Abstract
Significance: Acetaminophen (APAP) is one of the quantitively most consumed drugs worldwide. Although safe at therapeutic doses, intentional or unintentional overdosing occurs frequently causing severe liver injury and even liver failure. In the United States, 50% of all acute liver failure cases are caused by APAP overdose. However, only one antidote with a limited therapeutic window, N-acetylcysteine, is clinically approved. Thus, more effective therapeutic interventions are urgently needed.
Recent Advances: Although APAP hepatotoxicity has been extensively studied for almost 50 years, particular progress has been made recently in two areas. First, there is now a detailed understanding of involvement of oxidative and nitrosative stress in the pathophysiology, with identification of the reactive species involved, their initial generation in mitochondria, amplification through the c-Jun N-terminal kinase pathway, and the mechanisms of cell death. Second, it was demonstrated in human hepatocytes and through biomarkers in vivo that the mechanisms of liver injury in animals accurately reflect the human pathophysiology, which allows the translation of therapeutic targets identified in animals to patients.
Critical Issues: For progress, solid understanding of the pathophysiology of APAP hepatotoxicity and of a drug's targets is needed to identify promising new therapeutic intervention strategies and drugs, which may be applied to humans.
Future Directions: In addition to further refine the mechanistic understanding of APAP hepatotoxicity and identify additional drugs with complementary mechanisms of action to prevent cell death, more insight into the mechanisms of regeneration and developing of drugs, which promote recovery, remains a future challenge. Antioxid. Redox Signal. 35, 718–733.
Keywords: acetaminophen, drug hepatotoxicity, mitochondria, peroxynitrite, therapeutic approaches, Nrf2
Introduction
Acetaminophen (N-acetyl-p-aminophenol, APAP) is an analgesic and antipyretic used worldwide. At therapeutic doses, it is generally considered safe (74, 106), however, subclinical transient alanine aminotransferase (ALT) elevations can occur in susceptible patients (74). In very rare cases when multiple risk factors (chronic alcoholism, fasting) are combined, therapeutic doses have been suspected to cause severe liver injury (68, 72). In contrast, intentional or unintentional overdoses can cause liver injury and in severe cases even acute liver failure (47, 66, 119). The establishment of a mouse model of APAP-induced liver injury provided an early mechanistic insight into the toxicity, including the cytochrome P450-dependent formation of a reactive metabolite (85), which can be detoxified by cellular glutathione (GSH) (Fig. 1A) (86). However, after an overdose, hepatic GSH levels are depleted and the reactive metabolite, later identified as N-acetyl-p-benzoquinone imine (NAPQI) (21), covalently binds to sulfhydryl groups of proteins (54), a key event in the toxicity. Based on this early mechanistic understanding, N-acetylcysteine (NAC) was identified as an effective antidote (96, 108); NAC remains to this day the only FDA-approved drug against APAP overdose.
FIG. 1.
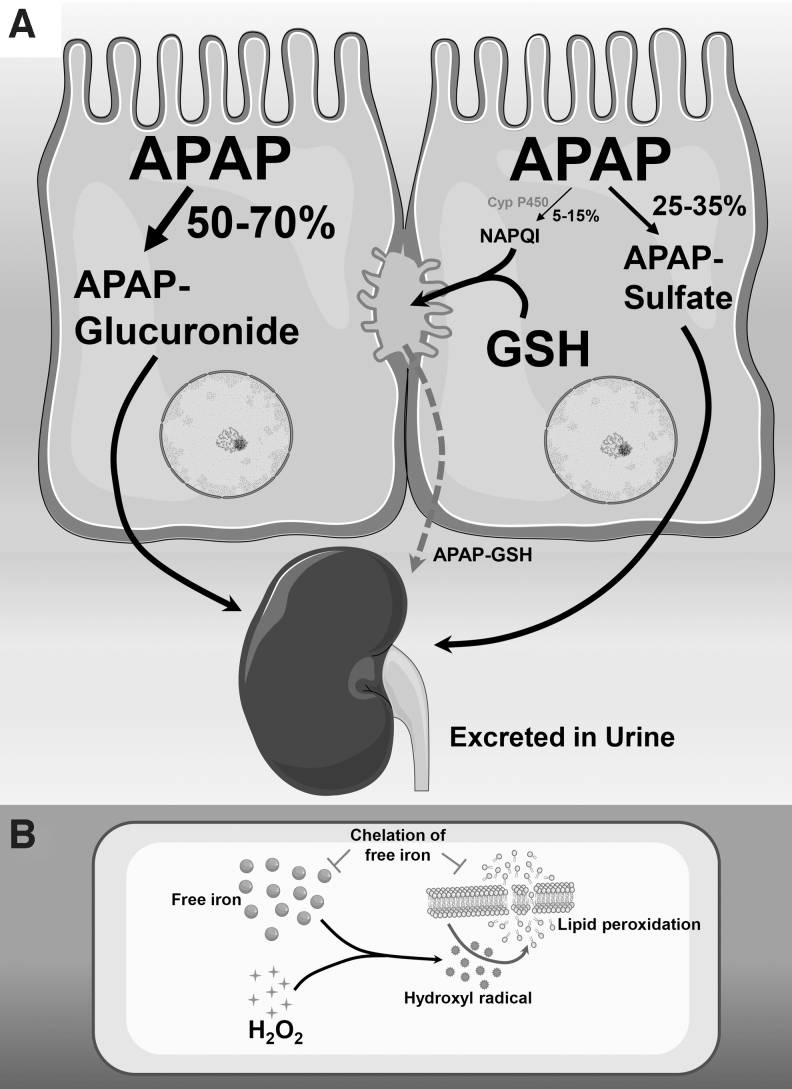
APAP metabolism and mechanism of lipid peroxidation. (A) Metabolic pathways for APAP in liver. Under therapeutic doses, APAP is predominantly metabolized to the glucuronide or sulfate forms, which are then excreted through the kidney. A small percentage of APAP undergoes cytochrome P450-mediated metabolism to a reactive metabolite NAPQI, which is efficiently scavenged by hepatic glutathione and initially excreted into bile, from where it is transported to the kidney for excretion. (B) Role of free iron in lipid peroxidation. Intracellular hydrogen peroxide generation from oxygen free radicals can react with available cellular free iron through the Fenton reaction to produce reactive hydroxyl radicals. These reactive species can target polyunsaturated membrane lipids to induce lipid peroxidation, which initiates a chain reaction with formation of lipid peroxides, which further degrade membrane lipids and ultimately cause membrane damage. The critical role of free iron in lipid peroxidation is illustrated by the protection afforded by chelation of free iron, which prevents lipid peroxidation. APAP, acetaminophen; NAPQI, N-acetyl-p-benzoquinone imine. These figures include templates from Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 generic license.
Over the years, an ever-increasing number of studies using mainly mice and mouse hepatocytes led to a more detailed understanding of the cell death mechanisms and overall organ injury (99). Importantly, experiments with human hepatocytes (81, 135) and the use of biomarkers in overdose patients (6, 7, 78, 79, 129) could confirm in humans most of the pathophysiology established in mice. The fact that APAP overdose is a significant clinical problem and that the mouse is a clinically highly relevant animal model (52) contributed to the widespread use of the murine APAP-induced liver injury model to investigate mechanisms of drug hepatotoxicity and to evaluate intervention strategies against drug-induced liver injury. In particular, the critical role of oxidant stress in the pathophysiology makes this the favorite model to test the efficacy of real or assumed antioxidants, especially from the natural product area (16, 120). Nevertheless, the mechanism of APAP toxicity is complicated and misinterpretations occur frequently. This review focuses in detail on the role of various reactive oxygen species (ROS) in the signaling mechanisms of APAP-induced cell death and discusses emerging novel therapeutic strategies that target oxidants and antioxidant pathways.
History of Oxidant Stress in APAP Hepatotoxicity
The early studies of the mechanisms of APAP hepatotoxicity focused only on protein adduct formation as key event in the toxicity (54, 85). However, a few years later, Wendel and coworkers introduced the concept of cytochrome P450-dependent oxidant stress and lipid peroxidation (LPO) as the central mechanism of APAP-induced cell death (126, 127). This caused a significant controversy. Mitchell and colleagues argued against the idea of reactive oxygen formation during APAP metabolism. Any oxidant stress involving enhanced release of superoxide and hydrogen peroxide (H2O2) would result in increased glutathione disulfide (GSSG) generation during detoxification by glutathione peroxidase. However, no enhanced GSSG formation was observed during the metabolism phase after APAP overdose in rats or mice (67, 118).
In addition, the pathophysiological relevance of LPO was questioned when chelation of iron, which facilitates the formation of hydroxyl radicals or lipid alkoxyl radicals (Fig. 1B), attenuated LPO but had no effect on APAP-induced liver injury (141). Some of these contradictory findings could be reconciled when considering that the extreme susceptibility of Wendel's mice was caused by feeding the animals a diet high in soybean oil (polyunsaturated fatty acids) and deficient in vitamin E (126, 127). In addition, it was recognized that there was an oxidant stress in mitochondria after the drug metabolism phase (10, 46, 123), which correlated with mitochondrial protein adduct formation and toxicity (122).
A few years later, evidence for the formation of peroxynitrite in the centrilobular area was provided (39); not unexpected, the peroxynitrite formation occurs inside mitochondria (20). Together, these data suggest that the cytochrome P450-dependent formation of NAPQI causes protein adduct formation in mitochondria, thereby triggering an oxidant stress and a peroxynitrite formation (49). As discussed below, the real mechanisms of oxidant stress involvement in the pathophysiology are more complicated. The basic concept that NAPQI formation and protein adducts are the initiating event of the toxicity, which causes an oxidant stress that is critical for the cell death mechanism, led to the conclusion that countless interventions that protected in this model did so because of their antioxidant effect. Unfortunately, the critical upstream event (P450-mediated NAPQI formation) is mostly ignored, which then raises concerns that the compound may act as an inhibitor of cytochrome P450 and not as an antioxidant. Thus, it is essential to understand the detailed mechanisms of the oxidative and nitrosative stress involvement in the pathophysiology to successfully target these events with therapeutic intervention strategies.
Sources of Reactive Oxygen and Nitrogen Radicals After APAP
Cytochrome P450
The fact that APAP can be metabolized mainly through cytochrome P450 2E1 (Cyp2E1) to form the reactive metabolite NAPQI focused attention on this enzyme as a direct source of ROS after an APAP overdose. This assumption was supported by studies demonstrating that cytochrome P450-mediated drug metabolism in vitro could directly generate superoxide and H2O2 during the enzyme reaction (65), and such Cyp2E1-mediated ROS production was implicated in alcohol-induced liver injury (63). It was thus proposed that cytochrome P450-mediated ROS generation after APAP led to LPO and subsequent liver injury in the mouse (126). However, direct evidence of cytochrome P450-mediated ROS production in vivo is lacking and even in animal models of APAP hepatotoxicity, no increase in oxidized glutathione was evident after APAP (118), questioning the role of cytochrome P450 as a primary source of ROS in vivo. Thus, although Cyp2E1 has been implicated in ROS production in a number of studies, the vast majority are in vitro or in cell culture, and the relevance of these to in vivo ROS production is not established (36).
Interestingly, measurement of F2 isoprostanes, which are a more reliable marker of ROS generation in vivo, showed no increase when rats were treated with the Cyp2E1 inducer isoniazid (24), and Cyp2e1 knockout mice were shown to have very similar levels of liver, brain, and urinary isoprostanes as the wild-type animals (25). In the context of APAP hepatotoxicity, the time line of ROS production also argues against a significant contribution from cytochrome P450 enzymes. Measurement of free radical production using 2′,7′-dichlorofluorescein fluorescence in primary mouse hepatocytes only showed elevations after APAP metabolism, when GSH levels were depleted at 3–4 h after APAP exposure (10). Alternate measurement of ROS by examination of peroxynitrite in the metabolically competent human hepatoma cell line HepaRG also showed elevations only after APAP metabolism (81).
Another point arguing against ROS production during cytochrome P450-mediated APAP metabolism is the fact that although rats metabolize APAP and produce protein adducts, no relevant oxidative stress or liver injury is generally detectable (80). Some studies using rats typically report a minor increase in plasma ALT and liver malondialdehyde levels after a high overdose of APAP, suggesting minor liver injury and some oxidant stress (110). This supports the low susceptibility of rats to APAP overdose compared with mice or humans and indicates a minor oxidant stress at later time points, but not during drug metabolism. Although more recent studies using nanosensors have detected H2O2 formation during APAP metabolism in vivo, which was blunted by cytochrome P450 inhibitors (115), the biological relevance of these minor elevations in ROS is not confirmed, and again, direct evidence of cytochrome P450-mediated ROS production in vivo is lacking. Taken together, current evidence does not support the notion that direct ROS production through cytochrome P450 enzymes plays an important role in APAP hepatotoxicity.
Early mitochondrial superoxide production
Although it was well established that excess NAPQI formed after an APAP overdose depletes cytosolic and mitochondrial glutathione stores in the liver and subsequently binds to cysteine moieties of cellular proteins (17, 77), the importance of the mitochondria was only apparent when experiments were conducted comparing APAP with its nontoxic (in mice) regioisomer, 3′-hydroxyacetanilide (AMAP). A comparison of protein adducts of the reactive metabolites between the two treatments identified mitochondrial adducts in APAP, but not in AMAP-treated mice (76, 89, 122). The importance of mitochondrial adducts was further reiterated by more recent experiments in human hepatocytes, where AMAP was found to be cytotoxic and its reactive metabolite also formed mitochondrial adducts (136).
Early analysis of mitochondrial targets for protein binding identified a number of candidates such as glutathione peroxidase 1 (GPx1) (123) as well as the α subunit of ATP synthase (97), which result in decreased GPx1 activity and compromised mitochondrial ATP production (46, 123), respectively. While these modifications ultimately induce mitochondrial oxidant stress (23, 46, 83), the direct molecular events mediated by mitochondrial ROS after NAPQI protein adduct formation and the importance of this to downstream cellular signaling after APAP were not well established.
Our recent data provide additional insight into these mechanisms, revealing the nuanced role of mitochondrial ROS in mediating cellular signaling at various phases of APAP-hepatotoxicity. Examining very early changes in mitochondrial adduct formation and superoxide formation 15–60 min after APAP in mice, we demonstrated that the initial production of superoxide after NAPQI adduct formation in mitochondria occurs from respiratory complex III, which releases superoxide toward the intermembrane space and into the cytosol accompanied by elevations in H2O2 (90). This superoxide generation occurs without change in mitochondrial respiratory rates or coupling indicating lack of catastrophic mitochondrial damage at this stage. This early superoxide generation also occurs before detectable peroxynitrite generation in hepatocytes, indicating that mitochondrial generation of ROS occurs in stages with varying consequences throughout the initial time line after NAPQI-adduct formation (Fig. 2).
FIG. 2.
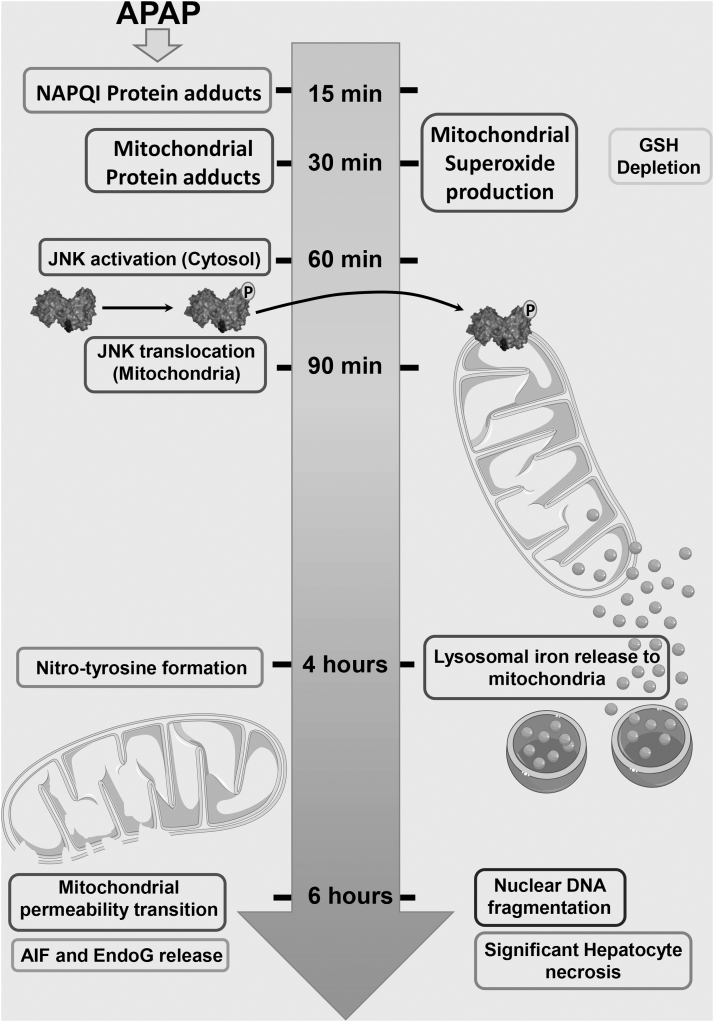
Time course of changes after an APAP overdose. In a typical time course in the mouse after a moderate overdose of 300 mg/kg APAP, NAPQI-protein adducts are detectable within 15 min in the liver and mitochondrial protein adducts by 30 min post-APAP. This is accompanied by enhanced mitochondrial superoxide production and hepatic glutathione depletion. Activation of the MAP kinase JNK is evident by 60 min within the cytosol and its translocation to mitochondria by 90 min. The resultant amplification of mitochondrial injury and nitrotyrosine modification of proteins are evident by 4 h post-APAP, at which point lysosomal iron release to mitochondria is also evident. By 6 h post-APAP, complete induction of the mitochondrial membrane permeability transition, release of intramitochondrial proteins such as AIF and EndoG, nuclear DNA fragmentation, and significant hepatocyte necrosis are evident. AIF, apoptosis-inducing factor; EndoG, endonuclease G; JNK, c-Jun N-terminal kinase; MAP, mitogen activated protein. This figure includes templates from Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 generic license.
Mitochondrial ROS after c-Jun N-terminal kinase translocation
The early directional release of superoxide from mitochondrial respiratory complex III into the mitochondrial intermembrane space and subsequently the cytosol activates a mitogen activated protein (MAP) kinase cascade (98), which results in activation a translocation of the c-Jun N-terminal kinase (JNK) from the cytosol to the mitochondria (37) and ultimately compromises mitochondrial respiration (23, 83). JNK translocation to mitochondria amplifies the mitochondrial oxidant stress through Src-mediated inhibition of the mitochondrial electron transport chain (132), which then has far-reaching consequences (Fig. 3A). Mitochondrial ROS generation subsequent to JNK translocation seems to predominantly be focused on respiratory complex I, which is a crucial site of ROS formation (35, 44, 64) and can be modulated by formation of respiratory super complexes (75). Complex I activity was increased significantly after an APAP overdose in vivo in mice, with a strong correlation between severity of liver injury and complex I activity (28). Interventions targeting complex I such as metformin treatment were able to prevent oxidative stress and liver injury in mice (28); in addition, exposure of APAP-treated human HepaRG cells to metformin attenuated cell injury and partially mitochondrial dysfunction (28). Interestingly, a recent in vitro study using human hepatocytes also revealed that mitochondrial respiration through complex I substrates was uniquely inhibited by APAP treatment as well (95), suggesting that the upregulation of complex I seen in vivo could be an adaptive response to overcome this inhibition of respiration. This futile increase in complex I activity could then contribute to the detrimental generation of free radicals after JNK translocation.
FIG. 3.
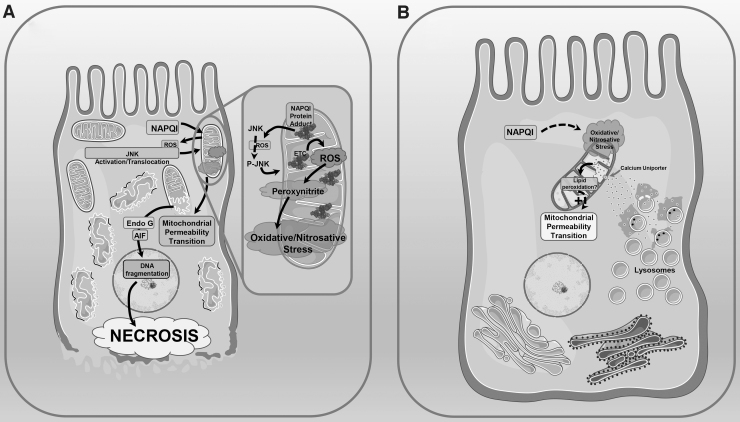
Mitochondrial free radical production and lipid peroxidation in APAP pathophysiology. (A) Mitochondrial oxidant stress is central to APAP pathophysiology. Cytochrome P450-mediated generation of the reactive metabolite NAPQI from APAP results in formation of mitochondrial protein adducts (insert), which induce mild generation of ROS from the mitochondrial ETC into the cytosol. This ultimately activates the MAP kinase JNK, which translocates to the mitochondria and amplifies ROS production from the mitochondrial ETC. Reaction of ETC-generated superoxide with nitric oxide results in generation of peroxynitrite and the subsequent oxidative and nitrosative stress results in induction of the mitochondrial permeability transition. This causes release of mitochondrial intermembrane proteins such as EndoG and AIF into the cytosol. These proteins then translocate to the nucleus and initiate DNA fragmentation, which ultimately results in hepatocyte necrosis. (B) Limited mitochondrial lipid peroxidation may be involved in induction of the mitochondrial permeability transition after APAP. Parallel to the events detailed above, APAP exposure also results in lysosomal instability and release of free iron, which is taken up by mitochondria through the calcium uniporter. This elevation in free iron within mitochondria in the milieu of oxidative/nitrosative stress due to JNK translocation could result in local lipid peroxidation within the mitochondria, which facilitates induction of the mitochondrial permeability transition and subsequent cellular damage as described in Figure 1. ETC, electron transport chain; ROS, reactive oxygen species. These figures include templates from Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 generic license.
Direct evidence of elevations in mitochondrial superoxide has been demonstrated in primary mouse hepatocytes after APAP exposure (139), as well as in the human hepatoma cell line HepaRG (81). This was corroborated by in vivo data demonstrating elevated superoxide production in liver mitochondria isolated from APAP-treated mice (26). All these data confirm that mitochondrial superoxide production after JNK translocation is an important feature of APAP hepatotoxicity. However, what are the consequences of APAP-induced elevations in mitochondrial superoxide? Superoxide generated from the mitochondrial electron transport chain is typically scavenged by mitochondrial antioxidant defenses such as manganese superoxide dismutase (MnSOD), resulting in the formation of H2O2. This can be detoxified by a variety of antioxidant enzymes such as glutathione peroxidase or catalase (50) or by direct reaction with antioxidant molecules such as glutathione (60, 70). However, JNK-mediated electron transport chain dysfunction results in significant elevation in mitochondrial superoxide generation, creating a milieu that allows its reaction with nitric oxide within the mitochondria to generate the more potent nitrating species peroxynitrite (98). Nitrotyrosine staining, a marker for peroxynitrite-mediated protein modification, appears in hepatocytes within 2 h after an APAP overdose (61), by which time JNK activation and mitochondrial translocation are clearly evident (137). Interestingly, it has also been shown that nitration and a decrease in activity of MnSOD also parallel this time course (1), illustrating the functional consequence of elevated peroxynitrite formation. The relevance of compromised MnSOD function in this context is further reiterated in the increased susceptibility of mice with a partial MnSOD deficiency to peroxynitrite and protein carbonyl formation, exacerbating APAP hepatotoxicity (32, 100). Peroxynitrite formation after an APAP overdose has wide-ranging consequences to mitochondrial function, including modification of mitochondrial DNA (20).
The importance of mitochondrial oxidant stress toward APAP pathophysiology is further illustrated by the physiological consequences of its modulation. Faster GSH recovery in mitochondria due to higher induction of GSH-synthesizing enzymes (gclc) after APAP exposure leads to enhanced scavenging of peroxynitrite and ROS, which was shown to contribute to the lower susceptibility of female mice to APAP-induced liver injury (30). From an intervention standpoint, supplementation of mitochondrial GSH was also able to accelerate scavenging of peroxynitrite and H2O2, thus protecting against APAP hepatotoxicity (9, 53, 60). More directly, scavenging superoxide with the mitochondrial-targeted SOD mimetic Mito-TEMPO (125) provided significant protection against APAP-induced mitochondrial oxidant stress and liver injury (26). Protection was also evident on scavenging peroxynitrite with resveratrol (27, 40), reiterating the importance of mitochondrial oxidative and nitrosative stress on APAP pathophysiology. These concepts are now being translated to the clinic with the recent safety trial of the MnSOD mimetic, calmangafodipir, as a therapeutic option in APAP-overdose patients (88).
Role of xanthine oxidase and NADPH quinone dehydrogenase
Another source of free radicals suggested to be involved during APAP overdose was xanthine oxidase (XO), with early data demonstrating conversion of liver xanthine dehydrogenase to oxidase after an APAP overdose, with treatment with the XO inhibitor, allopurinol, providing protection against oxidative stress and APAP-induced liver injury (46). However, it was subsequently evident that the dose of allopurinol required for partial (25 mg/kg) or complete protection (100 mg/kg) against APAP hepatotoxicity was much higher than doses of 5–10 mg/kg that resulted in complete XO inhibition, with a lower dose (where XO was inhibited) having no effect on the injury (46). Mechanistic investigation of the protection against APAP hepatotoxicity by these high allopurinol doses revealed that these effects were independent of XO and were mediated by a preconditioning effect of allopurinol metabolism leading to transcriptional induction of protective mediators such as metallothionein proteins (130). Metallothionein can scavenge NAPQI and ROS (111), resulting in attenuation of APAP-induced liver injury. Although other protective mechanisms of allopurinol pretreatment cannot be excluded, these data conclusively indicate that XO is an unlikely source of ROS during APAP hepatotoxicity.
Another enzyme more recently implicated in APAP pathophysiology is NAD(P)H quinone dehydrogenase 2 (NQO2) (84), which was suggested to play a role merely due to its binding to APAP in vitro and in HeLa cells, where it modulated levels of APAP-induced ROS (84). The relevance of these findings to APAP hepatotoxicity is highly questionable since experiments were not conducted in hepatocytes, and elevations in cytosolic ROS, which would result in enhanced GSSG excretion in the bile, were absent after APAP overdose in vivo (46, 118). Moreover, the large amount of evidence indicating mitochondrial ROS generation in vivo (32, 100), as well as the robust protection by mitochondrial-targeted antioxidants (26, 29), clearly indicates the biological relevance of mitochondrial ROS in APAP pathophysiology.
Iron and LPO in APAP-induced liver injury
Early studies in the 1980s evaluated the role of iron in induction of LPO in the context of comparing mouse models of APAP hepatotoxicity with carbon tetrachloride (CCl4)-induced liver injury. As mentioned earlier, it was clearly determined that while LPO does require the presence of Fe2+-ions, and is involved in CCl4 toxicity, it was not involved in APAP-induced liver damage (142). It was also subsequently shown that treatment with the iron chelator deferoxamine did not offer protection against APAP hepatotoxicity, while preventing CCl4 toxicity (116), indicating that LPO is not the primary mechanism of APAP toxicity. Further studies confirmed that APAP overdose alone does not induce LPO in vivo, and even though LPO was evident when animals were either supplemented with iron or depleted of GSH with phorone along with APAP, these changes did not influence the extent of hepatotoxicity (141). This requirement for a blunted antioxidant response on the background of APAP overdose for detection of LPO was further reiterated in vitamin E-depleted animals as discussed earlier (127).
Subsequent studies in the 1990s also confirmed that while iron chelation showed some decrease in liver injury at earlier time points, no difference in APAP hepatotoxicity was evident at 24 h after APAP (114). Although another study in rats suggested that pretreatment with an iron chelator decreased APAP hepatotoxicity (113), the rat is not a good model for APAP hepatotoxicity because most rat strains are much less sensitive to APAP-induced liver injury than mice or humans (52, 80). Although a subsequent in vitro study using mouse hepatocytes indicated that iron potentiated APAP-induced ROS production (87), the hepatocytes used were cultured for 2 weeks before APAP exposure. Since primary mouse hepatocytes rapidly lose the metabolic ability in culture, NAPQI generation in these cells is probably compromised, and hence, interpretation is questionable. Thus, intensive investigation in the 1980s and 1990s clearly confirmed that alterations in cellular iron and induction of LPO were not biologically relevant mechanisms of APAP hepatotoxicity. While it could be argued that the earlier measurements of LPO using ethane and pentane exhalation were less sensitive, those methods were relatively specific and the lack of appreciable LPO in APAP hepatotoxicity has subsequently been confirmed using more sensitive measurements. Unfortunately, the recent change in semantics labeling iron-mediated, LPO-induced cell death as ferroptosis resulted in a few studies, which completely neglected the earlier mentioned literature on iron and LPO in APAP pathophysiology and implicated ferroptosis in APAP-induced liver injury. As we have recently discussed (48, 51), attributing this nomenclature to APAP-induced hepatocyte death is inaccurate and rather naive and does not advance the understanding of APAP pathophysiology.
Having said that, nuanced investigation of the role of LPO constrained within subcellular organelles such as the mitochondria could have benefits and provide additional mechanistic insight in this context. The intracellular labile iron pool, which can participate in the Fenton reaction and facilitate LPO, is tightly controlled within the cell and trafficked between organelles such as the mitochondria, lysosomes, and the nucleus (73). As discussed earlier, mitochondrial ROS generation and dysfunction are central to APAP pathophysiology, but APAP overdose also induces lysosomal instability (133). In vitro studies in isolated mouse hepatocytes have implicated the mobilization of chelatable iron from lysosomes in the induction of mitochondrial permeability transition (MPT) after APAP (62).
Mechanistically, it was demonstrated that mitochondrial uptake of chelatable iron through the calcium uniporter promotes MPT as evidenced by the protection of the lysosomal iron chelator starch, Desferal, or inhibition of the calcium uniporter by either Ru360 or minocycline (Fig. 3B) (41). Suppression of this lysosomal iron mobilization by minocycline was also able to attenuate APAP hepatotoxicity in vivo (42). So how can these findings be reconciled with the earlier data from the 1980s with regard to iron chelation, LPO, and APAP hepatotoxicity? One possibility is that APAP-induced changes in chelatable iron within specific subcellular compartments such as the mitochondria induce very localized LPO (131), which is unable to propagate beyond the organelle due to the robust antioxidant defenses such as vitamin E. This limited localized LPO could have nuanced effects on the organellar function of relevance to APAP pathophysiology and need targeted studies to be further investigated.
Therapeutic Interventions That Target ROS
Due to the central role of oxidative and nitrosative stress in the pathophysiology of APAP hepatotoxicity, interventions targeting the formation or elimination of reactive oxygen and nitrogen species could be important therapeutic approaches. The following chapters outline the mechanisms of protection of actual antidotes and promising drug candidates under development.
N-acetylcysteine
NAC is the only clinically approved antidote against APAP overdose (107). The original observation that a reactive metabolite depleted hepatic GSH and subsequently initiated toxicity by protein adduct formation (86) provided the rationale for the use of sulfhydryl reagents that replenished glutathione levels of the liver and promoted the detoxification of NAPQI (18). However, this beneficial effect was dependent on the early administration of NAC after the APAP overdose, that is, generally within less than 8 h after the overdose in humans (108). Thus, the fact that NAC was also at least partially effective at later time points (117) suggested that additional mechanisms of protection could be present. As discussed, a selective oxidant stress and peroxynitrite formation in the mitochondrial matrix is central to the toxicity (20, 46, 61). Thus, if NAC or GSH administration is delayed until after the drug metabolism phase, the enhanced formation of GSH in the cytosol and the import of GSH into mitochondria restores the capacity to scavenge peroxynitrite and H2O2 in the mitochondrial matrix and effectively protects against APAP-induced cell death (Fig. 4) (9, 53, 60, 112).
FIG. 4.
NAC protects by replenishing glutathione with cysteine being critical for protection. (A) Time course of hepatic glutathione+glutathione disulfide (GSH+GSSG) levels after treatment with 300 mg/kg APAP in mice. Animals also received vehicle (saline), 0.65 mmol/kg GSH, or 0.65 mmol/kg NAC iv 1.5 h after APAP. (B) Plasma ALT indicative of liver injury after treatment of mice with 300 mg/kg APAP for 6 h. Animals also received saline as vehicle or 0.65 mmol/kg NAC (l-NAC), 1.95 mmol/kg NAC (h-NAC), a mixture of 3 amino acids (0.65 mmol/kg of glycine, glutamic acid, and NAC) (3AS) required for GSH synthesis or a mixture of 2 amino acids (0.98 mmol/kg glycine and glutamic acid) (2AS) iv 1.5 h after APAP. Data represent mean ± standard error of n = 4–5 animals per time point. *p < 0.05 (compared with APAP/saline). Data adapted from Ref. (112). ALT, alanine aminotransferase; NAC, N-acetylcysteine.
However, it is important to recognize that both NAC and GSH (after degradation) supply cysteine for the intracellular de novo synthesis of GSH in hepatocytes and do not act directly with NAPQI or ROS (19, 128). In addition, excess NAC, a glucogenic amino acid, is being converted to glucose, which feeds reducing equivalents into the Krebs cycle and via pyruvate carboxylase the substrate oxaloacetate (112). Together, this supports mitochondrial bioenergetics as indicated by the higher cellular ATP levels after excess NAC treatment (112). Furthermore, these findings demonstrate that NAC treatment supports multiple mechanisms of protection at different stages of pathophysiology, which is the reason for the substantial therapeutic window of the antidote in patients (117). However, due to the potential side effects of NAC including anaphylactic reactions at early time points (94) and even delayed regeneration during prolonged treatment at later time points (140), there is a need for additional therapeutics.
4-Methylpyrazole
4-Methylpyrazole (4MP, fomepizole) is a clinically approved antidote against methanol and ethylene glycol poisoning due to its inhibitory activity of alcohol dehydrogenase (13, 82). However, it was recognized that 4MP can also inhibit cytochrome P450 enzymes in vitro, especially Cyp2E1 (38), which is the dominant enzyme responsible for the oxidative metabolism of APAP (34, 36). Thus, cotreatment with 4MP eliminated APAP-induced liver injury in mice in vivo (3). Based on the dramatic reduction of both oxidative metabolite and protein adduct formations under these conditions, the protection was mainly caused by the inhibition of Cyp2E1 both in the liver (Fig. 5) (3) and in the kidney (4). However, a delayed treatment with 4MP, that is, after the drug metabolism is over in the mouse, again showed an impressive protection clearly independent of the inhibitory effect on Cyp2E1 (2). Under these conditions, 4MP did not affect protein adduct formation but effectively prevented the activation of JNK and the mitochondrial translocation of P-JNK (2), which resulted in the elimination of the mitochondrial oxidative and nitrosative stress, mitochondrial dysfunction, and nuclear DNA fragmentation (Fig. 5) (2). However, under conditions of severe APAP overdose, where Cyp2E1-mediated metabolism could persist for longer durations, it is possible that 4MP could have dual benefits—preventing the formation of NAPQI by inhibiting Cyp2E1 and blocking the amplification of mitochondrial dysfunction by preventing JNK activation. Molecular modeling could confirm that 4MP can bind to both the substrate binding site of Cyp2E1 and the ATP binding site of JNK1 and JNK2 (2). Importantly, the significant therapeutic window of 4MP as shown in mice could also be reproduced in primary human hepatocytes (3). Furthermore, the almost complete elimination of oxidative metabolite formation was demonstrated in human volunteers taking a mild overdose of APAP (55). Because of the efficacy in preclinical models and in human volunteers, and the observation that 4MP is clinically used against methanol poisoning for almost 20 years with minimal side effects (102), a prospective randomized double-blind clinical trial to show efficacy in overdose patients is needed to obtain official FDA approval as a new antidote against APAP hepatotoxicity. Nevertheless, based on the discussed information, 4MP has already been used successfully off-label in patients with severe overdoses (58, 101, 134).
FIG. 5.
Mechanism of protection by 4MP in APAP hepatotoxicity. 4MP has distinct mechanistic targets along the temporal course of APAP pathophysiology (D) when given either as a cotreatment (A–C) or post-treatment (E–G). When given together with APAP, 4MP predominantly protects by inhibition of P450-mediated generation of NAPQI. This is reflected in the significant protection against elevation in ALT (A) and formation of protein adducts (B) at 6 h. Since upstream events are blocked, there is also a complete attenuation of JNK activation seen after APAP at this time point (C). When given as a post-treatment 90 min after a 300 mg/kg dose, which corresponds to a time point after early JNK activation (D), robust attenuation of liver injury is evident at both 3 and 6 h post-APAP (E). While NAPQI adducts at 3 h are not significantly influenced by the delayed 4MP treatment (F), some effect is evident at 6 h, probably due to modulation of adduct clearance by autophagy. Since the post-treatment is subsequent to JNK activation, no significant effect is seen at the earlier 3-h time point (G), but the presence of 4MP prevents maintenance of JNK activation as evident in the protection seen at 6 h (G). Data adapted from Refs. (2, 3). 4MP, 4-methylpyrazole.
Calmangafodipir
Previous studies using partial MnSOD-deficient mice showed the essential role of this enzyme in protecting against APAP hepatotoxicity (32, 100). The therapeutic potential of SOD in mitochondria was confirmed when animals exposed to an APAP overdose were completely protected when treated with an SOD mimetic targeted to mitochondria (Mito-Tempo) (26, 29). The observation that Mito-Tempo did not affect mechanisms upstream of mitochondria but eliminated nitrotyrosine staining showed that the main effect was the enhanced dismutation of superoxide, which prevented peroxynitrite formation (26). The SOD mimetic Tempo was only partially effective and required five-times the dose of Mito-Tempo (26). Together, these findings support the hypothesis that an SOD mimetic, especially when targeted to mitochondria, could potentially be an effective therapeutic agent against APAP-induced liver injury (Fig. 6). This is particularly important because peroxynitrite inactivates endogenous MnSOD during APAP toxicity, thus hampering endogenous antioxidant defenses (1).
FIG. 6.
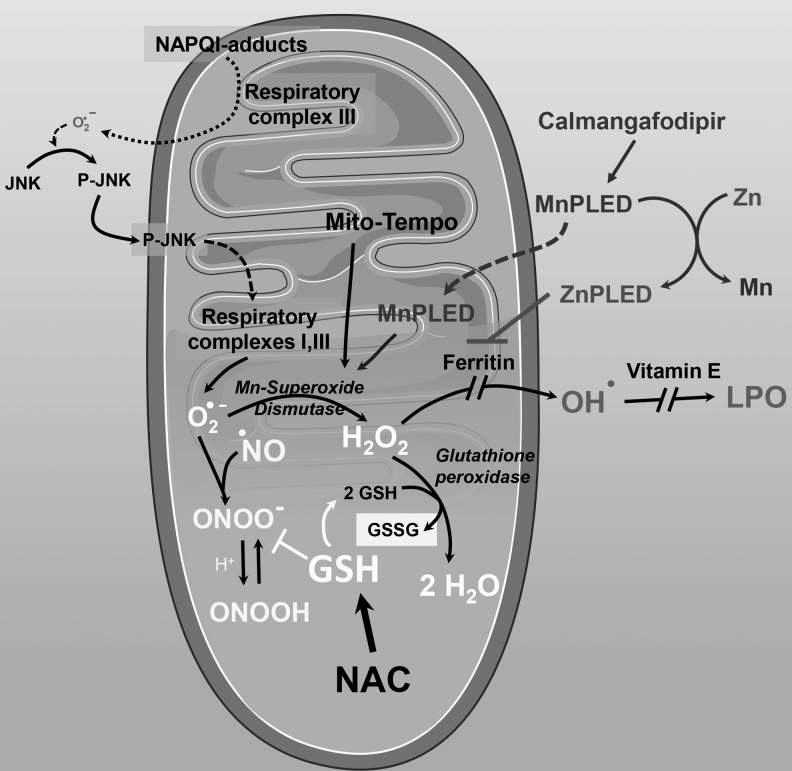
APAP-induced superoxide production and mechanism of protection by Mito-Tempo, NAC, and calmangafodipir. APAP protein adduct formation on mitochondria initiates a signaling cascade, which ultimately results in elevated superoxide production from mitochondrial respiratory complexes I and III. Superoxide is dismutated by MnSOD to form hydrogen peroxide, which is further converted to water by glutathione peroxidase using reduced glutathione for the reaction, generating glutathione disulfide. If MnSOD scavenging of superoxide is compromised, as occurs after APAP overdose, generated superoxide can react with nitric oxide to form the reactive peroxynitrite. This reaction can be blocked by reduced glutathione, which is synthesized in the cytosol from administered NAC, which also supplies glutathione for use by glutathione peroxidase. An alternate fate for hydrogen peroxide in the presence of free iron would be conversion to the hydroxyl radical, which can be prevented by iron chelators such as ferritin. Generation of hydroxyl radicals will then cause lipid peroxidation, which again can be prevented by lipid soluble antioxidants such as vitamin E. Mito-Tempo and the calmangafodipir derivative MnPLED are SOD mimetics, facilitating conversion of superoxide to hydrogen peroxide and preventing formation of peroxynitrite. In addition, the calmangafodipir derivative ZnPLED can also function as an iron chelator to prevent lipid peroxidation. MnPLED, manganese pyridoxyl ethyldiamine; MnSOD, manganese superoxide dismutase; SOD, superoxide dismutase. This figure includes templates from Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 generic license.
Calmangafodipir is a derivative of the original compound manganese dipyridoxyl diphosphate (MnDPDP, mangafodipir), which has been shown to protect against APAP hepatotoxicity in mice (11) because of its known SOD mimetic activities (8, 14). The SOD activity of mangafodipir depends on the presence of Mn2+ in the complex (57). Because Mn2+ in mangafodipir can easily be replaced by Zn2+, the resulting excess Mn2+ in circulation can cause toxicity by accumulation in the brain and, due to the biliary excretion, also in the liver (12). To avoid this toxicity, 80% of the Mn2+ in mangafodipir was replaced by Ca2+ (56). This new compound, calmangafodipir ([Ca4Mn(DPDP)5]), is less toxic and has superior therapeutic efficacy compared with mangafodipir (56).
Similar to mangafodipir (MnDPDP), calmangafodipir is rapidly dephosphorylated in plasma to MnPLED (manganese pyridoxyl ethyldiamine) (124), which also has SOD mimetic activities and is considered the actual lipophilic metabolite that can be taken up into cells (56). Interestingly, DPDP and PLED have also a very high affinity for iron (Fe3+), which makes them effective iron chelators and inhibitors of the Fenton reaction (56). The Mn2+ metal ion in MnDPDP as well as calmangafodipir can be replaced by Zn2+ in plasma (124) due to the 1000 times higher affinity of DPDP for Zn2+ compared with Mn2+ (56); however, all Zn metabolites (ZnDPDP, ZnPLED) still possess the iron chelation properties but are no longer SOD mimetics (14). Taken together, treatment with mangafodipir or calmangafodipir in vivo results in rapid dephosphorylation to MnPLED, which is an SOD mimetic and iron chelator, and, in part, to ZnPLED, which is only an iron chelator (Fig. 6) (53).
Based on these properties and the previous study with mangafodipir (11), it was hypothesized that calmangafodipir might be effective against APAP hepatotoxicity. Preliminary studies in mice confirmed the efficacy of calmangafodipir in preventing liver injury when administered up to 6 h after APAP (22). In addition, calmangafodipir was assessed in a Phase I/II safety trial in APAP-overdose patients (88). In this study, patients treated with NAC were also administered calmangafodipir. Due to the early NAC treatment, there was no relevant liver injury, and thus, any beneficial effect of calmangafodipir could not be evaluated. Nevertheless, the drug was well tolerated in the overdose patients in the presence of NAC, suggesting that this intervention is safe to use in this patient population. A randomized double-blind Phase III trial is warranted to evaluate the clinical efficacy of calmangafodipir in APAP-overdose patients. In addition, more preclinical studies are necessary to better understand the mechanisms of action of calmangafodipir.
NF-E2-related factor 2 activators
Activation of the transcription factor NF-E2-related factor 2 (Nrf2) is a critical adaptation response of cells to stress, especially oxidative stress. Nrf2 is located in the cytosol bound to the cytoskeletal anchor protein kelch-like ECH associated protein 1 (KEAP1) (45). Upon oxidative stress or exposure to electrophiles, Nrf2 dissociates from Keap1 and translocates to the nucleus and binds to the antioxidant response element, where it can induce a large number of genes that are involved in stress response (heme oxygenase 1 [HMOX1]), iron metabolism (ferritin), glutathione homeostasis (e.g., glutamate/cysteine ligase and glutathione synthetase), and drug metabolism [e.g., NAD(P)H quinone oxidoreductase 1, NQO1, and UDP-glucuronosyltransferases] (5).
NQO1 is a flavoprotein that metabolizes quinones to hydroquinones and thus has multiple protective roles (105). Along with glutathione S-transferase, NQO1 has been shown to be involved in the detoxification of APAP by regulating GSH homeostasis (15). The relevance of NQO1 to APAP hepatotoxicity is illustrated by the higher sensitivity of NQO1-deficient mice to APAP-mediated liver injury, with larger areas of necrosis and increased nitrotyrosine adducts and oxidative stress (43). Early depletion of glutathione was similar in KO and WT mice, indicating that generation of NAPQI is not affected by NQO1 deficiency (43). The p53-mediated protection against APAP hepatotoxicity by doxorubicin administration also involved upregulation of NQO1, due to activation of the Nrf2 pathway (121), and this has been suggested to be sirtuin 6 dependent (143).
Each of the other Nrf2-regulated genes mentioned has also been shown to be protective in the APAP hepatotoxicity model. Thus, Nrf2 activation is a prominent response to an APAP overdose (33) and the pathophysiological relevance was shown by the dramatically increased susceptibility of Nrf2 gene-deficient mice (31) and the marked protection of Keap1-deficient mice with chronic Nrf2 activation (Fig. 7) (93). In addition, chronic cellular stress such as observed with deficiency of the autophagy process when Atg5, an essential protein for the extension of membranes forming autophagic vesicles, is knocked out, leads to Nrf2 activation with enhanced GSH synthesis (91). These animals, despite chronic cellular stress, are remarkably resistant to acute APAP toxicity (91). However, persistent activation of Nrf2 during chronic stress promotes inflammation, fibrosis, and tumor formation in the liver (92).
FIG. 7.
Oxidant stress-mediated Nrf2 activation has far-reaching consequences. Under homeostatic conditions, Nrf2 is bound to its cytosolic anchor protein Keap1, which sequesters it in the cytoplasm. APAP overdose-induced mitochondrial dysfunction causes ROS production and oxidative stress, which modifies sulfhydryl groups on Keap1 resulting in its dissociation from Nrf2, which then translocates to the nucleus. Nrf2 binds to the ARE and induces transcription of a large number of genes listed, which all have protective roles in the APAP hepatotoxicity model. ARE, antioxidant response element; Keap1, kelch-like ECH associated protein 1; Nrf2, NF-E2-related factor 2. This figure includes templates from Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 generic license.
Based on this mechanistic background, short-term activation of Nrf2 may be a promising therapeutic strategy to counteract the acute stress during APAP hepatotoxicity especially because it induces a spectrum of defense mechanisms. In fact, pretreatment with selective Nrf2 activators, such as 2-cyano-3,12 dioxooleana-1,9 diene-28-imidazolide (CDDO-Im) (69), caused Nrf2 activation and induced Nrf2-dependent genes such as gclc, Nqo1, and Hmox1 and protected against APAP toxicity (104). This beneficial effect of CDDO-Im was not observed in Nrf2-deficient mice, suggesting that the Nrf2-induced gene expression was responsible for the protection (104). Pretreatment with oleanolic acid also caused Nrf2 activation and Nrf2-dependent gene induction, and effectively protected against APAP toxicity (103). However, oleanolic acid also partially protected Nrf2 null mice, which was caused by the upregulation of metallothionein, an Nrf2-independent gene (103). These results demonstrate a number of issues related to the use of Nrf2 activators in APAP toxicity and potentially other liver diseases. First, Nrf2 activators work best when animals are pretreated for several days. This is clinically not relevant as therapeutic agents are needed to be used after drug exposure. Second, most compounds used to activate Nrf2 may have off-target effects, which need to be considered in the interpretation of the data. If the off-target effect is the induction of another protective gene, it is not a problem. However, if the off-target effect is inhibition of P450-dependent drug metabolism, the protection may be independent of Nrf2 gene activation and of limited clinical relevance, especially as pretreatment. Although the interest in Nrf2 activation as a mechanism of action for natural products has been dramatically increased in the last few years (71, 138), these issues need to be carefully evaluated for every compound under consideration to obtain reliable and accurate mechanistic information, which may be clinically relevant.
Antioxidants
Numerous compounds have been considered to act as direct antioxidants in the APAP hepatotoxicity model. However, in most cases, there are some concerns regarding this conclusion. As discussed, many exogenous agents such as NAC, GSH, and other sulfhydryl-containing compounds promote the synthesis of the endogenous antioxidant GSH, which is an effective scavenger of peroxynitrite and a cosubstrate for GPx1 detoxifying H2O2. GSH synthesis can also be stimulated by IL-4 treatment (109). Due to the importance of these water-soluble oxidants in APAP toxicity, endogenous GSH is an effective antidote (9, 53, 60, 112). On the contrary, the lipid-soluble antioxidant tocopherol acetate (vitamin E) showed no relevant protection even when the levels of vitamin E in the liver membranes are increased sevenfold above baseline (59). This finding is not surprising because there is at best limited LPO after an APAP overdose under normal circumstances, which makes it less likely that a lipid-soluble antioxidant can be effective.
Despite this insight into the pathophysiology, numerous compounds, including most natural products, are considered to be acting as antioxidants (16, 120). This conclusion is mainly based on the observation that the protection with these compounds correlates with reduced LPO and other more or less specific parameters of oxidant stress (16, 120). However, there are very serious concerns with most of these interpretations. First, despite pretreatment, in none of the cases is it investigated whether there is accumulation of the assumed antioxidant in the liver or intracellular compartments. In order for an exogenous compound to be able to make a difference as an antioxidant, it has to effectively compete against the multiple layers of endogenous antioxidants and defense systems. Second, most of the conclusions in these studies are only based on correlations, which neither prove causality nor exclude any off-target effects. In many cases, interference with the metabolic activation of APAP may be the main reason for reduced oxidant stress, not a direct antioxidant effect. Third, most antioxidants will require pretreatment to allow for sufficient compound levels to accumulate, which again is not clinically relevant for treating a patient with a drug overdose. Thus, an attempt to directly enhance the antioxidant capacity by pretreatment with any drug is the therapeutic approach with the lowest chances of success.
Conclusions
Extensive efforts to investigate the mechanisms of APAP-induced liver injury in preclinical models and the translation to patients have resulted in a better understanding of the role of reactive oxygen and nitrogen species in the pathophysiology. In particular, the initiation of the mitochondrial oxidant stress by protein adducts in mitochondria, the amplification by the JNK pathway, and the central role of MnSOD in the mitochondrial matrix to limit peroxynitrite formation revealed promising therapeutic targets for the existing antidote NAC (scavenging NAPQI and peroxynitrite) and the emerging antidotes 4MP (P450 inhibitor and JNK inhibitor) and calmangafodipir (SOD mimetic and iron chelator). Strengthening endogenous antioxidant defense systems through Nrf2-dependent gene activation was shown to be a promising approach to limit APAP toxicity in animals; however, the need for pretreatment questions the clinical utility. On the contrary, directly targeting oxidants through treatment with poorly characterized antioxidants is not a realistic approach even in preclinical models.
Abbreviations Used
- 4MP
4-methylpyrazole
- AIF
apoptosis-inducing factor
- ALT
alanine aminotransferase
- AMAP
3′-hydroxyacetanilide
- APAP
acetaminophen
- ARE
antioxidant response element
- CCl4
carbon tetrachloride
- CDDO-Im
2-cyano-3,12 dioxooleana-1,9 diene-28-imidazolide
- Cyp2E1
cytochrome P450 2E1
- EndoG
endonuclease G
- ETC
electron transport chain
- GPx
glutathione peroxidase
- GSH
glutathione
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- HMOX1
heme oxygenase 1
- IL-4
interleukin-4
- JNK
c-Jun N-terminal kinase
- KEAP1
kelch-like ECH associated protein 1
- LPO
lipid peroxidation
- MAP
mitogen activated protein
- MnDPDP
manganese dipyridoxyl diphosphate
- MnPLED
manganese pyridoxyl ethyldiamine
- MnSOD
manganese superoxide dismutase
- MPT
mitochondrial permeability transition
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinone imine
- NQO
NAD(P)H quinone dehydrogenase
- Nrf2
NF-E2-related factor 2
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- XO
xanthine oxidase
Authors' Contributions
Both authors contributed equally to the writing of the review.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Work in this laboratory was supported by the National Institutes of Health grant R01 DK102142, McNeil Consumer Health, Inc., and the National Institute of General Medical Sciences (P20 GM103549 and P30 GM118247) from the National Institutes of Health to Hartmut Jaeschke and R01 grant DK125465 to Anup Ramachandran.
References
- 1. Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, and Hinson JA. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther 337: 110–116, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akakpo JY, Ramachandran A, Duan L, Schaich MA, Jaeschke MW, Freudenthal BD, Ding WX, Rumack BH, and Jaeschke H. Delayed treatment with 4-methylpyrazole protects against acetaminophen hepatotoxicity in mice by inhibition of c-Jun n-terminal kinase. Toxicol Sci 170: 57–68, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akakpo JY, Ramachandran A, Kandel SE, Ni HM, Kumer SC, Rumack BH, and Jaeschke H. 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum Exp Toxicol 37: 1310–1322, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akakpo JY, Ramachandran A, Orhan H, Curry SC, Rumack BH, and Jaeschke H. 4-Methylpyrazole protects against acetaminophen-induced acute kidney injury. Toxicol Appl Pharmacol 409: 115317, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aleksunes LM and Manautou JE. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol Pathol 35: 459–473, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, Bateman DN, Goldring CE, and Park BK. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology 58: 777–787, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, Possamai LA, Bruce M, McPhail M, Starling C, Wagner B, Barnardo A, Pomplun S, Auzinger G, Bernal W, Heaton N, Vergani D, Thursz MR, and Wendon J. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology 56: 735–746, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Asplund A, Grant D, and Karlsson JO. Mangafodipir (MnDPDP)-and MnCl2-induced endothelium-dependent relaxation in bovine mesenteric arteries. J Pharmacol Exp Ther 271: 609–614, 1994. [PubMed] [Google Scholar]
- 9. Bajt ML, Knight TR, Farhood A, and Jaeschke H. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther 307: 67–73, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Bajt ML, Knight TR, Lemasters JJ, and Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetylcysteine. Toxicol Sci 80: 343–349, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Bedda S, Laurent A, Conti F, Chéreau C, Tran A, Tran-Van Nhieu J, Jaffray P, Soubrane O, Goulvestre C, Calmus Y, Weill B, and Batteux F. Mangafodipir prevents liver injury induced by acetaminophen in the mouse. J Hepatol 39: 765–772, 2003. [DOI] [PubMed] [Google Scholar]
- 12. Borg DC and Cotzias GC. Manganese metabolism in man: rapid exchange of MN56 with tissue as demonstrated by blood clearance and liver uptake. J Clin Invest 37: 1269–1278, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brent J. Fomepizole for ethylene glycol and methanol poisoning. N Engl J Med 360: 2216–2223, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Brurok H, Ardenkjaer-Larsen JH, Hansson G, Skarra S, Berg K, Karlsson JO, Laursen I, and Jynge P. Manganese dipyridoxyl diphosphate: MRI contrast agent with antioxidative and cardioprotective properties? Biochem Biophys Res Commun 254: 768–772, 1999. [DOI] [PubMed] [Google Scholar]
- 15. Chan K, Han XD, and Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci U S A 98: 4611–4616, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang L, Xu D, Zhu J, Ge G, Kong X, and Zhou Y. Herbal therapy for the treatment of acetaminophen-associated liver injury: recent advances and future perspectives. Front Pharmacol 11: 313, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, and Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol 143: 1–12, 1997. [DOI] [PubMed] [Google Scholar]
- 18. Corcoran GB, Racz WJ, Smith CV, and Mitchell JR. Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J Pharmacol Exp Ther 232: 864–872, 1985. [PubMed] [Google Scholar]
- 19. Corcoran GB and Wong BK. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: studies with N-acetyl-D-cysteine in mice. J Pharmacol Exp Ther 238: 54–61, 1986. [PubMed] [Google Scholar]
- 20. Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, and Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther 315: 879–887, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Dahlin DC, Miwa GT, Lu AY, and Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A 81: 1327–1331, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dear JW, Morrison E, Henriksen D, and Nasstrom J. Calmangafodipir is a new treatment for late stage liver toxicity after acetaminophen overdose. Hepatology 66 (Suppl 1): 4A–5A, 2017.28370190 [Google Scholar]
- 23. Donnelly PJ, Walker RM, and Racz WJ. Inhibition of mitochondrial respiration in vivo is an early event in acetaminophen-induced hepatotoxicity. Arch Toxicol 68: 110–118, 1994. [DOI] [PubMed] [Google Scholar]
- 24. Dostalek M, Brooks JD, Hardy KD, Milne GL, Moore MM, Sharma S, Morrow JD, and Guengerich FP. In vivo oxidative damage in rats is associated with barbiturate response but not other cytochrome P450 inducers. Mol Pharmacol 72: 1419–1424, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Dostalek M, Hardy KD, Milne GL, Morrow JD, Chen C, Gonzalez FJ, Gu J, Ding X, Johnson DA, Johnson JA, Martin MV, and Guengerich FP. Development of oxidative stress by cytochrome P450 induction in rodents is selective for barbiturates and related to loss of pyridine nucleotide-dependent protective systems. J Biol Chem 283: 17147–17157, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Du K, Farhood A, and Jaeschke H. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch Toxicol 91: 761–773, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Du K, McGill MR, Xie Y, Bajt ML, and Jaeschke H. Resveratrol prevents protein nitration and release of endonucleases from mitochondria during acetaminophen hepatotoxicity. Food Chem Toxicol 81: 62–70, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Du K, Ramachandran A, Weemhoff JL, Chavan H, Xie Y, Krishnamurthy P, and Jaeschke H. Editor's highlight: metformin protects against acetaminophen hepatotoxicity by attenuation of mitochondrial oxidant stress and dysfunction. Toxicol Sci 154: 214–226, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Du K, Ramachandran A, Weemhoff JL, Woolbright BL, Jaeschke AH, Chao X, Ding WX, and Jaeschke H. Mito-tempo protects against acute liver injury but induces limited secondary apoptosis during the late phase of acetaminophen hepatotoxicity. Arch Toxicol 93: 163–178, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du K, Williams CD, McGill MR, and Jaeschke H. Lower susceptibility of female mice to acetaminophen hepatotoxicity: role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase. Toxicol Appl Pharmacol 281: 58–66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, and Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci 59: 169–177, 2001. [DOI] [PubMed] [Google Scholar]
- 32. Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, and Manabe S. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol 37: 193–200, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Goldring CE, Kitteringham NR, Elsby R, Randle LE, Clement YN, Williams DP, McMahon M, Hayes JD, Itoh K, Yamamoto M, and Park BK. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology 39: 1267–1276, 2004. [DOI] [PubMed] [Google Scholar]
- 34. Gonzalez FJ. The 2006 Bernard B. Brodie Award Lecture. Cyp2e1. Drug Metab Dispos 35: 1–8, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Grivennikova VG and Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta 1757: 553–561, 2006. [DOI] [PubMed] [Google Scholar]
- 36. Guengerich FP. Cytochrome P450 2E1 and its roles in disease. Chem Biol Interact 322: 109056, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, and Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem 283: 13565–13577, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hazai E, Vereczkey L, and Monostory K. Reduction of toxic metabolite formation of acetaminophen. Biochem Biophys Res Commun 291: 1089–1094, 2002. [DOI] [PubMed] [Google Scholar]
- 39. Hinson JA, Pike SL, Pumford NR, and Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol 11: 604–607, 1998. [DOI] [PubMed] [Google Scholar]
- 40. Holthoff JH, Woodling KA, Doerge DR, Burns ST, Hinson JA, and Mayeux PR. Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem Pharmacol 80: 1260–1265, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hu J, Kholmukhamedov A, Lindsey CC, Beeson CC, Jaeschke H, and Lemasters JJ. Translocation of iron from lysosomes to mitochondria during acetaminophen-induced hepatocellular injury: protection by starch-desferal and minocycline. Free Radic Biol Med 97: 418–426, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu J and Lemasters JJ. Suppression of iron mobilization from lysosomes to mitochondria attenuates liver injury after acetaminophen overdose in vivo in mice: protection by minocycline. Toxicol Appl Pharmacol 392: 114930, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hwang JH, Kim YH, Noh JR, Gang GT, Kim KS, Chung HK, Tadi S, Yim YH, Shong M, and Lee CH. The protective role of NAD(P)H:quinone oxidoreductase 1 on acetaminophen-induced liver injury is associated with prevention of adenosine triphosphate depletion and improvement of mitochondrial dysfunction. Arch Toxicol 89: 2159–2166, 2015. [DOI] [PubMed] [Google Scholar]
- 44. Ishihara G, Kawamoto K, Komori N, and Ishibashi T. Molecular hydrogen suppresses superoxide generation in the mitochondrial complex I and reduced mitochondrial membrane potential. Biochem Biophys Res Commun 522: 965–970, 2020. [DOI] [PubMed] [Google Scholar]
- 45. Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, and Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76–86, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther 255: 935–941, 1990. [PubMed] [Google Scholar]
- 47. Jaeschke H. Acetaminophen: dose-dependent drug hepatotoxicity and acute liver failure in patients. Dig Dis 33: 464–471, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jaeschke H, Adelusi OB, and Ramachandran A. Ferroptosis and acetaminophen hepatotoxicity—are we going down another rabbit hole? Gene Expr 2021. [Epub ahead of print]; DOI: 10.3727/105221621X16104581979144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jaeschke H, Knight TR, and Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett 144: 279–288, 2003. [DOI] [PubMed] [Google Scholar]
- 50. Jaeschke H and Ramachandran A. Antioxidant defense mechanisms. In: Comprehensive Toxicology, 3rd ed, vol. 2, edited by McQueen CA. Oxford: Elsevier Ltd., 2018, pp. 277–295. [Google Scholar]
- 51. Jaeschke H and Ramachandran A. Does acetaminophen hepatotoxicity involve apoptosis, inflammatory liver injury, and lipid peroxidation? J Biochem Mol Toxicol 35: e22718, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jaeschke H, Xie Y, and McGill MR. Acetaminophen-induced Liver Injury: from Animal Models to Humans. J Clin Transl Hepatol 2: 153–161, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. James LP, McCullough SS, Lamps LW, and Hinson JA. Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci 75: 458–467, 2003. [DOI] [PubMed] [Google Scholar]
- 54. Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, and Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther 187: 195–202, 1973. [PubMed] [Google Scholar]
- 55. Kang AM, Padilla-Jones A, Fisher ES, Akakpo JY, Jaeschke H, Rumack BH, Gerkin RD, and Curry SC. The effect of 4-methylpyrazole on oxidative metabolism of acetaminophen in human volunteers. J Med Toxicol 16: 169–176, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karlsson JO, Ignarro LJ, Lundström I, Jynge P, and Almén T. Calmangafodipir [Ca4Mn(DPDP)5], mangafodipir (MnDPDP) and MnPLED with special reference to their SOD mimetic and therapeutic properties. Drug Discov Today 20: 411–421, 2015. [DOI] [PubMed] [Google Scholar]
- 57. Karlsson JO, Kurz T, Flechsig S, Näsström J, and Andersson RG. Superior therapeutic index of calmangafodipir in comparison to mangafodipir as a chemotherapy adjunct. Transl Oncol 5: 492–502, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kiernan EA, Fritzges JA, Henry KA, and Katz KD. A case report of massive acetaminophen poisoning treated with a novel “Triple Therapy”: N-acetylcysteine, 4-methylpyrazole, and hemodialysis. Case Rep Emerg Med 2019: 9301432, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Knight TR, Fariss MW, Farhood A, and Jaeschke H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci 76: 229–236, 2003. [DOI] [PubMed] [Google Scholar]
- 60. Knight TR, Ho YS, Farhood A, and Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther 303: 468–475, 2002. [DOI] [PubMed] [Google Scholar]
- 61. Knight TR, Kurtz A, Bajt ML, Hinson JA, and Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci 62: 212–220, 2001. [DOI] [PubMed] [Google Scholar]
- 62. Kon K, Kim JS, Uchiyama A, Jaeschke H, and Lemasters JJ. Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes. Toxicol Sci 117: 101–108, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Koop DR. Alcohol metabolism's damaging effects on the cell: a focus on reactive oxygen generation by the enzyme cytochrome P450 2E1. Alcohol Res Health 29: 274–280, 2006. [PMC free article] [PubMed] [Google Scholar]
- 64. Kushnareva Y, Murphy AN, and Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J 368: 545–553, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kuthan H and Ullrich V. Oxidase and oxygenase function of the microsomal cytochrome P450 monooxygenase system. Eur J Biochem 126: 583–588, 1982. [DOI] [PubMed] [Google Scholar]
- 66. Lancaster EM, Hiatt JR, and Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol 89: 193–199, 2015. [DOI] [PubMed] [Google Scholar]
- 67. Lauterburg BH, Smith CV, Hughes H, and Mitchell JR. Biliary excretion of glutathione and glutathione disulfide in the rat. Regulation and response to oxidative stress. J Clin Invest 73: 124–133, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee WM and Kaplowitz N. Alcohol, fasting, and therapeutic dosing of acetaminophen: a perfect storm. Hepatology 73: 1634–1636, 2021. [DOI] [PubMed] [Google Scholar]
- 69. Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, and Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res 65: 4789–4798, 2005. [DOI] [PubMed] [Google Scholar]
- 70. Liu P, Fisher MA, Farhood A, Smith CW, and Jaeschke H. Beneficial effects of extracellular glutathione against endotoxin-induced liver injury during ischemia and reperfusion. Circ Shock 43: 64–70, 1994. [PubMed] [Google Scholar]
- 71. Liu J, Wu KC, Lu YF, Ekuase E, and Klaassen CD. Nrf2 protection against liver injury produced by various hepatotoxicants. Oxid Med Cell Longev 2013: 305861, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Louvet A, Ntandja Wandji LC, Lemaître E, Khaldi M, Lafforgue C, Artru F, Quesnel B, Lassailly G, Dharancy S, and Mathurin P. Acute liver injury with therapeutic doses of acetaminophen: a prospective study. Hepatology 73: 1945–1955, 2021. [DOI] [PubMed] [Google Scholar]
- 73. Lv H and Shang P. The significance, trafficking and determination of labile iron in cytosol, mitochondria and lysosomes. Metallomics 10: 899–916, 2018. [DOI] [PubMed] [Google Scholar]
- 74. Maeda M, Tanaka R, Aso M, Sakamoto Y, Song I, Ochiai M, Saito Y, Maekawa K, Arakawa N, Ohno Y, and Kumagai Y. Hepatic adaptation to therapeutic doses of acetaminophen: an exploratory study in healthy individuals. Clin Ther 42: 1276–1291, 2020. [DOI] [PubMed] [Google Scholar]
- 75. Maranzana E, Barbero G, Falasca AI, Lenaz G, and Genova ML. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal 19: 1469–1480, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Matthews AM, Hinson JA, Roberts DW, and Pumford NR. Comparison of covalent binding of acetaminophen and the regioisomer 3'-hydroxyacetanilide to mouse liver protein. Toxicol Lett 90: 77–82, 1997. [DOI] [PubMed] [Google Scholar]
- 77. McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, and Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol 269: 240–249, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, and Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest 122: 1574–1583, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McGill MR, Staggs VS, Sharpe MR, Lee WM, and Jaeschke H; Acute Liver Failure Study Group. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology 60: 1336–1345, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McGill MR, Williams CD, Xie Y, Ramachandran A, and Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol 264: 387–394, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, and Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology 53: 974–982, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McMartin KE. Antidotes for alcohol and glycol toxicity: translating mechanisms into treatments. Clin Pharmacol Ther 88: 400–404, 2010. [DOI] [PubMed] [Google Scholar]
- 83. Meyers LL, Beierschmitt WP, Khairallah EA, and Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol 93: 378–387, 1988. [DOI] [PubMed] [Google Scholar]
- 84. Miettinen TP and Björklund M. NQO2 is a reactive oxygen species generating off-target for acetaminophen. Mol Pharm 11: 4395–4404, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, and Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther 187: 185–194, 1973. [PubMed] [Google Scholar]
- 86. Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, and Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187: 211–217, 1973. [PubMed] [Google Scholar]
- 87. Moon MS, Richie JP, and Isom HC. Iron potentiates acetaminophen-induced oxidative stress and mitochondrial dysfunction in cultured mouse hepatocytes. Toxicol Sci 118: 119–127, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Morrison EE, Oatey K, Gallagher B, Grahamslaw J, O'Brien R, Black P, Oosthuyzen W, Lee RJ, Weir CJ, Henriksen D, and Dear JW; POP Trial Investigators. Principal results of a randomised open label exploratory, safety and tolerability study with calmangafodipir in patients treated with a 12 h regimen of N-acetylcysteine for paracetamol overdose (POP trial). EBioMedicine 46: 423–430, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Myers TG, Dietz EC, Anderson NL, Khairallah EA, Cohen SD, and Nelson SD. A comparative study of mouse liver proteins arylated by reactive metabolites of acetaminophen and its nonhepatotoxic regioisomer, 3'-hydroxyacetanilide. Chem Res Toxicol 8: 403–413, 1995. [DOI] [PubMed] [Google Scholar]
- 90. Nguyen NT, Du K, Akakpo JY, Umbaugh DS, Jaeschke H, and Ramachandran A. Mitochondrial protein adduct and superoxide generation are prerequisites for early activation of c-jun N-terminal kinase within the cytosol after an acetaminophen overdose in mice. Toxicol Lett 338: 21–31, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, and Ding WX. Liver-specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol Sci 127: 438–450, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ni HM, Woolbright BL, Williams J, Copple B, Cui W, Luyendyk JP, Jaeschke H, and Ding WX. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol 61: 617–625, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, and Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun 339: 79–88, 2006. [DOI] [PubMed] [Google Scholar]
- 94. Pakravan N, Waring WS, Sharma S, Ludlam C, Megson I, and Bateman DN. Risk factors and mechanisms of anaphylactoid reactions to acetylcysteine in acetaminophen overdose. Clin Toxicol (Phila) 46: 697–702, 2008. [DOI] [PubMed] [Google Scholar]
- 95. Piel S, Chamkha I, Dehlin AK, Ehinger JK, Sjövall F, Elmér E, and Hansson MJ. Cell-permeable succinate prodrugs rescue mitochondrial respiration in cellular models of acute acetaminophen overdose. PLoS One 15: e0231173, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, and Proudfoot AT. Intravenous N-acetylcystine: the treatment of choice for paracetamol poisoning. Br Med J 2: 1097–1100, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Qiu Y, Benet LZ, and Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem 273: 17940–17953, 1998. [DOI] [PubMed] [Google Scholar]
- 98. Ramachandran A, Duan L, Akakpo JY, and Jaeschke H. Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: current understanding and future perspectives. J Clin Transl Res 4: 75–100, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ramachandran A and Jaeschke H. Acetaminophen hepatotoxicity. Semin Liver Dis 39: 221–234, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ramachandran A, Lebofsky M, Weinman SA, and Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 251: 226–233, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rampon G, Wartman H, Osmon S, and Scalzo A. Use of fomepizole as an adjunct in the treatment of acetaminophen overdose: a case series. Toxicol Commun 4: 1–4, 2020. [Google Scholar]
- 102. Rasamison R, Besson H, Berleur MP, Schicchi A, and Mégarbane B. Analysis of fomepizole safety based on a 16-year post-marketing experience in France. Clin Toxicol (Phila) 58: 742–747, 2020. [DOI] [PubMed] [Google Scholar]
- 103. Reisman SA, Aleksunes LM, and Klaassen CD. Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem Pharmacol 77: 1273–1282, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Reisman SA, Buckley DB, Tanaka Y, and Klaassen CD. CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol Appl Pharmacol 236: 109–114, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ross D and Siegel D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol 41: 101950, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rumack B, Heard K, Green J, Albert D, Bucher-Bartelson B, Bodmer M, Sivilotti ML, and Dart RC. Effect of therapeutic doses of acetaminophen (up to 4 g/day) on serum alanine aminotransferase levels in subjects consuming ethanol: systematic review and meta-analysis of randomized controlled trials. Pharmacotherapy 32: 784–791, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rumack BH and Bateman DN. Acetaminophen and acetylcysteine dose and duration: past, present and future. Clin Toxicol (Phila) 50: 91–98, 2012. [DOI] [PubMed] [Google Scholar]
- 108. Rumack BH, Peterson RC, Koch GG, and Amara IA. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med 141: 380–385, 1981. [DOI] [PubMed] [Google Scholar]
- 109. Ryan PM, Bourdi M, Korrapati MC, Proctor WR, Vasquez RA, Yee SB, Quinn TD, Chakraborty M, and Pohl LR. Endogenous interleukin-4 regulates glutathione synthesis following acetaminophen-induced liver injury in mice. Chem Res Toxicol 25: 83–93, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sakeran MI, Zidan N, Rehman H, Aziz AT, and Saggu S. Abrogation by Trifolium alexandrinum root extract on hepatotoxicity induced by acetaminophen in rats. Redox Rep 19: 26–33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Saito C, Yan HM, Artigues A, Villar MT, Farhood A, and Jaeschke H. Mechanism of protection by metallothionein against acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 242: 182–190, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Saito C, Zwingmann C, and Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 51: 246–254, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sakaida I, Kayano K, Wasaki S, Nagatomi A, Matsumura Y, and Okita K. Protection against acetaminophen-induced liver injury in vivo by an iron chelator, deferoxamine. Scand J Gastroenterol 30: 61–67, 1995. [DOI] [PubMed] [Google Scholar]
- 114. Schnellmann JG, Pumford NR, Kusewitt DF, Bucci TJ, and Hinson JA. Deferoxamine delays the development of the hepatotoxicity of acetaminophen in mice. Toxicol Lett 106: 79–88, 1999. [DOI] [PubMed] [Google Scholar]
- 115. Shuhendler AJ, Pu K, Cui L, Uetrecht JP, and Rao J. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat Biotechnol 32: 373–380, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Siegers CP, Steffen B, and Younes M. Antidotal effects of deferrioxamine in experimental liver injury—role of lipid peroxidation. Pharmacol Res Commun 20: 337–343, 1988. [DOI] [PubMed] [Google Scholar]
- 117. Smilkstein MJ, Knapp GL, Kulig KW, and Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med 319: 1557–1562, 1988. [DOI] [PubMed] [Google Scholar]
- 118. Smith CV and Jaeschke H. Effect of acetaminophen on hepatic content and biliary efflux of glutathione disulfide in mice. Chem Biol Interact 70: 241–248, 1989. [DOI] [PubMed] [Google Scholar]
- 119. Stravitz RT and Lee WM. Acute liver failure. Lancet 394: 869–881, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Subramanya SB, Venkataraman B, Meeran MFN, Goyal SN, Patil CR, and Ojha S. Therapeutic potential of plants and plant derived phytochemicals against acetaminophen-induced liver injury. Int J Mol Sci 19: 3776, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sun J, Wen Y, Zhou Y, Jiang Y, Chen Y, Zhang H, Guan L, Yao X, Huang M, and Bi H. p53 attenuates acetaminophen-induced hepatotoxicity by regulating drug-metabolizing enzymes and transporter expression. Cell Death Dis 9: 536, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tirmenstein MA and Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J Biol Chem 264: 9814–9819, 1989. [PubMed] [Google Scholar]
- 123. Tirmenstein MA and Nelson SD. Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides. J Biol Chem 265: 3059–3065, 1990. [PubMed] [Google Scholar]
- 124. Toft KG, Hustvedt SO, Grant D, Martinsen I, Gordon PB, Friisk GA, Korsmo AJ, and Skotland T. Metabolism and pharmacokinetics of MnDPDP in man. Acta Radiol 38: 677–689, 1997. [DOI] [PubMed] [Google Scholar]
- 125. Trnka J, Blaikie FH, Smith RA, and Murphy MP. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic Biol Med 44: 1406–1419, 2008. [DOI] [PubMed] [Google Scholar]
- 126. Wendel A and Feuerstein S. Drug-induced lipid peroxidation in mice—I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol 30: 2513–2520, 1981. [DOI] [PubMed] [Google Scholar]
- 127. Wendel A, Feuerstein S, and Konz KH. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. Biochem Pharmacol 28: 2051–2055, 1979. [DOI] [PubMed] [Google Scholar]
- 128. Wendel A and Jaeschke H. Drug-induced lipid peroxidation in mice—III. Glutathione content of liver, kidney and spleen after intravenous administration of free and liposomally entrapped glutathione. Biochem Pharmacol 31: 3607–3611, 1982. [DOI] [PubMed] [Google Scholar]
- 129. Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, and Jaeschke H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol 275: 122–133, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Williams CD, McGill MR, Lebofsky M, Bajt ML, and Jaeschke H. Protection against acetaminophen-induced liver injury by allopurinol is dependent on aldehyde oxidase-mediated liver preconditioning. Toxicol Appl Pharmacol 274: 417–424, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wimborne HJ, Hu J, Takemoto K, Nguyen NT, Jaeschke H, Lemasters JJ, and Zhong Z. Aldehyde dehydrogenase-2 activation decreases acetaminophen hepatotoxicity by prevention of mitochondrial depolarization. Toxicol Appl Pharmacol 396: 114982, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Win S, Than TA, Min RW, Aghajan M, and Kaplowitz N. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology 63: 1987–2003, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Woolbright BL, Ramachandran A, McGill MR, Yan HM, Bajt ML, Sharpe MR, Lemasters JJ, and Jaeschke H. Lysosomal instability and cathepsin B release during acetaminophen hepatotoxicity. Basic Clin Pharmacol Toxicol 111: 417–425, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Woolum JA, Hays WB, and Patel KH. Use of fomepizole, n-acetylcysteine, and hemodialysis for massive acetaminophen overdose. Am J Emerg Med 38: 692.e5–692.e7, 2020. [DOI] [PubMed] [Google Scholar]
- 135. Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, and Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol 279: 266–274, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Xie Y, McGill MR, Du K, Dorko K, Kumer SC, Schmitt TM, Ding WX, and Jaeschke H. Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes. Toxicol Appl Pharmacol 289: 213–222, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, and Jaeschke H. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol 286: 1–9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Xu D, Xu M, Jeong S, Qian Y, Wu H, Xia Q, and Kong X. The role of Nrf2 in liver disease: novel molecular mechanisms and therapeutic approaches. Front Pharmacol 9: 1428, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yan HM, Ramachandran A, Bajt ML, Lemasters JJ, and Jaeschke H. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol Sci 117: 515–523, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yang R, Miki K, He X, Killeen ME, and Fink MP. Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit Care 13: R55, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Younes M, Cornelius S, and Siegers CP. Ferrous ion supported in vivo lipid peroxidation induced by paracetamol—its relation to hepatotoxicity. Res Commun Chem Pathol Pharmacol 51: 89–99, 1986. [PubMed] [Google Scholar]
- 142. Younes M and Siegers CP. The role of iron in the paracetamol- and CCl4-induced lipid peroxidation and hepatotoxicity. Chem Biol Interact 55: 327–334, 1985. [DOI] [PubMed] [Google Scholar]
- 143. Zhou Y, Fan X, Jiao T, Li W, Chen P, Jiang Y, Sun J, Chen Y, Chen P, Guan L, Wen Y, Huang M, and Bi H. SIRT6 as a key event linking P53 and NRF2 counteracts APAP-induced hepatotoxicity through inhibiting oxidative stress and promoting hepatocyte proliferation. Acta Pharm Sin B 11: 89–99, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]