Abstract
Background
Topical corticosteroids are the most frequently prescribed dermatological treatment and are often used by pregnant women with skin conditions. However, little is known about their safety in pregnancy.
Objectives
To assess the effects of topical corticosteroids on pregnancy outcomes in pregnant women.
Search methods
This is an update of a review previously published in 2009. We updated our searches of the following databases to July 2015: the Cochrane Skin Group Specialised Register, the Cochrane Pregnancy and Childbirth Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 6), MEDLINE, EMBASE, and LILACS. We also searched five trials registers and checked the reference lists of included studies, published reviews, articles that had cited the included studies, and one author's literature collection, for further references to relevant RCTs.
Selection criteria
Randomised controlled trials and cohort studies of topical corticosteroids in pregnant women, as well as case‐control studies comparing maternal exposure to topical corticosteroids between cases and controls when studies reported pre‐specified outcomes. The primary outcomes included mode of delivery, major congenital abnormality, birth weight, and preterm delivery (delivery before 37 completed weeks gestation); the secondary outcomes included foetal death, minor congenital abnormality, and low Apgar score (less than seven at 5 min).
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two authors independently applied selection criteria, extracted data, and assessed the quality of the included studies. A third author was available for resolving differences of opinion. A further author independently extracted data from included studies that were conducted by authors of this systematic review.
Main results
We included 7 new observational studies in this update, bringing the total number to 14, including 5 cohort and 9 case‐control studies, with 1,601,515 study subjects.
Most studies found no causal associations between maternal exposure to topical corticosteroids of any potency and pregnancy outcomes when compared with no exposure. These outcomes included: mode of delivery (risk ratio (RR) 1.04, 95% confidence interval (CI) 0.95 to 1.15, 1 cohort study, n = 9904, low quality evidence); congenital abnormalities, including orofacial cleft or cleft palate and hypospadias (where the urethral opening is on the underside of the penis) (RR 0.82, 95% CI 0.34 to 1.96, 2 cohort studies, n = 9512, low quality evidence; and odds ratio (OR) 1.07, 95% CI 0.71 to 1.60, 1 case‐control study, n = 56,557); low birth weight (RR 1.08, 95% CI 0.86 to 1.36; n = 59,419, 4 cohort studies; very low quality evidence); preterm delivery (RR 0.93, 95% CI 0.81 to 1.08, 4 cohort studies, n = 59,419, low quality evidence); foetal death (RR 1.02, 95% CI 0.60 to 1.73, 4 cohort studies, n = 63,885, very low quality evidence); and low Apgar score (RR 0.84, 95% CI 0.54 to 1.31, 1 cohort study, n = 9220, low quality evidence).
We conducted stratified analyses of mild or moderate potency, and potent or very potent topical corticosteroids, but we found no causal associations between maternal exposure to topical corticosteroid of any potency and congenital abnormality, orofacial clefts, preterm delivery, or low Apgar score. For low birth weight, although the meta‐analysis based on study‐level data was not significant for either mild to moderate corticosteroids (pooled RR 0.90, 95% CI 0.74 to 1.09, 3 cohort studies, n > 55,713) or potent to very potent corticosteroids (pooled RR 1.58, 95% CI 0.96 to 2.58, 4 cohort studies, n > 47,651), there were significant differences between the two subgroups (P = 0.04). The results from three of the individual studies in the meta‐analysis indicated an increased risk of low birth weight in women who received potent to very potent topical corticosteroids. Maternal use of mild to moderate potency topical steroids was associated with a decreased risk of foetal death (pooled RR 0.70, 95% CI 0.64 to 0.77, 2 studies, n = 48,749; low quality evidence), but we did not observe this effect when potent to very potent topical corticosteroids were given during pregnancy (pooled RR 1.14, 95% CI 0.69 to 1.88, 3 studies, n = 37,086, low quality evidence).
We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group approach to rate the overall quality of the evidence. Data from observational studies started at low quality. We further downgraded the evidence because of imprecision in low birth weight and inconsistency in foetal death. Lower quality evidence resulted in lower confidence in the estimate of effect for those outcomes.
Authors' conclusions
This update adds more evidence showing no causal associations between maternal exposure to topical corticosteroids of all potencies and pregnancy outcomes including mode of delivery, congenital abnormalities, preterm delivery, foetal death, and low Apgar score, which is consistent with the previous version of this review. This update provides stratified analyses based on steroid potency; we found no association between maternal use of topical corticosteroids of any potency and an increase in adverse pregnancy outcomes, including mode of delivery, congenital abnormality, preterm delivery, foetal death, and low Apgar score. Similar to the previous version of the review, this update identified a probable association between low birth weight and maternal use of potent to very potent topical corticosteroids, especially when the cumulative dosage of topical corticosteroids throughout the pregnancy is very large, which warrants further investigation. The finding of a possible protective effect of mild to moderate topical corticosteroids on foetal death could also be examined.
Plain language summary
Safety of topical steroids in pregnancy
Review question
Is it safe to use topical steroids (steroid creams or ointments) in pregnancy?
Background
Topical steroids are the most commonly used medicines for skin conditions. Pregnant women may need topical steroids to treat skin conditions, but it is unclear if they are safe or harmful during pregnancy. We aimed to examine the safety of topical steroids in pregnancy.
Study characteristics
We updated the review that was previously published in 2009. We examined the research published up to July 2015 and found seven new studies. All in all, this updated review included a total of 14 observational studies that assessed 1,601,515 pregnancies. Observational studies are generally regarded as less rigorous than randomised controlled clinical trials. The funding source was from academic or governmental institutions in 10 studies and was not reported in 4 studies.
Key results
We found no associations between mothers' use of topical steroids of any potency and type of delivery, birth defects, premature births, or low Apgar score.
There is some evidence indicating a relation between low birth weight and maternal use of potent or very potent topical steroids, especially when high doses are used in pregnancy, and this may warrant more research. On the other hand, maternal use of mild or moderate topical corticosteroids is not related to low birth weight. We even found that mild or moderately potent topical steroids protect against death of the baby, but this was not seen when the mothers used potent or very potent topical steroids. This finding needs further examination.
Quality of evidence
The overall quality of evidence is low because all available studies were observational. The high quality study design of the randomised controlled trial that allocates participants to receive either topical corticosteroids or no treatment is not generally feasible in pregnant women due to ethical concerns about possible exposure of the foetus to an experimental treatment.
Where we further downgraded the quality of the evidence to 'very low', it was because we had detected variation in the results from the studies that we found, which means that we have low confidence in our estimates of the effects for our outcomes.
Summary of findings
Summary of findings for the main comparison. Topical corticosteroids compared with no topical corticosteroids for pregnant women.
| Topical corticosteroids compared with no topical corticosteroids for pregnant women | ||||||
|
Participants or population: pregnant women Settings: ranging from single hospital to multinational congenital abnormality register Intervention: topical corticosteroids Comparison: no topical corticosteroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Unexposed group (in cohort study)/control group (in case‐control study) | Exposed group (in cohort study)/case group (in case‐control study) | |||||
| Mode of delivery (risk for either assisted or cesarean delivery) | 18.29% | 18.89% | RR 1.04, 95% CI 0.95 to 1.15 | 9904 (1 cohort study) | ++OOa low | Only 1 study (Chi 2013) provided detailed data |
| Congenital abnormality | Cohort studies: 3.58% in the unexposed group Case‐control study: 0.17% in the control group |
Cohort studies: 2.94% in the exposed group Case‐control study: 0.18% in the control group |
Cohort studies: RR 0.82, 95% CI 0.34 to 1.96 Case‐control study: OR 1.07, 95% CI 0.71 to 1.60 |
9433 (1 cohort study); 56,557 (1 case‐control study) | ++OOa low | The RR in 1 cohort study (Mahé 2007) was not estimable due to no events in either the exposed or unexposed group |
| Orofacial cleft | Cohort studies: incidence of orofacial cleft ranged from 0.10% to 0.16% Case‐control studies: prevalence of exposure ranged across the control groups from 0.19% to 18.75% |
Cohort studies: incidence of orofacial cleft ranged from 0.13% to 0.21% Case‐control studies: prevalence of exposure in the case groups was 0.04% to 1.72% |
Cohort studies: RR 1.12, 95% CI 0.54 to 2.33 Case‐control studies: OR 1.20, 95% CI 0.68 to 2.13 |
40,605 (2 cohort studies); 641,917 (8 case‐control studies) | ++OOa low | Consistent results except 1 case‐control with a high risk of bias |
| Low birth weight | Cohort studies: 0.16% to 10.71% | Cohort studies: 0.18% to 30.43% | RR 1.08, 95% CI 0.86 to 1.36 | 59,419 (4 cohort studies) | +OOOb very low | 3 studies showed an increased risk of low birth weight in those who received potent or very potent topical corticosteroids |
| Preterm delivery | Cohort studies: 0.76% to 6.40% | Cohort studies: 0% to 6.61% | RR 0.93, 95% CI 0.81 to 1.08 | 59,419 (4 cohort studies) | ++OOa low | — |
| Foetal death | Cohort studies: 0% to 9.27% | Cohort studies: 0% to 7.11% | RR 1.02, 95% CI 0.60 to 1.73 | 63,885 (4 cohort studies) | +OOOc very low | — |
| Low Apgar score | Cohort study: 0% to 1.30% | Cohort study: 0% to 1.06% | RR 0.84, 95% CI 0.54 to 1.31 | 9,220 (1 cohort study) | ++OOa low | — |
| *The basis for the assumed risk is the prevalence of the outcome in the control group. The corresponding risk is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE Working Group grades of evidence
High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality (+OOO): We are very uncertain about the estimate. RR: risk ratio; OR: odds ratio; CI: Confidence interval. | ||||||
a The default level of the quality of the evidence for observational studies is low. bDowngraded one level due to imprecision. cDowngraded one level due to inconsistency.
Summary of findings 2. Mild/moderate topical corticosteroids versus no topical corticosteroids for pregnant women.
| Mild to moderate topical corticosteroids versus no topical corticosteroids for pregnant women | ||||||
|
Participants or population: pregnant women Settings: population‐based Intervention: mild or moderate topical corticosteroids Comparison: no topical corticosteroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Unexposed group (in cohort study)/control group (in case‐control study) | Exposed group (in cohort study)/case group (in case‐control study) | |||||
| Congenital abnormality | Not reported | Not reported | RR 0.93, 95% CI 0.23 to 3.80 | > 9263 (1 cohort study) | ++OOa low | — |
| Orofacial cleft | 0.10% to 0.16% | 0.13% to 0.14% | RR 0.95, 95% CI 0.40 to 2.28 | 38,446 (2 cohort studies) | ++OOa low | — |
| Low birth weight | 0.55% to 4.80% | 0.50% to 4.53% | RR 0.90, 95% CI 0.74 to 1.09 | > 55,713 (3 cohort studies) | ++OOa low | 1 study did not report the number of women who received mild or moderate topical corticosteroids |
| Preterm delivery | 0.76% to 2.32% | 0.75% to 2.19% | RR 0.88, 95% CI 0.75 to 1.03 | > 55,713 (3 cohort studies) | ++OOa low | 1 study did not report the number of women who received mild or moderate topical corticosteroids |
| Foetal death | 0.47% to 9.27% | 0.37% to 6.46% | RR 0.70, 95% CI 0.64 to 0.77 | 48,749 (2 cohort studies) | ++OOa low | — |
| Low Apgar score | 1.30% | 0.95% | RR 0.73, 95% CI 0.45 to 1.20 | 8756 (2 cohort studies) | ++OOa low | — |
| *The basis for the assumed risk is the prevalence of the outcome in the control group. The corresponding risk is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE Working Group grades of evidence
High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality (+OOO): We are very uncertain about the estimate. RR: risk ratio; OR: odds ratio; CI: Confidence interval. | ||||||
a The default level of the quality of the evidence for observational studies is low.
Summary of findings 3. Potent/very potent topical corticosteroids compared with no topical corticosteroids for pregnant women.
| Potent or very potent topical corticosteroids compared with no topical corticosteroids for pregnant women | ||||||
|
Participants or population: pregnant women Settings: ranging from a single hospital to a population‐based database Intervention: potent or very potent topical corticosteroids Comparison: no topical corticosteroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Unexposed group (in cohort study)/control group (in case‐control study) | Exposed group (in cohort study)/case group (in case‐control study) | |||||
| Congenital abnormality | 0% to 3.6% | 0% to unknown | RR 0.56, 95% CI 0.14 to 2.28 | > 9342 (2 cohort studies) | ++OOa low | 1 study did not report the number of women who received potent or very potent topical corticosteroids |
| Orofacial cleft | 0.10% to 0.16% | 0.21% to 0.36% | RR 1.50, 95% CI 0.59 to 3.82 | 36,348 (2 cohort studies) | ++OOa low | — |
| Low birth weight | 0.55% to 10.71% | 1.21% to 30.43% | RR 1.58, 95% CI 0.96 to 2.58 | > 47,651 (4 cohort studies) | ++OOa low | 1 study did not report the number of women who received potent or very potent topical corticosteroids |
| Preterm delivery | 0% 6.4% | 0.97% to 3.57% | RR 1.05, 95% CI 0.85 to 1.31 | > 47,651 (4 cohort studies) | ++OOa low | — |
| Foetal death | 0% to 9.27% | 0% to 8.76% | RR 1.14, 95% CI 0.69 to 1.88 | 37,086 (3 cohort studies) | ++OOa low | — |
| Low Apgar score | 0% to 1.30% | 0% to 1.34% | RR 1.03, 95% CI 0.52 to 2.03 | 7514 (2 cohort studies) | ++OOa low | — |
| *The basis for the assumed risk is the prevalence of the outcome in the control group. The corresponding risk is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE Working Group grades of evidence
High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality (+OOO): We are very uncertain about the estimate. RR: risk ratio; OR: odds ratio; CI: Confidence interval. | ||||||
aThe default level of the quality of the evidence for observational studies is low.
Background
Description of the intervention
Corticosteroids have four pharmacological properties: they cause constriction of blood vessels and decrease cell proliferation, immunosuppression, and anti‐inflammatory effects (Baumann 1999). Topical corticosteroids are the most frequently prescribed dermatologic treatment and are often preferred to systemic corticosteroids because they are assumed to be associated with less systemic effects (Baumann 1999). Topical corticosteroids are the principal therapy for eczematous dermatoses (Berth‐Jones 2004), and they are also effective in treating inflammatory dermatoses such as discoid lupus erythematosus (Jessop 2000), bullous pemphigoid (Khumalo 2005), and chronic palmoplantar pustulosis (Marsland 2006). Women with these chronic dermatoses may continuously need a topical corticosteroid during pregnancy. Moreover, women with specific dermatoses of pregnancy, e.g., atopic eruption of pregnancy, polymorphic eruption of pregnancy, and pemphigoid gestationis, also require topical corticosteroids (Ambros‐Rudolph 2006). However, little is known about the effects of topical corticosteroids on the foetus.
The maternal skin conditions in pregnancy where topical corticosteroids are required may be classified into two main categories according to their pathological mechanism.
Autoimmune dermatoses such as autoimmune bullous dermatoses, in particular pemphigoid gestationis and lupus erythematosus.
Immunological and inflammatory dermatoses such as atopic eruption of pregnancy, polymorphic eruption of pregnancy, seborrhoeic dermatitis, and psoriasis.
In general, the aforementioned maternal skin conditions do not affect pregnancy outcomes, although pemphigoid gestationis is associated with an increase in small‐for‐gestational‐age children (Ambros‐Rudolph 2006), and systemic lupus erythematosus is associated with an increase in preterm delivery, foetal growth restriction, and stillbirth (Cunningham 2005). The Apgar score is a measure of the physical condition (breathing, heart rate, muscle tone, reflexes and skin colour) of newborns shortly after birth; a score of less than seven at 5 min is a poor indicator of survival (Casey 2001).
How the intervention might work
Studies have shown that corticosteroids cause birth defects and other adverse effects of the foetus in animals. Systemic corticosteroids induced cleft palate in rabbits, mice, rats, and hamsters (Nanda 1970; Nasjleti 1967; Shah 1976; Walker 1967). The incidence of sex organ defects in mice correlated with the dose of corticosteroids applied topically to the eyes (Ballard 1977). In juvenile rhesus monkeys, prenatal administration of dexamethasone caused a permanent loss of hippocampal neurons and an elevation of baseline and poststress cortisol concentrations in the blood (Uno 1994). Prenatal administration of one to four doses of betamethasone 0.5 mg per kg at 7‐day intervals, starting from three weeks before delivery, reduced the birth weight of lambs by 15% after one dose, 19% after two doses, and 27% after three and four doses (Ikegami 1997).
Whether systemic corticosteroid exposure in humans is teratogenic is controversial, and conflicting reports have appeared in the literature over the last two decades. A population‐based case‐control study of 662 infants with orofacial cleft and 734 controls showed that systemic corticosteroid use during the periconceptional period was associated with orofacial cleft (Carmichael 1999). Another case‐control study on 1184 infants with non‐syndromic orofacial cleft (i.e., orofacial cleft without associated congenital malformations, believed to be caused by multifactorial environmental and genetic factors with a low risk of familial occurrence; Edwards 2003) also showed a significant association between first trimester exposure to systemic corticosteroids and cleft lip (Rodriguez‐Pinil 1998). By contrast, a cohort study did not find significant differences in the incidence of major anomaly between 111 infants of mothers with first trimester exposure to systemic corticosteroids and 172 unexposed infants (Park‐Wyllie 2000). Another cohort study comparing 311 exposed and 790 non‐exposed women detected no significant differences in the rates of major anomalies, non‐genetic major anomalies, or congenital heart defects (Gur 2004). Nevertheless, both studies found a lowered gestational age at delivery, an increase in preterm delivery, and reduced birth weight in the exposed group (Gur 2004; Park‐Wyllie 2000).
The systemic effects of topical corticosteroids depend largely on the extent of skin absorption, which varies from 0.7% to 7% through intact skin (Sifton 2002). However, topical corticosteroids are often prescribed for inflammatory dermatoses, where the skin barrier is disrupted and skin absorption is enhanced. This could possibly lead to systemic effects and might have an impact on the foetus (Chi 2011b). The absorption from hydrocortisone cream 1% during exacerbation of atopic dermatitis was 11 to 31 times that in remission (Turpeinen 1988). Although hydrocortisone is the weakest corticosteroid, skin application of hydrocortisone cream 1% beyond one month was shown to suppress the adrenal glands in people with severe skin disease (Turpeinen 1989). Clobetasol propionate ointment 0.05%, the most potent topical corticosteroid available, can cause adrenal suppression at doses as low as 2 g per day for one week (Sifton 2002).
The foetotoxic effects of corticosteroids depend on their ability to cross the placenta (Chi 2011b). The principal enzyme that metabolises corticosteroids is 11‐beta‐hydroxysteroid dehydrogenase‐2, or 11βHSD2 (Sun 1998). This enzyme converts the active form cortisol (also known as hydrocortisone) to biologically inactive cortisone, acting as a barrier in pregnancy and protecting the foetus from potential harm by regulating the amount of maternal cortisol that passes through the placenta to reach the foetal compartment (Sun 1998). Based on the weak potency and high metabolism in the placenta, hydrocortisone is often presumed safe in pregnancy (Chi 2011b). However, a human study on maternal‐foetal cortisol transfer conducted before abortion illustrated that 15% of 3H‐cortisol passed through the placenta without being metabolised (Murphy 1974). Another human study demonstrated a linear relationship between maternal and foetal serum cortisol levels (Gitau 1998; Gitau 2001). Therefore, administration of hydrocortisone in pregnancy may still affect the foetus.
The ability to cross the placental barrier varies among other corticosteroids. Only 10% to 13% of prednisolone crosses the placenta to reach the foetus (Beitins 1972). By contrast, betamethasone, methylprednisolone, and dexamethasone are much less metabolised by 11βHSD2: around 30%, 45%, and 67% cross the placenta, respectively (Anderson 1981; Ballard 1975; Petersen 1980; Smith 1988). Fluticasone and budesonide are not metabolised by placental 11βHSD2 (Murphy 2007) and therefore cross the placenta unhindered.
To the best of our knowledge, there are no human studies evaluating the amounts of topical corticosteroids that reach the foetus after topical application, but animal studies have found that corticosteroids are present in the foetal blood after topical application. Considerable amounts of betamethasone 17,21‐dipropionate appeared in the foetal blood of mice and rabbits after topical application to their mothers' skin (Yamada 1981). Furthermore, corticosteroids are teratogenic not only through systemic administration but also through topical application in animals. For example, diflorasone diacetate cream induced cleft palate when applied topically to the chest skin of pregnant rats at a dose of 0.001 mg per kg per day, which is about 30% of the human topical dose. When the application dose was increased to 0.5 mg per kg per day, the treated rats had a higher rate of foetal death than the untreated controls (Taro 1999). Rabbits receiving a topical dose of diflorasone diacetate 0.016 mg per kg per day had depressed foetal growth, external anomalies (31.9%), cleft palate (22.2%), and visceral defects (45.5%) (Narama 1984).
Around 40 topical corticosteroids are commercially available (Baumann 1999; Berth‐Jones 2004; Hengge 2006; Mehta 2006). The aforementioned variations in placental metabolism and their differences in potency and skin absorption suggest that they may have varying degrees of adverse effects on the mother and foetus.
Why it is important to do this review
Treatment decisions are almost always a trade‐off between potential benefit and harm. Lack of information and clarity about the risk of topical corticosteroids increases therapeutic uncertainty and often results in under‐prescribing, even in situations when treatment is required and considered safe for use in pregnancy (Chi 2011b). On the other hand, many people are excessively nervous about the adverse effects of corticosteroids anyway. 'Steroid phobia' may be increased during pregnancy because of concerns for possible foetal harm, resulting in unnecessary suffering in pregnant women due to under‐treatment (Chi 2011b). Thus, there is a need for clear guidance.
Pharmacology references such as the British National Formulary and Thompson Micromedex do not give specific advice on prescribing topical corticosteroids for pregnant women (Mehta 2006; Thomson Healthcare 2009). Topical corticosteroids are often only labelled in the prescribing information as "should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus" (GlaxoSmithKline 2002; Schering 2003). A general assumption is that use of low‐potency topical corticosteroids, like hydrocortisone, is safe during pregnancy, but this may be ill‐founded. On the other hand, despite the lack of sufficient safety data, some women still use topical corticosteroids during pregnancy; a survey of the UK General Practice Research Database showed that over 3% of 81,975 pregnant women had been prescribed a topical corticosteroid during early pregnancy (Hardy 2006).
Thus, a systematic review of the safety of topical corticosteroid use during pregnancy is required to bring together the relevant evidence for people wishing to make a well‐informed decision. This review aimed to summarise the best evidence of adverse perinatal effects of topical corticosteroids. We did not consider other aspects of the safety of topical corticosteroids, for example their local and systemic adverse effects, as they are not specific to pregnant women.
A previous version of this review included seven observational studies (Chi 2009). The data available at that time were limited and inconclusive, failing to detect an association between topical corticosteroids and congenital abnormality, preterm delivery, or stillbirth, but the findings suggested an association of very potent topical corticosteroids with low birth weight. We therefore decided to update this review to take into account new evidence that has emerged.
Objectives
To assess the effects of topical corticosteroids on pregnancy outcomes in pregnant women.
Methods
Criteria for considering studies for this review
Types of studies
Owing to ethical concerns, randomised controlled trials (RCTs) of drugs are not carried out in pregnant women for fear of maternal exposure to an experimental drug that may harm the foetus, unless the clinical trial focuses on a pregnancy‐related condition such as labour induction (Meadows 2001). Therefore, when we started working on this review, we expected that there would be few or no RCTs of topical corticosteroid use in pregnant women. Furthermore, although RCTs are the gold standard for investigating the effects of interventions, they are not a good tool for detecting adverse outcomes that are rare, prone to occur in a specific group of people, or take a long time to develop (Higgins 2011). This review therefore included cohort and case‐control studies as well. The inclusion criteria for each type of study were as follows.
All RCTs that exclusively recruited pregnant women, tested topical corticosteroids during pregnancy, and reported pregnancy outcomes or adverse events. We did not include RCTs that recruited pregnant women only as a subset.
All cohort studies that evaluated pregnancy outcomes or adverse events after exposure to topical corticosteroids in pregnancy. We included both prospective and retrospective cohort studies.
All case‐control studies that compared exposure to topical corticosteroids during pregnancy between cases with any of the outcomes of interest and the control group.
Types of participants
Any pregnant women with a skin condition requiring topical corticosteroid treatment.
Types of interventions
In RCTs: If we had found relevant RCTs, the intervention group would have received one or more topical corticosteroids during pregnancy. The comparators would have been placebo, no treatments, or any treatments other than corticosteroids (e.g., topical emollients, other non‐corticosteroid topical medicines, and oral antihistamines). If we had analysed the effects on congenital abnormality by topical corticosteroids, the intervention group would have been restricted to women who received topical corticosteroids in the first trimester of gestation.
In cohort studies: The exposed group received one or more topical corticosteroids during pregnancy. The unexposed group was composed of either pregnant women with a skin condition not exposed to topical corticosteroids or pregnant women from the general population not exposed to topical corticosteroids. When we analysed the effects of topical corticosteroids on congenital abnormality, the exposed group was restricted to women who received topical corticosteroids in the first trimester of gestation.
In case‐control studies: The case group consisted of any women and their children with any of the outcomes of interest. The control group consisted of any women and their children without that outcome. Some case‐control studies of congenital abnormality may have used a control group consisting of children with congenital abnormalities other than the abnormality of interest. If the comparison congenital abnormalities resulted from a similar embryo‐pathological mechanism to the abnormality of interest, we excluded such studies. When assessing the effects of topical corticosteroids on congenital abnormality, we only compared maternal exposure to topical corticosteroids in the first trimester of gestation between the two groups.
Types of outcome measures
Primary outcomes
Maternal outcomes
Mode of delivery: normal vaginal delivery, assisted delivery, or cesarean section.
Outcomes in children
Major congenital abnormality: structural‐morphological birth defect that is either fatal or causing handicap or death if untreated (Czeizel 2005)
Outcomes related to foetal growth: birth weight, body length, foetal growth restriction, or other
Preterm delivery (delivery before 37 completed weeks' gestation)
Secondary outcomes
Foetal death
Mild congenital abnormality: structural‐morphological birth defect requiring medical intervention but with good life expectancy, such as congenital dislocation of the hip or undescended testis (Czeizel 2005)
Apgar score < 7 at 5 min (Casey 2001)
Timing of outcome assessment
In RCTs and cohort studies, the follow‐up had to be long enough for the outcomes to develop, be measured, and be recorded. Mode of delivery, foetal growth‐related measures, preterm delivery, and low Apgar score are amenable to assessment immediately after birth. By contrast, congenital abnormalities may not be diagnosed or recorded until some time has passed. We thus included all relevant studies irrespective of the length of follow‐up, but we addressed the length of follow‐up of the children when assessing the methodological quality of the studies on congenital abnormality.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
For this update, we revised the search strategies for the Skin Group Specialised Register, CENTRAL, MEDLINE, EMBASE, and LILACS. We searched the following databases up to 9 July 2015.
The Cochrane Skin Group Specialised Register, using the search strategy in Appendix 1.
The Cochrane Pregnancy and Childbirth Group Specialised Register, by contacting the Trials Search Co‐ordinator (searched to July 2013). The topic list was used for searching as described in Appendix 2.
The Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 6), using the strategy in Appendix 3.
MEDLINE via Ovid (from 1946) using the strategy in Appendix 4.
EMBASE via Ovid (from 1974) using the strategy in Appendix 5.
LILACS (Latin American and Caribbean Health Sciences Information Database, from 1982) using the strategy in Appendix 6.
In MEDLINE we searched for cohort and case‐control studies as well as RCTs, using the BMJ Clinical Evidence filter available at http://www.york.ac.uk/inst/crd/intertasc/observational.htm (see Appendix 4). In LILACS we searched for cohort, case‐control, and controlled clinical trials, using the filters available within the database.
We also searched the following trials registers on 10 July 2015 using the terms 'pregnancy','pregnant', 'topical steroid' and 'topical corticosteroid'.
The ISRCTN registry (www.controlled‐trials.com), using the strategy in Appendix 7.
The US National Institutes of Health Ongoing Trials Register (clinicaltrials.gov), using the strategy in Appendix 8.
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au), using the strategy in Appendix 9.
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch), using the strategy in Appendix 10.
The EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/), using the strategy in Appendix 11.
Searching other resources
Handsearching
We handsearched the literature collection of one author (CC) on 10 July 2015.
Reference lists
We scanned the bibliographies of the included studies and published reviews for relevant references. We also used SCI‐EXPANDED on 21 July 2014 to identify the articles that had cited the included studies and scanned for further relevant studies. When we updated our search for this review on 10 July 2015, our institution no longer had a subscription to SCI‐EXPANDED, so we could not update the citation lists.
Correspondence
We planned to correspond with the original researchers to identify unpublished or ongoing trials and observational studies.
Data collection and analysis
Some parts of the Methods section of this review use text that was originally published in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included 'Summary of findings' tables in our review to summarise the essential primary and secondary outcomes and assessed the quality of the body of evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias).
Selection of studies
Two authors (CC and SW) independently checked all the titles and abstracts identified from the searches. If it was clear that the study did not refer to a study on the use of topical corticosteroids in pregnant women, we excluded it. The same two authors independently assessed the full‐text version of each remaining study to determine whether it met the pre‐defined selection criteria. We resolved any differences in opinion through discussion within the review team. We listed excluded studies in the Characteristics of excluded studies tables after having read the full text.
Data extraction and management
Two authors (CC and SW) independently extracted the data using a specially designed data extraction form. A third team member (FW) was available for resolving any differences of opinion.
Assessment of risk of bias in included studies
RCTs
If we had found relevant RCTs, we would have evaluated the following components that have been shown to result in biased estimates of intervention effects and recorded them in the 'Risk of bias' tables in the Characteristics of included studies (Higgins 2011).
The method of randomisation sequence generation.
The method of allocation concealment; we would have judged 'adequate' if the assignment was sufficiently unpredictable.
Blinding of participants and investigators.
Blinding of outcome assessors.
Incomplete outcome data: we would have assessed how many participants were lost to follow up in each arm, whether the reasons for the losses were adequately reported, and whether all participants were analysed in the groups to which they were originally randomised (intention‐to‐treat principle).
Selective reporting: we would have assessed the possibility of selective outcome reporting.
-
Other sources of biases: we would have assessed:
the maternal skin conditions for which topical corticosteroids were required and the degree of certainty that the women had a skin condition;
the baseline assessment of the participants for age, duration of disease, location involved, and severity of the skin condition(s);
drug identity, source, dose, duration of treatment, and adequacy of instructions;
whether the outcome measures were described and their assessment was standardised;
whether previous treatments for skin conditions were discontinued;
whether concomitant treatments for skin conditions were permitted or standardised;
the use and appropriateness of statistical analyses, where tabulated data could not be extracted from the original publication.
Non‐randomised studies
We used the Newcastle‐Ottawa Scale (NOS) (Wells 2006) only as a checklist to describe quality to provide the readers with a better understanding of the diverse methods used. Below, we summarise the considerations relevant to cohort and case‐control studies.
Cohort studies
Representativeness of the exposed cohort (including method of recruitment, clinical setting, and proportion of eligible mothers or children recruited)
Selection of the unexposed cohort
Ascertainment of exposure to topical corticosteroids (e.g., how the exposure was defined and whether over‐the‐counter topical corticosteroids were available)
Demonstration that outcome of interest was not present at start of study
Comparability of the cohorts (control for potential confounders, e.g., maternal skin condition, comorbidity, maternal age, smoking and drinking habit, family history of congenital abnormality, exposure to other medications, and socioeconomic status)
Assessment of the outcomes
Sufficient length of the follow‐up for outcomes to occur
Adequacy of the follow‐up of the cohorts
Case‐control studies
Adequacy of the case definition
Representativeness of the cases
Selection of the controls (including method of recruitment and source)
Definition of the controls
Comparability of the cases and controls (control for potential confounders, e.g., maternal skin condition, comorbidity, maternal age, smoking and drinking habit, family history of congenital abnormality, exposure to other medications, and socioeconomic status)
Ascertainment of exposure to topical corticosteroids (e.g., how the exposure was defined and whether over‐the‐counter topical corticosteroids were available)
Standard and valid method of ascertainment of exposure for the cases and controls
Non‐response rate
Measures of treatment effect
Dichotomous data
For dichotomous outcomes in RCTs (if found) and cohort studies, we expressed the results as risk ratios (RR) and 95% confidence intervals (CI). We expressed dichotomous outcomes in case‐control studies as odds ratios (OR) and 95% CIs. We expressed the results as 'number needed to treat for an additional harmful outcome (NNTH)' where appropriate for a range of plausible control event rates.
Continuous data
We expressed continuous outcomes as mean difference (MD) and 95% CIs.
Unit of analysis issues
The study subjects in the included studies were the unit of analysis. For the following types of studies, we would have used appropriate analytical techniques, and we would not have pooled these studies with studies of other designs.
Cluster‐randomised trials
We would have used the technique described in Chapter 16.3 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Cross‐over trials
We excluded cross‐over RCTs.
Studies with multiple treatment groups
If a study contained multiple intervention groups, we would have made pair‐wise comparisons of topical corticosteroids of similar potency or active components versus no treatments, placebo, treatments other than topical corticosteroids, or another topical corticosteroid.
Dealing with missing data
We contacted the investigators or funders for missing data when the studies were published in the previous 15 years. When participants dropped out, and the trialists adopted the per‐protocol analysis, we would have used the intention‐to‐treat analysis to recalculate the results.
Assessment of heterogeneity
We assessed the heterogeneity of studies with different designs (i.e., RCTs, cohort studies, and case‐control studies) separately. We used the I2 statistic in examining the statistical heterogeneity. If the I2 statistic was less than 80% with reasonable clinical homogeneity, we applied meta‐analysis techniques as appropriate.
Assessment of reporting biases
We assessed the reporting biases of studies with different designs separately. We planned to test publication bias by using a funnel plot when adequate data were available for topical corticosteroids of similar potency or for a similar active component.
Data synthesis
For studies with topical corticosteroids of similar potency or active components, we conducted a meta‐analysis to calculate a weighted treatment effect across trials using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We discussed issues of heterogeneity, such as study design, selection of the control group in case‐control studies, maternal skin condition and severity, maternal comorbidity, similarities and differences in the types of interventions. We performed further subgroup analyses where adequate data were available.
The originally planned subgroups were different maternal skin conditions (polymorphic eruption of pregnancy, pemphigoid gestationis, etc.) and maternal ages as described by the original researchers. However, these data were unavailable.
As we expected, the risk of adverse effects of topical corticosteroids may be related to the strength, so for this update we conducted a subgroup analysis based on corticosteroid potency (mild to moderate versus potent to very potent), as defined by the British National Formulary (Mehta 2006); see Table 4.
1. Potency of topical corticosteroidsa.
|
Mild Hydrocortisone 0.10%‐2.50% Hydrocortisone acetate 0.1% Fluocinolone acetonide 0.0025% |
|
Moderate Alclometasone dipropionate 0.05% Betamethasone valerate 0.025% Clobetasone butyrate 0.05% Fludroxycortide (flurandrenolone) 0.0125% Fluocinolone acetonide 0.00625% Fluocortolone 0.25% |
|
Potent Hydrocortisone butyrate 0.10% Beclometasone dipropionate 0.025% Betamethasone valerate 0.10%‐0.12% Fluocinolone acetonide 0.025% Fluprednidene acetate 0.10% Fluocinonide 0.05% Diflucortolone valerate 0.10% Fluticasone propionate 0.005%‐0.05% Mometasone furoate 0.10% Triamcinolone acetonide 0.10% Betamethasone dipropionate 0.05%‐0.064% |
|
Very potent Diflucortolone valerate 0.30% Halcinonide 0.10% Clobetasol propionate 0.05% |
aThe listed potency of the topical corticosteroid preparations is according to the British National Formulary (Mehta 2006).
Sensitivity analysis
We conducted a sensitivity analysis to examine the intervention effects after excluding poor quality studies. We defined poor quality studies as those rated at a high risk of bias for one or more key domains.
GRADE
We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system to describe the quality of the evidence and the strength of recommendation (GRADE 2013; Guyatt 2011). GRADE has been adopted postprotocol to rate the quality of evidence. We expressed the quality of evidence on a four‐point adjectival scale from 'high' to 'very low'. We downgraded it if there was unexplained, clinically important heterogeneity or if the study methodology had a risk of bias, the evidence was indirect, there was important uncertainty around the estimate of effect, or there was evidence for publication bias. Therefore, it was possible to grade the evidence at a very low quality if several of these concerns were present.
Other
Where there was uncertainty, we contacted the original researchers for clarification. A consumer (ED) worked with us to help ensure the relevance and readability of the final review.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
As shown in Figure 1, our update searches identified 441 additional records. We screened out 431 references based on titles and abstracts. Of the remaining 10 records, we excluded 4 (see Characteristics of excluded studies). We included 6 records reporting 7 new studies (one publication reporting 2 studies: Skuladottir 2014b; Skuladottir 2014c), along with 7 studies from the previous review, bringing the total number of studies that we included in the quantitative and qualitative analyses to 14.
1.
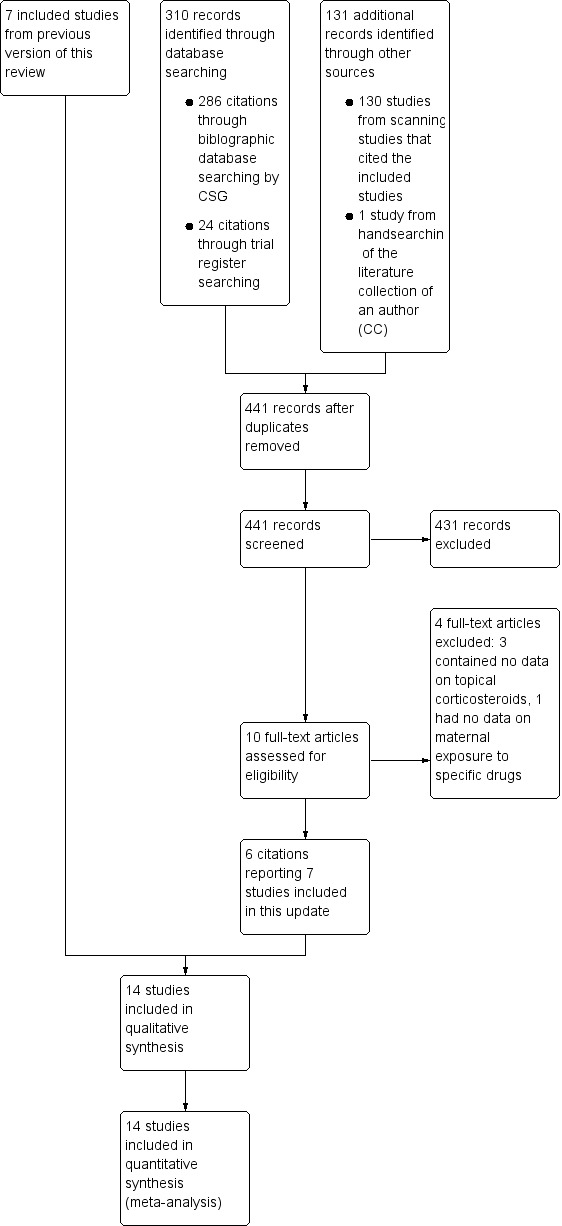
Study flow diagram.
Included studies
We included 14 studies involving 1,601,515 study subjects.
In addition to the 7 studies included in the previous version of the review (Carmichael 2007; Czeizel 1997;Edwards 2003; Källén 2003; Mahé 2007; Mygind 2002; Pradat 2003), we included 7 new studies that met our inclusion criteria for this update (Carmichael 2009; Chi 2011a; Chi 2013; Hviid 2011; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c).
Skuladottir 2014a was an extension of the Carmichael 2007 study on orofacial cleft, with the latter reporting data collected from 1997 to 2002 and the former reporting those collected from 2003 to 2009. Carmichael 2009 used the same data source as Carmichael 2007 and Skuladottir 2014a, but it examined another outcome (hypospadias) using data collected from 1997 to 2004. We describe the details of the 14 included studies in the Characteristics of included studies tables.
Design
Of the 14 included studies, 5 were cohort studies (Chi 2011a; Chi 2013; Hviid 2011; Mahé 2007; Mygind 2002), and the other 9 were case‐control studies (Carmichael 2007; Carmichael 2009; Czeizel 1997; Edwards 2003; Källén 2003; Pradat 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c). The original researchers reported the Skuladottir 2014c study as a cohort study; however, we judged it to be a case‐control study after examining the full text.
Sample size
The 5 cohort studies that we included (Chi 2011a; Chi 2013; Hviid 2011; Mahé 2007; Mygind 2002) enrolled 35,503 women, 2658 women, 22,480 women, 28 women, and 363 women exposed to topical corticosteroids during pregnancy, respectively. However, the Mygind 2002 study did not report the respective number of women who received mild or moderate and potent to very potent topical corticosteroids. We requested detailed data from the original researchers of the Mahé 2007 study, but they could only provide valid data for 23 exposed women for analysis. Six of the included case‐control studies (Carmichael 2007; Edwards 2003; Källén 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c) enrolled 1769, 48, 1044, 2372, 573, and 184 children with orofacial cleft, respectively. The other two case‐control studies (Czeizel 1997; Pradat 2003) enrolled 20,830 and 11,150 children with congenital abnormality, but only 1223 and 982 of them had orofacial cleft, respectively. The Carmichael 2009 study included 1165 children with moderate to severe hypospadias.
Setting
The settings of the included studies ranged from a multinational project (Pradat 2003) to a single country or local population‐based register (Carmichael 2007; Carmichael 2009; Chi 2011a; Chi 2013; Czeizel 1997; Hviid 2011; Källén 2003; Mygind 2002; Skuladottir 2014a; Skuladottir 2014c) to a single hospital (Edwards 2003; Mahé 2007; Skuladottir 2014b).
Study subjects
The study subjects in all of the included cohort studies (Chi 2011a; Chi 2013; Hviid 2011; Mahé 2007; Mygind 2002) were pregnant women. In the Danish study (Mygind 2002), the study subjects were restricted to primiparous women carrying a single foetus. The cases of the included case‐control studies had congenital abnormality, orofacial cleft, or hypospadias (Carmichael 2007; Carmichael 2009; Czeizel 1997; Edwards 2003; Källén 2003; Pradat 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c).
Interventions
The interventions in all included studies were topical corticosteroids. In the Senegalese study (Mahé 2007), the women used very potent topical corticosteroids (predominantly clobetasol propionate, mean dosage: 600 g (range 120 to 1700) during the whole pregnancy) for skin lightening. In Chi 2011a, 64.9% of the exposed women received topical corticosteroids for steroid‐responsive dermatoses. The mean amounts of potent to very potent topical corticosteroids prescribed during the whole pregnancy was 83.5 g (range 10 to 2800 g) and 64 g (range 15 to 490 g) in the Chi 2011a and Chi 2013 studies, respectively. In two case‐control studies (Czeizel 1997; Edwards 2003), topical corticosteroids were used mainly for allergic dermatoses such as urticaria and eczema. In the Australian study (Edwards 2003), seven out of the nine women in the case (orofacial cleft) group used potent topical corticosteroids. Only one woman in the control group used hydrocortisone, the weakest topical corticosteroid. The other nine studies did not report the indications for topical corticosteroids (Carmichael 2007; Carmichael 2009; Hviid 2011; Källén 2003; Pradat 2003; Mygind 2002; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c).
Outcomes
Due to the limitation of study design, the only outcome that could be measured in all nine case‐control studies was congenital abnormality, orofacial cleft, or hypospadias (Carmichael 2007; Carmichael 2009; Czeizel 1997; Edwards 2003; Källén 2003; Pradat 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c). Investigators in only four cohort studies measured the other outcomes, including mode of delivery, birth weight, preterm delivery, stillbirth, and low Apgar score (Chi 2011a; Chi 2013; Mahé 2007; Mygind 2002). The other cohort study, Hviid 2011, only assessed orofacial cleft.
We analysed orofacial cleft separately, as it is an expected possible associated outcome. When detailed data were available, we further analysed the two categories of orofacial cleft, (i.e., cleft lip with or without cleft palate, and isolated cleft palate), separately because they are considered aetiologically distinct (Stanier 2004).
Edwards 2003 used a classification of orofacial cleft different from ours and divided the cases as cleft palate ± lip and isolated cleft palate (see Effects of interventions). We thus used the published data to calculate the case number of cleft lip with or without cleft palate and used Review Manager software (RevMan 2014) to recalculate all the crude ORs and 95% CIs for consistency.
Excluded studies
We excluded four studies identified from searches after obtaining the full text (see the Characteristics of excluded studies tables).
Studies awaiting assessment
We did not identify any studies that we could not classify.
Ongoing studies
We did not find any relevant ongoing studies.
Risk of bias in included studies
We present the summarised risk of bias across all included studies in Figure 2 and the respective 'Risk of bias' item for each included study in Figure 3.
2.
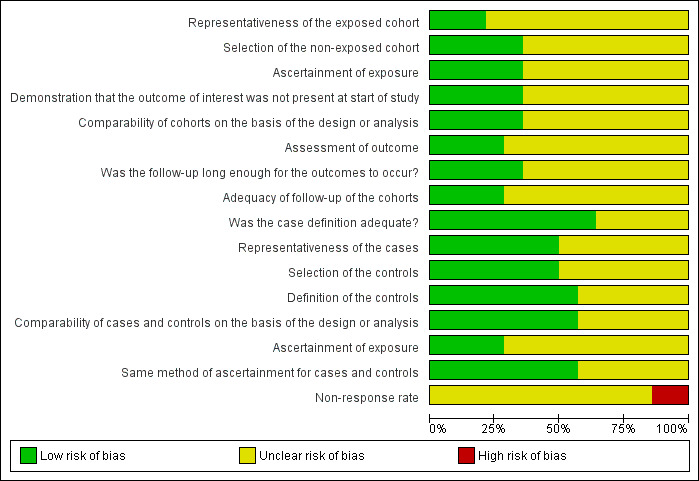
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
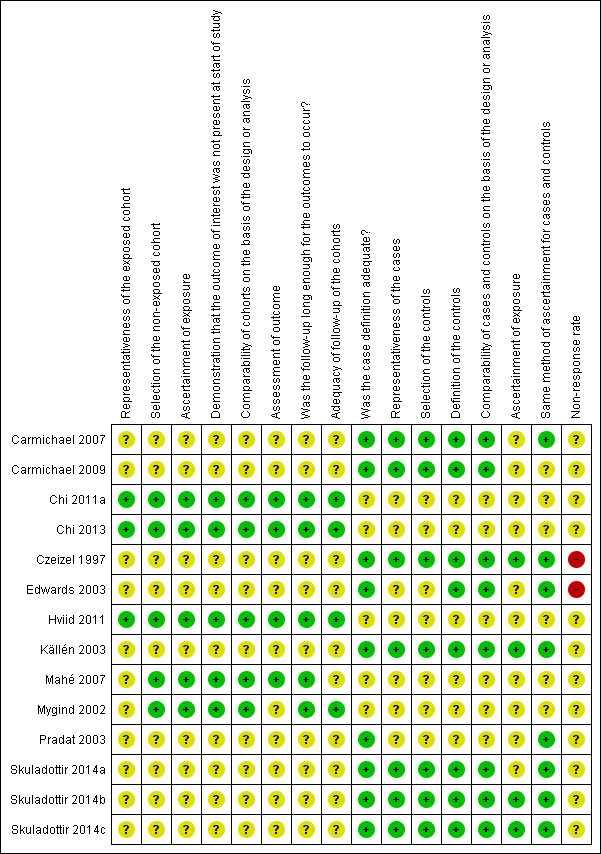
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Cohort studies
The 5 cohort studies were Chi 2011a, Chi 2013, Hviid 2011, Mahé 2007, and Mygind 2002.
Representativeness of the exposed cohort: the setting of the Mygind 2002 study was a local population, but investigators only recruited primiparous women carrying a single foetus. The setting of the Mahé 2007 study was a single maternity centre. These settings limited the external validity of the studies. The other three cohort studies used population‐based data and thus had a better generalisability (Chi 2011a; Chi 2013; Hviid 2011).
Selection of the unexposed cohort: all of the five included cohort studies drew the unexposed cohort from the same source as the exposed cohort so were judged at low risk of bias.
Ascertainment of exposure to topical corticosteroids: four studies used record linkage to the prescription database (Chi 2011a; Chi 2013; Hviid 2011; Mygind 2002). However, whether the women adhered to the prescribed corticosteroids and whether they used over‐the‐counter topical corticosteroids or topical corticosteroids from previous prescriptions or even from their relatives or friends was unknown. The Senegalese study used structured interviews (Mahé 2007). All were judged at low risk of bias.
Demonstration that outcome of interest was not present at start of study: all of the included cohort studies had a low risk of bias for this item.
Comparability of the cohorts: Mygind 2002 controlled for potential confounders, including maternal age and smoking, but it did not assess potential confounding by indication. Though Mahé 2007 and Hviid 2011 did not control for potential confounders, they found no significant differences in the potential confounders such as maternal socioeconomic and education levels, age, and parity between women who used very potent topical corticosteroids and those who did not. All of the exposed women in the Mahé 2007 study used topical corticosteroids for skin lightening. In the Chi 2011a and Chi 2013 studies, there were differences in potential confounders between the exposed and control group, but these confounders were adjusted in the statistical analyses.
Assessment of the outcomes: all of the included cohort studies used record linkage to clinical records or birth registry (Chi 2011a; Chi 2013; Hviid 2011; Mahé 2007; Mygind 2002). None of them reported record validation for a sample of the exposed women without the outcomes. In the Mygind 2002 study, the records of congenital abnormality in the register were not entirely accurate. Among the five children registered as having a congenital abnormality in the exposed group, two actually did not have any abnormalities according to their hospital records.
Sufficient length of the follow‐up for outcomes to occur: all studies had a sufficient length of follow‐up until delivery or foetal death (Chi 2011a; Chi 2013; Hviid 2011; Mahé 2007; Mygind 2002).
Adequacy of the follow‐up of the cohorts: Chi 2013, Hviid 2011, and Mygind 2002 had complete follow‐up because the records were from birth registries. In Mahé 2007, which was prospective, 10 out of 99 (10.10%) women were lost to follow up. The incidence of foetal growth restriction in Chi 2011a was 0.59%, which was lower than the usual reported rate of 3% to 7% (Romo 2009). The low incidence in Chi 2011a may have reduced the statistical power leading to false‐negative results; however, this study found a significant association between maternal exposure to potent or very potent topical corticosteroids and foetal growth restriction.
Case‐control studies
The 9 case‐control studies were: Czeizel 1997; Edwards 2003; Källén 2003; Pradat 2003; Carmichael 2007; Carmichael 2009; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c.
Case definition: When assessing the teratogenic risk of topical corticosteroids, the definition of the case varied among the case‐control studies that were included. Czeizel 1997 defined a 'case' as having an isolated congenital abnormality or unidentified multiple congenital abnormalities, excluding those with some mild congenital abnormalities, minor anomalies, or congenital abnormality syndromes of known origin. Fourteen congenital abnormality groups performed stratified analysis. Edwards 2003 only included cases with non‐syndromic orofacial cleft. Skuladottir 2014a excluded cases of orofacial cleft that were believed to be the result of another defect. On the other hand, Källén 2003 and Skuladottir 2014c did not exclude cases with a syndrome diagnosis. Carmichael 2007 and Skuladottir 2014b included all cases of orofacial cleft in primary analyses and excluded syndromic cleft in a sensitivity analysis. The Malformation Drug Exposure Surveillance (MADRE) project also included cases with multiple malformations, syndromes, and even known causes like chromosomal defects (Pradat 2003). Carmichael 2009 included cases of second‐ or third‐degree hypospadias.
Representativeness of the cases: The source of cases in six studies was from a congenital malformation register (Carmichael 2007; Carmichael 2009; Czeizel 1997; Källén 2003; Pradat 2003; Skuladottir 2014a) and was limited to a cleft palate clinic in Edwards 2003 and to two specialised surgical centres for orofacial cleft in Skuladottir 2014b. In Skuladottir 2014c, investigators identified cases with orofacial cleft through the Medical Birth Registry of Norway. Pradat 2003 only included children with congenital malformations and a positive history of maternal first trimester drug exposure, excluding children with congenital malformations but without a history of maternal first‐trimester drug exposure; thus we judged it at unclear risk of bias for this item.
Selection of the controls (including method of recruitment and source): Czeizel 1997, Källén 2003, Skuladottir 2014b, and Skuladottir 2014c selected the controls from a national birth registry. Pradat 2003 selected controls from a multicentre database, and Edwards 2003 used hospital controls. The NOS considers that the use of hospital controls denotes an 'unclear' risk of bias. In the Carmichael 2007, Carmichael 2009, and Skuladottir 2014a studies, the controls without major congenital malformations were randomly selected from birth certificates or birth hospitals.
Definition of the controls: The controls were defined as those without congenital abnormalities (Carmichael 2007; Carmichael 2009; Czeizel 1997; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c) or without orofacial cleft (Edwards 2003; Källén 2003). In the Pradat 2003 study, the controls were infants with congenital malformations other than orofacial cleft and a history of maternal first‐trimester drug intake.
Comparability of the cases and controls: Eight studies controlled the potential confounders (Carmichael 2007; Carmichael 2009; Czeizel 1997; Edwards 2003; Källén 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c). Pradat 2003 used the MADRE database, which recruits children with birth defects from many congenital abnormality registers around the world; the researchers from this study treated the children from the different registers as study subjects from different studies and calculated the OR and 95% CI by using the Mantel‐Haenszel method to adjust the data that each register provided about the study subjects.
Ascertainment of exposure to topical corticosteroids: In six studies, investigators ascertained corticosteroid exposure retrospectively by interviews (Carmichael 2007; Carmichael 2009; Edwards 2003; Pradat 2003; Skuladottir 2014a) or questionnaire (Skuladottir 2014b). Czeizel 1997 used a prenatal log book, questionnaire, and interview for ascertaining exposure. In Källén 2003, midwives prospectively gathered data on drug exposure (mainly in first trimester) at the first antenatal care visit (usually week 10 to 12). In the Skuladottir 2014c study, investigators also prospectively collected data on maternal drug exposure using questionnaires completed at gestational weeks 15, 22, and 30.
Standard and valid method of ascertainment of exposure for the cases and controls: All nine case‐control studies used the same ascertainment method for the cases and controls. However, there was a 4.3 months' delay in the interview of case mothers in the Carmichael 2009 study.
Non‐response rate: In the Australian study (Edwards 2003), the non‐response rate for the case and control groups was very high at 70% and 85.8%, respectively. In the Hungarian study (Czeizel 1997), the non‐response rate was 18% and 35% for the case and control groups, respectively. In the case group, regional nurse visits to non‐respondents decreased the non‐response rate by 10%. These two studies were judged at high risk of bias. In Carmichael 2009, the non‐response rate in the case group was 23%, but the exact non‐response rate in the mothers of male‐only controls was not available. The non‐response rate was unavailable in six other studies (Carmichael 2007; Källén 2003; Pradat 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c).
Effects of interventions
See: Table 1; Table 2; Table 3
We have addressed the effects of the interventions according to our pre‐specified outcomes. The interventions in all included studies were topical corticosteroids.
Primary outcomes
Maternal outcomes
Mode of delivery
Of the included studies, only two cohort studies assessed the mode of delivery and found no significant differences between the exposed and control groups (Chi 2013; Mahé 2007). Mahé 2007 did not provide exact statistics. Women who received topical corticosteroids during pregnancy did not have an increased risk for either assisted or cesarean delivery (RR 1.04, 95% CI 0.95 to 1.15, 1 cohort study, n = 9904) in the Chi 2013 study (Analysis 1.1). The quality of the evidence was rated as low for this outcome.
1.1. Analysis.

Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 1 Assisted or cesarean delivery (cohort study).
Outcomes in children
Congenital abnormality
We originally planned to analyse major and minor congenital abnormalities separately, but did not find any studies that reported them separately. Therefore, we grouped the two outcomes together.
Cohort studies
Only two cohort studies assessed this outcome (Mahé 2007; Mygind 2002). One cohort study, Mygind 2002, did not find significant differences in the risk for congenital abnormality between women who received topical corticosteroids 30 days before conception or during the first trimester and those who did not (RR 0.82, 95% CI 0.34 to 1.96, 1 cohort study, n = 9433; low quality evidence; Analysis 1.2). Another cohort study, Mahé 2007 (n = 79), did not have any children with a congenital abnormality in the exposed or unexposed groups (Analysis 1.2).
1.2. Analysis.
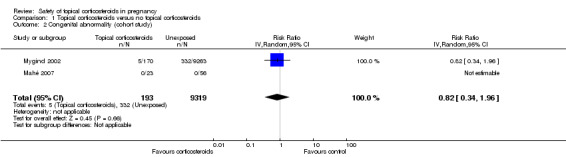
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 2 Congenital abnormality (cohort study).
Case‐control studies
One case‐control study, Czeizel 1997, did not find significant differences in maternal use of topical corticosteroids in the first three months of gestation between children with 14 congenital abnormality groups and their controls (OR 1.07, 95% CI 0.71 to 1.60, n = 56,557; Analysis 1.3). We rated the quality of the evidence as low.
1.3. Analysis.
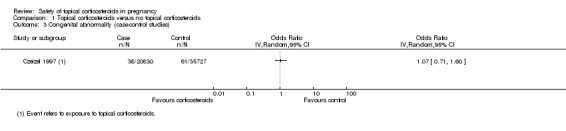
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 3 Congenital abnormality (case‐control studies).
Orofacial cleft
Cohort studies
Three cohort studies assessed orofacial cleft, its two categories, or both (i.e., cleft lip with or without cleft palate and cleft palate alone) (Chi 2011a; Chi 2013; Hviid 2011). We rated the quality of evidence as low for orofacial cleft in the cohort studies.
Orofacial cleft: Chi 2011a and Chi 2013 found no associations between orofacial cleft and maternal exposure to topical corticosteroids in the first 12 gestational weeks (pooled RR 1.12, 95% CI 0.54 to 2.33, 2 studies, n = 40,605; Analysis 1.4).
Cleft lip with or without cleft palate (Analysis 1.5): Neither Chi 2011a nor Chi 2013 found any association between maternal exposure to topical corticosteroids and cleft lip with or without cleft palate (adjusted RR 1.20, 95% CI 0.40 to 3.61, n= 32,642; and 4.79, 95% CI 0.43 to 52.71, n = 7963, respectively). Hviid 2011 found a link between maternal exposure to topical corticosteroids in the first 12 gestational weeks and cleft lip with or without cleft palate (adjusted RR 1.45, 95% CI 1.03 to 2.05, n= 832,636); however, the study authors found no dose‐response nor potency‐response relationship and concluded it to be a spurious finding resulting from multiple comparisons. We thus did not undertake a meta‐analysis.
Cleft palate alone: None of the three studies found any associations between maternal exposure to topical corticosteroids and cleft palate alone (pooled RR 1.31, 95% CI 0.82 to 2.11, 3 studies, n = 873,241; Analysis 1.6).
1.4. Analysis.
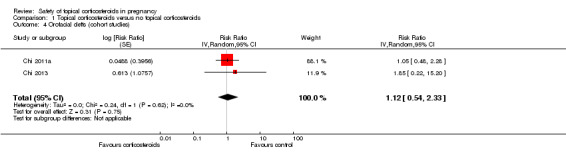
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 4 Orofacial clefts (cohort studies).
1.5. Analysis.
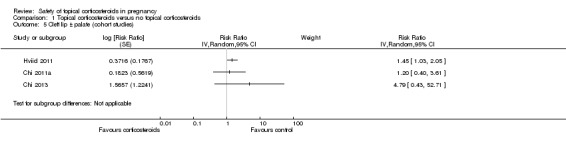
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 5 Cleft lip ± palate (cohort studies).
1.6. Analysis.
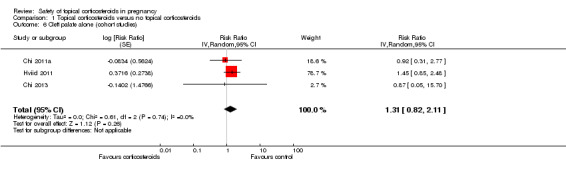
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 6 Cleft palate alone (cohort studies).
Case‐control studies
Eight of the included case‐control studies assessed this outcome (Carmichael 2007; Czeizel 1997; Edwards 2003; Källén 2003;Pradat 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c). We rated the quality of the evidence for the outcome of orofacial cleft as low for the case‐control studies.
Orofacial cleft (Analysis 1.7): One case‐control study, Edwards 2003, reported a significant association between orofacial cleft and first‐trimester use of topical corticosteroids (adjusted OR 18.65, 95% CI 1.29 to 270.1, n = 106). However, the other seven case‐control studies found no such association. The pooled OR was 1.20 (95% CI 0.68 to 2.13, 8 studies, n = 641,917).
Cleft lip with or without cleft palate (Analysis 1.8): Edwards 2003 found a significant association between first‐trimester use of topical corticosteroids with cleft lip with or without cleft palate (crude OR 13.57, 95% CI 1.50 to 123.05, n = 84), but the other seven case‐control studies did not. The pooled OR was 1.52 (95% CI 0.84 to 2.75, 8 studies, n = 639,654).
Cleft palate alone (Analysis 1.9): Again, Edwards 2003 found significant associations between first‐trimester use of topical corticosteroids and cleft palate alone (crude OR 12.67, 95% CI 1.33 to 120.72, n = 80), unlike the rest of the case‐control studies. The pooled OR was 1.20 (95% CI 0.57 to 2.54, 8 studies, n = 637,450).
1.7. Analysis.
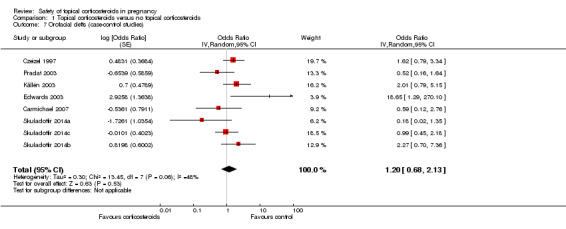
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 7 Orofacial clefts (case‐control studies).
1.8. Analysis.
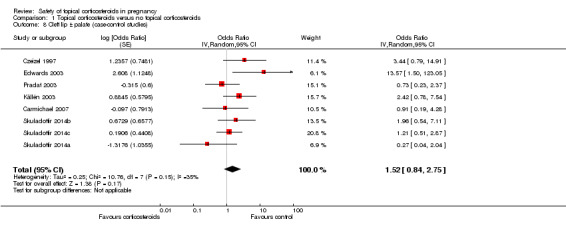
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 8 Cleft lip ± palate (case‐control studies).
1.9. Analysis.
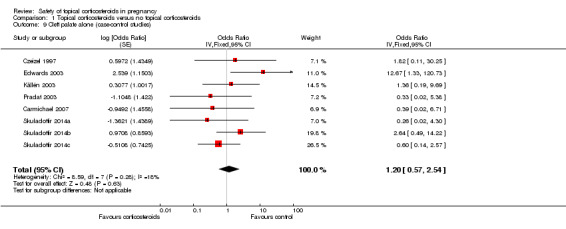
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 9 Cleft palate alone (case‐control studies).
Hypospadias
One study, Carmichael 2009, only assessed this outcome, and we thus decided to analyse it separately. Czeizel 1997 also reported relevant data for this outcome. Both Carmichael 2009 and Czeizel 1997 found an association between hypospadias and first‐trimester use of topical corticosteroids (pooled OR 0.45, 95% CI 0.19 to 1.09, 2 studies, n = 42,618; Analysis 1.10). In our analyses, there was limited or suggested evidence for an effect, although this did not reach statistical significance.
1.10. Analysis.
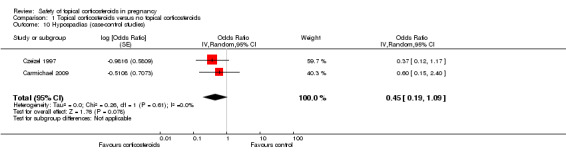
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 10 Hypospadias (case‐control studies).
Birth weight
A total of 4 cohort studies assessed low birth weight (i.e., birth weight < 2500 g) or foetal growth restriction (defined as small‐for‐dates, birth weight < 2500 g, or birth weight < 10th percentile) (Chi 2011a; Chi 2013; Mahé 2007; Mygind 2002). When we ignored the potency of corticosteroids, maternal exposure to topical corticosteroids was not associated with low birth weight or foetal growth restriction (RR 1.08, 95% CI 0.86 to 1.36, 4 studies, n = 59,419; Analysis 1.11). We rated the quality of evidence as very low, given that the default level of the quality of the evidence for observational studies is low, and we downgraded the evidence one further level due to imprecision in these results.
1.11. Analysis.
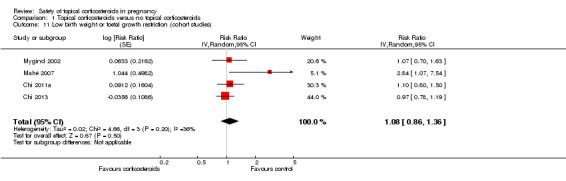
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 11 Low birth weight or foetal growth restriction (cohort studies).
Preterm delivery
A total of 4 cohort studies assessed preterm delivery (Chi 2011a; Chi 2013; Mahé 2007; Mygind 2002), and none found significant differences in the risk for preterm delivery between the exposed and unexposed women (pooled RR 0.93, 95% CI 0.81 to 1.08, 4 studies, n = 59,419; Analysis 1.12). The quality of the evidence was assessed as low.
1.12. Analysis.
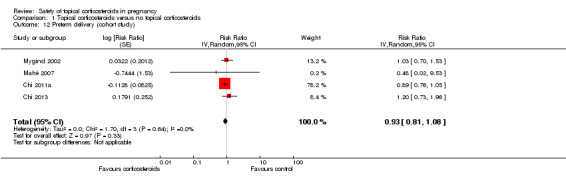
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 12 Preterm delivery (cohort study).
Secondary outcomes
Foetal death
A total of four cohort studies assessed foetal death (Chi 2011a; Chi 2013; Mahé 2007; Mygind 2002), but none found any increased risk among women who received topical corticosteroids during pregnancy (pooled RR 1.02, 95% CI 0.60 to 1.73, 4 studies, n = 63,885; Analysis 1.13). The quality of the evidence was assessed as very low, given that the default level of quality for observational studies is low and we downgraded one further level due to inconsistency in the results (I2 = 60%). The direction of effects also varies, and imprecision is present in that the confidence intervals are very wide.
1.13. Analysis.
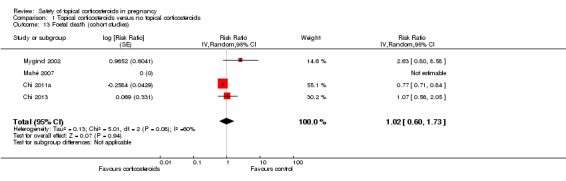
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 13 Foetal death (cohort studies).
Mild congenital abnormality
As stated in the primary outcomes for the children, due to the lack of studies reporting major and minor congenital abnormalities separately, we grouped the two outcomes together and reported as 'congenital abnormality' in the primary outcomes.
Low Apgar score
Only two cohort studies provided data relevant to this outcome. Mahé 2007 found no children with a low Apgar score from mothers who had used very potent topical corticosteroids or from those who did not. Chi 2013 found no significant differences in low Apgar score between women who received and did not receive topical corticosteroids during pregnancy (RR 0.84, 95% CI 0.54 to 1.31, 1 study, n = 9220; Analysis 1.14). We rated the quality of the evidence as low.
1.14. Analysis.
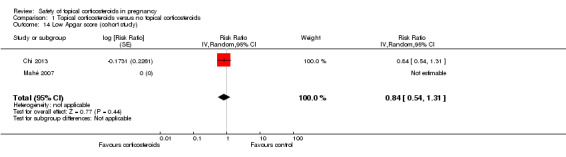
Comparison 1 Topical corticosteroids versus no topical corticosteroids, Outcome 14 Low Apgar score (cohort study).
Stratified analysis by corticosteroid potency
Primary outcomes (in children)
Congenital abnormality
The stratified analysis of the Mygind 2002 and Mahé 2007 cohort studies (Analysis 2.1) showed that when compared with women who did not receive topical corticosteroids, there were no significant differences in the risk for congenital abnormality in those who received mild or moderate topical corticosteroids (adjusted RR 0.93, 95% CI 0.23 to 3.80, n > 9263 (the Mygind 2002 study did not report the respective number of women who received mild or moderate and potent or very potent topical corticosteroids)) and those who received potent or very potent topical corticosteroids (RR 0.56, 95% CI 0.14 to 2.28, n > 9342). The quality of the evidence was rated as low. We found no significant differences for tests between the subgroups (P = 0.62).
2.1. Analysis.
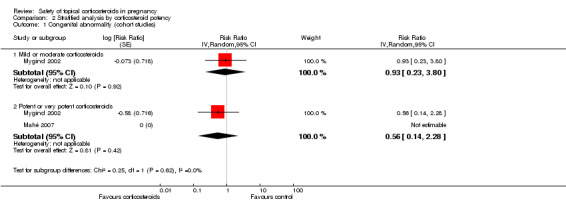
Comparison 2 Stratified analysis by corticosteroid potency, Outcome 1 Congenital abnormality (cohort studies).
A stratified analysis of cohort studies (Analysis 2.2) found no associations between orofacial cleft and maternal exposure to any potency of topical corticosteroids in the first 12 gestational weeks. There were no significant differences for tests between the subgroups (P = 0.49). For the outcome of orofacial cleft, we rated the quality of the evidence as low.
2.2. Analysis.
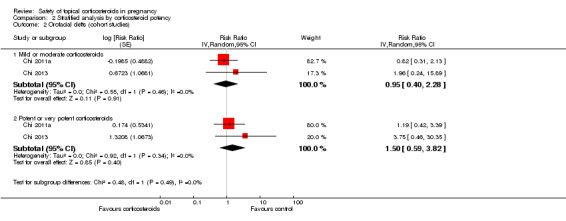
Comparison 2 Stratified analysis by corticosteroid potency, Outcome 2 Orofacial clefts (cohort studies).
Birth weight
The stratified analysis of cohort studies found no significantly increased risk for low birth weight in women who received mild or moderate topical corticosteroids when compared with those who did not receive topical corticosteroids (pooled RR 0.90, 95% CI 0.74 to 1.09, 3 studies, n > 55,713 (the Mygind 2002 study did not report the respective number of women who received mild or moderate and potent or very potent topical corticosteroids); Analysis 2.3). For this outcome we rated the quality of the evidence as low. However, there were significant differences between the subgroups (P = 0.04).
2.3. Analysis.
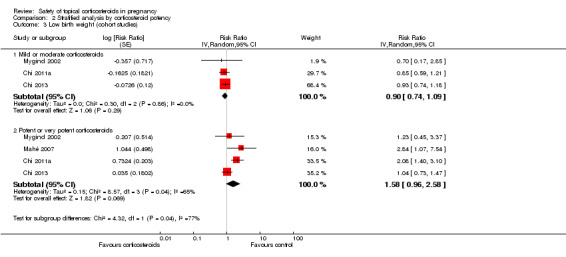
Comparison 2 Stratified analysis by corticosteroid potency, Outcome 3 Low birth weight (cohort studies).
We show the stratified analysis for potent to very potent topical corticosteroids in Analysis 2.3. Although the meta‐analysis based on study‐level data did not reach statistical significance (pooled RR 1.58, 95% CI 0.96 to 2.58, 4 studies, n > 47,651 (study subject number not fully reported in the Mygind 2002 study)), the results from individual studies indicated an increased risk of low birth weight in women who received potent or very potent topical corticosteroids. Mygind 2002 did not find an increased risk for low birth weight in women who received potent or very potent topical corticosteroids (adjusted RR 1.23, 95% CI 0.45 to 3.37, n > 9263), but the original researchers observed a trend indicating a dose‐response relationship between low birth weight and topical corticosteroids. Mahé 2007 and Chi 2011a demonstrated a significantly increased risk for low birth weight among women who used potent or very potent topical corticosteroids during pregnancy (RR 2.84, 95% CI 1.07 to 7.54, n = 79) and (RR 2.08, 95% CI 1.40 to 3.10, n = 30,372), respectively. Chi 2011a reported a 'number needed to treat for an additional harmful outcome (NNTH)' of 168. When not considering the quantity of corticosteroids, Chi 2013 did not identify a significantly increased risk for low birth weight among women who used potent or very potent topical corticosteroids during pregnancy (adjusted RR 1.04, 95% CI 0.73 to 1.47). However, an exploratory analysis reported in the Chi 2013 study found an increased risk for those who received a cumulative dose of more than 300 g of potent or very potent topical corticosteroids during the entire pregnancy (adjusted RR 7.74, 95% CI 1.49 to 40.11, n = 7937).
Preterm delivery
The stratified analysis of cohort studies showed that when compared with women who did not receive topical corticosteroids, there were no significant differences in the risk for preterm delivery in those who received mild or moderate versus potent or very potent topical corticosteroids (adjusted RR being 0.88, 95% CI 0.75 to 1.03, n > 55,713 (the Mygind 2002 study did not report the respective number of women who received mild or moderate and potent or very potent topical corticosteroids) and RR 1.05, 95% CI 0.85 to 1.31, n > 47,651, respectively) (Analysis 2.4). We rated the quality of the evidence as low. There were no significant differences between the subgroups (P = 0.19).
2.4. Analysis.
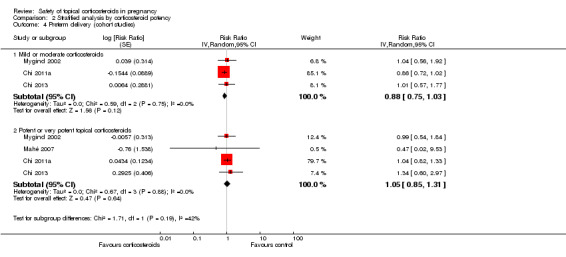
Comparison 2 Stratified analysis by corticosteroid potency, Outcome 4 Preterm delivery (cohort studies).
Secondary outcomes
Foetal death
The stratified analysis of the cohort studies according to corticosteroid potency (Analysis 2.5) found no increased risk of foetal death and a seemingly protective effect for mild to moderate topical corticosteroids on foetal death (pooled RR 0.70, 95% CI 0.64 to 0.77, 2 studies, n = 48,749; low quality evidence; Chi 2011a; Chi 2013) and for potent to very potent topical corticosteroids (pooled RR 1.14, 95% CI 0.69 to 1.88, 3 studies, n = 37,086; low quality evidence; Chi 2011a; Chi 2013; Mahé 2007). There were no significant differences between the subgroups (P = 0.06).
2.5. Analysis.
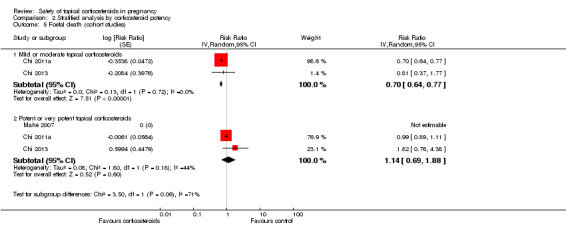
Comparison 2 Stratified analysis by corticosteroid potency, Outcome 5 Foetal death (cohort studies).
Low Apgar score
The stratified analysis of the cohort studies according to corticosteroid potency (Analysis 2.6) did not find an increase in low Apgar score in women who received mild or moderate topical corticosteroids (RR 0.73, 95% CI 0.45 to 1.20; 1 study n = 8756; low quality evidence; Chi 2013). This was also the case in those who received potent or very potent topical corticosteroids during pregnancy (RR 1.03, 95% CI 0.52 to 2.03, 2 studies, n = 7514; low quality evidence; Chi 2013; Mahé 2007). There were no differences between the subgroups (P = 0.43).
2.6. Analysis.
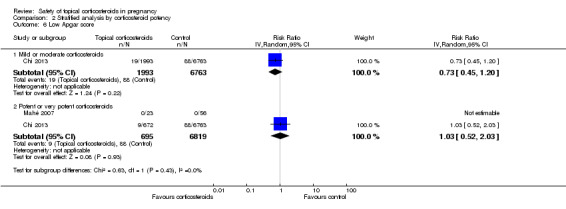
Comparison 2 Stratified analysis by corticosteroid potency, Outcome 6 Low Apgar score.
Sensitivity analysis after excluding poor‐quality studies
When we ran sensitivity analyses after excluding poor quality studies (i.e., Czeizel 1997 and Edwards 2003, which had a high risk of bias due to high non‐response rate), we found no significant associations between maternal exposure to topical corticosteroids and orofacial cleft (OR 0.98, 95% CI 0.53 to 1.82, 6 studies, n = 604,300; Analysis 3.1), its two categories: cleft lip with or without cleft palate (OR 1.20, 95% CI 0.73 to 1.97, 6 studies, n = 602,620; Analysis 3.2), or cleft palate alone (OR 0.84, 95% CI 0.37 to 1.93, 6 studies, n = 601,082; Analysis 3.3).
3.1. Analysis.
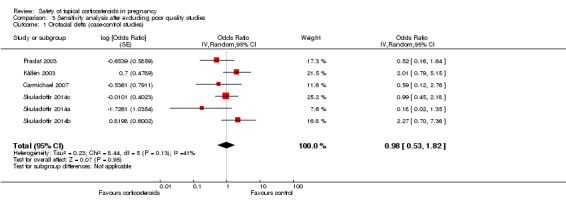
Comparison 3 Sensitivity analysis after excluding poor quality studies, Outcome 1 Orofacial clefts (case‐control studies).
3.2. Analysis.
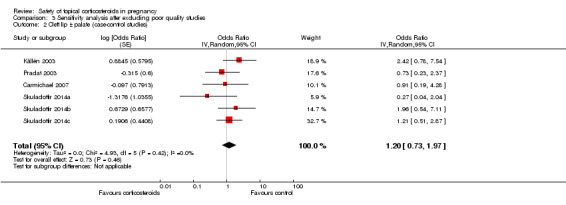
Comparison 3 Sensitivity analysis after excluding poor quality studies, Outcome 2 Cleft lip ± palate (case‐control studies).
3.3. Analysis.
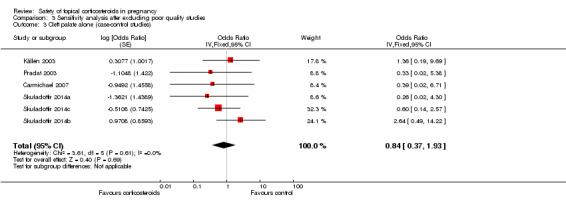
Comparison 3 Sensitivity analysis after excluding poor quality studies, Outcome 3 Cleft palate alone (case‐control studies).
Discussion
Summary of main results
After including seven new studies for this update, the overall quality of evidence from the included observational studies was still low (Table 1; Table 2; Table 3). We downgraded the evidence because we detected wide confidence interval values (imprecision) and clinical and statistical heterogeneity (inconsistency). Lower quality evidence resulted in lower confidence in the estimate of effect for those outcomes. Most of the studies did not find significant associations between maternal use of topical corticosteroids and pregnancy outcomes, including mode of delivery, congenital abnormality (including orofacial cleft), preterm delivery, and foetal death (Carmichael 2007; Carmichael 2009; Chi 2011a; Chi 2013; Czeizel 1997; Källén 2003; Mahé 2007; Mygind 2002; Pradat 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c). Although one small case‐control study, Edwards 2003, identified a significant association between topical corticosteroids and orofacial cleft, the study subjects were from a single hospital, and the statistical power was very low because of the small sample size (48 cases and 58 controls). Furthermore, the response rate for the case and control groups was only 25.3% and 14.2%, respectively. Hviid 2011 compared use of various forms of corticosteroids during pregnancy in those with and without orofacial cleft and found a link between maternal exposure to topical corticosteroids and cleft lip with or without cleft palate. However, the study authors found no dose‐response nor potency‐response relationship and concluded it was a spurious finding resulting from multiple comparisons. There was limited data from two case‐control studies suggesting an association between fewer hypospadias and maternal use of topical corticosteroids, but this did not reach statistical significance (Czeizel 1997; Carmichael 2009).
We were unable to conduct our originally planned subgroups of maternal skin conditions (polymorphic eruption of pregnancy, pemphigoid gestationis, etc.) and maternal ages because the data were unavailable from the investigators of studies included in this review. We elected to conduct a post hoc analysis based on steroid potency, as recent evidence suggests that potent topical corticosteroids are associated with low birth weight. The stratified meta‐analyses according to corticosteroid potency found no significant associations between low birth weight with maternal exposure to topical corticosteroids of any potency. However, the data from individual studies proposed a relationship between low birth weight and the potency and dose of topical corticosteroids as follows: two cohort studies found a significant association between maternal exposure to potent or very potent topical corticosteroids and low birth weight (Chi 2011a; Mahé 2007). In another cohort study, an exploratory analysis found an increased risk of low birth weight when the cumulative dose of potent or very potent topical corticosteroids exceeded 300 g during the entire pregnancy (Chi 2013).
The post hoc stratified analysis by steroid potency (from two studies: Chi 2011a and Chi 2013), found that mild or moderate topical corticosteroids had a seemingly protective effect of on foetal death (RR 0.70, 95% CI 0.64 to 0.77; Analysis 2.5). However, this finding was not supported by a dose‐response relationship.
Overall completeness and applicability of evidence
The body of evidence has substantially increased since our previous review, with the contribution from seven new studies. Due to the restriction of study design, the only outcomes measured in all nine case‐control studies were congenital abnormality, orofacial cleft, and hypospadias (Czeizel 1997; Carmichael 2007; Carmichael 2009; Edwards 2003; Källén 2003; Pradat 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c). Hviid 2011 was a cohort study but only assessed orofacial cleft. Only in the other four cohort studies were other pregnancy outcomes (such as birth weight and preterm delivery) investigated (Chi 2011a; Chi 2013; Mahé 2007; Mygind 2002).
When assessing the teratogenic risk of topical corticosteroids, the definition of 'case' varied substantially among the case‐control studies. The Hungarian study, Czeizel 1997, defined cases as children having an isolated congenital abnormality or unidentified multiple congenital abnormalities and excluded those with some mild congenital abnormalities, minor anomalies, or congenital abnormality syndromes of known origin. We performed stratified analyses on fourteen congenital abnormality groups. The other seven case‐control studies restricted cases to children having an orofacial cleft (Carmichael 2007; Edwards 2003; Källén 2003; Pradat 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c).
A total of 10 observational studies analysed cleft lip with or without cleft palate and cleft palate alone separately (Carmichael 2007; Chi 2011a; Chi 2013; Czeizel 1997; Edwards 2003; Hviid 2011; Pradat 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c).
Orofacial cleft is classified into syndromic and non‐syndromic clefts according to whether associated congenital abnormalities are present or not. Syndromic orofacial cleft occurs due to idiopathic, inherited, or chromosomal defects and presents with associated congenital abnormalities. Non‐syndromic cleft is believed to be the result of multifactorial environmental and genetic factors, with a low risk of familial occurrence (Edwards 2003). It is more likely to be subject to the influence of environmental insults, so many teratologic studies only included cases of non‐syndromic cleft. Edwards 2003 included only children with non‐syndromic orofacial cleft. Carmichael 2007, Hviid 2011, and Skuladottir 2014a only included non‐syndromic orofacial cleft and further excluded those with a recognised or strongly suspected single‐gene disorder or chromosomal abnormality. On the other hand, Källén 2003 and Skuladottir 2014c did not exclude children with a syndrome diagnosis. The original researcher of the Källén 2003 study argued that those with a syndrome diagnosis only occupied 5% in his series, that the quality of diagnosing clinical syndromes was occasionally doubtful, and that drug exposure may modify the phenotypic expression. Pradat 2003 also included in the case group children with multiple congenital abnormalities, syndromes, and even known causes like chromosomal defect.
In a clinical setting, it is sometimes difficult to distinguish non‐syndromic cleft from syndromic cleft. Orofacial cleft without associated malformations may be merely an incomplete manifestation of the syndromic cleft (Wong 2004). The difficulty in precisely identifying cases that are vulnerable to environmental influences can compromise the accuracy of risk assessment. An analysis on all clefts followed by a sensitivity test excluding cases of syndromic cleft, as performed in the Carmichael 2007 and Skuladottir 2014b studies, can resolve this problem. The results of the sensitivity test excluding syndromic cleft were very similar to the results without the exclusion.
The selection and definition of controls also varied among the case‐control studies. In the Czeizel 1997 study, the controls were healthy newborns selected from the national birth register matched for gender, birth week, and district of parents' residence. Källén 2003 study healthy controls from a national birth register. Carmichael 2007, Carmichael 2009, Hviid 2011, Skuladottir 2014a, Skuladottir 2014b, and Skuladottir 2014c randomly selected healthy controls from birth registries, birth certificates, or from birth hospitals. Edwards 2003 also used healthy controls matched by birth date, but the source was limited to the same hospital. On the other hand, Pradat 2003 merely enrolled malformed infants with a history of maternal first trimester drug exposure and used 'sick controls', i.e., infants with other congenital anomalies from the same database, for comparison. The influence on the direction of effects on risk assessment is unclear. The external validity is highly dependent on the robustness of the register.
The selection of study subjects in the cohort studies also differed. Mygind 2002 restricted their study subjects to primiparous women. Mahé 2007 only included women who used potent topical corticosteroids for skin lightening during pregnancy and excluded women receiving topical corticosteroids for a medical reason. These restrictions limit the external validity of the two studies. On the other hand, Chi 2011a and Chi 2013 included all pregnant women aged 15 to 44 years except for those with multifoetal pregnancy or pregnancy following assisted reproduction.
Quality of the evidence
We did not find any randomised controlled trials (RCTs) relevant to this review. The most likely reason for the absence of relevant RCTs stems from ethical concerns that result in the exclusion of pregnant women from clinical trials unless the objective is to investigate a pregnancy‐related condition (Meadows 2001). We only identified 14 relevant observational studies, including 5 cohort and 9 case‐control studies, with a total of 1,601,515 study subjects (Carmichael 2007; Carmichael 2009; Chi 2011a; Chi 2013; Czeizel 1997; Edwards 2003; Hviid 2011; Källén 2003; Pradat 2003; Mahé 2007; Mygind 2002; Skuladottir 2014a;Skuladottir 2014b; Skuladottir 2014c).
We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system to evaluate the quality of the evidence for outcomes reported in this review (Table 1; Table 2; Table 3). Because only observational studies were available, the body of evidence was assigned by default as 'low quality' according to the GRADE system (Higgins 2011). Regarding the outcome 'foetal death' in Table 1, we further downgraded the quality of evidence due to inconsistency. For the outcome of low birth weight, we also downgraded further to 'very low quality' on the basis of imprecision. The confidence intervals of the risk ratio (0.86 to 1.36) were quite narrow; however, they were wide for each study, and while the variations in the absolute data may reflect the variations in the baseline risk of different studies, we are still very uncertain about the effect estimate.
In the Mygind 2002 study, the records of congenital abnormality in the register were not entirely accurate. Among the five registered congenital abnormality cases in the exposed group, a review of their hospital records revealed that two babies actually did not have any congenital abnormalities. All of the remaining three correctly recorded cases belonged to malformations of the foot (club foot, flat foot, and metatarsus varus) instead of orofacial cleft as suggested by animal studies.
The measurement of exposure varied considerably among the included studies. Six case‐control studies retrospectively measured exposure (Carmichael 2007; Carmichael 2009; Edwards 2003; Pradat 2003; Skuladottir 2014a; Skuladottir 2014b). Recall bias may be introduced especially when the outcomes were already known (Altman 1991). The information of exposure may be more detailed, and the timing of exposure may be misleading in the case group since the mothers tend to attribute the adverse outcome to an explainable cause. On the other hand, the exposure in the controls is often underreported. Furthermore, as the length of time after exposure increases, detailed memory of exposure may be compromised, amplifying the risk of recall bias.
The Källén 2003 and Skuladottir 2014c studies prospectively collected data of first‐trimester drug use at antenatal visits. Prospective measurement can reduce recall bias, and well‐trained research staff can make vigorous attempts to acquire the details of women's adherence, dosage, potency, application, and duration of topical corticosteroids use from co‐operative study subjects. The Czeizel 1997 study used a variety of prospective and retrospective information sources, encompassing a prenatal log book, questionnaire, and interview, to assess drug exposure. The use of regional district nurse visits increased the response rate in the case group by 10%. This mechanism might have incurred further differences between the two groups. The original researcher did not perform a subgroup analysis; thus, the direction of its influence on pregnancy outcomes is not clear.
Chi 2011a, Chi 2013, Hviid 2011, and Mygind 2002 used linkage to the prescription database to provide the exposure data. Prescription databases have the advantage of providing details of exposure, such as the constituents and dosage of medicines, and the data are more accurate than study subjects' memory. However, prescription databases cannot offer data on study subjects' adherence and whether study subjects used over‐the‐counter topical corticosteroids, topical corticosteroids from previous prescriptions, or even prescriptions from their relatives or friends.
The most crucial period for foetal organogenesis is from the 4th to 10th week of gestation. Teratogen exposure during this period may cause major malformations. Foetal maturation and functional development continue after the 11th week, and certain organs remain vulnerable. Teratogen exposure may cause functional defects and minor malformations (Cunningham 2005). The critical period for the fusion of the lip and palate (from the primary and second palates, respectively) is from the 5th to 12th gestational week (Arosarena 2007). The examined timing of exposure differed among the studies on congenital abnormality or orofacial cleft. Most studies examined exposure in the first trimester of gestation (Edwards 2003; Källén 2003; Pradat 2003) or the first three months of gestation (Skuladottir 2014b). Mygind 2002 examined exposure from 30 days before conception until the end of the first trimester of gestation. Chi 2011a considered the possibility of prolonged use after obtaining topical corticosteroids and examined exposure from 85 days before conception until the 12th gestational week in the primary analysis and then conducted a sensitivity analysis by examining exposure from the last menstrual period to the 12th gestational week. Chi 2013 examined exposure from the last menstrual period to the 12th gestational week. Carmichael 2007 and Skuladottir 2014a examined exposure from 4 weeks before conception to 12 weeks after conception. Similarly, the Czeizel 1997 study only regarded exposure in the second and third months of gestation as crucial. Skuladottir 2014c assessed drug exposure from six months before pregnancy to the first 15 weeks of pregnancy.
Controlling for potential confounders is essential for observational studies, and most of our included studies performed this (Carmichael 2007; Carmichael 2009; Chi 2011a; Chi 2013; Czeizel 1997; Edwards 2003; Hviid 2011; Källén 2003; Mygind 2002; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c). The most common confounder controlled was smoking (in nine studies), followed by maternal age (in eight studies) and birth order (in four studies). Pradat 2003 controlled for no potential confounders but used the Mantel‐Haenszel method to adjust for the register that provided the cases. Mahé 2007 did not control for potential confounders in their analysis, although there were no significant differences between women who used very potent corticosteroids and those who did not in terms of potential confounders such as socioeconomic and education levels, age, and parity.
Maternal conditions indicated for topical corticosteroids may have a direct impact on pregnancy outcomes. For example, an increased risk for foetal growth restriction occurs in women with pemphigoid gestationis, whereas adverse pregnancy outcomes, including preterm delivery, intrapartal foetal distress, and stillbirths, happen more frequently in women with intrahepatic cholestasis of pregnancy (Ambros‐Rudolph 2006). On the other hand, there is no evidence indicating that eczema affects pregnancy outcomes (Weatherhead 2007). Only the Chi 2011a study controlled for the confounding by indication. Czeizel 1997 prescribed corticosteroid ointments for allergic dermatoses, such as urticaria and eczema, in both the case and control groups. The indications in all nine exposed cases were dermatitis or eczema in the Australian study (Edwards 2003). All the exposed women in the Senegalese study used topical corticosteroids for skin‐lightening (Mahé 2007). Thus, the maternal indications for topical corticosteroids in these studies were unlikely to affect pregnancy outcomes. Meanwhile, the indications for topical corticosteroids in pregnancy were unavailable in the other studies (Carmichael 2007; Chi 2013; Hviid 2011; Källén 2003; Mygind 2002; Pradat 2003; Skuladottir 2014a; Skuladottir 2014b; Skuladottir 2014c). In Carmichael 2007, a stratified analysis by indication was not undertaken because of a lack of relevant records in most women.
Concurrent use of other medications or nutritional supplements in pregnancy may also affect pregnancy outcomes and thus should be considered. For example, isotretinoin is a potent teratogen, while folic acid reduces the risk of neural tube defects and other congenital abnormalities (Briggs 2008). Four studies considered the concurrent use of other medications (Carmichael 2007; Chi 2011a; Chi 2013; Czeizel 1997; Edwards 2003; Källén 2003). Two cohort studies (Chi 2011a; Chi 2013) adjusted for maternal exposure to the US Food and Drug Administration (FDA) pregnancy risk category D or X medicines in analyses. Czeizel 1997 did not find any significant difference in the frequency of concurrent use of 10 medications. Two case‐control studies compared maternal intake of folic acid in early pregnancy and did not find significant differences (Carmichael 2007; Edwards 2003). Another case‐control study, Källén 2003, compared the frequency of use of 30 medications in the first trimester of gestation and also identified a significant association of orofacial cleft with naproxen. The Hviid 2011 study compared maternal exposure with five specified categories of medicines and found no differences between the exposed and control groups. By contrast, the other five studies did not consider concurrent use of other medications, and thus did not control for confounding (Mahé 2007; Mygind 2002; Pradat 2003; Skuladottir 2014b; Skuladottir 2014c).
Potential biases in the review process
We did not deliberately examine all trials on topical corticosteroids to find out if they contained any pregnant women. However, we contacted 11 pharmaceutical companies that have introduced an original topical corticosteroid product to provide relevant studies and did not obtain any relevant data. Furthermore, pregnant women are routinely excluded from clinical trials unless the objective is to assess a drug's efficacy on a pregnancy‐related condition. Thus, it is unlikely that we have missed relevant studies for this review.
Given the high degree of bias found in non‐randomised studies, which are the sole source of evidence, we expected some degree of statistical heterogeneity and only applied meta‐analysis techniques as appropriate. That is, we used the I2 statistic to examine the statistical heterogeneity, and when levels of statistical heterogeneity were high, we elected to pool the data only where there was reasonable clinical homogeneity to provide further evidence; however, we accept that this decision may limit the reliability and applicability of the findings.
Agreements and disagreements with other studies or reviews
We did not find specific reviews on the safety of topical corticosteroids in pregnancy but found three narrative reviews on dermatological treatments for pregnant women (Hale 2002; Leachman 2006; Zip 2006). None of these were systematic reviews, and none included any of the seven relevant studies identified in our previous review. The three reviews only quoted a case report of foetal growth restriction following maternal use of 40 mg/d of triamcinolone cream (Katz 1990), and they pointed to topical corticosteroids as having the FDA pregnancy risk class C. Thus, the data included in previous reviews were incomplete, and their conclusions were accordingly limited. Another European evidence‐based guideline on topical corticosteroids in pregnancy, Chi 2011b, was based on data from the previous version of this review and two studies included in this updated review (Chi 2009; Chi 2011a; Hviid 2011).
Although the meta‐analyses in this review did not reach statistical significance for the outcome of low birth weight, the results from individual studies indicated a potential for increased risk of low birth weight in women who received potent or very potent topical corticosteroids. In this review, we did not address dose‐response relationships, but other studies have explored whether there may be a dose‐response relationship between low birth weight and topical corticosteroids. Mygind 2002 observed a trend indicating a dose‐response relationship between low birth weight and topical corticosteroids. Chi 2011a found a significant dose‐response relationship between the quantity of potent or very potent topical corticosteroids and low birth weight (P = 0.025). Chi 2013 conducted an exploratory analysis on the associations of the cumulative dosage (in the entire pregnancy) of potent or very potent topical corticosteroids with low birth weight. That study found an increased risk for those who received more than 300 g of potent or very potent topical corticosteroids during pregnancy (adjusted RR 7.74, 95% CI 1.49 to 40.11, n = 7937). The available evidence for maternal use of potent or very potent topical corticosteroids may therefore suggest that such usage, especially in large quantities, could be associated with low birth weight, but more studies are needed to confirm this.
The stratified analysis in this review of foetal death by steroid potency found a possible protective effect of mild to moderate topical corticosteroids. However, this finding was not supported by a dose‐response relationship in Chi 2011a (for both mild to moderate and potent to very potent topical corticosteroids). It should not be inferred that mild to moderate topical corticosteroids may prevent foetal death in pregnant women with or without skin disorders. Further explorations of whether a dose‐response relationship exists were outside the scope of this review, so it is difficult to draw any firm conclusions, but further investigation may be warranted to determine whether nuances such as steroid potency and cumulative dose have differential effects on outcomes.
Authors' conclusions
Implications for practice.
The current evidence shows a small risk of low birth weight in pregnant women who receive potent or very potent topical corticosteroids, especially in large quantities. On the other hand, maternal use of mild or moderate topical corticosteroids is not related to low birth weight. The available evidence does not support a causal relationship between maternal use of topical corticosteroids (of any potency) and other pregnancy outcomes, including mode of delivery, congenital abnormality, preterm delivery, foetal death, and low Apgar score.
Implications for research.
Most of the previous studies purely assessed the risk for congenital abnormality or orofacial cleft. Only four studies had data on other pregnancy outcomes. More data on outcomes, such as preterm birth, foetal death, mode of delivery, birth weight, low Apgar score, or a selected core set of outcomes, as suggested by Devane 2007, should be collected and analysed in future research by adopting a cohort study design.
In this updated version of the review, we did not find a significantly increased risk of low birth weight with maternal exposure to potent or very potent topical corticosteroids in study‐level meta‐analysis. However, there is a probable association between low birth weight, steroid potency and potentially the cumulative dosage of topical corticosteroids throughout the pregnancy, and this warrants further investigation. The finding of a possible protective effect of mild or moderate topical corticosteroids on foetal death could also be examined.
Therefore, the effects of potency and dose of topical corticosteroids as well as the duration, site, and extent of application should be assessed in future research. The confounding from maternal indication for topical corticosteroids in pregnancy outcomes should be considered in order to mitigate its effect on the results.
What's new
| Date | Event | Description |
|---|---|---|
| 20 October 2015 | New search has been performed | We included 7 new observational studies in this update |
| 20 October 2015 | New citation required but conclusions have not changed | There has been no significant alteration to the conclusions of the previous version of the review |
Acknowledgements
We appreciate the Cochrane Pregnancy and Childbirth Group for their assistance in searching.
The Cochrane Skin Group editorial base wishes to thank Sue Jessop, who was the Cochrane Dermatology Editor for this review; Ben Carter, who was the Statistical Editor; Esther van Zuuren, who was Methods Editor; the clinical referees, Ashraf Nabhan and Alan Arbuckle; and the consumer referee, Anne Lyddiatt.
Appendices
Appendix 1. Skin Group Specialised Register strategy
((“Adrenal Cortex Hormone*” or “topical corticosteroid*" or “topical glucocorticoid*” or “topical steroid*” or “topical corticoid*” or Hydrocortisone or cortisol or “Fluocinolone Acetonide” or “alclometasone dipropionate” or Betamethasone or clobetasone or flurandrenolone or fludroxycortide or Fluocortolone or beclometasone or Fluprednidene or Fluocinonide or diflucortolone or fluticasone or mometasone or triamcinolone or halcinonide or clobetasol or diflorasone or amcinonide or desoximetasone or desonide or cortisone or methylprednisolone or prednisolone or budesonide or fluclorolone or flumethasone or prednicarbate or halobetasol or ulobetasol or “clocortolone pivalate” or fluocortin or halometasone) and (pregnan* or abnormalit* or obstetric* or labor or labour or fetal or foetal or fetus or foetus or birth or congenital or complication* or cleft or orofacial or teratogen* or toxic* or “birth weight” or birthweight or “body height” or “body length” or “crown rump length” or “intrauterine growth retardation” or “embryonic development” or “apgar score*” or prematurity or “premature birth” or “preterm delivery”)):ti,ab
Appendix 2. Pregnancy and Childbirth Group Specialised Register strategy
The Cochrane Pregnancy and Childbirth Group's Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
1. monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL); 2. weekly searches of MEDLINE; 3. weekly searches of Embase; 4. handsearches of 30 journals and the proceedings of major conferences; 5. weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the 'Specialized Register' section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Appendix 3. CENTRAL (Cochrane Library) strategy
#1 (corticosteroid* or steroid* or *cortisone or glucocorticoid* or *methasone or *metasone or *cinonide or *nisolone or *cinolone or *betasone or *betasol or corticoid or flurandrenolone or *cortolone or fluprednidene or fluticasone or fluclorolone or diflorasone or *desonide or fluprednidene or prednicarbate or fluocinolone or triamcinolone or halcinonide or ulobetasol or fluocortin):ti,ab and (topical or absorption or skin):ti,ab #2 MeSH descriptor: [Steroids] explode all trees #3 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees #4 (pregnan* or obstetric* or labor or labour or fetal or foetal or fetus or foetus or birth or congenital or cleft or teratogen* or toxic* or "birth weight" or birthweight or "body height" or "body length" or "crown rump length" or "intrauterine growth retardation" or "embryonic development" or "apgar score" or prematurity or "premature birth" or "preterm delivery"):ti,ab #5 MeSH descriptor: [Pregnancy] explode all trees #6 MeSH descriptor: [Abnormalities, Drug‐Induced] explode all trees #7 MeSH descriptor: [Teratogens] explode all trees #8 MeSH descriptor: [Pregnancy Complications] explode all trees #9 MeSH descriptor: [Fetal Growth Retardation] explode all trees #10 MeSH descriptor: [Cleft Lip] explode all trees #11 MeSH descriptor: [Cleft Palate] explode all trees #12 MeSH descriptor: [Congenital Abnormalities] explode all trees #13 MeSH descriptor: [Infant, Premature] explode all trees #14 (topical or absorption or skin):ti,ab #15 #2 and #14 #16 #3 and #14 #17 #1 or #15 or #16 #18 {or #4‐#13} #19 #17 and #18
Appendix 4. MEDLINE (Ovid) strategy
1. exp Cohort Studies/ 2. cohort$.tw. 3. controlled clinical trial.pt. 4. Epidemiologic Methods/ 5. limit 4 to yr=1966‐1989 6. exp case‐control studies/ 7. (case$ and control$).tw. 8. or/1‐3,5‐7 9. randomized controlled trial.pt. 10. controlled clinical trial.pt. 11. randomized.ab. 12. placebo.ab. 13. clinical trials as topic.sh. 14. randomly.ab. 15. trial.ti. 16. 9 or 10 or 11 or 12 or 13 or 14 or 15 17. exp animals/ not humans.sh. 18. 16 not 17 19. 8 or 18 20. exp Adrenal Cortex Hormones/ and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 21. exp Glucocorticoids/ and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 22. (topical corticosteroid$ or topical glucocorticoid$ or topical steroid$ or topical corticoid$).ti,ab. 23. exp Hydrocortisone/ and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 24. (hydrocortisone or cortisol).ti,ab. and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 25. hydrocortisone butyrate.ti,ab. 26. hydrocortisone valerate.ti,ab. 27. hydrocortisone aceponate.ti,ab. 28. exp Fluocinolone Acetonide/ and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 29. fluocinolone acetonide.ti,ab. and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 30. alclometasone dipropionate.ti,ab. 31. (exp Betamethasone/ or betamethasone.ti,ab.) and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 32. exp Betamethasone 17‐Valerate/ or "betamethasone adj2 valerate".ti,ab. 33. betamethasone dipropionate.ti,ab. 34. clobetasone.ti,ab. 35. exp Flurandrenolone/ or (flurandrenolone or fludroxycortide).ti,ab. 36. (exp Fluocortolone/ or fluocortolone.ti,ab.) and (exp Administration, Topical/ or exp Ointments/) 37. (exp Beclomethasone/ or beclometasone.ti,ab.) and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 38. fluprednidene.ti,ab. 39. exp Fluocinonide/ or fluocinonide.ti,ab. 40. exp Diflucortolone/ or diflucortolone.ti,ab. 41. fluticasone.ti,ab. and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 42. mometasone.ti,ab. and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 43. (exp Triamcinolone/ or exp Triamcinolone Acetonide/ or triamcinolone.ti,ab.) and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 44. exp Halcinonide/ or halcinonide.ti,ab. 45. exp Clobetasol/ or clobetasol.ti,ab. 46. diflorasone.ti,ab. 47. amcinonide.ti,ab. 48. exp Desoximetasone/ or desoximetasone.ti,ab. 49. exp Desonide/ or desonide.ti,ab. 50. (exp Cortisone/ or cortisone.ti,ab.) and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 51. (exp Methylprednisolone/ or methylprednisolone.ti,ab.) and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 52. methylprednisolone aceponate.ti,ab. 53. (exp Prednisolone/ or prednisolone.ti,ab.) and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 54. (exp Budesonide/ or budesonide.ti,ab.) and (exp Ointments/ or Dermatologic Agents/) 55. fluclorolone.ti,ab. 56. (exp Flumethasone/ or flumethasone.ti,ab.) and (exp Administration, Topical/ or exp Ointments/ or Dermatologic Agents/) 57. flumethasone pivalate.ti,ab. 58. prednicarbate.ti,ab. 59. (halobetasol or ulobetasol).ti,ab. 60. clocortolone pivalate.ti,ab. 61. fluocortin.ti,ab. 62. halometasone.ti,ab. 63. or/20‐62 64. ae.fs. 65. to.fs. 66. co.fs. 67. po.fs. 68. or/64‐67 69. exp Pregnancy/ 70. pregnan$4.ti,ab. 71. 69 or 70 72. exp Drug Toxicity/ or toxic$5.ti,ab. 73. exp Abnormalities, Drug‐Induced/ or exp Abnormalities/ 74. 72 or 73 75. 71 and 74 76. exp Teratogens/ or teratogen$.ti,ab. 77. exp Obstetric Labor Complications/ or exp Pregnancy Complications/ or exp Pregnancy Outcome/ 78. obstetric$ outcome$.ti,ab. 79. ((obstetric$ or labour or labor) adj2 complication$).ti,ab. 80. ((fetal adj outcome$) or (foetal adj outcome$)).ti,ab. 81. exp Birth Weight/ or (birth weight or birthweight).ti,ab. 82. (exp Body Height/ or body height.ti,ab. or body length.ti,ab.) and Infant/ 83. exp Crown‐Rump Length/ or crown rump length.ti,ab. 84. exp Fetal Growth Retardation/ or intrauterine growth retardation.ti,ab. 85. exp Fetal Development/ 86. exp Embryonic Development/ 87. exp Fetal Diseases/ 88. exp Apgar Score/ or Apgar score$.ti,ab. 89. exp Cleft Palate/ or exp Cleft Lip/ or (cleft palate or cleft lip or oral cleft or orofacial cleft).ti,ab. 90. (congenital anomal$ or congenital malformation$ or inborn error$ or congenital abnormalit$).ti,ab. 91. exp Congenital Abnormalities/ or exp Genetic Diseases, Inborn/ 92. ((congenital or hereditary or neonatal) adj2 (disease$ or abnormalit$)).ti,ab. 93. exp Heart Defects, Congenital/ or (congenital heart disease$ or congenital heart defect$).ti,ab. 94. exp Obstetric Labor, Premature/ or exp Premature Birth/ or preterm delivery.ti,ab. 95. exp Infant, Premature/ or prematurity.ti,ab. 96. or/75‐95 97. 63 and 68 98. 19 and 96 and 97
NB Lines 1‐8 are the BMJ Clinical Evidence MEDLINE (Ovid) cohort and case‐control search filter.
Appendix 5. EMBASE (Ovid) strategy
1. crossover procedure.sh. 2. double‐blind procedure.sh. 3. single‐blind procedure.sh. 4. (crossover$ or cross over$).tw. 5. placebo$.tw. 6. (doubl$ adj blind$).tw. 7. allocat$.tw. 8. trial.ti. 9. randomized controlled trial.sh. 10. random$.tw. 11. or/1‐10 12. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 13. human/ or normal human/ 14. 12 and 13 15. 12 not 14 16. 11 not 15 17. exp corticosteroid/ and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 18. exp glucocorticoid/ and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 19. (topical corticosteroid$ or topical glucocorticoid$ or topical steroid$ or topical corticoid$).ti,ab. 20. exp Hydrocortisone/ and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 21. (hydrocortisone or cortisol or hydrocortisone acetate).ti,ab. and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 22. (hydrocortisone butyrate or hydrocortisone valerate or hydrocortisone aceponate).ti,ab. 23. exp hydrocortisone butyrate/ or exp hydrocortisone valerate/ or exp hydrocortisone aceponate/ 24. exp fluocinolone acetonide/ and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 25. fluocinolone acetonide.ti,ab. and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 26. alclometasone dipropionate.ti,ab. or exp alclometasone dipropionate/ 27. (exp betamethasone/ or betamethasone.ti,ab.) and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 28. exp betamethasone dipropionate/ or exp betamethasone valerate/ 29. (betamethasone dipropionate or "betametasone adj2 valerate").ti,ab. 30. clobetasone butyrate/ or exp clobetasone/ or clobetasone.ti,ab. 31. flurandrenolone.ti,ab. or exp fludroxycortide/ or fludroxycortide.ti,ab. 32. (fluocortolone.ti,ab. or exp fluocortolone/) and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 33. (beclomethasone.ti,ab. or exp beclometasone/) and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 34. exp fluprednidene/ or fluprednidene.ti,ab. 35. exp Fluocinonide/ or fluocinonide.ti,ab. 36. exp diflucortolone/ or diflucortolone.ti,ab. 37. (fluticasone.ti,ab. or exp fluticasone/ or exp fluticasone propionate/) and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 38. (mometasone.ti,ab. or exp mometasone furoate/) and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 39. (triamcinolone.ti,ab. or exp triamcinolone/ or exp triamcinolone acetonide/) and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 40. halcinonide.ti,ab. or exp halcinonide/ 41. exp clobetasol propionate/ or clobetasol.ti,ab. or exp clobetasol/ or exp clobetasol butyrate/ 42. exp diflorasone diacetate/ or exp diflorasone/ or diflorasone.ti,ab. 43. amcinonide.ti,ab. or exp amcinonide/ 44. desoximetasone.ti,ab. or exp desoximetasone/ 45. exp Desonide/ or desonide.ti,ab. 46. (cortisone.ti,ab. or exp cortisone acetate/ or exp cortisone/) and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 47. (exp methylprednisolone/ or methylprednisolone.ti,ab. or exp methylprednisolone acetate/) and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 48. methylprednisolone aceponate.ti,ab. or exp methylprednisolone aceponate/ 49. (exp prednisolone/ or prednisolone.ti,ab. or exp prednisolone acetate/) and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 50. (exp Budesonide/ or budesonide.ti,ab.) and (exp ointment/ or dermatological agent/) 51. exp fluclorolone/ or fluclorolone.ti,ab. 52. (flumetasone.ti,ab. or exp flumetasone/ or flumethasone.ti,ab.) and (exp ointment/ or exp topical drug administration/ or exp dermatological agent/) 53. flumetasone pivalate.ti,ab. or exp flumetasone pivalate/ 54. prednicarbate.ti,ab. or exp prednicarbate/ 55. (ulobetasol or halobetasol).ti,ab. 56. clocortolone pivalate.ti,ab. or exp clocortolone pivalate/ 57. exp fluocortin/ or fluocortin.ti,ab. 58. halometasone.ti,ab. or exp halometasone/ 59. or/17‐58 60. (ae or to).fs. 61. 59 and 60 62. exp Pregnancy/ or pregnan$4.ti,ab. 63. exp teratogenic agent/ or teratogen$.ti,ab. 64. (exp Drug Toxicity/ or toxic$5.ti,ab.) and exp Pregnancy/ 65. exp labor complication/ or exp Pregnancy Complication/ or exp Pregnancy Outcome/ 66. obstetric$ outcome$.ti,ab. 67. ((obstetric$ or labour or labor) adj2 complication$).ti,ab. 68. ((fetal adj outcome$) or (foetal adj outcome$)).ti,ab. 69. exp Birth Weight/ or (birth weight or birthweight).ti,ab. 70. exp Body Height/ or body height.ti,ab. or body length.ti,ab. 71. exp Crown‐Rump Length/ or crown‐rump length.ti,ab. 72. exp intrauterine growth retardation/ or intrauterine growth retardation.ti,ab. 73. exp fetus development/ or exp embryo development/ or exp fetus disease/ 74. exp Apgar Score/ or Apgar score$.ti,ab. 75. exp Cleft Palate/ or exp Cleft Lip/ or (cleft palate or cleft lip or oral cleft or orofacial cleft).ti,ab. 76. exp Abnormalities, Drug‐Induced/ or exp Abnormalities/ 77. "Congenital, Hereditary, and Neonatal Diseases and Abnormalities"/ 78. (congenital anomal$ or congenital malformation or inborn error or congenital abnormalit$).ti,ab. 79. exp congenital malformation/ or exp "inborn error of metabolism"/ 80. ((congenital or hereditary or neonatal) adj2 (disease$ or abnormalit$)).ti,ab. 81. exp congenital heart malformation/ or congenital heart disease.ti,ab. 82. exp premature labor/ or preterm delivery.ti,ab. 83. exp prematurity/ or prematurity.ti,ab. 84. or/62‐83 85. 16 and 61 and 84
Appendix 6. LILACS strategy
(pregnan$ or embarazo or baby or bebe or obstetric$ or foetal or congenital or cleft or hendidura or teratogen$ or apgar or prematur$) and (steroid$ or esteroide$ or corticosteroid$ or corticoesteroide$ or corticoid$ or glucocorticoid$)
Appendix 7. ISRCTN registry strategy
Public title: pregnancy topical steroid (0)
Public title: pregnancy topical corticosteroid (0)
Public title: pregnant topical steroid (0)
Public title: pregnant topical corticosteroid (0)
Appendix 8. US National Institutes of Health Ongoing Trials Register strategy
"pregnancy" AND "topical steroid" (0)
"pregnancy" AND "topical corticosteroid" (2)
"pregnant" AND "topical steroid" (1)
"pregnant" AND "topical corticosteroid" (0)
Appendix 9. Australian New Zealand Clinical Trials Registry strategy
pregnancy topical steroid (0)
pregnancy topical corticosteroid (1)
pregnant topical steroid (0)
pregnant topical corticosteroid (0)
Appendix 10. World Health Organization International Clinical Trials Registry platform strategy
pregnancy AND topical steroid (0)
pregnancy AND topical corticosteroid (0)
pregnant AND topical steroid (0)
pregnant AND topical corticosteroid (0)
Appendix 11. EU Clinical Trials Register strategy
pregnancy AND topical steroid (8)
pregnancy AND topical corticosteroid (11)
pregnant AND topical steroid (1)
pregnant AND topical corticosteroid (0)
Data and analyses
Comparison 1. Topical corticosteroids versus no topical corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Assisted or cesarean delivery (cohort study) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Congenital abnormality (cohort study) | 2 | 9512 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.34, 1.96] |
| 3 Congenital abnormality (case‐control studies) | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4 Orofacial clefts (cohort studies) | 2 | Risk Ratio (Random, 95% CI) | 1.12 [0.54, 2.33] | |
| 5 Cleft lip ± palate (cohort studies) | 3 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 6 Cleft palate alone (cohort studies) | 3 | Risk Ratio (Random, 95% CI) | 1.31 [0.82, 2.11] | |
| 7 Orofacial clefts (case‐control studies) | 8 | Odds Ratio (Random, 95% CI) | 1.20 [0.68, 2.13] | |
| 8 Cleft lip ± palate (case‐control studies) | 8 | Odds Ratio (Random, 95% CI) | 1.52 [0.84, 2.75] | |
| 9 Cleft palate alone (case‐control studies) | 8 | Odds Ratio (Fixed, 95% CI) | 1.20 [0.57, 2.54] | |
| 10 Hypospadias (case‐control studies) | 2 | Odds Ratio (Random, 95% CI) | 0.45 [0.19, 1.09] | |
| 11 Low birth weight or foetal growth restriction (cohort studies) | 4 | Risk Ratio (Random, 95% CI) | 1.08 [0.86, 1.36] | |
| 12 Preterm delivery (cohort study) | 4 | Risk Ratio (Random, 95% CI) | 0.93 [0.81, 1.08] | |
| 13 Foetal death (cohort studies) | 4 | Risk Ratio (Random, 95% CI) | 1.02 [0.60, 1.73] | |
| 14 Low Apgar score (cohort study) | 2 | Risk Ratio (Random, 95% CI) | 0.84 [0.54, 1.31] |
Comparison 2. Stratified analysis by corticosteroid potency.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Congenital abnormality (cohort studies) | 2 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Mild or moderate corticosteroids | 1 | Risk Ratio (Random, 95% CI) | 0.93 [0.23, 3.80] | |
| 1.2 Potent or very potent corticosteroids | 2 | Risk Ratio (Random, 95% CI) | 0.56 [0.14, 2.28] | |
| 2 Orofacial clefts (cohort studies) | 2 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Mild or moderate corticosteroids | 2 | Risk Ratio (Random, 95% CI) | 0.95 [0.40, 2.28] | |
| 2.2 Potent or very potent corticosteroids | 2 | Risk Ratio (Random, 95% CI) | 1.50 [0.59, 3.82] | |
| 3 Low birth weight (cohort studies) | 4 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Mild or moderate corticosteroids | 3 | Risk Ratio (Random, 95% CI) | 0.90 [0.74, 1.09] | |
| 3.2 Potent or very potent corticosteroids | 4 | Risk Ratio (Random, 95% CI) | 1.58 [0.96, 2.58] | |
| 4 Preterm delivery (cohort studies) | 4 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Mild or moderate corticosteroids | 3 | Risk Ratio (Random, 95% CI) | 0.88 [0.75, 1.03] | |
| 4.2 Potent or very potent topical corticosteroids | 4 | Risk Ratio (Random, 95% CI) | 1.05 [0.85, 1.31] | |
| 5 Foetal death (cohort studies) | 3 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 Mild or moderate topical corticosteroids | 2 | Risk Ratio (Random, 95% CI) | 0.70 [0.64, 0.77] | |
| 5.2 Potent or very potent topical corticosteroids | 3 | Risk Ratio (Random, 95% CI) | 1.14 [0.69, 1.88] | |
| 6 Low Apgar score | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Mild or moderate corticosteroids | 1 | 8756 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.45, 1.20] |
| 6.2 Potent or very potent corticosteroids | 2 | 7514 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.52, 2.03] |
Comparison 3. Sensitivity analysis after excluding poor quality studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Orofacial clefts (case‐control studies) | 6 | Odds Ratio (Random, 95% CI) | 0.98 [0.53, 1.82] | |
| 2 Cleft lip ± palate (case‐control studies) | 6 | Odds Ratio (Random, 95% CI) | 1.20 [0.73, 1.97] | |
| 3 Cleft palate alone (case‐control studies) | 6 | Odds Ratio (Fixed, 95% CI) | 0.84 [0.37, 1.93] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carmichael 2007.
| Methods | Case‐control | |
| Participants | Inclusion: 1141 infants with cleft lip with or without cleft palate, 628 infants with cleft palate alone Exclusion: recognised or strongly suspected single‐gene disorders or chromosomal abnormalities Controls: 4143 control infants without major congenital malformations randomly selected from birth certificates or birth hospitals |
|
| Interventions | Topical corticosteroids | |
| Outcomes | Cleft lip with or without cleft palate, cleft palate alone | |
| Funding source | Centers for Disease Control and Prevention | |
| Notes | Country: USA Setting: population‐based (National Birth Defects Prevention Study) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Unclear risk | NA |
| Selection of the non‐exposed cohort | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Demonstration that the outcome of interest was not present at start of study | Unclear risk | NA |
| Comparability of cohorts on the basis of the design or analysis | Unclear risk | NA |
| Assessment of outcome | Unclear risk | NA |
| Was the follow‐up long enough for the outcomes to occur? | Unclear risk | NA |
| Adequacy of follow‐up of the cohorts | Unclear risk | NA |
| Was the case definition adequate? | Low risk | Cases of orofacial cleft received an additional review by one clinical geneticist to ensure that standard eligibility criteria were met |
| Representativeness of the cases | Low risk | Idenitifed from birth defect surveillance systems in 8 US states |
| Selection of the controls | Low risk | Controls were randomly selected from birth certificates or birth hospitals |
| Definition of the controls | Low risk | Infants without major congenital malformations |
| Comparability of cases and controls on the basis of the design or analysis | Low risk | Controlled for potential confounders including maternal race/ethnicity, education, intake of folic acid‐containing supplements or smoking during the month before or the first 3 months of pregnancy, and study centre |
| Ascertainment of exposure | Unclear risk | Interview not blinded to case/control status |
| Same method of ascertainment for cases and controls | Low risk | Same method of ascertainment used in both groups |
| Non‐response rate | Unclear risk | NA |
Carmichael 2009.
| Methods | Case‐control | |
| Participants | Inclusion: 1165 cases of second‐ or third‐degree hypospadias, that is, with the urethral opening at the penile shaft, scrotum, or perineum Exclusion: cases described as chordee alone, mild hypospadias (i.e., first‐degree, coronal, or glandular), hypospadias not otherwise specified, epispadias, or ambiguous genitalia without further description; infants with recognised single gene disorders, female karyotypes, or chromosomal abnormalities also excluded Controls: 3000 non‐malformed male controls |
|
| Interventions | Topical corticosteroids | |
| Outcomes | Hypospadias | |
| Funding source | Centers for Disease Control and Prevention | |
| Notes | Country: USA Setting: population‐based (National Birth Defects Prevention Study) from October 1997 to December 2004 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Unclear risk | NA |
| Selection of the non‐exposed cohort | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Demonstration that the outcome of interest was not present at start of study | Unclear risk | NA |
| Comparability of cohorts on the basis of the design or analysis | Unclear risk | NA |
| Assessment of outcome | Unclear risk | NA |
| Was the follow‐up long enough for the outcomes to occur? | Unclear risk | NA |
| Adequacy of follow‐up of the cohorts | Unclear risk | NA |
| Was the case definition adequate? | Low risk | Quote: "Each case received a final review by a single clinical geneticist (R.O.) to ensure that cases from each study center met standard eligibility criteria. This geneticist also classified each case as isolated, if there was no concurrent major anomaly or only a minor anomaly (e.g., sacral/pilonidal dimple), or multiple, if there was at least 1 unrelated accompanying major anomaly and in another organ system" |
| Representativeness of the cases | Low risk | Identified from birth defect surveillance systems in 10 US states |
| Selection of the controls | Low risk | Quote: "Each state randomly selected approximately 100 non‐malformed liveborn controls per study year from birth certificates" |
| Definition of the controls | Low risk | Non‐malformed male infants |
| Comparability of cases and controls on the basis of the design or analysis | Low risk | Controlled for potential confounders including maternal education, race/ethnicity, age, number of previous live births, folic acid‐containing supplement intake, smoking, body mass index, subfertility, and study site |
| Ascertainment of exposure | Unclear risk | Quote: "Maternal interviews were conducted using a standardized, computer‐based telephone questionnaire in English or Spanish, no earlier than 6 weeks and no later than 24 months after the infant's estimated date of delivery" |
| Same method of ascertainment for cases and controls | Unclear risk | Quote: "The mean time from delivery to interview was 13.2 months in the mothers of cases and 8.9 months in the mothers of controls" |
| Non‐response rate | Unclear risk | The non‐response rate in the case group was 23%. The non‐response rate in the mothers of all controls in the congenital abnormality registry was 25%, but the exact rate in the mothers of male‐only controls was unavailable |
Chi 2011a.
| Methods | Retrospective cohort study | |
| Participants | Inclusion: 35,503 pregnant women aged 15‐44 years, having registered with the practice with up‐to‐standard follow‐up for at least 6 months before last menstrual period, having 1 or more prescriptions for topical corticosteroids during the period from 85 days before last menstrual period (LMP) to delivery or foetal death Exclusion: women prescribed oral, injected, inhaled, ophthalmological, or haemorrhoidal corticosteroids during the same period; women with multifoetal pregnancies Controls: 48,630 unexposed women not having prescriptions for any corticosteroid preparations during the period from 85 days before LMP to delivery or foetal death |
|
| Interventions | Topical corticosteroids prescribed during the period from 85 days before LMP to delivery or foetal death. Exposure was defined as beginning at 85 days before LMP because women may use topical corticosteroids for some time after receiving the prescriptions | |
| Outcomes |
|
|
| Funding source | UK Medical Research Council, British Skin Foundation, John Fell Oxford University Press Research Fund, and Chang Gung Memorial Hospital, Chiayi | |
| Notes | Country: UK Setting: population‐based (UK General Practice Research Database) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | Obtained from the UK General Practices Research Database, which has the primary care records of over 3.5 million currently registered patients (5.5% of the UK population); broadly representative of pregnant women in UK population |
| Selection of the non‐exposed cohort | Low risk | Drawn from the same source as the exposed cohort |
| Ascertainment of exposure | Low risk | Quote: "The prescription records were used to identify the timing, potency, and dosage of topical corticosteroids prescribed" |
| Demonstration that the outcome of interest was not present at start of study | Low risk | The outcomes of interest did not happen at the start of the study |
| Comparability of cohorts on the basis of the design or analysis | Low risk | There were non‐clinically significant differences between the exposed and control cohorts in the potential cofounders, which had been adjusted in statistical analyses |
| Assessment of outcome | Low risk | Diagnostic codes used to identify the outcomes in the clinical records |
| Was the follow‐up long enough for the outcomes to occur? | Low risk | The study subjects followed up until delivery or foetal death |
| Adequacy of follow‐up of the cohorts | Low risk | Compared to the usual reported rate of 3‐7%, the incidence of foetal growth restriction of 0.59% in the study was low. "The low number of foetal growth restriction events could lead to loss of statistical power, resulting in underestimation of the true effect and type II error; i.e., a truly significant association is not detected. However, our study has detected a significant association between maternal exposure to potent/very potent topical corticosteroids and foetal growth restriction. We assumed that missed cases would have occurred equally in the exposed and unexposed groups. The way that data are recorded in the GPRD makes this a reasonable assumption" |
| Was the case definition adequate? | Unclear risk | NA |
| Representativeness of the cases | Unclear risk | NA |
| Selection of the controls | Unclear risk | NA |
| Definition of the controls | Unclear risk | NA |
| Comparability of cases and controls on the basis of the design or analysis | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Same method of ascertainment for cases and controls | Unclear risk | NA |
| Non‐response rate | Unclear risk | NA |
Chi 2013.
| Methods | Retrospective cohort study | |
| Participants | Inclusion: pregnant women aged 15 to 44 years who received ≥ 1 dispensed prescription(s) for topical corticosteroids during pregnancy Exclusion: women who had received ≥ 1 dispensed prescription(s) for any other form (systemic, injection, inhalation, or nasal) of corticosteroids during pregnancy; women with multifoetal pregnancy or pregnancy following assisted reproduction Controls: pregnant women aged 15 to 44 years who did not receive any dispensed prescription for any form of corticosteroids during pregnancy |
|
| Interventions | Dispensed prescriptions for topical corticosteroids during pregnancy | |
| Outcomes |
|
|
| Funding source | Wellbeing of Women and Chang Gung Memorial Hospital, Chiayi | |
| Notes | Country: UK Setting: population‐based (UK Health Informatics Centre datasets) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | Obtained from the UK Health Informatics Centre (HIC) datasets containing anonymised longitudinal medical records of everyone registered with the National Health Service (NHS) Tayside in Scotland; truly representative of average pregnant women (monofoetal pregnancies, not IVF) receiving topical steroids |
| Selection of the non‐exposed cohort | Low risk | Drawn from the same source as the exposed cohort (matching for maternal age (5‐year bands), as well as the calendar year of pregnancy |
| Ascertainment of exposure | Low risk | The pharmacy records identify the timing, potency, and dosage of topical corticosteroids dispensed from community pharmacies |
| Demonstration that the outcome of interest was not present at start of study | Low risk | None of the study subjects had the outcome of interest at the start of the study |
| Comparability of cohorts on the basis of the design or analysis | Low risk | There were significant differences between the exposed and control cohorts only in the proportions of subjects with asthma and receiving US FDA pregnancy risk category D or X drugs, which had been adjusted in statistical analysis |
| Assessment of outcome | Low risk | The birth registry and diagnostic codes in the clinical records used to identify the outcomes |
| Was the follow‐up long enough for the outcomes to occur? | Low risk | The study subjects were followed up till delivery or foetal death |
| Adequacy of follow‐up of the cohorts | Low risk | The HIC datasets had the data on all births |
| Was the case definition adequate? | Unclear risk | NA |
| Representativeness of the cases | Unclear risk | NA |
| Selection of the controls | Unclear risk | NA |
| Definition of the controls | Unclear risk | NA |
| Comparability of cases and controls on the basis of the design or analysis | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Same method of ascertainment for cases and controls | Unclear risk | NA |
| Non‐response rate | Unclear risk | NA |
Czeizel 1997.
| Methods | Case‐control | |
| Participants | Inclusion: 20,830 cases with isolated congenital abnormalities and unidentified multiple congenital abnormalities Exclusion: mild congenital abnormalities, minor anomalies, and congenital abnormality syndromes of known origin Controls: 35,727 newborns without congenital abnormalities as controls matched to each case (control: case = 2 to 1 until 1988, 3 to 1 thereafter) according to sex, birth week, and district of parents' residence from the national birth registry of the Central Statistical Office |
|
| Interventions | Topical corticosteroids used mainly for allergic dermatoses such as urticaria and eczema | |
| Outcomes | 14 congenital abnormality groups including neural tube defect, hydrocephaly, cleft lip with or without cleft palate, posterior cleft palate, ear congenital abnormalities, cardiovascular congenital abnormalities, intestinal atresia/stenosis, hypospadias, undescended testis, poly/syndactyly, limb deficiencies, clubfoot, other isolated congenital abnormalities, and multiple congenital abnormalities | |
| Funding source | Not reported | |
| Notes | Country: Hungary Setting: population‐based (using the data set Hungarian Case‐Control Surveillance of Congenital Abnormalities) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Unclear risk | NA |
| Selection of the non‐exposed cohort | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Demonstration that the outcome of interest was not present at start of study | Unclear risk | NA |
| Comparability of cohorts on the basis of the design or analysis | Unclear risk | NA |
| Assessment of outcome | Unclear risk | NA |
| Was the follow‐up long enough for the outcomes to occur? | Unclear risk | NA |
| Adequacy of follow‐up of the cohorts | Unclear risk | NA |
| Was the case definition adequate? | Low risk | Identified from Hungarian Congenital Abnormality Registry |
| Representativeness of the cases | Low risk | Population‐based setting |
| Selection of the controls | Low risk | Community controls |
| Definition of the controls | Low risk | No congenital abnormalities |
| Comparability of cases and controls on the basis of the design or analysis | Low risk | Controlled for potential confounders including maternal age, birth order, proportion of threatened and preterm birth, maternal disorders, and use of other drugs |
| Ascertainment of exposure | Low risk | Prenatal log book, questionnaire, and interview |
| Same method of ascertainment for cases and controls | Low risk | Same methods of ascertainment used in both groups |
| Non‐response rate | High risk | The non‐response rate for the case and control groups was 18% and 35%, respectively |
Edwards 2003.
| Methods | Case‐control | |
| Participants | Inclusion: 48 cases with non‐syndromic cleft lip or palate Exclusion: syndromic cleft Controls: 58 controls selected by date of birth as close as possible to that of cases |
|
| Interventions | Topical corticosteroids for dermatitis or eczema | |
| Outcomes | Cleft lip or palate | |
| Funding source | Not reported | |
| Notes | Country: Australia Setting: a single teaching hospital |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Unclear risk | NA |
| Selection of the non‐exposed cohort | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Demonstration that the outcome of interest was not present at start of study | Unclear risk | NA |
| Comparability of cohorts on the basis of the design or analysis | Unclear risk | NA |
| Assessment of outcome | Unclear risk | NA |
| Was the follow‐up long enough for the outcomes to occur? | Unclear risk | NA |
| Adequacy of follow‐up of the cohorts | Unclear risk | NA |
| Was the case definition adequate? | Low risk | All cases had been assessed by a geneticist and a paediatrician in the cleft palate clinic |
| Representativeness of the cases | Unclear risk | Cases were recruited from the cleft palate clinic in a teaching hospital |
| Selection of the controls | Unclear risk | Selected from the same hospital |
| Definition of the controls | Low risk | No cleft lip or palate |
| Comparability of cases and controls on the basis of the design or analysis | Low risk | Controlled for potential confounders including family income, family history of cleft, maternal age, birth length, and birth order |
| Ascertainment of exposure | Unclear risk | Telephone interview not blinded to case/control status |
| Same method of ascertainment for cases and controls | Low risk | Same methods of ascertainment used in both groups |
| Non‐response rate | High risk | The non‐response rate for the case and control groups was 70% and 85.8%, respectively |
Hviid 2011.
| Methods | Retrospective cohort study | |
| Participants | All live births in Denmark from 1 January 1996 to 30 September 2008 | |
| Interventions | All corticosteroid prescriptions given to women and filled during the first trimester (defined as the first 12 weeks after the start of pregnancy) identified from the Danish Prescription Drug Register | |
| Outcomes |
|
|
| Funding source | Danish Medical Research Council and Lundbeck Foundation | |
| Notes | Country: Denmark Setting: population‐based (the Danish Medical Birth Registry) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | Obtained from the Danish Medical Birth Registry which contains information on all live births in Denmark |
| Selection of the non‐exposed cohort | Low risk | Drawn from the same source as the exposed cohort |
| Ascertainment of exposure | Low risk | Quote: "Information on all corticosteroid prescriptions given to women in the cohort and filled during the period starting four weeks before pregnancy and ending at birth was obtained from the Danish Prescription Drug Register" |
| Demonstration that the outcome of interest was not present at start of study | Low risk | The outcomes of interest did not happen at the start of the study |
| Comparability of cohorts on the basis of the design or analysis | Low risk | No obvious differences in the year of birth, maternal age at start of pregnancy, maternal parity, maternal place of residence and origin, maternal level of education, socioeconomic status, smoking, history of orofacial clefts and birth defects among offspring, maternal diseases, and maternal drug use during the first trimester |
| Assessment of outcome | Low risk | Quote: "Infants with orofacial clefts (clefts) were identified through the National Hospital Discharge Register." "Clefts were subcategorized as cleft lip with or without cleft palate (ICD‐10 codes Q36 and Q37) and cleft palate alone (ICD‐10 code Q35). Only diagnoses made during the first year of life were included" |
| Was the follow‐up long enough for the outcomes to occur? | Low risk | Study subjects were followed up till delivery |
| Adequacy of follow‐up of the cohorts | Low risk | The Danish Medical Birth Registry had all the data on live births |
| Was the case definition adequate? | Unclear risk | NA |
| Representativeness of the cases | Unclear risk | NA |
| Selection of the controls | Unclear risk | NA |
| Definition of the controls | Unclear risk | NA |
| Comparability of cases and controls on the basis of the design or analysis | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Same method of ascertainment for cases and controls | Unclear risk | NA |
| Non‐response rate | Unclear risk | NA |
Källén 2003.
| Methods | Case‐control | |
| Participants | Inclusion: 1044 infants with orofacial cleft Exclusion: chromosome anomalies Total number of births for the study: 576,873 births |
|
| Interventions | Topical corticosteroids | |
| Outcomes | Orofacial cleft | |
| Funding source | KA Wallenberg Foundation | |
| Notes | Country: Sweden Setting: population‐based (Swedish Medical Birth Registry) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Unclear risk | NA |
| Selection of the non‐exposed cohort | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Demonstration that the outcome of interest was not present at start of study | Unclear risk | NA |
| Comparability of cohorts on the basis of the design or analysis | Unclear risk | NA |
| Assessment of outcome | Unclear risk | NA |
| Was the follow‐up long enough for the outcomes to occur? | Unclear risk | NA |
| Adequacy of follow‐up of the cohorts | Unclear risk | NA |
| Was the case definition adequate? | Low risk | The case was defined as having a diagnosis of orofacial cleft (but without a chromosome anomaly). The case group was identified from the Swedish Medical Birth Registry, supplemented with the Swedish Registry of Congenital Malformations and the Hospital Discharge Registry |
| Representativeness of the cases | Low risk | The Swedish Medical Birth Registry covers all of Sweden, although 1% to 2% of deliveries are missing in the register |
| Selection of the controls | Low risk | The controls were also identified from the Swedish Medical Birth Registry |
| Definition of the controls | Low risk | No orofacial clefts |
| Comparability of cases and controls on the basis of the design or analysis | Low risk | Comparisons of drug use were made as Mantel‐Haenszel odds ratio stratified for year of birth, maternal age, parity, smoking habits, and period of involuntary childlessness |
| Ascertainment of exposure | Low risk | Data on drug exposure (mainly in first trimester) were prospectively collected by midwives at first antenatal care visit (usually week 10 to 12) |
| Same method of ascertainment for cases and controls | Low risk | Same methods of ascertainment used in both groups |
| Non‐response rate | Unclear risk | NA |
Mahé 2007.
| Methods | Prospective cohort | |
| Participants | Inclusion: 28 women, who were 6 to 9 months pregnant and lived in the administrative district of the maternity centre, and who used potent topical corticosteroids for skin lightening during pregnancy (including 27 women using clobetasol propionate) Exclusion: receiving oral or topical corticosteroids treatment for a medical reason Controls: 60 women with no use of very potent topical corticosteroids during pregnancy (including 6 women using topical corticosteroids of other potency for skin lightening during pregnancy) |
|
| Interventions | Very potent topical corticosteroids at a mean quantity of 60 g per month | |
| Outcomes | Mode of delivery, birth weight, low birth weight, gestational age at delivery, Apgar score | |
| Funding source | Not reported | |
| Notes | Country: Senegal Setting: single maternity centre We requested detailed statistics from the original researchers, but they could only provide valid data of 79 women (including 23 exposed and 56 unexposed women) for analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Unclear risk | Enrolled from a maternity centre every alternate day |
| Selection of the non‐exposed cohort | Low risk | Drawn from the same source as the exposed cohort |
| Ascertainment of exposure | Low risk | Structured interview |
| Demonstration that the outcome of interest was not present at start of study | Low risk | None of the study subjects had the outcome of interest at the start of the study |
| Comparability of cohorts on the basis of the design or analysis | Low risk | No significant differences between women using very potent corticosteroids and those who did not, in terms of potential confounders such as socioeconomic and education levels, age, and parity |
| Assessment of outcome | Low risk | Outcomes obtained from delivery registers |
| Was the follow‐up long enough for the outcomes to occur? | Low risk | The study participants were followed up until delivery |
| Adequacy of follow‐up of the cohorts | Unclear risk | 10 out of 99 women (10.1%) lost to follow up |
| Was the case definition adequate? | Unclear risk | NA |
| Representativeness of the cases | Unclear risk | NA |
| Selection of the controls | Unclear risk | NA |
| Definition of the controls | Unclear risk | NA |
| Comparability of cases and controls on the basis of the design or analysis | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Same method of ascertainment for cases and controls | Unclear risk | NA |
| Non‐response rate | Unclear risk | NA |
Mygind 2002.
| Methods | Retrospective cohort | |
| Participants | Inclusion: 363 primiparous women carrying a single foetus who filled a prescription for topical corticosteroids 30 days before conception or during pregnancy Controls: 9263 primiparous women carrying a single foetus in the same region, receiving no prescriptions for topical corticosteroids 30 days before conception or during pregnancy |
|
| Interventions | Having filled a prescription for topical corticosteroids 30 days before conception or during pregnancy | |
| Outcomes |
|
|
| Funding source | Western Danish Research Forum for Health Sciences, Danish Medical Research Council, and Foundation of Hørslev | |
| Notes | Country: Denmark Setting: local population in North Jutland (using Danish Medical Birth register) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Unclear risk | Restricted to primiparous women |
| Selection of the non‐exposed cohort | Low risk | Drawn from the same population |
| Ascertainment of exposure | Low risk | Record linkage using the Population‐Based Prescription Database |
| Demonstration that the outcome of interest was not present at start of study | Low risk | None of the study subjects had the outcome of interest at the start of the study |
| Comparability of cohorts on the basis of the design or analysis | Low risk | Controlled for potential confounders including maternal age, gestational age, and smoking status |
| Assessment of outcome | Unclear risk | Information on congenital abnormality was obtained from the Regional Hospital Discharge Registry. The hospital records of children with congenital abnormality were reviewed. The records of congenital abnormality in the register were not entirely accurate. Among the 5 registered congenital abnormality cases in the exposed group, 2 children actually did not have any abnormalities after reviewing their hospital records. Data on other pregnancy outcomes were obtained from the Danish Medical Birth Registry |
| Was the follow‐up long enough for the outcomes to occur? | Low risk | The study subjects were followed up till delivery or foetal death |
| Adequacy of follow‐up of the cohorts | Low risk | Complete follow‐up because the data were from the Danish Medical Birth Registry |
| Was the case definition adequate? | Unclear risk | NA |
| Representativeness of the cases | Unclear risk | NA |
| Selection of the controls | Unclear risk | NA |
| Definition of the controls | Unclear risk | NA |
| Comparability of cases and controls on the basis of the design or analysis | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Same method of ascertainment for cases and controls | Unclear risk | NA |
| Non‐response rate | Unclear risk | NA |
Pradat 2003.
| Methods | Case‐control | |
| Participants | Inclusion: 982 infants with orofacial cleft and a maternal history of first trimester drug intake Controls: 10,168 infants with congenital malformations other than orofacial cleft and a history of maternal first trimester drug intake |
|
| Interventions | Topical corticosteroids | |
| Outcomes | Cleft palate or lip | |
| Funding source | Not reported | |
| Notes | Country: Multinational Setting: Multicentre database (Malformation Drug Exposure Surveillance, MADRE) All the infants in the MADRE database had a congenital malformation and a maternal history of first trimester drug intake |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Unclear risk | NA |
| Selection of the non‐exposed cohort | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Demonstration that the outcome of interest was not present at start of study | Unclear risk | NA |
| Comparability of cohorts on the basis of the design or analysis | Unclear risk | NA |
| Assessment of outcome | Unclear risk | NA |
| Was the follow‐up long enough for the outcomes to occur? | Unclear risk | NA |
| Adequacy of follow‐up of the cohorts | Unclear risk | NA |
| Was the case definition adequate? | Low risk | Based on reporting to the MADRE database |
| Representativeness of the cases | Unclear risk | Only children with congenital malformations and a positive history of maternal first trimester drug exposure were reported to the MADRE database. That is, children with congenital malformations but without a history of maternal first trimester drug exposure were not enrolled in the database |
| Selection of the controls | Unclear risk | Hospital controls |
| Definition of the controls | Unclear risk | Children with congenital malformations other than orofacial cleft and with a history of maternal first trimester drug intake |
| Comparability of cases and controls on the basis of the design or analysis | Unclear risk | Using the Mantel‐Haenszel method to adjust for register only |
| Ascertainment of exposure | Unclear risk | Based on self‐report |
| Same method of ascertainment for cases and controls | Low risk | Same methods of ascertainment used in both groups |
| Non‐response rate | Unclear risk | NA |
Skuladottir 2014a.
| Methods | Case‐control study | |
| Participants | Inclusion: 2372 cleft cases (1577 infants with cleft lip with or without cleft palate and 795 infants with cleft palate alone) Exclusion: recognised or strongly suspected single‐gene disorders or chromosomal abnormalities Controls: 5922 controls without major congenital malformations randomly selected from birth certificates or birth hospitals |
|
| Interventions | Topical corticosteroids | |
| Outcomes | Cleft lip with or without cleft palate, cleft palate alone | |
| Funding source | Centers for Disease Control and Prevention | |
| Notes | Country: USA Setting: multistate population‐based (National Birth Defects Prevention Study) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Unclear risk | NA |
| Selection of the non‐exposed cohort | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Demonstration that the outcome of interest was not present at start of study | Unclear risk | NA |
| Comparability of cohorts on the basis of the design or analysis | Unclear risk | NA |
| Assessment of outcome | Unclear risk | NA |
| Was the follow‐up long enough for the outcomes to occur? | Unclear risk | NA |
| Adequacy of follow‐up of the cohorts | Unclear risk | NA |
| Was the case definition adequate? | Low risk | Cases of orofacial cleft received an additional review by 1 clinical geneticist to ensure that standard eligibility criteria were met |
| Representativeness of the cases | Low risk | Identified from birth defect surveillance systems in 8 US states |
| Selection of the controls | Low risk | Controls were randomly selected from birth certificates or birth hospitals |
| Definition of the controls | Low risk | Infants without major congenital malformations |
| Comparability of cases and controls on the basis of the design or analysis | Low risk | Controlled for potential confounders including maternal race/ethnicity, education, intake of folic acid‐containing supplements or smoking during the month before or the first 3 months of pregnancy, and study centre |
| Ascertainment of exposure | Unclear risk | Interview not blinded to case/control status |
| Same method of ascertainment for cases and controls | Low risk | Same method of ascertainment used in both groups |
| Non‐response rate | Unclear risk | NA |
Skuladottir 2014b.
| Methods | Case‐control study | |
| Participants | Inclusion: 573 cleft cases (377 infants with cleft lip with or without cleft palate and 196 infants with cleft palate alone) Control group: 763 controls without major congenital malformations randomly selected from the Medical Birth Registry of Norway |
|
| Interventions | Topical corticosteroids | |
| Outcomes | Cleft lip with or without cleft palate, cleft palate alone | |
| Funding source | Western Norwegian Health Authorities | |
| Notes | Country: Norway Setting: The only 2 specialised surgical centres for oral cleft in Norway |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Unclear risk | NA |
| Selection of the non‐exposed cohort | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Demonstration that the outcome of interest was not present at start of study | Unclear risk | NA |
| Comparability of cohorts on the basis of the design or analysis | Unclear risk | NA |
| Assessment of outcome | Unclear risk | NA |
| Was the follow‐up long enough for the outcomes to occur? | Unclear risk | NA |
| Adequacy of follow‐up of the cohorts | Unclear risk | NA |
| Was the case definition adequate? | Low risk | The cases were infants in Norway referred for clefts surgery Quote: "Information for cases on accompanying birth defects or syndromes was obtained from three sources: (1) medical records at the hospital performing corrective surgery, (2) the Medical Birth Registry, and (3) the mothers' questionnaire" |
| Representativeness of the cases | Low risk | Quote: "In Norway, the treatment of all babies with CLP is carried out in two specialized surgical centers in Oslo and Bergen... the families of all newborn infants in Norway referred for clefts surgery were invited to participate in a case‐control study... A total of 653 infants with clefts were eligible for study, and 573 of their families (88%) agreed to participate. There were 1006 randomly selected live‐born nonmalformed controls eligible for study, and 763 of their families (76%) agreed to participate" |
| Selection of the controls | Low risk | Quote: "Controls were randomly selected from all live births during the same period, sampling from the Medical Birth Registry of Norway" |
| Definition of the controls | Low risk | Quote: "live‐born nonmalformed controls" |
| Comparability of cases and controls on the basis of the design or analysis | Low risk | Quote: "We adjusted for the following potential confounders: mother's education (six categories), work status in early pregnancy (yes or no), alcohol intake (total number of drinks during the first 3 months of pregnancy; none, 1‐3, 4‐6, and 7 +), smoking (none, passive only, 1‐5 cigarettes/d, 6‐10 cigarettes/d, and 11 + cigarettes/d), folic acid supplementation (none, < 400 μg/d, and 400 + μg/d), dietary folates (quartiles with cutoffs at 171, 214, and 264 μg/d), multivitamin supplementation (yes or no), and calendar year of baby's birth" |
| Ascertainment of exposure | Low risk | Quote: "All mothers in the case‐control study completed a self‐administered questionnaire after delivery covering demographic information and a wide range of exposures during pregnancy. In particular, mothers were asked detailed questions about their use of prescribed and over‐the‐counter medications during the first, second and third month of pregnancy." "Information on medications was collected for only the first 3 months of pregnancy" |
| Same method of ascertainment for cases and controls | Low risk | Same as above |
| Non‐response rate | Unclear risk | NA |
Skuladottir 2014c.
| Methods | Although the authors claimed this study (the Norwegian Mother and Child Cohort Study) had a cohort design, we judged it as a case‐control study after examining the full text | |
| Participants | Inclusion: 123 cases with cleft lip with or without cleft palate and 61 with cleft palate alone identified through the Medical Birth Registry of Norway Control group: 551 mothers randomly selected from the MoBa cohort | |
| Interventions | Topical corticosteroids | |
| Outcomes | Cleft lip with or without cleft palate, cleft palate alone | |
| Funding source | Western Norwegian Health Authorities | |
| Notes | Country: Norway Setting: population‐based |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Unclear risk | NA |
| Selection of the non‐exposed cohort | Unclear risk | NA |
| Ascertainment of exposure | Unclear risk | NA |
| Demonstration that the outcome of interest was not present at start of study | Unclear risk | NA |
| Comparability of cohorts on the basis of the design or analysis | Unclear risk | NA |
| Assessment of outcome | Unclear risk | NA |
| Was the follow‐up long enough for the outcomes to occur? | Unclear risk | NA |
| Adequacy of follow‐up of the cohorts | Unclear risk | NA |
| Was the case definition adequate? | Low risk | Quote: "Cases within the cohort were identified by linking all cohort members with the Medical Birth Registry, which includes information on all defects recorded during the newborn's hospital stay. For oral clefts, the sensitivity of the Medical Birth Registry is 94% for cleft lip with or without cleft palate and 57% for cleft palate only" |
| Representativeness of the cases | Low risk | The cases with orofacial cleft were identified through the Medical Birth Registry of Norway |
| Selection of the controls | Low risk | The control group was randomly selected from the same population‐based study |
| Definition of the controls | Low risk | Same as above |
| Comparability of cases and controls on the basis of the design or analysis | Low risk | Quote: "We adjusted for folic acid use (400 μg/d or none), smoking (none, passive only, and active smoker), mother's education (< high school and high school or more) and alcohol consumption (none or any)" |
| Ascertainment of exposure | Low risk | Quote: "Mothers in the cohort study were asked to complete self‐administered questionnaires at pregnancy week 15, 22, and 30. We used information from the 15‐week questionnaire, which focuses on maternal health and use of medications 6 months before pregnancy and during the first 15 weeks of pregnancy" |
| Same method of ascertainment for cases and controls | Low risk | Same as above |
| Non‐response rate | Unclear risk | Representativeness of the exposed cohort |
CLP: cleft lip or palate or both; FDA: Food and Drug Administration (USA); IVF: in vitro fertilisation; LMP: last menstrual period; MoBa: Norwegian Mother & Child Cohort Study (from Norwegian den norske Mor & barn‐undersøkelsen); NA: not applicable.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bjørn 2013 | Study on systemic and inhaled corticosteroids, with no data on topical corticosteroids |
| Bjørn 2014 | Study on systemic and inhaled corticosteroids, with no data on topical corticosteroids |
| Lin 2014 | Case‐control study on non‐syndromic cleft of the lip and palate, but lacking data relevant to topical corticosteroids |
| Zandi 2011 | Case‐control study on cleft lip and palate with no data on maternal exposure to specific drugs |
Differences between protocol and review
Differences between protocol and review
We originally planned to express all dichotomous outcomes as risk ratios (RR) and 95% confidence intervals (CI). However, we obtained some data from case‐control studies, and we decided to retain them as OR with 95% CIs.
We originally planned to analyse major and minor congenital abnormalities separately but did not find any studies that reported them separately. Therefore, we grouped the two outcomes together.
We did not originally plan 'Summary of Findings' tables, but we thought it important and included them in this review.
GRADE has been adopted postprotocol to rate the quality of evidence.
Differences between protocol and this review update
Types of studies: In the protocol we said, "We will not deliberately examine all trials of topical corticosteroids to find out if they contained any pregnant women, or contact the original researchers to enquire if any women became pregnant during the trial." In the review, we simplified this to "We did not include RCTs recruiting pregnant women only as a subset." and we have retained this phrase for this update.
Types of outcome measures: For this update of the review, we expanded the scope of the outcome 'stillbirth' to 'foetal death'.
Search methods for identification of studies, Electronic searches: For this update of the review, we decided not to search Cumulative Index to Nursing & Allied Health (CINAHL), British Nursing Index (BNI), or BIOSIS Previews because they did not produce any useful results in the 2009 searches. We updated the list of trials registers we searched to match current Skin Group searching practice.
Searching other resources, Reference lists: To increase the sensitivity of our search, we also used SCI‐EXPANDED on 21 July 2014 to identify and scan the articles that had cited the included studies, which we had not planned at the protocol stage.
Data collection and analysis, Assessment of risk of bias in included studies: We omitted from the protocol that we planned to assess 'selective reporting' in any RCTs. We will include this is future updates of this review if we find any RCTs that we can include.
Differences between the original 2009 review and this update
Types of outcome measures, Primary outcomes: The following phrase was in the review but has been moved to the Results, Included studies, Outcomes: "We analysed orofacial cleft separately as it is an expected possible associated outcome. When detailed data were available, we further analysed the two categories of orofacial cleft (i.e., cleft lip with or without cleft palate, and isolated cleft palate), separately, because they are considered aetiologically distinct (Stanier 2004)."
Search methods for identification of studies, Electronic searches: For this update, we revised the search strategies for the Skin Group Specialised Register, CENTRAL, MEDLINE, EMBASE, and LILACS to increase the sensitivity of searching cohort and case‐control studies. We did not search the EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/) in the original 2009 review, but for this update, we did search it in order to increase the sensitivity of our search.
Searching other resources, Handsearching: In the original 2009 review, we searched two electronic conference proceedings databases. When we updated this review, we decided this handsearching was less valuable than handsearching CC's private collection of literature relevant to the review topic.
Searching other resources, Correspondence: In the protocol, we said "We shall correspond with the original researchers to identify unpublished or ongoing trials and observational studies", but while conducting the previous review, we requested relevant studies from 11 pharmaceutical companies that had introduced an original topical corticosteroid product (Table 8). Only three companies replied to us, and all of them were unaware of any relevant data. For this update, we did not correspond with pharmaceutical companies.
2. Contact with manufacturers.
| Manufacturer | Products | Date of contact letter sent | Date of receiving reply | Reply |
| GlaxoSmithKline | Hydrocortisone, beclometasone dipropionate, betamethasone valerate, clobetasol propionate, clobetasone butyrate, fluticasone propionate | 15 August 2008 | 29 August 2008 | "Unfortunately we are not aware of any data..." |
| Astellas | Hydrocortisone, hydrocortisone butyrate | 15 August 2008 | — | — |
| Schering‐Plough | Alclometasone dipropionate, betamethasone dipropionate, mometasone furoate | 15 August 2008 | 26 August 2008 | An extensive search through Schering‐Plough UK Pharmacovigilance Database found 3 cases of pregnancy while taking/using betamethasone dipropionate, mometasone furoate, but none reported any follow‐up or outcomes. No relevant clinical studies done by them |
| Meadow | Diflucortolone valerate, fluocortolone | 15 August 2008 | — | — |
| Typharm | Fludroxycortide (flurandrenolone) | 15 August 2008 | — | — |
| GP Pharma (Derma UK) |
Fluocinolone acetonide, fluocinonide | 15 August 2008 | — | — |
| Ferndale | Fluprednidene acetate, hydrocortisone butyrate, hydrocortisone acetate | 15 August 2008 | — | — |
| Bristol‐Myers Squibb | Halcinonide, triamcinolone acetonide | 15 August 2008 | 5 September 2008 | Did not provide any relevant data but SPC for triamcinolone acetonide injection |
| TARO | Diflorasone diacetate, alclometasone dipropionate, amcinonide, betamethasone dipropionate, betamethasone valerate, clobetasol propionate, desonide, desoximetasone, fluocinonide, hydrocortisone, hydrocortisone butyrate, hydrocortisone valerate, mometasone furoate, triamcinolone acetonide | 15 August 2008 | — | — |
| Intendis | Hydrocortisone aceponate | 15 August 2008 | — | — |
| Dermik (Sanofi‐Aventis US) | Diflorasone diacetate, prednicarbate | 15 August 2008 | — | — |
SPC: statistical process control.
Data collection and analysis: we explained our inclusion of 'Summary of findings' tables in this review update.
Data collection and analysis, Assessment of risk of bias in included studies, RCTs: we did not find any RCTs so could not evaluate risk of bias.
Data collection and analysis, Assessment of risk of bias in included studies, Non‐randomised studies: We added another assessment for cohort studies, 'Demonstration that outcome of interest was not present at start of study', as well as another assessment for case‐control studies, 'Representativeness of the cases', and we omitted '... selection of the cases' to bring it in line with the original NOS checklist.
Data collection and analysis, Measures of treatment effect: We moved the following text from the Methods to the Results, Included studies, Outcomes: "Edwards 2003 used a classification of orofacial cleft different from ours and divided the cases as cleft palate ± lip and isolated cleft palate (see Effects of interventions). We thus used the published data to calculate the case number of cleft lip with or without cleft palate and used Review Manager software (RevMan 2014) to recalculate all the crude ORs and 95% CIs for consistency."
Data collection and analysis, Unit of analysis issues: We changed this from the plan in the protocol and review to meet the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data collection and analysis, Dealing with missing data: We could not carry out our plans due to the lack of relevant RCTs.
Data collection and analysis, Assessment of reporting biases: The small number of studies identified and the heterogeneity among them meant that it was not possible to use funnel plots to test for publication bias.
Data collection and analysis, Subgroup analysis and investigation of heterogeneity: We did not analyse our originally planned subgroups of maternal skin conditions (polymorphic eruption of pregnancy, pemphigoid gestationis, etc.) and maternal ages because the data were unavailable from the investigators of studies included in this review.
Results, Effects of interventions: We conducted a post hoc analysis of steroid dose potencies.
Results, Effects of interventions: We made a decision to report hypospadias separately because it was reported in the newly included study Skuladottir 2014a, which was an extension of the Carmichael 2007 study on orofacial cleft.
Contributions of authors
CC was the contact person with the editorial base, co‐ordinated contributions from the coauthors, and wrote the final draft of the review. CC and SW screened papers against eligibility criteria. CC obtained data on ongoing and unpublished studies. CC and SW appraised the quality of papers. CC extracted data for the review and sought additional information about papers. CC entered data into RevMan. CC, SW, GK, and FW analysed and interpreted data. CC worked on the Methods sections. CC drafted the clinical sections of the background and responded to the clinical comments of the referees. CC responded to the methodology and statistics comments of the referees. ED was the consumer coauthor and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. CB extracted data from two studies (Chi 2011a; Chi 2013), independently from the investigators of those two studies who are also authors of this Cochrane review. CC is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
Chang Gung Memorial Hospital, Chiayi, Taiwan.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Ching‐Chi Chi was involved in two studies included in this review (Chi 2011a; Chi 2013). Shu‐Hui Wang was a coauthor of a cohort study included in this review (Chi 2013). Fenella Wojnarowska was a coauthor of two cohort studies included in this review (Chi 2011a; Chi 2013) but was not involved in data extraction. Gudula Kirtschig has nothing to declare. Emily Davies has nothing to declare. Cathy Bennett is the proprietor of Systematic Research Ltd and received a consultancy fee from the Cochrane Skin Group for her work on this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Carmichael 2007 {published and unpublished data}
- Carmichael SL, Shaw GM, Ma C, Werler MM, Rasmussen SA, Lammer EJ. Maternal corticosteroid use and orofacial clefts. American Journal of Obstetrics & Gynecology 2007;197(6):585.e1‐585.e7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carmichael 2009 {published data only}
- Carmichael SL, Ma C, Werler MM, Olney RS, Shaw GM, National Birth Defects Prevention Study. Maternal corticosteroid use and hypospadias. Journal of Pediatrics 2009;155(1):39‐44. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chi 2011a {published data only}
- Chi CC, Mayon‐White RT, Wojnarowska FT. Safety of topical corticosteroids in pregnancy: a population‐based cohort study. Journal of Investigative Dermatology 2011;131(4):884‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chi 2013 {published data only}
- Chi CC, Wang SH, Mayon‐White R, Wojnarowska F. Pregnancy outcomes after maternal exposure to topical corticosteroids: a UK population‐based cohort study. JAMA Dermatology 2013;149(11):1274‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Czeizel 1997 {published and unpublished data}
- Czeizel AE, Rockenbauer M. Population‐based case‐control study of teratogenic potential of corticosteroids. Teratology 1997;56(5):335‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Edwards 2003 {published and unpublished data}
- Edwards MJ, Agho K, Attia J, Diaz P, Hayes T, Illingworth A, et al. Case‐control study of cleft lip or palate after maternal use of topical corticosteroids during pregnancy. American Journal of Medical Genetics Part A 2003;120A(4):459‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hviid 2011 {published data only}
- Hviid A, Molgaard‐Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. Canadian Medical Association Journal 2011;183(7):796‐804. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Källén 2003 {published and unpublished data}
- Källén B. Maternal drug use and infant cleft lip/palate with special reference to corticoids. Cleft Palate‐Craniofacial Journal 2003;40(6):624‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mahé 2007 {published and unpublished data}
- Mahé A, Perret JL, Ly F, Fall F, Rault JP, Dumont A. The cosmetic use of skin‐lightening products during pregnancy in Dakar, Senegal: a common and potentially hazardous practice. Transactions of the Royal Society of Tropical Medicine & Hygiene 2007;101(2):183‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mygind 2002 {published and unpublished data}
- Mygind H, Thulstrup AM, Pedersen L, Larsen H. Risk of intrauterine growth retardation, malformations and other birth outcomes in children after topical use of corticosteroid in pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2002;81(3):234‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pradat 2003 {published and unpublished data}
- Pradat P, Robert‐Gnansia E, Tanna GL, Rosano A, Lisi A, Mastroiacovo P, et al. First trimester exposure to corticosteroids and oral clefts. Birth Defects Research 2003;67(12):968‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Skuladottir 2014a {published data only}
- Skuladottir H, Wilcox AJ, Ma C, Lammer EJ, Rasmussen SA, Werler MM, et al. Corticosteroid use and risk of orofacial clefts. Birth Defects Research 2014;100(6):499‐506. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skuladottir 2014b {published data only}
- Skuladottir H, Wilcox A, McConnaughey R, Vindenes H, Lie RT. First‐trimester nonsystemic corticosteroid use and the risk of oral clefts in Norway. Annals of Epidemiology 2014;24(9):635‐40. [PUBMED: 25127739] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skuladottir 2014c {published data only}
- Skuladottir H, Wilcox A, McConnaughey R, Vindenes H, Lie RT. First‐trimester nonsystemic corticosteroid use and the risk of oral clefts in Norway. Annals of Epidemiology 2014;24(9):635‐40. [PUBMED: 25127739] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bjørn 2013 {published data only}
- Bjørn AM, Nielsen RB, Norgaard M, Nohr EA, Ehrenstein V. Risk of miscarriage among users of corticosteroid hormones: a population‐based nested case‐control study. Clinical Epidemiology 2013;5(1):287‐94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjørn 2014 {published data only}
- Bjørn AM, Ehrenstein V, Hundborg HH, Nohr EA, Sørensen HT, Nørgaard M. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. American Journal of Therapeutics 2014;21(2):73‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lin 2014 {published data only}
- Lin Y, Shu S, Tang S. A case‐control study of environmental exposures for nonsyndromic cleft of the lip and/or palate in eastern Guangdong, China. International Journal of Pediatric Otorhinolaryngology 2014;78(3):544‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zandi 2011 {published data only}
- Zandi M, Heidari A. An epidemiologic study of orofacial clefts in Hamedan City, Iran: a 15‐year study. Cleft Palate‐Craniofacial Journal 2011;48(4):483‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Altman 1991
- Altman DG. Practical Statistics for Medical Research. 1st Edition. London: Chapman & Hall, 1991. [Google Scholar]
Ambros‐Rudolph 2006
- Ambros‐Rudolph CM. Dermatoses of pregnancy [Schwangerschaftsdermatosen]. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology] 2006;4(9):748‐61. [MEDLINE: ; doi:10.1111/j.1610‐0387.2006.06008.x] [DOI] [PubMed] [Google Scholar]
Anderson 1981
- Anderson GG, Rotchell Y, Kaiser DG. Placental transfer of methylprednisolone following maternal intravenous administration. American Journal of Obstetrics & Gynecology 1981;140(6):699‐701. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arosarena 2007
- Arosarena OA. Cleft lip and palate. Otolaryngologic Clinics of North America 2007;40(1):27‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ballard 1975
- Ballard PL, Granberg P, Ballard RA. Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. Journal of Clinical Investigation 1975;56(6):1548‐54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballard 1977
- Ballard PD, Hearney EF, Smith MB. Comparative teratogenicity of selected glucocorticoids applied ocularly in mice. Teratology 1977;16(2):175‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baumann 1999
- Baumann L, Kerdel F. Topical glucocorticoids. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, et al. editor(s). Fitzpatrick's Dermatology in General Medicine. 5th Edition. New York: McGraw‐Hill, 1999:2713‐7. [Google Scholar]
Beitins 1972
- Beitins IZ, Bayard F, Ances IG, Kowarski A, Migeon CJ. The transplacental passage of prednisone and prednisolone in pregnancy near term. Journal of Pediatrics 1972;81(5):936‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berth‐Jones 2004
- Berth‐Jones J. Chapter 75. Topical therapy. In: Burns T, Breathnach SM, Cox N, Griffiths C editor(s). Rook's Textbook of Dermatology. 7th Edition. Oxford: Blackwell Publishing, 2004:1‐52. [Google Scholar]
Briggs 2008
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. Drugs in Pregnancy and Lactation. 8th Edition. Philadelphia: Lippincott Williams & Wilkins, 2008. [Google Scholar]
Carmichael 1999
- Carmichael SL, Shaw GM. Maternal corticosteroid use and risk of selected congenital anomalies. American Journal of Medical Genetics 1999;86(3):242‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Casey 2001
- Casey BM, McIntire DD, Leveno KJ. The continuing value of the Apgar score for the assessment of newborn infants. New England Journal of Medicine 2001;344(7):467‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chi 2011b
- Chi CC, Kirtschig G, Aberer W, Gabbud JP, Lipozenčić J, Kárpáti S, et al. Evidence‐based (S3) guideline on topical corticosteroids in pregnancy. British Journal of Dermatology 2011;165(5):943‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cunningham 2005
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap LC III, Wenstrom KD. Williams Obstetrics. 22nd Edition. New York: McGraw‐Hill, 2005. [Google Scholar]
Czeizel 2005
- Czeizel AE. Birth defects are preventable. International Journal of Medical Sciences 2005;2(3):91‐2. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Devane 2007
- Devane D, Begley CM, Clarke M, Horey D, O'Boyle C. Evaluating maternity care: A core set of outcome measures. Birth 2007;34(2):164‐172. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gitau 1998
- Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. The Lancet 1998;352(9129):707‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gitau 2001
- Gitau R, Fisk NM, Teixeira JM, Cameron A, Glover V. Fetal hypothalamic‐pituitary‐adrenal stress responses to invasive procedures are independent of maternal responses. Journal of Clinical Endocrinology & Metabolism 2001;86(1):104‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GlaxoSmithKline 2002
- GlaxoSmithKline. Cutivate cream prescribing information. Pharmaceutical Information 2002.
GRADE 2013
- Schünemann H, Brozek J, Guyatt G, Oxman A (editors). GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. [updated October 2013] The GRADE Working Group, 2013. Available from www.guidelinedevelopment.org/handbook.
Gur 2004
- Gur C, Diav‐Citrin O, Shechtman S, Arnon J, Ornoy A. Pregnancy outcome after first trimester exposure to corticosteroids: a prospective controlled study. Reproductive Toxicology 2004;18(1):93‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Hale 2002
- Hale EK, Pomeranz MK. Dermatologic agents during pregnancy and lactation: an update and clinical review. International Journal of Dermatology 2002;41(4):197‐203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hardy 2006
- Hardy JR, Leaderer BP, Holford TR, Hall GC, Bracken MB. Safety of medications prescribed before and during early pregnancy in a cohort of 81,975 mothers from the UK General Practice Research Database. Pharmacoepidemiology & Drug Safety 2006;15(8):555‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hengge 2006
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. Journal of the American Academy of Dermatology 2006;54(1):1‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011], The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ikegami 1997
- Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. American Journal of Respiratory & Critical Care Medicine 1997;156(1):178‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jessop 2000
- Jessop S, Whitelaw D, Jordaan F. Drugs for discoid lupus erythematosus. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002954] [DOI] [PubMed] [Google Scholar]
Katz 1990
- Katz VL, Thorp JM Jr, Bowes WA Jr. Severe symmetric intrauterine growth retardation associated with the topical use of triamcinolone. American Journal of Obstetrics & Gynecology 1990;162(2):396‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khumalo 2005
- Khumalo N, Kirtschig G, Middleton P, Hollis S, Wojnarowska F, Murrell D. Interventions for bullous pemphigoid. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD002292.pub2] [DOI] [PubMed] [Google Scholar]
Leachman 2006
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatologic Clinics 2006;24(2):167‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marsland 2006
- Marsland AM, Chalmers RJG, Hollis S, Leonardi‐Bee J, Griffiths CEM. Interventions for chronic palmoplantar pustulosis. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD001433.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meadows 2001
- Meadows M. Pregnancy and the drug dilemma. FDA Consumer 2001;35(3):16‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Mehta 2006
- Mehta DK. British National Formulary. British National Formulary. 2nd Edition. BMJ Publishing Group Ltd and RPS Publishing, 2006:BMJ Publishing Group Ltd and RPS Publishing 2006. [Google Scholar]
Murphy 1974
- Murphy BE, Clark SJ, Donald IR, Pinsky M, Vedady D. Conversion of maternal cortisol to cortisone during placental transfer to the human fetus. American Journal of Obstetrics & Gynecology 1974;118(4):538‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murphy 2007
- Murphy VE, Fittock RJ, Zarzycki PK, Delahunty MM, Smith R, Clifton VL. Metabolism of synthetic steroids by the human placenta. Placenta 2007;28(1):39‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nanda 1970
- Nanda R, Linden FP, Jansen HW. Production of cleft palate with dexamethasone and hypervitaminosis A in rat embryos. Experientia 1970;26(10):1111‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Narama 1984
- Narama I. Reproduction studies of diflorasone diacetate (DDA) 4. Teratogenicity study in rabbits by percutaneous administration. Pharmacometrics 1984;28(2):241‐50. [Google Scholar]
Nasjleti 1967
- Nasjleti CE, Avery JK, Spencer HH, Walden JM. Tritiated cortisone distribution and induced cleft palate in mice. Journal of Oral Therapeutics & Pharmacology 1967;4(1):71‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Park‐Wyllie 2000
- Park‐Wyllie L, Mazzotta P, Pastuszak A, Moretti ME, Beique L, Hunnisett L, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta‐analysis of epidemiological studies. Teratology 2000;62(6):385‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Petersen 1980
- Petersen MC, Nation RL, Ashley JJ, McBride WG. The placental transfer of betamethasone. European Journal of Clinical Pharmacology 1980;18(3):245‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodriguez‐Pinil 1998
- Rodriguez‐Pinilla E, Martinez‐Frias ML. Corticosteroids during pregnancy and oral clefts: a case‐control study. Teratology 1998;58(1):2‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Romo 2009
- Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatric Endocrinology Reviews 2009;6(Suppl 3):332‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Schering 2003
- Schering. ELOCON cream prescribing information. Pharmaceutical Information 2003.
Shah 1976
- Shah RM, Kilistoff A. Cleft palate induction in hamster fetuses by glucocorticoid hormones and their synthetic analogues. Journal of Embryology & Experimental Morphology 1976;36(1):101‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Sifton 2002
- Sifton DW. Physicians' Desk Reference. 6th Edition. Montvale: Medical Economics Company, 2002. [Google Scholar]
Smith 1988
- Smith MA, Thomford PJ, Mattison DR, Slikker W Jr. Transport and metabolism of dexamethasone in the dually perfused human placenta. Reproductive Toxicology 1988;2(1):37‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stanier 2004
- Stanier P, Moore GE. Genetics of cleft lip and palate: syndromic genes contribute to the incidence of non‐syndromic clefts. Human Molecular Genetics 2004;13(Spec No 1):R73‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sun 1998
- Sun K, Yang K, Challis JR. Glucocorticoid actions and metabolism in pregnancy: implications for placental function and fetal cardiovascular activity. Placenta 1998;19(5‐6):353‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taro 1999
- Taro Pharmaceuticals. Diflorasone diacetate cream prescribing information. Pharmaceutical Information 1999.
Thomson Healthcare 2009
- Thomson Healthcare. DRUGDEX® Evaluations. ThomsonMicromedex 2009; Vol. 140.
Turpeinen 1988
- Turpeinen M, Mashkilleyson N, Bjorksten F, Salo OP. Percutaneous absorption of hydrocortisone during exacerbation and remission of atopic dermatitis in adults. Acta Dermato‐Venereologica 1988;68(4):331‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Turpeinen 1989
- Turpeinen M. Adrenocortical response to adrenocorticotropic hormone in relation to duration of topical therapy and percutaneous absorption of hydrocortisone in children with dermatitis. European Journal of Pediatrics 1989;148(8):729‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Uno 1994
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, et al. Neurotoxicity of glucocorticoids in the primate brain. Hormones & Behavior 1994;28(4):336‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walker 1967
- Walker BE. Induction of cleft palate in rabbits by several glucocorticoids. Proceedings of the Society for Experimental Biology & Medicine 1967;125(4):1281‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weatherhead 2007
- Weatherhead S, Robson SC, Reynolds NJ. Eczema in pregnancy. BMJ (Clinical research ed.) 2007;335(7611):152‐4. [PUBMED: 17641349] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2006
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 23 November 2006).
Wong 2004
- Wong FK, Hagg U. An update on the aetiology of orofacial clefts. Hong Kong Medical Journal 2004;10(5):331‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Yamada 1981
- Yamada H, Nakano M, Ichihashi T. Fetal concentration after topical application of betamethasone 17,21‐dipropionate (S‐3440) ointment and teratogenesis in mice and rabbits. Pharmacometrics 1981;21(4):645‐55. [Google Scholar]
Zip 2006
- Zip C. A practical guide to dermatological drug use in pregnancy. Skin Therapy Letter 2006;11(4):1‐4. [MEDLINE: ] [PubMed] [Google Scholar]
References to other published versions of this review
Chi 2009
- Chi CC, Lee CW, Wojnarowska F, Kirtschig G. Safety of topical corticosteroids in pregnancy. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007346.pub2] [DOI] [PubMed] [Google Scholar]


