Abstract
Objectives: We aim at reporting the functional and oncological outcomes in men with localized prostate cancer who underwent individualized partial gland cryoablation of the prostate by using validated quality-of-life instruments.
Methods: We retrospectively reviewed our cryosurgery database between July 2003 and September 2019 for men who were treated with individualized partial gland cryoablation of the prostate at our tertiary care center. Baseline and periodic urinary and sexual function surveys were administered throughout the post-treatment period.
Results: A total of 82 men were included in the study. Median follow-up was 28 months (interquartile range: 10.5–59.3 months). A total of 71 men underwent primary individualized partial gland cryoablation, whereas 11 men underwent salvage partial gland ablation. Failure-free survival at 1 to 5 years was 98%, 89%, 84%, 75%, and 75% in the primary therapy group, and 100%, 80%, and 40% in the salvage group at 1 to 3 years, respectively. In the primary therapy group, all 71 patients remained free of pads at 3 months and throughout the follow-up period. Men who had undergone primary focal cryoablation had a higher post-treatment International Index of Erectile Function (IIEF) score, followed by men treated with primary hemi-cryoablation and primary subtotal cryoablation. The American Urological Association (AUA) symptom scores decreased regardless of the type of partial gland ablation performed, with subtotal ablation having the lowest score compared with hemiablation and focal cryoablation. No patient developed a fistula in the primary group, and 1 (9%) patient developed a fistula in the salvage group.
Conclusion: Individualized partial gland cryoablation of the prostate is able to achieve excellent oncological and functional outcomes in select men with localized prostate cancer.
Keywords: focal cryotherapy, salvage cryotherapy, localized prostate cancer, focal therapy
Introduction
In recent years, advancement in imaging and targeted prostate biopsy have challenged the boundaries for partial gland treatment of prostate cancer.1 Partial gland ablation or focal therapy of the prostate can be performed via different energy sources such as cryoablation, high-intensity focused ultrasound (US), irreversible electroporation, laser ablation, and photodynamic therapy.2–5 The goal of such therapy is to achieve comparable oncological outcomes, while causing less morbidity compared with whole gland treatment.2–5
In recent years, there have been multiple studies evaluating the efficacy of focal cryoablation for the treatment of prostate cancer, in both the primary and salvage settings.6–8 Many of these studies on cryoablation of the prostate treat predominantly grade group 1 (Gleason score 3 + 3) cancer and have very low adherence to follow-up biopsy rates after focal cryosurgery.9,10 Hence, endpoints such as disease-free survival or biochemical recurrence based on the Phoenix criteria have been utilized in lieu of tissue pathology to determine cancer survival outcomes after focal cryoablation. This can be misleading given that the malignant potential for low-grade localized prostate cancer is long/protracted and is considered by many as not being clinically significant.11,12 Further, many of these studies do not include functional outcomes measured by standardized erectile functional and lower urinary tract symptoms instruments.13
In this study, we aim at reporting the oncological outcomes after individualized partial gland cryoablation of the prostate at a single tertiary care center. We also sought to report the functional outcomes utilizing validated American Urological Association (AUA) symptom scores and International Index of Erectile Function (IIEF) questionnaires, over a period of 3 years.
Methods
This is an institution review board-approved retrospective review of a prospective quality improvement database of patients who have undergone individualized partial gland cryoablation of the prostate at a tertiary care center between July 2003 and September 2019. Inclusion criteria for the analysis were men who had a multiparametric magnetic resonance imaging and ultrasound (mpMRI/US) fusion-guided biopsy or a three-dimensional (3D) template mapping saturation biopsy of the prostate. Patients were deemed to be good candidates for individualized partial gland cryoablation, and informed consent was obtained preceding treatment. Before prostate cryoablation, baseline urinary and sexual functions were assessed by using self-administered validated instruments: the AUA symptom score and IIEF-5.14,15 Our technique, post-cryotherapy follow-up protocol and definition is listed in Appendix.
Clinical and demographic data
Variables of interest were age (in years), race (white, black, other), pre-treatment data including body mass index (kg/m2), prostate-specific antigen (PSA; ng/mL), grade group score before cryoablation (1–5), pre-cryotherapy androgen deprivation therapy (yes/no), pre-treatment IIEF-5 and AUA symptom scores, utilization of mpMRI, prostate size on mpMRI (cm3), number and size of lesions on MRI, location of lesion (laterality, anterior/posterior, zone), type of biopsy (fusion, 12-core and 3D transperineal mapping biopsy), prior treatment (external beam radiation therapy [EBRT], brachytherapy), post-treatment follow-up (months), post-cryotherapy IIEF-5 and IPSS scores, complications (fistula, incontinence, erectile dysfunction), PSA nadir, biopsy on follow-up, treatment failure, infield recurrence (IFR), additional therapies received, failure-free survival (FFS), metastasis-free survival (months), and cancer-specific survival (months). Metastasis was defined based on a bone scan and/or positron emission tomography (PET)-CT. IFR was defined as evidence of grade group 2 or higher cancer on post-therapy prostate biopsy inside the cryoablation zone. Out-of-field recurrence (OFR) was defined by having a positive biopsy in a location where cryoablation of the prostate was not performed. Urinary incontinence is defined by any degree of urinary leakage requiring pads in a patient who did not have any incontinence before cryoablation. Erectile dysfunction was defined as an inability to achieve an erection by a patient who was able to achieve erections before cryoablation with or without pharmacologic therapy.
Statistical analysis
Medians and their interquartile range (IQR) were used to summarize continuous variables, whereas categorical variables were summarized with counts and percentages. Mean and standard error was used for graphical depiction of IIEF-5 and AUA symptoms score over time. To determine whether missing data affected the results from the IIEF-5 and AUA symptom score instruments, we performed a sensitivity analysis by constructing a multiple imputations model for each of the questionnaires. Briefly, we generated 20 complete datasets and generalized linear regression models were fit to each of the 20 imputed datasets and pooled by using Rubin's rules. Identical models were fit to the complete case data for comparison. Survival analysis was depicted by using Kaplan-Meier plots. A multivariate Cox proportional hazards model was constructed with grade group, pretreatment PSA, and type of partial gland treatment (focal, hemiablation or subtotal cryoablation). Statistical significance was defined as p < 0.05. R 3.5.1 for Rstudio 1.1.456 was used for statistical analyses, with the key packages: mice, dplyr, ggplot2, reshape2, survivor, and VIM installed.16
Results
Primary therapy
Baseline characteristics
A total of 71 men with biopsy-proven localized prostate cancer were included in the analysis. Median follow-up was 28 months (IQR: 10.5–59.3 months). Patient characteristics are described in Table 1.
Table 1.
Demographics for Primary and Salvage Patients
| Primary (n = 71) | Salvage (n = 11) | |
|---|---|---|
| Age, years, median (IQR) | 66.7 (59.8–72.1) | 68.0 (64.6–68.2) |
| Race | ||
| White | 59 (83%) | 9 (82%) |
| Black | 10 (14%) | 2 (18%) |
| Others | 2 (3%) | 0 (0%) |
| Family history | ||
| None | 55 (78%) | 8 (73%) |
| First degree | 10 (14%) | 2 (18%) |
| Second degree | 6 (9%) | 1 (9%) |
| Smoking history | ||
| None | 36 (50%) | 5 (45%) |
| Former | 27 (38%) | 6 (54%) |
| Current | 8 (11%) | 0 (0%) |
| BMI, kg/m2, median (IQR) | 29.3 (25.6–31.6) | 32.2 (25.9–36.1) |
| PSA at time of biopsy, ng/mL, median (IQR) | 6.55 (4.80–8.85) | 4.99 (2.23–7.86) |
| Pre-cryotherapy ADT | ||
| Yes | 2 (2.8%) | 0 (0%) |
| No | 69 (97.2%) | 11 (100%) |
| Pre-cryotherapy erections | ||
| Yes | 43 (61%) | 4 (36%) |
| No | 28 (30%) | 7 (64%) |
| Pre-cryotherapy IIEF-5, median (IQR) | 19 (13–22) | 15 (6.5–19.5) |
| Pre-cryotherapy AUA symptoms score, median (IQR) | 6 (3–12) | 7 (3–9.5) |
| MRI | ||
| Yes | 62 (87%) | 8 (72%) |
| No | 9 (13%) | 3 (28%) |
| Pre-treatment prostate volume on MRI, cc, median (IQR) | 41 (29.3–56.5) | 34 (32–38.3) |
| Lesion(s) location on MRIa | ||
| Apex | 15 (30%) | 1 (33%) |
| Middle | 23 (46%) | 1 (33%) |
| Base | 12 (24%) | 1 (33%) |
| Anterior | 20 (40%) | 2 (66%) |
| Posterior | 30 (60%) | 1 (33%) |
| Lesion zone on MRI | ||
| Peripheral | 37 (74%) | 1 (33%) |
| Transition | 10 (20%) | 2 (67%) |
| Central | 3 (6) | 0 (0%) |
| Lesion size on MRI, cm3, median (IQR) | 1.25 (0.7–2.5) | 0.62 (—) |
| Type of biopsy | ||
| 12-Core | 23 (32.4%) | 0 |
| 18-Core | 0 | 5 (45.5%) |
| US/mpMRI fusion | 24 (33.8%) | 2 (18.1%) |
| 3D mapping biopsy | 24 (33.8%) | 4 (36.4%) |
| No. of positive cores, median (IQR) | 3 (1–4) | 3 (1–4) |
| Total number of cores, median (IQR) | 12 (7–26) | 16 (12–26) |
| Clinical stage | ||
| T1 | 66 (93%) | 10 (91%) |
| T2 | 5 (7%) | 1 (9%) |
| Baseline GG | ||
| GG1 | 30 (42.3%) | 2 (18%) |
| GG2 | 33 (46.5%) | 5 (46%) |
| GG3 | 5 (7.0%) | 1 (9%) |
| GG4 | 2 (2.8%) | 3 (27%) |
| GG5 | 1 (1.4%) | 0 |
| Gland treated | ||
| Focal | 56 (79%) | 6 (55%) |
| Hemi | 9 (13%) | 2 (18%) |
| Subtotal | 6 (8%) | 3 (27%) |
Values are rounded to the nearest whole number and may exceed or be less than 100%.
3D = three-dimensional; ADT = androgen deprivation therapy; AUA = American Urological Association; BMI = body mass index; GG = grade group; IIEF = International Index of Erectile Function; IQR = interquartile range; mpMRI = multiparametric magnetic resonance imaging; MRI = magnetic resonance imaging; PSA = prostate-specific antigen; US = ultrasound.
Biopsy and FFS after individualized partial gland cryoablation
The median PSA nadir was 0.82 ng/dL (IQR 0.4–1.8) after cryotherapy. A total of 42 (59%) of patients underwent a for-cause follow-up biopsy. Median time to biopsy was 474.5 days (IQR 393–558.5). Thirty-six of the 42 (85.7%) patients underwent an mpMRI of the prostate after cryotherapy. A total of 11 of the 36 (30.5%) of patients who underwent an MRI had a suspicious lesion in the ablation zone. Three of the 11 (27.3%) of lesions were positive for grade group 2 cancer on biopsy.
Of the 42 patients who underwent a post-treatment 12 core (including treatment targets) biopsy, 7 (16.7%) patients were found to have grade group 1 disease and 9 (21.4%) patients were found to have grade group 2 or higher disease. IFR was found in 8 (19%) patients (5 patients with grade group 2, 1 patient with grade group 4, and 2 patients with grade group 5). Out-of-field recurrence was found in 1 (2.4%) patient (grade group 2). Five of the nine patients with recurrence opted for a second partial gland cryoablation of the prostate, and four of the five patients who were retreated had their PSA nadir less than 1 ng/dL. The one patient whose PSA remained above 1 ng/dL was subsequently treated with EBRT and androgen deprivation therapy. The remaining four of the nine patients with recurrence were treated with whole gland therapy (three received EBRT, and one underwent a salvage prostatectomy).
Of the remaining 29 patients who did not undergo a biopsy, 12 (41.4%) of the 29 patients refused a standard, recommended follow-up biopsy given their PSA nadired <0.5 ng/dL. A total of 7 (24.1%) of the 29 patients who declined biopsy had a PSA that nadired between 0.5 and 1 ng/dL. Ten (34.5%) of the 29 patients declined a biopsy despite medical recommendation, even though their nadir PSA was >1 ng/dL.
FFS, definitive treatment and metastasis
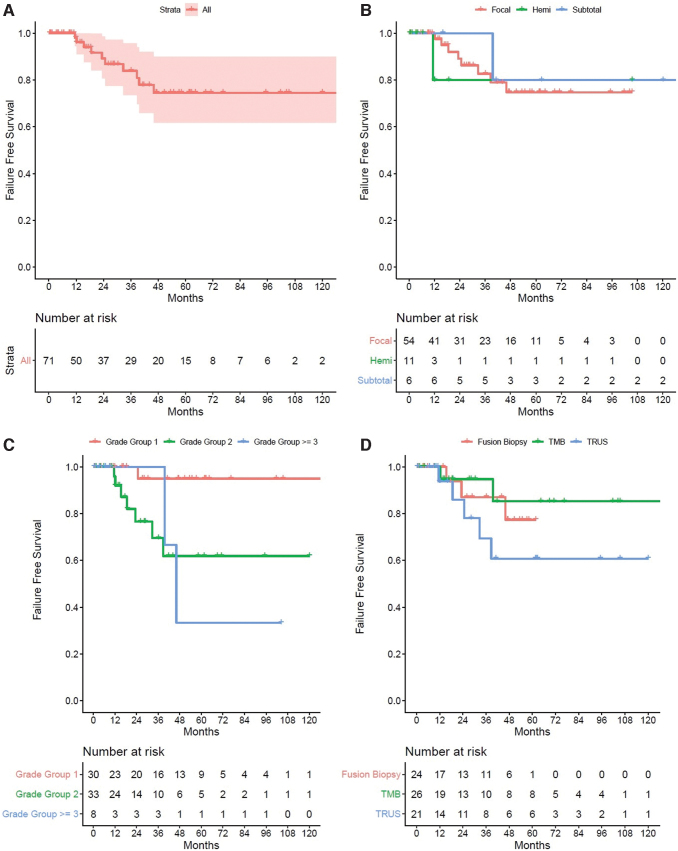
The FFS in the primary setting at years 1 to 5 was 98%, 89%, 84%, 75%, and 75% (Fig. 1A). When stratified by type of partial gland ablation in the primary setting, there was no statistically significant difference between treatment schematic, p = 0.36 (Fig. 1B). When stratified by grade group score, patients with grade group 1 had a higher FFS followed by grade group 2 and 3, p = 0.006 (Fig. 1C). When stratified by types of biopsy performed, patients who underwent a 3D transperineal prostate mapping biopsy had a higher FFS, followed by patients who underwent a fusion biopsy and standard 12 core biopsy (Fig. 1D). There were no patients who developed a metastasis or died from prostate cancer after partial gland ablation. On multivariate cox proportional hazards analysis, a higher-grade group was associated with a higher likelihood of treatment failure, p = 0.006 (hazard ratio 4.0 [95% confidence interval 1.47–10.7]).
FIG. 1.
(A) Kaplan-Meier plot of failure-free survival in patients undergoing primary partial gland ablation. (B) Kaplan-Meier plot of failure-free survival in patients undergoing primary partial gland ablation stratified by type of ablation. (C) Kaplan-Meier plot of failure-free survival in patients undergoing primary partial gland ablation stratified by grade group score. (D) Kaplan-Meier plot of failure-free survival in patients undergoing primary partial gland ablation stratified by type of biopsy. Color images are available online.
AUA symptoms score, IIEF-5 scores, urinary continence, and fistula rate
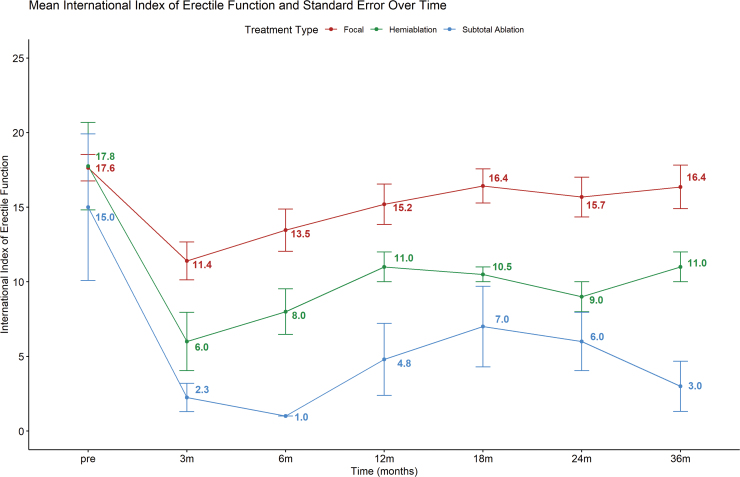
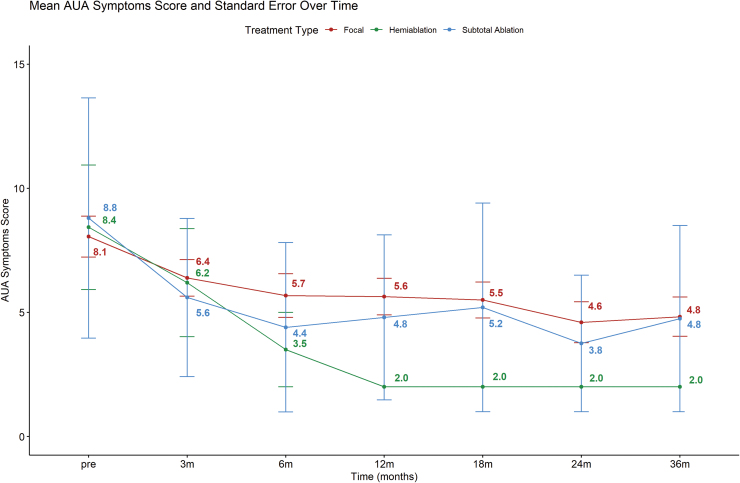
Urinary continence status in all 71 patients was available at baseline and at 3 months of follow-up. All 71 patients remained continent and free of pads at 3 months. No patient developed a fistula. Mean IIEF-5 score for patients' post-focal ablation, hemiablation, and subtotal ablation was 17.8, 17.6, and 15.0, respectively. Figure 2 depicts the mean IIEF-5 score over the course of 36 months. Evidently, patients who underwent focal gland ablation had a higher post-treatment IIEF-5 score compared with hemiablation and subtotal ablation of the prostate. Mean AUA symptom score for patients treated with focal ablation, hemiablation, and subtotal ablation was 8.0, 8.4, and 8.8 before partial gland cryoablation of the prostate (Fig. 3). The AUA symptoms score decreased across all three groups after partial cryoablation of the prostate, with hemiablation having the most improvement compared with subtotal gland ablation and focal ablation (Fig. 3).
FIG. 2.
Line plot of IIEF score over a period of 36 months. IIEF = International Index of Erectile Function. Color images are available online.
FIG. 3.
Line plot of AUA symptoms score over a period of 36 months. AUA, American Urological Association. Color images are available online.
Trifecta rate
A total of 41 (58%) patients were able to achieve erections that were firm enough for penetration before partial gland cryoablation of the prostate (Table 2). After partial gland ablation, 27 (81%) patients in the focal ablation group were able to maintain erections that were firm enough for penetration. Twenty-six (79%) patients in the focal ablation group were able to achieve all three factors (erections firm enough for penetration; no urinary incontinence; did not have an IFR, OFR, were able to avoid whole gland/systemic therapy, and did not die from prostate cancer).
Table 2.
Success of Focal Therapy at 5 Years
| Focal | Hemi | Subtotal | |
|---|---|---|---|
| Patients able to achieve erections firm enough for penetrative sex pretreatment | 33 | 3 | 5 |
| Patients able to achieve erections firm enough for penetrative sex post-treatment | 27 (81%) | 0 (0%) | 0 (0%) |
| IFR, OFR, received whole gland, systemic therapy, or death within 5 years after treatment | |||
| Yes | 6 (18%) | 0 (0%) | 0 (0%) |
| No | 27 (82%) | 3 (100%) | 5 (100%) |
| Incontinence | |||
| Yes | 0 (0%) | 0 (0%) | 0 (0%) |
| No | 33 (100%) | 3 (100%) | 5 (100%) |
| All three factors | 26/33 (79%) | 0/3 (0%) | 0/5 (0%) |
IFR = infield recurrence; OFR = out-of-field recurrence.
Sensitivity analysis
Sensitivity analysis was performed by using multiple imputations to address missing data for the IIEF-5 and AUA symptoms scores. Density plots were used to visualize imputed data, and the imputed values were, indeed, “plausible values.” The imputed line graph for IIEF-5 scores resulted in a similar pattern as Figure 2, with focal ablation showing the highest IIEF-5 at all time points, followed by hemiablation and subtotal ablation. Similarly, the imputed line graph for AUA symptoms score also showed lower values across all time points compared with AUA symptom scores before therapy.
Salvage ablation
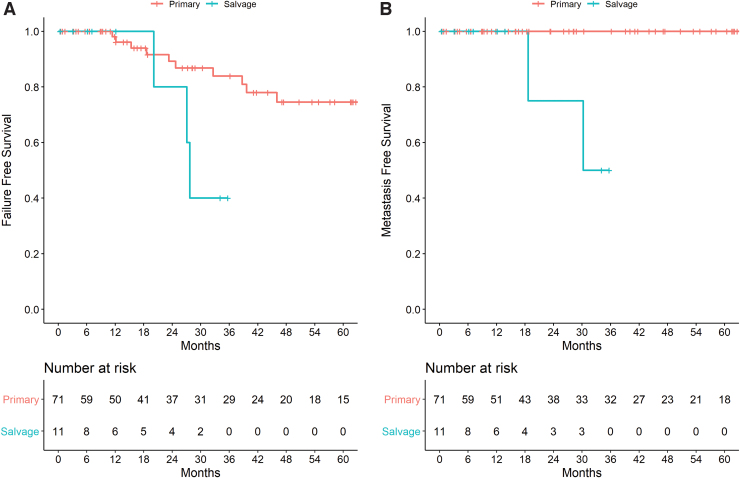
A total of 11 patients received salvage partial gland cryoablation of the prostate. Patient demographics are listed in Table 1. Median follow-up was 12 months (IQR 4.6–26.8). FFS was 100%, 80%, and 40% at 12, 24, and 36 months, respectively, whereas metastasis-free survival was 100%, 75%, and 50% at 12, 24, and 36 months, respectively (Fig. 4A, B). One patient developed incontinence requiring a sling procedure, and one patient developed a rectal–urethral fistula requiring a colonic diversion.
FIG. 4.
(A) Kaplan-Meier plot of failure-free survival in patients undergoing partial gland ablation stratified by primary or salvage therapy. (B) Kaplan-Meier plot of metastasis-free survival in patients undergoing partial gland ablation stratified by primary or salvage therapy. Color images are available online.
Discussion
Individualized partial gland cryoablation of the prostate is gaining traction as an exciting alternative therapy for localized prostate cancer. In this study, we sought to evaluate the oncological and functional outcomes of patients treated with partial gland cryoablation at our institution. We observe an FFS of 75% at 5 years for primary treatment and show that patients in the higher grade group have a higher likelihood of failure. We used very strict criteria to define failure, including IFR, OFR, any form of whole gland treatment, initiation of systematic therapy, or death.
Further, we found that patients who underwent 3D transperineal mapping biopsy (TMB) biopsies had a higher FFS compared with patients who underwent a fusion-guided biopsy and 12 core random biopsy, respectively. We believe that the 3D TMB biopsies result in a more accurate pathologic mapping, approximating a radical prostatectomy pathology, therefore resulting in less prostate cancer being missed as we had a better sense of the locations/size and boundaries of the tumor(s).
In terms of functional outcomes, we found that any form of partial gland cryoablation can result in a small decline of IIEF score in the immediate setting. We show that focal gland ablation resulted in the least decline of IIEF-5 score, followed by hemi-cryoablation and subtotal cryoablation of the prostate. The largest decline in IIEF-5 score occurred 3 months after partial gland cryoablation, and the IIEF-5 score seemed to improve and plateau between 12 and 18 months after a period of recovery. Similarly, other studies have documented that there is no statistically significant difference between sexual health inventory for men's scores 12 months after focal gland ablation compared with before focal therapy.17 In terms of AUA symptoms score, all men had an improvement in lower urinary tract symptoms and had lower AUA symptom scores after partial gland cryoablation. The AUA symptom score seems to plateau around 12 months. Interestingly, subtotal cryoablation and hemiablation of the prostate result in a decline of AUA symptom score of 32% and 80% at 3 years, respectively. Our study is unique, as it provides a longitudinal assessment of erectile function and lower urinary tract symptoms using validated instruments in men undergoing partial gland cryoablation for prostate cancer.
We defined success of partial gland cryoablation by three factors: (1) the ability to have penetrative sex after treatment; (2) remain continent and pad-free; and (3) not have any IFR, OFR, be able to avoid whole gland or systemic treatment and death related to prostate cancer after partial gland cryoablation of the prostate. We show that 79% of patients in the focal ablation group were able to achieve all three factors. Further review of the 19% of patients who had new-onset erectile dysfunction post-treatment had a posteriorly located tumor, with most being located in the apex of the prostate as verified by biopsy and mpMRI. Hence, it would be exceedingly challenging to preserve a margin of warm perfused tissue directly adjacent to the neurovascular bundle while attempting to eradicate cancer. Thus, the ability to preserve erectile function, in part, is dependent on location of the tumor around the neurovascular bundles. Regardless of this, the erectile dysfunction rate of 19% is slightly better than the published erectile dysfunction rate of 23% post-prostatectomy, especially in our cohort of patients with patients already having compromised erectile function before treatment where the median pretreatment IIEF-5 score was 17.6.18 Further, none of our patients treated with primary individualized partial gland cryoablation reported urinary incontinence, and lower urinary tract symptoms improved post cryoablation, regardless of the treatment template used. Urinary incontinence has been previously shown to be associated with a change in the patient's quality of life and distress for partner, especially in the post-prostatectomy cohort.19
Our study has several limitations that should be interpreted within context. First, selection bias is present due to the study's retrospective nature. Patients desired this treatment and from a patient selection viewpoint, they had a reasonably limited tumor burden that was able to be localized and treated by using both biopsy and mpMRI information. Also, many private insurers in the United States refuse to cover mpMRI for patients without a prior 12-core biopsy. Second, patient treatment templates were customized based on the location and volume of the malignant elements present. Third, there were several technical modifications over the course of the study period, as focal therapy is a technique in evolution. Fourth, only 59% of our patients underwent a follow-up biopsy. It remains challenging to convince men, particularly with a low stable PSA, unremarkable follow-up mpMRI, and good genitourinary function, who feel well, to commit to a biopsy, a procedure that is the more invasive means to monitor their follow-up. Last, given that our institution is a referral center, some patients are followed up locally due to travel distances, leading to early censoring in the Kaplan Meier plots. In fact, there were nine patients who chose to be followed locally, once their urethral catheter was removed on postoperative day 14. This could actually be considered a strength of the study, as omitting these patients from the dataset would result in a median follow-up of 51 months, and that is a better representation of this cohort. These nine patients resulted in 12% of missing data for the IIEF-5 and AUA symptoms score inventories. To ensure the result was accurate, we performed a sensitivity analysis by using multiple imputations to account for the missing data. Despite these limitations, we offer a very robust analysis of men treated with partial gland cryoablation at a tertiary care center with minimal missing data, using very strict criteria for treatment failure and functional outcomes.
Conclusion
In the appropriate clinical context, partial gland ablation of the prostate is able to achieve excellent genitourinary functional and oncological outcomes in men with localized prostate cancer. Prospective clinical trials with larger numbers and appropriate endpoints are needed to determine whether partial gland ablation of the prostate could be a substitute for radical therapy in select cases. Partial gland ablation may currently be an option for men who want to avoid radical therapy for localized prostate cancer.
Abbreviations Used
- 3D
three-dimensional
- ADT
androgen deprivation therapy
- AUA
American Urological Association
- BMI
body mass index
- EBRT
external beam radiation therapy
- FFS
failure-free survival
- GG
grade group
- IFR
infield recurrence
- IIEF
International Index of Erectile Function
- IQR
interquartile range
- mpMRI
multiparametric magnetic resonance imaging
- MRI
magnetic resonance imaging
- OFR
out-of-field recurrence
- PET
positron emission tomography
- PSA
prostate-specific antigen
- US
ultrasound
Appendix
Techniques
Multiparametric magnetic resonance imaging
Our institution's MRI protocol has been previously described.A1 Briefly, patients underwent a multiparametric MRI by using a 3T MRI scanner and a single channel endorectal coil. Imaging sequences included a T2-weighted sequence, diffusion weighted sequence, and a dynamic contrast enhancement sequence.
Multiparametric magnetic resonance imaging and ultrasound fusion-guided biopsy
We performed two to four image-targeted biopsies per lesion that were ≥ Prostate Imaging-Reporting and Data System (PIRADS) 3, along with a minimum of 12 core random systematic biopsy. All fusion biopsies were performed by using the UroNav system and DynaCAD software (Phillips, Andover, MA).
Three-dimensional template mapping saturation biopsy
Transperineal three-dimensional template mapping saturation biopsy was performed as described by Barzell and Melamed.A2 Briefly, we obtained a minimum of 32 cores in a systematic fashion covering the apex to the base of the prostate and both the right and left side of the prostate in a systematic fashion.A2–A4
Radiation failure
Our biopsy protocol for patients with radiation failure is to obtain a multiparametric magnetic resonance imaging and ultrasound (mpMRI/US) fusion-guided biopsy if a lesion is localized, 12-core systematic random biopsy, and 4 cores of each seminal vesical biopsy (2 cores at the base and 2 at the seminal vesicle tip).A5
Individualized partial gland cryoablation
Our individualized partial cryoablation technique has been previously described.A6 Briefly, cryo-needles were inserted under general or regional anesthesia while in the lithotomy position. Cryo-needle placement was confirmed by both axial and sagittal transrectal ultrasonography. The needles were inserted in a fashion that allowed sufficient overlap of adjacent ice balls for adequate coverage of the targeted region with lethal ice. Focal gland ablation entailed cryoablation of the targeted lesion with an ∼1 cm margin while sparing the neurovascular bundle. Hemiablation entailed freezing an entire half gland (left/right with the urethra representing the midline, or the anterior prostate) of the prostate and its corresponding neurovascular bundle. Subtotal ablation entailed freezing an entire half gland (left/right), while placing probes on the contralateral side to freeze part of the contralateral gland, producing an ice ball in a hockey-stick ablation pattern and resulting in the freezing of one or both neurovascular bundles. We decided to treat patients in a focal, hemi-ablation, or subtotal gland ablation manner based on the amount of tumor on biopsy as well as lesion volume on mpMRI of the prostate. We ablated the minimal amount of prostate tissue preserving a margin of 1 cm around the area of the lesion on MRI and positive biopsy. We are also more inclined to perform hemi-ablation of the prostate if the neurovascular bundle is already involved or is in close proximity to the lesion, and treatment would have ablated the neurovascular bundle anyway. If the lesion is at least 1 cm away from the neurovascular bundle, we typically perform a focal ablation and attempt to spare the neurovascular bundle. Two temperature thermistors were placed at Denonvillier's fascia and the urinary sphincter, respectively. The procedure was monitored with a combination of real-time ultrasonography and temperature thermometry. All patients underwent a dual freeze–thaw cycle.
Definition of failure-free survival, infield recurrence, and metastasis-free survival
Failure-free survival was defined as the absence of infield recurrence, out-of-field recurrence, absence of whole gland or systemic therapy, and death/mortality. Metastasis-free survival was defined as the duration from the date of treatment until last follow-up or the diagnosis of metastasis on bone scan or positron emission tomography (PET)/CT as indicated by the clinician. Cancer-specific survival was defined as the duration from the date of treatment until death due to prostate cancer, or last follow-up.
Post-cryosurgery monitoring
Follow-up comprised clinical examination and prostate-specific antigen (PSA) level determination at every 6 months for the subsequent 5 years, followed by a PSA level annually. mpMRI was obtained at 3 to 6 months, 12 to 24 months, and at 5 years after image-targeted cryoablation. A for-cause biopsy was performed in follow-up of the primary cohort if the post-cryoablation PSA exceeded 0.5 ng/mL. Fusion-targeted biopsy was performed on any suspicious lesion visualized on mpMRI. If no lesion was identified and if the patient's nadir PSA exceeded 0.5 ng/mL, a 12-core random biopsy including the previous area(s) of ablation was performed at 12 to 24 months and again at 5 years as per our previously published protocol.A7
Appendix References
- A1.Holtz JN, Silverman RK, Tay KJ, et al. New prostate cancer prognostic grade group (PGG): Can multiparametric MRI (mpMRI) accurately separate patients with low-, intermediate-, and high-grade cancer? Abdom Radiol (NY) 2018;43:702–712. [DOI] [PubMed] [Google Scholar]
- A2.Barzell WE, Melamed MR. Appropriate patient selection in the focal treatment of prostate cancer: The role of transperineal 3-dimensional pathologic mapping of the prostate—A 4-year experience. Urology 2007;70(Suppl 1):S27–S35. [DOI] [PubMed] [Google Scholar]
- A3.Aminsharifi A, Gupta RT, Huang J, Polascik TJ. Three-dimensional localization and targeting of prostate cancer foci with imaging and histopathologic correlation: Establishing a multidisciplinary team for quality improvement. Curr Opin Urol 2018;28:506–511. [DOI] [PubMed] [Google Scholar]
- A4.Chen VH, Mouraviev V, Mayes JM, et al. Utility of a 3-dimensional transrectal ultrasound-guided prostate biopsy system for prostate cancer detection. Technol Cancer Res Treat 2009;8:99–104. [DOI] [PubMed] [Google Scholar]
- A5.Kovac E, ElShafei A, Tay KJ, Mendez M, Polascik TJ, Jones JS. Five-year biochemical progression-free survival following salvage whole-gland prostate cryoablation: Defining success with nadir prostate-specific antigen. J Endourol 2016;30:624–631. [DOI] [PubMed] [Google Scholar]
- A6.Polascik TJ, Nosnik I, Mayes JM, Mouraviev V. Short-term cancer control after primary cryosurgical ablation for clinically localized prostate cancer using third-generation cryotechnology. Urology 2007;70:117–121. [DOI] [PubMed] [Google Scholar]
- A7.Tay KJ, Amin MB, Ghai S, et al. Surveillance after prostate focal therapy. World J Urol 2019;37:397–407. [DOI] [PubMed] [Google Scholar]
Author Disclosure Statement
No competing financial interests exist.
Funding Information
W.P.T. is supported by the Ruth L. Kirschstein NRSA Institutional Research Training Grant (T32-CA093245).
References
- 1. Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378:1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahdoot M, Lebastchi AH, Turkbey B, Wood B, Pinto PA. Contemporary treatments in prostate cancer focal therapy. Curr Opin Oncol 2019;31:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aminsharifi A, de la Rosette J, Polascik TJ. Focal therapy of prostate and kidney cancer. Curr Opin Urol 2018;28:491–492. [DOI] [PubMed] [Google Scholar]
- 4. Tay KJ, Schulman AA, Sze C, Tsivian E, Polascik TJ. New advances in focal therapy for early stage prostate cancer. Expert Rev Anticancer Ther 2017;17:737–743. [DOI] [PubMed] [Google Scholar]
- 5. Valerio M, Cerantola Y, Eggener SE, et al. New and established technology in focal ablation of the prostate: A systematic review. Eur Urol 2017;71:17–34. [DOI] [PubMed] [Google Scholar]
- 6. Truesdale MD, Cheetham PJ, Hruby GW, et al. An evaluation of patient selection criteria on predicting progression-free survival after primary focal unilateral nerve-sparing cryoablation for prostate cancer: Recommendations for follow up. Cancer J 2010;16:544–549. [DOI] [PubMed] [Google Scholar]
- 7. Onik G, Vaughan D, Lotenfoe R, Dineen M, Brady J. The “male lumpectomy”: Focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-up. Urol Oncol 2008;26:500–505. [DOI] [PubMed] [Google Scholar]
- 8. Tan WP, ElShafei A, Aminsharifi A, Khalifa AO, Polascik TJ. Salvage focal cryotherapy offers similar short-term oncologic control and improved urinary function compared with salvage whole gland cryotherapy for radiation-resistant or recurrent prostate cancer. Clin Genitourin Cancer 2020;18:e260–e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: A report from the national Cryo On-Line Database (COLD) Registry. BJU Int 2012;109:1648–1654. [DOI] [PubMed] [Google Scholar]
- 10. Ellis DS, Manny TB Jr., Rewcastle JC. Focal cryosurgery followed by penile rehabilitation as primary treatment for localized prostate cancer: Initial results. Urology 2007;70(6 Suppl):9–15. [DOI] [PubMed] [Google Scholar]
- 11. Johansson J-E, Andrén O, Andersson S-O, et al. Natural history of early, localized prostate cancer. JAMA 2004;291:2713–2719. [DOI] [PubMed] [Google Scholar]
- 12. Dall'Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: A systematic review of the literature. Eur Urol 2012;62:976–983. [DOI] [PubMed] [Google Scholar]
- 13. Tourinho-Barbosa RR, Sanchez-Salas R, Claros OR, et al. Focal therapy for localized prostate cancer with either high intensity focused ultrasound or cryoablation: A single institution experience. J Urol 2020;203:320–330. [DOI] [PubMed] [Google Scholar]
- 14. Cappelleri JC, Siegel RL, Glasser DB, Osterloh IH, Rosen RC. Relationship between patient self-assessment of erectile dysfunction and the sexual health inventory for men. Clin Ther 2001;23:1707–1719. [DOI] [PubMed] [Google Scholar]
- 15. Barry MJ, Fowler FJ Jr., O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992;148:1549–1557; discussion 64. [DOI] [PubMed] [Google Scholar]
- 16. RStudio Team. RStudio: Integrated Development for R. Boston, MA: RStudio, Inc., 2015. [Google Scholar]
- 17. Barqawi AB, Stoimenova D, Krughoff K, et al. Targeted focal therapy for the management of organ confined prostate cancer. J Urol 2014;192:749–753. [DOI] [PubMed] [Google Scholar]
- 18. Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA 2011;306:1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250–1261. [DOI] [PubMed] [Google Scholar]