Abstract
Background:
Frailty may be a risk factor for complications in inflammatory bowel diseases (IBD) patients. We examined the impact of treatment on IBD patients who were frail prior to treatment and identified predictors of post-treatment change in frailty.
Methods:
In an electronic health record-based cohort of IBD patients initiating anti-tumor necrosis factor (TNF)-α agents, we applied a validated claims-based frailty index to determine frailty in the one year prior to and after treatment initiation. We characterized treatment non-response using a composite outcome of IBD-related hospitalization, surgery, change in therapy, or initiation of systemic steroids. We constructed multivariable logistic regression models to identify determinants of post-treatment frailty.
Results:
The 1,210 patients initiating anti-TNF therapy had a median age of 30 years; 20% were ≥50 years. In the first year after anti-TNF initiation, 40% were non-responders. Many more treatment non-responders were frail in the year following treatment compared with treatment responders (27% v 7%, p<0.001). Pre-treatment frailty (OR 2.01, 95% CI 1.35 – 3.00) and prior IBD-related hospitalization (OR 1.63, 95% CI 1.15 – 2.30) were independently predictive of higher likelihood of post-treatment frailty. Therapy response was associated with a lower likelihood (OR 0.24, 95% CI 0.16 – 0.34) of post-treatment frailty. Nearly 85% of patients who were frail prior to treatment demonstrated improvement in frailty following treatment
Conclusions:
Response to anti-TNF therapy is an important determinant of post-treatment frailty in patients with IBD. Our findings suggest that effectively treating inflammatory states in older patients with IBD may improve frailty.
Keywords: Inflammatory Bowel Disease, Frailty, Function, Treatment, Anti-Tumor Necrosis Factor
Summary:
In this retrospective cohort study of 1,210 patients with inflammatory bowel disease initiating therapy with anti-tumor necrosis factor agents, we found patients who responded to therapy demonstrate an improvement in pre-treatment frailty.
INTRODUCTION
Inflammatory bowel diseases (IBD; Crohn’s disease (CD), ulcerative colitis (UC)) affect nearly 7 million people world-wide.1 They are associated with considerable morbidity, frequently resulting in irreversible bowel damage requiring hospitalizations or surgery. The availability of effective treatments has improved our ability to achieve clinical and endoscopic remission, contributing to a temporal reduction in the need for IBD-related surgery.2, 3 Yet, the potential for therapy-related complications such as serious infections and malignancy raises concerns regarding the use of such treatments in those perceived to be at a higher risk, particular older individuals and those with substantial comorbidity.4, 5
It is increasingly recognized that an individuals’ functional status rather than chronologic age may be a more important determinant of health outcomes.6, 7 Frailty, defined as a state of increased vulnerability from an aging-associated decline in reserve and function, is an important measure of functional status.8 The frailty syndrome was initially defined by Fried et al. as comprising at least three of the following features - low grip strength, fatigue, slowed walking speed, low physical activity, and unintentional weight loss.9 An individual was deemed to be in a ‘pre-frail’ state if one or two of these components were present. Complementarily, many studies of frailty in large databases are based on a deficit accumulation model which quantifies frailty as a proportion of abnormalities from a list of age-associated health deficits.10 In relation to IBD, we and others, have previously demonstrated that frailty is an important biologic determinant of outcomes including mortality, therapy-related infectious complications, and hospital readmission.11-13 However, prior work has not examined dynamic changes in frailty that may occur in patients with IBD, particularly in relation to treatment.
A leading hypothesis is that frailty is a down-stream sequela of chronic inflammatory processes.14 Furthermore, many of the features reported in the frailty phenotype, such as unintentional weight loss, fatigue and low strength, are common in the setting of active IBD. Therefore, while frailty may be a risk factor for disease- or treatment-related complications, it is conceivable that effective treatment of chronic inflammation may ameliorate frailty. With the rising burden of geriatric IBD in parallel with the recognition of the importance of early effective treatment to ensure optimal IBD outcomes, it is critically important to examine the impact of treating IBD on an individuals’ frailty.1, 15, 16 Using a large cohort of patients with IBD initiating anti-tumor necrosis factor-α (anti-TNF) biologic therapy, we examined the impact of treatment on frailty defined using a validated claims-based frailty index. Specifically, we examined the impact of treatment on individuals who were frail prior to treatment and identified predictors of post-treatment change in frailty.
METHODS
Study Population
The data source for this study consisted of an electronic health record (EHR)-based cohort of patients with CD or UC detailed previously.17-19 Briefly, from individuals receiving care within the MassGeneral Brigham healthcare system serving the greater Boston metropolitan area, we identified all patients with at least one International Classification of Diseases, 9th edition (ICD-9) code for CD or UC between 1996 and 2010. Using a combination of administrative codes (ICD-9 diagnosis or Current Procedural Terminology (CPT) procedure codes), electronic prescriptions for IBD-related medications, and free text narrative concepts identified using natural language processing, we developed a classification algorithm that had a positive predictive value ≥ 97% in identifying CD or UC. For this study, we included patients who had received a prescription for infliximab (IFX) or adalimumab (ADA) and had a follow-up data available for 1 year after treatment. In prior work, we demonstrated the ability to accurately identify therapy initiation in this cohort using the first electronic prescription date.20
Assessment of Frailty
The primary outcome of interest was frailty. To define this, we used a validated claims-based frailty index (CFI) developed by Kim et al.21 This score uses a combination of ICD-9 diagnosis codes, CPT codes for procedures, and HCPCS codes for supplies and services to calculate a composite score quantifying frailty. The CFI has good correlation with survey-based frailty assessments (rho=0.60) and other claims-based indices and is similar or superior to the Charlson comorbidity index (CCI) in predicting mortality and disability.
Here, we calculated the frailty index in the one year before (pre-treatment) and one year following (post-treatment) the first prescription for an anti-TNF agent. For this, we ascertained codes associated with any visit during the relevant time period. For ease of interpretation, we normalized the CFI score by presenting it in a range with a maximum value of 100 (which represented the highest CFI score in the entire cohort) [Normalized CFI = calculated CFI*100/maximum CFI in the cohort]. A higher value represented greater frailty. We classified patients as frail if their frailty score was in the highest quartile in the pre- or post-treatment period.
Change in Frailty
Among patients who had a CFI score above the median (more frail), we then examined change in frailty score post-treatment. We examined the impact of individual covariates including treatment response on this change in frailty.
Covariates
Covariates identified in the medical records included type of IBD, age at first anti-TNF prescription, sex, race and ethnicity and duration of follow-up in the system. We also noted if the patients had been hospitalized or undergone surgery prior to anti-TNF therapy. Patients were determined to be on combination therapy if they had a prescription for an immunomodulator at the time of anti-TNF initiation.
We classified therapy response used two methods. Firstly, we used an objective measure of therapy failure that included change in biologic therapy, a new prescription for systemic corticosteroids, IBD-related surgery, or IBD-related hospitalization in the year following anti-TNF initiation. Patients who did not meet these criteria were labeled ‘therapy responders’. A second, subjective measure of therapy response was our previously validated narrative non-response score.20 As described in prior work, this score was a sum of the narrative mentions of IBD-related symptoms identified using NLP in the year following therapy initiation. A higher score suggested the patient was more likely to be a non-responder. We also separately examined the number of narrative mentions of IBD-related symptoms including abdominal pain, rectal bleeding, diarrhea, and fatigue in the year following treatment initiation.
Statistical Analysis
Continuous variables were summarized using medians with inter-quartile ranges (IQRs) or means with standard deviations, while categorical variables were characterized using proportions and compared using the chi-square test. We first performed univariate logistic regression to calculate odds ratios (ORs) with 95% confidence intervals (CIs), identifying determinants of post-treatment frailty. Variables that were significant in this analysis at p < 0.10 were included in a final stepwise multivariable logistic regression model to identify determinants of post-treatment frailty. Variables significant at a two-sided p-value ≤ 0.05 were considered statistically significant. Among those who were frail pre-treatment, we examined the change in frailty post-treatment and defined factors impacting this change. We performed a priori specified subgroup analysis that stratified by age ≥50 years, sex, type of IBD, type of anti-TNF and pre-treatment frailty.
This study was approved by the MassGeneral Brigham Institutional Review Board.
RESULTS
Study Population
Our study cohort included 1,210 patients initiating anti-TNF with a median age at treatment initiation of 30 years (IQR 22 – 45 years). One-fifth (20%) of the cohort were aged 50 years or older (n=254) at anti-TNF initiation. Over half (54%) the cohort were women and most (88%) were white. Over two-thirds (71%) had CD. Most (77%) patients were initiated on IFX with 15% of patients having prior exposure to anti-TNF therapy. Only a small fraction of patients (9%) had undergone bowel resection previously while 46% had at least one prior IBD-related hospitalization. Over the one year of follow-up after anti-TNF initiation, 40% of patients (35% CD, 51% UC) were classified non-responders due to experiencing the composite adverse outcome of IBD-related hospitalization, surgery, change in therapy due to lack of efficacy, or initiation of systemic steroids.
The median pre-treatment frailty index was 8.1 (IQR −3.2 to 17.0, range −67.2 to 82.9). On univariate analysis, patients who were classified as frail pre-treatment were likely to be older (mean age 36.9 vs 33.9 years, p=0.010), have higher CCI (4.4 vs 2.5, p <0.001), and have required a prior IBD-related hospitalization (55% vs 44%, p=0.007). There was no difference in sex, race, type of IBD or prior bowel resection (Table 1). In the subgroup of 407 patients where the data were available, the mean pre-treatment C-reactive protein (CRP) was higher in those who were characterized as frail (39.9 vs 27.3mg/dL, p=0.030). There was no difference in the type of anti-TNF agent or use of combination therapy in frail compared to non-frail group.
Table 1:
Characteristics of patients with inflammatory bowel disease (IBD) initiating treatment with anti-tumor necrosis factor (TNF)-α agents, stratified by pre-treatment frailty
| Characteristic | Frail (n=189) N(%) |
Not frail (n=1,021) N(%) |
p-value |
|---|---|---|---|
| Median pre-treatment frailty index (IQR) | 22.0 (19.9 – 36.4) | 4.9 (−5.8 to 11.6) | <0.01 |
| Mean age at anti-TNF start (in years) (SD) | 36.9 (17.1) | 33.9 (15.8) | 0.01 |
| Sex | 0.07 | ||
| Female | 59 | 52 | |
| Male | 41 | 48 | |
| Race | 0.54 | ||
| White | 90 | 88 | |
| Non-white | 10 | 12 | |
| IBD type | 0.09 | ||
| Crohn’s disease | 76 | 70 | |
| Ulcerative colitis | 24 | 30 | |
| Mean Charlson Co-morbidity index (SD) | 4.4 (3.9) | 2.5 (2.8) | <0.01 |
| Prior anti-TNF therapy | 19 | 15 | 0.22 |
| Prior IBD-hospitalization | 55 | 44 | <0.01 |
| Prior IBD-surgery | 11 | 9 | 0.41 |
| Mean pre-treatment CRP (mg/dl) (SD) | 39.9 (61.7) | 27.3 (39.8) | 0.03 |
| Type of anti-TNF agent | 0.13 | ||
| Infliximab | 81 | 76 | |
| Adalimumab | 19 | 24 | |
| Combination therapy with immunomodulator | 35 | 33 | 0.54 |
SD: Standard Deviation
CRP: C-Reactive Protein
Therapy response is associated with a lower likelihood of post-treatment frailty
The median post-treatment frailty index was 9.1 (range −74.3 to 100). Over one-quarter (27%) of treatment non-responders were classified as frail in the year following treatment compared to only 7% of treatment responders (p <0.001) (Figure 1). Further supporting an association between therapy response and post-treatment frailty, those who were classified as frail post-treatment had higher likelihood of narrative non-response scores (indicating higher likelihood of therapy failure) (0.97 vs 0.42, p < 0.001), and more frequent narrative mentions of abdominal pain (70 vs 18, p < 0.001), diarrhea (9 vs 3, p < 0.001), rectal bleeding (19 vs 9, p < 0.001), and fatigue (2 vs 1, p=0.002) in the year following therapy initiation than those who were not frail.
Figure 1:
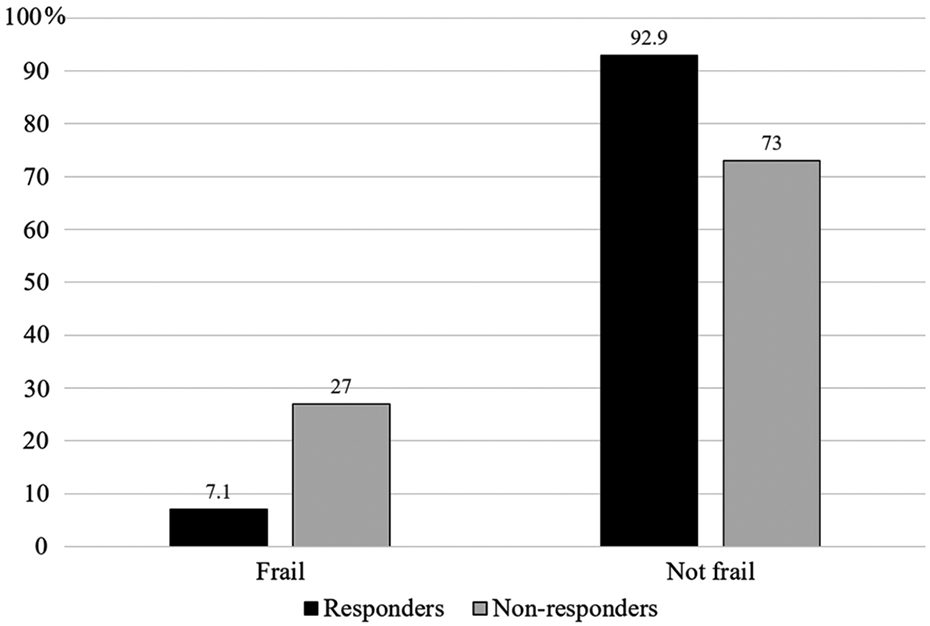
Post-treatment frailty status among treatment responders and non-responders in patients with inflammatory bowel diseases (IBD) initiating anti-tumor necrosis factor(TNF)-α therapy
On stepwise logistic regression, pre-treatment frailty (OR 2.01, 95% CI 1.35 – 3.00) and prior IBD related hospitalization (OR 1.63, 95% CI 1.15 – 2.30) were independently predictive of higher likelihood of post-treatment frailty. In contrast, therapy response was associated with a significantly lower likelihood (OR 0.24, 95% CI 0.16 – 0.34) of post-treatment frailty (Table 2). Introducing CCI in the model did not alter the association between therapy response and post-treatment frailty (OR 0.27, 95% CI 0.19 – 0.39). Repeating the analysis after excluding IBD-related hospitalization as a marker of therapy non-response did not alter our results.
Table 2:
Predictors of Post-treatment frailty on multivariable analysis
| Predictor | Odds ratio | 95% confidence intervals |
|---|---|---|
| Pre-treatment frailty | 2.01 | 1.35 – 3.00 |
| Prior IBD-related hospitalization | 1.63 | 1.15 – 2.30 |
| Treatment response | 0.24 | 0.16 – 0.34 |
Improvement in frailty in those who were frail pre-treatment
A total of 513 patients had pre-treatment frailty scores above the median. In this population, the mean pre-treatment frailty score decreased from 19.8 pre-treatment to 11.8 post-treatment (Δ=−7.96, 95% CI −9.53 to −6.39) (p < 0.001). The magnitude of improvement in frailty among those who had frailty scores above the median pre-treatment was similar in those over the age of 50 years at anti-TNF initiation (Δ= −8.85, 95% CI −19.95 to −2.0, p < 0.001) as in those younger than age 50 years (Δ= −7.75, 95% CI −17.60 to −0.87, p < 0.001). The improvement in frailty scores was also more striking among those with a high Charlson comorbidity index >2 at baseline (Δ=−10.31) than those with a low comorbidity burden (Δ=−5.72). Among those older than age 60 years at anti-TNF initiation, those who had a frailty index above the median in the year prior (n=42) experienced a mean improvement in frailty index by 8 points (Δ= −7.96, 95% CI −9.5 to −6.4, p=0.007) with a more striking improvement among therapy responders (Δ=−12.1, p=0.007).
The improvement in frailty was even more pronounced among those in the highest frailty quartile (pre: 29.4; post: 13.2, Δ= −16.2, p < 0.0001). Nearly 85% of patients who were frail prior to treatment demonstrated some improvement in frailty following treatment with half (45%) of the patients experiencing a ≥50% improvement in their frailty score in the year following treatment and 12% experiencing a ≥100% improvement.
DISCUSSION
It is increasingly recognized that restoration of normal functional status is an important goal of treatment of chronic inflammatory diseases, particularly in older patients who experience a disproportionate burden of disability adjusted life years. Here, we demonstrated that response to anti-TNF biologic therapy is an important determinant of post-treatment frailty in patients with CD or UC. Further, the improvement in frailty was particularly notable in those who were more frail prior to treatment including those over the age of 50 years while initiating therapy. Our findings suggest that effectively treating inflammatory states in older patients with IBD may improve frailty.
To our knowledge, there have been no prior studies examining the impact of biologic treatment on frailty outcomes in IBD or other immune-mediated diseases. However, a potential beneficial effect of such treatment strategies may be indirectly inferred from two separate observations. First, cross-sectional studies, primarily in those with rheumatoid arthritis (RA), demonstrate a strong association between disease activity and frailty. In a cross-sectional cohort of 100 patients with RA, the prevalence of frailty was only 6.7% among patients in remission, but 46.7% among those with high disease activity suggesting that inflammatory burden and frailty are correlated.22 Similarly, another cohort of patients with RA between the ages of 18 and 75 years suggested an independent effect of disease activity on frailty.23 In a cohort of 375 patients with RA between the ages of 40 and 79 years, frailty was associated higher disease activity scores.24
The second line of evidence supporting a potentially beneficial impact of treatment on frailty is from cross-sectional studies demonstrating a correlation between frailty and circulating markers of inflammation. In a study of 117 subjects aged 62-95 years, frailty was associated with elevated serum interleukin (IL)-6, soluble TNF-α Receptor-1, and soluble TNF-α Receptor-II.25 In a systematic review that comprised 35 studies which included 3,232 frail individuals, both frail and pre-frail patients had higher levels of CRP and IL-6 demonstrating the association between systemic inflammation and frailty.26 Other studies have demonstrated alterations in inflammatory pathways including in the T-cell compartment of the adaptive immune system and metabolomic changes such as changes in the kynurenine pathway in association with frailty.27, 28 Together, these lines of evidence suggest effective treatment of inflammation with anti-TNF biologic therapy in patients with IBD may have a beneficial effect on frailty, thereby improving functional status.
There is sparse literature examining frailty in IBD. Prior work has focused on the prevalence of frailty and its association with various disease outcomes including mortality, re-hospitalizations, and risk of treatment-related complications. In a large multi-institutional study, frailty was associated with increased mortality even after adjusting for age and comorbidity, demonstrating its independent effect as a modifier29. Two studies using national re-admission databases, while demonstrating the same association in the hospitalized IBD population, also demonstrated frailty to be associated with longer hospitalization stays and increased likelihood of re-hospitalization30, 31. We have previously demonstrated that among patients who were initiating immunomodulator or anti-TNF therapy, frailty prior to treatment predicted higher risk of infectious complications.12 However, the findings from the present study also suggest that treating frail patients with effective therapy may actually result in improvement in frailty. Further studies are essential to define the appropriate risk-benefit threshold for each patient on an individual level, weighing potential benefits against risks of such treatments. However, given the relatively low event rates of serious adverse events, our findings would suggest consideration for effective treatment even in frail patients with IBD as amelioration of the inflammatory burden may actually result in significant improvement in frailty.
We readily acknowledge several limitations to our work. First, since this was not a prospective trial, patients who were deemed candidates for biologic therapy may be different from the general IBD population. Second, as in any observational study, there is the potential for confounding though we adjusted for most potentially relevant variables including age and comorbidity. Third, there may be missing data from patients seeking care outside our healthcare system. However, the overall treatment response rate observed is similar to that reported from other observational cohorts and one would expect such bias to be non-differential. Since not all patients were systematically assessed for each contributing comorbidity in the pre- and post-treatment time periods and the contributing comorbidities differed between individuals, it was not possible to assess the most impactful elements that improved after treatment. The component(s) of the frailty phenotype that improve with effective treatment merit comprehensive examination in future studies. We also did not have information on the mechanism of anti-TNF failure. Finally, given the relatively younger age of an IBD cohort compared to other chronic diseases, it is possible that the magnitude of benefit was less striking given overall lower frailty scores at baseline. It is important for these studies to be conducted in large cohort, particularly of older patients, to assess the benefits of individual therapies as well as comparatively between different medical and surgical therapeutic options.
Certain caveats also need to be applied to the interpretation of the findings. Any change in frailty may not be attributable to biologic intervention alone. Institution of treatment could be accompanied by heightened medical attention to not only IBD but also other comorbidities and initiation of physical or nutritional therapy services which may contribute to impact. While these would not explain the fact that the improvement in frailty was more notable in those with a treatment response, as arguably all patients may have had access to the above services, there is a need for more nuanced prospective evaluation of both general and inflammation-targeted interventions and their impact on overall frailty as well as individual components of the frailty phenotype. The frailty score used was also primarily meant for research and may not be directly applicable in routine clinical practice. There is an important need to develop simple IBD-specific frailty scores for implementation in routine clinical care. In addition, there is need for longer term studies to examine the sustainability of impact of treatment on frailty.
In conclusion, we demonstrate that treatment of CD or UC with anti-TNF biologic therapy was associated with an improvement in frailty among those who responded to treatment. The greatest magnitude of benefit was noted in those were had higher frailty scores prior to treatment. Therapy for each patient needs to be individualized, balancing the potential adverse impact of frailty on treatment complications against the potential benefits and improvement in functional status with effective biologic therapy in those with CD or UC.
Acknowledgement:
Funding for this study was supported in part by Crohn’s and Colitis Foundation Career Development Award (568735 to BK)
Ananthakrishnan is supported by the National Institutes of Health, Crohn’s and Colitis Foundation, and the Chleck Family Foundation.
Footnotes
Conflict of Interest:
BK: Advisory board for Pfizer
WC: No conflicts of interest
ANA: Scientific Advisory Board for Ikena therapeutics and Gilead.
REFERENCES
- 1.Collaborators GBDIBD. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dittrich AE, Sutton RT, Haynes K, et al. Incidence Rates for Surgery in Crohn's Disease Have Decreased: A Population-based Time-trend Analysis. Inflamm Bowel Dis 2020. [DOI] [PubMed] [Google Scholar]
- 3.Barnes EL, Jiang Y, Kappelman MD, et al. Decreasing Colectomy Rate for Ulcerative Colitis in the United States Between 2007 and 2016: A Time Trend Analysis. Inflammatory Bowel Diseases 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cottone M, Kohn A, Daperno M, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol 2011;9:30–5. [DOI] [PubMed] [Google Scholar]
- 5.Toruner M, Loftus EV Jr., Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008;134:929–36. [DOI] [PubMed] [Google Scholar]
- 6.Pawelec G, Goldeck D, Derhovanessian E Inflammation, ageing and chronic disease. Curr Opin Immunol 2014;29:23–8. [DOI] [PubMed] [Google Scholar]
- 7.Ananthakrishnan AN, Shi HY, Tang W, et al. Systematic Review and Meta-analysis: Phenotype and Clinical Outcomes of Older-onset Inflammatory Bowel Disease. J Crohns Colitis 2016;10:1224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, et al. Frailty in Older Adults: Evidence for a Phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2001;56:M146–M157. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood K, Mitnitski A, Song X, et al. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc 2006;54:975–9. [DOI] [PubMed] [Google Scholar]
- 11.Kochar B, Cai W, Cagan A, et al. Frailty is independently associated with mortality in 11,001 patients with inflammatory bowel diseases. Aliment Pharmacol Ther 2020;52:311–318. [DOI] [PubMed] [Google Scholar]
- 12.Kochar B, Cai W, Cagan A, et al. Pretreatment Frailty Is Independently Associated With Increased Risk of Infections After Immunosuppression in Patients With Inflammatory Bowel Diseases. Gastroenterology 2020;158:2104–2111.e2. [DOI] [PubMed] [Google Scholar]
- 13.Faye A, Wen T, Colombel J, et al. Sa1836 FRAILTY AS A RISK FACTOR FOR HOSPITAL READMISSION IN PATIENTS WITH INFLAMMATORY BOWEL DISEASE: A NATIONWIDE STUDY. Gastroenterology 2020;158:S-445. [Google Scholar]
- 14.Ferrucci L, Fabbri E Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018;15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG Clinical Guideline. The American Journal of Gastroenterology 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 17.Ananthakrishnan AN, Cai T, Savova G, et al. Improving case definition of Crohn's disease and ulcerative colitis in electronic medical records using natural language processing: a novel informatics approach. Inflamm Bowel Dis 2013;19:1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ananthakrishnan AN, Cheng SC, Cai T, et al. Serum inflammatory markers and risk of colorectal cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12:1342–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ananthakrishnan AN, Cagan A, Cai T, et al. Diabetes and the risk of infections with immunomodulator therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2015;41:1141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ananthakrishnan AN, Cagan A, Cai T, et al. Identification of Nonresponse to Treatment Using Narrative Data in an Electronic Health Record Inflammatory Bowel Disease Cohort. Inflammatory Bowel Diseases 2016;22:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DH, Schneeweiss S, Glynn RJ, et al. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci 2018;73:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tada M, Yamada Y, Mandai K, et al. Correlation between frailty and disease activity in patients with rheumatoid arthritis: Data from the CHIKARA study. Geriatrics & Gerontology International 2019;19:1220–1225. [DOI] [PubMed] [Google Scholar]
- 23.Haider S, Grabovac I, Berner C, et al. Frailty in seropositive rheumatoid arthritis patients of working age: a cross-sectional study. Clin Exp Rheumatol 2019;37:585–592. [PubMed] [Google Scholar]
- 24.Kojima M, Kojima T, Waguri-Nagaya Y, et al. Depression, physical function, and disease activity associated with frailty in patients with rheumatoid arthritis. Mod Rheumatol 2020:1–8. [DOI] [PubMed] [Google Scholar]
- 25.Van Epps P, Oswald D, Higgins PA, et al. Frailty has a stronger association with inflammation than age in older veterans. Immun Ageing 2016;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Research Reviews 2016;31:1–8. [DOI] [PubMed] [Google Scholar]
- 27.Yao X, Li H, Leng SX Inflammation and immune system alterations in frailty. Clin Geriatr Med 2011;27:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westbrook R, Chung T, Lovett J, et al. Kynurenines link chronic inflammation to functional decline and physical frailty. JCI Insight 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochar B, Cai W, Cagan A, et al. Frailty is independently associated with mortality in 11 001 patients with inflammatory bowel diseases. Aliment Pharmacol Ther 2020;52:311–318. [DOI] [PubMed] [Google Scholar]
- 30.Faye AS, Wen T, Soroush A, et al. Increasing Prevalence of Frailty and Its Association with Readmission and Mortality Among Hospitalized Patients with IBD. Dig Dis Sci 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian AS, Nguyen NH, Elia J, et al. Frailty Is Independently Associated with Mortality and Readmission in Hospitalized Patients with Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


