Abstract
Background: Untreated hypothyroidism is associated with acquired von Willebrand syndrome, and hyperthyroidism is associated with increased thrombosis risk. However, the causal effects of thyroid function on hemostasis, coagulation, and fibrinolysis are unknown.
Methods: In a two-sample Mendelian randomization (MR) study with genome-wide association variants, we assessed causality of genetically predicted hypothyroidism (N = 134,641), normal-range thyrotropin (TSH; N = 54,288) and free thyroxine (fT4) (N = 49,269), hyperthyroidism (N = 51,823), and thyroid peroxidase antibody positivity (N = 25,821) on coagulation (activated partial thromboplastin time, von Willebrand factor [VWF], factor VIII [FVIII], prothrombin time, factor VII, fibrinogen) and fibrinolysis (D-dimer, tissue plasminogen activator [TPA], plasminogen activator inhibitor-1) from the CHARGE Hemostasis Consortium (N = 2583–120,246). Inverse-variance-weighted random effects were the main MR analysis followed by sensitivity analyses. Two-sided p < 0.05 was nominally significant, and p < 0.0011[ = 0.05/(5 exposures × 9 outcomes)] was Bonferroni significant for the main MR analysis.
Results: Genetically increased TSH was associated with decreased VWF [β(SE) = −0.020(0.006), p = 0.001] and with decreased fibrinogen [β(SE) = −0.008(0.002), p = 0.001]. Genetically increased fT4 was associated with increased VWF [β(SE) = 0.028(0.011), p = 0.012]. Genetically predicted hyperthyroidism was associated with increased VWF [β(SE) = 0.012(0.004), p = 0.006] and increased FVIII [β(SE) = 0.013(0.005), p = 0.007]. Genetically predicted hypothyroidism and hyperthyroidism were associated with decreased TPA [β(SE) = −0.009(0.024), p = 0.024] and increased TPA [β(SE) = 0.022(0.008), p = 0.008], respectively. MR sensitivity analyses showed similar direction but lower precision. Other coagulation and fibrinolytic factors were inconclusive.
Conclusions: In the largest genetic studies currently available, genetically increased TSH and fT4 may be associated with decreased and increased synthesis of VWF, respectively. Since Bonferroni correction may be too conservative given the correlation between the analyzed traits, we cannot reject nominal associations of thyroid traits with coagulation or fibrinolytic factors.
Keywords: coagulation, fibrinolysis, hemostasis, hyperthyroidism, hypothyroidism, thyroid hormone, thyroid peroxidase antibody, thyrotropin
Introduction
Hypothyroidism is characterized by increased thyrotropin (TSH) and decreased free thyroxine (fT4), whereas hyperthyroidism is characterized by decreased TSH and increased fT4. Von Willebrand factor (VWF) is secreted by vascular endothelium and platelets, and VWF promotes platelet aggregation and adhesion to the vascular endothelium in primary hemostasis (1). In observational studies, untreated hypothyroidism is associated with acquired von Willebrand syndrome (2,3), characterized by decreased concentrations of VWF and factor VIII (FVIII), and bleeding symptoms such as mild mucocutaneous bleeding (3) and menorrhagia, but rarely major hemorrhage (2–4). Possible underlying mechanisms for these findings include (i) a synthesis defect with downregulation of VWF and (ii) an underlying autoimmune mechanism (2). In favor of the synthesis defect is the restoration of VWF in hypothyroid patients when they reach the euthyroid state after levothyroxine replacement (3,5,6). Concordantly, in observational studies, elevated fT4 is associated with increased concentrations of VWF and FVIII (7–10), increased risk of venous thromboembolism (VTE) (8,11,12), and increased risk of stroke (13). However, effects of thyroid function on other measures of the coagulation and fibrinolytic system (activated partial thromboplastin time [APTT], prothrombin time [PT], factor VII [FVII], D-dimer, plasminogen activator inhibitor-1 [PAI-1], tissue plasminogen activator [TPA]) are still debated (9,10,14).
The genetic diversity of thyroid function and regulation by the hypothalamic–pituitary–thyroid pathway is reflected by the multiple genes implicated by genome-wide association studies (GWAS) in hypo- and hyperthyroidism (15–17), normal-range TSH and fT4 concentrations (16,18,19), and thyroid peroxidase antibodies (TPOAb, a marker of autoimmune thyroid suppression) (20,21). The Mendelian randomization (MR) design can be used to integrate this information to investigate the causal relevance of thyroid function on coagulation and fibrinolysis, avoiding the confounding and reverse causation in the traditional, that is, nongenetic observational designs. In MR studies, genetically predicted hypothyroidism and increased TSH are associated with decreased risk of atrial fibrillation (AF) (22,23) and decreased kidney function (24), and genetically increased TSH is also associated with decreased risk of ischemic stroke (25), increased risk of type 2 diabetes (26), and increased low-density lipoprotein concentration and blood pressure (26). However, genetically predicted thyroid function is not associated with bone mineral density (27), cerebral hemorrhage (23), or VTE (23). In this novel MR study, we hypothesize that thyroid function is causally associated with changes in coagulation and fibrinolysis. We investigated this using summary estimates on thyroid function from the ThyroidOmics Consortium (16) and 23andMe (17). We used summary statistics on outcomes from the Cohorts for Heart and Aging Research in Genetic Epidemiology (CHARGE) Hemostasis Group on coagulation (VWF, FVIII, APTT, fibrinogen, PT, FVII) and fibrinolysis (D-dimer, TPA, PAI-1) (28–34).
Materials and Methods
Genetic variants associated with thyroid function
For hypothyroidism, we used 18 single nucleotide polymorphisms (SNPs) from the European ancestry GWAS study by Pickrell et al. including 17,558 participants with hypothyroidism and 117,083 control participants from 23andMe (Table 1) (17). Hypothyroidism was determined by self-reported use of thyroid medication, self-reported increased TSH levels, or self-reported Hashimoto's thyroiditis (Supplementary Fig. S1 and Supplementary Table S1).
Table 1.
Characteristics of Cohorts and Consortia
| Trait | Consortium | European, % | N cohort | Sample size | Reference |
|---|---|---|---|---|---|
| Exposure | |||||
| Hypothyroidism | 23andMe | 100 | 1 | 17,558ca/117,083co | Pickrell et al. (17) |
| TSH | ThyroidOmics | 100 | 22 | 54,288 | Teumer et al. (16) |
| TPOAb positivity | — | 100 | 17 | 25,821 | Schultheiss et al. (20) |
| Hyperthyroidism | ThyroidOmics | 100 | 22 | 51,823 | Teumer et al. (16) |
| fT4 | ThyroidOmics | 100 | 19 | 49,269 | Teumer et al. (16) |
| Outcome | |||||
| APTT | CHARGE | 100 | 1 | 9240 | Tang et al. (28) |
| D-dimer | CHARGE | 100 | 13 | 21,052 | Smith et al. (34) |
| Fibrinogen | CHARGE | 100 | 34 | 120,246 | de Vries et al. (31) |
| FVII | CHARGE | 85.40 | 9 | 23,434 | de Vries et al. (33) |
| FVIII | CHARGE | 79.40 | 9 | 32,610 | Sabater-Lleal et al. (32) |
| PT | CHARGE | 100 | 3 | 2583 | Tang et al. (28) |
| PAI-1 | CHARGE | 100 | 8 | 19,599 | Huang et al. (29) |
| TPA | CHARGE | 100 | 14 | 26,929 | Huang et al. (30) |
| VWF | CHARGE | 91.80 | 18 | 46,354 | Sabater-Lleal et al. (32) |
Hypothyroidism was self-reported and included a confirmed diagnosis of hypothyroidism, increased TSH levels, or taking medication for hypothyroidism. For TSH and fT4, people were excluded if they reported use of thyroid medication (defined as ATC, code H03) or previous thyroid surgery. Hyperthyroidism was defined as TSH below the cohort-specific normal range. Controls were free of thyroid disease.
APTT, activated partial thromboplastin time; ATC, Anatomical Therapeutic Chemical; ca, cases; co, controls; fT4, free thyroxine; FVII, factor VII; FVIII, factor VIII; PAI-1, plasminogen activator inhibitor 1; PT, prothrombin time; TPA, tissue plasminogen activator; TPOAb, thyroid peroxidase antibody; TSH, thyrotropin; VWF, von Willebrand factor.
For TSH, we used the largest and most recent European ancestry meta-GWAS for normal-range TSH from the ThyroidOmics Consortium, an international consortium that studies the determinants and effects of thyroid diseases and thyroid function (16). The GWAS by Teumer et al. consisted of data from 22 independent cohorts with 54,288 participants for TSH. The study identified 60 SNPs and 1 indel (in 41 loci) associated with TSH (16). A priori, we excluded the indel, and two variants associated with TSH levels, which were highly pleiotropic (ABO-rs8176645) or had the same effect allele associated (p < 0.05) with both higher TSH levels and higher fT4 levels within the normal range (BCAS3-rs1157994). Several variants associated with TSH levels in the GWAS by Teumer et al. (16) have also been associated with autoimmune thyroid disease (AITD), including Hashimoto's thyroiditis, Graves' disease, and TPOAb positivity (16,21,35). To investigate pleiotropy, we identified variants (i) associated with AITD and (ii) variants not associated with AITD (Supplementary Tables S1 and S2). Only individuals with TSH values within their cohort-specific normal ranges were included in the GWAS. Participants with known thyroid disease were excluded. Details on laboratory analyses for each cohort are provided in the article by Teumer et al. (16).
For TPOAb, we used two European ancestry studies by Schultheiss et al. (20) and Medici et al. (21) of 17 cohorts with 25,821 participants (3009 TPOAb positive [11.7%], 22,812 TPOAb negative), and which identified 6 SNPs (Supplementary Methods section and Supplementary Table S1).
For hyperthyroidism (NSNP = 8), we used the European ancestry GWAS study by Teumer et al. (16) of 51,823 participants including 1840 participants with TSH below the cohort-specific normal range, which for most cohorts was below 0.4 mIU/L (Supplementary Table S1). Participants with known thyroid disease were excluded. For this study, we excluded the variant rs925488 in FOXE1 as the effect allele also increases TSH (16).
For normal-range fT4 (NSNP = 31) concentrations, we used the European ancestry GWAS study from ThyroidOmics Consortium published by Teumer et al. (16) (Supplementary Table S1) or proxy SNPs from previous fT4 GWAS studies (18,19). The study consists of 19 cohorts with 49,269 participants for fT4. Participants with known thyroid disease were excluded. Details on laboratory analyses for each cohort are provided in the article by Teumer et al. (16). As the genetic variants associated with fT4 levels form a highly heterogeneous group with potentially diverse effects on thyroxine (T4) and triiodothyronine (T3) tissue availability (36,37), we stratified fT4 variants into: (i) variants within the deiodinases loci (i.e., DIO1 and DIO2), which are enzymes involved in conversion of fT4 to the bioactive free triiodothyronine (fT3), and (ii) other (nondeiodinase) genetic variants associated with fT4 levels in the GWAS by Teumer et al. (16). There was only a limited overlap between GWAS TSH SNPs that were nominally associated with fT4 and vice versa (16).
For all thyroid traits, we included independent SNPs with minor allele frequency >1% previously identified in the GWAS studies with p < 5 × 10−8 (Supplementary Methods section and Supplementary Tables S1 and S2) (16,17,20). Instrument strength for each SNP in MR was estimated with an approximated F-statistic as a function of the magnitude and precision of the genetic effect: F = (βGX)2/(SEGX)2, where βGX is the per-allele genotype–phenotype association on the biomarker and SEGX is the standard error (SE) (38). Included SNPs had F above 10 as recommended to avoid weak instrument bias and commonly considered as sufficient strength threshold for valid genetic variants (39). β and SEs for TSH, fT4, and TPOAb are expressed in standard deviation (SD) units, whereas β and SEs for genetically predicted hypo- and hyperthyroidism are expressed as logodds.
Genetic estimates of thyroid function on coagulation and fibrinolysis
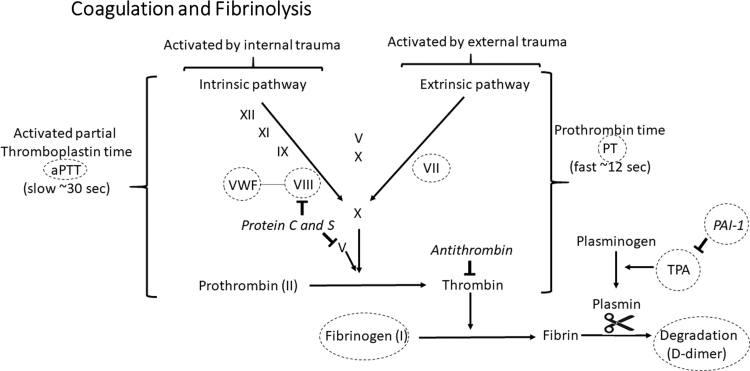
We examined TSH, hypothyroidism, TPOAb, fT4, and hyperthyroidism SNPs in the CHARGE Hemostasis data sets in relation to coagulation (VWF, FVIII, APTT, PT, FVII, fibrinogen) and fibrinolysis (D-dimer, TPA, PAI-1) (Fig. 1). APTT is test for the coagulation factors in the intrinsic and common pathways, whereas PT is a test for coagulation factors in the extrinsic and common pathways. In CHARGE, the number of cohorts varied from 1 to 34, and the total number of participants varied from 2583 to 120,246 depending on the biomarker. The majority of participants were European (Table 1 and Supplementary Table S3) (28–34).
FIG. 1.
A simplified overview of coagulation and fibrinolysis. Coagulation factors denoted Roman numbers are shown. Italicized words are inhibitors. The arrows show the activating direction of the coagulation cascade.  :: denotes inhibition. Dashed circles indicate the biomarkers investigated in this study. PAI-1, plasminogen activator inhibitor; TPA, tissue plasminogen activator; VWF, von Willebrand factor.
:: denotes inhibition. Dashed circles indicate the biomarkers investigated in this study. PAI-1, plasminogen activator inhibitor; TPA, tissue plasminogen activator; VWF, von Willebrand factor.
We extracted summary statistics for the thyroid function instruments, indexing SNP position according to build GRCh37.13. We aligned the effect of the outcome SNPs to the thyroid effect alleles with “effect allele” as the thyroid risk increasing allele. Preanalytical and analytical details for the measurement of the CHARGE Hemostasis biomarkers are listed in the GWAS articles and are summarized in Supplementary Table S3 (28–34). The beta and its SE for D-dimer, fibrinogen, FVII, FVIII, PAI-1, TPA, and VWF were all calculated on natural logarithm transformed trait, whereas the beta and its SE for APTT (28) was initially provided as a Z-score and converted to SD units (Supplementary Methods section), and based on international normalized ratio units for PT. We were not able to identify all thyroid exposure SNPs for the CHARGE coagulation outcomes, as genotyping and imputation were performed on reference panels using HapMap, while 1000G was used for TSH and fT4.
Statistical analysis
For each SNP, we calculated the instrumental variable ratio as the quotient of the SNP-outcome to SNP-exposure effects using the Wald estimator. We calculated the combined effect across all SNPs using inverse-variance weighted random effects (IVW-RE), which was the main analysis. We assessed the robustness of the IVW-RE in three complementary sensitivity analyses with different assumptions about horizontal pleiotropy: weighted median, weighted mode, and MR-Egger regression (40–43). The between-instrument heterogeneity Cochran's Q-statistic and the I2 index were used to assess heterogeneity in the meta-analysis (44). We used the MR-PRESSO to test and correct for possible bias from horizontal pleiotropy (45). We stratified TSH SNPs based on their previous association with autoimmune diseases, and fT4 SNPs based on their involvement in the enzymatic conversion of fT4 to fT3 (i.e., SNPs in DIO1 and DIO2 genes) (Supplementary Tables S1 andS2). We considered p < 0.05 as nominally significant. A Bonferroni correction was used to control for false-positive findings due to multiple comparisons, and with five thyroid traits (TSH, hypothyroidism, TPOAb positivity, fT4, hyperthyroidism) and nine biomarker outcomes, a two-sided p < 0.0011 [ = 0.05/(5 exposures × 9 outcomes)] was considered significant for the main IVW-RE analysis. We used the Stata package mrrobust for all analyses, except MR-PRESSO, which was performed in R. This study was based on aggregate data and did not require IRB approval.
Results
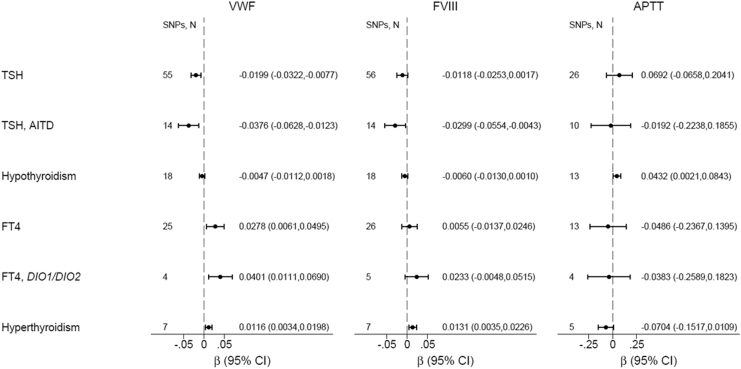
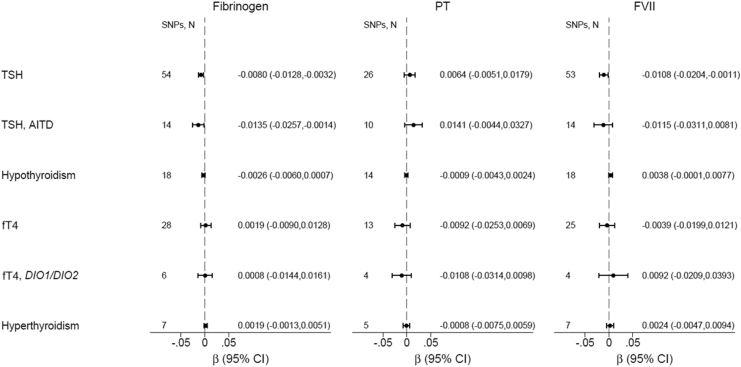
Genetically increased TSH was associated with decreased VWF [β(SE) = −0.020(0.006), p = 0.001] among all TSH SNPs (Fig. 2 and Supplementary Table S4) and [β(SE) = −0.038(0.013), p = 0.004] among autoimmune TSH SNPs (Supplementary Table S5); p-values were borderline significant after Bonferroni correction (Fig. 2 and Supplementary Tables S4–S6). Genetically increased normal-range TSH was associated with decreased fibrinogen [β(SE) = −0.008(0.002), p = 0.001], which was significant after Bonferroni correction (Fig. 2 and Supplementary Tables S4–S6). Genetically predicted hypothyroidism did not associate with decreased VWF [β(SE) = −0.005(0.003), p = 0.15] (Fig. 3 and Supplementary Table S7), although the direction was similar as to TSH. Genetically predicted TPOAb positivity did not associate with VWF (Supplementary Table S8). Genetically increased normal-range fT4 was nominally associated with increased VWF [β(SE) = 0.028(0.011), p = 0.012] (Fig. 2 and Supplementary Table S9). Genetically increased normal-range fT4 driven by DIO1 and DIO2 variants was nominally associated with increased VWF [β(SE) = 0.040(0.015), p = 0.007] (Fig. 2 and Supplementary Tables S10 and S11). Genetically predicted hyperthyroidism was nominally associated with increased VWF [β(SE) = 0.012(0.004), p = 0.006] and increased FVIII [β(SE) = 0.013(0.005), p = 0.007] (Fig. 2 and Supplementary Table S12).
FIG. 2.
Mendelian randomization of thyroid function on VWF, FVIII, and APTT. Results shown are based on inverse-variance-weighted random effects analyses. AITD, autoimmune thyroid disease; APTT, activated partial thromboplastin time; β, beta coefficient; DIO1/DIO2, Deiodinase 1 and Deiodinase 2 genes; FVIII, factor VIII; SNPs, N, number of single nucleotide polymorphisms for each analysis; TSH, thyrotropin.
FIG. 3.
Mendelian randomization of thyroid function on fibrinogen, PT, and FVII. Results shown are based on inverse-variance-weighted random effects analyses. fT4, free thyroxine; FVII, factor VII; PT, prothrombin time.
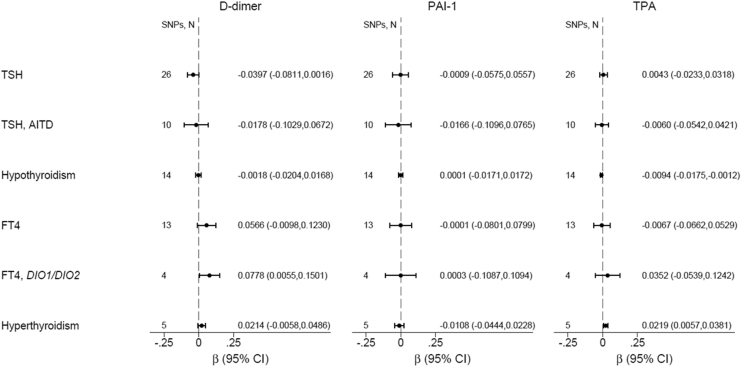
Genetically predicted hypothyroidism [β(SE) = −0.009(0.024), p = 0.024] and TPOAb positivity [β(SE) = −0.045(0.020), p = 0.026] were nominally associated with decreased TPA, whereas genetically predicted hyperthyroidism was nominally associated with increased TPA [β(SE) = 0.022(0.008), p = 0.008] (Fig. 4). Genetically increased TSH and fT4 were not associated with TPA.
FIG. 4.
Mendelian randomization of thyroid function on D-dimer, PAI-1 (plasminogen activator inhibitor) and tissue plasminogen activator (TPA). Results shown are based on inverse-variance-weighted random effects analyses.
The sensitivity analyses for the above-mentioned results showed similar direction but lower precision (Supplementary Tables S4–S12). For other coagulation and fibrinolytic factors, results were inconclusive (Figs. 2–4).
Discussion
This is the first MR study to investigate the causal effects of thyroid function on hemostasis, coagulation, and fibrinolysis. The hypothalamic–pituitary–thyroid axis is a hormonal feedback system with tight regulation and represents in an MR context an endogenous vertical pleiotropy. We found that genetically increased normal-range TSH, mainly driven by autoimmune variants, was associated with decreased VWF levels, and correspondingly genetically predicted hyperthyroidism and genetically increased normal-range fT4 driven by DIO1 and DIO2 variants were associated with increased VWF levels. The sensitivity analyses showed similar direction but with lower precision. Genetically predicted hypothyroidism and TPOAb positivity did not associate with increased VWF.
VWF is secreted from the vascular endothelium and platelets. VWF acts as a carrier for FVIII to protect it from degradation and acts as a bridge between platelets to promote platelet aggregation and adhesion to the vascular endothelium during vascular injury (1). Observationally, untreated hypothyroidism is associated with acquired von Willebrand syndrome (2,3), and concordantly, untreated hyperthyroidism is associated with increased concentrations of VWF and FVIII (7–10). The genetic correlation of FVIII and VWF is 83.5% (32); therefore, low VWF is often associated with a low FVIII activity and vice versa.
In the present MR study, we found a potential association that may be suggestive of a causal mechanism between thyroid function and VWF concentrations. The observed associations for genetically increased TSH on VWF were mirrored by genetically predicted hyperthyroidism and fT4. These findings serve as an internal validation of the results, despite that sensitivity analyses were not Bonferroni significant. Genetically predicted hyperthyroidism was the only thyroid trait associated with increased FVIII, further validating the observations for VWF (through the high genetic correlation between the two biomarkers). We were not able to find a causal association between genetically predicted hypothyroidism, a diagnosis that was partially based on self-reported levothyroxine treatment, and decreased VWF. This is also in accordance with the observational literature showing that VWF concentrations in hypothyroidism restore after levothyroxine replacement therapy and achievement of euthyroidism (3). Our findings that TSH was associated with decreased VWF, while fT4, particularly through deiodinase variants, was associated with increased VWF, are in line with the synthesis defect, that is, downregulation of VWF. We cannot exclude an underlying autoimmune mechanism, as the genetic TSH association was mainly driven by autoimmune variants. However, we were not able to find a causal association between genetically predicted TPOAb positivity and decreased VWF, but this could be due to the low number of genetic variants.
The genetic loci contributing to thyroid function did not include the VWF gene, and therefore, the observed MR association of increased TSH with decreased VWF does not qualify as von Willebrand disease, but may rather contribute to the variability and explain a low-level VWF phenotype (46). The observed effects of thyroid function on VWF as observed in this MR study may have physiological importance but weak clinical importance. Despite our suggestive MR finding that increased TSH is associated with decreased VWF and conversely increased fT4 was associated with increased VWF, previous MR studies of genetically increased TSH and fT4 did not find an association with intracerebral or subarachnoid hemorrhage (23) and VTE (23). Furthermore, in previous MR, genetically increased TSH was associated with decreased risk of AF (22) and decreased risk of stroke, but the latter association fully disappeared when controlling for AF (25), suggesting that other pleiotropic biological pathways between TSH and stroke other than AF are unlikely. Thus, despite the effect of increased VWF and FVIII on increased risk of ischemic stroke, VTE and coronary artery have been established (32); the suggestive effect in this MR of hyperthyroidism and increased fT4 on increased VWF and FVIII does not translate into a dominant clinical mechanism. Prospective studies are needed to address if patients with increased TSH and low VWF concentration will benefit from hemostatic treatment during surgical interventions, and if patients with decreased TSH and increased VWF concentration will benefit from VTE prophylaxis for surgical interventions.
Epidemiological evidence and directionality for the association of thyroid function on other measures of the coagulation and fibrinolytic system (increased concentrations of APTT, PT, FVII, D-dimer, PAI-1, TPA) are still debated (9,10,14). TPA is primarily synthesized and secreted by the vascular endothelium and is a biomarker of thromboembolic stroke (47) and major cardiovascular events in patients with atrial fibrillation (48). TPA does therefore not represent fibrinolysis alone, but also an increased activation of the coagulation cascade. In our MR study, genetically predicted hypothyroidism and TPOAb positivity were nominally associated with decreased TPA, whereas genetically predicted hyperthyroidism was nominally associated with increased TPA.
In this MR, the effect of thyroid function on other coagulation and fibrinolysis factors was null given the large number of tests and the corresponding Bonferroni standards for significance. Since Bonferroni correction, which assumes independence, was overly conservative, given the correlation between thyroid traits, and between coagulation outcomes, and statistical tests were correlated, we cannot completely reject nominal associations of thyroid traits with coagulation or fibrinolysis. The correlation structures for thyroid traits and coagulation outcomes are complex (49,50). Thyroid traits are (i) ordered (TSH before fT4) in the hypothalamic–pituitary–organ axis with negative feedback of fT4 on TSH, (ii) ranked as hypothyroidism, euthyroidism, or hyperthyroidism, and (iii) classified into autoimmunity or not (TPOAb positivity) (16,20). The coagulation system is (i) divided into the intrinsic and extrinsic systems, which are not independent and are regulated by the anticoagulation system, (ii) is an ordered sequence of dependent events, and (iii) commences in a common pathway (fibrinogen), which is regulated by the fibrinolytic (PAI-1, TPA) system with D-dimer as the breakdown product (28–34). The nine outcomes assessed both overall function and separate factors for the intrinsic (APTT) and the extrinsic (PT) systems (49,50). Finally, the five MR analyses were correlated as they use meta-analysis as the underlying model but have different assumptions and handling of heterogeneity (51).
We were unable to investigate the VWF activity using the platelet binding ristocetin (VWF:RCo) assay or the collagen binding activity (VWF:CB) assay, as GWAS for these analyses have not been published. However, as the VWF gene is not among the thyroid trait loci, a functional thyroid-associated VWF defect is unlikely. Therefore, in hypothyroid patients, low concentration of VWF can be detected using VWF:Ag assay, as in this study (46). Another potential limitation is population stratification, as genetic studies on VWF (and also FVIII and FVII) included trans-ethnic populations (79–92% of European ancestry), while genetic studies on all other coagulation, fibrinolysis, and all thyroid traits were limited to individuals of European ancestry. The number of individuals included in the GWAS for the outcomes varied from 2583 (PT) to 120,246 (fibrinogen), and due to the less dense imputation in the CHARGE compared with the ThyroidOmics Consortium (HapMap vs. 1000G reference panel), the number of SNPs obtained varied substantially. Thus, these MR analyses may be worth revisiting in the future with greater number of SNPs, which provide stronger instrumental variables.
In the largest studies currently available, genetically increased TSH and fT4 may be associated with decreased and increased synthesis of VWF, respectively. Bonferroni correction was conservative as thyroid traits, coagulation outcomes, and MR analyses were correlated, and thus, we cannot completely reject nominal associations of thyroid traits with coagulation or fibrinolytic factors.
Supplementary Material
Authors' Contributions
Conceptual idea: A.D.K., C.E., D.I.C., P.S.D.V. CHARGE Hemostasis Group: A.D.J., D.-A.T., D.I.C., J.H., M.S.-L., P.S.D.V., N.L.S. CHARGE look-up: P.S.D.V. ThyroidOmics Consortium: A.D.K., A.K., A.T., B.Å., C.E., D.I.C., E.M., M.M., R.B.T.M.S., S.B. Analyses: C.E. Statistical design and coding: A.D.K., C.E. Article draft: A.D.K., C.E., D.I.C., P.S.D.V. Critical revision and final approval: all authors.
Author Disclosure Statement
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute (NHLBI); the National Institutes of Health; or the U.S. Department of Health and Human Services.
Funding Information
M.S.-L. is supported by a Miguel Servet contract from the ISCIII Spanish Health Institute (CP17/00142) and co-financed by the European Social Fund. S.M. was funded by the National Heart, Lung, and Blood Institute (NHLBI) grant K24HL136852. A.D.J. is supported by the National Heart, Lung, and Blood Institute Intramural funding. P.S.D.V was supported by the American Heart Association grant number 18CDA34110116 and NHLBI grants R01HL141291, R01HL139553, and R01HL134894. The CHARGE Hemostasis Working Group is partially funded by NHLBI grants HL134894 and HL139553. D.-A.T. is partially supported by the EPIDEMIOM-VT Senior Chair from the University of Bordeaux initiative of excellence IdEX.
Supplementary Material
References
- 1. Randi AM, Smith KE, Castaman G. 2018. von Willebrand factor regulation of blood vessel formation. Blood 132:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manfredi E, van Zaane B, Gerdes VE, Brandjes DP, Squizzato A. 2008. Hypothyroidism and acquired von Willebrand's syndrome: a systematic review. Haemophilia 14:423–433. [DOI] [PubMed] [Google Scholar]
- 3. Stuijver DJ, Piantanida E, van Zaane B, Galli L, Romualdi E, Tanda ML, Meijers JC, Buller HR, Gerdes VE, Squizzato A. 2014. Acquired von Willebrand syndrome in patients with overt hypothyroidism: a prospective cohort study. Haemophilia 20:326–332. [DOI] [PubMed] [Google Scholar]
- 4. Krassas GE, Pontikides N, Kaltsas T, Papadopoulou P, Paunkovic J, Paunkovic N, Duntas LH. 1999. Disturbances of menstruation in hypothyroidism. Clin Endocrinol (Oxf) 50:655–659. [DOI] [PubMed] [Google Scholar]
- 5. Horacek J, Maly J, Svilias I, Smolej L, Cepkova J, Vizda J, Sadilek P, Fatorova I, Zak P. 2015. Prothrombotic changes due to an increase in thyroid hormone levels. Eur J Endocrinol 172:537–542. [DOI] [PubMed] [Google Scholar]
- 6. Homoncik M, Gessl A, Ferlitsch A, Jilma B, Vierhapper H. 2007. Altered platelet plug formation in hyperthyroidism and hypothyroidism. J Clin Endocrinol Metab 92:3006–3012. [DOI] [PubMed] [Google Scholar]
- 7. Elbers LP, Moran C, Gerdes VE, van Zaane B, Meijers J, Endert E, Lyons G, Chatterjee VK, Bisschop PH, Fliers E 2016 The hypercoagulable state in hyperthyroidism is mediated via the thyroid hormone beta receptor pathway. Eur J Endocrinol [Epub ahead of print]; DOI: 10.1530/EJE-15-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Debeij J, van Zaane B, Dekkers OM, Doggen CJ, Smit JW, van Zanten AP, Brandjes DP, Buller HR, Gerdes VE, Rosendaal FR, Cannegieter SC. 2014. High levels of procoagulant factors mediate the association between free thyroxine and the risk of venous thrombosis: the MEGA study. J Thromb Haemost 12:839–846. [DOI] [PubMed] [Google Scholar]
- 9. Stuijver DJ, van Zaane B, Romualdi E, Brandjes DP, Gerdes VE, Squizzato A. 2012. The effect of hyperthyroidism on procoagulant, anticoagulant and fibrinolytic factors: a systematic review and meta-analysis. Thromb Haemost 108:1077–1088. [DOI] [PubMed] [Google Scholar]
- 10. Van Zaane B, Squizzato A, Debeij J, Dekkers OM, Meijers JC, Van Zanten AP, Buller HR, Gerdes VE, Cannegieter SC, Brandjes DP. 2011. Alterations in coagulation and fibrinolysis after levothyroxine exposure in healthy volunteers: a controlled randomized crossover study. J Thromb Haemost 9:1816–1824. [DOI] [PubMed] [Google Scholar]
- 11. Debeij J, Dekkers OM, Asvold BO, Christiansen SC, Naess IA, Hammerstrom J, Rosendaal FR, Cannegieter SC. 2012. Increased levels of free thyroxine and risk of venous thrombosis in a large population-based prospective study. J Thromb Haemost 10:1539–1546. [DOI] [PubMed] [Google Scholar]
- 12. van Zaane B, Squizzato A, Huijgen R, van Zanten AP, Fliers E, Cannegieter SC, Buller HR, Gerdes VE, Brandjes DP. 2010. Increasing levels of free thyroxine as a risk factor for a first venous thrombosis: a case-control study. Blood 115:4344–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dekkers OM, Horvath-Puho E, Cannegieter SC, Vandenbroucke JP, Sorensen HT, Jorgensen JO. 2017. Acute cardiovascular events and all-cause mortality in patients with hyperthyroidism: a population-based cohort study. Eur J Endocrinol 176:1–9. [DOI] [PubMed] [Google Scholar]
- 14. Elbers LPB, Fliers E, Cannegieter SC. 2018. The influence of thyroid function on the coagulation system and its clinical consequences. J Thromb Haemost 16:634–645. [DOI] [PubMed] [Google Scholar]
- 15. Eriksson N, Tung JY, Kiefer AK, Hinds DA, Francke U, Mountain JL, Do CB. 2012. Novel associations for hypothyroidism include known autoimmune risk loci. PLoS One 7:e34442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teumer A, Chaker L, Groeneweg S, Li Y, Di Munno C, Barbieri C, Schultheiss UT, Traglia M, Ahluwalia TS, Akiyama M, Appel EVR, Arking DE, Arnold A, Astrup A, Beekman M, Beilby JP, Bekaert S, Boerwinkle E, Brown SJ, De Buyzere M, Campbell PJ, Ceresini G, Cerqueira C, Cucca F, Deary IJ, Deelen J, Eckardt KU, Ekici AB, Eriksson JG, Ferrrucci L, Fiers T, Fiorillo E, Ford I, Fox CS, Fuchsberger C, Galesloot TE, Gieger C, Gogele M, De Grandi A, Grarup N, Greiser KH, Haljas K, Hansen T, Harris SE, van Heemst D, den Heijer M, Hicks AA, den Hollander W, Homuth G, Hui J, Ikram MA, Ittermann T, Jensen RA, Jing J, Jukema JW, Kajantie E, Kamatani Y, Kasbohm E, Kaufman JM, Kiemeney LA, Kloppenburg M, Kronenberg F, Kubo M, Lahti J, Lapauw B, Li S, Liewald DCM, Lifelines Cohort S, Lim EM, Linneberg A, Marina M, Mascalzoni D, Matsuda K, Medenwald D, Meisinger C, Meulenbelt I, De Meyer T, Meyer Zu Schwabedissen HE, Mikolajczyk R, Moed M, Netea-Maier RT, Nolte IM, Okada Y, Pala M, Pattaro C, Pedersen O, Petersmann A, Porcu E, Postmus I, Pramstaller PP, Psaty BM, Ramos YFM, Rawal R, Redmond P, Richards JB, Rietzschel ER, Rivadeneira F, Roef G, Rotter JI, Sala CF, Schlessinger D, Selvin E, Slagboom PE, Soranzo N, Sorensen TIA, Spector TD, Starr JM, Stott DJ, Taes Y, Taliun D, Tanaka T, Thuesen B, Tiller D, Toniolo D, Uitterlinden AG, Visser WE, Walsh JP, Wilson SG, Wolffenbuttel BHR, Yang Q, Zheng HF, Cappola A, Peeters RP, Naitza S, Volzke H, Sanna S, Kottgen A, Visser TJ, Medici M. 2018. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat Commun 9:4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pickrell JK, Berisa T, Liu JZ, Segurel L, Tung JY, Hinds DA. 2016. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet 48:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, Bos SD, Deelen J, den Heijer M, Freathy RM, Lahti J, Liu C, Lopez LM, Nolte IM, O'Connell JR, Tanaka T, Trompet S, Arnold A, Bandinelli S, Beekman M, Bohringer S, Brown SJ, Buckley BM, Camaschella C, de Craen AJ, Davies G, de Visser MC, Ford I, Forsen T, Frayling TM, Fugazzola L, Gogele M, Hattersley AT, Hermus AR, Hofman A, Houwing-Duistermaat JJ, Jensen RA, Kajantie E, Kloppenburg M, Lim EM, Masciullo C, Mariotti S, Minelli C, Mitchell BD, Nagaraja R, Netea-Maier RT, Palotie A, Persani L, Piras MG, Psaty BM, Raikkonen K, Richards JB, Rivadeneira F, Sala C, Sabra MM, Sattar N, Shields BM, Soranzo N, Starr JM, Stott DJ, Sweep FC, Usala G, van der Klauw MM, van Heemst D, van Mullem A, Vermeulen SH, Visser WE, Walsh JP, Westendorp RG, Widen E, Zhai G, Cucca F, Deary IJ, Eriksson JG, Ferrucci L, Fox CS, Jukema JW, Kiemeney LA, Pramstaller PP, Schlessinger D, Shuldiner AR, Slagboom EP, Uitterlinden AG, Vaidya B, Visser TJ, Wolffenbuttel BH, Meulenbelt I, Rotter JI, Spector TD, Hicks AA, Toniolo D, Sanna S, Peeters RP, Naitza S. 2013. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet 9:e1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor PN, Porcu E, Chew S, Campbell PJ, Traglia M, Brown SJ, Mullin BH, Shihab HA, Min J, Walter K, Memari Y, Huang J, Barnes MR, Beilby JP, Charoen P, Danecek P, Dudbridge F, Forgetta V, Greenwood C, Grundberg E, Johnson AD, Hui J, Lim EM, McCarthy S, Muddyman D, Panicker V, Perry JR, Bell JT, Yuan W, Relton C, Gaunt T, Schlessinger D, Abecasis G, Cucca F, Surdulescu GL, Woltersdorf W, Zeggini E, Zheng HF, Toniolo D, Dayan CM, Naitza S, Walsh JP, Spector T, Davey Smith G, Durbin R, Richards JB, Sanna S, Soranzo N, Timpson NJ, Wilson SG; UK0K Consortium. 2015. Whole-genome sequence-based analysis of thyroid function. Nat Commun 6:5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schultheiss UT, Teumer A, Medici M, Li Y, Daya N, Chaker L, Homuth G, Uitterlinden AG, Nauck M, Hofman A, Selvin E, Volzke H, Peeters RP, Kottgen A. 2015. A genetic risk score for thyroid peroxidase antibodies associates with clinical thyroid disease in community-based populations. J Clin Endocrinol Metab 100:E799–E807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medici M, Porcu E, Pistis G, Teumer A, Brown SJ, Jensen RA, Rawal R, Roef GL, Plantinga TS, Vermeulen SH, Lahti J, Simmonds MJ, Husemoen LL, Freathy RM, Shields BM, Pietzner D, Nagy R, Broer L, Chaker L, Korevaar TI, Plia MG, Sala C, Volker U, Richards JB, Sweep FC, Gieger C, Corre T, Kajantie E, Thuesen B, Taes YE, Visser WE, Hattersley AT, Kratzsch J, Hamilton A, Li W, Homuth G, Lobina M, Mariotti S, Soranzo N, Cocca M, Nauck M, Spielhagen C, Ross A, Arnold A, van de Bunt M, Liyanarachchi S, Heier M, Grabe HJ, Masciullo C, Galesloot TE, Lim EM, Reischl E, Leedman PJ, Lai S, Delitala A, Bremner AP, Philips DI, Beilby JP, Mulas A, Vocale M, Abecasis G, Forsen T, James A, Widen E, Hui J, Prokisch H, Rietzschel EE, Palotie A, Feddema P, Fletcher SJ, Schramm K, Rotter JI, Kluttig A, Radke D, Traglia M, Surdulescu GL, He H, Franklyn JA, Tiller D, Vaidya B, de Meyer T, Jorgensen T, Eriksson JG, O'Leary PC, Wichmann E, Hermus AR, Psaty BM, Ittermann T, Hofman A, Bosi E, Schlessinger D, Wallaschofski H, Pirastu N, Aulchenko YS, de la Chapelle A, Netea-Maier RT, Gough SC, Meyer Zu Schwabedissen H, Frayling TM, Kaufman JM, Linneberg A, Raikkonen K, Smit JW, Kiemeney LA, Rivadeneira F, Uitterlinden AG, Walsh JP, Meisinger C, den Heijer M, Visser TJ, Spector TD, Wilson SG, Volzke H, Cappola A, Toniolo D, Sanna S, Naitza S, Peeters RP. 2014. Identification of novel genetic Loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet 10:e1004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellervik C, Roselli C, Christophersen IE, Alonso A, Pietzner M, Sitlani CM, Trompet S, Arking DE, Geelhoed B, Guo X, Kleber ME, Lin HJ, Lin H, MacFarlane P, Selvin E, Shaffer C, Smith AV, Verweij N, Weiss S, Cappola AR, Dorr M, Gudnason V, Heckbert S, Mooijaart S, Marz W, Psaty BM, Ridker PM, Roden D, Stott DJ, Volzke H, Benjamin EJ, Delgado G, Ellinor P, Homuth G, Kottgen A, Jukema JW, Lubitz SA, Mora S, Rienstra M, Rotter JI, Shoemaker MB, Sotoodehnia N, Taylor KD, van der Harst P, Albert CM, Chasman DI. 2019. Assessment of the relationship between genetic determinants of thyroid function and atrial fibrillation: a Mendelian randomization study. JAMA Cardiol 4:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larsson SC, Allara E, Mason AM, Michaelsson K, Burgess S. 2019. Thyroid function and dysfunction in relation to 16 cardiovascular diseases. Circ Genom Precis Med 12:e002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellervik C, Mora S, Ridker PM, Chasman DI. 2020. Hypothyroidism and kidney function: a Mendelian randomization study. Thyroid 30:365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marouli E, Kus A, Del Greco MF, Chaker L, Peeters R, Teumer A, Deloukas P, Medici M 2020 Thyroid function affects the risk of stroke via atrial fibrillation: a Mendelian randomization study. J Clin Endocrinol Metab 105:2634–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kus A, Marouli E, Del Greco MF, Chaker L, Bednarczuk T, Peeters RP, Teumer A, Medici M, Deloukas P. 2020. Variation in normal range thyroid function affects serum cholesterol levels, blood pressure, and type 2 diabetes risk: a Mendelian randomization study. Thyroid 31:721–731. [DOI] [PubMed] [Google Scholar]
- 27. van Vliet NA, Noordam R, van Klinken JB, Westendorp RG, Bassett JD, Williams GR, van Heemst D. 2018. Thyroid stimulating hormone and bone mineral density: evidence from a two-sample Mendelian randomization study and a candidate gene association study. J Bone Miner Res 33:1318–1325. [DOI] [PubMed] [Google Scholar]
- 28. Tang W, Schwienbacher C, Lopez LM, Ben-Shlomo Y, Oudot-Mellakh T, Johnson AD, Samani NJ, Basu S, Gogele M, Davies G, Lowe GD, Tregouet DA, Tan A, Pankow JS, Tenesa A, Levy D, Volpato CB, Rumley A, Gow AJ, Minelli C, Yarnell JW, Porteous DJ, Starr JM, Gallacher J, Boerwinkle E, Visscher PM, Pramstaller PP, Cushman M, Emilsson V, Plump AS, Matijevic N, Morange PE, Deary IJ, Hicks AA, Folsom AR. 2012. Genetic associations for activated partial thromboplastin time and prothrombin time, their gene expression profiles, and risk of coronary artery disease. Am J Hum Genet 91:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang J, Sabater-Lleal M, Asselbergs FW, Tregouet D, Shin SY, Ding J, Baumert J, Oudot-Mellakh T, Folkersen L, Johnson AD, Smith NL, Williams SM, Ikram MA, Kleber ME, Becker DM, Truong V, Mychaleckyj JC, Tang W, Yang Q, Sennblad B, Moore JH, Williams FM, Dehghan A, Silbernagel G, Schrijvers EM, Smith S, Karakas M, Tofler GH, Silveira A, Navis GJ, Lohman K, Chen MH, Peters A, Goel A, Hopewell JC, Chambers JC, Saleheen D, Lundmark P, Psaty BM, Strawbridge RJ, Boehm BO, Carter AM, Meisinger C, Peden JF, Bis JC, McKnight B, Ohrvik J, Taylor K, Franzosi MG, Seedorf U, Collins R, Franco-Cereceda A, Syvanen AC, Goodall AH, Yanek LR, Cushman M, Muller-Nurasyid M, Folsom AR, Basu S, Matijevic N, van Gilst WH, Kooner JS, Hofman A, Danesh J, Clarke R, Meigs JB, Consortium D, Kathiresan S, Reilly MP, Consortium CA, Klopp N, Harris TB, Winkelmann BR, Grant PJ, Hillege HL, Watkins H, Consortium CD, Spector TD, Becker LC, Tracy RP, Marz W, Uitterlinden AG, Eriksson P, Cambien F; CARDIoGRAM Consortium, Morange PE, Koenig W, Soranzo N, van der Harst P, Liu Y, O'Donnell CJ, Hamsten A. 2012. Genome-wide association study for circulating levels of PAI-1 provides novel insights into its regulation. Blood 120:4873–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang J, Huffman JE, Yamakuchi M, Trompet S, Asselbergs FW, Sabater-Lleal M, Tregouet DA, Chen WM, Smith NL, Kleber ME, Shin SY, Becker DM, Tang W, Dehghan A, Johnson AD, Truong V, Folkersen L, Yang Q, Oudot-Mellkah T, Buckley BM, Moore JH, Williams FM, Campbell H, Silbernagel G, Vitart V, Rudan I, Tofler GH, Navis GJ, Destefano A, Wright AF, Chen MH, de Craen AJ, Worrall BB, Rudnicka AR, Rumley A, Bookman EB, Psaty BM, Chen F, Keene KL, Franco OH, Bohm BO, Uitterlinden AG, Carter AM, Jukema JW, Sattar N, Bis JC, Ikram MA; Cohorts for Heart and Aging Research in Genome Epidemiology Consortium Neurology Working Group, Sale MM, McKnight B, Fornage M, Ford I, Taylor K, Slagboom PE, McArdle WL, Hsu FC, Franco-Cereceda A, Goodall AH, Yanek LR, Furie KL, Cushman M, Hofman A, Witteman JC, Folsom AR, Basu S, Matijevic N, van Gilst WH, Wilson JF, Westendorp RG, Kathiresan S, Reilly MP, Consortium CA, Tracy RP, Polasek O, Winkelmann BR, Grant PJ, Hillege HL, Cambien F, Stott DJ, Lowe GD, Spector TD, Meigs JB, Marz W, Eriksson P, Becker LC, Morange PE, Soranzo N, Williams SM, Hayward C, van der Harst P, Hamsten A, Lowenstein CJ, Strachan DP, O'Donnell CJ; CHARGE Consortium Hemostatic Factor Working Group. 2014. Genome-wide association study for circulating tissue plasminogen activator levels and functional follow-up implicates endothelial STXBP5 and STX2. Arterioscler Thromb Vasc Biol 34:1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Vries PS, Chasman DI, Sabater-Lleal M, Chen MH, Huffman JE, Steri M, Tang W, Teumer A, Marioni RE, Grossmann V, Hottenga JJ, Trompet S, Muller-Nurasyid M, Zhao JH, Brody JA, Kleber ME, Guo X, Wang JJ, Auer PL, Attia JR, Yanek LR, Ahluwalia TS, Lahti J, Venturini C, Tanaka T, Bielak LF, Joshi PK, Rocanin-Arjo A, Kolcic I, Navarro P, Rose LM, Oldmeadow C, Riess H, Mazur J, Basu S, Goel A, Yang Q, Ghanbari M, Willemsen G, Rumley A, Fiorillo E, de Craen AJ, Grotevendt A, Scott R, Taylor KD, Delgado GE, Yao J, Kifley A, Kooperberg C, Qayyum R, Lopez LM, Berentzen TL, Raikkonen K, Mangino M, Bandinelli S, Peyser PA, Wild S, Tregouet DA, Wright AF, Marten J, Zemunik T, Morrison AC, Sennblad B, Tofler G, de Maat MP, de Geus EJ, Lowe GD, Zoledziewska M, Sattar N, Binder H, Volker U, Waldenberger M, Khaw KT, McKnight B, Huang J, Jenny NS, Holliday EG, Qi L, McEvoy MG, Becker DM, Starr JM, Sarin AP, Hysi PG, Hernandez DG, Jhun MA, Campbell H, Hamsten A, Rivadeneira F, McArdle WL, Slagboom PE, Zeller T, Koenig W, Psaty BM, Haritunians T, Liu J, Palotie A, Uitterlinden AG, Stott DJ, Hofman A, Franco OH, Polasek O, Rudan I, Morange PE, Wilson JF, Kardia SL, Ferrucci L, Spector TD, Eriksson JG, Hansen T, Deary IJ, Becker LC, Scott RJ, Mitchell P, Marz W, Wareham NJ, Peters A, Greinacher A, Wild PS, Jukema JW, Boomsma DI, Hayward C, Cucca F, Tracy R, Watkins H, Reiner AP, Folsom AR, Ridker PM, O'Donnell CJ, Smith NL, Strachan DP, Dehghan A. 2016. A meta-analysis of 120 246 individuals identifies 18 new loci for fibrinogen concentration. Hum Mol Genet 25:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sabater-Lleal M, Huffman JE, de Vries PS, Marten J, Mastrangelo MA, Song C, Pankratz N, Ward-Caviness CK, Yanek LR, Trompet S, Delgado GE, Guo X, Bartz TM, Martinez-Perez A, Germain M, de Haan HG, Ozel AB, Polasek O, Smith AV, Eicher JD, Reiner AP, Tang W, Davies NM, Stott DJ, Rotter JI, Tofler GH, Boerwinkle E, de Maat MPM, Kleber ME, Welsh P, Brody JA, Chen MH, Vaidya D, Soria JM, Suchon P, van Hylckama Vlieg A, Desch KC, Kolcic I, Joshi PK, Launer LJ, Harris TB, Campbell H, Rudan I, Becker DM, Li JZ, Rivadeneira F, Uitterlinden AG, Hofman A, Franco OH, Cushman M, Psaty BM, Morange PE, McKnight B, Chong MR, Fernandez-Cadenas I, Rosand J, Lindgren A; INVENT Consortium; MEGASTROKE Consortium of the International Stroke Genetics Consortium, Gudnason V, Wilson JF, Hayward C, Ginsburg D, Fornage M, Rosendaal FR, Souto JC, Becker LC, Jenny NS, Marz W, Jukema JW, Dehghan A, Tregouet DA, Morrison AC, Johnson AD, O'Donnell CJ, Strachan DP, Lowenstein CJ, Smith NL. 2019. Genome-wide association transethnic meta-analyses identifies novel associations regulating coagulation factor VIII and von Willebrand factor plasma levels. Circulation 139:620–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Vries PS, Sabater-Lleal M, Huffman JE, Marten J, Song C, Pankratz N, Bartz TM, de Haan HG, Delgado GE, Eicher JD, Martinez-Perez A, Ward-Caviness CK, Brody JA, Chen MH, de Maat MPM, Franberg M, Gill D, Kleber ME, Rivadeneira F, Soria JM, Tang W, Tofler GH, Uitterlinden AG, van Hylckama Vlieg A, Seshadri S, Boerwinkle E, Davies NM, Giese AK, Ikram MK, Kittner SJ, McKnight B, Psaty BM, Reiner AP, Sargurupremraj M, Taylor KD, Consortium I, Consortium MCotISG, Fornage M, Hamsten A, Marz W, Rosendaal FR, Souto JC, Dehghan A, Johnson AD, Morrison AC, O'Donnell CJ, Smith NL. 2019. A genome-wide association study identifies new loci for factor VII and implicates factor VII in ischemic stroke etiology. Blood 133:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith NL, Huffman JE, Strachan DP, Huang J, Dehghan A, Trompet S, Lopez LM, Shin SY, Baumert J, Vitart V, Bis JC, Wild SH, Rumley A, Yang Q, Uitterlinden AG, Stott DJ, Davies G, Carter AM, Thorand B, Polasek O, McKnight B, Campbell H, Rudnicka AR, Chen MH, Buckley BM, Harris SE, Peters A, Pulanic D, Lumley T, de Craen AJ, Liewald DC, Gieger C, Campbell S, Ford I, Gow AJ, Luciano M, Porteous DJ, Guo X, Sattar N, Tenesa A, Cushman M, Slagboom PE, Visscher PM, Spector TD, Illig T, Rudan I, Bovill EG, Wright AF, McArdle WL, Tofler G, Hofman A, Westendorp RG, Starr JM, Grant PJ, Karakas M, Hastie ND, Psaty BM, Wilson JF, Lowe GD, O'Donnell CJ, Witteman JC, Jukema JW, Deary IJ, Soranzo N, Koenig W, Hayward C. 2011. Genetic predictors of fibrin D-dimer levels in healthy adults. Circulation 123:1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brcic L, Baric A, Gracan S, Brekalo M, Kalicanin D, Gunjaca I, Torlak Lovric V, Tokic S, Radman M, Skrabic V, Miljkovic A, Kolcic I, Stefanic M, Glavas-Obrovac L, Lessel D, Polasek O, Zemunik T, Barbalic M, Punda A, Boraska Perica V. 2019. Genome-wide association analysis suggests novel loci for Hashimoto's thyroiditis. J Endocrinol Invest 42:567–576. [DOI] [PubMed] [Google Scholar]
- 36. Kus A, Chaker L, Teumer A, Peeters RP, Medici M. 2020. The genetic basis of thyroid function: novel findings and new approaches. J Clin Endocrinol Metab 105:dgz225. [DOI] [PubMed] [Google Scholar]
- 37. Medici M, Peeters RP, Teumer A, Taylor P. 2019. The importance of high-quality Mendelian randomisation studies for clinical thyroidology. Lancet Diabetes Endocrinol 7:665–667. [DOI] [PubMed] [Google Scholar]
- 38. Gill D, Del Greco MF, Rawson TM, Sivakumaran P, Brown A, Sheehan NA, Minelli C. 2017. Age at menarche and time spent in education: a Mendelian randomization study. Behav Genet 47:480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. 2008. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27:1133–1163. [DOI] [PubMed] [Google Scholar]
- 40. Bowden J, Davey Smith G, Burgess S. 2015. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44:512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bowden J, Davey Smith G, Haycock PC, Burgess S. 2016. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. 2016. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol 45:1961–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. 2017. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med 36:1783–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Greco MF, Minelli C, Sheehan NA, Thompson JR. 2015. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med 34:2926–2940. [DOI] [PubMed] [Google Scholar]
- 45. Verbanck M, Chen CY, Neale B, Do R. 2018. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leebeek FW, Eikenboom JC. 2016. Von Willebrand's disease. N Engl J Med 375:2067–2080. [DOI] [PubMed] [Google Scholar]
- 47. Ridker PM, Hennekens CH, Stampfer MJ, Manson JE, Vaughan DE. 1994. Prospective study of endogenous tissue plasminogen activator and risk of stroke. Lancet 343:940–943. [DOI] [PubMed] [Google Scholar]
- 48. Freynhofer MK, Draxler DF, Gruber SC, Bruno V, Hochtl T, Fellner B, Jakl-Kotauschek G, Wojta J, Pabinger-Fasching I, Huber K, Ay C. 2013. Endogenous t-PA-antigen is an independent predictor of adverse cardiovascular events and all-cause death in patients with atrial fibrillation. J Thromb Haemost 11:1069–1077. [DOI] [PubMed] [Google Scholar]
- 49. Lippi G, Franchini M, Poli G, Salvagno GL, Montagnana M, Guidi GC. 2007. Is the activated partial thromboplastin time suitable to screen for von Willebrand factor deficiencies? Blood Coagul Fibrinolysis 18:361–364. [DOI] [PubMed] [Google Scholar]
- 50. Kim SY, Kim JE, Kim HK, Kim I, Yoon SS, Park S. 2013. Influence of coagulation and anticoagulant factors on global coagulation assays in healthy adults. Am J Clin Pathol 139:370–379. [DOI] [PubMed] [Google Scholar]
- 51. Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, Evans DM, Smith GD. 2017. Recent developments in Mendelian randomization studies. Curr Epidemiol Rep 4:330–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.