Abstract
Background:
Non-prescribed opioid use is illegal in Vietnam. People who are apprehended for use of non-prescribed opioids may be arrested and incarcerated or sent to compulsory rehabilitation centers. For those on medication to treat opioid use disorder (MOUD), incarceration in either setting may disrupt treatment. This study estimates the effects of incarceration and compulsory rehabilitation on MOUD and HIV treatment outcomes in Vietnam.
Methods:
Data are from a clinical trial testing the effects of MOUD on HIV viral suppression in six Vietnamese HIV clinics. Participants were assessed quarterly for 12 months. We assessed the associations between incarceration or compulsory rehabilitation during months 0–9 and study outcomes of receipt of MOUD, HIV clinic engagement, and antiretroviral therapy prescription during months 9–12, among those who were released by month 9 of the study, using logistic regression and zero-inflated negative binomial models.
Results:
At nine months, 25 of 258 participants (9.7%) were incarcerated or sent to compulsory rehabilitation at least once and completed the month 9 assessment. Of those, 19 (76.0%) did not receive MOUD in months 9 through 12. Both incarceration and compulsory rehabilitation were negatively associated with subsequent receipt of MOUD (aOR=0.05, 95% CI= (0.01, 0.24); 0.14 (0.04, 0.50), respectively) and HIV clinic engagement (aOR=0.13, 95% CI= (0.03, 0.71); 0.09 (0.02, 0.39), respectively). In the final three months of the study, participants who were incarcerated had 42.5 fewer days of MOUD (95% CI= 23.1, 61.9), and participants in compulsory rehabilitation had 46.1 fewer days of MOUD (95% CI=33.8, 58.4) than those not incarcerated or in compulsory rehabilitation.
Conclusion:
Our findings suggest that both incarceration and compulsory rehabilitation disrupt MOUD and HIV treatment among people with HIV and Opioid Use Disorder in Vietnam. Prioritization of evidence-based strategies to support engagement in care for people who use drugs could potentially expand HIV and Opioid Use Disorder treatment access and curb substance use more effectively than reliance on incarceration or compulsory rehabilitation.
Keywords: Vietnam, Opioid-related disorders, prisons, HIV infections, buprenorphine, methadone
1. Introduction
Globally, 10.4 million people are incarcerated (United States Department of Justice National Institute of Corrections, 2015). Vietnam incarcerates 128 per 100,000 people, versus the United States which incarcerates 655 per 100,000 people (World Prison Brief, 2019). Similar to many countries, Vietnam approaches incarceration as a tool to deter drug use by individuals in the community (Windle, 2016). While the government began shifting to expand evidence-based treatment and harm reduction strategies for people who use drugs in Vietnam in 2006 (Jardine et al., 2012; Nguyen et al., 2012), incarceration remains common, and some jurisdictions require police quotas for certain offenses (Jardine et al., 2012). In Vietnam, a separate system of compulsory rehabilitation centers, also known as “06 centers”, exist with the goal of curbing drug use. While research does not support compulsory rehabilitation center’s ability to achieve this goal (Vuong et al., 2018), they remain a common mechanism to encourage abstinence from substance use.
In 2018, over 225,000 people were registered as people who use drugs in Vietnam (Regional Report on South East Asia). People who use opioids in Vietnam and are detained by police may enter two paths: people may be arrested and incarcerated in a jail or prison, or they may be sent to a compulsory rehabilitation at an “06 center”. Incarceration is overseen by the Ministry of Justice, whereas compulsory rehabilitation is overseen by the Ministry of Labor (Windle, 2016) and is more likely if the person previously did not adhere to medication for Opioid Use Disorder (OUD). 06 centers were established in 1993 when Vietnam passed Resolution 06/CP, requiring compulsory treatment for drug use (Giang et al., 2013). At the centers, people undergo non-medically supervised drug detoxification, and then receive vocational training and perform manual labor for up to two to four years (Hammett et al., 2008; Tomori et al., 2014; Vuong et al., 2012). Some using drugs enroll in 06 rehabilitation centers voluntarily or are referred to local authorities by family members and are detained for shorter time periods.
Both incarceration and 06 center use have been shown to be ineffective at treating or deterring drug use (HIV/AIDS & (UNAIDS), 2012; Human Rights Watch, 2011; Vuong et al., 2018). For people who have initiated medications for treatment of opioid use disorder (MOUD), incarceration and compulsory rehabilitation may be particularly disruptive, as MOUD is not available in most prisons or 06 centers (UNAIDS, 2016b; Vuong et al., 2018). Antiretroviral therapy (ART) is allowed at 06 centers but uptake is variable (UNAIDS, 2016b; Vuong et al., 2018). There may be additional challenges to reengaging in MOUD and HIV treatment after detention. For patients on methadone treatment, research in the United States has shown that withdrawal from methadone during incarceration adversely effects reengagement in MOUD after leaving prison (Maradiaga et al., 2016). In a separate study of people discharged from methadone treatment for any reason, only 36% of people had reengaged in treatment at 9 months post-discharge (Coviello et al., 2011). Studies in the United States have demonstrated low rates of reengagement in HIV care following release from prison, even among people who were on ART during incarceration (Baillargeon et al., 2009; Loeliger et al., 2018). Similarly, in Thailand, incarceration among people with HIV and OUD was negatively associated with initiation of ART (Schleifer, 2008). Three additional studies identified incarceration as positively associated with HIV incidence (Hayashi et al., 2009; Suntharasamai et al., 2009; Thomson et al., 2009). Globally, 80% of studies have identified negative effects of incarceration on HIV prevention or care (DeBeck et al., 2017). To date, no research has explored reengagement on MOUD or ART after incarceration or 06 compulsory rehabilitation in Vietnam.
The BRAVO study randomized people with HIV and opioid use disorder to receive HIV clinic-based buprenorphine/naloxone (BUP/NX, intervention) or referral to methadone treatment (MT, control) in Vietnam from July 2015 through January 2019 (Korthuis, 2020). The majority (84%) of participants reported prior incarceration, 64% reported prior compulsory rehabilitation, and incarceration or compulsory rehabilitation was the leading cause of study loss-to follow-up (Korthuis, 2020). Some study participants also experienced incarceration and/or compulsory rehabilitation during the 12 months of study participation. The objective of this study was to assess the effects of incarceration or compulsory rehabilitation, versus no incarceration or compulsory rehabilitation, during the trial on receipt of MOUD and HIV treatment in Vietnam.
2. Methods
2.1. Study setting and design
This study uses data from the “Buprenorphine to Improve HIV Care Engagement and Outcomes (BRAVO) Randomized Trial” (ClinicalTrials.gov NCT01936857) (Korthuis, 2020), which tested two strategies for treatment of opioid use disorder (OUD) in Vietnam among people living with HIV between July 2015 and February 2019. We trained HIV clinic physicians at six Vietnamese HIV clinics with high OUD prevalence (four in Hanoi, one in Tanh Hoa Province, and one in Bac Giang Province) to treat OUD using HIV clinic-based BUP/NX or refer participants to local methadone treatment (MT) programs. Additionally, clinic nursing staff, pharmacists, and counselors were trained regarding changes in treatment protocols. We then recruited people with HIV and untreated moderate-to-severe opioid use disorder (OUD) who were randomized to receive HIV clinic-based BUP/NX or referral to MT for treatment of OUD. The primary trial outcome in the BRAVO trial was achievement of HIV viral suppression at 12 months follow-up. This study was conducted in partnership with Hanoi Medical University, the Provincial AIDS Control authorities of Hanoi, Thanh Hoa and Bac Giang, and Oregon Health & Science University in Portland, Oregon. The study was approved by Institutional Review Boards at Oregon Health & Science University and Hanoi Medical University, and the Socialist Republic of Vietnam’s Ministry of Health Ethics Review Committee.
2.2. Participants
Participants were eligible for inclusion in BRAVO if they were HIV positive, had moderate-to-severe OUD as defined by DSM-5 (American Psychiatric Association), a positive urine drug screen for opioids at the time of enrollment, interest in receiving treatment for OUD, were age 18 or older, and were willing to practice birth control, if female. Interest in OUD treatment was assessed by HIV clinic physicians, who offered study participation with chance to receive buprenorphine or referral to methadone treatment. HIV clinic physicians were trained to offer methadone referral to patients desiring treatment but not wanting to participate in the RCT. Participants already receiving antiretroviral therapy (ART) were allowed to enroll.
Participants were ineligible if they had a known hypersensitivity to buprenorphine or naloxone, an aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level greater than five times the upper limit of normal, were currently pregnant or breastfeeding, had a serious medical or psychiatric illness in the past 30 days that precluded safe participation in the opinion of the study physician, or had received MT within the past 30 days.
For this analysis, only participants who were released from incarceration or an 06 center before their 9-month assessment were included. Those remaining incarcerated or in compulsory rehabilitation during months 9 to 12 (i.e., those without the opportunity to re-engage in community MOUD treatment or HIV care) were excluded, though patients re-incarcerated after month 9 were included. Participants incarcerated for the first time between months 9 and 12 were counted as not incarcerated. Participants who were incarcerated or in an 06 center were compared to patients who were not incarcerated or in an 06 center between months 0 and 9 of the study (Figure 1).
Figure 1.
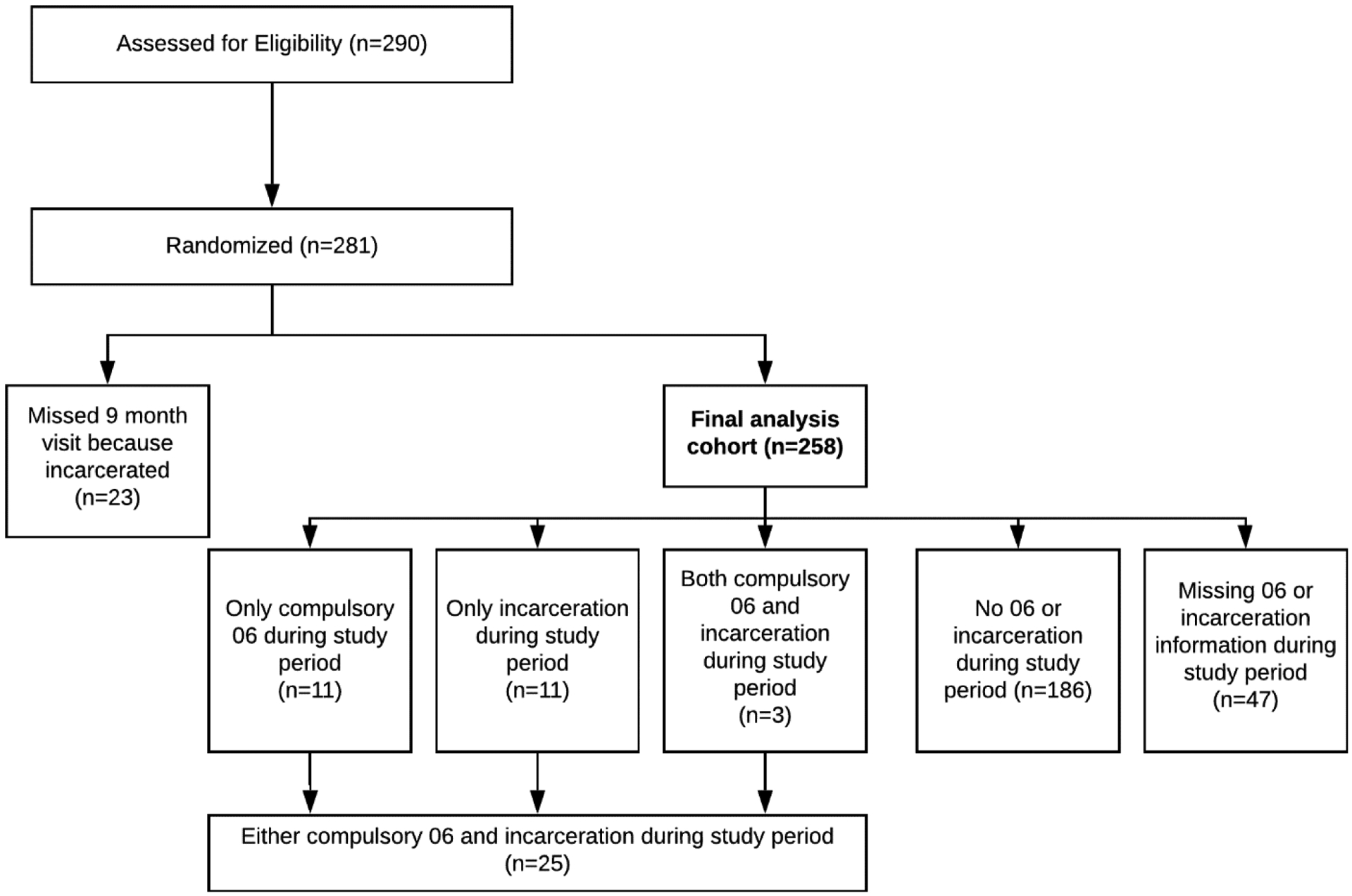
Analysis cohort from the BRAVO trial, 2015 to 2019
2.3. Measures
Research assistants administered surveys to participants in confidential settings using secured, web-based electronic data entry. All survey instruments were administered in Vietnamese. Participants were assessed every three months from baseline to study completion (12 months), for a total of five assessment time points.
We collected demographic information including age, sex, marital status, and education level. Baseline surveys also assessed if the participant had ever been arrested and convicted or placed in a compulsory rehabilitation center, the number of days the participant had participated in illegal acts for money in the previous 30 days (those who reported at least one day were considered to have participated in illegal acts and those who reported zero days were considered to not have participated in illegal acts), and the participant’s Depression Anxiety Stress Subscale (DASS) (Lovibond & Lovibond, 1995; Tran et al., 2013) and Multidimensional Scale of Perceived Social Support score (MSPSS) (Zimet et al., 1988; Zimet et al., 1990). Baseline lab values included urine drug screen for opioids, methamphetamine or amphetamines, CD4 count, and HIV RNA PCR (<200 copies/mL classified as viral suppression).
2.3.1. Receipt of MOUD and ART
Outcome variables were 1) receipt of MOUD, 2) HIV clinic engagement, and 3) active ART prescription; each was dichotomous. We used chart abstraction to identify if the participant 1) received BUP/NX or MT (receipt of MOUD), 2) had engaged in at least one HIV clinic visit (HIV clinic engagement), and 3) had an active antiretroviral therapy prescription (active ART prescription) and, between 9 to 12 months. We also analyzed the number of days participants had an active MOUD prescription during that time window based on review of BUP/NX and MT dosing logs.
2.4. Incarceration and compulsory rehabilitation
The independent variables were 1) incarceration (arrest, jail, or prison) or 2) compulsory 06 center rehabilitation during months 0 through 9 of study participation. At each visit, participants were asked if they 1) had been arrested, charged, and convicted in the past 3 months (incarceration), or if they 2) had spent time in compulsory rehabilitation in the previous 3 months (compulsory 06 center rehabilitation). If participants missed a study visit, we asked friends and family members, who had been previously identified by the research participant as someone they would allow us to contact in a detailed locator form collected at the time of written consent, why the person missed the study visit. We derived two exposure variables from this information: 1) incarceration during the first 9 months of the study and 2) compulsory rehabilitation during the first 9 months.
2.5. Data analysis
We used separate logistic regression models to evaluate the effect of 1) incarceration and 2) compulsory rehabilitation on the binary study outcomes of 1) receipt of MOUD, 2) HIV clinic engagement, and 3) active ART prescription. We specified covariates listed above a priori, based on prior literature and study team experience in Vietnam, and evaluated covariates for collinearity. We evaluated continuous covariates (age, DASS score, MSPSS score) for linearity in the log-odds and planned to transform covariates that were not approximately linear.
We used zero-inflated negative binomial (ZINB) models to evaluate the effects of incarceration and compulsory rehabilitation on the continuous study outcome (number of days of MOUD retention). Data cleaning and analyses were conducted in Stata version 16 (StataCorp, 2019).
We used complete case analysis to evaluate bivariate associations, and multiple imputation to handle missing values in adjusted logistic regression models (King et al., 2020). We did not conduct logistic regression analyses using complete case, as we did not believe data was Missing Completely At Random (King et al., 2020). We included all covariates considered for our model in our multiple imputation models, and ran each logistic regression model using 100 imputed data sets.
3. Results
Participants were predominately male (96.5%) with HIV viral suppression at baseline (66.9%), and had been either incarcerated (82.9%) or sent to compulsory rehabilitation (60.1%) at least once before the study (Table 1). Twenty-three participants missed their 9 months study visit because of incarceration or compulsory rehabilitation stay and were dropped from the analysis. From the study start through nine months, 25 of 258 participants (9.7%) were incarcerated (n=14) or sent to compulsory rehabilitation (n=14) at least once. Of those, 19 (76%) did not receive buprenorphine or methadone between months 9 and 12 (Figure 1, Table 2).
Table 1.
Baseline participant demographics in the BRAVO trial compared by 0 to 9 month incarceration and compulsory rehabilitation status, 2015 to 2019
| All participants (n=258) | Incarceration or compulsory rehabilitation (n=25) | No incarceration or compulsory rehabilitation (n=186) | Lost-to-follow-up (n=47) | p-value (chi-squared/t-test) | |
|---|---|---|---|---|---|
| Age | 38.5 (6.1) | 36.6 (5.1) | 39.1 (5.7) | 36.9 (7.6) | 0.03 |
| Sex (male) | 249 (96.5%) | 25 (100%) | 179 (96.2%) | 45 (95.7%) | >0.99 |
| Randomization group (MT) | 130 (50.3%) | 12 (48.0%) | 101 (54.3%) | 17 (36.2%) | 0.67 |
| 9th grade education | 106 (41.1%) | 10 (40.0%) | 82 (44.1%) | 14 (29.8%) | 0.83 |
| Married | 105 (40.7%) | 7 (28.0%) | 80 (43.0%) | 18 (38.2%) | 0.20 |
| History of incarceration at baseline | 214 (82.9%) | 22 (88.0%) | 154 (82.8%) | 38 (80.9%) | 0.77 |
| History of 06 at baseline | 155 (60.1%) | 18 (72.0%) | 122 (65.6%) | 25 (53.2%) | 0.28 |
| Virally suppressed (n=257) | 172 (66.9%) | 19 (76.0%) | 133 (71.5%) | 20 (42.6%) | 0.81 |
| Methamphetamine use | 23 (8.9%) | 2 (8.0%) | 14 (7.5%) | 7 (14.9%) | >0.99 |
| Antiretroviral therapy prescription (n=248) | 174 (70.2%) | 21 (84.0%) | 136 (73.1%) | 17 (36.2%) | 0.46 |
| Any illegal acts for money (n=254) | 2 (0.8%) | 0 | 2 (1.1%) | 0 | >0.99 |
| DASS (n=253) | 13.4 (9.9) | 16.4 (11.8) | 12.9 (9.7) | 13.6 (9.3) | 0.11 |
| MSPSS (n=243) | 3.8 (0.7) | 3.6 (0.6) | 3.8 (0.7) | 3.7 (0.7) | 0.28 |
Table 2.
Study incarceration exposures, outcomes, and unadjusted odds ratios using complete case analysis between 9 and 12 months, BRAVO trial, 2015–2019
| All participants (n=258) | MOUD (n=240) | HIV Clinic Engagement (n=258) | Active ART prescription (n=236) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (n=148) | No (n=92) | Yes (n=210) | No (n=48) | Yes (n=207) | No (n=29) | ||||||
| n (%) | n (%) | n (%) | OR (95% CI) | n (%) | n (%) | OR (95% CI) | n (%) | n (%) | OR (95% CI) | ||
| Incarcerated | Yes | 14 (5.4%) | 2 (1.4%) | 12 (13.0%) | 0.06 (0.01, 0.27) | 11 (5.2%) | 3 (6.3%) | 0.22 (0.05, 0.91) | 12 (5.8%) | 2 (6.9%) | 0.33 (0.07, 1.69) |
| No | 194 (75.2%) | 141 (95.3%) | 50 (54.3%) | - | 183 (87.1%) | 11 (22.0%) | - | 181 (87.4%) | 10 (34.5%) | - | |
| Missing | 50 (19.4%) | 5 (2.0%) | 30 (32.6%) | - | 16 (7.6%) | 34 (70.8%) | - | 14 (6.8%) | 17 (58.6%) | - | |
| Compulsory Rehabilitation | Yes | 14 (5.4%) | 4 (2.7%) | 10 (10.9%) | 0.15 (0.05, 0.50) | 11 (5.2%) | 3 (6.3%) | 0.20 (0.05, 0.83) | 13 (6.3%) | 1 (3.4%) | 0.80 (0.10, 6.68) |
| No | 193 (74.8%) | 138 (93.2%) | 52 (56.5%) | - | 183 (87.1%) | 10 (20.8%) | - | 179 (86.5%) | 11 (37.9%) | - | |
| Missing | 51 (19.8%) | 6 (4.1%) | 30 (32.6%) | - | 16 (7.6%) | 35 (72.9%) | - | 15 (7.2%) | 17 (58.6%) | - | |
Logistic regression models adjusted for age, sex, education, marital status, randomization group (office-based buprenorphine/naloxone versus MT) and the following baseline covariates: ART prescription, history of incarceration, history of compulsory rehabilitation, methamphetamine use, depression (DASS), social support (MSPSS). We found that both incarcerated (adjusted odds ratio [aOR] 0.05, 95% CI 0.01, 0.24) and compulsory rehabilitation (aOR 0.14, 95% CI 0.04, 0.50) were negatively associated with subsequent receipt of MOUD (Table 3). Similarly, being incarcerated (aOR=0.13, 95% CI= (0.03, 0.71) or sent to compulsory rehabilitation (0.09 (0.02, 0.39) negatively impacted HIV clinic engagement. There were similar associations between incarceration and decreased likelihood of ART prescription which did not reach the threshold for statistical significance (incarcerated: aOR=0.24, 95% CI = (0.04, 1.35); compulsory rehabilitation: aOR= 0.31, 95% CI = (0.05, 1.88)).
Table 3.
Adjusted logistic regression results examining incarceration or compulsory rehabilitation from 0 to 9 months on MOUD retention, HIV clinic engagement, and ART prescription from 9 to 12 months
| Incarceration model aOR (95% CI) | Compulsory rehabilitation model aOR (95% CI) | |
|---|---|---|
| MOUD Retention | 0.05 (0.01, 0.24) | 0.14 (0.04, 0.50) |
| HIV Clinic Engagement | 0.13 (0.03, 0.71) | 0.09 (0.02, 0.39) |
| ART Prescription | 0.24 (0.04, 1.35) | 0.31 (0.05, 1.88) |
All analyses adjusted for age, sex, education, marital status, randomization group (office-based buprenorphine/naloxone versus MT) and the following baseline covariates: ART prescription, history of incarceration, history of compulsory rehabilitation, methamphetamine use, depression (DASS), social support (MSPSS).
Zero-inflated negative binomial models suggested 42.5 fewer days of MOUD between 9 and 12 months for participants who were incarcerated between 0 and 9 months (95% CI= 23.1, 61.9) (Table 4). Similarly, participants in compulsory rehabilitation had 46.1 fewer days of MOUD (95% CI=33.8, 58.4).
Table 4.
Number of days of medication exposure between 9 and 12 months from zero-inflated negative binomial models, by incarceration or 06 exposure status
| Yes (number of days) | No (number of days) | Mean difference | |
|---|---|---|---|
| Incarceration | 12.3 | 54.8 | 42.5 (23.1, 61.9) |
| Compulsory rehabilitation | 9.3 | 55.5 | 46.1 (33.8, 58.4) |
4. Discussion
In this study, we found that incarceration and compulsory rehabilitation similarly and substantially decreased the odds of reinitiating MOUD and HIV clinic visits upon release. Participants who were incarcerated or in compulsory rehabilitation centers during the first nine months of the study were nearly 10 times less likely to use MOUD after release than those not incarcerated or in compulsory rehabilitation. Similarly, both incarceration and compulsory rehabilitation decreased the number of days participants were on MOUD following release.
Our findings suggest that both incarceration and compulsory rehabilitation decrease engagement in substance use disorder care for participants prescribed MOUD. Our data further suggest that people who undergo compulsory rehabilitation experience interruptions in OUD and HIV treatment to the same degree as those incarcerated. In both settings, providing access to MOUD is essential, as providing access to MOUD to people who are incarcerated can prevent death post-release (Marsden et al., 2017). MOUD was not available in Vietnamese prisons or compulsory rehabilitation centers in participating provinces during the BRAVO trial, though recent pilot studies of methadone in some prisons have emerged in Vietnam (UNODC, 2015). However, lack of access to MOUD in prison and compulsory rehabilitation settings alone likely does not explain the lower use of MOUD after discharge from these settings, as most participants had previously initiated MOUD. Jourdey et al. describe a conceptual model that elucidates causes of post-release opioid-related overdose, which is the leading cause of death for people released from incarceration in the U.S., Sweden, and Australia (Joudrey et al., 2019).
In their model, the authors highlight that the incarceration itself is an adverse exposure for post-discharge outcomes, underlying the potential interaction of other important predictors (e.g., HIV infection, prior trauma). While some of the effect of decreased MOUD uptake following release may come directly from MOUD-related access challenges and attitudes, structural factors may contribute, as well, and suggest potentially modifiable policy interventions. For example, the requirement to abstain from drug use following both incarceration and compulsory rehabilitation is likely to impede reengagement in MOUD upon release, as patients must acknowledge heroin use to enroll. Currently, there are no direct pathways to treatment for people newly released from either setting. Interventions to improve direct linkages to MOUD following release could improve outcomes.
Exposure to incarceration may similarly impact post-discharge HIV care. A large meta-analysis found that for people with HIV who largely had access to ART during incarceration, HIV care engagement and ART use following release both rapidly decreased, often to levels below pre-incarceration levels (Iroh et al., 2015). The authors identify that co-occurring substance use may contribute to decreased engagement in HIV care, highlighting the uniquely vulnerable group of people with HIV and a substance use disorders who are incarcerated. These patients who may be at high risk of poor transitions and reengagement with both substance use disorder and HIV care (Chitsaz et al., 2013). In Vietnam, ART is available in prisons and 06 centers, though uptake is variable. Site differences in ART availability may explain why some patients were able to maintain ART therapy in our trial, and why it did not reach statistical significance in models. However, the lower rates of HIV clinic engagement post-prison or 06 release versus those who were not in prison or in 06 is worrying, and it is possible that the impact of decreased HIV care engagement may have impacted patients after the conclusion of the study.
Importantly, self-reported illegal activities were rare and did not differ by incarceration or 06 status in our study. While social desirability bias may be at play, notions that participants were arrested because they were involved in higher levels of criminal activity are not supported by our data. Punishment is not an effective strategy for reducing long-term substance use. Evidence-based interventions to engage people in care instead of incarcerating them or sending them to 06 centers can help reduce crime, improve public health, and restore people who use drugs to full participation in their communities (UN Office on Drugs and Crime, 2007). For those incarcerated or in compulsory rehabilitation, medication for both OUD and HIV should be provided based on evidence-based protocols, in stigma-free settings. However, opportunities to engage in HIV care and OUD care may not be enough to reengage participants after release. Additional work should evaluate pathways to continuing to reduce incarceration and compulsory rehabilitation in Vietnam and globally.
There were several limitations of this research. First, Vietnam is a middle-income country with resources devoted to improving care for people with OUD and HIV. These study results may not be generalizable to countries where incarceration or treatment pathways are different from Vietnam’s. Second, participants who were released before their 9-month study visit may be different than participants who remained incarcerated beyond 9 months. Third, information from close contacts about incarceration and detention status may be less reliable than information from prison logs, or other sources, directly. Fourth, sample size among those who were incarcerated or in compulsory rehabilitation was small. Additional work is needed to understand potential differences in impact of incarceration or compulsory rehabilitation in different populations, and stratified by important variables, including gender and by comorbid substance use disorders. Fifth, research participants were recruited from HIV clinics, who are likely different from patients who were not seen at HIV clinics prior to incarceration or compulsory rehabilitation. However, this sample would only serve to increase the proportion of patients on ART in the months after release from incarceration or compulsory rehabilitation in our study. Future work should also evaluate the impact of incarceration or compulsory rehabilitation on initiation of ART. Sixth, because our exposure of interest was incarceration or compulsory rehabilitation in the first 9 months of the study, we classified participants who were only incarcerated or in compulsory rehabilitation in the final 3 months of the study as not incarcerated or in compulsory rehabilitation. Because it is unlikely the participants received MOUD, our measures of association and estimates of difference in MOUD days may underestimate disparities. Finally, we were unable to capture exactly how long people were incarcerated in our study; there may be a dose response to incarceration that we were unable to assess.
5. Conclusion
Our analysis suggests that, similar to incarceration, compulsory rehabilitation center detention may be at odds with Vietnam’s goal of increasing access to medications for opioid use disorder and supporting patient engagement in HIV care (UNAIDS, 2016a). People who were incarcerated or in compulsory rehabilitation during the first 9 months of study had markedly decreased use of MOUD and decreased engagement in HIV care during the final 3 months of the study. After release, they fared no better than study participants who were incarcerated in prison settings with no therapeutic model. Countries that use incarceration and compulsory rehabilitation to deter and treat substance use should consider less punitive policies, incarcerate fewer people for substance use, and support alternative pathways to health other than compulsory rehabilitation. Support for expansion of evidence-based programs and policies for treatment of drug use may facilitate better engagement and retention in HIV and substance use treatment.
Highlights.
Nearly 10% of participants were incarcerated or in compulsory rehabilitation
Of those, 75% did not receive medication for OUD at follow-up
Both exposures were negatively associated with MOUD receipt & HIV clinic engagement
Funding:
This research was supported through grants from the National Institutes of Health National Institute on Drug Abuse (R01DA037441, UG1DA015815) and National Center for Advancing Translational Sciences (UL1TR002369), which provided support of REDCap, the web application this study used for data collection.
Conflicts of Interest:
No authors have financial conflicts of interest. Dr. Korthuis serves as principal investigator for NIH-funded trials that accept donated study medications from Alkermes (extended release naltrexone) and Indivior (buprenorphine-naloxone). Dr. Waddell is an investigator on multiple NIH-sponsored trials that received in kind study drug from Indivior and Alkermes. Dr. Waddell is PI of a CDC-sponsored trial which received in-kind study drug from Alkermes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnosttic and statistical manual of mental disorders: DSM 5 (5th ed.). American Psychiatric Publishing, Inc. [Google Scholar]
- Baillargeon J, Giordano TP, Rich JD, Wu ZH, Wells K, Pollock BH, & Paar DP (2009, Feb 25). Accessing antiretroviral therapy following release from prison. JAMA, 301(8), 848–857. 10.1001/jama.2009.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitsaz E, Meyer JP, Krishnan A, Springer SA, Marcus R, Zaller N, Jordan AO, Lincoln T, Flanigan TP, Porterfield J, & Altice FL (2013, Oct). Contribution of substance use disorders on HIV treatment outcomes and antiretroviral medication adherence among HIV-infected persons entering jail. AIDS Behav, 17 Suppl 2, S118–127. 10.1007/s10461-013-0506-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coviello DM, Zanis DA, Wesnoski SA, Lynch KG, & Drapkin M (2011, Mar). Characteristics and 9-month outcomes of discharged methadone maintenance clients. J Subst Abuse Treat, 40(2), 165–174. 10.1016/j.jsat.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBeck K, Cheng T, Montaner JS, Beyrer C, Elliott R, Sherman S, Wood E, & Baral S (2017, Aug). HIV and the criminalisation of drug use among people who inject drugs: a systematic review. Lancet HIV, 4(8), e357–e374. 10.1016/S2352-3018(17)30073-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giang LM, Ngoc LB, Hoang VH, Mulvey K, & Rawson RA (2013, Dec). Substance use disorders and HIV in Vietnam since Doi Moi (Renovation): an overview. J Food Drug Anal, 21(4), S42–S45. 10.1016/j.jfda.2013.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammett TM, Wu Z, Duc TT, Stephens D, Sullivan S, Liu W, Chen Y, Ngu D, & Des Jarlais DC (2008, Jan). ‘Social evils’ and harm reduction: the evolving policy environment for human immunodeficiency virus prevention among injection drug users in China and Vietnam. Addiction, 103(1), 137–145. 10.1111/j.1360-0443.2007.02053.x [DOI] [PubMed] [Google Scholar]
- Hayashi K, Milloy MJ, Fairbairn N, Kaplan K, Suwannawong P, Lai C, Wood E, & Kerr T (2009, Dec 30). Incarceration experiences among a community-recruited sample of injection drug users in Bangkok, Thailand. BMC Public Health, 9, 492. 10.1186/1471-2458-9-492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIV/AIDS, J. U. N. P. o., & (UNAIDS). (2012). Joint UN Statement Calls for the Closure of Compulsory Drug Detention and Rehabilitation Centers http://www.unaids.org/en/resources/presscentre/featurestories/2012/march/20120308adetentioncenters
- Human Rights Watch. (2011). The Rehab Archipelago: Forced Labor and Other Abuses in Drug Detention Centers in Southern Vietnam. http://www.hrw.org/sites/default/files/reports/vietnam0911ToPost.pdf
- Iroh PA, Mayo H, & Nijhawan AE (2015, Jul). The HIV Care Cascade Before, During, and After Incarceration: A Systematic Review and Data Synthesis. Am J Public Health, 105(7), e5–16. 10.2105/AJPH.2015.302635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine M, Crofts N, Monaghan G, & Morrow M (2012, Jul 9). Harm reduction and law enforcement in Vietnam: influences on street policing. Harm Reduct J, 9, 27. 10.1186/1477-7517-9-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joudrey PJ, Khan MR, Wang EA, Scheidell JD, Edelman EJ, McInnes DK, & Fox AD (2019, Apr 15). A conceptual model for understanding post-release opioid-related overdose risk. Addict Sci Clin Pract, 14(1), 17. 10.1186/s13722-019-0145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Englander H, Priest KC, Korthuis PT, & McPherson S (2020, Mar 5). Addressing Missing Data in Substance Use Research: A Review and Data Justice-based Approach. J Addict Med. 10.1097/ADM.0000000000000644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, King C, Cook RR, Tong KT, Kunkel L, Bart G, Nguyen T, Thanh Thuy D, Bielavitz S, Nguyen DB, Nguyen TMT, Giang LM (2020). HIV Clinic-Based Buprenorphine versus Methadone Maintenance Referral for Treatment of Opioid Use Disorder in Vietnam HIV Clinics: a Non-Blinded, Randomized Non-inferiority Trial The Lancet HIV. https://doi.org/InPress [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeliger KB, Meyer JP, Desai MM, Ciarleglio MM, Gallagher C, & Altice FL (2018, Oct). Retention in HIV care during the 3 years following release from incarceration: A cohort study. PLoS Med, 15(10), e1002667. 10.1371/journal.pmed.1002667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond SH, & Lovibond PF (1995). Manual for the Depression Anxiety Stress Scales (Vol. 2). Psychology Foundation of Australia. [Google Scholar]
- Maradiaga JA, Nahvi S, Cunningham CO, Sanchez J, & Fox AD (2016, Mar). “I Kicked the Hard Way. I Got Incarcerated.” Withdrawal from Methadone During Incarceration and Subsequent Aversion to Medication Assisted Treatments. J Subst Abuse Treat, 62, 49–54. 10.1016/j.jsat.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden J, Stillwell G, Jones H, Cooper A, Eastwood B, Farrell M, Lowden T, Maddalena N, Metcalfe C, Shaw J, & Hickman M (2017, Aug). Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction, 112(8), 1408–1418. 10.1111/add.13779 [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Nguyen LT, Pham MD, Vu HH, & Mulvey KP (2012). Methadone maintenance therapy in Vietnam: an overview and scaling-up plan. Adv Prev Med, 2012, 732484. 10.1155/2012/732484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regional Report on South East Asia. https://data.consilium.europa.eu/doc/document/ST-13786-2019-INIT/en/pdf
- Schleifer R, Kaplan K, Suwannawong P. (2008). Deadly denial: barriers to HIV/AIDS treatment for people who use drugs in Thailand. Oral Abstract Session: AIDS 2008 - XVII International AIDS Conference. [Google Scholar]
- StataCorp. (2019). Stata Statistical Software: Release 16. StataCorp LLC. [Google Scholar]
- Suntharasamai P, Martin M, Vanichseni S, van Griensven F, Mock PA, Pitisuttithum P, Tappero JW, Sangkum U, Kitayaporn D, Gurwith M, Choopanya K, & Bangkok Vaccine Evaluation G (2009, Feb). Factors associated with incarceration and incident human immunodeficiency virus (HIV) infection among injection drug users participating in an HIV vaccine trial in Bangkok, Thailand, 1999–2003. Addiction, 104(2), 235–242. 10.1111/j.1360-0443.2008.02436.x [DOI] [PubMed] [Google Scholar]
- Thomson N, Sutcliffe CG, Sirirojn B, Keawvichit R, Wongworapat K, Sintupat K, Aramrattana A, & Celentano DD (2009, Jul). Correlates of incarceration among young methamphetamine users in Chiang Mai, Thailand. Am J Public Health, 99(7), 1232–1238. 10.2105/AJPH.2008.136648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomori C, Go VF, Tuan le N, Huong NM, Binh NT, Zelaya CE, Celentano DD, Dat do T, & Quan VM (2014, Sep). “In their perception we are addicts”: social vulnerabilities and sources of support for men released from drug treatment centers in Vietnam. Int J Drug Policy, 25(5), 897–904. 10.1016/j.drugpo.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TD, Tran T, & Fisher J (2013). Validation of the depression anxiety stress scales (DASS) 21 as a screening instrument for depression and anxiety in a rural community-based cohort of northern Vietnamese women. BMC Psychiatry, 13, 24. https://doi.org/1471-244X-13-24[pii]10.1186/1471-244X-13-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN Office on Drugs and Crime. (2007). Handbook of basic principles and promising practices on Alternatives to Imprisonment (Criminal Justice Handbook Series, Issue. https://www.un.org/ruleoflaw/files/alternativestoimprisonment_handbook%20of%20basic%20principles.pdf [Google Scholar]
- UNAIDS. (2016a). Viet Nam is the first country in Asia to commit to new HIV treatment targets. https://www.unaids.org/en/resources/presscentre/featurestories/2016/november/20161125_vietnam
- UNAIDS. (2016b). Viet Nam opens its first opioid substitution therapy service for prisoners. https://www.unaids.org/en/resources/presscentre/featurestories/2016/november/20161125_vietnam
- United States Department of Justice National Institute of Corrections. (2015). World Prison Population List Eleventh Edition. Retrieved July 27, 2020 from https://nicic.gov/world-prison-population-listeleventh-edition
- UNODC. (2015). Viet Nam opens the first methadone maintenance therapy service unit for prisoners. https://www.unodc.org/southeastasiaandpacific/en/vietnam/2015/10/prisoners/story.html
- Vuong T, Ali R, Baldwin S, & Mills S (2012, Jul). Drug policy in Vietnam: a decade of change? Int J Drug Policy, 23(4), 319–326. 10.1016/j.drugpo.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Vuong T, Ritter A, Shanahan M, Ali R, Nguyen N, Pham K, Vuong TTA, & Le GM (2018, Apr). Outcomes of compulsory detention compared to community-based voluntary methadone maintenance treatment in Vietnam. J Subst Abuse Treat, 87, 9–15. 10.1016/j.jsat.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Windle J (2016). A Slow March from Social Evil to Harm Reduction: Drugs and Drug Policy in Vietnam. Foreign Policy at Brookings. https://www.brookings.edu/wp-content/uploads/2016/07/WindleVietnam-final.pdf
- World Prison Brief. (2019). Retrieved July 27, 2020 from https://www.prisonstudies.org/highest-to-lowest/prison-population-total
- Zimet G, Dahlem N, Zimet S, & Farley G (1988, March/01). The Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment - J PERSONAL ASSESS, 52, 30–41. 10.1207/s15327752jpa5201_2 [DOI] [Google Scholar]
- Zimet GD, Powell SS, Farley GK, Werkman S, & Berkoff KA (1990, Winter). Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess, 55(3–4), 610–617. 10.1080/00223891.1990.9674095 [DOI] [PubMed] [Google Scholar]


