Abstract
We present a case of maxillary sinusitis in a diabetic female caused by the basidiomycete fungus Schizophyllum commune. Identification of the isolate was hampered by its atypical features. Subcultures formed sterile medusoid structures from nonclamped mycelia until spontaneous dikaryotization resulted in the development of characteristic fan-shaped fruiting bodies. Identification was confirmed by the presence of spicules formed on the hyphae and by vegetative compatibility with known isolates.
Case report.
Schizophyllum commune is a basidiomycetous fungus that is being reported with increasing frequency as a cause of sinusitis and invasive disease in both immunocompetent and immunocompromised hosts. We present a case of maxillary sinusitis in a diabetic female whose infection was successfully treated with surgery alone. S. commune infection may be misdiagnosed or not recognized because presentation of infection and histopathological findings can be suggestive of sinusitis caused by Aspergillus species and because the fungus can be difficult to identify in culture when atypical isolates are encountered.
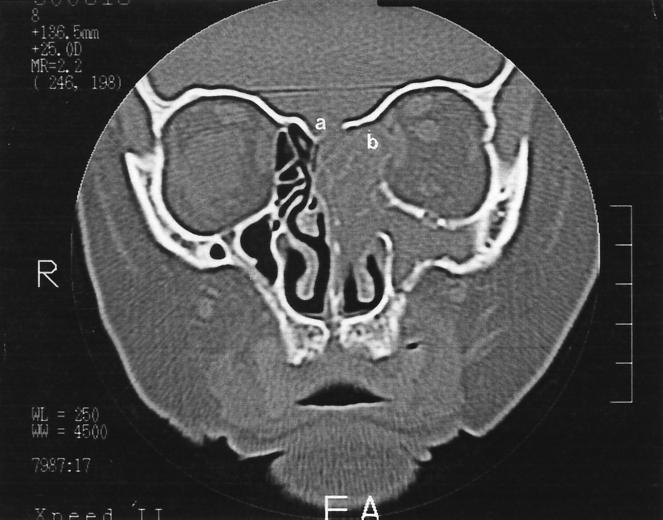
A 62-year-old woman presented with a 4-month history of discomfort over her left frontal and maxillary sinuses. This was associated with anosmia and a left-sided purulent anterior nasal and postnasal discharge. She had completed a 10-day course of doxycyline with minimal symptomatic improvement. She was a non-insulin-dependent diabetic on metformin (850 mg) and gliclazide (40 mg), both administered twice a day. She had smoked 10 cigarettes per day for the last 30 years. There was no history of previous surgery, facial trauma, distant travel, or drug abuse. On physical examination, a purulent discharge was noted in the left middle meatus. The right middle meatus was clear. The remaining examination of the head and neck revealed no significant findings. Her hemoglobin was 15.3 g/liter, her hematocrit was 0.45, and the leukocyte count was 7.4 × 109/liter with a normal leukocyte differential. The chest X-ray was unremarkable. A bone density computerized tomography (CT) scan of her sinuses showed extensive left paranasal sinusitis (Fig. 1). There was thinning of the bony margins about the medial aspect of the left orbit and about portions of the cribriform plate. The left ethmoid sinus was expanded, in keeping with the development of a mucocele. In soft-tissue CT scans, areas of calcification were present within the ethmoid sinuses. A swab specimen of the middle meatus was obtained, and the patient was commenced on erythromycin for 1 week. The swab recovered Escherichia coli but no fungi. Following erythromycin treatment there was a reduction in the nasal discharge and an improvement in the patient’s sense of smell.
FIG. 1.
Coronal bone density CT of the sinuses, showing opaque left paranasal sinuses and expansion of the left ethmoid sinus, in keeping with the development of a mucocele. There is evidence of thinning and bony erosion about portions of the cribriform plate (a) and the medial aspects of the left orbital wall (b).
A presumptive clinical diagnosis of Aspergillus sinusitis was made. The patient underwent left-sided endoscopic sinus surgery with opening of the left frontal, maxillary, and ethmoid sinuses. Thick mucus was present in the left maxillary and sphenoid sinuses. The frontal sinus was found to be normal. Inspissated mucus was removed from the ethmoid sinuses and sent for culture and histological examination. No antifungal agents were prescribed. The patient’s postoperative course was unremarkable. By 1 week there had been a dramatic resolution of symptoms, and by 4 weeks the cavity had healed well, with no evidence of residual infection. At her 1-year review the patient was free of disease.
Specimens sent for histological studies showed extensive eosinophilic infiltration with Charcot-Leyden crystals. There was no tissue invasion. Examination after Gomori methenamine silver staining showed septate, nondichotomously branching fungal hyphae. Direct microscopy of a KOH preparation of the surgical specimen showed the presence of hyaline septate hyphae with rare dichotomous branching, suggestive of an Aspergillus species.
Mycology.
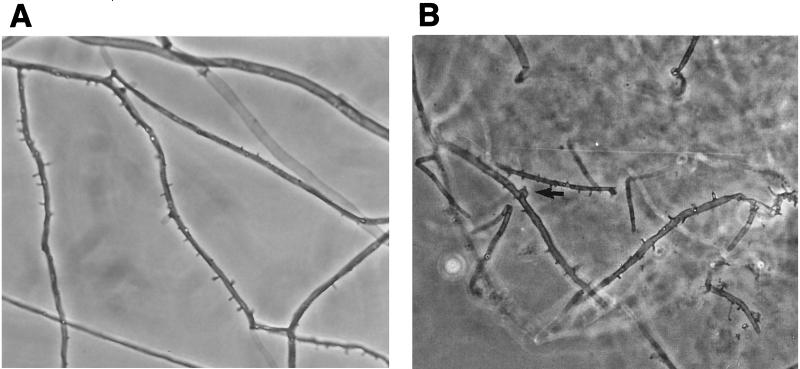
Culture at 27 and 37°C on Sabouraud dextrose agar containing gentamicin yielded a rapidly growing, white to pale buff, densely woolly fungus with a pale-brown reverse. It produced a strong disagreeable odor. There was no growth on medium containing cycloheximide. No bacteria were isolated under aerobic or anaerobic conditions. Microscopic examination of the mold showed hyphae of various widths (2 to 6.5 μm). Some hyphae bore small peglike projections (spicules) (Fig. 2A), but there was no evidence of clamp connections. The isolate was held for several months and subcultured on different media, but it failed to sporulate under any conditions. It remained unidentified until a connection between the spicules and S. commune was made by one of us (L.S.) (19). Further study showed the isolate to be tolerant of benomyl (2 μg/ml), consistent with this basidiomycete (19, 21). Further evaluation of the isolate was done at Auckland Hospital, Auckland, New Zealand, and at the University of Alberta Microfungus Collection and Herbarium, Edmonton, Alberta, Canada, where the isolate was deposited as accession no. UAMH 8729.
FIG. 2.
Microscopic appearance of hyphae of S. commune UAMH 8729. (A) Hyphae of the primary isolate formed spicules but lacked clamp connections at the septa. (B) Clamp connections formed on hyphae in the contact zone between a pairing of the case isolate and an isolate obtained from a single basidiospore (UAMH 7694). The arrow indicates a clamp connection. Magnification, ×580.
Cultures of the fungus on potato dextrose agar grown in the light of a window sill produced many small, tube-like structures measuring 1 to 2 mm in diameter by 2 to 4 mm in height and having a central cavity (Fig. 3A). Some of these developed into irregular to cup-shaped fruiting bodies (3 to 5 mm in diameter by 3 to 5 mm in height) (Fig. 3B), but they failed to develop into the typical fan-shaped basidiocarp of S. commune or to produce basidiospores. Similar aberrant fruiting bodies of S. commune have been described previously as medusoid (14). Moreover, no clamp connections were found in either the medusoid fruiting bodies or the mycelial mat, although occasional septa showed rounded protuberances.
FIG. 3.
Medusoid fruiting bodies of S. commune UAMH 8729 formed on potato dextrose agar placed in the light of a window sill. (A) Infertile tube-like structures (arrow). Magnification, ×2. (B) Infertile, larger, irregular to almost cup-shaped fruiting bodies.
To confirm the identity of this fungus as S. commune, the case isolate was tested for compatibility by growing it together with each of three single-basidiospore isolates of S. commune, UAMH 7693, 7694, and 7695, as described previously (21). Between each pairing of the case isolate and a single-basidiospore isolate, clamp connections were observed at the contact zone between advancing mycelia (Fig. 2B), thereby demonstrating dikaryotization and partial compatibility and confirming the identification. No basidiocarps were formed in pairings. No clamp connections were observed when the case isolate was grown alone on different media and under different conditions, except in two separate subcultures on potato dextrose agar, one incubated in the window and one incubated in a translucent container. Sectors of each of these cultures spontaneously developed clamps after approximately 3.5 months. Clamped and nonclamped hyphae were mounted in DAPI (4′,6-diamidino-2-phenylindole; Sigma-Aldrich Canada Ltd., Oakville, Ontario) and examined by fluorescence microscopy. The nonclamped hyphae contained a single nucleus per cell (monokaryon), while the clamped hyphae contained two nuclei per cell (dikaryon). Potato dextrose agar subcultures of the sectors having clamped hyphae developed typical fan-shaped basidiocarps and basidiospores after 3 weeks of incubation, in addition to tube-like medusoid structures similar to those observed in the original cultures.
Discussion.
S. commune occurs worldwide on a wide range of dead deciduous trees and uncommonly on vegetation such as hay, where its fan-shaped basidiocarps are easily identified (4, 19). Although S. commune is ubiquitous in nature, there are only rare reports of its association with human infections, including those of the brain, lung, and mouth (5, 15, 16, 20, 21). Animal studies have shown that S. commune is a fungal agent of relatively low virulence causing a progressive low-grade infection, with death occurring in some very young animals, and in which the progress of infection is influenced by the age of the host, the size of the inoculum, and prior treatment with an immunosuppressive agent (5, 6).
The incidence of fungal sinusitis, particularly in immunocompetent patients, appears to be increasing (23, 24). Although many cases of sinusitis have been attributed to the genus Aspergillus, several reports document the emerging role of S. commune in chronic or allergic sinusitis (2, 3, 8, 11, 17, 22) or other allergic pathologies (1, 7). Because the hyphae of this basidiomycete may appear similar to those of Aspergillus species in mucosal specimens, accurate diagnosis of S. commune infection relies on recognition of the fungus in culture.
Clinically and in the laboratory, confusion with Aspergillus infection can occur in the initial presentation and the microscopy of the specimen. Based on our patient’s history, nasal endoscopy, and CT scan, the initial clinical diagnosis was that of indolent invasive Aspergillus sinusitis mimicking a mucocele (18). The hyphae in the tissue also resembled those of Aspergillus species in being septate and light colored and in lacking clamp connections. In a study of human and animal S. commune infection, Greer (5) observed the presence of two kinds of hyphae in tissue, narrower filaments, 3 to 6 μm wide, and broader ones, up to 15 μm in width, each of which bore clamps and, rarely, spicules. However, in most reports concerning S. commune infection, clamps were either overlooked at initial examination or not found, and hyphae were of the narrower type, and then more similar to those of aspergilli (1, 15, 17, 21). Thus, isolation and accurate identification of the fungus are important in making the correct diagnosis of S. commune infection.
Dikaryotic isolates of S. commune are fairly readily recognized due to the presence of spicules and clamp connections on the hyphae and the development of fruiting bodies producing basidiospores. However, dikaryotic isolates may fail to fruit in the dark or on certain media or may lose their fruiting ability. Monokaryotic isolates are more difficult to recognize because they lack clamps and sometimes also spicules (19, 21) or they may demonstrate anomalous behavior, as in our case isolate. The unusual aspects of UAMH 8729 included (i) development of sterile medusoid structures from nonclamped mycelia, (ii) spontaneous development of clamps only in two subcultures that had been held for several months, and (iii) demonstration of typical fan-shaped basidiocarps mixed with medusoid forms once dikaryotized.
Basidiocarps of S. commune are produced by a heterokaryotic mycelium (containing distinct nuclear types) formed by the interaction of two compatible haploid mycelia (monokaryotic; i.e., derived from a single spore). The genetics of fruiting is complex and involves two compatibility factors, A and B, each having two loci and multiple alleles. Raper (13) has reported numerous deviations from normal sexual behavior, particularly under laboratory conditions. Raper and Krongelb (14) described several types of aberrant fruiting, including the medusoid type, in which elongated tube-like structures are sterile or fertile at the tips (i.e., forming basidiospores). Our patient’s isolate demonstrated sterile tube-like structures until spontaneous dikaryotization occurred, and then it produced more-typical basidiocarps forming basidiospores. Kern and Uecker (8) also described a medusoid variant causing sinusitis in a diabetic female, but their isolate differed from ours in forming clamp connections at an early stage of development and in forming medusoid structures that produced basidiospores.
Hyphal spicules may be considered a confirmatory feature in an otherwise sterile isolate, but difficulties are encountered when they are absent (i.e., in some monokaryons) or when personnel overlook them or confuse them with microconidia (Fig. 2A). The latter possibility has been advanced recently by Sigler and Kennedy (20), who suggested that isolates identified as Myriodontium keratinophilum (10) or Chrysosporium species (9) from cases of sinusitis may actually represent misidentified S. commune. The spicules of the latter could be confused especially with the conidia formed at the ends of narrow pegs, as occurs in M. keratinophilum. Based on the observations of Nobles, who defined the cultural characteristics of many basidiomycetes, the presence of spicules or spinulose projections on the hyphae has long been regarded as a special hyphal feature of S. commune, by far the commonest species of the genus (4). Spinulose hyphae have been reported in another species recently transferred to the genus (12), but the cultural features and distribution of other species of the genus are less well defined (4).
Clinical laboratories should not overlook basidiomycetes as potential opportunistic pathogens. It is likely that infections caused by S. commune are misdiagnosed or are not recognized because of the lack of familiarity of clinicians with this fungus and the difficulty many laboratories may have in identifying this basidiomycete. Any white, rapidly growing, sterile isolate with septate hyaline hyphae should be suspected as S. commune if (i) it grows well at 37°C; (ii) it forms a dense, tough (i.e., difficult to cut), woolly colony (but note that colonies of monokaryons are flat and thinly cottony); (iii) it is susceptible to cycloheximide (400 μg/ml); (iv) it tolerates benomyl (2 μg/ml); and (v) it has a pronounced and disagreeable odor. Confirmatory findings include (i) the presence of spicules on some hyphae (they may be absent in monokaryons), (ii) the presence of clamps at some septa, and (iii) formation of fan-shaped basidiocarps (lacking in monokaryons) when grown in the light. A monokaryon may be confirmed by demonstrating partial compatibility between it and single-basidiospore isolates (i.e., formation of clamp connections at the interface between paired mycelia). By keeping these features in mind, we hope that others will be able to correctly identify atypical isolates of this fungus so that a better understanding of its role in clinical disease will emerge.
Acknowledgments
L.S. acknowledges financial assistance from the Natural Sciences and Engineering Research Council of Canada.
We acknowledge assistance with nuclear staining from K. Mallett, Northern Forestry Centre, Edmonton, Alberta, Canada.
REFERENCES
- 1.Amitani R, Nishimura K, Niimi A, Kobayashi H, Nawada R, Murayama T, Taguchi H, Kuze F. Bronchial mucoid impaction due to the monokaryotic mycelium of Schizophyllum commune. Clin Infect Dis. 1996;22:146–148. doi: 10.1093/clinids/22.1.146. [DOI] [PubMed] [Google Scholar]
- 2.Catalano P, Lawson W, Bottone E, Lebenger J. Basidiomycetous (mushroom) infection of the maxillary sinus. Otolaryngol Head Neck Surg. 1990;102:183–185. doi: 10.1177/019459989010200217. [DOI] [PubMed] [Google Scholar]
- 3.Clark S, Campbell C K, Sandison A, Choa D I. Schizophyllum commune: an unusual isolate from a patient with allergic fungal sinusitis. J Infect. 1996;32:147–150. doi: 10.1016/s0163-4453(96)91436-x. [DOI] [PubMed] [Google Scholar]
- 4.Ginns J, Lefebvre M N L. Lignicolous corticioid fungi (Basidiomycota) of North America. St. Paul, Minn: American Phytopathological Society Press; 1993. [Google Scholar]
- 5.Greer D L. Basidiomycetes as agents of human infections: a review. Mycopathologia. 1977;65:133–139. doi: 10.1007/BF00447184. [DOI] [PubMed] [Google Scholar]
- 6.Greer D L, Bolanos B. Pathogenic potential of Schizophyllum commune isolated from a human case. Sabouraudia. 1973;11:233–244. [PubMed] [Google Scholar]
- 7.Kamai K, Unno H, Nagao K, Kuriyama T, Nishimura K, Miyaji M. Allergic bronchopulmonary mycosis caused by the basidiomycetous fungus Schizophyllum commune. Clin Infect Dis. 1994;18:305–309. doi: 10.1093/clinids/18.3.305. [DOI] [PubMed] [Google Scholar]
- 8.Kern M E, Uecker F A. Maxillary sinus infection caused by the homobasidiomycetous fungus Schizophyllum commune. J Clin Microbiol. 1986;23:1001–1005. doi: 10.1128/jcm.23.6.1001-1005.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy F E, Larson J T, George E, Maisel R H. Invasive Chrysosporium infection of the nose and paranasal sinuses in an immunocompromised host. Otolaryngol Head Neck Surg. 1991;104:384–388. doi: 10.1177/019459989110400317. [DOI] [PubMed] [Google Scholar]
- 10.Maran A G D, Kwong K, Milne L J R, Lamb D. Frontal sinusitis caused by Myriodontium keratinophilum. Br Med J. 1985;290:207. doi: 10.1136/bmj.290.6463.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marlier S, de Jaureguiberry J P, Aguilon P, Carloz E, Duval J L, Jaubert D. Sinusite chronique due a Schizophyllum commune au cours du SIDA. Presse Med. 1993;22:1107. [PubMed] [Google Scholar]
- 12.Nakasone K. Morphological and molecular studies on Auriculariopsis albomellea and Phlebia albida and a reassessment of A. ampla. Mycologia. 1996;88:762–775. [Google Scholar]
- 13.Raper J R. Life cycles, basic patterns of sexuality and sexual mechanisms. In: Ainsworth G S, Sussman A S, editors. The fungi. III. New York, N.Y: Academic Press; 1966. pp. 473–511. [Google Scholar]
- 14.Raper J R, Krongelb G S. Genetic and environmental aspects of fruiting in Schizophyllum commune Fr. Mycologia. 1958;50:707–740. [Google Scholar]
- 15.Restrepo A, Greer D L, Robledo M, Osorio O, Mondragon H. Ulceration of the palate caused by a basidiomycete Schizophyllum commune. Sabouraudia. 1973;11:201–204. [PubMed] [Google Scholar]
- 16.Rihs J D, Padhye A A, Good C B. Brain abscess caused by Schizophyllum commune: an emerging basidiomycete pathogen. J Clin Microbiol. 1996;34:1628–1632. doi: 10.1128/jcm.34.7.1628-1632.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal J, Katz R, DuBois D B, Morrissey A, Machicao A. Chronic maxillary sinusitis associated with the mushroom Schizophyllum commune in a patient with AIDS. Clin Infect Dis. 1992;14:46–48. doi: 10.1093/clinids/14.1.46. [DOI] [PubMed] [Google Scholar]
- 18.Rumans T M, Jones M, Ramirez S. Fungal sinusitis presenting as an ethmoid mucocoele. Am J Rhinol. 1995;9:247–249. [Google Scholar]
- 19.Sigler L, Abbott S P. Characterizing and conserving diversity of filamentous basidiomycetes from human sources. Microbiol Cult Collect. 1997;13:21–27. [Google Scholar]
- 20.Sigler L, Kennedy M J. Aspergillus, Fusarium, and other opportunistic moniliaceous fungi. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C: American Society for Microbiology; 1999. pp. 1212–1241. [Google Scholar]
- 21.Sigler L, de la Maza L M, Tan G, Egger K N, Sherburne R K. Diagnostic difficulties caused by a nonclamped Schizophyllum commune isolate in a case of fungus ball of the lung. J Clin Microbiol. 1995;33:1979–1983. doi: 10.1128/jcm.33.8.1979-1983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigler L, Estrada S, Montealegre A, Jaramilo E, Arango E, de Bedouf C, Restrepo A. Maxillary sinusitis caused by Schizophyllum commune and experience with treatment. J Med Vet Mycol. 1997;35:365–370. [PubMed] [Google Scholar]
- 23.Stammberger H. Special problems. In: Stammberger H, editor. Functional endoscopic sinus surgery. B.C. Philadelphia, Pa: Decker; 1991. p. 398. [Google Scholar]
- 24.Zieske L A, Kopke R D, Hamill R. Dematiaceous fungal sinusitis. Otolaryngol Head Neck Surg. 1991;105:567–577. doi: 10.1177/019459989110500408. [DOI] [PubMed] [Google Scholar]