
Purpose
Since non-adherence (NA) to intravitreal therapy with VEGF drugs is one of the most important modifiable factors compromising treatment outcome of nAMD, the purpose of this study was to investigate the contributing factors and barriers during long-term nAMD treatment.
Methods
Barriers and potential reasons for NA were prospectively measured using the Adherence Barriers Questionnaire Intravitreal Therapy (ABQ-IVT). A random sample of patients receiving intravitreal therapy was drawn based on data for different treatment periods. Three age-sex matched groups included the treatment periods of ≤30 months (group 1), between >30 months and ≤60 months (group 2), and >60 months (group 3). The occurrence of gaps between treatments and/or OCT visits was evaluated.
Results
NA with gaps of >56 days after the scheduled appointment was detected in 39%, 89%, and 100% of patients in group 1, 2, and 3, respectively (groups 1 and 2 vs group 3, p < 0.001). Two or more of such gaps were observed in 6%, 72%, and 94% of patients in group 1, 2, and 3, respectively. The overall ABQ-IVT score showed corresponding differences between the groups: 25.89 ± 7.68 (group 1, 95% CI 22.07–29.71), 34.72 ± 10.32 (group 2, 95% CI: 29.59–38.86), and 33.28 ± 9.04 (group 3, 95% CI 28.78–37.77). Accordingly, the score was inversely correlated with the number of regular follow-up visits in groups 2 and 3 (Pearson correlation coefficient r = −0.65 (p = 0.003) and r = −0.5 (p = 0.034), respectively). Within the groups of longer treatment duration, univariate logistic regression analysis showed higher odds of time commitment and challenge accompanying person to be relevant barriers.
Conclusion
NA is an arising problem with increasing duration of intravitreal therapy. Treatment barriers, detected by the ABQ-IVT, might change or increase during the course of the treatment.
Keywords: non-adherence, anti-VEGF, treatment barriers, age-related macular degeneration, ABQ-IVT
Introduction
Intensive therapy with repeated intravitreal injections (IVIs) and frequent follow-up examination is a challenge for individuals affected by neovascular age-related macular degeneration (nAMD) and the subsequent loss of vision.1 Although monthly injections of anti-vascular endothelial growth factor (VEGF) drugs showed favorable mean increases in visual acuity,2,3 the rigid schedule of monthly injections is a great burden not only for older patients but also for caregivers and physicians.4 Nevertheless, less frequent treatments might often be sufficient, either by dosing as needed (PRN) or by adjusting the re-treatment intervals, when considering disease activity.
However, even within controlled trials, switching from monthly treatment to a PRN regimen and missing study visits showed a less favorable visual outcome.5–7 Furthermore, a trend towards lower visual acuity was observed using the less intensive regimen (average of four injections) in the second year.8 In contrast to the randomized trials, the real-life studies have demonstrated that rare treatment is associated with significantly worse results.9,10 In the course of the disease, the treatment could be selectively focused on eyes with better visual acuity and higher lesion activity. Nevertheless, studies with long-term follow-up reported a further decline in the number of injections, followed or at least accompanied by further visual loss.11–14 Thus, a single or repeated delay of the treatment can have negative effects on the stability of visual function, while most of the current anti-VEGF preparations have effective levels of action of 4–6 weeks.15–17
Optimal outcomes of therapy for nAMD require not only efficacious treatment but also adherence to intravitreal therapy and visits, including optical coherence tomography (OCT) examinations. Patient adherence is defined as following the treatment plan on doctor’s advice after shared decision-making.18,19 Non-adherence (NA) is simultaneously influenced by more than one barrier, including socioeconomic factors, the health care team/system, disease, treatment and patient-related factors.20,21
Solving the issues related to each of these factors may have a strong influence on achieving better treatment outcomes. However, there is limited information on the frequency of these different barriers in dependence of the duration and course of the treatment. Therefore, the aim of this study was to evaluate these factors during long-term therapy.
Materials and Methods
Study Design
This cross-sectional study was initiated to prospectively assess the frequency of relevant treatment barriers. The random sample of patients was taken from the medical records of patients with nAMD with different treatment periods at a tertiary center (Center for Ophthalmology, University of Tübingen, Germany). This work adhered to the tenets of the Declaration of Helsinki. The study protocol with verbal informed consent using a telephone questionnaire was approved by the Institutional Ethics Committee of the University of Tübingen (101/2019BO2).
Inclusion/Exclusion Criteria
The inclusion criteria included treatment-naive patients with nAMD. They were treated with an upload of three monthly IVIs of anti-VEGF therapy (ranibizumab, aflibercept or bevacizumab). The criteria for retreatment included either a reduction in visual acuity or macular hemorrhage or changes measured using optical coherence tomography, such as a presence of subretinal fluid or pigment epithelial detachment, persistent or increased central retinal thickness, an increase in intraretinal cystoid macular edema. The interval of OCT examinations fluctuated between six and twelve weeks (as recommended by the German Ophthalmic Society and the German Retina Society).22 The exclusion criteria were eyes with any prior treatment for nAMD (including intravitreal pegaptanib sodium, laser photocoagulation, and verteporfin photodynamic therapy). No restrictions were placed on baseline visual acuity or lesion size.
Study Collective and Data Acquisition
The records of 60 patients who were treated between February 2009 and May 2020 were extracted. According to the date of the first injection, which was defined as the baseline date, the patients were assigned to three age-sex matched groups: group 1 with follow-up of ≤ 30 months; group 2 with follow-up between > 30 months and ≤ 60 months; and group 3 with follow-up of > 60 months. NA was defined as the occurrence of gaps between treatments and/or OCT visits of > 56 days after the scheduled appointment.23 Patients without any gaps were classified as adherent.
The following data were collected: best-corrected visual acuity (BCVA) measured with the use of the Snellen chart at the baseline visit and at each year of follow-up, as well as at the last visit; number of anti-VEGF injections and visits with OCT at each year of follow-up and at the last visit; the distance in kilometers between the home and treatment center (subgroup analysis compared patients with a distance of ≤ 25 km and > 25 km); disease activity defined as dry AMD (no signs of disease activity); switching of intravitreal therapy (use of one, two or three different anti-VEGF agents during the follow-up period); and number of non-persistent patients.
Adherence Barriers Questionnaire Intravitreal Therapy (ABQ-IVT)
Study subjects participated in a telephone questionnaire with the use of the Adherence Barriers Questionnaire Intravitreal Therapy (ABQ-IVT), which is a validated instrument for measurement of NA.24 It consists of 17 items formulated as statements (4-point Likert scale), with a total score from 17 to 58. The possible answers of the 4-point Likert scale were “strongly agree,” “generally agree,” “strongly disagree,” and “generally disagree,” which were scored from 1 to 4 depending of the formulation of each item. A higher score indicated a higher influence of barriers and, therefore, a higher level of NA. In the analysis of the ABQ-IVT, NA (barrier) was defined as a Morisky adherence score > 2.25 The ABQ-IVT was conducted in the German language by the first author (BS) independent of the care team and took approximately 10–15 minutes to complete.
Statistical Analysis
Descriptive statistics were used for group analysis. Data are presented as mean ± standard deviation (SD) or confidence interval (CI) for continuous variables and number of patients (n) and percentages for categorical variables. Data were compared using the analysis of variance with Bonferroni correction (for continuous variables that were normally distributed) or the Kruskal–Wallis test (for non-normally distributed variables) for independent samples (between groups) and the χ2 test for categorical variables, where appropriate. In addition, the Pearson (for continuous variables) or Spearman (for ordinal variables) correlation coefficient was used.
The data set was depersonalized for data collection and then anonymized for statistical analysis. For the purpose of statistical analysis, Snellen visual acuity was converted to logMAR visual acuity. The visual acuity of hand motion was converted to logMAR 2.3.26 The subgroup analysis included the change of VA in the study eye between the baseline and last follow-up visit classified as a gain of ≥ 3 lines, a change of 3 lines, or a loss of 3 lines, as well as the study eye better than the fellow eye. In case of treatment in both eyes, each eye was analyzed independently, and all results reported in this study were per eye.
Univariate logistic regression analysis was performed to assess the differences regarding significantly important barriers to intravitreal therapy between groups. A p value of less than 0.05 was considered as indicating a statistically significant difference. All statistical analyses were performed with commercial software (GNU PSPP version 0.10.2-g654fff).
Results
Group Characteristics
Baseline demographics, age, sex, and visual acuity of the study eye at baseline (Table 1) and at the end of follow-up did not differ with regard to the treatment duration (group assignment). Six patients could not be reached by phone, presumably because contact information was no longer current. However, since there was no refusal to participate in the small samples (20 per group), the patients, lost to follow-up (n=6) were distributed evenly among the three groups. In addition, the ratio of bilateral treatment, frequency of a change in medication and travel distance were not significantly different between groups (p > 0.1 for all comparisons). The subgroup analysis, including the proportion of eyes with a change in VA of the study eye (between baseline and the end of follow-up) showed a significant tendency regarding a greater loss of vision (≥ 3 lines) in group 2 when compared to group 1 only (13.04% in group 1 vs 37.04% in group 2, p = 0.071). The study eye better than the fellow eye showed a significant difference but only between groups 1 and 2 (p = 0.044). The mean follow-up was 26.78 ± 4.37 months (95% CI 24.60–28.95) in group 1, 41.17 ± 8.04 months (95% CI 37.17–45.16) in group 2, and 92.94 ± 26.43 months (95% CI 79.80–106.09) in group 3 (p < 0.001 between groups).
Table 1.
Description of Study Groups
| Group 1 | Group 2 | Group 3 | p value | |
|---|---|---|---|---|
| Agea, mean (SD), years | 77.44 (5.64) | 76.50 (7.64) | 73.50 (7.36) | 0.214 |
| Gender, female, No (%) | 9 (50%) | 9 (50%) | 9 (50%) | 1.000 |
| VA study eye baseline, [95% CI] | 0.55 [0.32–0.77] | 0.47 [0.29–0.66] | 0.69 [0.44–0.94] | 0.354 |
| VA study eye at the last follow-up visit [95% CI] | 0.60 [0.37–0.82] | 0.65 [0.45–0.85] | 0.69 [0.48–0.90] | 0.569 |
| VA fellow eye baseline | 0.15 [0.03–0.28] | 0.56 [−0.04–1.17] | 0.36 [−0.01–0.72] | 0.195 |
| Both eyes treated, No (%) | 5 (27.77%) | 9 (50%) | 6 (33.3%) | 0.356 |
| Study eye better than fellow eye, No (%) | 7 (38.88%) | 13 (72.22%) | 10 (55.55%) | 0.132 |
| Disease activity at last visit, No (%) | 1 (5.55%) | 3 (16.66%) | 10 (55.55%) | 0.289 |
| Number of visits after 24 months | 16.61 [15.25–17.97] | 14.00 [12.47–15.53] | 11.22 [9.55–12.89] | < 0.001 |
| Total number of visits | 19.61 [17.55–21.61] | 22.33 [17.22–27.45] | 42.94 [37.19–48.69] | < 0.001 |
| Number of IVIs after 24 months | 18.11 [16.45–19.77] | 15.12 [13.72–16.51] | 7.89 [5.89–9.88] | < 0.001 |
| Total number of IVIsb | 20.67 [19.35–21.99] | 22.00 [20.92–23.08] | 40.11 [32.21–48.01] | < 0.001 |
Notes: aAccording to the date of the first injection, bOnly study eye.
Abbreviations: CI, confidence interval; IVI, intravitreal injection; SD, standard deviation; VA, visual acuity.
The number of anti-VEGF injections and OCT visits during 24 months and the whole follow-up period were significantly different between all groups (p < 0.001) (Table 1). Bonferroni-adjusted post hoc analysis revealed significant differences between groups regarding the number of anti-VEGF injections and OCT visits during 24 months (p < 0.05 between groups 1 and 2, 1 and 3, and 2 and 3) and the total number of anti-VEGF injections and OCT visits (p < 0.001 between groups 1 vs. 3 and 2 vs. 3), except between groups 1 vs. 2, which was related to the total number of anti-VEGF injections and OCT visits (p = 1.000).
At the end of follow-up, there were no significant differences regarding disease activity between groups 1 and 2 (active nAMD in 17 and 15 patients, respectively, and inactive nAMD in one and three patients, respectively [p = 0.29]). In group 3, there were 10 patients with inactive nAMD and eight patients with active nAMD at the end of follow-up (p < 0.05 between groups 1 vs. 3, and 2 vs. 3).
The Occurrence of Gaps and Treatment Discontinuation
NA with gaps between treatments and/or follow-up visits of > 56 days after the scheduled appointment occurred in 11 (38.88%) patients in group 1 in comparison to 16 (88.88%) patients in group 2 and 18 (100%) patients in group 3, respectively (p < 0.001 between groups, without significant difference between groups 2 and 3). The median duration of treatment gaps was 0 days (mean ± SD: 45.56 ± 55.3 days) in group 1, 114 days (122.78 ± 77.86) in group 2, and 112 days (112.33 ± 41.84) in group 3 (p < 0.001 between all groups, without significant difference between groups 2 vs. 3). The frequency of gaps of > 56 days after the scheduled appointment was two or more gaps in 5.55% (n = 1) of patients in group 1, 72.22% (n = 13) of patients in group 2, and 94.44% (n = 17) of patients in group 3 (p < 0.001 between all groups, without significant difference between groups 2 and 3). The number of non-persistent patients was slightly different between the groups (group 1: none; group 2: eight; and group 3: three).
Response to the ABQ-IVT Questionnaire
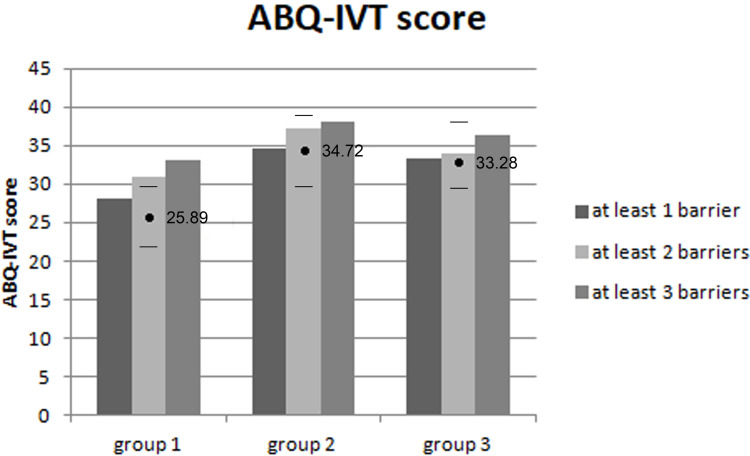
The overall score on the ABQ-IVT was significantly different between all groups (p = 0.011): 25.89 ± 7.68 (95% CI 22.07–29.71) in group 1, 34.72 ± 10.32 (95% CI 29.59–38.86) in group 2, and 33.28 ± 9.04 (95% CI 28.78–37.77) in group 3. Bonferroni-adjusted post hoc analysis revealed a significant difference between groups 1 and 2 (p = 0.016) and a significant tendency between groups 1 and 3 (p = 0.054), without a significant difference between groups 2 and 3 (p = 1.000) (Figure 1).
Figure 1.
The overall ABQ-IVT score differed significantly between groups (p = 0.011). The mean value ± CI for each group (group 1: ≤30 months, group 2: between >30 months and ≤60 months, group 3: >60 months).
The score was negatively associated with the number of follow-up visits in groups 2 and 3 (Pearson correlation coefficient r = −0.65 [p = 0.003] and r = −0.5 [p=0.034], respectively). In group 1, there was no association with the number of visits, but a positive correlation between the overall ABQ-IVT score and distance between home and treating center was observed (Pearson correlation coefficient r = 0.59 [p = 0.011]).
Identified Barriers to Intravitreal Therapy
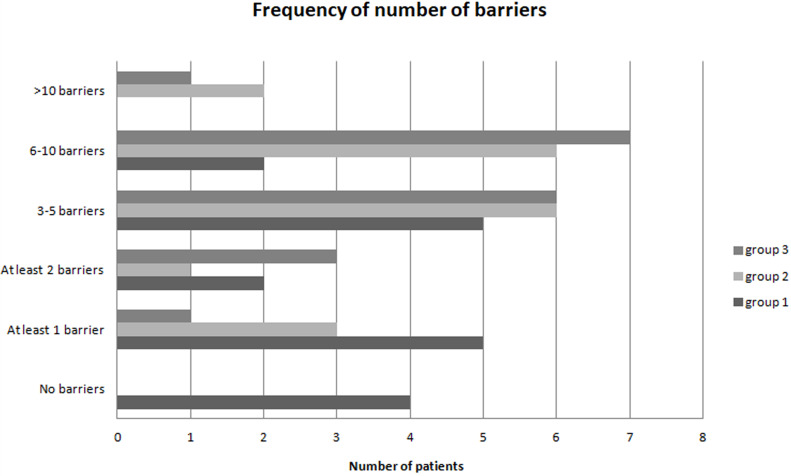
Each of the 17 barriers was rated as important by ≥ 5.55% of patients in group 1, except for three barriers: “lack of support,” “private/professional obligations” and “too old for therapy to be worthwhile.” In group 1, four (22.22%) patients reported having no barriers to adherence, five (27.77%) patients reported having one barrier to adherence, and nine (50%) patients reported having multiple barriers to adherence. In groups 2 and 3, all patients had barriers to adherence. Multiple barriers were reported in 15 (83.33%) patients in group 2 and in 17 (94.44%) patients in group 3, respectively (Figure 2).
Figure 2.
Frequency of number of barriers in three groups: group 1 with follow-up ≤ 30 months; group 2 with follow-up between > 30 months and ≤ 60 months; and group 3 with follow-up > 60 months.
Comparing Barriers Between Groups
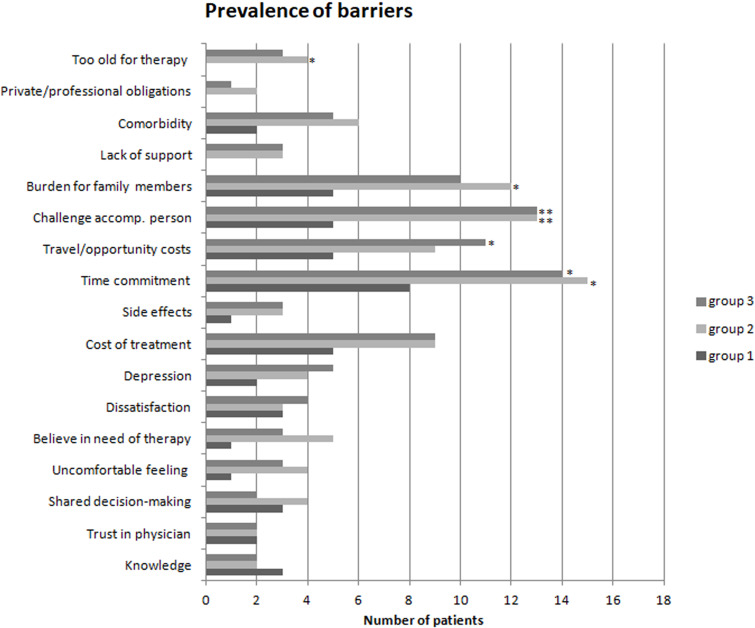
The first most important barrier was “time commitment” in eight (44.44%) patients in group 1, 15 (83.33%) patients in group 2, and 14 (77.77%) patients in group 3 (p < 0.05 between all groups, without significant difference between groups 2 and 3). The second most important barrier was “challenge accompanying person” in five (27.77%) patients in group 1 and in 13 (72.22%) patients both in groups 2 and 3 (p < 0.05 between all groups, without significant difference between groups 2 and 3). The third most important barrier was “burden for family members” in five (27.77%) patients in group 1, 12 (66.66%) patients in group 2, and 10 (55.55%) patients in group 3 (p = 0.056 between groups, without significant difference between groups 2 and 3). The prevalence of barriers is shown in Figure 3.
Figure 3.
Prevalence of barriers to intravitreal therapy in three groups: group 1 with follow-up ≤ 30 months; group 2 with follow-up between > 30 months and ≤ 60 months; and group 3 with follow-up > 60 months. The item score of > 2 was defined as a barrier. *p < 0.05, **p < 0.01.
Abbreviations: accomp, accompanying; oblig, obligations.
Univariate logistic regression analysis revealed that the following barriers were associated with higher odds of NA in groups 2 and 3, when compared to the patients with a shorter treatment history (group 1): “time commitment,” “challenge accompanying person” and “burden for family members” (Table 2). In addition, one barrier “travel/opportunity costs” differed significantly between groups 1 and 3 (p = 0.044) (Table 2).
Table 2.
Univariate Analysis for Barriers to Intravitreal Therapy in Groups 2 and 3 Compared to Group 1
| Barriers | Odds Ratio [95% CI] Group 2 | p value Group 2 | Odds Ratio [95% CI] Group 3 | p value Group 3 |
|---|---|---|---|---|
| Time commitment | 6.25 [1.33–29.43] | 0.020 | 4.37 [1.03–18.63] | 0.046 |
| Travel/opportunity costs | 2.60 [0.65–10.38] | 0.176 | 4.09 [1.01–16.58] | 0.049 |
| Challenge accompanying person | 6.76 [1.57–29.07] | 0.01 | 6.76 [1.57–29.07] | 0.01 |
| Burden for family members | 6.76 [1.57–29.07] | 0.01 | 3.25 [0.81–13.03] | 0.096 |
In the subgroup analysis of patients who discontinued treatment, the most frequent barrier was “challenge accompanying person” in 100% of patients (8 of 8 patients in group 2 and 3 of 3 patients in group 3). The second most frequent barrier was “burden for family members” (7 of 8 patients in group 2 and 3 of 3 patients in group 3). The following patient narratives were reported: “My brother/daughter/husband always brought me to the hospital.”
“Due to my general condition, I came by patient transport service only.”
“I have to rely on my husband, I have nobody else.”
Discussion
Intravitreal therapy cannot work if you do not receive them and appear at follow-up visits. According to the World Health Organization (WHO), only 50% of patients with chronic diseases adhere to treatment recommendations in developed countries.19,27,28 Historically, the research in ophthalmology was mainly based on NA to glaucoma medication.29–31 The invasive character of anti-VEGF treatment and necessity for frequent visits are also related to NA and subsequently worse visual outcomes.10 Recently, a group of experts succeeded in formulating meaningful proposals for a definition of NA.32 However, the extent of adherence to intravitreal therapy and their relevant key drivers are still not fully understood or at least inadequately captured within prospective studies.20,21 Anti-VEGF treatment should be “respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions”.33
In this cross-sectional study, 54 patients with nAMD treated with a different duration of intravitreal anti-VEGF therapy consisting of three age/sex-matched groups were characterized (≤ 30 months; > 30 months and ≤ 60 months; and > 60 months). While the group with the shortest treatment history showed the best adherence (only 39% NA in comparison to 89 and 100%), the duration and frequency of NA were significantly different between group 1 and the two other groups (p < 0.001). The number of non-persistent patients was greater in group 2 as compared to that in group 3. In other studies, treatment discontinuation was reported between 32 and 50% in patients with nAMD treated with anti-VEGF agents.34,35 In addition, the number of NA patients in group 1 is in accordance with the results of Ehlken et al, who used the same definition of unintended gaps (> 56 days between treatments/follow-ups).23 Similarly, 39% of patients with nAMD did not comply with anti-VEGF treatment and follow-up for 1 year in a study from Polat et al.36 In other studies, a slightly lower percentage (about 25%) of NA in patients with nAMD was shown.37–39 However, one- third of patients with at least one missed hospital appointment (MHA) was even recorded in the 1-year findings from the IVAN randomized trial.40 Furthermore, up to 95% of patients with nAMD were defined as non-adherent (no treatment or follow-up for at least 6 weeks) after analysis up to 24 months retrospectively and 12 months prospectively in 23 treatment centers in Germany. These data are comparable to our results in groups 2 and 3 with a follow-up longer than 30 months.41
In a 5-year study of adherence to ranibizumab treatment for nAMD, Boulanger-Scemama et al identified three major predictors using a 7-item questionnaire by phone or email in 58 patients: the long distance between home and hospital, dissatisfaction with the results of intravitreal therapy and the burden of regular follow-up visits.42 In other studies, patients with nAMD who had a long journey distance discontinued anti-VEGF treatment significantly more frequently than those who lived near the clinic, especially within 100 km.43–47 Besides travel costs, dependence on relatives, higher age and poor visual acuity at baseline were associated with higher risk for NA.23,36,43,48
In this study, the ABQ-IVT was used as a method for measurement of adherence and identification of possible barriers. The ABQ-IVT score was significantly lower in patients with follow-up of < 30 months in comparison to groups with a longer follow-up period. Multiple barriers were indicated by 83% of patients in group 2 and by 94% of patients in group 3. However, only 50% of patients reported having multiple barriers in group 1 without correlation between the ABQ-IVT score and number of OCT visits. Furthermore, the ABQ-IVT score negatively correlated with the number of OCT visits both in groups 2 and 3. These two groups were characterized by two barriers “challenge accompanying person” and “time commitment” with significantly higher odds ratios in comparison to the first group with the shortest follow-up. In addition, there was a significant tendency for “burden for family members” in group 2 and for “travel/opportunity costs” in group 3. The burden placed on family and time burden were also factors affecting patient adherence in a study by Boyle et al.43 Many patients are dependent on relatives, who often have to take time off for the visits. Mobility or needing help to keep follow-up visits was one of the major impediments to adherence in a 5-year real-world study by Boulanger-Scemama et al.42 Moreover, higher rates of NA were reported by caretakers compared to patients with nAMD treated with anti-VEGF.39 Other factors affecting adherence were long distance from home to hospital, poor baseline visual acuity, higher age, fear of injections, and serious comorbidities.23,36,43,48,49 In our analysis, only in group 1 with the shortest follow-up, patients with a distance of > 25 km between home and hospital had more barriers than patients with a distance of ≤ 25 km. Moreover, patients in group 2 had a significantly better visual acuity in the study eye as compared to that in the fellow eye, and there was a distinct trend toward worse visual outcomes compared to patients in group 1, which is indicative of a higher risk of NA.23,50,51 No correlation between higher age or comorbidity or fear of anti-VEGF injections and NA were observed. Currently, only higher age was a frequent factor associated with NA.21 However, Oishi et al reported a higher risk for NA in otherwise younger Japanese patients (OR 0.94, 95% CI 0.89–0.99).37 The evidence for an association of comorbidity with NA was inconsistent, and discomfort of anti-VEGF injections has not been proven to be a relevant risk factor in the systemic review from Ehlken et al.21 Moreover, McGrath et al even showed a higher rate of NA in healthy Australian patients.44
This study has several major limitations. Overall, the sample is too small to make reliable statements, representative of larger cohorts. Apart from a possible selection bias, it has to be considered that NA might be influenced by the re-treatment scheme used. There is a reasonable basis to believe that the now more commonly used “Treat and Extend” regimen is not only associated with a low number of visits but may achieve better adherence.
Derivations from real-life data are usually limited by administering injection numbers that are (too) low.10,23 However, even in group 3 with the longest follow-up period, the number of IVIs and OCT exams per year were higher than in most of the previous non-interventional studies.9,23,52 Learning curves of the tertiary center in recent years or a change in philosophy regarding re-treatment cannot be completely ruled out as a possible influence on the reported barriers. However, it is unlikely that the differences between the groups are due to this development alone, especially since major changes had already taken place before 2015.The ABQ-IVT was administered by phone call interview; therefore, recall bias cannot be entirely ruled out. Nevertheless, the strength to this study is the analysis of adherence using the ABQ-IVT with a wide range of barriers among patients with three different follow-up periods.
In conclusion, this study showed that the duration and frequency of NA might differ between patients with shorter and longer duration of nAMD treatment. Considering potential confounding factors of a longer treatment history, future research should focus on important barriers, such as the need of accompanying persons and time commitment in order to develop and test potential countermeasures. The likelihood of NA seems to correlate with the number of barriers. Interventions optimizing adherence should be focused on training and support programs. The ABQ-IVT is a good instrument that provides better insight into the treatment burden independently of physicians and treatment events. Future research is warranted to improve intravitreal treatment adherence. Even when agents with potentially longer duration or drug delivery devices are available, it still will remain important to correctly assess NA in patients.
Acknowledgment
We thank Nicolai Knaupp for graphic assistance.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
None of the authors has a financial or property interest in any material or method mentioned in this manuscript. B. Sobolewska has received a travel grant from Galderma, Novartis, and Santen; and F. Ziemssen has received consulting fees from Alimera, Allergan, Bayer HealthCare, Boehringer-Ingelheim, Novartis, Oxurion and Roche/Genentech, as well as speaker fees from Allergan/AbbVie, Bayer HealthCare, Novartis and Roche. The authors report no other conflicts of interest in this work.
References
- 1.Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila). 2017;6(6):493–494. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Kaiser PK, for ANCHOR Study Group; et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655 [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld PJ, Brown DM; for MARINA Study Group, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 4.Spooner KL, Mhlanga CT, Hong TH, Broadhead GK, Chang AA. The burden of neovascular age-related macular degeneration: a patient’s perspective. Clin Ophthalmol. 2018;12:2483–2491. doi: 10.2147/OPTH.S185052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maguire MG, Martin DF, Ying GS, et al.; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. 5-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(8):1751–1761. doi: 10.1016/j.ophtha.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramakrishnan MS, Yu Y, VanderBeek BL. Association of visit adherence and visual acuity in patients with neovascular age-related macular degeneration: secondary analysis of the comparison of age-related macular degeneration treatment trial. JAMA Ophthalmol. 2020;138(3):237–242. doi: 10.1001/jamaophthalmol.2019.4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201. doi: 10.1016/j.ophtha.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 9.Ziemssen F, Eter N, Fauser S, et al. Retrospektive Untersuchung der Anti-VEGF-Behandlungsrealität und Wirksamkeit bei Patienten mit neovaskulärer altersabhängiger Makuladegeneration (nAMD) in Deutschland: behandlungsrealität von Ranibizumab bei nAMD in Deutschland. [Retrospective investigation of anti-VEGF treatment reality and effectiveness in patients with neovascular age-related macular degeneration (AMD) in Germany: treatment reality of ranibizumab for neovascular AMD in Germany]. Ophthalmologe. 2015;112(3):246–254. German. [DOI] [PubMed] [Google Scholar]
- 10.Holz FG, Tadayoni R, Beatty S, et al. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol. 2016;100(12):1623–1628. doi: 10.1136/bjophthalmol-2015-308166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horner F, Lip PL, Clark H, Chavan R, Sarmad A, Mushtaq B. Real-world visual and clinical outcomes for patients with neovascular age-related macular degeneration treated with intravitreal ranibizumab: an 8-year observational cohort (AMD8). Clin Ophthalmol. 2019;13:2461–2467. doi: 10.2147/OPTH.S218378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K; for SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120(11):2292–2299. doi: 10.1016/j.ophtha.2013.03.046 [DOI] [PubMed] [Google Scholar]
- 13.Gillies M, Arnold J, Bhandari S, et al. Ten-year treatment outcomes of neovascular age-related macular degeneration from two regions. Am J Ophthalmol. 2020;210:116–124. doi: 10.1016/j.ajo.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 14.Jacob J, Brié H, Leys A, et al. Six-year outcomes in neovascular age-related macular degeneration with ranibizumab. Int J Ophthalmol. 2017;10(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yalamanchili SP, Maatouk CM, Enwere DU, et al. The short-term effect of a single lapse in anti-vascular endothelial growth factor treatment for diabetic macular edema within routine clinical practice. Am J Ophthalmol. 2020;219:215–221. doi: 10.1016/j.ajo.2020.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong Teo KY, Saxena N, Gan A, et al. Detrimental effect of delayed re-treatment of active disease on outcomes in neovascular age-related macular degeneration: the RAMPS study. Ophthalmol Retina. 2020;4(9):871–880. doi: 10.1016/j.oret.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 17.Hutton-Smith LA, Gaffney EA, Byrne HM, Caruso A, Maini PK, Mazer NA. Theoretical insights into the retinal dynamics of vascular endothelial growth factor in patients treated with ranibizumab, based on an ocular pharmacokinetic/pharmacodynamic model. Mol Pharm. 2018;15(7):2770–2784. doi: 10.1021/acs.molpharmaceut.8b00280 [DOI] [PubMed] [Google Scholar]
- 18.DeBusk RF, Miller NH, Superko HR, et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120(9):721–729. doi: 10.7326/0003-4819-120-9-199405010-00001 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO). Adherence to Long-Term Therapy: Evidence for Action. Geneva: WHO; 2003. [Google Scholar]
- 20.Okada M, Mitchell P, Finger RP, et al. Non-adherence or non-persistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2021;128(2):234–247. doi: 10.1016/j.ophtha.2020.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehlken C, Ziemssen F, Eter N, et al. Systematic review: non-adherence and non-persistence in intravitreal treatment. Graefes Arch Clin Exp Ophthalmol. 2020;258(10):2077–2090. doi: 10.1007/s00417-020-04798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deutsche Ophthalmologische Gesellschaft (DOG); Retinologische Gesellschaft e.V. (RG); Berufsverband der Augenärzte Deutschlands e.V. (BVA). Therapie bei der neovaskulären altersabhängigen Makuladegenertion: Stand Feburar 2020 [Statement of the German Ophthalmological Society (DOG), the German Retina Society (GRS), and the Professional Association of German Ophthalmologists (BVA) on anti-VEGF treatment in neovascular age-related macular degeneration: status February 2020]. Ophthalmologe. 2021;118(Suppl1):31–39. German. doi: 10.1007/s00347-020-01188-1 [DOI] [PubMed] [Google Scholar]
- 23.Ehlken C, Helms M, Boehringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2018;12:13–20. doi: 10.2147/OPTH.S151611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller S, Junker S, Wilke T, et al. Questionnaire for the assessment of adherence barriers of intravitreal therapy: the ABQ-IVT. Int J Retina Vitreous. 2021;7(1):43. doi: 10.1186/s40942-021-00311-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247(1):137–142. doi: 10.1007/s00417-008-0926-0 [DOI] [PubMed] [Google Scholar]
- 27.Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2002;2:CD000011. [DOI] [PubMed] [Google Scholar]
- 28.Sackett DL, Haynes RB, Gibson ES, Taylor DW, Roberts RS, Johnson AL. Patient compliance with antihypertensive regimens. Patient Couns Health Educ. 1978;1(1):18–21. doi: 10.1016/S0738-3991(78)80033-0 [DOI] [PubMed] [Google Scholar]
- 29.Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308–1316. doi: 10.1016/j.ophtha.2015.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClelland JF, Bodle L, Little JA. Investigation of medication adherence and reasons for poor adherence in patients on long-term glaucoma treatment regimes. Patient Prefer Adherence. 2019;13:431–439. doi: 10.2147/PPA.S176412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118(12):2398–2402. doi: 10.1016/j.ophtha.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada M, Wong TY, Mitchell P, et al. Defining nonadherence and nonpersistence to anti–vascular endothelial growth factor therapies in neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139(7):769–776. doi: 10.1001/jamaophthalmol.2021.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vennedey V, Hower KI, Hillen H, Ansmann L, Kuntz L, Stock S; For the Cologne Research and Development Network (CoRe-Net). Patients’ perspectives of facilitators and barriers to patient-centred care: insights from qualitative patient interviews. BMJ Open. 2020;10(5):e033449. doi: 10.1136/bmjopen-2019-033449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subhi Y, Sørensen TL. Neovascular age-related macular degeneration in the very old (≥90 years): epidemiology, adherence to treatment, and comparison of efficacy. J Ophthalmol. 2017;2017:7194927. doi: 10.1155/2017/7194927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroeder M, Westborg I, Adrian ML. Twelve percent of 6142 eyes treated for neovascular age‐related macular degeneration (nAMD) presented with low visual outcome within 2 years. Analysis from the Swedish Macula Registry (SMR). Acta Ophthalmol. 2020;98(3):274–278. doi: 10.1111/aos.14239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polat O, Inan S, Özcan S, et al. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in patients with age-related macular degeneration. Turk J Ophthalmol. 2017;47(4):205–210. doi: 10.4274/tjo.28003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oishi A, Mandai M, Nishida A, Hata M, Matsuki T, Kurimoto Y. Remission and dropout rate of anti-VEGF therapy for age-related macular degeneration. Eur J Ophthalmol. 2011;21(6):777–782. doi: 10.5301/EJO.2011.7430 [DOI] [PubMed] [Google Scholar]
- 38.Karampelas M, Pefkianaki M, Rees A, et al. Missed hospital appointments of patients receiving ranibizumab therapy for neovascular age-related macular degeneration. Ophthalmol Ther. 2015;4(1):43–49. doi: 10.1007/s40123-015-0031-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varano M, Eter N, Winyard S, et al. Current barriers to treatment for wet age- related macular degeneration (wAMD): findings from the wAMD patient and caregiver survey. Clin Ophthalmol. 2015;9:2243–2250. doi: 10.2147/OPTH.S92548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Study Investigators IVAN, Chakravarthy U, Harding SP, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–1411. doi: 10.1016/j.ophtha.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 41.Ehlken C, Wilke T, Bauer-Steinhusen U, Agostini HT, Hasanbasic Z, Müller S. Treatment of neovascular age-related macular degeneration patients with vascular endothelial growth factor inhibitors in everyday practice: identification of health care constraints in Germany-the PONS Study. Retina. 2018;38(6):1134–1144. doi: 10.1097/IAE.0000000000001681 [DOI] [PubMed] [Google Scholar]
- 42.Boulanger-Scemama E, Querques G, About F, et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol. 2015;38(7):620–627. doi: 10.1016/j.jfo.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 43.Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C, Rees G. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med. 2018;23(2):127–140. doi: 10.1080/13548506.2016.1274040 [DOI] [PubMed] [Google Scholar]
- 44.McGrath LA, Lee LR. Characteristics of patients who drop out from ranibizumab therapy. Asia Pac J Ophthalmol (Phila). 2013;2(5):295–299. doi: 10.1097/APO.0b013e31829dc65a [DOI] [PubMed] [Google Scholar]
- 45.Heimes B, Gunnemann F, Ziegler M, et al. Compliance of age related macular degeneration patients undergoing anti-VEGF therapy: analysis and suggestions for improvement. Ophthalmologe. 2016;113(11):925–932. doi: 10.1007/s00347-016-0275-z [DOI] [PubMed] [Google Scholar]
- 46.Chang A, Stokes J, Priestman L, Holmes C, Said P. Impact of a patient support program on patient beliefs about neovascular age-related macular degeneration and persistence to anti-vascular endothelial growth factor therapy. Patient Prefer Adherence. 2021;15:511–521. doi: 10.2147/PPA.S293941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiam M, Kunselman AR, Chen MC. Characteristics associated with new patient appointment no-shows at an academic ophthalmology department in the United States. Am J Ophthalmol. 2021;229:210–219. doi: 10.1016/j.ajo.2021.02.020 [DOI] [PubMed] [Google Scholar]
- 48.Westborg I, Rosso A. Risk factors for discontinuation of treatment for neovascular age-related macular degeneration. Ophthalmic Epidemiol. 2018;25(2):176–182. doi: 10.1080/09286586.2017.1397701 [DOI] [PubMed] [Google Scholar]
- 49.McClard CK, Wang R, Windham V, et al. Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII): development of a patient-reported measure to assess treatment burden of repeat intravitreal injections. BMJ Open Ophthalmol. 2021;6(1):e000669. doi: 10.1136/bmjophth-2020-000669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abu-Yaghi NE, Abed AM, Khlaifat DF, et al. Factors affecting compliance to anti-vascular endothelial growth factor treatment of diabetic macular edema in a cohort of Jordanian patients. Clin Ophthalmol. 2020;14:921–929. doi: 10.2147/OPTH.S248661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fajnkuchen F, Delyfer MN, Conrath J, Baillif S, Mrejen S, Srour M. Expectations and fears of patients with diabetes and macular edema treated by intravitreal injections. Acta Diabetol. 2020;57(9):1081–1091. doi: 10.1007/s00592-020-01513-9 [DOI] [PubMed] [Google Scholar]
- 52.Wachtlin J, Spital G, et al.; Ocean Study Group. Use of imaging modalities in real life: impact on visual acuity outcomes of ranibizumab treatment for neovascular age-related macular degeneration in Germany. J Ophthalmol. 2020;2020:8024258. doi: 10.1155/2020/8024258 [DOI] [PMC free article] [PubMed] [Google Scholar]