Abstract
Background: Preterm delivery (PTD) and poor fetal growth are major contributors to neonatal mortality and morbidity that can extend from birth onward. Although overt maternal nutrient deficiencies are associated with adverse pregnancy outcomes, such deficiencies are rare in developed countries. However, some evidence suggests that even within the normal range, higher levels of antioxidant nutrients are protective against adverse pregnancy outcomes.
Materials and Methods: Using data from the prospective Pregnancy Outcomes and Community Health (POUCH) Study (n = 301 preterm; n = 246 term), we examined associations between maternal blood levels of selected antioxidants and pregnancy outcomes. Serum collected at 16–27 weeks' gestation was analyzed for carotenoids, retinol, and α- and γ-tocopherol. Using weighted polytomous regression, these nutrient concentrations were assessed in relation to (1) PTD (<37 weeks gestation) overall and grouped as spontaneous or medically indicated; and (2) small for gestational age (SGA) defined as birthweight-for-gestational age <10th percentile of a national reference population.
Results: Women with total serum carotenoids in the upper quartile (Q4) had significantly lower odds of medically indicated PTD compared with women in the lower quartiles (Q1–Q3) even after adjustment for maternal characteristics (aOR = 0.4; 95% CI: 0.2–0.9). Odds ratios for SGA were consistently ≤0.5 among women with any of the serum nutrients in Q4 as compared with Q1–Q3, but final models did not reach statistical significance.
Conclusion: Results support the possibility that high maternal serum antioxidants and/or the larger dietary or lifestyle pattern they represent may play a protective role in preventing adverse pregnancy outcomes.
Keywords: pregnancy, antioxidant vitamins, carotenoids, preterm, birthweight
Introduction
In the United States, preterm delivery (PTD) (<37 weeks completed gestation) and poor fetal growth (as estimated by small for gestational age, SGA) occur in ∼9.9% and 8.2% of live births, respectively.1,2 Both PTD and SGA, which can be overlapping, represent strong predictors of neonatal mortality and morbidity.3,4 Globally, risk factors for both PTD and SGA include numerous maternal micronutrient deficiencies,5,6 although such deficiencies are not common in developed countries. In the United States, risk factors for these adverse birth outcomes include African American race/ethnicity, maternal smoking, nulliparity, and lean maternal body mass index.7
While the biological causes of PTD and SGA are not well understood, nutrient status potentially affects several of the putative mechanisms, including infection, uterine muscle contractility, inflammation, and oxidative stress.8 The demands of pregnancy impose substantial oxidative and inflammatory stress on the maternal system.9 Antioxidants can partially mitigate damage from oxidative reactions originating from both physiological (e.g., pregnancy) and pathological (e.g., smoking) pathways.10 Additionally, antioxidants can enhance immune responses and decrease inflammatory responses. Because of these properties, antioxidants have been hypothesized to play a part in the prevention of adverse pregnancy conditions such as preeclampsia11 and pregnancy outcomes, including PTD12,13 and SGA.14–17
In this study, we focus on serum carotenoids and vitamins A and E, which are all fat-soluble compounds with antioxidant properties. Vitamin A is required for cell differentiation and growth, and its deficiency (overt or subclinical) has been associated with an increased risk of PTD and maternal anemia.18 Vitamin E is essential for normal reproductive physiology and its deficiency is linked to PTD.19 Vitamins A and E are essential dietary micronutrients, and some carotenoids have provitamin A activity (i.e., biologic conversion to vitamin A).20,21
Serum levels of carotenoids and other antioxidants reflect dietary intake and individual host factors (e.g., absorption, transport, and metabolism).22–25 Serum carotenoids are notable because they are more reflective of dietary intake than other nutrients that are homeostatically controlled. The best food sources of carotenoids are yellow/orange and dark green vegetables,26 whereas vitamin A is derived mainly from animal products (meat and dairy), and vitamin E from vegetable oils and nuts.27 To the extent that serum levels of antioxidant nutrients represent dietary intake, they represent a modifiable factor in gravid health.
This study assesses the relations among selected fat-soluble serum antioxidant vitamins and two pregnancy outcomes, PTD and SGA (used in this study as a proxy for poor fetal growth). Data are from a prospective pregnancy cohort—the Pregnancy Outcomes and Community Health (POUCH) Study. We hypothesized that serum concentrations of selected antioxidants (i.e., carotenoids, vitamin A [measured as retinol], and vitamin E [measured as α- and γ-tocopherol]) would be inversely associated with risk of PTD (overall and grouped by spontaneous or medically indicated) and SGA (defined as birthweight-for-gestational age <10th percentile of a national reference population).
Materials and Methods
Study design: POUCH study cohort and subcohort
The POUCH Study is a prospective pregnancy cohort designed to elucidate pathways to adverse pregnancy outcomes. The study received Institutional Review Board approval at Michigan State University, the Michigan Department of Community Health (now known as the Michigan Department of Health and Human Services), and nine community hospitals. Women were provided initial information about the POUCH Study through their prenatal care providers. Eligibility criteria included: 16th–27th week of pregnancy, maternal serum alpha-fetoprotein (MSAFP) screening, prenatal care at one of 52 clinics in 5 Michigan communities between 1998 and 2004, singleton pregnancy with no known chromosomal abnormality or birth defect, maternal age ≥15 years, no prepregnancy diabetes mellitus, and ability to complete study interviews in English.
Women who met the eligibility criteria and expressed interest in the study constituted the sampling frame. A stratified random sample was used to oversample African American women and those with unexplained high MSAFP levels (≥ 2 multiples of the median), as these groups are known to be at increased risk for adverse pregnancy outcomes.28 Of the 3,038 women recruited into the cohort, 3,019 (99%) were followed through delivery. A stratified comparison of maternal characteristics between POUCH Study participants and all mothers delivering within the five study communities during the same period (data derived from birth certificates) showed that the study sample was predominantly reflective of women with live births in the communities at large.
To conserve resources, some costly data elements (e.g., placental examinations, medical record abstraction, assays of stored biologic samples) were obtained from only a subsample of POUCH Study participants, referred to as the subcohort. The subcohort of 1,371 women includes all PTD, all pregnancies with high MSAFP and a stratified random sample of term deliveries with oversampling of African American women as described in detail elsewhere.29 To account for the cohort and subcohort sampling strategy, inverse-probability sampling weights are used in all POUCH Study analyses. For example, African American women, women with high MSAFP, and women with PTD who were oversampled into the subcohort are assigned a ‘smaller weight’ so that they represent their proportion in the eligible population agreeing to participate in the original POUCH Study.
Data collection and variable specification
At enrollment (16–27 weeks' gestation), participants met with a study nurse who obtained consent, conducted interviews, measured body weight, and collected biologic specimens, including nonfasting blood. Participants also completed self-administered questionnaires. Interviews and questionnaires provided the covariate data used in this study, that is, maternal age, self-identified race/ethnicity, medical insurance (Medicaid yes/no), maternal education level, parity, and smoking history. The number of women whose race/ethnicity was neither non-Hispanic White nor African American were too few to constitute additional groups. Their other maternal characteristics and pregnancy outcomes most closely paralleled that of non-Hispanic Whites in the POUCH Study and were therefore combined with this group in the race/ethnicity variable.
Pregnancy outcomes were determined from labor and delivery records by trained study staff. PTD was defined as birth before 37 completed weeks' gestation. Gestational week at delivery was calculated using date of last menstrual period (LMP). Alternatively, in instances where the gestational age estimate based on the LMP differed from the ultrasound estimate by more than 2 weeks, the ultrasound estimate was used. Early ultrasound (≤ 25 weeks) data were available for 93% of the participants.
PTD was categorized into subtypes based on clinical presentations noted in the medical record review: (1) spontaneous labor (defined as cervix dilated ≥2 cm and regular contractions) or preterm premature rupture of the membranes (PPROM—defined as rupture of membranes before or simultaneous with the onset of labor); and (2) medically indicated, defined as delivery that began by induction or Cesarean section in the absence of spontaneous labor or rupture of membranes as an initiating event. Using a national birthweight reference population that is sex specific for singleton births,30 birthweight for gestational age was modeled as SGA at <10%tile, appropriate for gestational age (AGA), and large for gestational age (LGA) at >90%tile.
Laboratory analyses
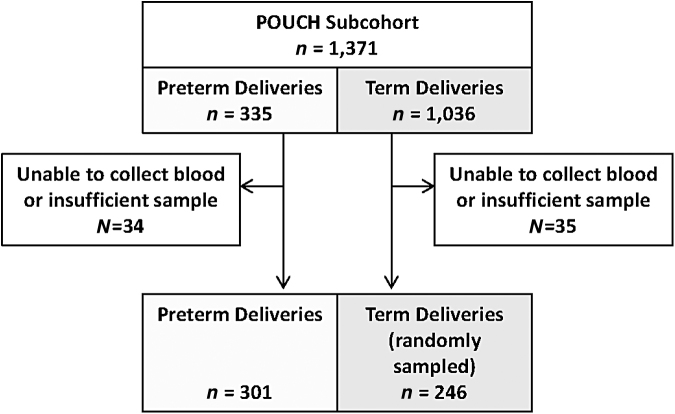
Nonfasting blood samples were drawn at enrollment (range 16–27 weeks' gestation), centrifuged within 45 minutes of collection, aliquoted (1 mL), and stored at −80°C. Subcohort stored serum specimens were shipped to the Heinz Nutrition Laboratory at the University of Pittsburgh for multiple assays, including lipid analyses described elsewhere.31 All serum specimens were transported on dry ice and protected from light. Procedures conducted in the laboratory were performed under subdued lighting in amber tubes. The final assays completed were of the antioxidant nutrients presented in this study, which were performed on specimens with sufficient remaining serum after two freeze/thaw cycles: all preterm (n = 301) and a random sample of term (n = 246). The sample size for term births was dictated by available resources (Fig. 1).
FIG. 1.
Flow Diagram of POUCH Study Participants Eligible for Maternal Serum Antioxidant Analyses. POUCH, pregnancy outcomes and community health.
Antioxidants were measured using an isocratic high-performance liquid chromatography (HPLC) procedure with UV detection developed by Chromsystems Diagnostics (Munich, Germany). The HPLC consists of Waters (Milford, MA) modules: two 515 pumps, a 717 Plus autosampler, and a 2,996 photodiode array detector. The tocopherols, retinol, and carotenoids were detected at 290, 325, and 453 nm, respectively. Calibrators and controls were run with each set of samples and the intra-assay coefficients of variation ranged from 3.1% to 8.0%.
Statistical analyses
As noted above, the POUCH Study cohort and subcohort were constructed by probability sampling from the original sampling frame. Sampling weights were used in all analyses to produce results that are generalizable to the sampling frame and maintain correct standard errors for hypothesis testing and confidence intervals. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Descriptive statistics for maternal characteristics and serum individual and total carotenoids, retinol, and α- and γ-tocopherol concentrations were calculated using mean and 95% confidence intervals (CI) for continuous variables and percentages for categorical variables. We assessed correlations between individual carotenoids and summed individual carotenoid concentrations to obtain total carotenoids. Distributions of the serum nutrient concentrations were skewed; therefore, all analyses used least squares (LS) geometric mean of log-transformed serum nutrient concentrations (SAS SurveyReg procedure, incorporating sampling weights). For presentation, results are expressed on the original scale after back transforming the LS geometric mean (± 95% CI).
We applied weighted polytomous regression (Proc Surveylogistic) to examine the odds of pregnancy outcomes associated with maternal serum nutrient concentrations. Our gestational age range (16–27 weeks) was limited to mid pregnancy, but because serum antioxidant status has been shown to vary over the course of pregnancy,32 we included gestational age at serum collection in all models. Additionally, maternal race/ethnicity was included in all models to account for potential confounding. Initial models included other covariates for which the mean nutrient concentrations were significantly different among maternal characteristic groups, that is, age, education, Medicaid enrollment, parity, season of enrollment, smoking status at enrollment, and BMI at enrollment. Final adjusted models included only covariates that met the following prespecified criteria: (1) remained significant in the multivariable model (along with gestational age at serum collection and race/ethnicity); and (2) removal did not alter the other main effect estimates by >10%. Serum nutrient concentrations were analyzed in quartiles using values from women with normal MSAFP and term deliveries to determine quartile cutpoints (<25th percentile = Q1; 26th – 49.9th percentile = Q2; 50th– 74.9th percentile = Q3; ≥ 75th percentile = Q4). After finding a protective effect in the highest quartile indicative of a threshold effect, we dichotomized the distribution of each nutrient (Q4 vs. Q1–Q3 combined).
In a second series of analyses, final adjusted models for carotenoids and tocopherols incorporated an additional covariate, serum total cholesterol, to account for potential confounding by blood lipid concentrations. The rationale for adjusting for serum cholesterol is to avoid under- or overestimating concentrations of the lipophilic compounds, carotenoids, and tocopherols, which circulate in blood primarily transported by lipoproteins.32–35 For example, tocopherols have no specific plasma transport protein, but they are transported in plasma lipoproteins and their distribution parallels that of total lipids.35 Plasma vitamin E levels have been shown to increase during pregnancy in response to the physiologic hyperlipidemia of pregnancy, such that the ratio of tocopherol to β-lipoprotein remains unchanged, supporting a role of vitamin E as a protective molecule inhibiting lipoprotein oxidation.33
Results
Maternal characteristics reflected the source population in this community-based Michigan cohort with 43% using Medicaid insurance (Table 1). Most participants (56%) were between 20 and 29 years at enrollment, with 16% under 20 years and 28% age 30 years or older. Race/ethnicity distribution was 25% African American and 75% White/Other. Slightly over half (52%) had more than a high school education, whereas 26% had a high school degree or equivalent and 22% had an education level of less than 12th grade.
Table 1.
Mean Serum Antioxidant Biomarker Concentrations by Maternal Characteristics in the POUCH Study (N = 547)1
| n (%)2 | Total carotenoids (ng/mL) |
Retinol (μg/mL) |
α-Tocopherol (μg/mL) |
γ-Tocopherol (μg/mL) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| Maternal age (y) | |||||||||
| <203 | 98 (15.5) | 942 | (843–1053) | 0.28 | (0.25–0.31) | 8.56 | (7.99–9.16) | 1.49 | (1.31–1.70) |
| 20–29 | 301 (56.2) | 849 | (782–922) | 0.31 | (0.29–0.33) | 8.86 | (8.42–9.31) | 1.63 | (1.51–1.76) |
| ≥30 | 148 (28.3) | 892 | (782–1017) | 0.334 | (0.30–0.35) | 9.35 | (8.69–10.1) | 1.47 | (1.30–1.66) |
| Race/ethnicity | |||||||||
| White/Others3 | 342 (75.4) | 875 | (809–947) | 0.33 | (0.31–0.34) | 9.27 | (8.85–9.70) | 1.54 | (1.44–1.66) |
| African American | 205 (24.6) | 874 | (810–943) | 0.264 | (0.25–0.27) | 8.034 | (7.69–8.39) | 1.62 | (1.50–1.75) |
| Maternal education (y) | |||||||||
| <123 | 135 (21.7) | 851 | (756–959) | 0.29 | (0.28–0.32) | 8.40 | (7.92–8.92) | 1.63 | (1.45–1.84) |
| 12 | 160 (26.1) | 786 | (702–879) | 0.31 | (0.29–0.33) | 8.56 | (8.01–9.14) | 1.69 | (1.51–1.88) |
| >12 | 252 (52.2) | 934 | (853–1022) | 0.32 | (0.30–0.33) | 9.394 | (8.88–9.92) | 1.48 | (1.36–1.60) |
| Medicaid6 | |||||||||
| No3 | 250 (56.6) | 905 | (829–988) | 0.32 | (0.30–0.34) | 9.30 | (8.83–9.80) | 1.51 | (1.39–1.64) |
| Yes | 296 (43.4) | 837 | (768–912) | 0.295 | (0.28–0.31) | 8.514 | (8.10–8.93) | 1.63 | (1.51–1.76) |
| Gestational age at enrollment (wks) | |||||||||
| <203 | 103 (16.1) | 823 | (737–920) | 0.33 | (0.31–0.36) | 8.35 | (7.70–9.04) | 1.74 | (1.55–1.95) |
| 20–24 | 371 (67.9) | 870 | (806–940) | 0.315 | (0.29–0.32) | 8.93 | (8.58–9.30) | 1.505 | (1.40–1.62) |
| 25–27 | 73 (16.0) | 951 | (801–1129) | 0.29 | (0.26–0.33) | 9.675 | (8.57–10.90) | 1.65 | (1.44–1.90) |
| Parity6 | |||||||||
| None3 | 242 (46.6) | 988 | (907–1076) | 0.31 | (0.29–0.33) | 9.29 | (8.81–9.80) | 1.41 | (1.31–1.52) |
| ≥ 1 | 304 (53.4) | 7864 | (724–854) | 0.31 | (0.29–0.33) | 8.66 | (8.25–9.09) | 1.714 | (1.57–1.86) |
| Enrollment date | |||||||||
| Spring3 | 139 (25.2) | 906 | (813–1009) | 0.33 | (0.30–0.35) | 9.04 | (8.45–9.67) | 1.57 | (1.48–1.67) |
| Summer | 150 (27.3) | 915 | (816–1026) | 0.30 | (0.28–0.32) | 9.14 | (8.47–9.86) | 1.54 | (1.24–1.83) |
| Fall | 125 (22.7) | 848 | (745–965) | 0.31 | (0.29–0.34) | 8.73 | (8.26–9.23) | 1.59 | (1.43–1.76) |
| Winter | 133 (24.9) | 828 | (718–954) | 0.30 | (0.28–0.33) | 8.85 | (8.13–9.63) | 1.56 | (1.37–1.77) |
| Smoking at enrollment | |||||||||
| No3 | 454 (88.9) | 886 | (827–948) | 0.31 | (0.30–0.32) | 9.00 | (8.65–9.37) | 1.57 | (1.48–1.67) |
| Yes | 93 (11.1) | 792 | (707–888) | 0.28 | (0.25–0.32) | 8.51 | (7.88–9.19) | 1.51 | (1.24–1.83) |
| BMI (kg/m2) at enrollment | |||||||||
| Quartile 13 | 137 (24.2) | 1030 | (900–1178) | 0.29 | (0.27–0.32) | 8.76 | (8.14–9.42) | 1.27 | (1.13–1.43) |
| Quartile 2 | 136 (27.9) | 980 | (889–1080) | 0.31 | (0.29–0.33) | 9.56 | (9.00–10.15) | 1.525 | (1.37–1.69) |
| Quartile 3 | 137 (26.0) | 910 | (839–986) | 0.33 | (0.30–0.35) | 9.60 | (8.87–10.38) | 1.754 | (1.57–1.95) |
| Quartile 4 | 137 (21.9) | 6044 | (532–687) | 0.31 | (0.29–0.33) | 7.754 | (7.32–8.21) | 1.784 | (1.59–1.99) |
Least square geometric mean ± 95%confidence intervals (CI) of the log values, which were retransformed back to non-log values for presentation here.
Weighted for the cohort and subcohort sampling design; weighted percentages reflect prevalence in sampled population.
Referent group.
p ≤ 0.01 for mean difference from the referent group, bold text used for clarity.
p ≤ 0.05 for mean difference from the referent group, bold text used for clarity.
Missing data: Medicaid (n = 1); Parity (n = 1).
Most (68%) were enrolled between 20 and 24 weeks gestation and 47% were nulliparous. The reasons specified for medically indicated PTD were determined by study obstetricians who reviewed prenatal and labor and delivery records. Many women had more than one specified reason for medically indicated PTD but we created a hierarchical list of primary reasons as follows: 37% maternal hypertension, 10% intrauterine growth restriction, 8% oligohydramnios, 3% abruption, 24% grouped as other maternal indications, and 18% grouped as other fetal indications.
Mean (i.e., LS geometric mean) serum nutrient concentrations are presented by maternal characteristics in Table 1. While there was wide variation in total carotenoid concentrations (overall mean ± SD = 956 ± 441, range 230–3,432 ng/mL), mean subgroup values did not vary significantly by most maternal characteristics assessed. However, mean serum carotenoids were highest in primiparous women and women with lower BMI (p < 0.01 for both). There was also wide variation in retinol and tocopherol concentrations (overall mean ± SD were: 0.32 ± 0.10, range 0.09–0.79 μg/mL for retinol; 9.22 ± 2.78, range 3.49–23.22 μg/mL for α-tocopherol; and 1.73 ± 0.80, range 0.35–5.44 μg/mL for γ-tocopherol). Mean serum retinol concentrations were highest in women ≥30 years and in women whose blood was sampled earlier in gestation (p < 0.05). Both retinol and α-tocopherol concentrations were highest in women in the White/Other race/ethnicity category, and in those with private insurance (p < 0.01 except where noted). Additionally, mean serum α-tocopherol concentrations were higher in women with more than a high school education (p < 0.01) as well as in women whose blood was sampled later in gestation (p < 0.05). Mean serum γ-tocopherol concentrations were higher in multiparous women and higher in those with higher BMIs (p < 0.01). None of the serum antioxidant nutrient concentrations varied significantly by smoking status or season of enrollment.
Correlations among retinol, α- and γ-tocopherol, the individual carotenoids, and total cholesterol are shown in Table 2. The highest correlation between individual carotenoids was 0.55 between β-cryptoxanthin and its immediate precursor β-carotene and, the lowest was 0.30 between β-carotene and lycopene. Correlations of each individual carotenoid with total carotenoids was high, ranging from 0.61 for α-cryptoxanthin to 0.81 for β-carotene.
Table 2.
Pearson Correlations Between Serum Antioxidant Biomarkers and Cholesterol in the POUCH Study (N = 547)1,2
| Retinol (μg/mL) | α-Tocopherol (μg/mL) | γ-Tocopherol (μg/mL) | β-Carotene (ng/mL) | α-Cryptoxanthin (ng/mL) | β-Cryptoxanthin (ng/mL) | Lycopene (ng/mL) | Lutein/Zeaxanthin (ng/mL) | Total carotenoid (ng/mL) | Total cholesterol (mg/dL) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Retinol | 1.00 | |||||||||
| α-tocopherol | 0.38 1 | 1.00 | ||||||||
| γ-tocopherol | 0.08 | 0.29 1 | 1.00 | |||||||
| β-carotene | 0.11 1 | 0.34 1 | −0.18 1 | 1.00 | ||||||
| α-Cryptoxanthin | 0.13 1 | 0.31 1 | 0.01 | 0.35 1 | 1.00 | |||||
| β-Cryptoxanthin | 0.09 1 | 0.20 1 | −0.06 | 0.55 1 | 0.39 1 | 1.00 | ||||
| Lycopene | 0.08 | 0.32 1 | 0.03 | 0.30 1 | 0.49 1 | 0.32 1 | 1.00 | |||
| Lutein/Zeaxanthin | 0.09 1 | 0.44 1 | 0.05 | 0.41 1 | 0.47 1 | 0.45 1 | 0.39 1 | 1.00 | ||
| Total carotenoid | 0.14 1 | 0.45 1 | −0.07 | 0.81 1 | 0.61 1 | 0.69 1 | 0.69 1 | 0.72 1 | 1.00 | |
| Total cholesterol | 0.06 | 0.46 1 | 0.31 1 | 0.23 1 | 0.27 1 | 0.19 1 | 0.34 1 | 0.36 1 | 0.37 | 1.00 |
p ≤ 0.05 for correlation (r), bold text used for clarity.
Weighted for the cohort and subcohort sampling design; weighted percentages reflect prevalence in sampled population.
Mean serum carotenoid concentrations are shown in Table 3 by pregnancy outcome. It is apparent across all pregnancy outcomes that α- and β-cryptoxanthin had the lowest concentrations, whereas lutein/zeaxanthin and lycopene had the highest. Within pregnancy outcome categories, lower mean (95%CI) concentrations of β-cryptoxanthin were significantly associated with medically indicated PTD [65.9 ng/mL (58.0–74.9)] compared with term delivery [77.8 ng/mL (71.3–84.9)] and with SGA [60.0 ng/mL (51.2–70.3)] compared with AGA [7.8 ng/mL (70.3–83.9)]. Lower mean (95%CI) concentrations of lutein/zeaxanthin were also significantly associated with SGA [173.2 ng/mL (145.1–206.8)] compared with AGA [215.9 ng/mL (204.2–228.3)]. In contrast, higher mean (95%CI) concentrations of β-carotene and α-cryptoxanthin were significantly associated with LGA [205.8 ng/mL (152.9–277.0) and 47.0 ng/mL (38.7–57.1), respectively] compared with AGA [136.6 ng/mL (123.3–151.4) and 32.5 ng/mL (30.4–34.6), respectively].
Table 3.
Mean Serum Individual Carotenoid Concentrations by Pregnancy Outcome in the POUCH Study (N = 547)1,2
| |
β-Carotene (ng/mL) |
α-Cryptoxanthin (ng/mL) |
β-Cryptoxanthin (ng/mL) |
Lycopene (ng/mL) |
Lutein/Zeaxanthin (ng/mL) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%)3 | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | |
| Pregnancy outcome | |||||||||||
| Preterm | 301 (10.7) | 127.2 | (115.1– 140.4) | 33.0 | (31.2– 34.9) | 69.4 | (64.4– 74.8) | 223.5 | (207.3– 241.0) | 215.0 | (203.8– 226.8) |
| Term4 | 246 (89.3) | 142.6 | (128.8– 157.8) | 33.1 | (31.0– 35.3) | 77.8 | (71.4– 84.9) | 231.3 | (213.0– 251.1) | 212.5 | (201.6– 224.1) |
| Pregnancy outcome subtype | |||||||||||
| Spontaneous | 122 (4.4) | 133.9 | (114.7– 156.3) | 33.7 | (31.1– 36.5) | 69.1 | (61.4– 77.8) | 224.7 | (201.1– 251.2) | 223.5 | (205.5– 243.1) |
| PPROM | 82 (2.9) | 126.5 | (107.0– 149.7) | 33.2 | (29.8– 37.0) | 74.1 | (65.5– 83.8) | 225.5 | (201.2– 252.8) | 215.2 | (195.7– 236.6) |
| Medically indicated | 97 (3.4) | 119.6 | (100.6– 142.1) | 31.9 | (29.0– 35.1) | 65.9 | (58.0– 74.9)6 | 220.1 | (196.1– 247.1) | 204.4 | (186.7– 223.8) |
| Term4 | 246 (89.3) | 142.6 | (128.8– 157.8) | 33.1 | (31.0– 35.3) | 77.8 | (71.3– 84.9) | 231.3 | (213.0–251.1) | 212.5 | (201.5– 224.1) |
| Size for gestational age5 | |||||||||||
| AGA4 | 458 (81.8) | 136.6 | (123.3– 151.4) | 32.5 | (30.4– 34.6) | 76.8 | (70.3– 83.9) | 226.3 | (208.1– 246.1) | 215.9 | (204.2– 228.3) |
| SGA (<10th %tile) | 58 (7.8) | 131.1 | (102.3– 167.8) | 29.0 | (25.1– 33.6) | 60.0 | (51.2– 70.3)6 | 232.7 | (190.9– 283.7) | 173.2 | (145.1– 206.8)6 |
| LGA (>90th %tile) | 30 (10.4) | 205.8 | (152.9– 277.0)6 | 47.0 | (38.7– 57.1)7 | 106.9 | (76.4– 149.6) | 263.5 | (220.0– 315.7) | 244.6 | (210.1– 284.7) |
Least square geometric means ±95% confidence intervals (CI) of log values, retransformed back to non-log values for presentation.
Adjusted for: maternal race (White/Other vs. African American), parity (≥1 vs. none), gestational age, and BMI at the time of enrollment.
Weighted percentages reflect oversampling of African Americans and preterm into the subcohort.
Referent group.
Birthweight missing (n = 1).
p < 0.05; bold text used for clarity.
p < 0.01; bold text used for clarity.
PPROM, preterm premature rupture of membranes; AGA, appropriate for gestational age; SGA, small for gestational age; LGA, large for gestational age.
Because individual carotenoids were highly correlated with total carotenoids, subsequent analyses focus on total carotenoids. As seen in Table 4, high serum carotenoids (≥ 75th percentile or Q4) were associated with lower odds of medically indicated PTD. While the number of women in Q4 carotenoids with medically indicated PTD was not large (n = 10), the strength of this association was substantial, an estimated 60% lower odds, that is, adjusted odds ratio (aOR) = 0.4; 95% CI: 0.2–0.9). Upon further adjustment for serum cholesterol (not shown in table), the association was slightly stronger (aOR = 0.3; 95% CI: 0.1–0.9). The primary reason listed in the medical record for medically indicated PTD in our sample was: hypertension (n = 36); intrauterine growth retardation (n = 11); oligohydramnios (n = 8); abruption (n = 3); other maternal indication (n = 23); and other fetal indication (n = 16). In sensitivity analyses that removed women with preeclampsia (n = 31), results were similar (data not shown). Odds ratios for the relation between high serum carotenoids and SGA were ≤0.5 in all models but confidence intervals in two of the three models included 1.0 (i.e., the unadjusted model and the adjusted model that included serum cholesterol).
Table 4.
Odds Ratios of Adverse Pregnancy Outcomes by Maternal Serum Concentrations of Vitamin A Compounds in the POUCH Study (N = 547)1,2
| |
Total carotenoids (ng/mL) |
Retinol (μg/mL) |
||||||
|---|---|---|---|---|---|---|---|---|
| Q4 vs. |
Q1–3 |
|
|
Q4 vs. |
Q1–Q3 |
|
|
|
| (≥1,206) |
(<1,206) |
Unadjusted |
Adjusted |
(≥0.37) |
(<0.37) |
Unadjusted |
Adjusted |
|
| Pregnancy outcome | N | n | OR (95% CI) | OR2 (95% CI) | n | n | OR (95% CI) | OR2 (95% CI) |
| Term (referent) | 63 | 183 | 1.0 | 1.0 | 66 | 180 | 1.0 | 1.0 |
| Preterm (<37 weeks) | 61 | 240 | 0.7 (0.4–1.1) | 0.7 (0.4–1.1) | 94 | 207 | 1.0 (0.7–1.5) | 1.1 (0.7–1.6) |
| Medically Indicated | 10 | 87 | 0.4 (0.2–0.7) | 0.4 (0.2–0.9) | 34 | 63 | 1.2 (0.7–2.0) | 1.3 (0.7–2.3) |
| Spontaneous or PPROM | 51 | 153 | 0.9 (0.5–1.4) | 0.8 (0.5–1.3) | 60 | 144 | 0.9 (0.6–1.4) | 1.0 (0.6–1.6) |
| Size for gestational age3 | ||||||||
| AGA (referent) | 109 | 349 | 1.0 | 1.0 | 138 | 320 | 1.0 | 1.0 |
| SGA (<10th percentile) | 7 | 51 | 0.5 (0.2–1.3) | 0.2 (0.1–0.8) | 13 | 45 | 0.3 (0.1–0.8) | 0.4 (0.1–1.2) |
| LGA (>90th percentile) | 8 | 22 | 1.2 (0.4–3.3) | 1.9 (0.5–6.5) | 9 | 21 | 1.7 (0.7–4.5) | 1.5 (0.5–4.1) |
95% CI does not include 1; bold text used for clarity.
All analyses were weighted for the cohort and subcohort sampling design; Q1–Q3 combined (<75th %tile) was used as the referent category when calculating odds ratios.
Adjusted for: maternal race (White/Other vs. African American); parity (≥1 vs. none); gestational age and BMI at the time of enrollment.
Birthweight missing (n = 1).
OR, odds ratio.
Serum retinol levels exhibited no significant association with PTD (Table 4). For SGA, odds ratios were <0.5 in all models among women in Q4 versus Q1–3, but the confidence interval for the adjusted model included 1.0. Similarly, results shown in Table 5 indicate no significant association between serum α-tocopherol or γ-tocopherol levels and PTD. However, again among women in Q4 versus Q1–3 for both α-tocopherol and γ-tocopherol, odds ratios for SGA were <0.5 in all models, but the confidence intervals included 1.0 after serum cholesterol was added to the models (aORs = 0.5; 95% CI: 0.2–1.6 for α-tocopherol and 0.3; 95% CI: 0.1–1.5 for γ-tocopherol).
Table 5.
Odds Ratios of Adverse Pregnancy Outcomes by Maternal Serum Concentrations of Vitamin E Compounds in the POUCH Study (N = 547)1,2
| |
α-Tocopherol (μg/mL) |
γ-Tocopherol (μg/mL) |
||||||
|---|---|---|---|---|---|---|---|---|
| Q4 vs. |
Q1–3 |
|
|
Q4 vs. |
Q1–3 |
|
|
|
| (≥<10.44) |
(<10.44) |
Unadjusted |
Adjusted |
(≥2.14) |
(<2.14) |
Unadjusted |
Adjusted |
|
| Pregnancy outcome | N | N | OR (95% CI) | OR2 (95% CI) | n | n | OR (95% CI) | OR2 (95% CI) |
| Term (referent) | 64 | 182 | 1.0 | 1.0 | 60 | 186 | 1.0 | 1.0 |
| Preterm (<37 weeks) | 85 | 216 | 1.0 (0.6–1.4) | 1.1 (0.7–1.6) | 73 | 228 | 1.0 (0.7–1.6) | 1.1 (0.7–1.7) |
| Medically indicated | 18 | 79 | 0.6 (0.3–1.1) | 0.6 (0.3–1.2) | 21 | 76 | 0.9 (0.5–1.6) | 0.8 (0.4–1.5) |
| Spontaneous or PPROM | 67 | 137 | 1.2 (0.7–1.8) | 1.3 (0.8–2.0) | 52 | 152 | 1.1 (0.7–1.8) | 1.2 (0.8–2.0) |
| Size for gestational age3 | ||||||||
| AGA (referent) | 132 | 326 | 1.0 | 1.0 | 113 | 345 | 1.0 | 1.0 |
| SGA (<10th percentile) | 8 | 50 | 0.2 (0.1–0.7) | 0.3 (0.1–0.8) | 11 | 47 | 0.3 (0.1–0.7) | 0.3 (0.1–0.8) |
| LGA (>90th percentile) | 9 | 21 | 1.6 (0.6–4.3) | 1.9 (0.7–5.2) | 9 | 21 | 1.3 (0.5–3.6) | 1.0 (0.3–2.9) |
95% CI does not include 1; bold text used for clarity.
All analyses were weighted for the cohort and subcohort sampling design; Q1–Q3 combined (<75th %tile) was used as the referent category when calculating odds ratios.
Adjusted for: maternal race (White/Other vs. African American); parity (≥1 vs. none); gestational age and BMI at the time of enrollment.
Birthweight missing (n = 1).
Discussion
In this prospective study of pregnancy outcomes, high concentrations of serum carotenoids measured in mid pregnancy were significantly associated with a lower risk for medically indicated PTD. This relation was not observed for the other serum antioxidant nutrients examined (i.e., retinol, α- and γ-tocopherol). Protective associations also were observed among high serum concentrations of total carotenoids, retinol, α- and γ-tocopherol, and the risk of SGA, although these associations were not consistent among individual carotenoids and did not remain statistically significant in adjusted models.
Our results are largely consistent with existing literature assessing the relations among antioxidants and PTD and/or SGA. We are aware of only one other prospective pregnancy cohort12,14 in a developed country (i.e., where few women have overt micronutrient deficiencies) that assessed associations among serum retinol, tocopherols, and carotenoids measured at mid pregnancy and the birth outcomes PTD and SGA. Results from that Canadian pregnancy cohort demonstrated a link between high concentrations of plasma carotenoids, but not retinol or tocopherols, and lower risk of spontaneous PTD; women with medically indicated PTD were not included in the cohort.12 In the same cohort, high concentrations of plasma carotenoids were also associated with lower risk of SGA, whereas high concentrations of retinol were associated with higher risk of SGA.14
A 1994 case/control study of 80 pregnant women reported significantly lower serum β-carotene levels measured after birth, but no differences in retinol or α-tocopherol, among women who experienced preterm premature rupture of membranes (PPROM) in comparison to serum levels in the control women.36 An important difference to note between the aforementioned study and the results presented here is the timing of blood collection. In the present prospective cohort study as well as the other described above,12,14 the blood was collected mid pregnancy, whereas in the case/control study that collected blood after birth,36 the lower serum β-carotene could be indicative of a depletion of β-carotene due to potentially increased antioxidant needs associated with PPROM.
In studies of nonpregnant adults, body composition has been shown to be inversely related to serum carotenoid concentrations,37,38 which is consistent with the POUCH Study results described in this study. The decreased risk for medically indicated PTD with high serum carotenoid concentrations in the POUCH Study remained significant after controlling for BMI.
Much of the focus in research on nutrient status during pregnancy includes vitamin and/or mineral supplementation, which may or may not be applicable to dietary intake of whole foods. Carotenoids are naturally occurring lipid-soluble plant pigments that provide the red, orange, and yellow colors in fruits and vegetables. Serum carotenoids have been shown to be a good marker of the relative consumption of fruit and vegetable intake within populations39,40 and have been shown to increase significantly in response to fruit/vegetable behavioral interventions.41,42 Fruit and vegetable intake is often associated with other healthy dietary behaviors, and accordingly, serum carotenoids have been shown to be related to multiple summary measures of dietary intake indicating they are a good marker for healthy dietary patterns.43 Therefore, protective associations with high serum carotenoids may be related to direct effects or because healthy dietary patterns are part of a larger, potentially synergistic effect.
The mechanism by which carotenoids or other antioxidants may be directly associated with pregnancy outcomes is unclear; however, some fraction of PTD and SGA may arise from insufficient placental perfusion, either due to problems with placentation or later-onset vascular events.44 Thus, our results support the possibility that this pathway may be protected by a threshold of maternal serum carotenoids, similar to mechanistic hypotheses posed to describe cardioprotective effects of carotenoids in nonpregnant adults.45 Alternatively, the placental perfusion pathway may be protected by other unmeasured correlates of high serum carotenoids.
Major strengths of this study are the carefully collected prospective data, the expert adjudication of pregnancy outcomes using medical records, high participant retention through delivery, and low rates of missing data.28 Women were sampled from community prenatal clinics and represent a diverse socioeconomic spectrum. Additionally, antioxidants and lipids were assessed using state-of-the-art laboratory methods.
The single serum measurement at mid pregnancy to characterize a woman's nutrient status may be considered a limitation of this study, although serum carotenoids are more representative of habitual dietary intake than many nutrients.46 Because we did not assess dietary intake or collect detailed dosage information about vitamin/mineral supplementation in the present study, we were unable to determine the extent to which the serum carotenoids or other antioxidant nutrients are reflective of usual dietary intake in our sample. Furthermore, with only one serum measure at mid pregnancy, we could not know whether we captured the critical time period during pregnancy that may most influence the pregnancy outcomes studied here. Maternal lipid levels have been shown to vary by fetal sex,47 suggesting that stratification by fetal sex may be important, however, given our sample size, this was not feasible. Similarly, there may be additional confounding factors that we did not capture, and we were limited by small sample sizes in certain cells, most notably cells with women in the highest quartile of serum antioxidant nutrients and medically indicated PTD or SGA infant (sample sizes ranged from 7 to 13 women). Although our odds ratios were consistently between 0.2 and 0.5 for all the serum antioxidant nutrients in relation to SGA, 95% confidence intervals were wide.
Conclusion
While there are well-known adverse effects of overt micronutrient deficiency during pregnancy, less is known about the potential benefits of above average intakes of micronutrients and other associated phytochemicals found in foods. Maternal serum antioxidant nutrients and/or the larger dietary and/or lifestyle pattern they represent may be partially protective against pathology leading to adverse pregnancy outcomes. Serum carotenoids are reflective of dietary intake and are therefore potentially modifiable. Dietary guidelines for pregnant women already include recommendations for intakes of food groups high in carotenoids (e.g., vegetables and fruits) and our results support the importance of efforts to assist women in adhering to these guidelines.
Acknowledgments
The authors thank the women who participated in the POUCH Study and their families as well as the entire POUCH Study team, with special thanks to Monal R. Shroff, PhD, who provided comments on an earlier version of this article. The authors thank Ms. Rona de la Vega for expert laboratory analyses. None of the authors had a personal or financial conflict of interest.
Author Confirmation Statement
This analysis was designed collaboratively by J.M.K., C.B.H., and Y.T. with data from the POUCH Study. Extensive data analyses were carried out by Y.T. The article was written primarily by J.M.K. under the guidance of C.B.H. Substantial review, comments, and final approval were provided by C.B.H., B.L.B., R.W.E., and J.B.S.
Author Disclosure Statement
No competing potential, actual, financial, or other interests exist for any of the authors.
Funding Information
This work was supported in part by the National Institute of Child Health and Human Development and the National Institute of Nursing Research (R01 HD-34543), the March of Dimes Foundation (20-FY98-0697 through 20-FY04-37), the Thrasher Research Foundation (02816-7), and the Centers for Disease Control and Prevention (U01-DP000143-01).
References
- 1. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final data for 2017. Natl Vital Stat Rep 2018;67:1–50. [PubMed] [Google Scholar]
- 2. Zhang X, Joseph KS, Kramer MS. Decreased term and postterm birthweight in the United States: Impact of labor induction. Am J Obstet Gynecol 2010;203:124 e121–e127. [DOI] [PubMed] [Google Scholar]
- 3. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379:2151–2161. [DOI] [PubMed] [Google Scholar]
- 4. Zeitlin JA, Ancel PY, Saurel-Cubizolles MJ, Papiernik E. Are risk factors the same for small for gestational age versus other preterm births? Am J Obstet Gynecol 2001;185:208–215. [DOI] [PubMed] [Google Scholar]
- 5. Black RE. Micronutrients in pregnancy. Br J Nutr 2001;85 Suppl 2:S193–S197. [DOI] [PubMed] [Google Scholar]
- 6. Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008;371:243–260. [DOI] [PubMed] [Google Scholar]
- 7. Catov JM, Bodnar LM, Ness RB, Markovic N, Roberts JM. Association of periconceptional multivitamin use and risk of preterm or small-for-gestational-age births. Am J Epidemiol 2007;166:296–303. [DOI] [PubMed] [Google Scholar]
- 8. Dunlop AL, Kramer MR, Hogue CJ, Menon R, Ramakrishan U. Racial disparities in preterm birth: An overview of the potential role of nutrient deficiencies. Acta Obstet Gynecol Scand 2011;90:1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol 2010;42:1634–1650. [DOI] [PubMed] [Google Scholar]
- 10. Chelchowska M, Ambroszkiewicz J, Gajewska J, Laskowska-Klita T, Leibschang J. The effect of tobacco smoking during pregnancy on plasma oxidant and antioxidant status in mother and newborn. Eur J Obstet Gynecol Reprod Biol 2011;155:132–136. [DOI] [PubMed] [Google Scholar]
- 11. Williams MA, Woelk GB, King IB, Jenkins L, Mahomed K. Plasma carotenoids, retinol, tocopherols, and lipoproteins in preeclamptic and normotensive pregnant Zimbabwean women. Am J Hypertens 2003;16:665–672. [DOI] [PubMed] [Google Scholar]
- 12. Kramer MS, Kahn SR, Platt RW, et al. Antioxidant vitamins, long-chain fatty acids, and spontaneous preterm birth. Epidemiology 2009;20:707–713. [DOI] [PubMed] [Google Scholar]
- 13. Villar J, Merialdi M, Gulmezoglu AM, et al. Nutritional interventions during pregnancy for the prevention or treatment of maternal morbidity and preterm delivery: An overview of randomized controlled trials. J Nutr 2003;133(5 Suppl 2):1606S–1625S. [DOI] [PubMed] [Google Scholar]
- 14. Cohen JM, Kahn SR, Platt RW, Basso O, Evans RW, Kramer MS. Small-for-gestational-age birth and maternal plasma antioxidant levels in mid-gestation: A nested case-control study. BJOG 2015;122:1313–1321. [DOI] [PubMed] [Google Scholar]
- 15. Osorio JC, Cruz E, Milanes M, et al. Influence of maternal redox status on birth weight. Reprod Toxicol 2011;31:35–40. [DOI] [PubMed] [Google Scholar]
- 16. Merialdi M, Carroli G, Villar J, et al. Nutritional interventions during pregnancy for the prevention or treatment of impaired fetal growth: An overview of randomized controlled trials. J Nutr 2003;133(5 Suppl 2):1626S–1631S. [DOI] [PubMed] [Google Scholar]
- 17. McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best practice & research. Clin Obstet Gynaecol 2009;23:779–793. [DOI] [PubMed] [Google Scholar]
- 18. Radhika MS, Bhaskaram P, Balakrishna N, Ramalakshmi BA, Devi S, Kumar BS. Effects of vitamin A deficiency during pregnancy on maternal and child health. BJOG 2002;109:689–693. [DOI] [PubMed] [Google Scholar]
- 19. Gagne A, Wei SQ, Fraser WD, Julien P. Absorption, transport, and bioavailability of vitamin E and its role in pregnant women. Journal of obstetrics and gynaecology Canada: JOGC 2009;31:210–217. [DOI] [PubMed] [Google Scholar]
- 20. Hebert JR, Hurley TG, Hsieh J, et al. Determinants of plasma vitamins and lipids: The Working Well Study. Am J Epidemiol 1994;140:132–147. [DOI] [PubMed] [Google Scholar]
- 21. Strobel M, Tinz J, Biesalski HK. The importance of beta-carotene as a source of vitamin A with special regard to pregnant and breastfeeding women. Eur J Nutr 2007;46 Suppl 1:I1–I20. [DOI] [PubMed] [Google Scholar]
- 22. Campbell DR, Gross MD, Martini MC, Grandits GA, Slavin JL, Potter JD. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol Biomarkers Prev 1994;3:493–500. [PubMed] [Google Scholar]
- 23. Kardinaal AF, van 't Veer P, Brants HA, van den Berg H, van Schoonhoven J, Hermus RJ. Relations between antioxidant vitamins in adipose tissue, plasma, and diet. Am J Epidemiol 1995;141:440–450. [DOI] [PubMed] [Google Scholar]
- 24. Stryker WS, Kaplan LA, Stein EA, Stampfer MJ, Sober A, Willett WC. The relation of diet, cigarette smoking, and alcohol consumption to plasma beta-carotene and alpha-tocopherol levels. Am J Epidemiol 1988;127:283–296. [DOI] [PubMed] [Google Scholar]
- 25. Yong LC, Forman MR, Beecher GR, et al. Relationship between dietary intake and plasma concentrations of carotenoids in premenopausal women: Application of the USDA-NCI carotenoid food-composition database. Am J Clin Nutr 1994;60:223–230. [DOI] [PubMed] [Google Scholar]
- 26. Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res 2007;55:207–216. [DOI] [PubMed] [Google Scholar]
- 27. Jenab M, Salvini S, van Gils CH, et al. Dietary intakes of retinol, beta-carotene, vitamin D and vitamin E in the European Prospective Investigation into Cancer and Nutrition cohort. Eur J Clin Nutr 2009;63(Suppl 4):S150–S178. [DOI] [PubMed] [Google Scholar]
- 28. Holzman C, Bullen B, Fisher R, Paneth N, Reuss L. Pregnancy outcomes and community health: The POUCH study of preterm delivery. Paediatr Perinat Epidemiol 2001;15(Suppl 2):136–158. [DOI] [PubMed] [Google Scholar]
- 29. Jones NM, Holzman C, Friderici KH, et al. Interplay of cytokine polymorphisms and bacterial vaginosis in the etiology of preterm delivery. J Reprod Immunol 2010;87:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mudd LM, Holzman CB, Catov JM, Senagore PK, Evans RW. Maternal lipids at mid-pregnancy and the risk of preterm delivery. Acta Obstet Gynecol Scand 2012;91:726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Belo L, Caslake M, Santos-Silva A, et al. LDL size, total antioxidant status and oxidised LDL in normal human pregnancy: A longitudinal study. Atherosclerosis 2004;177:391–399. [DOI] [PubMed] [Google Scholar]
- 33. Alvarez JJ, Montelongo A, Iglesias A, Lasuncion MA, Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J Lipid Res 1996;37:299–308. [PubMed] [Google Scholar]
- 34. Ford L, Farr J, Morris P, Berg J. The value of measuring serum cholesterol-adjusted vitamin E in routine practice. Ann Clin Biochem 2006;43(Pt 2):130–134. [DOI] [PubMed] [Google Scholar]
- 35. Kayden HJ, Traber MG. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J Lipid Res 1993;34:343–358. [PubMed] [Google Scholar]
- 36. Barrett BM, Sowell A, Gunter E, Wang M. Potential role of ascorbic acid and beta-carotene in the prevention of preterm rupture of fetal membranes. Int J Vitamin Nutr Res 1994;64:192–197. [PubMed] [Google Scholar]
- 37. Beydoun MA, Shroff MR, Chen X, Beydoun HA, Wang Y, Zonderman AB. Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys. J Nutr 2011;141:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harari A, Coster ACF, Jenkins A, et al. Obesity and insulin resistance are inversely associated with serum and adipose tissue carotenoid concentrations in adults. J Nutr 2020;150:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brevik A, Andersen LF, Karlsen A, Trygg KU, Blomhoff R, Drevon CA. Six carotenoids in plasma used to assess recommended intake of fruits and vegetables in a controlled feeding study. Eur J Clin Nutr 2004;58:1166–1173. [DOI] [PubMed] [Google Scholar]
- 40. Peng YM, Peng YS, Lin Y, Moon T, Roe DJ, Ritenbaugh C. Concentrations and plasma-tissue-diet relationships of carotenoids, retinoids, and tocopherols in humans. Nutr Cancer 1995;23:233–246. [DOI] [PubMed] [Google Scholar]
- 41. Lanza E, Schatzkin A, Daston C, et al. Implementation of a 4-y, high-fiber, high-fruit-and-vegetable, low-fat dietary intervention: Results of dietary changes in the Polyp Prevention Trial. Am J Clin Nutr 2001;74:387–401. [DOI] [PubMed] [Google Scholar]
- 42. McEligot AJ, Rock CL, Flatt SW, Newman V, Faerber S, Pierce JP. Plasma carotenoids are biomarkers of long-term high vegetable intake in women with breast cancer. J Nutr 1999;129:2258–2263. [DOI] [PubMed] [Google Scholar]
- 43. Lipsky LM, Cheon K, Nansel TR, Albert PS. Candidate measures of whole plant food intake are related to biomarkers of nutrition and health in the US population (National Health and Nutrition Examination Survey 1999–2002). Nutr Res 2012;32:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol 2008;61:1254–1260. [DOI] [PubMed] [Google Scholar]
- 45. Agarwal M, Parameswari RP, Vasanthi HR, Das DK. Dynamic action of carotenoids in cardioprotection and maintenance of cardiac health. Molecules 2012;17:4755–4769. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Rock CL, Swendseid ME, Jacob RA, McKee RW. Plasma carotenoid levels in human subjects fed a low carotenoid diet. J Nutr 1992;122:96–100. [DOI] [PubMed] [Google Scholar]
- 47. Pecks U, Rath W, Maass N, et al. Fetal gender and gestational age differentially affect PCSK9 levels in intrauterine growth restriction. Lipids Health Dis 2016;15:193. [DOI] [PMC free article] [PubMed] [Google Scholar]