Abstract
Purpose
To compare the refractive predictability of intraoperative aberrometry (IA, ORA, Alcon) and Barrett True-K/Universal II formulas for intraocular lens (IOL) power calculations in post-corneal refractive surgery and normal eyes.
Methods
Retrospective study of normal and post-corneal refractive surgery eyes that underwent cataract surgery with IA at tertiary academic center. Preoperatively, IOL power calculations were performed using Barrett Universal II (normal eyes) or Barrett True-K (post-corneal refractive surgery eyes) formulas. Intraoperatively, aphakic IA measurements were used for IOL power calculations. Mean absolute refractive prediction error (MAE) and the percentage of eyes with prediction error within ±0.50, ±0.75 and ±1.00 D were calculated. Refractive predictability was also evaluated in short, normal, and long eyes.
Results
Two hundred and seventy-three eyes were included in the analysis. No statistically significant differences were observed between the MAE of preoperative formulas and IA for post-hyperopic laser vision correction (LVC), post-myopic LVC, post-radial keratotomy (RK) and normal eyes. For prediction error within ±0.5 D in post-corneal refractive surgery eyes, range of agreement between Barrett True-K and IA ranged from 28% (7/25) of the time in post-RK eyes to 49% (40/81) of the time in post-hyperopic LVC; the corresponding value for Barrett Universal II/IA was 62% (64/103) in normal eyes. When there was disagreement, IA outperformed Barrett True-K in post-hyperopic LVC eyes and Barrett formula outperformed IA in post-myopic LVC, post-RK, and normal eyes.
Conclusion
IA appears to be comparable to Barrett formulas for IOL power calculations in post-corneal refractive surgery and normal eyes. In post-hyperopic LVC, IA yields better results compared to Barrett True-K formula; in real-life scenarios, IA reveals statistical advantage over the Barrett True-K no history formula for eyes post-hyperopic LVC.
Keywords: intraoperative aberrometry, eyes post refractive surgery, refractive outcomes, Barrett True-K formula
Introduction
Despite improvements in intraocular lens (IOL) formulas and intraoperative diagnostics, achieving the targeted refraction postoperatively remains a challenge.1 Previous studies have reported that 55–75% of patients without any history of ocular surgery achieve refractive outcomes within ±0.50 D of their intended target.2–4 In post-refractive surgery eyes, the percentage of patients achieving those results range from 40% to 69%.5–8
Over the past decade, several formulas were developed to improve the precision of IOL power calculation in post-corneal refractive surgery eyes, including the Haigis-L,9 Shammas,10 Masket,11 and Barrett True-K formulas.12 Several studies have reported on the superiority of the Barrett formulas (Barrett Universal II and Barrett True-K) compared to other preoperative calculation methods in predicting IOL power in eyes with or without previous refractive surgery.13–16
Intraoperative aberrometry (IA) was designed to increase the accuracy of IOL power calculation and to reduce residual refractive error after cataract surgery. The ORA System with VerifEye+ (Alcon Surgical Inc.) can calculate IOL power based on aphakic spherical equivalent, preoperative axial length (AL) and corneal power.6 Although results are comparable in normal eyes,17 it has been shown to improve outcomes in long18 and short19 eyes with no prior refractive surgery history. Previous reports analyzing the refractive prediction accuracy of IA in previous myopic laser vision correction (LVC)6,20 and radial keratotomy (RK)8,16 eyes have also reported good outcomes, however, outcomes in eyes with previous hyperopic LVC need to be assessed.
The present study aims to compare the refractive prediction accuracy of IA and preoperative calculations using the Barrett True-K no history formula in post-hyperopic LVC, post-myopic LVC, post-RK and Barrett Universal II in normal eyes.
Methods
This retrospective study included 170 consecutive post-corneal refractive surgery eyes of 110 patients and 103 consecutive normal eyes of 81 patients that underwent cataract surgery and in-the-bag IOL implantation at one single academic center. All surgeries were performed by 2 surgeons (KMR, GOW) at the Storm Eye Institute at the Medical University of South Carolina (MUSC) between January 2015 and December 2019. De-identified data, including age, gender, laterality of the operated eye, type of prior refractive surgery (if any), preoperative biometry, postoperative manifest refraction 1 month after surgery and best-corrected visual acuity (BCVA) were collected. All postoperative measurements were performed at the Storm Eye Institute. Data was analyzed in accordance with the tenets of the Declaration of Helsinki and its amendments. The study was approved by the MUSC Institutional Review Board (IRB) and permission from the Medical University of South Carolina was obtained to use its database. Direct patient consent was waived for this research.
Inclusion and Exclusion Criteria
This study included all eyes of patients with or without previous corneal refractive surgery (LASIK, PRK, or RK, with 4 to 16 incisions) who underwent uncomplicated cataract surgery or refractive lens exchange. The predicted spherical equivalent refraction (as determined by Barrett Universal II for normal eyes or Barrett True-K no history for post-corneal refractive surgery eyes) and IA for the implanted IOL were analyzed. Exclusion criteria were eyes with incomplete data, amblyopia, corneal ectasia/keratoconus, severe dry eye, and corneal pathologies (corneal dystrophy, corneal scars). Eyes with poorly dilating small pupils (<5mm) requiring additional procedures, including iris hooks, insertion of capsular tension rings or pupil extension rings, were also excluded. All eyes which did not achieve a BCVA of at least 0.3 logMar (20/40) were excluded from the study.
IOL Power and Prediction Error Calculations
Preoperative biometry data (IOLMaster 500 software version v.7.7 or IOLMaster 700, Carl Zeiss Meditec) were entered into the Barrett Universal II calculator for normal eyes (available at: https://calc.apacrs.org/barrett_universal2105/) and into the Barrett True K calculator for post-refractive surgery eyes (available at: http://calc.apacrs.org/Barrett_True_K_Universal_2105/).
Intraoperative aberrometry was performed in the aphakic state after cortical removal and before IOL implantation. The anterior chamber was inflated with cohesive viscoelastic (Provisc, Alcon) to a normotensive level (verified to be between 15 and 21 mmHg), with a Barraquer tonometer (Ocular Instruments Inc.). Prior to IA, care was taken to ensure that the ocular surface was uniformly hydrated and there was no distortion from eyelid squeezing, eye motion, or the eyelid speculum. The aphakic measurements of the SE refraction were used by IA to calculate the recommended IOL power and predicted refraction for the implanted IOL power.
The type and power of the IOL implanted, postoperative SE refraction and predicted postoperative SE refraction for the implanted IOL, as determined by Barrett Universal II or True-K and IA, were obtained from the AnalyzOR database. The prediction accuracy for Barrett Universal II/True-K and IA was assessed in terms of PE that was calculated as the difference between the achieved postoperative spherical equivalent refraction (manifest) and the spherical equivalent refraction predicted by each of the formulas tested.
Statistical Analysis
Data were analyzed using SPSS software (version 17.0; SPSS Inc.). Quantitative variables were described using mean ± standard deviation and range, where appropriate. The normality of the data was checked using histograms and Q–Q plots, as well as with the Shapiro–Wilk test and Kolmogorov–Smirnov test, as applicable. The prediction accuracy of Barrett Universal II/True-K and IA were compared using mean absolute prediction error (MAE) and median numerical error (MNE) as well as the difference in the percentage of eyes with an absolute PE ≤0.50 D, ≤0.75 D, ≤1.00 D in each of the four groups (post-hyperopic LVC, post-myopic LVC, post-RK and normal group).
To analyze correlated binary data such as two eyes of a same patient, regression model such as GEE was used. It takes account of the inter-eye correlation by estimating the covariance among all the residuals from a single subject, assuming the residuals from a subject are correlated. A p value of <0.05 was considered statistically significant. The variances in the mean numerical refractive prediction errors (MNE) were tested using the F-test for variances to assess the consistency of the prediction performance by the two methods. Additional analyses, including the refractive PE of Barrett True-K and IA for the implanted IOL AL, were performed. The eyes were stratified into short (<22.0 mm), normal (≥22.0 and <24.5 mm) and long (≥24.5 mm) eyes based on their AL.21 To evaluate relationships between absolute PE and AL, scatter plots were also prepared.
Results
This retrospective study included case records of 64 post-hyperopic LVC, 81 post-myopic LVC, 25 post-RK, and 103 normal eyes. Patient demographic and other clinical data are summarized in Table 1. Table 2 shows the IOL types, models and A-constant used in this study.
Table 1.
Demographic and Biometry Data of the Study Population
| Group | Post-Hyperopic LVC (Group 1) | Post-Myopic LVC (Group 2) | Post-RK (Group 3) | Non-Refractive Surgery (Group 4) |
|---|---|---|---|---|
| Eyes, (N) | 64 | 81 | 25 | 103 |
| Age (Years); Mean ± SD (Range) | 68.8 ± 7.3 (49 to 87) | 65.1 ± 5.8 (52 to 75) | 65.8 ± 6.4 (49 to 73) | 63.8 ± 11.7 (25 to 81) |
| Preoperative MRSE; Mean ± SD (Range) | 0.62 ± 1.45 (−4.00 to 3.38) | −0.77 ± 1.69 (−5.13 to 1.63) | 1.08 ± 2.39 (−4.50 to 5.88) | −1.64 ± 4.35 (−15.88 to 7.25) |
| Axial length (mm); Mean ± SD (Range) | 23.01 ± 0.93 (20.91 to 25.22) | 25.47 ± 1.29 (22.95 to 29.17) | 25.62 ± 1.08 (23.75 to 27.79) | 24.32 ± 1.91 (19.76 to 29.46) |
| Average Keratometry (D); Mean ± SD (Range) | 45.21 ± 1.88 (41.45 to 49.97) | 41.18 ± 2.10 (34.89 to 45.64) | 38.54 ± 2.25 (34.67 to 42.67) | 44.41 ± 2.02 (40.38 to 49.22) |
| IOL power implanted (D); Mean ± SD (Range) | 21.50 ± 2.91 (12.50 to 27.00) | 20.06 ± 2.17 (15.00 to 24.00) | 22.68 ± 2.12 (18.00 to 27.00) | 18.6 ± 6.32 (2.00 to 33.5) |
| Anterior chamber depth (mm); Mean ± SD (Range) | 3.00 ± 0.44 (2.43 to 5.07) | 3.40 ± 0.35 (2.81 to 4.01) | 3.55 ± 0.41 (2.83 to 4.58) | 3.30 ± 0.53 (2.19 to 5.00) |
| Postoperative MRSE; Mean ± SD (Range) | −0.23 ± 0.73 (−2.25 to 1.25) | −0.22 ± 0.94 (−3.00 to 2.25) | −0.06 ± 0.98 (−1.88 to 2.13) | −0.16 ± 0.84 (−3.38 to 2.63) |
Abbreviations: SD, standard deviation; MRSE, manifest refraction spherical equivalent; D, diopter; LVC, laser vision correction; RK, radial keratotomy.
Table 2.
Intraocular Lens Types, Models and A-Constant Implanted in Each Group
| Types and Models of IOL | A-Constant | Post-Hyperopic LVC | Post-Myopic LVC | Post-RK | Non-Refractive Surgery |
|---|---|---|---|---|---|
| Eyes, (N) | 64 | 81 | 25 | 103 | |
| Mx60E/Mx60T, (N) | 119.1 | 49 | 10 | 8 | 21 |
| ZCBOO/ZCT, (N) | 119.3 | 7 | 59 | 7 | 21 |
| ZXROO/ZXT, (N) | 119.3 | 2 | 7 | 8 | 20 |
| AR40E, (N) | 118.7 | – | 1 | – | 1 |
| SN60WF, (N) | 118.9 | – | 1 | – | 6 |
| SN6ATx, (N) | 119.2 | 7 | 2 | 2 | 22 |
| SV25Tx, (N) | 119.5 | – | 1 | – | 6 |
| TFNT00/ TFNTx, (N) | 119.1 | – | – | – | 6 |
Abbreviations: LVC, laser vision correction; RK, radial keratotomy.
Postoperatively, the MAE for IOL power calculations performed with preoperative data (Barrett Universal II and Barrett True-K) and IA were computed and compared. No statistically significant differences were observed between the MAE of preoperative formulas and IA for any of the four groups (post-hyperopic LVC, post-myopic LVC, post-RK and normal eyes) (Table 2). The percentage of eyes with predicted spherical equivalent refraction within ±0.5 D, as calculated by Barrett formulas vs IA, was 47% (30/64) vs 56% (36/64) in post-hyperopic LVC eyes, 68% (55/81) vs 54% (44/81) in post-myopic LVC eyes, 52% (13/25) vs 36% (9/25) in post-RK eyes, and 67% (69/103) vs 66% (68/103) in normal eyes. However, the differences between the two methods were not statistically significant (Table 3). Median numerical prediction error (MNE) was statistically significantly lower using IA compared to Barrett True-K (0.10 ± 0.64, 0.3 ± 0.65, p = 0.02) in post-hyperopic LVC eyes; results in post-myopic LVC (0.08 ± 0.80, −0.06 ± 0.82, p = 0.07), post-RK eyes (0.64 ± 0.96, 0.42 ± 0.88, p=0.2), and normal eyes (0.08 ± 0.77, 0.09 ± 0.86, p = 0.69) were not statistically different (Table 3).
Table 3.
Comparison of the Mean Absolute Prediction Error (MAE), Median Numerical Error (MNE) and Percentage Eyes with Absolute Prediction Error Within 0.5 D, 0.75 D and 1.0 D Obtained with Barrett Formulas (Barrett True K/Universal II) and IA in Post-Hyperopic LVC, Post-Myopic LVC, Post-RK and Non-Refractive Surgery Eyes
| Corneal Profile | No. of Eyes | Prediction Error | Barrett True K/Universal II | IA | p-value |
|---|---|---|---|---|---|
| Post-hyperopic LVC | 64 | MNE ± SD | 0.4±0.65 | 0.15±0.64 | 0.02 |
| MAE ± SD | 0.61 ± 0.42 | 0.51 ± 0.40 | 0.207 | ||
| % eyes with AE ≤0.5 D | 47% | 56% | 0.289 | ||
| % eyes with AE ≤0.75 D | 64% | 73% | 0.254 | ||
| % eyes with AE ≤1.0 D | 88% | 89% | 0.784 | ||
| Post-myopic LVC | 81 | MNE ± SD | −0.1±0.82 | 0.05±0.80 | 0.07 |
| MAE ± SD | 0.54 ± 0.62 | 0.57 ± 0.57 | 0.767 | ||
| % eyes with AE ≤0.5 D | 68% | 54% | 0.077 | ||
| % eyes with AE ≤0.75 D | 77% | 75% | 0.854 | ||
| % eyes with AE ≤1.0 D | 83% | 83% | 1.000 | ||
| Post-RK | 25 | MNE ± SD | 0.2±0.88 | 0.52±0.96 | 0.2 |
| MAE ± SD | 0.71 ± 0.68 | 0.89 ± 0.76 | 0.372 | ||
| % eyes with AE ≤0.5 D | 52% | 36% | 0.257 | ||
| % eyes with AE ≤0.75 D | 64% | 56% | 0.564 | ||
| % eyes with AE ≤1.0 D | 80% | 68% | 0.337 | ||
| Non-refractive surgery | 103 | MNE ± SD | 0.06±0.86 | 0.03±0.77 | 0.69 |
| MAE ± SD | 0.54 ± 0.69 | 0.50 ± 0.60 | 0.675 | ||
| % eyes with AE ≤0.5 D | 67% | 66% | 0.883 | ||
| % eyes with AE ≤0.75 D | 82% | 78% | 0.490 | ||
| % eyes with AE ≤1.0 D | 89% | 84% | 0.304 |
Abbreviations: MAE, mean absolute error; MNE, median numerical error; AE, absolute error; SD, standard deviation; LVC, laser vision correction; RK, radial keratotomy; IA, intraoperative aberrometry; D, diopter.
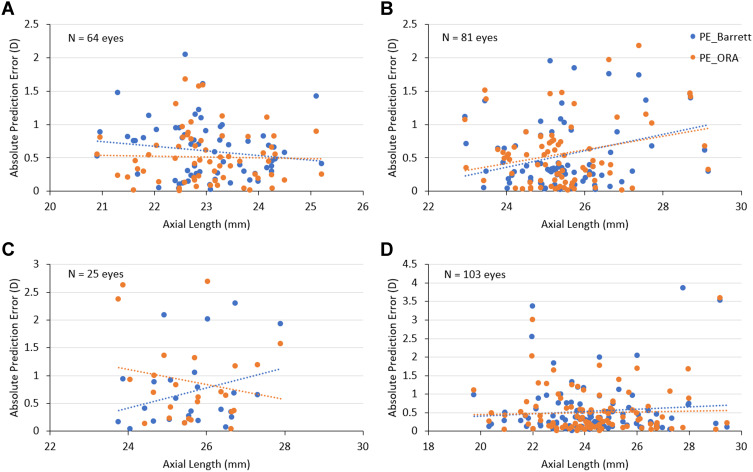
The effect of AL and the implanted IOL power on the PE of both methods was evaluated using scatter plots. In post-hyperopic LVC eyes, absolute PE with Barrett True-K tended to increase as AL decreased and absolute PE with IA showed no change with changing AL. A scattergram of post-myopic LVC eyes showed that the PE for both methods increased with increasing AL. Post-RK eyes revealed contrasting trends: ie, with increasing AL, the absolute PE increased with Barrett True-K and decreased with IA, and in normal eyes, AL had no effect on the PE for either method (Figure 1).
Figure 1.
Scatterplots depicting the relationship between AL and refractive prediction error with Barrett formula (Barrett True-K/Universal II) and IA. (A) Post-hyperopic LVC group; (B) post-myopic LVC group; (C) post-RK group; (D) normal (without prior refractive surgery) group.
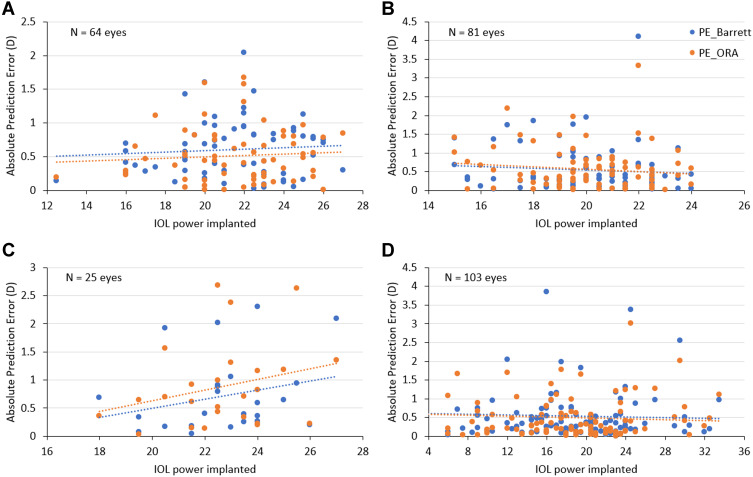
No effect of IOL power on the PE of either method was noted for post-hyperopic LVC and normal eyes, while in post-myopic LVC eyes, the PE decreased slightly with increased IOL power. In post-RK eyes, the PE increased with increasing IOL power (Figure 2).
Figure 2.
Scatterplots depicting the relationship between IOL power implanted and refractive prediction error with Barrett formula (Barrett True-K/Universal II) and IA. (A) Post-hyperopic LVC group; (B) post-myopic LVC group; (C) post-RK group; (D) normal (without prior refractive surgery) group.
Refractive prediction accuracy was also analyzed after stratifying the eyes in each of the four groups according to their AL (short, normal, and long eyes). In post-hyperopic LVC eyes with short AL, MAE was statistically significantly lower with IA compared to Barrett True-K (0.38 vs 0.82; p < 0.001). The proportion of eyes with absolute PE within ±0.5 D was statistically significantly higher with IA compared to Barrett True-K (67% (6/9) vs 11% (1/9); p = 0.03). In eyes with normal AL, the prediction accuracy of both methods was equivalent (54% (28/52) vs 54% (28/52)), whereas in long eyes, IA had a better prediction accuracy than Barrett True-K (67% (2/3) vs 33% (1/3)). This difference did not reach statistical significance. In post-myopic LVC and post-RK eyes with normal and long AL, the MAE was lower with Barrett True-K compared to IA; there was no statistically significant difference in the prediction accuracy of the two methods. In non-refractive surgery eyes with normal and long AL, MAE was lower with IA and for eyes with short AL, the refractive prediction accuracy of Barrett Universal II was better. However, the difference was not statistically significant (Table 4).
Table 4.
Mean Absolute Prediction Error (MAE) and Percentage Eyes with Absolute Prediction Error Within 0.5 D, 0.75 D and 1.0 D Obtained with Barrett Formulas (Barrett True K/Universal II) and IA After Stratifying Eyes in the Four Groups (Post-Hyperopic LVC, Post-Myopic LVC, Post-RK and Non-Refractive Surgery) According to Their Axial Length
| Corneal Profile | Axial Length | No. of Eyes | Prediction Error | Barrett True K/Universal II | IA | p-value |
|---|---|---|---|---|---|---|
| Post-hyperopic LVC | Short (<22 mm) | 9 | MAE ± SD | 0.82 ± 0.34 | 0.38 ± 0.23 | 0.001 |
| % eyes with AE ≤0.5 D | 11% | 67% | 0.030 | |||
| % eyes with AE ≤0.75 D | 44% | 89% | 0.067 | |||
| % eyes with AE ≤1.0 D | 78% | 100% | NA | |||
| Normal (22 to 24.5) | 52 | MAE ± SD | 0.56 ± 0.42 | 0.54 ± 0.42 | 0.849 | |
| % eyes with AE ≤0.5 D | 54% | 54% | 1.000 | |||
| % eyes with AE ≤0.75 D | 67% | 71% | 0.671 | |||
| % eyes with AE ≤1.0 D | 90% | 87% | 0.541 | |||
| Long (≥24.5) | 3 | MAE ± SD | 0.80 ± 0.54 | 0.43 ± 0.40 | 0.255 | |
| % eyes with AE ≤0.5 D | 33% | 67% | 0.423 | |||
| % eyes with AE ≤0.75 D | 67% | 67% | 1.000 | |||
| % eyes with AE ≤1.0 D | 67% | 100% | NA | |||
| Post-myopic LVC | Normal (22 to 24.5) | 15 | MAE ± SD | 0.47 ± 0.38 | 0.58 ± 0.44 | 0.458 |
| % eyes with AE ≤0.5 D | 60% | 47% | 0.466 | |||
| % eyes with AE ≤0.75 D | 87% | 80% | 0.626 | |||
| % eyes with AE ≤1.0 D | 87% | 80% | 0.626 | |||
| Long (≥24.5) | 66 | MAE ± SD | 0.56 ± 0.66 | 0.57 ± 0.60 | 0.932 | |
| % eyes with AE ≤0.5 D | 70% | 56% | 0.106 | |||
| % eyes with AE ≤0.75 D | 74% | 74% | 1.000 | |||
| % eyes with AE ≤1.0 D | 82% | 83% | 0.819 | |||
| Post-RK | Normal (22 to 24.5) | 4 | MAE ± SD | 0.38 ± 0.40 | 1.51 ± 1.19 | 0.015 |
| % eyes with AE ≤0.5 D | 75% | 25% | 0.178 | |||
| % eyes with AE ≤0.75 D | 75% | 25% | 0.178 | |||
| % eyes with AE ≤1.0 D | 100% | 50% | 0.429 | |||
| Long (≥24.5) | 21 | MAE ± SD | 0.77 ± 0.71 | 0.77 ± 0.62 | 0.991 | |
| % eyes with AE ≤0.5 D | 48% | 38% | 0.534 | |||
| % eyes with AE ≤0.75 D | 62% | 62% | 1.000 | |||
| % eyes with AE ≤1.0 D | 76% | 71% | 0.726 | |||
| Non-refractive surgery | Short (<22 mm) | 10 | MAE ± SD | 0.57 ± 0.74 | 0.62 ± 0.58 | 0.863 |
| % eyes with AE ≤0.5 D | 80% | 60% | 0.337 | |||
| % eyes with AE ≤0.75 D | 80% | 70% | 0.608 | |||
| % eyes with AE ≤1.0 D | 90% | 80% | 0.538 | |||
| Normal (22 to 24.5) | 48 | MAE ± SD | 0.50 ± 0.57 | 0.44 ± 0.56 | 0.593 | |
| % eyes with AE ≤0.5 D | 67% | 69% | 0.827 | |||
| % eyes with AE ≤0.75 D | 79% | 81% | 0.798 | |||
| % eyes with AE ≤1.0 D | 90% | 85% | 0.539 | |||
| Long (≥24.5) | 45 | MAE ± SD | 0.57 ± 0.81 | 0.54 ± 0.66 | 0.834 | |
| % eyes with AE ≤0.5 D | 64% | 64% | 1.000 | |||
| % eyes with AE ≤0.75 D | 84% | 76% | 0.295 | |||
| % eyes with AE ≤1.0 D | 89% | 84% | 0.537 |
Abbreviations: MAE, mean absolute error; AE, absolute error; SD, standard deviation; LVC, laser vision correction; RK, radial keratotomy; IA, intraoperative aberrometry; D, diopter; No information for short eyes in myopic LASIK/PRK and Prior RK groups.
For absolute PE ≤0.5 D, Barrett True-K agreed with IA in 34% (22/64) of the time in eyes post-hyperopic LVC, 49% (40/81) of the time in eyes post-myopic LVC and in 28% (7/25) to 49% (40/81) of the time in eyes post-RK, and Barrett Universal II and IA agreed 62% (64/103) of the time in normal eyes (Table 5). In instances where there was disagreement (absolute prediction error >0.5 D in at least one of the formulas), IA outperformed Barrett True-K in post-hyperopic LVC eyes and Barrett True-K/Universal II outperformed IA in post-myopic LVC, post-RK and normal eyes.
Table 5.
Agreements and Disagreements in the Refractive Prediction Accuracy of Barrett Formulas and IA in Post-Corneal Refractive Surgery Eyes and Non-Refractive Surgery Eyes
| Percentage of Eyes Where Barrett True K/Universal II and IA Resulted in Similar Predictive Accuracy (Absolute Prediction Error ≤0.5 D) | Percentage of Eyes Where Refractive Prediction Accuracy of Barrett True K/Universal II and IA Differed (Absolute Prediction Error >0.5 D by at Least One of the Formulas) | ||
|---|---|---|---|
| Corneal Profile | Both Barrett True K/Universal II and IA Agreed | Refractive Prediction Accuracy of IA Was Better | Refractive Prediction Accuracy of Barrett True K/Universal II Was Better |
| Post-hyperopic LVC | 34% | 39% | 27% |
| Post-myopic LVC | 49% | 19% | 32% |
| Post-RK | 28% | 24% | 48% |
| Non-refractive surgery | 62% | 16% | 22% |
Abbreviations: LVC, laser vision correction; RK, radial keratotomy; IA, intraoperative aberrometry; D, diopter.
No adverse events, device deficiencies or quality complaints were found in the case records during the execution of this investigator-initiated trial.
Discussion
Various methods have been proposed to improve the accuracy of IOL power calculation and refractive outcomes following lens surgery in normal and post-refractive surgery eyes.1,6,8,17,21 The present study compared the refractive prediction accuracy of the Barrett formula and IA in post-corneal refractive surgery (post-hyperopic LVC, post-myopic LVC, post-RK) as well as normal eyes. Our study suggests that intraoperative aberrometry improves accuracy in eyes post-hyperopic LVC.
Previous retrospective studies in post-myopic LVC and in normal eyes have reported IA to provide equal or better postoperative refractive outcomes.1,6,17–19,22 In post-RK, IA has been described to have inferior outcomes compared to preoperative calculation.8 No previous publications have investigated the performance of IA in post-hyperopic LVC in a large cohort. We demonstrated that the Barrett Universal II and True K formulas and IA perform comparably in predicting postoperative spherical equivalent in most groups, except in post-hyperopic LVC eyes when using numerical prediction error (MNE), which may translate to real-life examples. The spread of a mean numerical refractive PE (variance) for Barrett True-K and IA was also statistically comparable in all four groups, indicating similar consistency of the IOL prediction performance by the two methods. These findings corroborate the findings of previously published studies.8,15,21
To determine if prediction accuracy of the two methods is affected by AL, the correlation between absolute PE and AL was assessed. While there are studies that evaluate the effect of AL on the prediction accuracy of IOL formulas in eyes without previous LVC and in post-myopic LVC eyes,23 to the best of our knowledge, this is the first study to also stratify the results by AL in post-hyperopic LVC and post-RK eyes. Similar to previous reports,23 the scattergrams between absolute PE and AL revealed that both methods performed well, with no superiority of one over the other, across the range of AL in post-myopic LVC and normal eyes. In post-hyperopic LVC eyes, absolute PE with Barrett True-K tended to increase as AL decreased whereas the absolute PE with IA showed no change with changing AL. In post-myopic LVC and post-RK eyes, the trend observed within the full dataset was maintained for normal and long AL eyes, with Barrett True-K having a lower MAE. This shows a possible benefit of IA in IOL predictability in patients post-hyperopic LVC, specially in those with extreme axial length eyes, where even modern formulas may not provide a pristine refractive outcome. Nevertheless, given none of the other groups yielded similar outcomes, this finding is more likely to be related to the previous hyperopic LVC in these eyes than the extreme AL. Furthermore, care should be taken when extrapolating this result to real life given the small sample in each group.
In normal eyes, refractive outcomes within ± 0.50 D of the intended target have been reported to be as high as 75–84% using only preoperative biometry and 82% using IA.1 In eyes without prior refractive surgery with short AL eyes, Barrett Universal II was observed to have similar or greater accuracy than IA.19 In axial myopia, IA has been shown to improve IOL calculation accuracy to 80%.18 In this study, a similarity to previous reports could be observed and although it did not reach statistical significancy, Barrett Universal II tended to perform better in short eyes (lower MAE) and IA yielded better results in long eyes. Furthermore, we noticed that IA tended to perform better in normal AL eyes.
As expected, the difference between the MAE for IA and the Barrett formula was lowest in normal eyes with virgin corneas. In post-corneal refractive surgery eyes, measurement inaccuracies pose additional challenges for ophthalmologists attempting to calculate IOL power.24,25 Intraoperative aberrometry may help to reduce errors in IOL power calculation, especially in those with extremes in axial length or prior refractive surgery26,28 by measuring the eye’s refractive power in the aphakic state, thus accounting for both the anterior and posterior curvature and refractive index of the cornea.22 The precision and quality of the intraoperative wavefront aberrometry measurements, however, may be affected by its lower repeatability,24 the need to optimize IOP, hydration and wound integrity, the presence of bubbles in the visual axis, globe distortion due to eyelid speculum pressure, and patient cooperation.25 In the present study, corneal wound hydration and pressure on the globe from the lid speculum were closely observed and care was taken to avoid any related measurement errors.
The Barrett True-K formula (based on Barrett Universal II formula) uses a theoretical model to calculate a modified K from the measured K as well as the posterior corneal power in post-refractive surgery eyes. It helps increase the accuracy of the IOL power calculations, although the mathematical formula behind this method has not been published.27 There is evidence to support that measured posterior corneal curvature may improve the prediction accuracy of IOL calculations. A recent study has shown slight improvement in PE using measured posterior curvature values in virgin eyes.29 Another study reported the refractive prediction accuracy of the standard Haigis formula combined with total keratometry to be comparable with regression-based Haigis-L or Barrett True-K formulas combined with standard keratometry values in eyes with previous corneal refractive surgery.14 The incorporation of directly measured posterior corneal power reportedly improves refractive outcomes of the Barrett True-K formula in post-myopic LVC, but it remains to be validated how it would perform in post-hyperopic LVC or post-RK eyes.13,23
One limitation of this study is its retrospective nature. A prospective study using optimized IOL constants would be more robust; however, we believe that our study represents real-world scenarios and mimics the population that undergo cataract surgery. Second, we included both eyes for some patients because of the small sample size in some groups, which is not ideal. Nevertheless, we did use a statistical method to account for potentially correlated errors.
One may argue that RLE eyes and cataract surgery eyes might not be considered equivalent and may have wide variations in lens thickness (LT), ACD, aqueous depth (AQD) and effective lens position (ELP). However, although recent studies have reported the expected correlation among lens volume (LV) and LT with aging,30 others observed that neither LT nor lens volume or lens vaulting had a strong correlation with postoperative ACD.31,32 In fact, we have observed that the greatest predictor for postoperative ELP using swept-source OCT biometry (IOL Master 700, Carl Zeiss) is actually a metric called lens meridian position (LMP – distance from the corneal epithelium to the intersection of the anterior and posterior lens) measured by intraoperative spectral-domain OCT (Catalys® Precision System, Johnson & Johnson Vision); in these eyes, ELP displayed no correlation to intraoperative lens volume (Gouvea, et al, unpublished data, 2021).
In conclusion, the results of the present study suggest that both Barrett True-K and IA are effective methods for calculating IOL power in post-corneal refractive surgery as well as normal eyes. In face of newer-generation formulas, intraoperative aberrometry may have negligible benefits in standard cases, but it tends to be more accurate in post-hyperopic LVC eyes.
Acknowledgements
The authors would like to thank Jan Beiting and Raman Bedi, MD for statistical analysis and editorial support.
Funding Statement
This is a investigator initated study grant for statistical analysis supported by Alcon Surgical, Inc., USA.
Disclosure
Dr Larissa Gouvea reports grants from Alcon Lab, during the conduct of the study. Professor Wallace Chamon reports personal fees from Johnson & Johnson, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Cionni RJ, Dimalanta R, Breen M, Hamilton C. A large retrospective database analysis comparing outcomes of intraoperative aberrometry with conventional preoperative planning. J Cataract Refract Surg. 2018;44:1230–1235. [DOI] [PubMed] [Google Scholar]
- 2.Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63–71. doi: 10.1016/j.jcrs.2010.07.032 [DOI] [PubMed] [Google Scholar]
- 3.Behndig A, Montan P, Stenevi U, Kugelberg M, Zetterstrom C, Lundstrom M. Aiming for emmetropia after cataract surgery: Swedish National Cataract Register study. J Cataract Refract Surg. 2012;38:1181–1186. doi: 10.1016/j.jcrs.2012.02.035 [DOI] [PubMed] [Google Scholar]
- 4.Gale RP, Saldana M, Johnston RL, Zuberbuhler B, McKibbin M. Benchmark standards for refractive outcomes after NHS cataract surgery. Eye. 2009;23(1):149–152. doi: 10.1038/sj.eye.6702954 [DOI] [PubMed] [Google Scholar]
- 5.Canto AP, Chhadva P, Cabot F, et al. Comparison of IOL power calculation methods and intraoperative wavefront aberrometer in eyes after refractive surgery. J Refract Surg. 2013;29(7):484–489. doi: 10.3928/1081597X-20130617-07 [DOI] [PubMed] [Google Scholar]
- 6.Ianchulev T, Hoffer KJ, Yoo SH, et al. Intraoperative refractive biometry for predicting intraocular lens power calculation after prior myopic refractive surgery. Ophthalmology. 2014;121(1):56–60. doi: 10.1016/j.ophtha.2013.08.041 [DOI] [PubMed] [Google Scholar]
- 7.McCarthy M, Gavanski GM, Paton KE, Holland SP. Intraocular lens power calculations after myopic laser refractive surgery: a comparison of methods in 173 eyes. Ophthalmology. 2011;118(5):940–944. doi: 10.1016/j.ophtha.2010.08.048 [DOI] [PubMed] [Google Scholar]
- 8.Curado SX, Hida WT, Vilar CMC, Ordones VL, Chaves MAP, Tzelikis PF. Intraoperative aberrometry versus preoperative biometry for IOL power selection after radial keratotomy: a Prospective Study. J Refract Surg. 2019;35:656–661. doi: 10.3928/1081597X-20190913-01 [DOI] [PubMed] [Google Scholar]
- 9.Haigis W. Intraocular lens calculation after refractive surgery for myopia: Haigis-L formula. J Cataract Refract Surg. 2008;34:1658–1663. doi: 10.1016/j.jcrs.2008.06.029 [DOI] [PubMed] [Google Scholar]
- 10.Shammas HJ, Shammas MC. No-history method of intraocular lens power calculation for cataract surgery after myopic laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:31–36. doi: 10.1016/j.jcrs.2006.08.045 [DOI] [PubMed] [Google Scholar]
- 11.Masket S, Masket SE. Simple regression formula for intraocular lens power adjustment in eyes requiring cataract surgery after excimer laser photoablation. J Cataract Refract Surg. 2006;32:430–434. doi: 10.1016/j.jcrs.2005.12.106 [DOI] [PubMed] [Google Scholar]
- 12.Abulafia A, Hill WE, Koch DD, Wang L, Barrett GD. Accuracy of the Barrett True-K formula for intraocular lens power prediction after laser in situ keratomileusis or photorefractive keratectomy for myopia. J Cataract Refract Surg. 2016;42:363–369. doi: 10.1016/j.jcrs.2015.11.039 [DOI] [PubMed] [Google Scholar]
- 13.Savini G, Hoffer KJ, Barrett GD. Results of the Barrett True-K formula for IOL power calculation based on Scheimpflug camera measurements in eyes with previous myopic excimer laser surgery. J Cataract Refract Surg. 2020;46:1016–1019. doi: 10.1097/j.jcrs.0000000000000205 [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Spektor T, de Souza RG, Koch DD. Evaluation of total keratometry and its accuracy for intraocular lens power calculation in eyes after corneal refractive surgery. J Cataract Refract Surg. 2019;45:1416–1421. doi: 10.1016/j.jcrs.2019.05.020 [DOI] [PubMed] [Google Scholar]
- 15.Dawson VJ, Patnaik JL, Miller C, Lynch AM, Christopher KL. Comparison of refractive prediction for intraoperative aberrometry and Barrett True K no history formula in cataract surgery patients with prior radial keratotomy. Invest Ophthalmol Vis Sci. 2020;61:1659. [DOI] [PubMed] [Google Scholar]
- 16.Davison JA, Potvin R. Preoperative measurement vs intraoperative aberrometry for the selection of intraocular lens sphere power in normal eyes. Clin Ophthalmol. 2017;11:923–929. doi: 10.2147/OPTH.S135659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill DC, Sudhakar S, Hill CS, et al. Intraoperative aberrometry versus preoperative biometry for intraocular lens power selection in axial myopia. J Cataract Refract Surg. 2017;43(4):505–510. doi: 10.1016/j.jcrs.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 18.Sudhakar S, Hill DC, King TS, et al. Intraoperative aberrometry versus preoperative biometry for intraocular lens power selection in short eyes. J Cataract Refract Surg. 2019;45(6):719–724. doi: 10.1016/j.jcrs.2018.12.016 [DOI] [PubMed] [Google Scholar]
- 19.Fram NR, Masket S, Wang L. Comparison of intraoperative aberrometry, OCT-based IOL formula, Haigis-L, and masket formulae for IOL power calculation after laser vision correction. Ophthalmology. 2015;122(6):1096–1101. doi: 10.1016/j.ophtha.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 20.Srivannaboon S, Chirapapaisan C, Chirapapaisan N, Lertsuwanroj B, Chongchareon M. Accuracy of Holladay 2 formula using IOLMaster parameters in the absence of lens thickness value. Graefes Arch Clin Exp Ophthalmol. 2013;251:2563–2567. doi: 10.1007/s00417-013-2439-8 [DOI] [PubMed] [Google Scholar]
- 21.Chen AJ, Long CP, Lu T, Garff K, Heichel CW. Accuracy of intraoperative aberrometry and modern preoperative biometry for IOL power selection in post-refractive surgery patients. 2020 ASCRS Annual Meeting; 2020; ASCRS. [Google Scholar]
- 22.Zhang Z, Thomas LW, Leu SY, Carter S, Garg S. Refractive outcomes of intraoperative wavefront aberrometry versus optical biometry alone for intraocular lens power calculation. Indian J Ophthalmol. 2017;65:813–817. doi: 10.4103/ijo.IJO_163_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christopher K, Patnaik J, Miller C, et al. Accuracy of intraoperative aberrometry, Barrett True-K with and without posterior cornea measurements, Shammas-PL, and Haigis-L formulas after myopic refractive surgery. J Refract Surg. 2021;37(1):60–68. doi: 10.3928/1081597X-20201030-02 [DOI] [PubMed] [Google Scholar]
- 24.Giorgiev S, Hirnschall N, Fisus AD, et al. Repeatability of intraoperative Hartmann-Shack wavefront sensing in cataract surgery. J Cataract Refract Surg. 2021;47:902–906. [DOI] [PubMed] [Google Scholar]
- 25.Savini G, Hoffer KJ. Intraocular lens power calculation in eyes with previous corneal refractive surgery. Eye Vis. 2018;5:18. doi: 10.1186/s40662-018-0110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemmati HD, Gologorsky D, Pineda R 2nd. Intraoperative wavefront aberrometry in cataract surgery. Semin Ophthalmol. 2012;27(5–6):100–106. doi: 10.3109/08820538.2012.708809 [DOI] [PubMed] [Google Scholar]
- 27.Wang XZ, Cui R, Song XD, Yun B, Qian J, Ding N. Comparison of the accuracy of intraocular lens power calculation formulas for eyes after corneal refractive surgery. Ann Transl Med. 2020;8:871. doi: 10.21037/atm-20-4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christopher KL, Patnaik JL, Ifantides C, et al. Time utilization and refractive prediction enhancement associated with intraoperative aberrometry use during cataract surgery. Clin Ophthalmol. 2021;15:531–539. doi: 10.2147/OPTH.S287573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabian E, Wehner W. Prediction accuracy of total keratometry compared to standard keratometry using different intraocular lens power formulas. J Refract Surg. 2019;35:362–368. doi: 10.3928/1081597X-20190422-02 [DOI] [PubMed] [Google Scholar]
- 30.Waring IV GO, Chang DH, Rocha KM, Gouvea L, Pennati R. Correlation of intraoperative optical coherence tomography of crystalline lens diameter, thickness, and volume with biometry and age. Am J Ophthalmol. 2021;225:147–156. doi: 10.1016/j.ajo.2020.12.021 [DOI] [PubMed] [Google Scholar]
- 31.Yoo YS, Whang WJ, Hwang KY, et al. Use of the crystalline lens equatorial plane as a new parameter for predicting postoperative intraocular lens position. Am J Ophthalmol. 2019;198:17–24. doi: 10.1016/j.ajo.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 32.Norrby S, Bergman R, Hirnschall N, et al. Prediction of the true IOL position. Br J Ophthalmol. 2017;101(10):1440–1446. doi: 10.1136/bjophthalmol-2016-309543 [DOI] [PubMed] [Google Scholar]