Abstract
Background: Despite screening's effectiveness in reducing cervical cancer incidence and mortality, disparities in cervical cancer screening uptake remain, with lower rates documented among uninsured and low-income individuals. We examined perceived financial barriers to, and the perceived cost burden of, cervical cancer screening.
Materials and Methods: We surveyed 702 low-income, uninsured or publicly insured women ages 25–64 years in North Carolina, U.S., who were not up to date on cervical cancer screening according to national guidelines. Participants were asked about perceived financial barriers to screening and how much they perceived screening would cost. We used multivariable logistic regression to assess the sociodemographic predictors of perceived financial barriers.
Results: Seventy-two percent of participants perceived financial barriers to screening. Screening appointment costs (71%) and follow-up/future treatment costs (44%) were most commonly reported, followed by lost pay due to time missed from work (6%) and transportation costs (5%). In multivariable analysis, being uninsured (vs. publicly insured), younger (25–34 vs. 50–64 years), White (vs. Black), and not reporting income data were associated with perceiving screening costs and future treatment costs as barriers to screening. Participants reported wide-ranging estimates of the perceived out-of-pocket cost of screening ($0–$1300), with a median expected cost of $245.
Conclusions: The majority of our sample of low-income women perceived substantial financial barriers to screening, particularly related to screening appointment costs and potential follow-up/future treatment costs. Providing greater cost transparency and access to financial assistance may reduce perceived financial barriers to screening, potentially increasing screening uptake among this underserved population. Clinicaltrials.gov registration number NCT02651883.
Keywords: cervical cancer, cancer screening, human papillomavirus, under-screened populations, health disparities, financial barriers
Introduction
Disparities in cervical cancer incidence and mortality persist by poverty level, insurance status, race, and ethnicity, despite availability of effective and cost-effective cervical cancer screening.1,2 These disparities are, in part, driven by inequities in screening access and uptake,3,4 as over half of new cervical cancer cases are attributed to insufficient screening.5 Lower rates of screening uptake have been documented among uninsured and low-income individuals.6–15 In 2018, 81% of U.S. women 21–65 years of age reported being up to date for cervical cancer screening as per national screening guidelines—having had a Papanicolaou (Pap) test within the past 3 years or human papillomavirus (HPV) test within the past 5 years.15,16 However, as of 2015, only 64% of uninsured women, 78% of publicly insured women, and 75% of low-income women had been screened in accordance with national screening guidelines, suggesting that more work is needed to increase screening coverage in these medically underserved groups.17
Perceived financial barriers likely affect cervical cancer screening adherence, particularly within medically underserved groups who are financially vulnerable. Financial barriers, often conceptualized as a subset of structural barriers, have been defined as cost-related factors inhibiting a patient's ability to pay for health care services or that discourage providers from caring for them.18 U.S. women consistently report cost as a barrier to cervical cancer screening, particularly among low-income and other medically underserved populations.19–22 The prevalence of perceived cost barriers to cervical cancer screening ranges from around 20% to over 60% among low-income, uninsured women.23–26 Qualitative analyses have provided additional insight into the types of cost barriers to screening individuals perceive. In addition to screening appointment and laboratory test costs,27,28 other perceived cost barriers may stem from potential follow-up procedures or treatment,29,30 taking time off work,19,24,29,31,32 childcare,31,33 and transportation.24,27,31,34
The Health Belief Model posits that perceived barriers, influenced by sociodemographic factors, reduce an individual's propensity to engage in health behaviors, such as cervical cancer screening.35 A deeper understanding of the prevalence of perceived financial barriers and the factors associated with the report of these barriers is urgently needed. Prior quantitative analyses of barriers to cervical cancer screening have asked individuals whether they perceived cost, generally, to be a barrier to screening.23,25,36 However, to effectively tailor interventions aiming to alleviate financial barriers, it is critical to differentiate between the different types of cost barriers. Additionally, an understanding of the sociodemographic predictors of perceiving financial barriers—which, to date, have only been assessed in a small sample of Latina women living in Utah36—should inform this work. Therefore, the objective of this study was to further understand the types of perceived financial barriers to, and perceived cost burden of, cervical cancer screening, as reported by low-income women in North Carolina who reported not being up to date with cervical cancer screening recommendations.
Materials and Methods
Participants
Participants were enrolled in the MyBodyMyTest Phase Three (MBMT-3) Study, a two-arm randomized controlled trial examining the effect of mailed HPV self-testing on cervical cancer screening among under-screened women.
Participants were recruited for study enrollment between April 2016 and December 2019. Recruitment methods included printed (flyers, posters, etc.), online (Facebook, Craigslist), and radio advertisements; referral through the NC United Way 2-1-1 social assistance helpline; and in-person enrollment at community events and through community organizations.37 Participants were eligible for the study if they were between the ages of 25 and 64, were not pregnant, had an intact cervix (no history of hysterectomy), had income ≤250% of the U.S. federal poverty level (FPL), were uninsured or enrolled in Medicaid or Medicare, and were living within the catchment area of a study-associated clinic (covering 22 North Carolina counties). In addition, women were eligible only if they self-reported not having a Pap test in 4 years or more and not having an HPV test in 6 years or more, since these women are considered overdue for screening according to national U.S. guidelines.16
Procedures
Potential participants completed an eligibility screener by phone when recruited through advertisements or the United Way hotline, or in-person in the case of in-person recruitment. Eligible women received informed consent forms via mail. They then completed a baseline survey by phone following enrollment. Participants received a $25 incentive for completing this survey, in addition to a potential additional $55 for completion of the study follow-up and exit surveys. No incentives were given for the completion of screening. The University of North Carolina Institutional Review Board (IRB) approved the study procedures.
Measures
Data are drawn from the eligibility screener and baseline questionnaire administered as a part of the MBMT-3 study. The eligibility screener included sociodemographic characteristics such as race, ethnicity, education, and health insurance status. The baseline survey assessed perceived barriers to cervical cancer screening using the following question: “What are some reasons that you haven't had a Pap smear recently?” Response options included “cost” and “no insurance,” in addition to other nonfinancial barriers (multiple responses were allowed). For each reason selected, participants then indicated what about that reason made it difficult to be screened. We coded these open-ended responses as belonging to discrete categories. Primary outcome variables for this analysis were defined as whether participants reported each of the following specific financial barriers: cost of screening test or appointment (screening cost), cost of follow-up care or treatment (future treatment cost), cost of taking time off work (lost pay), and cost of getting to and from the appointment (transportation cost). Responses did not include any other financial reasons.
Additionally, the survey assessed perceived cost burden through open-ended items asking participants to estimate the cost of various aspects of the cervical cancer screening process, including “a Pap smear appointment and laboraory tests,” “transportation and parking for the appointment,” “paying someone to watch children or others you take care of,” and “lost pay due to time off work.” Participants had the option of responding “don't know” to each perceived cost question. The survey also assessed whether participants had ever heard of the Breast and Cervical Cancer Control Program (BCCCP), asking, “Low-income women who don't have insurance can get free breast and cervical cancer screening through a government program. It's called the Breast and Cervical Cancer Control Program, or “BEE-cep” (BCCCP). Have you ever heard of this program before?”
Participant sociodemographic characteristics were assessed as potential predictors of perceived financial barriers. The selection of predictors was guided by the Health Belief Model,35 and informed by prior literature documenting characteristics associated with the report of financial barriers36 and cervical cancer screening completion.6,7,9,10,38–40 Given that cost-related barriers mediate access to health care,35,41,42 characteristics associated with screening noncompliance were considered as potential predictors of perceived financial barriers. Potential predictors include demographics (age, marital status, sexual orientation), social factors (race, ethnicity, education, employment status, primary language), and resources (poverty level, health insurance status, receipt of social assistance, rurality). Receipt of social assistance refers to receipt of food stamps, housing assistance, welfare payments, supplemental security income, or disability payments. Poverty level was calculated from household size and annual income, using the FPLs set for the year in which the survey was completed. Rurality was determined using the 2006 Rural–Urban Commuting Area (RUCA) codes on the basis of participant zip codes.43
Analytic strategy
Participants missing financial barriers data were excluded from the analytic sample (2%, 16/729). Additionally, for predictors included in the multivariate analysis with less than 10 missing responses, participants with missing data were excluded from the analytic sample (2%, 11/729). Excluded participants (n = 27) did not differ from the final analytic sample on the four sociodemographic characteristics with full response rates (Supplementary Table S2).
Chi-square test statistics were used to assess differences in sociodemographic characteristics for the two most commonly perceived financial barriers. Multivariable logistic regression models were constructed to predict reporting screening cost and future treatment cost as barriers to screening. Characteristics related to participant resources (i.e., health insurance, poverty level, employment status) were prioritized for inclusion in the model due to their direct conceptual relationship with perceived financial barriers.44 Additionally, sociodemographic characteristics that may influence how an individual's resources or belief in the importance of screening influence their report of financial barriers were included (i.e., age, race, education). Receipt of social assistance was excluded due to multicollinearity with health insurance.
In the results of this analysis, average marginal effects for each explanatory variable can be interpreted as the average difference in the predicted probability of reporting the perceived financial barrier of interest, holding all other covariates constant, across all observations in the analytic sample.45 Standard errors (SEs) for the marginal effects were estimated by applying the Delta method using the “margins” command in STATA 16.1 (StataCorp, College Station, TX).46 No collinearity in the final models was detected. Chi-square tests and multivariable models were not constructed for the report of lost pay and transportation cost barriers due to the low prevalence of these outcomes.
Wilcoxon rank sum tests were used to compare participants' perceived cost burden by insurance status and report of perceived financial barriers. Because of the presence of outliers in perceived cost burden responses for the appointment and laboratory tests, screening cost estimates were winsorized by capping perceived costs above the 95th percentile at this value.
Results
Study participants
Most participants were uninsured (78%), unemployed (57%), and living at or below the FPL (55%). The majority of women identified as non-Hispanic or non-Latina ethnicity (91%) and identified as either Black (48%) or White (41%). Almost 95% of participants spoke English as their primary language. The age distribution was relatively uniform, with 33% between 25 and 34 years, 38% between 35 and 49, and 29% between 50 and 64. A slight majority of women (53%) were single and had never been married (Supplementary Table S1).
Perceived financial barriers to screening
Seventy-two percent (506/702) of participants perceived one or more financial barriers to screening, and the majority of participants (75%) reported that they strongly or somewhat agreed with the following statement, “If I needed to get cervical cancer screening, it would cost more than I could pay.” The most commonly reported perceived financial barriers reported in participant open-ended responses were out-of-pocket costs associated with the screening appointment (71%) and future treatment (44%). Lost pay due to time missed from work (6%) and transportation costs (5%) were less commonly identified as perceived financial barriers to cervical cancer screening (Fig. 1). Forty-seven percent of participants perceived two or more distinct financial barriers to screening (Fig. 2). Concerns about out-of-pocket screening costs and future treatment costs were most commonly reported together among participants perceiving two distinct barriers, and almost all participants (98%) who reported at least one financial barrier reported screening costs as a perceived barrier.
FIG. 1.
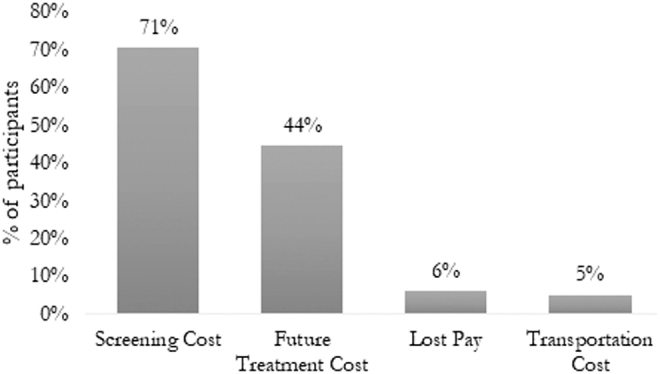
Perceived financial barriers to cervical cancer screening (N = 702). This figure shows the prevalence of perceived financial barriers to screening. Perceived barriers were assessed using the following survey question: “What are some reasons that you haven't had a Pap smear recently?” Answer options included “cost” and “no insurance,” in addition to other nonfinancial barriers. Participants were asked to mark all that apply. For each reason selected, participants were then asked to indicate, more specifically, what about that reason made it difficult to be screened. Open-ended responses were qualitatively coded as belonging to the discrete categories reported in the above figure. Pap, Papanicolaou.
FIG. 2.
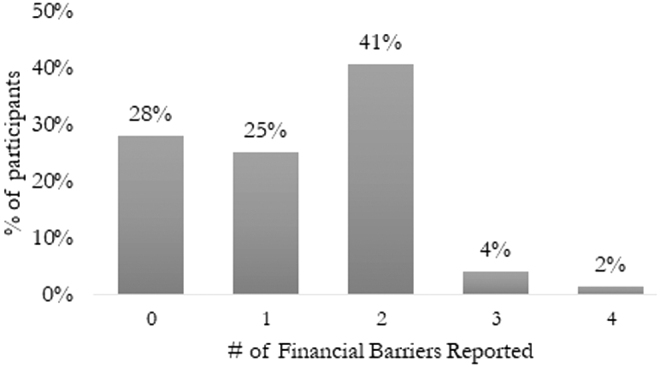
Number of perceived financial barriers to cervical cancer screening (N = 702). This figure represents the number of financial barriers perceived by participants, included screening costs (Pap test appointment and laboratory costs), future treatment costs (including follow-up screening for abnormal results), lost pay due to time missed from work, and transportation costs.
When assessing screening and treatment cost barriers across sociodemographic characteristics, in almost all groups, over 60% of participants reported screening costs as a barrier, and over 40% reported treatment costs as a barrier (Table 1). In bivariate analysis, insurance status was associated with reporting screening (p < 0.001) and future treatment (p < 0.001) costs as perceived barriers to screening (Table 1). Racial identity and not receiving social assistance were also associated with reporting screening and future treatment costs as perceived barriers to screening. Additionally, participant age and employment status were both associated with perceiving screening cost as a barrier, but not future treatment cost (Table 1).
Table 1.
Unadjusted Associations Between Sociodemographic Characteristics and Perceived Financial Barriers to Cervical Cancer Screening in the MyBodyMyTest-3 Study (N = 702)
| Characteristic | Perceived financial barriers No. perceiving barrier (% in category) |
|||
|---|---|---|---|---|
| Screening costsa | p b | Future treatment costs | p b | |
| N (%) | 495 (71%) | 312 (44%) | ||
| Age (years) | 0.004 | 0.26 | ||
| 25–34 | 157 (68%) | 106 (46%) | ||
| 35–49 | 209 (77%) | 126 (47%) | ||
| 50–64 | 129 (64%) | 80 (40%) | ||
| Race | <0.001 | <0.001 | ||
| Black | 219 (65%) | 125 (37%) | ||
| White | 228 (79%) | 154 (53%) | ||
| Other or not reportedc | 48 (62%) | 33 (42%) | ||
| Ethnicity | 0.65 | 0.60 | ||
| Non-Hispanic/Latina | 449 (70%) | 282 (44%) | ||
| Hispanic/Latina | 46 (73%) | 30 (48%) | ||
| Poverty | 0.040 | 0.004 | ||
| ≤100% FPL | 261 (67%) | 165 (43%) | ||
| >100%–250% FPL | 200 (73%) | 119 (43%) | ||
| Not reported (≤250% FPL) | 34 (85%) | 28 (70%) | ||
| Health insurance | <0.001 | <0.001 | ||
| Uninsured | 435 (79%) | 281 (51%) | ||
| Publicly insuredd | 60 (39%) | 31 (20%) | ||
| Receipt of social assistancee | 0.002 | 0.036 | ||
| No | 278 (76%) | 177 (48%) | ||
| Yes | 211 (65%) | 131 (40%) | ||
| Employment status | 0.010 | 0.41 | ||
| Unemployed | 266 (67%) | 172 (43%) | ||
| Employed | 229 (76%) | 140 (46%) | ||
| Education | 0.30 | 0.15 | ||
| High school diploma, GED, or less | 209 (69%) | 124 (41%) | ||
| Some college or more | 193 (70%) | 126 (46%) | ||
| Bachelor's degree or more | 93 (76%) | 62 (51%) | ||
| Primary language | 0.42 | 0.71 | ||
| English | 466 (70%) | 294 (44%) | ||
| Non-Englishf | 29 (76%) | 18 (47%) | ||
| Sexual orientation | 0.77 | 0.27 | ||
| Heterosexual/straight | 446 (71%) | 274 (44%) | ||
| Gay/lesbian | 10 (63%) | 10 (63%) | ||
| Bisexual | 35 (70%) | 24 (48%) | ||
| Marital status | 0.25 | 0.97 | ||
| Single/never married | 258 (69%) | 168 (45%) | ||
| Married/living with a partner | 109 (76%) | 64 (45%) | ||
| Divorced/separated/widowed | 128 (70%) | 80 (44%) | ||
| Ruralityg | 0.49 | 0.48 | ||
| Urban | 444 (70%) | 278 (44%) | ||
| Rural | 49 (74%) | 32 (49%) | ||
Bold p-values indicate p < 0.05.
Totals may not add up to full sample size due to missing data.
Screening cost includes screening appointment costs and associated laboratory test costs.
p-Values calculated using chi-squared test or Fisher's exact test if cell size less than 5.
Includes American Indian or Alaska Native (n = 12), Asian (n = 6), Native Hawaiian or Pacific Islander (n = 2), Mixed race (n = 31), and not reported (n = 27).
Includes Medicaid (n = 140), dual Medicaid/Medicare (n = 12), and Medicare only (n = 4).
Includes food stamps, housing assistance, welfare payments, SSI, or disability payments.
Includes Spanish (n = 32), Tongan, French, Creole, Mandarin, Russian, and Portuguese.
Defined using the 2006 RUCA codes on the basis of participant zip codes: rural (RUCA ≥4), urban (RUCA <4).
FPL, federal poverty level; GED, General Education Development, RUCA, Rural–Urban Commuting Area; SSI, supplemental security income.
In multivariate analysis, being uninsured, versus publicly insured, was associated with a 39% point (SE = 4.3, p < 0.001) increase in the predicted probability of reporting screening costs as a perceived barrier (Table 2). Being uninsured was also associated with a 31% point (SE = 3.9, p < 0.001) increase in the predicted probability of reporting future treatment costs as a perceived barrier. White participants, compared with their Black counterparts, had an 8% point (SE = 3.4, p = 0.01) higher predicted probability of perceiving screening costs as a barrier and a 12% point (SE = 3.9, p = 0.002) higher predicted probability of perceiving future treatment costs as a barrier. Additionally, individuals missing FPL data, most commonly due to not reporting income, were more likely than individuals falling below the FPL to perceive both screening costs (p = 0.04) and future treatment costs (p = 0.004) as barriers. Older individuals between age 50 and 64 were less likely than younger participants less than age 35 to perceive barriers of both screening costs (p = 0.03) and future treatment costs (p = 0.03) (Table 2).
Table 2.
Multivariate Associations Between Sociodemographic Characteristics and Perceived Financial Barriers to Cervical Cancer Screening (N = 702)
| Perceived financial barriers Average Marginal Effect (SE) |
||
|---|---|---|
| Screening cost | Future treatment cost | |
| Age (years) | ||
| 25–34 | Ref. | Ref. |
| 35–49 | 0.06 (0.04) | −0.02 (0.04) |
| 50–64 | −0.09* (0.04) | −0.10* (0.05) |
| Race | ||
| Black | Ref. | Ref. |
| White | 0.08* (0.03) | 0.12** (0.04) |
| Other or not reported | −0.07 (0.06) | 0.03 (0.06) |
| Education | ||
| High school diploma, GED, or less | Ref. | Ref. |
| Some college or Associate's degree | −0.002 (0.03) | 0.04 (0.04) |
| Bachelor's degree or more | 0.04 (0.04) | 0.08 (0.05) |
| Health insurance status | ||
| Public insurance | Ref. | Ref. |
| Uninsured | 0.39** (0.04) | 0.31** (0.04) |
| Employment status | ||
| Unemployed | Ref. | Ref. |
| Employed | 0.04 (0.03) | 0.01 (0.04) |
| FPL | ||
| ≤100% FPL | Ref. | Ref. |
| >100%–250% FPL | −0.04 (0.04) | −0.06 (0.04) |
| Not reported | 0.13* (0.06) | 0.22** (0.08) |
p < 0.01, *p < 0.05.
Multivariable logistic regression used to estimate average marginal effects (SEs reported in parentheses). Average marginal effects represent the average difference in the predicted probability of perceiving screening cost, or future treatment cost, as a barrier to cervical cancer screening holding all other covariates constant, across all observations in the analytic sample.
SE, standard error.
Perceived cost burden
Participants' total perceived out-of-pocket cost burden for cervical cancer screening—including the clinic appointment, lost pay, childcare, and transportation—ranged from $0 to $1300, with a median of $245 and interquartile range (IQR) of $135–$375 among participants reporting complete responses (n = 597). On average, participants reporting one or more financial barriers to screening perceived the total out-of-pocket cost of screening to be higher than those who did not report any financial barriers (median: $265 vs. $194, p < 0.0001). Uninsured participants also reported the total perceived cost of screening to be higher than publicly insured participants (median: $265 vs. $125, p < 0.0001). When asked about the BCCCP as a resource for free screening among low-income women without insurance, the vast majority (94% [662/702]) reported that they had not heard of the program.
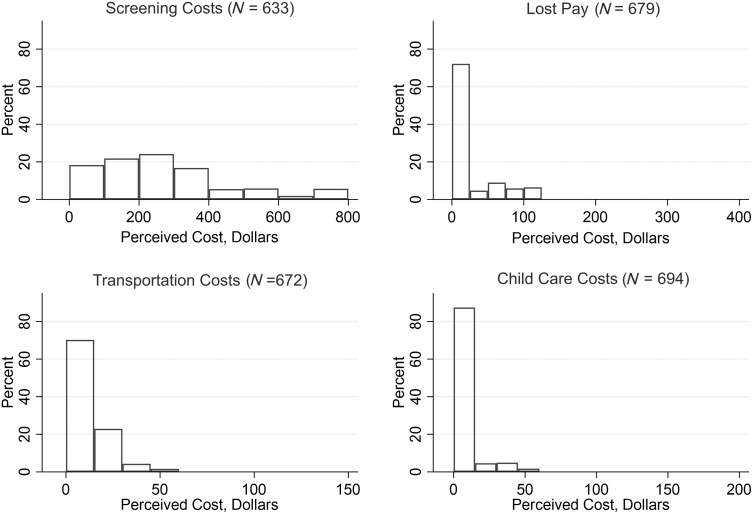
The majority of participants' perceived out-of-pocket cost burden stemmed from the cost of the screening appointment and laboratory tests, with a median perceived cost of $200 and responses ranging from $0 to $800 (Fig. 3). Of note, over 9% of participants reported that they did not know how much the screening procedure would cost. Almost 90% of participants reported nonzero transportation costs, with a median of $10 (IQR $5–$15). Smaller proportions of participants reported perceived out-of-pocket costs stemming from lost pay (30%) or childcare (13%). Among those who reported perceived costs associated with lost pay, the median was $70 (IQR $50–$100). Among individuals reporting childcare costs, the median was $30 (IQR $20–$40) (Table 3).
FIG. 3.
Perceived cost burden of cervical cancer screening among MBMT-3 participants. This figure shows the distribution of perceived costs stemming from each of four components of cervical cancer screening. Screening costs were winsorized at the 95th percentile due to outliers. MBMT-3, MyBodyMyTest Phase Three.
Table 3.
Perceived Cost Burden of Cervical Cancer Screening (N = 702)
| Expected out-of-pocket costs | Median (IQR): full sample | n (%) reporting nonzero costa | Median (IQR): among participants reporting nonzero cost |
|---|---|---|---|
| Screening costsb | $200 ($100–$300) | 601 (94.9%) | $200 ($110–$300) |
| Lost payc | $0 ($0–$40) | 201 (29.6%) | $70 ($50–$100) |
| Transportation costsd | $10 ($5–$15) | 604 (89.9%) | $10 ($5–$15) |
| Childcare costse | $0 ($0–$0) | 93 (13.4%) | $30 ($20–$40) |
Percentage of participants reporting an expected cost (participants who refused to report cost or did not know cost were excluded from the denominator).
Sixty-nine participants (9.8%) reported that they did not know this expected cost.
Twenty-two participants (3.1%) reported that they did not know this expected cost.
Thirty participants (4.3%) reported that they did not know this expected cost.
Eight participants (1.1%) reported that they did not know this expected cost.
IQR, interquartile range.
Discussion
Among a sample of low-income women in North Carolina, U.S. overdue for cervical cancer screening, the majority (72%) perceived one or more financial barriers to screening. Participants most commonly reported the perceived cost of the clinic appointment as a barrier and also attributed the highest perceived out-of-pocket screening costs to this component. Nonmedical costs, such as transportation, caregiving expenses, and lost wages due to time off work, were less often perceived as barriers. Being uninsured (vs. publicly insured), younger (age 25–34 vs. 50–64), White (vs. Black), and not reporting income data were associated with reporting perceived financial barriers to screening stemming from the clinic appointment and potential future treatment costs. Additionally, uninsured participants perceived higher screening costs than their publicly insured counterparts.
While our findings are largely in line with prior analyses of barriers to cervical cancer screening, our focus on rarely or never-screened women from low-income households, a group at heightened risk of cervical cancer incidence and mortality,47 is highly novel. With regard to age, our findings are in line with a prior analysis of barriers to cervical cancer screening, which found that older women were less likely to perceive financial barriers but more likely to report putting screening off or not feeling a need to be screened.36 The higher perceived financial barriers among White women compared with Black women observed in this analysis, viewed in light of comparable reported cervical cancer screening rates among Black (85.6%) and White (85.0%) individuals in the United States,48 may suggest that other nonfinancial barriers to screening are more potent or immediate for Black (vs. White) women. Racism has led to systemic inequities in access to and quality of care for Black individuals in the United States, which may influence psychosocial factors involved in an individual's likelihood of being screened (e.g., medical mistrust) 49,50; how or whether cancer risk factors and screening options are communicated51; and other structural access barriers to screening.30 Although the sample of participants who did not report FPL was less than 10%, the association with perceived financial barriers may suggest that individuals who did not provide income information are particularly in financially precarious situations, thus impacting their perceived barriers to screening.
Most notably, this study illustrates the importance of the availability and awareness of health insurance and other financial resources to reduce perceived financial barriers to screening. Insurance status heavily influences the actual out-of-pocket costs incurred from the cervical cancer screening appointment and laboratories, which may influence perceived cost burden and barriers. The U.S. Affordable Care Act (ACA) requires that most health plans, including Medicaid, cover recommended preventive services, including cancer screening, without cost sharing.52,53 Studies assessing the effects of this cost-sharing provision on preventive services uptake, however, have shown mixed results,14,54 with one analysis finding no effect on Pap test rates in low-income women.55 This may, in part, be explained by discrepancies between actual and perceived costs. Regardless of the actual costs, individuals' perceived costs influence perceived barriers and, consequently, screening uptake. The majority (84%) of publicly insured participants in our analysis estimated nonzero clinic appointment and laboratory test costs. This cost overestimation may be due to low health insurance literacy and miscommunication about health insurance policies, which is associated with delaying or forgoing preventive care.56
In contrast, uninsured individuals do not receive the preventive care protections mandated by the ACA and may be liable for full charges of the screening appointment, as well as potential follow-up of abnormal results, posing significant financial barriers to uptake. Almost 11% of North Carolinians remain uninsured post-ACA, as the state has not yet expanded Medicaid coverage.57 Although free and low-cost screening programs (e.g., BCCCP) are available to most low-income, uninsured individuals, our findings reflect a general lack of awareness of BCCCP, with 95% of participants having never heard of this program before. Although it is possible that participants were aware of other no-cost screening programs, or were familiar with BCCCP, but did not know it by name, this finding is in line with prior analyses' identification of not having heard of free screening programs as a barrier to screening.19,26,58 Furthermore, prior studies have found that national free screening programs only serve a small percentage of those eligible, leaving many low-income, uninsured individuals unscreened.59–61 Moreover, both insured and uninsured women are subject to nonmedical costs, such as transportation and lost wages due to time away from work, which may limit uptake of cervical cancer screening. This points to the need for available and accessible financial assistance mechanisms beyond insurance or free screening programs.
Our findings of high perceived costs and other financial barriers to cervical cancer screening among both uninsured and publicly insured women are in line with a 2010 analysis of Ohio Appalachian women, in which 80% reported not knowing the cost of cervical cancer screening and 40% of reported costs were overestimated.26 In addition to highlighting concerns about health insurance literacy and free screening program awareness, cost overestimation may be due to participants associating clinic appointments with nonscreening-related tests and expensive bills, a theme identified in prior work.30,62,63 Although we did not ask participants to estimate the perceived cost of potential follow-up testing or treatment in the case of abnormal results, 45% reported this as a barrier to screening, suggesting that the threat of future costs may factor into perceived cost burden as well.29,30
Several limitations should be considered in the interpretation of these findings. First, women surveyed for this analysis self-selected for entry by responding to recruitment efforts; as such, participants may have been more interested in getting screened for cervical cancer than the general population of low-income women overdue for cervical cancer screening. This may suggest that our sample experienced heightened financial barriers to screening compared with the general population. Additionally, this analysis was limited to low-income women living in North Carolina, making the generalizability of study findings to other contexts not yet known; however, the identification and recruitment of low-income women overdue for cervical cancer screening, a population less often reached for research efforts, is a major strength of this study. Although our analysis is unique in its in-depth examination of perceived financial barriers to cervical cancer screening, the full range of barriers—including psychosocial barriers, such as prior screening experiences or anticipated discomfort, and knowledge-related barriers—must be considered in designing policies and interventions to increase screening uptake. Another structural barrier that must be considered is not having a usual source of care.21,64,65 While reducing the cost of screening through insurance coverage expansion is necessary, it is not sufficient to increase screening without also ensuring access to a usual source of preventive care.
Conclusion
Our findings highlight the nature of perceived financial barriers to, and cost burden of, cervical cancer screening in a particularly at-risk population. The majority of low-income women overdue for cervical cancer screening in our sample perceived financial barriers associated with the cost of the clinic appointment and potential future treatment costs. Given the role of insurance status in predicting an individual's likelihood of perceiving financial barriers, expanding insurance coverage to low-income women, whether through expanded Medicaid eligibility or increased enrollment assistance, may be an effective way to reduce perceived and actual financial barriers, thereby increasing screening uptake. Additionally, centralized communication surrounding the availability of free screening programs for low-income, uninsured women is important to ensure maximal use of these programs. Finally, although less often reported as barriers, costs stemming from transportation, lost pay, and childcare were reported by a substantial number of participants; as such, financial support services that reduce the burden of these nonmedical costs are important to implement and evaluate. At-home HPV self-collection, which has been shown to be comparably effective to in-clinic testing and highly acceptable,66–70 may be one way to eliminate such nonmedical costs and thus reduce financial barriers, among other structural barriers, to screening.71,72 In the ongoing MBMT-3 study, we are assessing direct and indirect costs to patients in both arms of the trial: HPV self-collection and telephone reminders.37 To effectively increase screening uptake among low-income, under-screened women, perceived financial barriers must be considered as they relate to medical and nonmedical components of screening costs.
Supplementary Material
Acknowledgments
The authors are grateful to all of the women who participated in this study. They are also appreciative of the following study team members for their contribution to this study (in alphabetical order): Konyinsope Adewumi, Liz Aguilera, Anna Baker, Erin Brabble, Johana Bravo De Los Rios, Alexandra Bukowski, Jessica Islam, Jocelyn Kim, Yesenia Merino, Victoria Monge, Randolph Qiao, Rohanit Singh, Alison Swiatlo, Ruth Tesfalidet, Olivia Vaz, Abigail Warmack, Autumn Watson, Erin Young, Erica Zeno, and Stephanie Zentz. They would also like to thank the American Sexual Health Association, the Mecklenburg County Health Department, Charlotte Community Health Clinic, Inc., Albemarle Regional Health Services, Sampson County Health Department, Cone Health Cancer Center, Stedman Wade Health Services, Inc., Advance Community Health Clinic, and their many community outreach partners.
Author Disclosure Statement
J.S.S. has received research grants and consultancies from Hologic, Becton Dickenson, and Trovagene for the past 5 years. A.C.D.M. has had some conference travel expenses covered by Hologic in the last 5 years. S.B.W. has received research grants from Pfizer paid to her institution for unrelated work. All other authors declare no conflicts of interest.
Funding Information
The National Institutes of Health (NIH) sponsored the MyBodyMyTest-3 study (5R01CA183891-03). NIH did not have any role in the study design; collection, management, analysis, and interpretation of the data; writing of the article; or the decision to submit the report for publication. L.P.S. and C.B.B. are supported by a Cancer Care Quality Training Program grant, for which S.B.W. is mentor and PI, UNC-CH, Grant No. T32-CA-116339.
Supplementary Material
References
- 1. CDC. HPV-Associated Cervical Cancer Rates by Race and Ethnicity. Available at: https://www.cdc.gov/cancer/hpv/statistics/cervical.htm. Published 2019. Updated August 2, 2019. Accessed October 17, 2019.
- 2. Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer 2017;123:1044–1050. [DOI] [PubMed] [Google Scholar]
- 3. Landy R, Pesola F, Castañón A, Sasieni P. Impact of cervical screening on cervical cancer mortality: Estimation using stage-specific results from a nested case-control study. Br J Cancer 2016;115:1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dalton HJ, Farley JH. Racial disparities in cervical cancer: Worse than we thought. Cancer 2017;123:915–916. [DOI] [PubMed] [Google Scholar]
- 5. Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: Attributable factors in the screening process. J Natl Cancer Inst 2005;97:675–683. [DOI] [PubMed] [Google Scholar]
- 6. Chen HY, Kessler CL, Mori N, Chauhan SP. Cervical cancer screening in the United States, 1993–2010: Characteristics of women who are never screened. J Womens Health (Larchmt) 2012;21:1132–1138. [DOI] [PubMed] [Google Scholar]
- 7. Akinlotan MA, Weston C, Bolin JN. Individual- and county-level predictors of cervical cancer screening: A multi-level analysis. Public Health 2018;160:116–124. [DOI] [PubMed] [Google Scholar]
- 8. Carruth AK, Browning S, Reed DB, Skarke L, Sealey L. The impact of farm lifestyle and health characteristics: Cervical cancer screening among southern farmwomen. Nurs Res 2006;55:121–127. [DOI] [PubMed] [Google Scholar]
- 9. Coughlin SS, King J, Richards TB, Ekwueme DU. Cervical cancer screening among women in metropolitan areas of the United States by individual-level and area-based measures of socioeconomic status, 2000 to 2002. Cancer Epidemiol Biomarkers Prev 2006;15:2154–2159. [DOI] [PubMed] [Google Scholar]
- 10. Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Soc Sci Med 2008;66:260–275. [DOI] [PubMed] [Google Scholar]
- 11. Ma GX, Toubbeh JI, Wang MQ, Shive SE, Cooper L, Pham A. Factors associated with cervical cancer screening compliance and noncompliance among Chinese, Korean, Vietnamese, and Cambodian women. J Natl Med Assoc 2009;101:541–551. [DOI] [PubMed] [Google Scholar]
- 12. Miles-Richardson S, Allen S, Claridy MD, Booker EA, Gerbi G. Factors associated with self-reported cervical cancer screening among women aged 18 years and older in the United States. J Commun Health 2017;42:72–77. [DOI] [PubMed] [Google Scholar]
- 13. Welch C, Miller CW, James NT. Sociodemographic and health-related determinants of breast and cervical cancer screening behavior, 2005. J Obstet Gynecol Neonatal Nurs 2008;37:51–57. [DOI] [PubMed] [Google Scholar]
- 14. Garrido CO, Coşkun RA, Lent AB, Calhoun E, Harris RB. Use of cervical cancer preventive services among US women aged 21–29: An assessment of the 2010 Affordable Care Act rollout through 2018. Cancer Causes Control 2020;31:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. NCI. Cancer Trends Progress Report: Cervical Cancer Screening. Available at: https://progressreport.cancer.gov/detection/cervical_cancer. Published 2020. Updated March 2020 Accessed December 15, 2020.
- 16. USPSTF. Final Recommendation Statement: Cervical Cancer: Screening 2018. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/cervical-cancer-screening2 Accessed April 15, 2020.
- 17. White A, Thompson TD, White MC, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen NN, Moran MB, Frank LB, Ball-Rokeach SJ, Murphy ST. Understanding cervical cancer screening among Latinas through the lens of structure, culture, psychology and communication. J Health Commun 2018;23:661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown DR, Wilson RM, Boothe MA, Harris CE. Cervical cancer screening among ethnically diverse black women: Knowledge, attitudes, beliefs, and practices. J Natl Med Assoc 2011;103:719–728. [DOI] [PubMed] [Google Scholar]
- 20. Adunlin G, Cyrus JW, Asare M, Sabik LM. Barriers and facilitators to breast and cervical cancer screening among immigrants in the United States. J immigr Minor Health 2019;21:606–658. [DOI] [PubMed] [Google Scholar]
- 21. Ackerson K, Gretebeck K. Factors influencing cancer screening practices of underserved women. J Am Acad Nurse Pract 2007;19:591–601. [DOI] [PubMed] [Google Scholar]
- 22. Luft H, Perzan M, Mitchell R, Schmidt A. An integrative literature review of barriers and facilitators to cervical cancer screening among refugee women in the United States. Health Care Women Int [Epub ahead of print] 2020:1–21. [DOI] [PubMed] [Google Scholar]
- 23. Lea CS, Perez-Heydrich C, Des Marais AC, et al. Predictors of cervical cancer screening among infrequently screened women completing human papillomavirus self-collection: My Body My Test-1. J Womens Health (Larchmt) 2019;28:1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coronado GD, Thompson B, Koepsell TD, Schwartz SM, McLerran D. Use of Pap test among Hispanics and non-Hispanic whites in a rural setting. Prev Med 2004;38:713–722. [DOI] [PubMed] [Google Scholar]
- 25. Akinlotan M, Bolin JN, Helduser J, Ojinnaka C, Lichorad A, McClellan D. Cervical cancer screening barriers and risk factor knowledge among uninsured women. J Community Health 2017;42:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McAlearney AS, Song PH, Rhoda DA, et al. Ohio Appalachian women's perceptions of the cost of cervical cancer screening. Cancer 2010;116:4727–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katz ML, Wewers ME, Single N, Paskett ED. Key informants' perspectives prior to beginning a cervical cancer study in Ohio Appalachia. Qual Health Res 2007;17:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andrasik MP, Rose R, Pereira D, Antoni M. Barriers to cervical cancer screening among low-income HIV-positive African American women. J Health Care Poor Underserved 2008;19:912–925. [DOI] [PubMed] [Google Scholar]
- 29. Baezconde-Garbanati L, Murphy ST, Moran MB, Cortessis VK. Reducing the excess burden of cervical cancer among Latinas: Translating science into health promotion initiatives. Calif J Health Promot 2013;11:45–57. [PMC free article] [PubMed] [Google Scholar]
- 30. Sage SK, Hawkins-Taylor C, Crockett RA, Balls-Berry JE. “Girl, just pray…”: Factors that influence breast and cervical cancer screening among black women in Rochester, MN. J Natl Med Assoc 2020;112:454–467. [DOI] [PubMed] [Google Scholar]
- 31. Boom K, Lopez M, Daheri M, et al. Perspectives on cervical cancer screening and prevention: Challenges faced by providers and patients along the Texas-Mexico border. Perspect Public Health 2019;139:199–205. [DOI] [PubMed] [Google Scholar]
- 32. Lee MC. Knowledge, barriers, and motivators related to cervical cancer screening among Korean-American women. A focus group approach. Cancer Nurs 2000;23:168–175. [DOI] [PubMed] [Google Scholar]
- 33. Logan L, McIlfatrick S. Exploring women's knowledge, experiences and perceptions of cervical cancer screening in an area of social deprivation. Eur J Cancer Care (Engl) 2011;20:720–727. [DOI] [PubMed] [Google Scholar]
- 34. Fletcher FE, Buchberg M, Schover LR, et al. Perceptions of barriers and facilitators to cervical cancer screening among low-income, HIV-infected women from an integrated HIV clinic. AIDS Care 2014;26:1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Janz NK, Becker MH. The Health Belief Model: A decade later. Health Educ Q 1984;11:1–47. [DOI] [PubMed] [Google Scholar]
- 36. Lai D, Bodson J, Warner EL, Ayres S, Mooney R, Kepka D. Younger age and health beliefs associated with being overdue for Pap Testing among Utah Latinas who were non-adherent to cancer screening guidelines. J Immigr Minor Health 2017;19:1088–1099. [DOI] [PubMed] [Google Scholar]
- 37. Spees LP, Des Marais A, Wheeler SB, et al. Impact of human papillomavirus (HPV) self-collection on subsequent cervical cancer screening completion among under-screened US women: MyBodyMyTest-3 protocol for a randomized controlled trial. Trials 2019;20:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ackermann SP, Brackbill RM, Bewerse BA, Cheal NE, Sanderson LM. Cancer screening behaviors among U.S. women: Breast cancer, 1987–1989, and cervical cancer, 1988–1989. MMWR CDC Surveill Summ 1992;41:17–25. [PubMed] [Google Scholar]
- 39. Hughes MC, Hannon PA, Harris JR, Patrick DL. Health behaviors of employed and insured adults in the United States, 2004–2005. Am J Health Promot 2010;24:315–323. [DOI] [PubMed] [Google Scholar]
- 40. Herrera DG, Schiefelbein EL, Smith R, Rojas R, Mirchandani GG, McDonald JA. Cervical cancer screening in the US-Mexico border region: A binational analysis. Matern Child Health J 2012;16 Suppl 2:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Andersen RM. National health surveys and the behavioral model of health services use. Med Care 2008;46:647–653. [DOI] [PubMed] [Google Scholar]
- 42. Rosenstock IM. The Health Belief Model and preventive health behavior. Health Educ Monogr 1974;2:354–386. [Google Scholar]
- 43. Rural-Urban Commuting Area Codes. In: Center WRHR, ed. University of Washington, 2006. Available at: https://depts.washington.edu/uwruca/ruca-data.php Accessed April 7, 2020.
- 44. Campbell DJ, Manns BJ, Leblanc P, Hemmelgarn BR, Sanmartin C, King-Shier K. Finding resiliency in the face of financial barriers: Development of a conceptual framework for people with cardiovascular-related chronic disease. Medicine (Baltimore) 2016;95:e5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gauvin J-P. Quick look at the margins command. 2012. Available at: https://www.academia.edu/2010847/A_Quick_Look_at_the_Margins_Command Accessed March 13, 2020.
- 46. Norton EC, Wang H, Ai C. Computing interaction effects and standard errors in logit and probit models. Stata J 2004;4:154–167. [Google Scholar]
- 47. Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: Over six decades of changing patterns and widening inequalities. J Environ Public Health 2017;2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goding Sauer A, Siegel RL, Jemal A, Fedewa SA. Current prevalence of major cancer risk factors and screening test use in the United States: Disparities by education and race/ethnicity. Cancer Epidemiol Biomarkers Prev 2019;28:629–642. [DOI] [PubMed] [Google Scholar]
- 49. Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: A systematic review. Am J Public Health 2015;105:e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cooper LA, Beach MC, Johnson RL, Inui TS. Delving below the surface: Understanding how race and ethnicity influence relationships in health care. J Gen Intern Med 2006;21(Suppl 1):S21–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown DR, Wilson RM, Boothe MA, Harris CE. Cervical cancer screening among ethnically diverse black women: Knowledge, attitudes, beliefs, and practices. J Natl Med Assoc 2011;103:719–728. [DOI] [PubMed] [Google Scholar]
- 52. Ku L, Paradise J, Thompson V. Data Note: Medicaid's Role in Providing Access to Preventive Care for Adults. KFF. Available at: https://www.kff.org/medicaid/issue-brief/data-note-medicaids-role-in-providing-access-to-preventive-care-for-adults/. Published 2017. Updated May 17, 2017. Accessed October 17, 2019.
- 53. Lee LK, Chien A, Stewart A, et al. Women's coverage, utilization, affordability, and health after the ACA: A review of the literature. Health Aff (Millwood) 2020;39:387–394. [DOI] [PubMed] [Google Scholar]
- 54. Tummalapalli SL, Keyhani S. Changes in preventative health care after Medicaid expansion. Med Care 2020;58:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Alharbi A, Khan MM, Horner R, Brandt H, Chapman C. Impact of removing cost sharing under the Affordable Care Act (ACA) on mammography and Pap test use. BMC Public Health 2019;19:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tipirneni R, Politi MC, Kullgren JT, Kieffer EC, Goold SD, Scherer AM. Association between health insurance literacy and avoidance of health care services owing to cost. JAMA Netw Open 2018;1:e184796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. KFF. Health Insurance Coverage of the Total Population. Kaiser Family Foundation. Available at: https://www.kff.org/other/state-indicator/total-population/?currentTimeframe=0&selectedRows=%7B%22states%22:%7B%22north-carolina%22:%7B%7D%7D,%22wrapups%22:%7B%22united-states%22:%7B%7D%7D%7D&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22desc%22%7D. Published 2017. Accessed November 10, 2019.
- 58. Silvera SAN, Bandera EV, Jones BA, Kaplan AM, Demisse K. Knowledge of, and beliefs about, access to screening facilities and cervical cancer screening behaviors among low-income women in New Jersey. Cancer Causes Control 2020;31:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tangka FK, Howard DH, Royalty J, et al. Cervical cancer screening of underserved women in the United States: Results from the National Breast and Cervical Cancer Early Detection Program, 1997–2012. Cancer Causes Control 2015;26:671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tangka FK, O'Hara B, Gardner JG, et al. Meeting the cervical cancer screening needs of underserved women: The National Breast and Cervical Cancer Early Detection Program, 2004–2006. Cancer Causes Control 2010;21:1081–1090. [DOI] [PubMed] [Google Scholar]
- 61. Highlights of GAO-21-35, a report to the Chairman of the Committee on Finance, U.S. Senate. Federal Programs Provide Screening and Treatment for Breast and Cervical Cancer United States Government Accountability Office; October 2020. Available at: https://www.gao.gov/assets/720/710372.pdf Accessed December 14, 2020.
- 62. Boyer LE, Williams M, Callister LC, Marshall ES. Hispanic women's perceptions regarding cervical cancer screening. J Obstet Gynecol Neonatal Nurs 2001;30:240–245. [DOI] [PubMed] [Google Scholar]
- 63. Pignone MP, Crutchfield TM, Brown PM, et al. Using a discrete choice experiment to inform the design of programs to promote colon cancer screening for vulnerable populations in North Carolina. BMC Health Serv Res 2014;14:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. DeVoe JE, Fryer GE, Phillips R, Green L. Receipt of preventive care among adults: Insurance status and usual source of care. Am J Public Health 2003;93:786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hall IJ, Tangka FKL, Sabatino SA, Thompson TD, Graubard BI, Breen N. Patterns and trends in cancer screening in the United States. Prev Chronic Dis 2018;15:E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nelson EJ, Maynard BR, Loux T, Fatla J, Gordon R, Arnold LD. The acceptability of self-sampled screening for HPV DNA: A systematic review and meta-analysis. Sex Transm Infect 2017;93:56–61. [DOI] [PubMed] [Google Scholar]
- 67. Petignat P, Faltin DL, Bruchim I, Tramer MR, Franco EL, Coutlee F. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol Oncol 2007;105:530–535. [DOI] [PubMed] [Google Scholar]
- 68. Koliopoulos G, Nyaga VN, Santesso N, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev 2017;8:CD008587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Des Marais AC, Zhao Y, Hobbs MM, et al. Home self-collection by mail to test for human papillomavirus and sexually transmitted infections. Obstet Gynecol 2018;132:1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Anderson C, Breithaupt L, Des Marais A, et al. Acceptability and ease of use of mailed HPV self-collection among infrequently screened women in North Carolina. Sex Transm Infect 2018;94:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Smith JS, Des Marais AC, Deal AM, et al. Mailed human papillomavirus self-collection with Papanicolaou test referral for infrequently screened women in the United States. Sex Transm Dis 2018;45:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Madzima TR, Vahabi M, Lofters A. Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women: Focused literature review. Can Fam Physician 2017;63:597–601. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.