Abstract
Background
The amygdala is vital in processing psychological stress and predicting vulnerability or resilience to stress-related disorders. This study aimed to build the link between functional magnetic resonance imaging data obtained before the stress event and the subsequent stress-related depressive symptoms.
Methods
Neuroimaging data obtained before the coronavirus disease 2019 pandemic from 39 patients with major depressive disorder (MDD) and 61 health controls (HCs) were used in this study. The participants were divided retrospectively into four groups in accordance with the severity of depressive symptoms during the pandemic: remitted patients, non-remitted patients, depressed HCs (HCd) and non-depressed HCs (HCnd). Seed-based resting-state functional connectivity (rsFC) analyses of the amygdala and its subregions, including the centromedial (CM), the basolateral and the superficial (SF), were performed.
Results
Vulnerability to depression was suggested by decreased rsFC between the left CM amygdala and the bilateral lingual gyrus in the HCd group compared with the HCnd group, and decreased rsFC of the left CM or right SF amygdala with the precuneus and the postcentral gyrus in the HCd group compared with patients with MDD. No evidence supported the rsFC of the amygdala or its subregions as a biomarker for the resilience of patients with MDD to stress under antidepressant treatment.
Limitations
Smaller sample size and no longitudinal neuroimaging data.
Conclusions
Our findings suggested that the rsFC of amygdala subregions may represent a neurobiological marker of vulnerability to depression following stress.
Keywords: Amygdala, COVID-19, Vulnerability, Depression, Resting-state functional connectivity
1. Introduction
The coronavirus disease 2019 (COVID-19) was first reported in China at the end of 2019, and it spread rapidly across China and many countries worldwide. During its early stage, rapid transmission of COVID-19 and lack of effective treatment and mass isolation measures caused common psychological problems, such as depression, anxiety, fear and other stress-related problems, amongst those infected, their close contacts, medical workers and the general public (He et al., 2021; Kang et al., 2020; Wu et al., 2021; Xiang et al., 2020a). Undoubtedly, COVID-19 has become an important new psychological stressor (Vinkers et al., 2020). Many psychiatric patients have been infected, and they are regarded as highly vulnerable to contracting COVID-19 (Xiang et al., 2020b, 2020c). Moreover, psychiatric patients are more susceptible to the COVID-19 stressor due to their disordered mental states (Vinkers et al., 2020). However, the reaction of individuals to stress varies (Faye et al., 2018). Some patients with major depressive disorder (MDD) can continue remission under the protection of drug treatment when they are exposed to stress, whereas others cannot. Similarly, some normal individuals are more vulnerable to stress and experience mental health problems under stress, whereas others do not (Osorio et al., 2017; Vinkers et al., 2020). Vulnerability and resilience are two different outcomes that can emerge from stressful events (Hegde and Mitra, 2020). Individuals who can cope better with stress exhibit resilience, which refers to the capability to counter the negative effects of stress (Hegde and Mitra, 2020). When faced with stress, low resilience (i.e. vulnerability) can predict high risk of mental disorders, such as depression and anxiety (Faye et al., 2018). Therefore, exploring individual differences in vulnerability or resilience to stress is necessary to boost mental resilience for dealing successfully with the COVID-19 stressor.
The amygdala plays an important role in many psychiatric disorders, such as depression, anxiety and post-traumatic stress disorder (PTSD); it has been extensively studied in psychopathology as a hub region that underlies emotional processing, including emotional regulation, perception and memory (Sergerie et al., 2008). Most notably, previous studies have highlighted the importance of the amygdala in processing psychological stress. Convergent studies have determined that stress exposure exerts a significant effect on amygdala function. A study about resting-state functional connectivity (rsFC) found that stress-exposed people exhibited decreased rsFC between the amygdala and the superior temporal gyrus (STG) relative to health controls (HCs) (Liu et al., 2021). A research on Chinese earthquake survivors reported that the PTSD group presented increased positive rsFC between the amygdala and the medial prefrontal cortex relative to a trauma-exposed control group without PTSD (Zhang et al., 2016). Veterans with PTSD demonstrated increased rsFC between the amygdala and the insula compared with combat controls without PTSD (Sripada et al., 2012). Previous studies have also suggested the role of the amygdala in vulnerability or resilience to stress. Leaver et al. (2018) reported that increased amygdala function during rest was associated with low emotional resilience (i.e. vulnerability to stress). However, another study indicated that increased amygdala reactivity may reflect resilience enhancement or stress buffering (Yamamoto et al., 2017). Although these studies suggest the influence of stress exposure on the amygdala and link the amygdala with vulnerability or resilience to stress, whether amygdala activity is a neurobiological marker for vulnerability or resilience to stress still needs to be determined. To this end, it is necessary to build the link between functional characteristics of the amygdala measured by functional magnetic resonance imaging (fMRI) data obtained before the stress event (e.g. COVID-19 pandemic) and the subsequent stress-related behaviors, such as depression.
The amygdala is a heterogeneous nucleus that is part of the limbic system. Previous neuroimaging studies have regarded the amygdala as a unitary structure; however, increasing evidence suggests that the subregions of the amygdala exhibit distinct structures and specialised functions (Brown et al., 2014; Bzdok et al., 2013; Kerestes et al., 2017; Liu et al., 2021; Roy et al., 2009). The amygdala includes three major subregions, namely, the centromedial (CM), basolateral (BL) and superficial (SF) amygdala, which modulate different functions by extensively connecting with different cortical and subcortical regions (Bzdok et al., 2013; LeDoux, 2007). The CM amygdala, which is the primary output region of the amygdala (Kerestes et al., 2017; Sah et al., 2003), sends projections to the brain stem, hypothalamic and striatal regions; it is associated with motor behaviour and response preparation (Bzdok et al., 2013). The BL amygdala receives afferents from the cortical and subcortical regions, including the hippocampus and the thalamus; it is associated with learning process (Bzdok et al., 2013; LeDoux, 2003). The SF amygdala, which is adjacent to the BL amygdala, is connected with the insula, striatum and olfactory cortical areas; it is associated with olfactory and emotional stimuli (Bzdok et al., 2013; Price, 2003). The distinct connectivity patterns of amygdala subregions have been reported by using task-based or resting-state fMRI in patients with MDD or PTSD and healthy volunteers (Bzdok et al., 2013; Liu et al., 2021; Roy et al., 2009; Tang et al., 2019).
RsFC is a fundamental method for aiding the understanding of brain network alterations, that is, exploring the correlations of activities amongst different brain regions in mental disorders (Deco and Kringelbach, 2014). By using rsFC, a recent typhoon-related PTSD study found that the PTSD group exhibited increased rsFC between the BL amygdala and the prefrontal cortices compared with the trauma-exposed control group, and both of the groups presented reduced rsFC between the BL amygdala and the STG compared with the HC group (Liu et al., 2021). However, similar to studies on vulnerability or resilience to stress (Cisler et al., 2013; Kaiser et al., 2018; Thomason et al., 2015), all of these studies are cross-sectional research and failed to elucidate whether the abnormal rsFC of amygdala subregions is a risk or consequence of stress exposure due to the unpredictability of stress events. Thus, the identification of neurobiological vulnerability (i.e. the opposite of resilience) markers prior to stress exposure is meaningful for predicting whether people will have an onset of depressive symptoms following the COVID-19 pandemic.
In the current study, we used rsFC to explore whether the abnormal rsFC of the amygdala and its subregions assessed prior to the COVID-19 pandemic is associated with depressive symptoms following stress exposure. To our knowledge, this work is the first to explore a pre-existing risk neural marker of amygdala subregions for predicting vulnerability to depression following the COVID-19 pandemic. We aimed to test two specific hypotheses in this research.
Hypothesis 1: In HCs with depressive symptoms (i.e. the HCd group) after stress (i.e. the COVID-19 pandemic), the rsFCs of the amygdala or its subregions before stress were different from those in HCs without depressive symptoms (i.e. the HCnd group), possibly indicating vulnerability to psychological stress (i.e. the opposite of resilience). However, the rsFC patterns in the HCd group were also different from those in patients with MDD before stress.
Hypothesis 2: In MDD patients who experienced remission (i.e. the MDDr group) under antidepressant treatment during the pandemic, the rsFCs of the amygdala or its subregions before stress were different from those in MDD patients without remission (i.e. the MDDnr group). This hypothesis may be helpful in identifying the neural basis for the resilience to stress of MDD patients under antidepressant treatment.
For complete statistics, we also compared MDD patients with the HCnd group to explore the abnormity in the rsFC of the amygdala and its subregions.
2. Methods
2.1. Subjects
The initial samples were obtained from an ongoing fMRI study to explore resting-state brain imaging changes after antidepressant therapy. This study included 85 patients with MDD and 113 HCs. This dataset provided an ‘in case of not seeking’ opportunity for the implementation of the current research. All the participants completed fMRI scans prior to the COVID-19 pandemic (from June 13, 2018 to December 24, 2019). All patients were recruited from Beijing Anding Hospital, Capital Medical University. They were diagnosed in accordance with the Mini International Neuropsychiatric Interview (M.I.N.I.) 5.0.0 (Sheehan et al., 1998), a short structured clinical interview based on DSM-IV. The recruited patients scored ≥14 on the 17-item Hamilton Depression Rating Scale (Hamilton, 1967) and ≥11 on the 16-item Quick Inventory of Depressive Symptomatology (QIDS) (Liu et al., 2014, 2013). The patients were drug-naive or off psychotropic drugs for at least 14 days before the study. They agreed to take escitalopram or duloxetine for treatment. The exclusion criteria included the following: (1) present or previous history of other psychiatric disorders, (2) serious neurological disease or head trauma, (3) severe somatic diseases, (4) alcohol or drug dependence, (5) evident suicidal ideation or behaviour, (6) pregnancy or lactation and (7) any contraindications to undergo MRI scan (such as metal clips or implants). The included HCs were also assessed by the M.I.N.I. 5.0.0 to exclude any DSM-IV Axis I diagnosis. They reported no history of psychiatric illness and no family history of major psychiatric illness in first-degree relatives. All healthy participants met the same additional exclusion criteria as the patients with MDD. Thus, they were free of neurological disease or head trauma, severe somatic disease and alcohol or drug dependence and were not pregnant or lactating. They were matched with the patients in terms of age and gender, and they scored ≤5 on QIDS when they were recruited.
During the COVID-19 pandemic, we sent online questionnaires (from February 25, 2020 to February 28, 2020) to all the patients and HCs to determine their mental states and provided timely online mental health services to those in need. The assessment questionnaires consisted of QIDS (Liu et al., 2014, 2013), Patient Health Questionnaire-9 (PHQ-9) (Kroenke et al., 2001), Generalised Anxiety Disorder-7 (GAD-7) (Spitzer et al., 2006), Perceived Stress Scale (PSS) (Wang et al., 2011) and a self-developed Stress Behavior Scale induced by COVID-19 (SBSC). We received 100 completed questionnaires from 39 patients with MDD and 61 HCs. In accordance with the online QIDS score of >5, the participants were retrospectively divided into four groups: MDD patients with remission (MDDr), MDD patients without remission (MDDnr), HCs with depressive symptoms (HCd) and HCs without depressive symptoms (HCnd). All procedures used in this study were approved by the Human Research and Ethics Committee of Beijing Anding Hospital, Capital Medical University. All participants provided informed consent before this study.
2.2. MRI data acquisition
All imaging data were collected by using a 3.0T Siemens Prisma MRI scanner with a 64-channel phased-array head coil at the Radiology Department of Beijing Anding Hospital, Capital Medical University. Structural and functional images were acquired for each subject. The subjects were instructed to remain awake with their eyes closed and to focus on nothing in particular. Functional images were obtained using an echo-planar imaging sequence: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; flip angle (FA) = 90°; matrix = 64 × 64; field of view (FOV) = 200 × 200 mm2; number of slices = 33, with a thickness of 3.5 mm; gap = 0.7 mm and voxel size = 3.13 × 3.13 × 4.2 mm3. Two hundred volumes of resting-state fMRI were collected. T1 structural images were obtained using a magnetisation-prepared rapid acquisition gradient echo sequence: TR = 2530 ms; TE = 1.85 ms; FA = 15°; matrix = 256 × 256; FOV = 256 × 256 mm2; number of slices = 192, with a thickness of 1 mm and voxel size = 1 × 1 × 1 mm3.
2.3. fMRI data preprocessing
DPABI v4.5 (http://rfmri.org/dpabi), a data processing assistant toolkit (Yan et al., 2016) whose functional implementation is required to be based on Statistical Parametric Mapping (SPM 12, http://www.fil.ion.ucl.ac.uk/spm), was used to preprocess all MRI images. To maintain signal equilibrium, the first five volumes of resting-state fMRI were removed. Then, the preprocessing steps included corrections for slice timing; corrections for head motion; segmentation for structural images; nuisance variable regression consisting of the Friston 24-parameter model of head motion (Friston et al., 1996), signals of white matter and cerebrospinal fluid (the first five principal components of signals were extracted) and linear and quadratic trends; spatial normalisation to the Montreal Neurological Institute (MNI) space through the segmentation of T1 images (Ashburner and Friston, 2005); resampling of each voxel to 2 × 2 × 2 mm3; smoothing with a full width at half maximum kernel of 4 mm and temporal band-pass filtering (0.01–0.1 Hz). In addition, a volume-based framewise displacement (FD) was calculated to quantify head motion (Power et al., 2012). The averaged time series of the volume-based FD was used to compute mean FD (Power et al., 2012). Considering the potential effect of head motion on rsFC, we excluded participants with mean FD values of more than three standard deviations. One patient was excluded under this criterion. We used scrubbing regressors of head motion, in which time points with a threshold of FD > 0.5 mm and one backward and two forward frames were identified as ‘bad’ points and modelled as separate regressors in the regression model of the realigned resting fMRI data whilst removing nuisance covariates (Yan et al., 2013). Participants who had at most 100 ‘bad’ points of data were allowed. Therefore, 38 patients and 61 HCs were included for the subsequent analyses.
2.4. Seed-based rsFC analysis of the amygdala and its subregions
The SPM Anatomy Toolbox was used to form the seeds of amygdala subregions (i.e. CM, BL and SF amygdala), which were derived from histological properties of the amygdala on the basis of the cytoarchitectonic assessment of 10 human postmortem brain (Amunts et al., 2005; Eickhoff et al., 2005). We used maximum probability maps to keep each voxel nonoverlapping and assign each voxel exclusively to a single region. The three subregions were resampled to 2 × 2 × 2 mm3, and the result was used to analyse rsFC. The voxels of each subregion of the amygdala were as follows: the CM amygdala left 43 voxels, right 11 voxels; the BL amygdala left 237 voxels, right 215 voxels; the SF amygdala left 36 voxels, right 48 voxels.
The rsFC analysis was computed using DPABI v4.5. The time series of each voxel were extracted within the seed. The mean time series were calculated by averaging the time series of each voxel within the seed region. Then, Pearson's correlation coefficients were computed between the mean time series of each seed and the time series of each voxel within the brain. After conducting Fisher r-to-z translation, z-score maps were acquired. These maps were used to conduct second-level analysis.
2.5. Statistical analysis
We conducted two sample t-tests between groups on the basis of our hypotheses. To test Hypothesis 1, we firstly compared the differences between the HCd and HCnd groups to investigate whether the rsFC of the amygdala and its subregions in the HCd group was different from those in the HCnd group before stress, indicating vulnerability to psychological stress (i.e. the opposite of resilience). We further compared the differences in the rsFC of the amygdala and its subregions between the HCd group and the MDDr or MDDnr group to validate whether the rsFC patterns in the HCd group were different from those in patients with MDD before stress. To test Hypothesis 2, we compared the differences between the MDDr and MDDnr groups to clarify the neural basis of MDD patients’ resilience under antidepressant treatment. For complete data analyses, we also compared the MDDr or MDDnr group with the HCnd group.
The demographics and clinical traits of the four groups were compared via two-sample t-test or χ2 test, which were performed using IBM SPSS Statistics 23. The two-sample t-test in SPM 12 was used to calculate the differences of rsFC in the amygdala and its subregions between any two groups. In this step, we controlled for age, gender, education level and mean FD as covariates. We set the threshold to p < 0.001 at the voxel level and corrected the family-wise error rate (FWE) to p < 0.05 at the cluster level.
3. Results
3.1. Sample characteristics
The demographic variables and clinical traits were summarised in Table 1 . No significant differences were found in terms of age, gender and education level between any two groups (ps > 0.05). Before stress, 18 participants had taken escitalopram in the MDDr group; 19 participants had taken escitalopram, and 1 participant had taken duloxetine in the MDDnr group. No difference in the distribution of antidepressant treatment type was found between the two groups (p > 0.05). The QIDS and PHQ-9 scores of the HCd or HCnd groups were significantly lower than those of the MDDr and MDDnr groups (ps < 0.001). No differences were found in the QIDS and PHQ-9 scores between the MDDr and MDDnr groups (ps > 0.05), demonstrating similar severity of illness amongst the two patient groups before stress. During the COVID-19 pandemic, the QIDS scores were significantly different between any two groups (ps < 0.05). The PHQ-9, GAD-7 and PSS scores of the HCd and MDDnr groups were all significantly higher than those of the HCnd and MDDr groups (ps < 0.05). However, no differences in the PHQ-9, GAD-7 and PSS scores were noted between the HCd and MDDnr groups or between the MDDr and HCnd groups (ps > 0.05). The SBSC scores of the HCd and MDDnr groups were all significantly higher than those of the HCnd group (ps < 0.05).
Table 1.
Demographic variables and clinical information.
|
Mean ± SD |
pvalue |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCd | HCd | HCd | MDDr | MDDr | MDDnr | |||||
| vs. | vs. | vs. | vs. | vs. | vs. | |||||
| MDDr | MDDnr | HCd | HCnd | HCnd | MDDr | MDDnr | MDDnr | HCnd | HCnd | |
| Age | 27.78 ± 5.93 | 26.75 ± 8.32 | 25.80 ± 4.60 | 25.41 ± 3.26 | 0.721 | 0.300 | 0.693 | 0.667 | 0.124 | 0.494 |
| Gender (M/F) | 8/10 | 5/15 | 5/10 | 12/34 | 0.832 | 0.722 | 0.712 | 0.307 | 0.154 | 0.926 |
| Education level a | 2/12/4 | 3/14/3 | 0/11/4 | 1/31/14 | 1.000 | 0.700 | 0.335 | 0.892 | 0.280 | 0.067 |
| Treatment (E/D) | 18/0 | 19/1 | − | − | − | − | − | 1.000 | − | − |
| Mean FD | 0.17 ± 0.09 | 0.16 ± 0.08 | 0.13 ± 0.07 | 0.13 ± 0.07 | 0.951 | 0.212 | 0.246 | 0.827 | 0.094 | 0.131 |
| ‘Bad’ points | 6.83 ± 11.22 | 5.75 ± 8.55 | 4.07 ± 5.51 | 2.30 ± 6.14 | 0.327 | 0.391 | 0.511 | 0.738 | 0.120 | 0.114 |
| QIDS | 15.17 ± 4.18 | 17.50 ± 3.07 | 1.47 ± 1.19 | 1.50 ± 1.31 | 0.931 | <0.001⁎ | <0.001⁎ | 0.056 | <0.001⁎ | <0.001⁎ |
| QIDS b | 2.89 ± 1.64 | 12.90 ± 3.63 | 9.80 ± 4.95 | 1.67 ± 1.52 | <0.001⁎ | <0.001⁎ | 0.040⁎ | <0.001⁎ | 0.007⁎ | <0.001⁎ |
| PHQ-9 | 15.83 ± 5.63 | 17.55 ± 4.40 | 1.93 ± 1.53 | 2.04 ± 1.81 | 0.833 | <0.001⁎ | <0.001⁎ | 0.299 | <0.001⁎ | <0.001⁎ |
| PHQ-9 b | 2.28 ± 1.53 | 9.50 ± 3.79 | 9.07 ± 4.30 | 1.46 ± 2.10 | <0.001⁎ | <0.001⁎ | 0.754 | <0.001⁎ | 0.136 | <0.001⁎ |
| GAD-7 b | 1.17 ± 1.69 | 4.85 ± 3.59 | 4.73 ± 3.96 | 0.96 ± 1.70 | 0.002⁎ | <0.004⁎ | 0.928 | <0.001⁎ | 0.657 | <0.001⁎ |
| PSS b | 10.67 ± 4.75 | 17.35 ± 6.71 | 15.47 ± 6.11 | 11.04 ± 5.86 | 0.015⁎ | 0.016⁎ | 0.400 | 0.001⁎ | 0.791 | <0.001⁎ |
| SBSC b | 23.00 ± 7.88 | 27.50 ± 10.68 | 28.20 ± 11.25 | 21.39 ± 8.81 | 0.045⁎ | 0.144 | 0.852 | 0.152 | 0.502 | 0.018⁎ |
Notes: aEducation level was divided into three categories: junior high school, senior high school and junior college/bachelor's degree/master's degree or above.
bThese questionnaires were measured during the COVID-19 pandemic. ⁎p < 0.05. Abbreviations: MDDr: MDD patients with remission; MDDnr: MDD patients without remission; HCd: HCs with depressive symptoms; HCnd: HCs without depressive symptoms; M: male; F: female; E: escitalopram; D: duloxetine; QIDS: 16-item Quick Inventory of Depressive Symptomatology; PHQ-9: Patient Health Questionnaire-9; GAD-7: Generalised Anxiety Disorder-7; PSS: Perceived Stress Scale; SBSC: self-developed Stress Behavior Scale induced by COVID-19.
3.2. rsFC of the amygdala and its subregions
3.2.1. rsFC of the CM amygdala
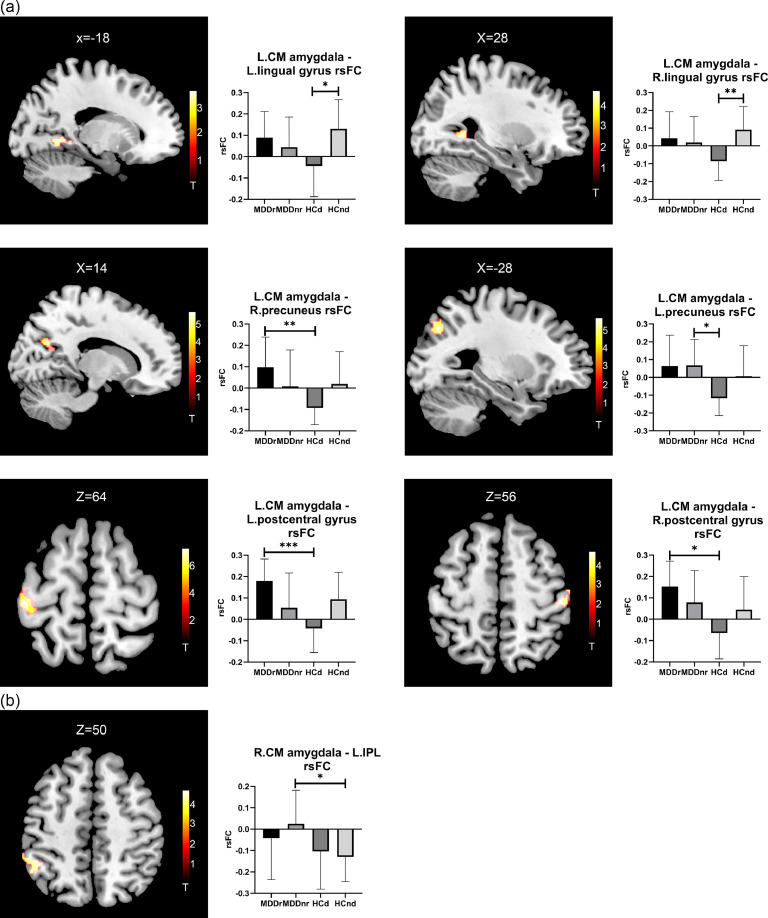
As shown in Fig. 1 , the HCd group exhibited decreased rsFC between the left CM amygdala and the bilateral lingual gyrus compared with the HCnd group. The HCd group also presented decreased rsFC between the left CM amygdala and the right precuneus compared with the MDDr group and decreased rsFC between the left CM amygdala and the left precuneus compared with the MDDnr group. We also observed that the HCd group exhibited decreased rsFC between the left CM amygdala and the bilateral postcentral gyrus compared with the MDDr group. These group differences support our hypothesis that the rsFC of the amygdala and its subregions in the HCd group is different from those in the HCnd group and patients with MDD before stress.
Fig. 1.
Brain regions showing significant rsFC differences with the CM amygdala amongst the four groups. The left part shows the significant brain regions, and the right part shows the averaged rsFC of the four groups through bar graphs. (a) Regions showing significant rsFC differences with the left CM amygdala amongst the four groups. (b) Regions showing significant rsFC differences with the right CM amygdala amongst the four groups. Abbreviations: CM: centromedial; IPL: inferior parietal lobule. *p < 0.05, **p < 0.01, ***p < 0.001.
However, no group differences in the rsFC of the left or right CM amygdala were found between the MDDr and MDDnr groups.
In addition, we found that the MDDnr group presented increased rsFC between the right CM amygdala and the left inferior parietal lobule (IPL) compared with the HCnd group (Fig. 1 and Table 2 ).
Table 2.
Group differences in the rsFC of the amygdala and its subregions.
| Seeded region | Hemisphere | Area | BA | Cluster size | MNI coordinates | Peak T values | Cluster-level pFWE |
|---|---|---|---|---|---|---|---|
| Left CM amygdala | |||||||
| HCd < HCnd | Left | Lingual gyrus | 19 | 111 | −14 −52 −8 | 3.87 | 0.037 |
| Right | Lingual gyrus | 30 | 147 | 28 −52 2 | 4.97 | 0.009 | |
| HCd < MDDr | Right | Precuneus | 31/7 | 176 | 14 −66 26 | 6.02 | 0.002 |
| Left | Postcentral gyrus | 3/4 | 406 | −44 −26 64 | 6.20 | <0.001 | |
| Right | Postcentral gyrus | 3/1 | 104 | 48 −24 52 | 4.51 | 0.030 | |
| HCd < MDDnr | Left | Precuneus | 19/7 | 115 | −28 −72 38 | 5.30 | 0.022 |
| Right CM amygdala | |||||||
| MDDnr > HCnd | Left | IPL | 40 | 145 | −46 −48 48 | 4.33 | 0.010 |
| Right SF amygdala | |||||||
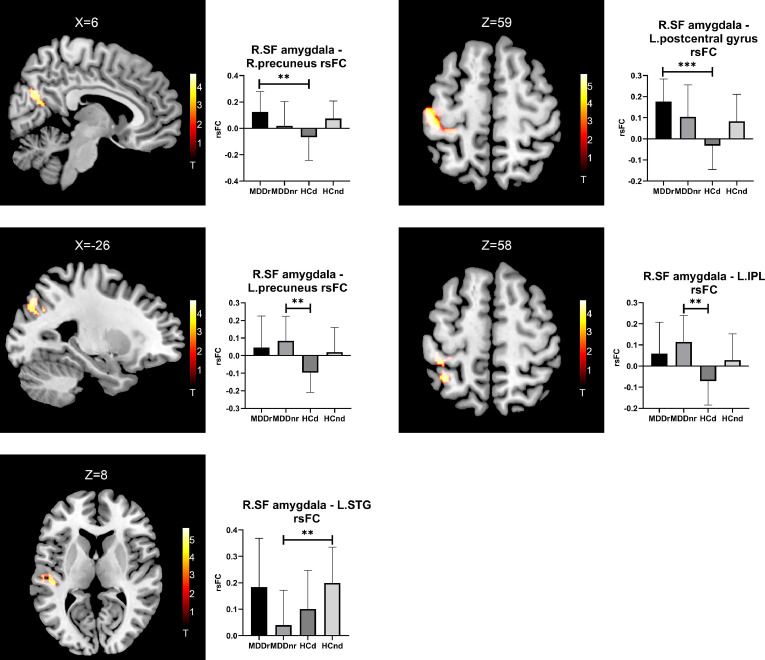
| HCd < MDDr | Right | Precuneus | 31/7 | 152 | 6 −68 28 | 4.63 | 0.004 |
| Left | Postcentral gyrus | 3/4 | 457 | −44 −28 60 | 5.82 | <0.001 | |
| HCd < MDDnr | Left | Precuneus | 19/7 | 153 | −26 −76 40 | 4.74 | 0.005 |
| Left | IPL | 40/3 | 174 | −36 −50 58 | 4.65 | 0.002 | |
| MDDnr < HCnd | Left | STG | 41 | 172 | −48 −24 4 | 5.10 | 0.003 |
| Left amygdala | |||||||
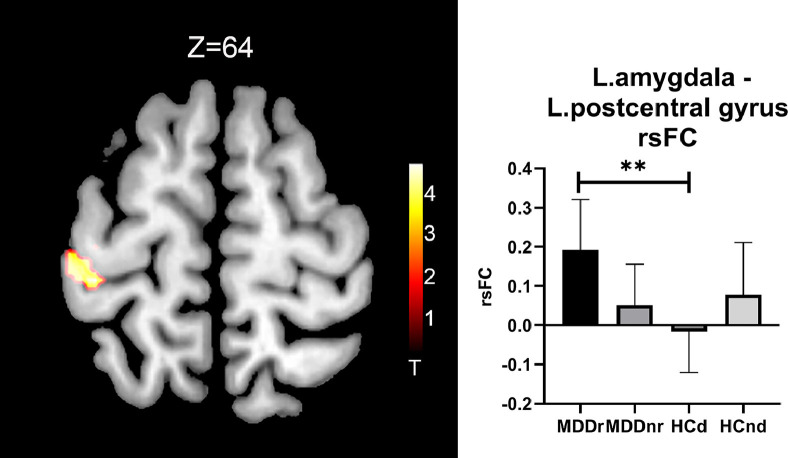
| HCd < MDDr | Left | Postcentral gyrus | 3/4 | 135 | −40 −30 62 | 4.95 | 0.008 |
Notes: Only the results that remained from the multiple comparison corrections (voxel-level threshold of puncorrected < 0.001, cluster-level threshold of pFWE < 0.05) are listed in this table.
Abbreviations: MDDr: MDD patients with remission; MDDnr: MDD patients without remission; HCd: HCs with depressive symptoms; HCnd: HCs without depressive symptoms; CM: centromedial;
SF: superficial; IPL: inferior parietal lobule; STG: superior temporal gyrus.
3.2.2. rsFC of the SF amygdala
No group differences in the rsFC of the SF amygdala were found between the HCd and HCnd groups. However, the HCd group demonstrated decreased rsFC between the right SF amygdala and the right precuneus and the left postcentral gyrus compared with the MDDr group, and decreased rsFC between the right SF amygdala and the left precuneus and the left IPL compared with the MDDnr group.
No group differences in the rsFC of the left or right SF amygdala were found between the MDDr and MDDnr groups.
In addition, the MDDnr group presented decreased rsFC between the right SF amygdala and the left STG compared with the HCnd group (Fig. 2 and Table 2).
Fig. 2.
Brain regions showing significant rsFC differences with the SF amygdala amongst the four groups. The left part shows the significantly different brain regions, and the right part shows the averaged rsFC of the four groups through bar graphs. Abbreviations: SF: superficial; IPL: inferior parietal lobule; STG: superior temporal gyrus. ** p < 0.01, *** p < 0.001.
3.2.3. rsFC of the BL amygdala
No significant differences in the rsFC of the left or right BL amygdala were found between any two groups.
3.2.4. rsFC of the entire amygdala
The HCd group showed decreased rsFC between the left amygdala and the left postcentral gyrus compared with the MDDr group. No other significant differences in the rsFC of the left or right amygdala were found between any two groups (Fig. 3 and Table 2).
Fig. 3.
Brain regions showing significant rsFC differences with the left amygdala amongst the four groups. The left part shows the significantly different brain regions, and the right part shows the averaged rsFC of the four groups through bar graphs. ** p < 0.01.
4. Discussion
In the current study, we provided novel information that the rsFC of amygdala subregions prior to the COVID-19 pandemic was associated with subsequent depressive state following the pandemic, suggesting the neural basis of individual differences in vulnerability to stress. In particular, we found that the HCd group exhibited decreased rsFC of an amygdala subregion compared with the HCnd group and decreased rsFC of the amygdala or its subregions compared with MDD patients. These findings support Hypothesis 1 and suggest that although these participants appeared healthy, as indicated by their clinical assessments before stress, they may have vulnerability to stress and thus develop depressive symptoms after being exposed to severe psychological stress, such as the COVID-19 pandemic. However, we did not find evidence to support Hypothesis 2 because no group differences in the rsFC of the amygdala and its subregions were found between the MDDr and MDDnr groups before stress. In addition, we observed abnormalities in the rsFC of amygdala subregions in the MDDnr group but not in the MDDr group compared with the HCnd group, providing evidence for the heterogeneity of patients with MDD.
4.1. rsFC of the amygdala or its subregions as a biomarker for vulnerability to depression
Consistent with our hypothesis, we found that the HCd group presented decreased rsFC between the left CM amygdala and the bilateral lingual gyrus compared with the HCnd group. In anatomy, the amygdala sends back projections to the lingual gyrus, which participates in visual processing, social cognition and processing and self-reference (Cheng et al., 2018; Lan et al., 2015). Abnormal connectivity between the amygdala and the lingual gyrus has been observed in patients with MDD (Cheng et al., 2018), although inconsistent findings have been reported. For example, a meta-analysis found that adult patients with MDD exhibited increased amygdala connectivity with the left lingual gyrus, but adolescent patients with MDD presented decreased amygdala connectivity with the right lingual gyrus compared with HCs (Tang et al., 2018). However, another rsFC analysis that was not included in the meta-analysis found reduced rsFC between the amygdala and the lingual gyrus in adult patients with MDD compared with HCs (Cheng et al., 2018). In our study, the HCd group presented similar changes in the rsFC of the amygdala and the lingual gyrus as those in a previous study (Cheng et al., 2018), suggesting that these participants may have a biological trait similar to patients with MDD. Our study also found that this biological trait is related to a specific subregion of the amygdala (i.e. the left CM amygdala), rather than the entire amygdala. Yuan et al. (2019) reported that PTSD+MDD patients demonstrated different CM amygdala rsFC compared with PTSD patients. This result is consistent with our finding that the depressive biological trait is related to the CM amygdala. However, few studies have reported abnormal rsFC between the CM amygdala and the lingual gyrus in patients with MDD or other mental disorders. A recent study that compared male combat veterans with and without impulsive aggression observed a significant difference in rsFC between the CM amygdala and the lingual gyrus (Varkevisser et al., 2017).
We also found that the HCd group exhibited decreased rsFC of the left CM or right SF amygdala with the precuneus compared with MDD patients (including the MDDr and MDDnr groups). The precuneus, which is located in the posteromedial portion of the parietal lobe, participates in self-centred mental imagery strategies and facilitates successful episodic memory retrieval (Cavanna and Trimble, 2006). Consistent with our findings, previous studies have reported that patients with MDD presented increased rsFC between the amygdala and the precuneus compared with HCs (Cullen et al., 2014; Tang et al., 2018). However, Ramasubbu et al. (2014) demonstrated reduced rsFC between the left amygdala and the precuneus in patients with MDD. Our finding suggests that the HCd group still differed from patients with MDD before stress. The impaired rsFC between the amygdala and the precuneus has been suggested to be related to rumination, a recursive affective response to negative events that is frequently observed in patients with MDD (Cooney et al., 2010). The differences in rsFC between the CM/SF amygdala and the precuneus between the HCd group and patients with MDD may be related to rumination. However, this speculation must be tested in the future.
We also found that the HCd group presented decreased rsFC between the amygdala or its subregions (the left CM and right SF) and the postcentral gyrus compared with the MDDr group before stress. The postcentral gyrus, a primary somatosensory cortex, participates in perceiving proprioception and emotional regulation (Kropf et al., 2019). Consistent with our findings, Yue et al. (2013) reported that late-onset depressed patients demonstrated increased rsFC between the left amygdala and the right postcentral gyrus compared with HCs. However, inconsistent findings have also been reported. For example, Cheng et al. (2018) observed decreased rsFC between the amygdala and the postcentral gyrus in patients with MDD compared with HCs. Our finding suggests that the HCd group differed from patients with MDD before stress, particularly the MDDr group. A previous study reported that stronger negative rsFC between the amygdala and the postcentral gyrus was associated with better emotional regulation (Pagliaccio et al., 2015). The differences in rsFC between the amygdala or its subregions (the left CM and right SF) and the postcentral gyrus between the HCd and MDDr groups may be related to different emotional regulation capabilities. This hypothesis must be verified in a future study.
In summary, these findings suggest that a proportion of healthy participants before stress may have specific rsFC of amygdala subregions that may be helpful in identifying the vulnerable biomarker for depression risk when exposed to severe stress, such as the COVID-19 pandemic, amongst healthy populations.
4.2. No evidence for the rsFC of the amygdala or its subregions as a biomarker for the resilience of patients with MDD to stress under antidepressant treatment
We did not find evidence to support that the rsFC of the amygdala or its subregions before stress in patients with MDD who experienced remission (i.e. the MDDr group) following the COVID-19 pandemic under antidepressant treatment was different from that in patients with MDD without remission (i.e. the MDDnr group). Thus, the biomarker for the resilience of patients with MDD to stress under antidepressant treatment could not be determined in the current study. We speculated that the rsFC of the amygdala or its subregions was a diathesis factor rather than a state factor in patients with MDD, and other biological indicators of stress resilience under antidepressant treatment may exist in patients with MDD. In a recent review, the author pointed out that hippocampal neurogenesis may be a promising biomarker of stress resilience in patients with MDD (Park, 2019); however, no antidepressant protection factors were mentioned. A future study must verify this speculation and focus on exploring the antidepressant protection mechanism on stress resilience.
In addition, we found that the MDDnr group exhibited increased rsFC between the right CM amygdala and the left IPL, but decreased rsFC between the right SF amygdala and the left STG compared with the HCnd group. However, no differences were found between the MDDr and HCnd groups. The IPL is the core component of the central executive network (Igelstrom and Graziano, 2017), which is associated with MDD (Che et al., 2020; Liang et al., 2013). The STG is a part of the temporal lobe region, and its abnormal rsFC is associated with anhedonia in MDD (Yang et al., 2017). A meta-analysis demonstrated that adult patients with MDD presented decreased rsFC between the amygdala and the STG (Tang et al., 2018). This finding is consistent with our result. However, in a repetitive transcranial magnetic stimulation (rTMS) study, the authors found that patients with MDD showed lower rsFC between the amygdala and the IPL compared with HCs before rTMS, but their rsFC increased after treatment (Chen et al., 2020). The inconsistency of these results may reflect the heterogeneity of patients with MDD. That is, the MDDr group is similar to the HCnd group, but the MDDnr group is dissimilar to the HCnd group.
The current study has three limitations. Firstly, the sample size was small, particularly in the HCd group. The small sample size may be one of the reasons why we could not find group differences in the rsFC of the BL amygdala, the abnormality of which has been frequently observed in previous MDD and PTSD studies (Liu et al., 2021; Tang et al., 2019). Moreover, the negative finding between the MDDr group and the MDDnr group may also be due to the small sample size. In the future, studies with a large sample size are necessary to validate and extend our current findings. Although this type of dataset, with MRI scans prior to stress exposure and depressive assessments during stress exposure, is opportunity what you cannot seek for, it is possible to explore pre-existing risk biomarkers prior to stress exposure by utilising available MRI cohorts or platforms. These cohorts or platforms include the UK Biobank Project (https://www.ukbiobank.ac.uk/), the Human Connectome Project (https://www.humanconnectome.org/), the Behavioral Brain Research Project of Chinese Personality and the Southwest University Longitudinal Imaging Multimodal Project (He et al., 2021) and so on, in which MRI images have been collected before the COVID-19 pandemic and thus can be rapidly deployed for future stress events, such as the COVID-19 pandemic (Holmes et al., 2020). Secondly, although we observed depressive symptoms in some of the pre-defined healthy controls (i.e. the HCd group) during the COVID-19 pandemic, we could not make a conclusion that there is a causal relationship between the COVID-19 pandemic and depression only based on the temporal sequence of the two events (appearance of depressive symptoms after the COVID-19 pandemic in pre-defined HCs). However, 53 amongst the 61 HCs also took part in a face-to-face clinical follow-up visit after 12 weeks of the baseline (from September 24, 2018 to January 15, 2020). The visit furthermore verified that these HCs were free of depression, indicated by a QIDS score ≤5, prior to the COVID-19 pandemic. Therefore, we think that the depressive symptoms in the HCs are most likely linked to the COVID-19 pandemic. Unfortunately, we could not clarify if the participants in the HCd group were clinically depressed patients because of the lack of definitive psychiatric diagnosis for healthy participants during the COVID-19 pandemic due to the implementation of epidemic prevention and control measures. Thirdly, we could not acquire neuroimaging data during the COVID-19 pandemic due to massive isolation measures. Such data can help us understand whether exposure to the COVID-19 stressor is associated with changes in the rsFC of the brain. Future longitudinal studies will be helpful in investigating vulnerable or resilient neural function changes when facing stressful events.
5. Conclusions
We used the rsFC of the amygdala and its subregions to predict vulnerability to depression for the first time following the COVID-19 pandemic, suggesting the neural basis of individual differences in vulnerability to stress. We found that the HCd group exhibited decreased rsFC between the left CM amygdala and the bilateral lingual gyrus compared with the HCnd group, and decreased rsFC of the left CM or right SF amygdala with the precuneus and the postcentral gyrus compared with the patient groups, suggesting a neural marker of vulnerability to depression following stress in previously healthy participants. No differences in the rsFC of the amygdala or its subregions were found between the two patient groups. Abnormalities in the rsFC of amygdala subregions were found in the MDDnr group but not in the MDDr group compared with the HCnd group. In summary, our findings suggested that the abnormal connectivity of amygdala subregions may represent a neurobiological marker of vulnerability to depression following the COVID-19 pandemic. This finding may have important implications for offering early mental health interventions to general populations.
CRediT authorship contribution statement
Shudong Zhang: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Jian Cui: Data curation, Conceptualization, Formal analysis. Zhifang Zhang: Conceptualization, Formal analysis, Supervision. Yun Wang: Supervision. Rui Liu: Supervision. Xiongying Chen: Supervision. Yuan Feng: Data curation. Jingjing Zhou: Data curation. Yuan Zhou: Conceptualization, Formal analysis, Supervision, Writing – review & editing. Gang Wang: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflicts of interest of any kind.
Acknowledgments
Acknowledgements
The authors gratefully acknowledge all the participants in this study and thank Yuening Jin and Yuwen He for their technical support.
Funding
This work was supported by the National Key Research & Development Program of China (2016YFC1307200); the National Natural Science Foundation of China (82071531, 81901372, 81901368 and 81771473); Capital's Funds for Health Improvement and Research (2020-4-2125) and Scientific Research Project of Beijing Educational Committee (KM202010025010).
References
- Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N.J., Habel U., Schneider F., Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. (Berl.) [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Brown V.M., LaBar K.S., Haswell C.C., Gold A.L., Mid-Atlantic M.W., McCarthy G., Morey R.A. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39:351–359. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D., Laird A.R., Zilles K., Fox P.T., Eickhoff S.B. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum. Brain Mapp. 2013;34:3247–3266. doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Che K., Mao N., Li Y., Liu M., Ma H., Bai W., Xu X., Dong J., Li Y., Shi Y., Xie H. Altered spontaneous neural activity in peripartum depression: a resting-state functional magnetic resonance imaging study. Front. Psychol. 2020;11:656. doi: 10.3389/fpsyg.2020.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.J., Gu C.Z., Zhai N., Duan H.F., Zhai A.L., Zhang X. Repetitive transcranial magnetic stimulation improves amygdale functional connectivity in major depressive disorder. Front. Psychiatry. 2020;11:732. doi: 10.3389/fpsyt.2020.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Rolls E.T., Qiu J., Xie X., Lyu W., Li Y., Huang C.C., Yang A.C., Tsai S.J., Lyu F., Zhuang K., Lin C.P., Xie P., Feng J. Functional connectivity of the human amygdala in health and in depression. Soc. Cogn. Affect. Neurosci. 2018;13:557–568. doi: 10.1093/scan/nsy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., James G.A., Tripathi S., Mletzko T., Heim C., Hu X.P., Mayberg H.S., Nemeroff C.B., Kilts C.D. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol. Med. 2013;43:507–518. doi: 10.1017/S0033291712001390. [DOI] [PubMed] [Google Scholar]
- Cooney R.E., Joormann J., Eugene F., Dennis E.L., Gotlib I.H. Neural correlates of rumination in depression. Cogn. Affect. Behav. Neurosci. 2010;10:470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Westlund M.K., Klimes-Dougan B., Mueller B.A., Houri A., Eberly L.E., Lim K.O. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 2014;71:1138–1147. doi: 10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Kringelbach M.L. Great expectations: using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron. 2014;84:892–905. doi: 10.1016/j.neuron.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Faye C., McGowan J.C., Denny C.A., David D.J. Neurobiological mechanisms of stress resilience and implications for the aged population. Curr. Neuropharmacol. 2018;16:234–270. doi: 10.2174/1570159X15666170818095105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- He L., Wei D., Yang F., Zhang J., Cheng W., Feng J., Yang W., Zhuang K., Chen Q., Ren Z., Li Y., Wang X., Mao Y., Chen Z., Liao M., Cui H., Li C., He Q., Lei X., Feng T., Chen H., Xie P., Rolls E.T., Su L., Li L., Qiu J. Functional connectome prediction of anxiety related to the COVID-19 pandemic. Am. J. Psychiatry. 2021;178:530–540. doi: 10.1176/appi.ajp.2020.20070979. [DOI] [PubMed] [Google Scholar]
- Hegde A., Mitra R. Environment and early life: decisive factors for stress-resilience and vulnerability. Stress Brain Health Across Life Course. 2020:155–185. doi: 10.1016/bs.irn.2019.12.002. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., O'Connor R.C., Perry V.H., Tracey I., Wessely S., Arseneault L., Ballard C., Christensen H., Cohen Silver R., Everall I., Ford T., John A., Kabir T., King K., Madan I., Michie S., Przybylski A.K., Shafran R., Sweeney A., Worthman C.M., Yardley L., Cowan K., Cope C., Hotopf M., Bullmore E. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7:547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelstrom K.M., Graziano M.S.A. The inferior parietal lobule and temporoparietal junction: a network perspective. Neuropsychologia. 2017;105:70–83. doi: 10.1016/j.neuropsychologia.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Clegg R., Goer F., Pechtel P., Beltzer M., Vitaliano G., Olson D.P., Teicher M.H., Pizzagalli D.A. Childhood stress, grown-up brain networks: corticolimbic correlates of threat-related early life stress and adult stress response. Psychol. Med. 2018;48:1157–1166. doi: 10.1017/S0033291717002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L., Li Y., Hu S., Chen M., Yang C., Yang B.X., Wang Y., Hu J., Lai J., Ma X., Chen J., Guan L., Wang G., Ma H., Liu Z. The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus. Lancet Psychiatry. 2020;7:e14. doi: 10.1016/S2215-0366(20)30047-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerestes R., Chase H.W., Phillips M.L., Ladouceur C.D., Eickhoff S.B. Multimodal evaluation of the amygdala's functional connectivity. Neuroimage. 2017;148:219–229. doi: 10.1016/j.neuroimage.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf E., Syan S.K., Minuzzi L., Frey B.N. From anatomy to function: the role of the somatosensory cortex in emotional regulation. Braz. J. Psychiatry. 2019;41:261–269. doi: 10.1590/1516-4446-2018-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan C.C., Tsai S.J., Huang C.C., Wang Y.H., Chen T.R., Yeh H.L., Liu M.E., Lin C.P., Yang A.C. Functional connectivity density mapping of depressive symptoms and loneliness in non-demented elderly male. Front. Aging Neurosci. 2015;7:251. doi: 10.3389/fnagi.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver A.M., Yang H., Siddarth P., Vlasova R.M., Krause B., St Cyr N., Narr K.L., Lavretsky H. Resilience and amygdala function in older healthy and depressed adults. J. Affect. Disord. 2018;237:27–34. doi: 10.1016/j.jad.2018.04.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr. Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Liang M.J., Zhou Q., Yang K.R., Yang X.L., Fang J., Chen W.L., Huang Z. Identify changes of brain regional homogeneity in bipolar disorder and unipolar depression using resting-state FMRI. PLoS ONE. 2013;8:e79999. doi: 10.1371/journal.pone.0079999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Xiang Y.T., Lei H., Wang Q., Wang G., Ungvari G.S., Morris D.W., Zhu X.Z., Lai K.Y., Zhong B.L., Wong S.Y., Zhang L., Zhang Q., Zou Y.C., Xiao L., Zhao Q., Li Y., Wu J., Zhang G.F., Chiu H.F. Guidance on the conversion of the Chinese versions of the quick inventory of depressive symptomatology-self-report (C-QIDS-SR) and the Montgomery-Asberg Scale (C-MADRS) in Chinese patients with major depression. J. Affect. Disord. 2014;152-154:530–533. doi: 10.1016/j.jad.2013.09.023. [DOI] [PubMed] [Google Scholar]
- Liu J., Xiang Y.T., Wang G., Zhu X.Z., Ungvari G.S., Kilbourne A.M., Lai K.Y., Zhong B.L., Zhang L., Zhang Q., Zou Y.C., Xiao L., Zhao Q., Li Y., Wu J., Zhang G.F., Chiu H.F. Psychometric properties of the Chinese versions of the quick inventory of depressive symptomatology - clinician rating (C-QIDS-C) and self-report (C-QIDS-SR) J. Affect. Disord. 2013;147:421–424. doi: 10.1016/j.jad.2012.08.035. [DOI] [PubMed] [Google Scholar]
- Liu T., Ke J., Qi R., Zhang L., Zhang Z., Xu Q., Zhong Y., Lu G., Chen F. Altered functional connectivity of the amygdala and its subregions in typhoon-related post-traumatic stress disorder. Brain Behav. 2021;11:e01952. doi: 10.1002/brb3.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio C., Probert T., Jones E., Young A.H., Robbins I. Adapting to stress: understanding the neurobiology of resilience. Behav. Med. 2017;43:307–322. doi: 10.1080/08964289.2016.1170661. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D., Luby J.L., Bogdan R., Agrawal A., Gaffrey M.S., Belden A.C., Botteron K.N., Harms M.P., Barch D.M. Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. J. Abnorm. Psychol. 2015;124:817–833. doi: 10.1037/abn0000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.C. Neurogenesis and antidepressant action. Cell Tissue Res. 2019;377:95–106. doi: 10.1007/s00441-019-03043-5. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L. Comparative aspects of amygdala connectivity. Ann. N. Y. Acad. Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Ramasubbu R., Konduru N., Cortese F., Bray S., Gaxiola-Valdez I., Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front. Psychiatry. 2014;5:17. doi: 10.3389/fpsyt.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly A.M., Uddin L.Q., Gotimer K., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P., Faber E.S., Lopez De Armentia M., Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B., Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Garfinkel S.N., Wang X., Sripada C.S., Welsh R.C., Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J. Psychiatry Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Li H., Lu L., Wang Y., Zhang L., Hu X., Bu X., Hu X., Gao Y., Gong Q., Huang X. Anomalous functional connectivity of amygdala subregional networks in major depressive disorder. Depress. Anxiety. 2019;36:712–722. doi: 10.1002/da.22901. [DOI] [PubMed] [Google Scholar]
- Tang S., Lu L., Zhang L., Hu X., Bu X., Li H., Hu X., Gao Y., Zeng Z., Gong Q., Huang X. Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: a comparative meta-analysis. EBioMedicine. 2018;36:436–445. doi: 10.1016/j.ebiom.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Marusak H.A., Tocco M.A., Vila A.M., McGarragle O., Rosenberg D.R. Altered amygdala connectivity in urban youth exposed to trauma. Soc. Cogn. Affect. Neurosci. 2015;10:1460–1468. doi: 10.1093/scan/nsv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkevisser T., Gladwin T.E., Heesink L., van Honk J., Geuze E. Resting-state functional connectivity in combat veterans suffering from impulsive aggression. Soc. Cogn. Affect. Neurosci. 2017;12:1881–1889. doi: 10.1093/scan/nsx113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkers C.H., van Amelsvoort T., Bisson J.I., Branchi I., Cryan J.F., Domschke K., Howes O.D., Manchia M., Pinto L., de Quervain D., Schmidt M.V., van der Wee N.J.A. Stress resilience during the coronavirus pandemic. Eur. Neuropsychopharmacol. 2020;35:12–16. doi: 10.1016/j.euroneuro.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Chen J., Boyd J.E., Zhang H., Jia X., Qiu J., Xiao Z. Psychometric properties of the Chinese version of the perceived stress scale in policewomen. PLoS ONE. 2011;6:e28610. doi: 10.1371/journal.pone.0028610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Jia X., Shi H., Niu J., Yin X., Xie J., Wang X. Prevalence of mental health problems during the COVID-19 pandemic: a systematic review and meta-analysis. J. Affect. Disord. 2021;281:91–98. doi: 10.1016/j.jad.2020.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y.T., Yang Y., Li W., Zhang L., Zhang Q., Cheung T., Ng C.H. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7:228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y.T., Jin Y., Cheung T. Joint international collaboration to combat mental health challenges during the coronavirus disease 2019 pandemic. JAMA Psychiatry. 2020;77:989–990. doi: 10.1001/jamapsychiatry.2020.1057. [DOI] [PubMed] [Google Scholar]
- Xiang Y.T., Zhao Y.J., Liu Z.H., Li X.H., Zhao N., Cheung T., Ng C.H. The COVID-19 outbreak and psychiatric hospitals in China: managing challenges through mental health service reform. Int. J. Biol. Sci. 2020;16:1741–1744. doi: 10.7150/ijbs.45072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Toki S., Siegle G.J., Takamura M., Takaishi Y., Yoshimura S., Okada G., Matsumoto T., Nakao T., Muranaka H., Kaseda Y., Murakami T., Okamoto Y., Yamawaki S. Increased amygdala reactivity following early life stress: a potential resilience enhancer role. BMC Psychiatry. 2017;17:27. doi: 10.1186/s12888-017-1201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., Colcombe S., Craddock R.C., Di Martino A., Li Q., Zuo X.N., Castellanos F.X., Milham M.P. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Wang X.D., Zuo X.N., Zang Y.F. DPABI: data processing & analysis for (Resting-State) brain imaging. Neuroinformatics. 2016;14:339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Yang X.H., Tian K., Wang D.F., Wang Y., Cheung E.F.C., Xie G.R., Chan R.C.K. Anhedonia correlates with abnormal functional connectivity of the superior temporal gyrus and the caudate nucleus in patients with first-episode drug-naive major depressive disorder. J. Affect. Disord. 2017;218:284–290. doi: 10.1016/j.jad.2017.04.053. [DOI] [PubMed] [Google Scholar]
- Yuan M., Pantazatos S.P., Zhu H., Li Y., Miller J.M., Rubin-Falcone H., Zanderigo F., Ren Z., Yuan C., Lui S., Gong Q., Qiu C., Zhang W., John Mann J. Altered amygdala subregion-related circuits in treatment-naive post-traumatic stress disorder comorbid with major depressive disorder. Eur. Neuropsychopharmacol. 2019;29:1092–1101. doi: 10.1016/j.euroneuro.2019.07.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., Yuan Y., Hou Z., Jiang W., Bai F., Zhang Z. Abnormal functional connectivity of amygdala in late-onset depression was associated with cognitive deficits. PLoS ONE. 2013;8:e75058. doi: 10.1371/journal.pone.0075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang J., Wang L., Li R., Zhang W. Altered resting-state functional connectivity of the amygdala in Chinese earthquake survivors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;65:208–214. doi: 10.1016/j.pnpbp.2015.10.003. [DOI] [PubMed] [Google Scholar]